Introduction
Lung cancer is the most common malignancy, its
mortality ranks first, and more than a million of patients die from
lung cancer each year world-wide. Although surgery, radio- and
chemotherapy, and other treatments all were used for lung cancer,
but the therapeutic results are limited. Therefore, the combined
therapy strategy used to kill the tumor cells has become a hotspot
in the field of cancer treatment research. Cancer treated by
genetic means combined with radiotherapy is a method proposed
recently (1). The enhancements of
tumor-killing gene expression and radiotherapy produce synergistic
effects to inhibit tumors.
With the aim to improve the clinical outcomes of
locally advanced cancer, various radiotherapeutic approaches have
been implemented. Tumor gene-radiotherapy is a strategy proposed
for a large quantity of genes inducing tumor cell apoptosis,
inhibiting oncogenic activity, reducing angiogenic activity to
sensitize cells to radiation. Usually, tumor cell apoptosis
resistances is a central hallmark of carcinogenesis, consequently
the efficacy of all anti-tumor treatments is limited by the
presence of cells displaying alterations in their apoptotic
machinery (2). Selective induction
of apoptosis in malignant cells may represent a central therapeutic
strategy in radiation oncology (3).
Tumor necrosis factor related apoptosis inducing ligand (TRAIL) was
coloned from human lymphocytes and cardiac muscle cDNA by Wiley
et al (4). In recent years,
by virtue of its pro-apoptotic effect on cancer cells, but not
normal cells, TRAIL has been considered a promising candidate for
tumor gene therapy, even tumor gene-radiotherapy (5–8).
Gene-radiotherapy based on TRAIL has significant synergistic
effect, which has improved curative effect in breast cancer
treatment of preclinical experiments and I–II stage clinical trials
(9). Some studies confirm that
ionizing radiation can upregulate the expression levels of TRAIL
and death receptors (DR4 and DR5) and promote the enhancement of
tumor cell apoptosis. DR4 and DR5 receptors have pro-apoptotic
activity, but decoy receptors DcR1 and DcR2, and osteoprotegerin
have none (7). DR4 and DR5 are the
only proapoptotic TRAIL-receptors, that may regulate apoptosis by
caspase-8 or caspase-10 to caspase-3 pathways (10).
Gene expression enhancement mediated radiation
inducible promoter became impossible in tumor gene-radiotherapy,
the early growth response 1 (Egr1), also known as NGFI-A, zif268,
TIS8, cef5 and krox24, has been shown to have the characteristics,
it contains 6 serum response elements, [CC(A/T)6GG],
which are essential for Egr1 gene activation by radiation (11). In 1992, Weichselbaum and his
collegues concluded that Egr1 promoter could be linked to encoding
region of exogenous genes to activate the transcription and to
enhance protein expression levels by ionizing radiation (12). One by one, many studies have
confirmed the radiation-inducible characteristics of Egr1 promoter,
the exogenous genes included TNF-α, IFN-γ, endostatin and TRAIL,
etc, gene-radiotherapy based on Egr1 promoter became a hotspot of
radiation oncology (8,13–15).
It is well known that hypoxia is a common phenomenon in solid tumor
development, it causes tumor radio- and chemotherapy resistance. In
addition, Egr1 promoter inducible activity by radiation was greatly
reduced under hypoxic condition, which was caused by oxygen-free
radical reduction (16,17). Hypoxia response elements (HREs) are
important hypoxic response regulatory sequences and sensitivity
enhancers, and have higher activity in solid tumor. Some studies
constructed HRE and Egr1 dual-sensitive promoter, and gene
transcriptional activity was enhanced significantly under hypoxic
conditions, which might make hypoxia a contributing factor for
radiotherapy treatment (18,19).
In order to achieve the maximum effects of tumor
gene-radiotherapy, in the current study, pro-apoptotic gene human
secreted TRAIL (hsTRAIL) was mediated by dual sensitivity promoter
HRE/Egr1 to form recombinant expression plasmid pc-H/E-hsT, and
pc-E-hsT was used as a control. After transient transfection into
human lung adenocarcinoma A549 cells, the radiosensitivity under
normoxic and hypoxic condition was evaluated, to determine whether
pc-H/E-hsT may serve as a novel gene-radiotherapeutic agent for
cancer treatment.
