Introduction
Hepatocellular carcinoma (HCC) is one of the major
health problems worldwide, ranking as the third leading cause of
cancer-related mortality globally and the second in China (1). High hepatitis B virus (HBV) load and
chronic hepatitis B infection increase the risk of developing HCC
(2), while high HBV viral load is
also associated with recurrence for patients with hepatitis B
virus-related HCC (HBV-related HCC) although the best therapeutic
choices are early-stage tumors and preserved hepatic function,
liver resection and liver transplantation (3,4).
Therefore, drug with both antitumor and anti-HBV effect will be
important for patients with HBV-related HCC.
Natural products have played pivotal roles in the
drug discovery and development process. This is particularly
evident in the field of cancer therapeutics, where >50% of the
approved drugs introduced from 1981 to 2002 were of natural origin
(5). It has been noted that natural
products may embody more ‘privileged structures’ than purely
synthetic chemical libraries (6)
and they are a rich resource of new chemical motifs. Therefore, the
natural product-based drug discovery program remains an important
avenue toward the development of small-molecule therapeutics for
cancer as well as other diseases (7). Pseudolaric acid B (PAB) is a novel
diterpene acid isolated from the root bark of Pseudolarix
kaempferi Gordon, known as ‘Tu-Jin-Pi’ Chinese herb, which has
been safely used for centuries in traditional Chinese medicine for
treating skin inflammation (8). PAB
has antitumor effect through different mechanism in a number of
cancer cell lines (8–13) which might be related to a unique
polyhydroazulene with a trans substitution pattern at the
junction sites which has not been found in any other natural
products (14), however, it is
still not known whether PAB possesses antiviral ability, especially
anti-HBV.
In the present study, we confirmed that PAB had a
new effect of inhibiting secretion of HBV and PAB was a candidate
drug for anti-HBV and to treat HBV-related HCC.
Materials and methods
Materials
PAB, purchased from the National Institute for the
Control of Pharmaceutical and Biological Products (Beijing, China),
was dissolved in dimethyl sulfoxide (DMSO) to make a stock
solution. DMSO concentration was kept below 0.01% in all the cell
cultures, and did not exert any detectable effect on cell growth or
cell death. Propidium iodode (PI), Hoechst 33258, RNase A and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma Chemical (St. Louis, MO, USA).
Pro-caspase 3 antibody (SC-65497) and secondary antibodies (goat
anti-rabbit or mouse) were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Rabbit Histone H3 antibody (A01502-40) was
from GenScript Corp. (Piscataway, NJ, USA). Diagnostic kit for
hepatitis B virus surface antigen (ELISA) was from Shanghai Kehua
Bio-Engineering, Co., Ltd. (Shanghai, China).
Cell culture
Human carcinoma HepG2 and HepG2215 were obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and the cells were cultured in Dulbeccos modified Eagles medium
(DMEM; HyClone Laboratories, Logan, UT, USA) supplemented with 10%
fetal calf serum (FBS; Gibco, Grand Island, NY, USA), and
maintained at 37°C with 5% CO2 in a humidified
atmosphere.
Plasmid transfection and drug
treatment
The pCMV ayw HBV proviral construct was previously
described (15). Following the
manufacturers protocol of Lipofectamine 2000, we transfect 1 µg of
plasmid together with 3 µl of Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) into cells in a 12-well plate, 12 h later, 4 µM
PAB were treated for 12 h, then the supernatant or cells were
collected for HBV surface antigen (HBsAg) detection.
HBsAg detection by ELISA
After 4 µM PAB treatment for 12 h, the cultured
medium was examined for HBV surface antigen (HBsAg) with ELISA kits
(Shanghai Kehua Bio-Engineering). The samples (50 µl/well) were
incubated in the 96-well microplate at 37°C for 1 h, followed by 50
µl horseradish peroxidase-conjugated primary antibodies for 30 min,
and then 50 µl substrate for 10 min and then adding 50 µl
termination buffer to end the reaction. HBsAg in supernatants and
cells are shown in the figures.
Flow cytometric analysis of cell
cycle
HepG2215 cells or HepG2 cells (1.0×106)
were harvested and rinsed with phosphate-buffered saline (PBS). The
cell pellets were fixed in 70% ethanol at 4°C overnight. After
washing twice with PBS, the cells were stained with 1.0 ml of PI
solution containing PI 50 mg/l, RNase A 1 g/l, and 0.1% Triton
X-100 in sodium citrate 3.8 mM, followed by incubation on ice in
the dark condition for 30 min. The samples were analyzed by a
FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ,
USA).
Observation of morphologic changes by
light microscopy
HepG2215 cells (5×105 cells/well) were
cultured in 6-wells plate for 24 h. Then, 4 µM PAB were treated for
0, 6, 12, 24 and 36 h, morphologic changes were observed by phase
contrast microscopy (Leica Biosystems GmbH, Nusslich, Germany).
Observation of nuclear morphologic
changes
HepG2215 cells (5×105) were placed on the
coverslips in a 6-well plate. After 24 h of cell culture, they were
treated with 4 µM PAB for 0, 6, 12, 24 and 36 h, then were washed
by PBS, fixed in 3.7% formaldehyde for 1 h, then were stained with
5 mg/l Hoechst 33258 for 30 min. Nuclear changes were observed by
fluorescence microscopy at excitation wavelength 350 nm with
emission filter 460 nm (Leica Biosystems GmbH).
Determination of DNA fragmentation by
agarose gel electrophoresis
Cells were trypsinized after PAB treatment for 0, 6,
12, 24 and 36 h, and both adherent and floating cells were
collected by centrifugation at 1,000 × g for 5 min. It was done
according to the protocol (12).
Western blot analysis of protein
expression
Cells (1×106) were cultured in 25-ml
culture bottle, and then were treated with 4 µM PAB for 0, 6, 12,
24 and 36 h. Both adherent and floating cells were collected and
frozen at −80°C. Western blot analysis was performed for total
proteins as follows (12).
Cell growth inhibition test
The inhibition of cell growth was determined by MTT
test. HepG2 and HepG2215 cells (1.0×104 cells/well) were
seeded into 96-well culture plates (Nunc A/S, Roskilde, Denmark).
After 24 h of incubation, different concentration of PAB was added
to the plates. Following incubation, cell growth was measured at
different time-points by addition of 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
5 mg/ml) at 37°C for 3 h, and DMSO (150 µl) was added to dissolve
the formazan crystals. Absorbance was measured at 492 nm with
enzyme-linked immunosorbent assay plate reader (Bio-Rad
Laboratories, Hercules, CA, USA). The percentage of inhibition was
calculated as follows: Inhibitory ratio (%) =
[A492(control) - A492
(sample)]/[A492(control) - A492(blank)] ×
100%.
Statistical analysis
All data represent at least three independent
experiments, and are expressed as mean ± SD. Statistical
comparisons were made using the Students t-test. P-values of
<0.001 were considered to represent a statistically significant
difference.
Results
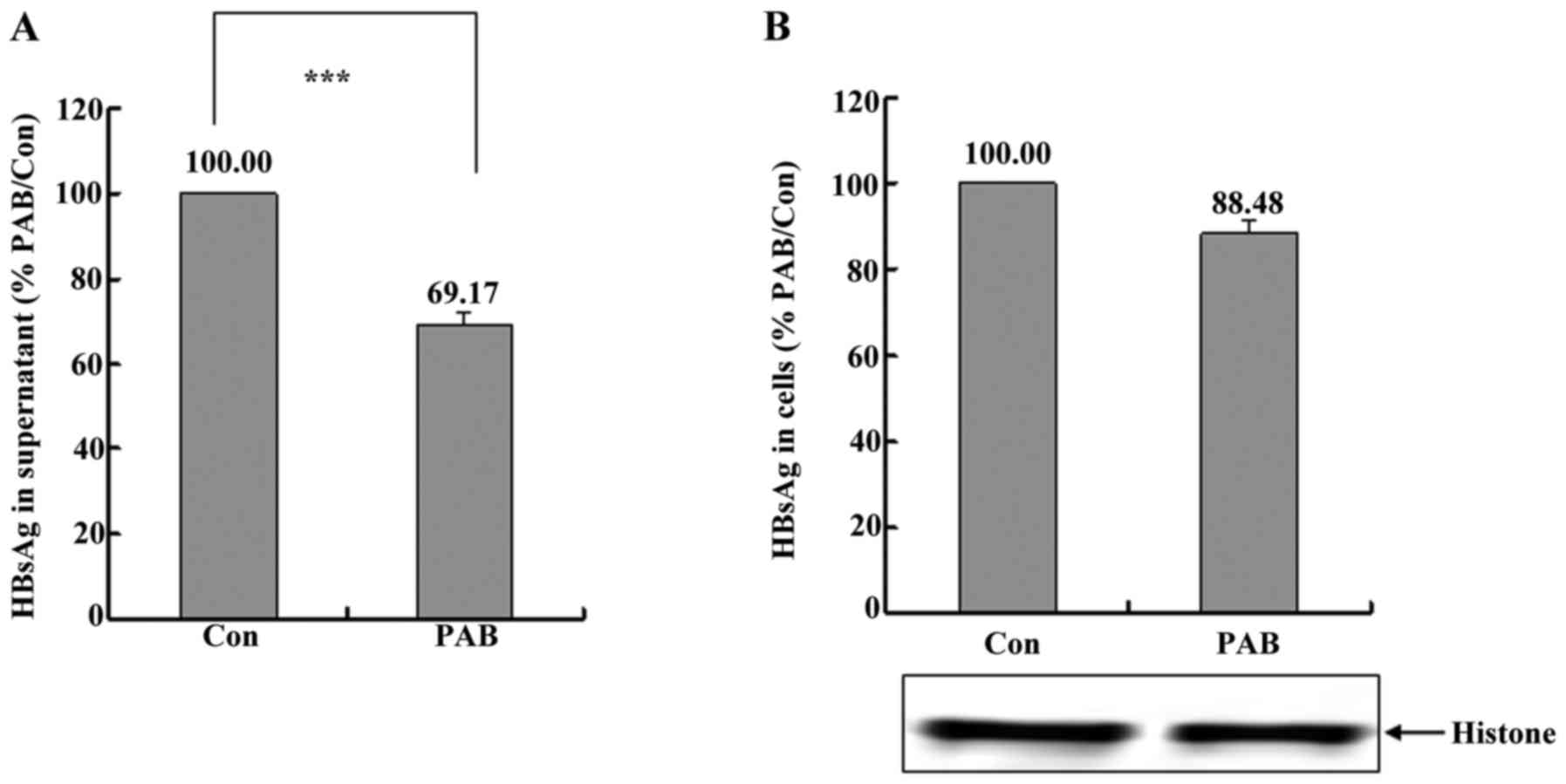
PAB inhibited the secretion of HBV in
HepG2215 cell line
HBV secretion was related to ocurrence and
recurrence of hepatocellular carcinoma (3,4), so
the present study detected the secretion of HBV after PAB
treatment. Firstly, it was found that at 12 h PAB obviously
decreased the level of HBV in supernatant through detecting HBsAg,
and supernatant HBsAg after PAB treatment was 69.17±2.81% of
control group (Fig. 1A). Meanwhile
it was also found that intracellular HBsAg was not affected
obviously after PAB treatment compared to control group on
condition that PAB group had the same cell number with control
group (Fig. 1B). Therefore, PAB
inhibited the secretion of HBV in HepG 2215 cell line.