Materials and methods
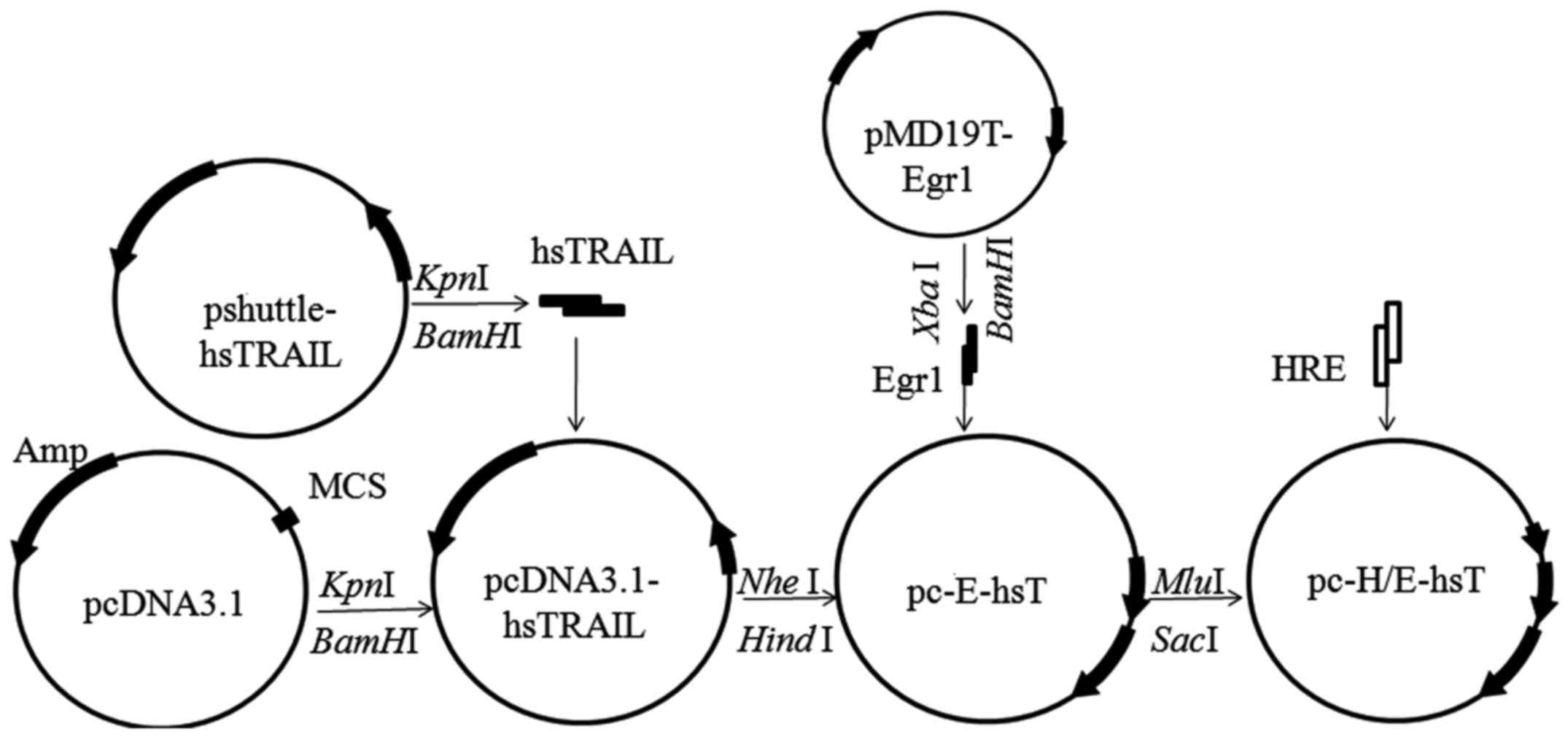
Construction of recombinant
plasmid
The pshuttle-hsTRAIL and pMD19T-Egr1 plasmids were
constructed and kindly given by Dr Yan-bo Li, School of Public
Health and Family Medicine, Capital Medical University. Egr1
fragment was obtained from pMD19T-Egr1 vector digested by
XbaI and HindIII, hsTRAIL fragment was obtained from
pshuttle-hsTRAIL vector digested by KpnI and BamHI,
HRE dual-strand containing MluI and SacI restriction
site were by Biochemical synthesis (Sangon Biotech Co., Ltd.,
Shanghai, China). The pc-E-shT and pc-H/E-shT plasmids were
constructed by gene recombination technique as shown in Fig. 1.
Cell culture and transfection
Human lung adenocarcinoma A549 cells were obtained
from American Type Culture Collection (ATCC, Manassas, VA, USA).
A549 cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) (Invitrogen, Carlsbad, CA, USA). DMEM was supplemented with
10% fetal bovine serum (FBS) (Invitrogen), penicillin (Invitrogen)
(100 U/ml), streptomycin sulfate (Invitrogen) (100 µg/ml), at 37°C
in a humidified 5% CO2 atmosphere. The cells were
transfected with the Lipofectamine™ 2000 (Invitrogen) reagent
according to the manufacturer's protocol. Cells were incubated for
6 h, then the transfection medium were replaced by fresh complete
growth medium.
Cell hypoxia and irradiation
A549 cells were transfected with pc-E-hsT and
pc-H/E-hsT as described above. After 24 h, CoCl2 (Sigma,
St. Louis, MO, USA) were added into the wells of plate at 150
µmol/l final concentration to study hypoxia, after 24 h, cells were
exposed to X-rays using an X-ray generator (model XSZ-Z20/20,
Dandong, Liaoning, China) with 200 kV and 18 mA. The dose rate of
irradiation was 0.342 Gy/min, 4 Gy total dose was used (8).
RT-PCR and ELISA
A549 cells were seeded into 6-well plates by
5×105 cells/well, after transfection, hypoxia and
irradiation as described above, culture supernatant were collected
to measure TRAIL concentration by enzyme-linked immunosorbent assay
(ELISA). While total RNA were extracted using TRIzol reagent
(Invitrogen). RNA of each sample (200 ng) was synthesized into cDNA
by RT-PCR kits (Takara, Dalian, China). Following primers were
synthesized by Takara, GAPDH forward: 5′-TATTGGGCGCCTGGTCACCA-3′,
reverse: 5′-CCACCTTCTTGATGTCATCA-3′; amplicons were 187 bp; hsTRAIL
forward: 5′-CATCTATTCCCAAACATACTT-3′, reverse:
5′-CCCTTGATAGATGGAATAGA-3′, amplicons were 746 bp. The PCR reaction
were performed: 94°C for 5 min; 94°C for 30 sec, 62°C for 30 sec,
and 72°C for 45 sec, 30 cycles; 72°C for 10 min. Amplified products
were separated in 1% agrose gels and visualized using ethidium
bromide staining. Band intensities were quantified using a gel
imaging instrument and Quantity One software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The hsTRAIL concentration in the culture
supernatants were measured by ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Colony forming assay
A549 cells were seeded into 60-mm dish by
5×105/dish, after transfection and hypoxia as described
above. Cells were digested, then they were seeded again into 60-mm
dish at 1×102/dish (0 Gy), 2×102/dish (2 Gy),
6×102/dish (4 Gy), 2×103/dish (6 Gy),
5×103/dish (8 Gy) and 5×104/dish (10 Gy). The
DMEM was replaced every 2 days for 10 days. Cells were fixed with
methanol and dyed with Giemsa, the colonies were counted. The
survival fraction (SF) was calculated upon the rate of treated
group colony number to untreated group colony number. Mean lethal
dose D0 value was calculated using linear correlation
and regression analysis. A smaller D0 value indicates a
higher radiosensitivity.