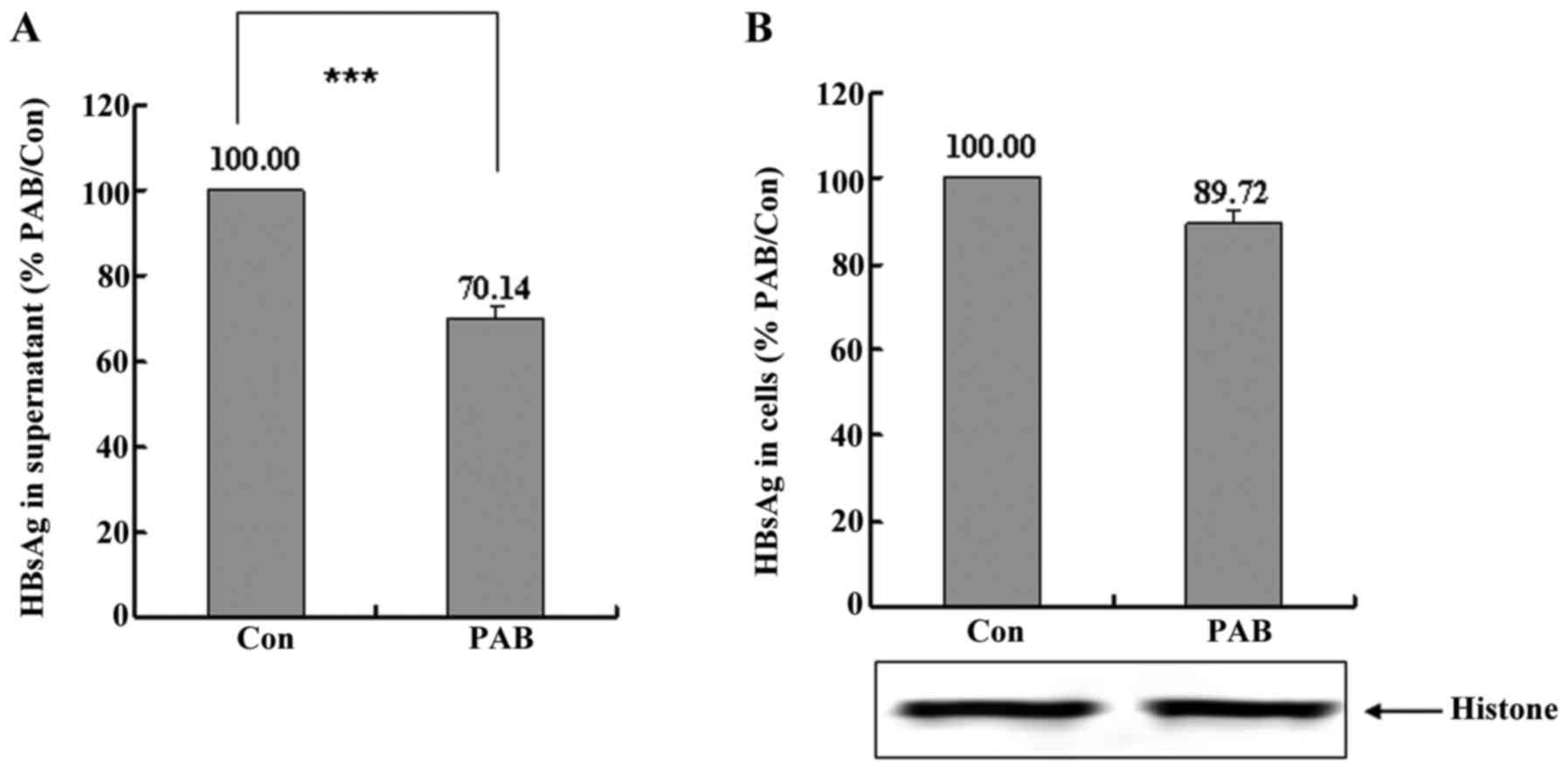
PAB inhibits the secretion of HBV in
HepG2 cell line transfected HBV gene
For further confirming the effect of PAB on HBV
secretion, we transfected HBV plasmid into HepG2 cells, and found
that PAB also inhibited HBV secretion in transfect HepG2 cells, and
supernatant HBsAg after PAB treatment was 70.14±2.84% of control
group (Fig. 2A), and the inhibitory
effect of PAB on intracellular HBsAg was not observed compared to
control group as the PAB group had the same cell number than the
control group (Fig. 2B). Therefore,
PAB inhibited the secretion of HBV in HepG2 cell transfected HBV
gene.
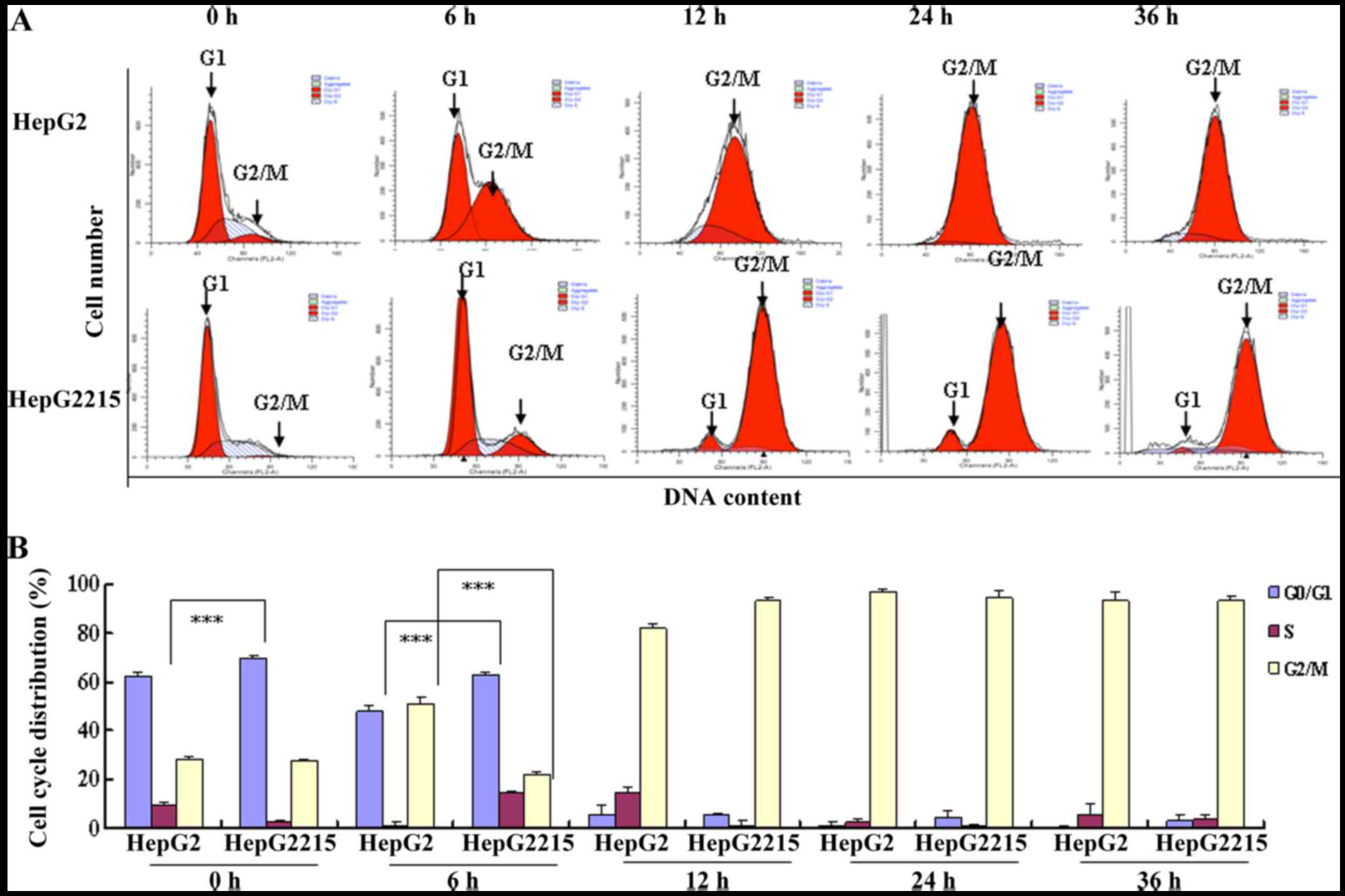
PAB induces G2/M arrest of HepG2 and
HepG2215 cells
The drug affecting cell cycle status favored by
virus inhibited viral production (16), so we detected cell cycle
distribution after PAB treatment. After 4 µM PAB treatment for 6,
12, 24 and 36 h, the DNA amount was obviously doubled compared with
the control group in both HepG2 and HepG2215 cells, indicating the
PAB-treated cells were arrested at the G2/M phase (Fig. 3). It was observed that more HepG2215
cells (69.66±0.94%) arrested in G0/G1 phase than HepG2 cells
(62.3±1.98%) in normal situation. In addition, at 6 h of PAB
treatment, G0/G1 ratio of HepG2 and HepG2215 was 47.75±2.52 and
63.2±1.12%, respectively, while G2/M ratio of HepG2 and HepG2215
was 50.79±2.36 and 22.16±1.30%, respectively (Fig. 3B), indicating that HBV infection
induced G0/G1 arrest, and G0/G1 arrest-induced by HBV retarded the
entry of G2/M-induced by PAB. Therefore, PAB might affect cell
cycle status favored by HBV to inhibit HBV secretion.