Flow cytometry
Apoptotic rate was measured by Annexin V staining of
externalized phosphatidylserin in apoptotic cells using FCM
(Becton-Dickinson Co., Franklin Lakes, NJ, USA) with Annexin V/FITC
kit (KeyGen Biotech, Nanjing, China). Briefly, A549 cells were
seeded into 24-well plate by 1×105/well, after
transfection, hypoxia treatment and irradiation as described above,
cells were harvested and washed with phosphate-buffered saline
(PBS) 3 times, and stained with Annexin V-FITC and propidium iodide
(PI) (KeyGen Biotech) for 10 min at room temperature according to
the protocol of the manufacturer. The apoptotic cells were analyzed
by FCM, which display Annexin V+/PI− (early
apoptosis) or Annexin V+/PI+ staining (late
apoptosis) (20). There were 3
replicate wells per group. The experiment was performed in
triplicate.
TUNEL assay
A549 cells were seeded into 24-well plate with clean
coverslip by 5×104/well, after transfection, hypoxia
treatment and irradiation as described above, the coverslip were
removed and fixed with 4% paraformaldehyde, TUNEL (terminal
deoxynucleotidyl transferase-mediated dUTP-biotin nick end
labeling) (KeyGen Biotech) reaction was performed according to the
manufacturer's protocol. The cells were stained with
diaminobenzidine (DAB) for 10 min and counterstained with Mayrow
hematoxylin. TUNEL positive cells from 100 cells in one visual
field under a microscope were counted, 3 fields were randomly
selected for one sample.
Western blotting
A549 cells were seeded into 6-well plates at
5×105 cells/well, after transfection, hypoxia and
irradiation as described above, cells were collected into Eppendorf
tubes, total proteins were extracted with cold lysis buffer (10
mmol/l Tris-HCl, pH 7.4; 1 mmol/l EDTA, pH 8.0; 0.1 mol/l NaCl; 1
µg/ml aprotinin; 100 µg/ml PMSF), and the protein concentration was
determined by using Coomassie brilliant blue protein assay from
Jiancheng Bioengineering Institute (Nanjing, China). For western
blotting, 25 µg protein was separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then
transferred to nitrocellulose membranes. The membrane was blocked
in PBS containing 5% free-fat milk for 1 h, then incubated at 4°C
with anti-DR4, anti-DR5, caspase-3 and β-actin antibody overnight
(1:200, 1:200, 1:150 and 1:500, Santa Cruz Biotechnology, Inc.
Santa Cruz, CA, USA), and then incubated with horseradish
peroxidase-conjugated secondary antibody (1:2000, Pierce, Rockford,
IL, USA) at 37°C for 1 h. Bound antibodies were visualized by
chemiluminescence reagents.
Statistical analysis
All statistical analyses were performed by SPSS12.0
(statistical package for the social science program 12.0) (SPSS
Inc., Chicago, IL, USA). The data are presented as mean ± SD and
subjected to one-way ANOVA followed by Student's t-test, and
P<0.05 was considered significant.
Results
Characterization of TRAIL expression
following hypoxia and irradiation
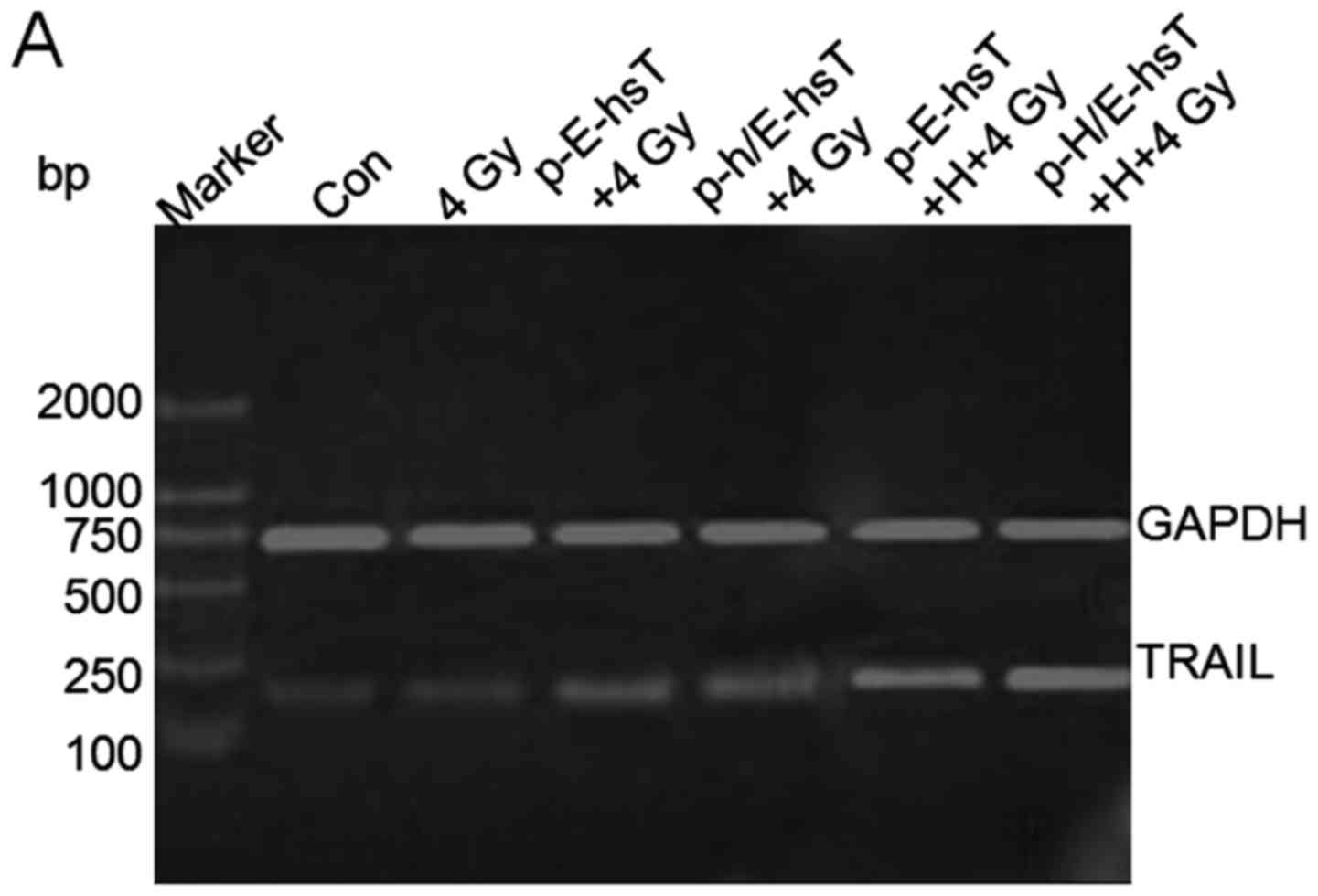
To characterize TRAIL expression following hypoxia
and irradiation, TRAIL mRNA amplication products were made using
DNA electrophoresis (Fig. 2A). As
compared with control, TRAIL mRNA significantly increased after 4
Gy irradiation (P<0.001), and recombinant plasmids combined with
4 Gy irradiation increased TRAIL mRNA expression significantly
(P<0.001), furthermore, after cells were transfected with
recombinant plasmids, they were treated by hypoxia and irradiation,
TRAIL mRNA increased the most (P<0.001), even compared with 4 Gy
(P<0.05, P<0.001) (Fig. 2B).