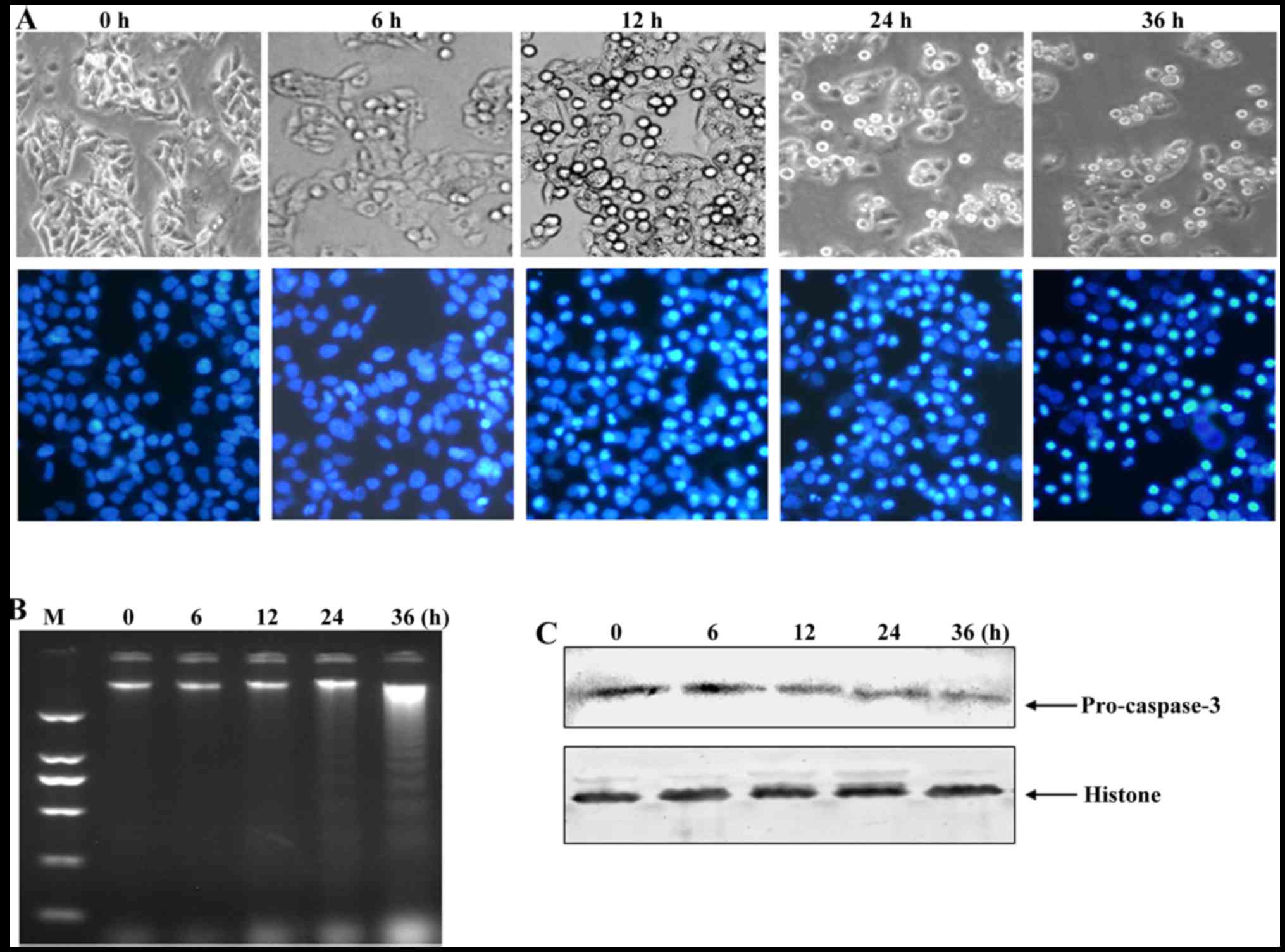
PAB induces apoptosis of HepG2215
cells
Cell apoptosis help to kill host cells of HBV
(12), and macrophages to engulf
apoptotic bodies (17) with HBV,
thus, we detected the occurrence of apoptosis after PAB treatment.
After 4 µM PAB treatment for 12 h, the number of floating cells was
increased (upper panel of Fig. 4A)
and the number of cells with bright blue condensed nuclei (low
panel of Fig. 4A) were increased in
HepG2215 cells. In addition, at 24 and 36 h after PAB treatment,
there were obvious DNA ladder in agarose gel electrophoresis
analysis of HepG2215 cells (Fig.
4B). The expression of procaspase-3 was decreased after PAB
treatment in HepG2215 cells (Fig.
4C). Therefore, PAB induced apoptosis of HepG2215 cells.
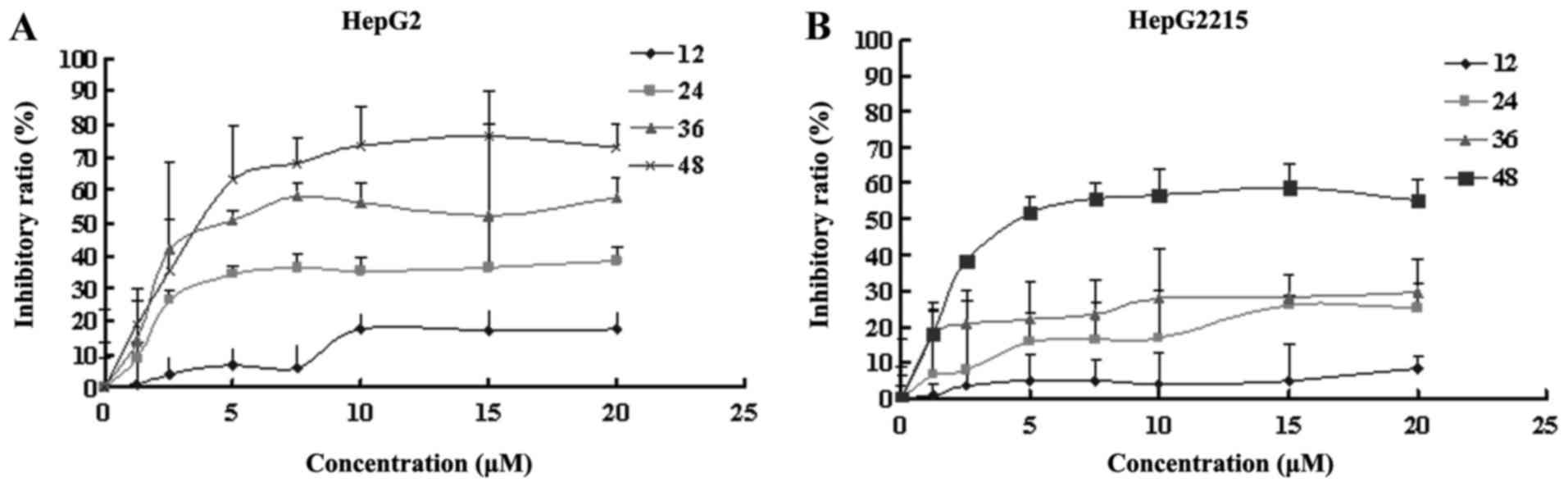
The inhibitory effect of PAB on HepG2
and HepG2215 growth
To detect the growth inhibition of PAB-exposed HepG2
and HepG2215 cells to confirm the effect of HBV on the drug
sensitivity, the cells were treated with various doses of PAB, from
0.4 to 20 µM for 12, 24, 36 and 48 h. PAB showed potent suppressive
effect on HepG2 (Fig. 5A) and
HepG2215 (Fig. 5B) cells, and the
IC50 values of PAB in HepG2 and HepG2215 cells at 36 h
were 8.58 and 103.44 µM, at 48 h were 4.31 and 8.06 µM,
respectively. Therefore, the cytotoxicity of PAB on HepG2 and
HepG2215 cells was increased with the increased dose and time, and
the inhibitory ratio of PAB on HepG2 was more obvious than HepG2215
(Fig. 6).
Discussion
Hepatocellular carcinoma (HCC) is a serious
malignancy, and frequently found in refractory cancers in China,
chronic infections of HBV could aid the development and recurrence
of HCC (3,4), and antivirus therapy would prevent
development and recurrence of HBV-related HCC. It was reported that
pseudolaric acid B (PAB) possessed selective anti-proliferative
effect in human cancer cells but not in normal cells in
vitro and in vivo (18,19),
therefore, we investigated the effect of PAB on HBV secretion for
HBV-related HCC treatment.
In the present study, HepG2215 cell line was used as
a model to study HBV-related HCC because HepG2215 was stably
transfected in the HBV genome into HepG2 cells (20), which is a widely used cell line in
the study of the life cycle of HBV and antiviral research (21–23).
In this study, PAB inhibited HBV secretion, but did not decrease
the level of intracellular HBV in HepG2215, indicating that PAB had
the ability of an anti-virus through inhibiting the HBV secretion.
Furthermore, to confirm the effect of PAB on HBV secretion, we
transfected HBV gene into HepG2 cell line, and we obtained similar
results as in HepG2215, namely PAB inhibited HBV secretion.
Therefore, it was concluded that PAB could inhibit HBV
secretion.
As part of their pathogenic mechanism, many viruses
facilitate their own growth by interacting with factors that
regulate the host cell cycle. Examples can be found among DNA
viruses, retroviruses and RNA viruses. For the DNA viruses, for
example, some small DNA viruses such as simian virus 40 (24), adenovirus (25,26),
and human papillomavirus (27),
which lack their own polymerases, for using host polymerase promote
the entrance of cells into the S phase from the G1 phase. Other
large DNA viruses, such as herpesviruses, can induce cell cycle
arrest in the G0/G1 phase to avoid competition for cellular DNA
replication resources (28).
Besides the DNA viruses, cell cycle regulation has been observed
for retroviruses (29,30). Furthermore, RNA viruses, for example
infectious bronchitis virus (IBV) induces an S and G2/M-phase
arrest to favor viral replication (31,32).
Then, we analyzed the inhibitory mechanism of PAB on HBV secretion
on the cell cycle, it was found that more HepG2215 cells were in
G0/G1 phase than HepG2 from the analysis of the cell cycle,
indicating that HBV induced G0/G1 arrest, which was consistent with
a previous report (33). As through
regulating the host cell cycle, virus replication could be affected
(16), we analyzed whether PAB
affected the status of cell cycle favored by HBV. In addition, it
was confirmed that PAB induced G2/M arrest in HepG2215 cells, which
proved that PAB changed the cell cycle status favored by HBV. It
was noted that at 6 h after PAB treatment, more HepG2 entered G2/M
cell cycle than HepG2215, namely HepG2215 still stayed in G0/G1,
indicating HBV struggled to let cells stay in G0/G1 phase after PAB
treatment. Therefore, cell cycle arrest was a mechanism to inhibit
HBV secretion.
Apoptosis is a mechanism to kill cancer cells,
apoptotic cells were fragmented into apoptotic bodies which still
had integrated membrane, and would be engulfed by macrophagy cells.
We found that after PAB treatment, chromatin condensation,
condensed cell floating, chromosomal DNA fragmentation and
procaspase-3 cleavage, all of which are apoptotic markers appeared
in HepG2215 cells. It was speculated that HBV-related HCC cell
apoptosis would be in favor of viral elimination because HBV virus
was packaged in apoptotic bodies which was engulfed directly by
immune cells, and HBV-related HCC cell apoptosis kill the host of
HBV to eradicate HBV.
In addition, the present study found that PAB
exerted potent inhibitory effect on HCC HepG2 cells and HBV-related
HCC HepG2215 cells, and PAB had stronger inhibitory ability on
HepG2 than HepG2215. Therefore, it is speculated that HBV infection
endowed cancer cells drug tolerance to some extent.