In addition, secreted TRAIL concentration in culture supernatant
was measured by ELISA, as compared with control group, 4 Gy
irradiation alone increased protein concentration significantly
(P<0.05), the combination with plasmids increased the protein
concentration the most (P<0.001), while there were no obvious
difference between the two plasmids. Secreted TRAIL concentration
was increased the most in cells treated by recombinant plasmids
transfection, hypoxia and irradiation (P<0.001), even compared
with 4 Gy (P<0.001) (Fig.
2B).
Difference of radiosensitivity between
transfected pc-E-hsT and pc-H/E-hsT cells under hypoxic and
normoxic condition
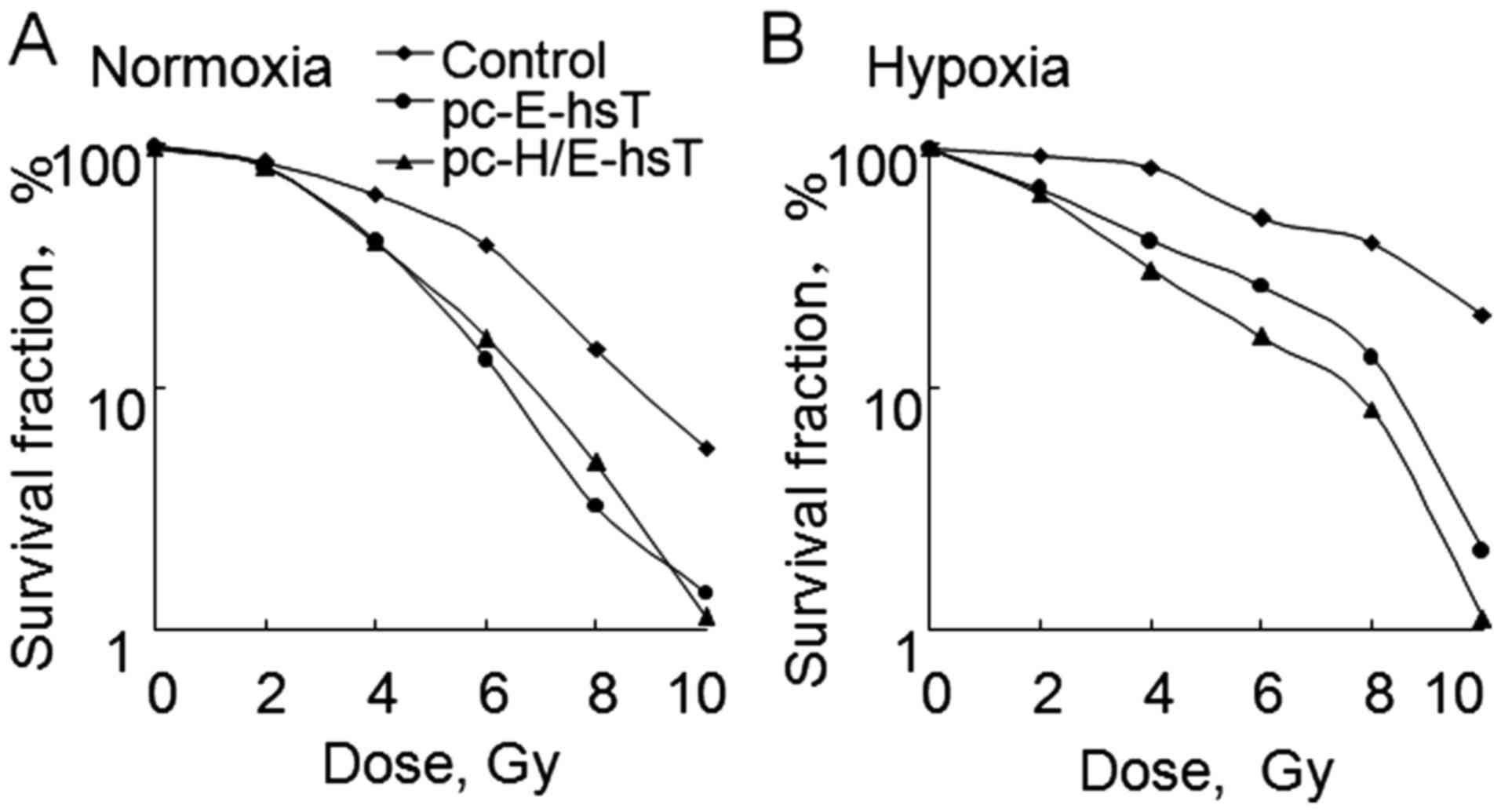
In order to compare the difference of
radiosensitivity between transfected pc-E-hsT and pc-H/E-hsT cells
under hypoxic and normoxic condition, cologenic assay was
introduced (21). The survival
curves were constructed and D0 value were determined.
Under normoxia condition, the survival curve for cells transfected
with pc-E-hsT and pc-H/E-hsT plasmids had much steeper slopes, and
there was no obvious difference between two plasmids, D0
value of control, pc-E-hsT and pc-H/E-hsT group was 3.26, 1.91 and
1.89 Gy, respectively (Fig. 3A). In
addition, under hypoxia condition, the survival curve for control
cells, cells transfected with pc-E-hsT and pc-H/E-hsT plasmids
gradually became steeper, D0 value of control, pc-E-hsT
and pc-H/E-hsT group was 4.81, 2.54 and 1.13 Gy, respectively
(Fig. 3B). These results indicated
that hypoxia can cause A549 cell radioresistance, pc-E-hsT can
increase radiosensitivity, even in hypoxia condition, while
pc-H/E-hsT can overcome radioresistance induced by hypoxia
increasing radiosensitivity the most.
Transfection of cell apoptosis rates
induced by hypoxia and irradiation
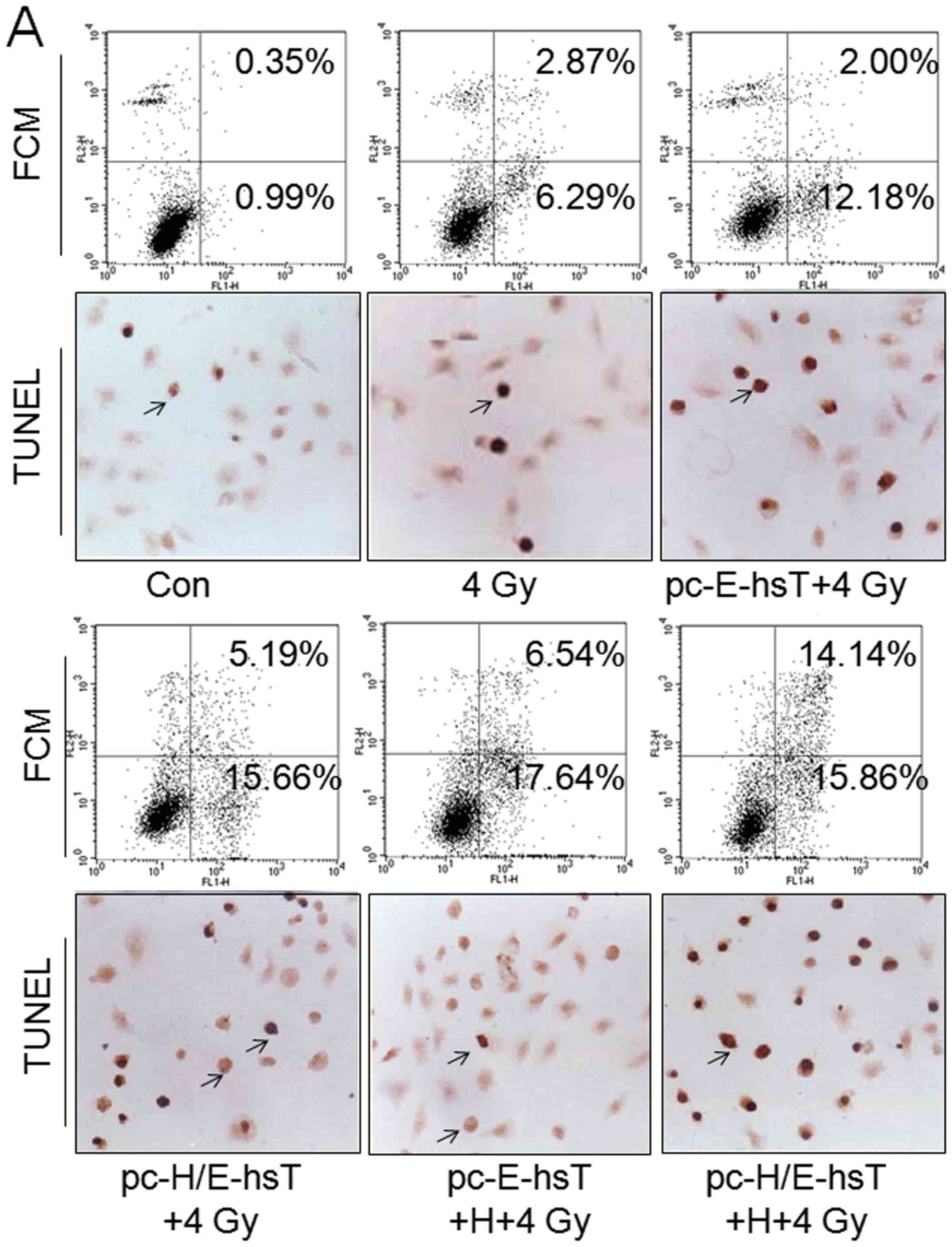
Cell apoptosis was attributed to the
radiosensitivity of cancer cells (22), therefore, apoptotic rate was
measured to identify the hypothesis. The cell apoptotic rate was
subsequently calculated based on the combined percentages of early
apoptosis (Annexin V+/PI−) and late apoptosis
(Annexin V+/PI+) detected by flow cytometry
and TUNEL (Fig. 4A). Quantification
of apoptotic rate found that as compared with control group, 4 Gy
irradiation could significantly induce apoptosis increase
(P<0.001), and the roles of combination with pc-E-hsT and
pc-H/E-hsT plasmid transfection, hypoxia and irradiation was more
obvious (P<0.001), even as compared with 4 Gy (P<0.05,
P<0.001), but there were no obvious difference between the
plasmids. Based on transfection of plasmids, cells treated with
hypoxia and irradiation, hypoxia did not increase pc-E-hsT
transfected cells apoptotic rate, but promoted pc-H/E-hsT
transfected cells. Additionally, apoptotic rate measured by TUNEL
was similar with FCM (Fig. 4B).