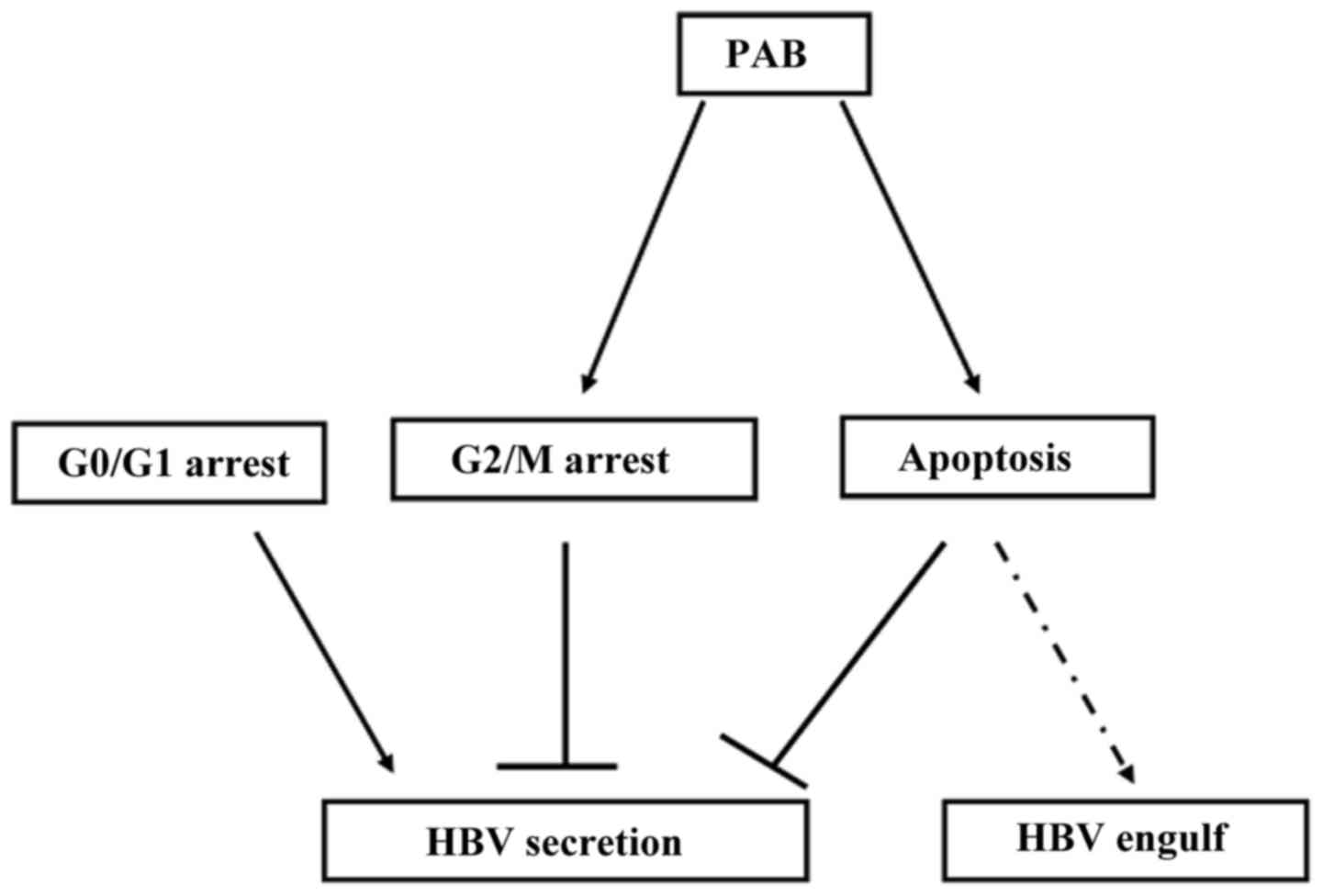
The detailed mechanism of PAB of anti-HBV is still
not clear, but it was speculated that: i) PAB as a depolymerization
of tubulin drug inhibited the polymerization of tubulin (34) which was important for HBV production
and secretion; ii) PAB induced G2/M cell cycle arrest, but G0/G1
arrest was required by HBV secretion; and iii) PAB induced
apoptosis which could damage some important proteins, organelles,
or others that were required for secretion and thus apoptosis would
be in favor of engulfing the virus by macrophages.
Uncovering the phenomenon of PAB inhibiting HBV
secretion might lead to its use as an anticancer treatment of
HBV-related HCC.
Acknowledgements
The present study was supported by funding from the
National Natural Science Foundation of China (81301416), the
Postdoctoral Science Foundation of China (2014M561302, 2015T80299),
the Norman Bethune Program of Jilin University (2015202), the Jilin
Provincial Science and Technology Department (20140204004YY and
20160414025GH), and the Department of Human Resources and Social
Security of Jilin Province (2016014).
References
|
1
|
Hadziyannis SJ, Tassopoulos NC, Heathcote
EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z,
Ma J, et al: Adefovir Dipivoxil 438 Study Group: Long-term therapy
with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for
up to 5 years. Gastroenterology. 131:1743–1751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sangiovanni A, Del Ninno E, Fasani P, De
Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R and Colombo
M: Increased survival of cirrhotic patients with a hepatocellular
carcinoma detected during surveillance. Gastroenterology.
126:1005–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sherman M: Recurrence of hepatocellular
carcinoma. N Engl J Med. 359:2045–2047. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman DJ, Cragg GM and Snader KM: Natural
products as sources of new drugs over the period 1981–2002. J Nat
Prod. 66:1022–1037. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Breinbauer R, Vetter IR and Waldmann H:
From protein domains to drug candidates-natural products as guiding
principles in the design and synthesis of compound libraries. Angew
Chem Int Ed Engl. 41:2879–2890. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou BN, Ying BP, Song GQ, Chen ZX, Han J
and Yan YF: Pseudolaric acids from Pseudolarix kaempferi. Planta
Med. 47:35–38. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong X, Wang M, Tashiro S, Onodera S and
Ikejima T: Involvement of JNK-initiated p53 accumulation and
phosphorylation of p53 in pseudolaric acid B induced cell death.
Exp Mol Med. 38:428–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong XF, Wang MW, Tashiro S, Onodera S and
Ikejima T: Pseudolaric acid B induces apoptosis through p53 and
Bax/Bcl-2 pathways in human melanoma A375-S2 cells. Arch Pharm Res.