Taken together, plasmid carrying Egr1 promoter played inducible
roles under normoxia and hypoxia, but promoter HRE only under
hypoxia.
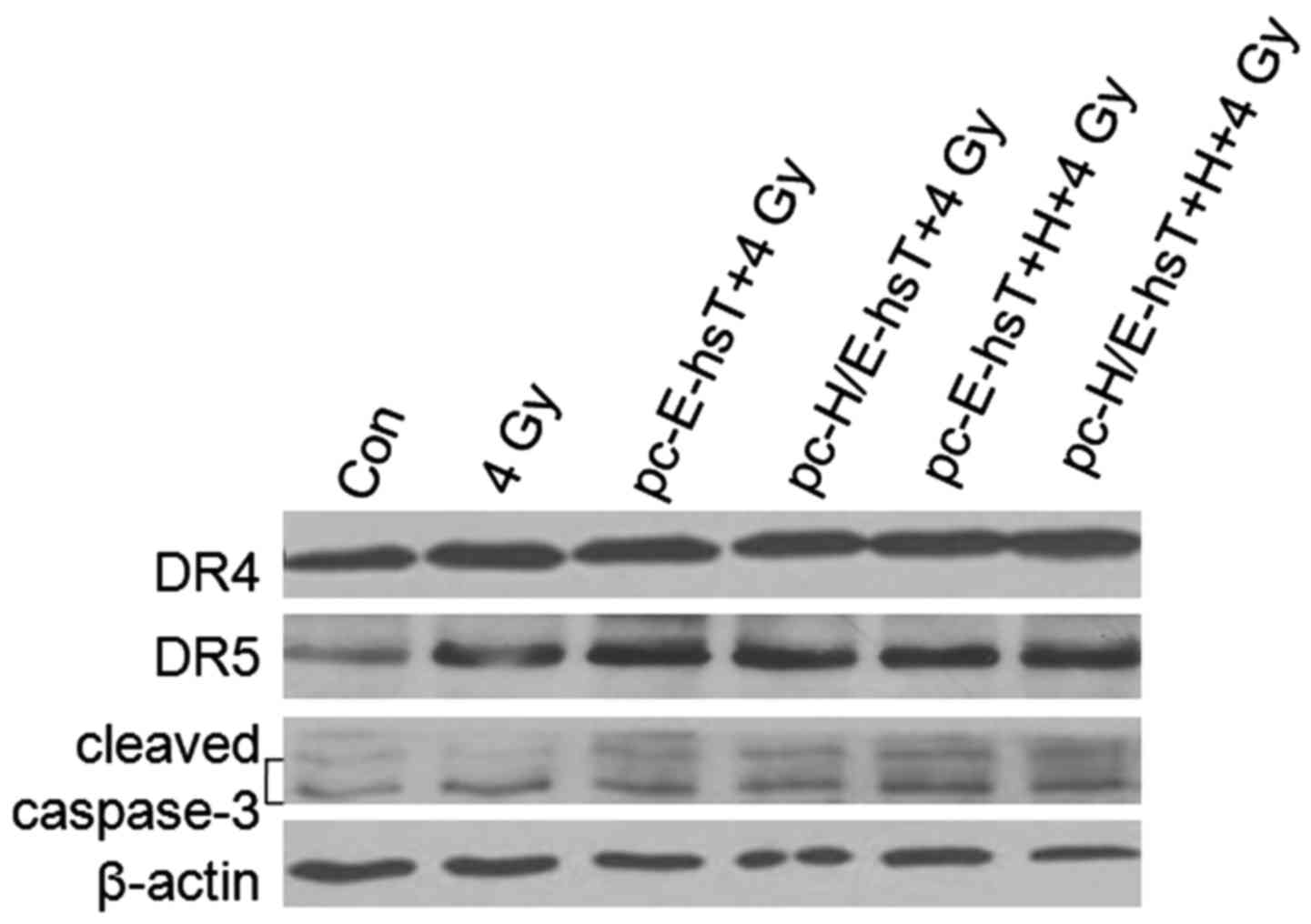
DR4, DR5 and caspase-3 expression
levels after transfection, hypoxia and irradiation
TRAIL and DR4, DR5 receptors regulated apoptosis
upon caspase-8 or caspase-10 to caspase-3 pathways, caspase-3
cleaved fragments, then caused cell apoptosis (10). As shown (Fig. 5), 4 Gy irradiation caused DR4, DR5
and cleaved caspase-3 increase, DR4 had not obvious change in
pc-E-hsT and pc-H/E-hsT transfected cells under normoxic and
hypoxic condition. DR5 and cleaved caspase-3 increased mostly in
pc-H/E-hsT transfected cells under hypoxic condition. These results
also indicated TRAIL overexpression could induce A549 cell
apoptosis, and its regulation was by TRAIL and DR5 to caspase-3
pathway, but not DR4.
Discussion
Tumor gene-radiotherapy is a method put forward in
recent years to treat tumors. It is used to transfer an exogenous
gene into cells, which can kill tumor cells and enhance
radiosensitivity. It is synergistic on gene and radiation in local
radiotherapy (22). It had been
reported that Egr1 was transcriptionally induced by radiation
exposure, and the radiation inducible role was conferred by serum
response or CC(A/T)6GG elements in its promoter region (12,23).
Therefore, the expression for exogenous gene controlled by Egr1
promoter could be enhanced by ionizing radiation temporally,
spatially and dose-dependently (24). However, Egr1 promoter inducible
activity by radiation was greatly reduced under hypoxic condition,
which was caused by oxygen-free radical reduction (16,17).