28:68–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Li X, Tashiro S, Onodera S and
Ikejima T: Bcl-2 family proteins were involved in pseudolaric acid
B-induced autophagy in murine fibrosarcoma L929 cells. J Pharmacol
Sci. 107:295–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu JH, Cui Q, Jiang YY, Yang W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces apoptosis,
senescence, and mitotic arrest in human breast cancer MCF-7. Acta
Pharmacol Sin. 28:1975–1983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu JH, Wang HJ, Li XR, Tashiro S, Onodera
S and Ikejima T: Protein tyrosine kinase, JNK, and ERK involvement
in pseudolaric acid B-induced apoptosis of human breast cancer
MCF-7 cells. Acta Pharmacol Sin. 29:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma G, Chong L, Li XC, Khan IA, Walker LA
and Khan SI: Selective inhibition of human leukemia cell growth and
induction of cell cycle arrest and apoptosis by pseudolaric acid B.
J Cancer Res Clin Oncol. 136:1333–1340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu R, Zhang X, Zhang W, Fang Y, Zheng S
and Yu XF: Association of human APOBEC3 cytidine deaminases with
the generation of hepatitis virus B × antigen mutants and
hepatocellular carcinoma. Hepatology. 46:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Zhang L, Ren P, Zhong T, Li Z, Wang
Z, Li J, Liu X, Zhao K, Zhang W, et al: Enterovirus 71 mediates
cell cycle arrest in S phase through non-structural protein 3D.
Cell Cycle. 14:425–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sciorati C, Rigamonti E, Manfredi AA and
Rovere-Querini P: Cell death, clearance and immunity in the
skeletal muscle. Cell Death Differ. 23:927–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan M, Zheng B, Yi F, Rasul A, Gu Z, Li
T, Gao H, Qazi JI, Yang H and Ma T: Pseudolaric Acid B induces
caspase-dependent and caspase-independent apoptosis in u87
glioblastoma cells. Evid Based Complement Alternat Med.
2012:9575682012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong VK, Chiu P, Chung SS, Chow LM, Zhao
YZ, Yang BB and Ko BC: Pseudolaric acid B, a novel
microtubule-destabilizing agent that circumvents multidrug
resistance phenotype and exhibits antitumor activity in vivo. Clin
Cancer Res. 11:6002–6011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sells MA, Chen ML and Acs G: Production of
hepatitis B virus particles in Hep G2 cells transfected with cloned
hepatitis B virus DNA. Proc Natl Acad Sci USA. 84:1005–1009. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding XR, Yang J, Sun DC, Lou SK and Wang
SQ: Whole genome expression profiling of hepatitis B
virus-transfected cell line reveals the potential targets of
anti-HBV drugs. Pharmacogenomics J. 8:61–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li GQ, Xu WZ, Wang JX, Deng WW, Li D and
Gu HX: Combination of small interfering RNA and lamivudine on
inhibition of human B virus replication in HepG2.2.15 cells. World
J Gastroenterol. 13:2324–2327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xin XM, Li GQ, Guan XR, Li D, Xu WZ, Jin
YY and Gu HX: Combination therapy of siRNAs mediates greater
suppression on hepatitis B virus cccDNA in HepG2.2.15 cell.
Hepatogastroenterology. 55:2178–2183. 2008.PubMed/NCBI
|
|
24
|
DeCaprio JA, Ludlow JW, Figge J, Shew JY,
Huang CM, Lee WH, Marsilio E, Paucha E and Livingston DM: SV40
large tumor antigen forms a specific complex with the product of
the retinoblastoma susceptibility gene. Cell. 54:275–283. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eckner R, Ewen ME, Newsome D, Gerdes M,
DeCaprio JA, Lawrence JB and Livingston DM: Molecular cloning and
functional analysis of the adenovirus E1A-associated 300-kD protein
(p300) reveals a protein with properties of a transcriptional
adaptor. Genes Dev. 8:869–884. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Howe JA, Mymryk JS, Egan C, Branton PE and
Bayley ST: Retinoblastoma growth suppressor and a 300-kDa protein
appear to regulate cellular DNA synthesis. Proc Natl Acad Sci USA.
87:5883–5887. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Werness BA, Levine AJ and Howley PM:
Association of human papillomavirus types 16 and 18 E6 proteins
with p53. Science. 248:76–79. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flemington EK: Herpesvirus lytic
replication and the cell cycle: Arresting new developments. J
Virol. 75:4475–4481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goh WC, Rogel ME, Kinsey CM, Michael SF,
Fultz PN, Nowak MA, Hahn BH and Emerman M: HIV-1 Vpr increases
viral expression by manipulation of the cell cycle: A mechanism for
selection of Vpr in vivo. Nat Med. 4:65–71. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He J, Choe S, Walker R, Di Marzio P,
Morgan DO and Landau NR: Human immunodeficiency virus type 1 viral
protein R (Vpr) arrests cells in the G2 phase of the cell cycle by
inhibiting p34cdc2 activity. J Virol. 69:6705–6711. 1995.PubMed/NCBI
|
|
31
|
Dove B, Brooks G, Bicknell K, Wurm T and
Hiscox JA: Cell cycle perturbations induced by infection with the
coronavirus infectious bronchitis virus and their effect on virus
replication. J Virol. 80:4147–4156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li FQ, Tam JP and Liu DX: Cell cycle
arrest and apoptosis induced by the coronavirus infectious
bronchitis virus in the absence of p53. Virology. 365:435–445.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Zhao R, Wu Y, Kong D, Zhang L, Wu
D, Li C, Zhang C, Yu Z and Jin X: Hepatitis B virus induces G1
phase arrest by regulating cell cycle genes in HepG2.2.15 cells.
Virol J. 8:2312011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tong YG, Zhang XW, Geng MY, Yue JM, Xin
XL, Tian F, Shen X, Tong LJ, Li MH, Zhang C, et al: Pseudolarix
acid B, a new tubulin-binding agent, inhibits angiogenesis by
interacting with a novel binding site on tubulin. Mol Pharmacol.
69:1226–1233. 2006. View Article : Google Scholar : PubMed/NCBI
|