Therefore, some strategies to enhance Egr1 radiation inducible
characteristics were applied. Such as an adenoviral vector carrying
Egr1 promoter, more efficient transferring system that controls the
expression of tumor necrosis factor-α (Ad-Egr1-TNF-α) was shown to
enhance anti-tumor response, TNF-α was preferentially activated in
tumors by ionizing radiation using the system (25).
In addition, the presence or absence of molecular
oxygen is known to influence the biological effect of ionizing
radiation; cells obtain radioresistance under hypoxic conditions
(26). Although tumor hypoxia is
one of the major obstacles in radiotherapy, we can take advantage
of it as a tumor-specific therapeutic target to improve tumor
radiation resistance, then significantly promoting tumor killing
effects. HRE/Egr1 dual sensitive promoter depending on HRE
inducible features under hypoxic condition could effectively
improve Egr1 promoter transcription ability. In the present study,
we successfully constructed TRAIL gene expression system regulated
by HRE/Egr1 chimeric promoters.
Adverse side effects that can arise from the
non-specific killing of normal cells with high doses of radiation
limits the clinical application of radiotherapy (27). Consequently, strategies that
sensitize tumor cells to radiation and decrease the necessary
radiation dose, while increasing treatment specificity, are
essential for achieving successful radiotherapy. Tumor necrosis
factor-related apoptosis inducing ligand (TRAIL) was found coloned
from human lymphocytes and cardiac muscle cDNA by Wiley et
al, it is known as apoptosis-2 ligand (Apo-2L), and is a member
of TNF superfamily (4). In recent
years, by virtue of its pro-apoptotic effect on cancer cells, but
not normal cells, TRAIL has been considered a promising candidate
for tumor genetherapy, even tumor gene radiotherapy (5–8).
Targeting TRAIL therapy, either alone or in conjunction with other
therapies, has been extensively used for tumor treatment. In
comparison with the soluble recombinant TRAIL protein, TRAIL gene
therapy may overcome protein instability and resistance, and
neighboring cancer cells untransfected by TRAIL can be killed via
bystander effect (28,29). In the present study, human secreted
TRAIL (hsTRAIL) was mediated by HRE and Egr1 together, mRNA and
ELISA results showed that radiation alone can not induce increase
of TRAIL expression, inducible activity of Egr1 has no obvious
difference under normoxic and hypoxic conditions. TRAIL expression
may increase under hypoxic condition with additional regulation of
HRE, indicated that HRE/Egr1 chimeric promoters can improve the
inefficient Egr1 under hypoxic condition.
Cell viability, proliferation and apoptosis rates
are all factors attributed to radiosensitivity of cancer cells
(30). Therefore, we investigated
whether overexpression of TRAIL can sensitize lung cancer cells to
radiation using a clonogenic assay and cell apoptosis by FCM
(31). Under normoxic condition,
sensitization ability of pc-E-hsT and pc-H/E-hsT plasmids on A549
cells was basically similar, but under hypoxic condition,
sensitization ability of pc-H/E-hsT plasmids was stronger than that
of pc-E-hsT. Furthermore, taking into account the role of apoptosis
on radiosensitivity, changes induced by radiation was analyzed, and
FCM and TUNEL results showed that radiation induced cell apoptosis,
TRAIL overexpression mediated by Egr1 promoter induced more
apoptosis. Moreover, if TRAIL overexpression was mediated by Egr1
and HRE together, radiation-induced apoptosis occurred mostly under
hypoxic condition. On the contrary, hypoxia was beneficial for
cancer radiotherapy. In addition, TRAIL as well as DR4 and DR5
receptors regulated apoptosis upon caspase-8 or caspase-10 to
caspase-3 pathways, caspase-3 was cleaved into active fragments,
then caused cell apoptosis (10).
In the present study, DR5 receptor played pro-apoptosis role with
TRAIL, and DR4 receptor pro-apoptosis role was similar.
Subsequently, caspase-3 was cleaved into active fragments, this
also indicated apoptotic characteristics as described above.
Collectively, TRAIL overexpression co-regulated by
Egr1 and HRE promoters may enhance A549 cell radiosensitivity under
hypoxic condition, which have important implication for clinical
radiation treatment of lung cancer. Moreover, radiosensitivity
enhancement of A549 cells is related to TRAIL-mediated apoptosis
depending on TRAIL and DR5 to caspase-3 pathways. Thus, our results
and conclusions provide theoretical and experimental bases for
future clinical application of hypoxic lung cancer
radiotherapy.
Acknowledgements
This project was supported by the National Natural
Science Foundation of China (no. 81372929), Young Scholars Program
of Norman Bethune Health Science Center of Jilin University
(2013202017) and Basic Research and Operating Expenses of Jilin
University (200903116). The pshuttle-hsTRAIL and pMD19T-Egr1
plasmids were kindly given by Dr Yan-bo Li, School of Public Health
and Family Medicine, Capital Medical University.
References
|
1
|
Grade M, Wolff HA, Gaedcke J and Ghadimi
BM: The molecular basis of chemoradiosensitivity in rectal cancer:
Implications for personalized therapies. Langenbecks Arch Surg.
397:543–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woynarowska BA, Roberts K, Woynarowski JM,
MacDonald JR and Herman TS: Targeting apoptosis by
hydroxymethylacylfulvene in combination with gamma radiation in
prostate tumor cells. Radiat Res. 154:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perlstein B, Finniss SA, Miller C,
Okhrimenko H, Kazimirsky G, Cazacu S, Lee HK, Lemke N, Brodie S,
Umansky F, et al: TRAIL conjugated to nanoparticles exhibits
increased anti-tumor activities in glioma cells and glioma stem
cells in vitro and in vivo. Neuro Oncol. 15:29–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tucker-Kellogg L, Shi Y, White JK and
Pervaiz S: Reactive oxygen species (ROS) and sensitization to
TRAIL-induced apoptosis, in Bayesian network modelling of HeLa cell
response to LY303511. Biochem Pharmacol. 84:1307–1317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niemoeller OM and Belka C: Radiotherapy
and TRAIL for cancer therapy. Cancer Lett. 332:184–193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YB, Guo CX, Wang ZC, Dong LH, Guan F,
Liu Y, Wang HF, Sun ZW and Gong SL: Radiosensitization of breast
cancer cells by TRAIL-endostatin-targeting gene therapy. Neoplasma.
60:613–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: Are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sprick MR, Rieser E, Stahl H, Grosse-Wilde
A, Weigand MA and Walczak H: Caspase-10 is recruited to and
activated at the native TRAIL and CD95 death-inducing signalling
complexes in a FADD-dependent manner but can not functionally
substitute caspase-8. EMBO J. 21:4520–4530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Datta R, Rubin E, Sukhatme V, Qureshi S,
Hallahan D, Weichselbaum RR and Kufe DW: Ionizing radiation
activates transcription of the EGR1 gene via CArG elements. Proc
Natl Acad Sci USA. 89:10149–10153. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weichselbaum RR, Hallahan DE, Sukhatme VP
and Kufe DW: Gene therapy targeted by ionizing radiation. Int J
Radiat Oncol Biol Phys. 24:565–567. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu LL, Smith MJ, Sun BS, Wang GJ, Redmond
HP and Wang JH: Combined IFN-gamma-endostatin gene therapy and
radiotherapy attenuates primary breast tumor growth and lung
metastases via enhanced CTL and NK cell activation and attenuated
tumor angiogenesis in a murine model. Ann Surg Oncol. 16:1403–1411.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W and Li XY: Anti-tumor effect of
pEgr-interferon-γ-endostatin gene-radiotherapy in mice bearing
Lewis lung carcinoma and its mechanism. Chin Med J (Engl).
118:296–301. 2005.PubMed/NCBI
|
|
15
|
Li ZL, Liang S, Wang ZC, Li YB, Guo CX,
Fang F, Gong SL and Lin CH: Expression of Smac induced by the Egr1
promoter enhances the radiosensitivity of breast cancer cells.
Cancer Gene Ther. 21:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weichselbaum RR, Kufe DW, Advani SJ and
Roizman B: Molecular targeting of gene therapy and radiotherapy.
Acta Oncol. 40:735–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pines A, Bivi N, Romanello M, Damante G,
Kelley MR, Adamson ED, D'Andrea P, Quadrifoglio F, Moro L and Tell
G: Cross-regulation between Egr-1 and APE/Ref-1 during early
response to oxidative stress in the human osteoblastic HOBIT cell
line: Evidence for an autoregulatory loop. Free Radic Res.
39:269–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang WD, Chen ZT, Li R, Li DZ, Duan YZ and
Cao ZH: Enhanced efficacy of radiation-induced gene therapy in mice
bearing lung adenocarcinoma xenografts using hypoxia responsive
elements. Cancer Sci. 96:918–924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greco O, Joiner MC, Doleh A, Powell AD,
Hillman GG and Scott SD: Hypoxia- and radiation-activated Cre/loxP
‘molecular switch’ vectors for gene therapy of cancer. Gene Ther.
13:206–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donadelli M, Pozza E Dalla, Scupoli MT,
Costanzo C, Scarpa A and Palmieri M: Intracellular zinc increase
inhibits p53(−/−) pancreatic adenocarcinoma cell growth by
ROS/AIF-mediated apoptosis. Biochim Biophys Acta. 1793:273–280.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pauwels B, Korst AE, de Pooter CM, Pattyn
GG, Lambrechts HA, Baay MF, Lardon F and Vermorken JB: Comparison
of the sulforhodamine B assay and the clonogenic assay for in vitro
chemoradiation studies. Cancer Chemother Pharmacol. 51:221–226.
2003.PubMed/NCBI
|
|
22
|
Ding M, Li R, He R, Wang X, Yi Q and Wang
W: p53 activated by AND gate genetic circuit under radiation and
hypoxia for targeted cancer gene therapy. Cancer Sci.
106:1163–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao XM, Koski RA, Gashler A, McKiernan M,
Morris CF, Gaffney R, Hay RV and Sukhatme VP: Identification and
characterization of the Egr-1 gene product, a DNA-binding zinc
finger protein induced by differentiation and growth signals. Mol
Cell Biol. 10:1931–1939. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min FL, Zhang H and Li WJ: Current status
of tumor radiogenic therapy. World J Gastroenterol. 11:3014–3019.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bickenbach KA, Veerapong J, Shao MY,
Mauceri HJ, Posner MC, Kron SJ and Weichselbaum RR: Resveratrol is
an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer
Gene Ther. 15:133–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang TJ and Ho AY: Radiation therapy in
the management of breast cancer. Surg Clin North Am. 93:455–471.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seol JY, Park KH, Hwang CI, Park WY, Yoo
CG, Kim YW, Han SK, Shim YS and Lee CT: Adenovirus-TRAIL can
overcome TRAIL resistance and induce a bystander effect. Cancer
Gene Ther. 10:540–548. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Ouyang W, Wu F, Cao CH, Wang K, Liao
ZK, Zhong YH, Zhou FX, Liu SQ, Xia L, et al: Enhanced
radiosensitivity of SW480 cells via TRAIL up-regulation mediated by
Egr-1 promoter. Oncol Rep. 22:765–771. 2009.PubMed/NCBI
|
|
30
|
Cheng G, Kong D, Hou X, Liang B, He M,
Liang N, Ma S and Liu X: The tumor suppressor, p53, contributes to
radiosensitivity of lung cancer cells by regulating autophagy and
apoptosis. Cancer Biother Radiopharm. 28:153–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pauwels B, Korst AE, de Pooter CM, Pattyn
GG, Lambrechts HA, Baay MF, Lardon F and Vermorken JB: Comparison
of the sulforhodamine B assay and the clonogenic assay for in vitro
chemoradiation studies. Cancer Chemother Pharmacol. 51:221–226.
2003.PubMed/NCBI
|