Introduction
The pathogenesis of hepatocellular carcinoma (HCC)
is closely linked with chronic inflammation and the level of
interleukin-6 (IL-6). Serum IL-6 level is higher in HCC patients
than in healthy adults (1),
demonstrating that inflammation is a critical risk factor for the
formation and progression of HCC. IL-6 rapidly activates the signal
transducer and activator of transcription 3 (STAT3), which is a
major mediator regulating signal transduction from IL-6 to the
nucleus and inducing the transcription of proliferation associated
genes (2). Phosphorylated STAT3 has
been implicated in abnormal oncogenic processes, such as
initiation, proliferation, angiogenesis, and progression in HCC
(3). Owing to these carcinogenetic
roles of the IL-6/STAT3 signaling pathway, which promotes the
development of HCC, STAT3 is often considered an attractive target
for liver cancer therapy. In addition, in our previous study, we
found that male-specific W4P mutations in the preS1 region
contribute to carcinogenesis and male predominance in HCC via
activation of the IL-6/STAT3 signaling axis. Ectopic expression of
W4P mutant HBV large antigen (LHB) was sufficient to induce
transformation of NIH3T3 fibroblast cells, emphasizing the crucial
role of the IL-6/STAT3 signaling pathway in HCC carcinogenesis
(4).
Artemisia is one of the most important genera
of the Asteraceae or Compositae family, the largest
family of flowering plants with over 15,000 species (5). The genus Artemisia consists of
more than 400 species, some of which exhibit medicinal activities
for a variety of diseases such as malaria, rabies, tonsillitis,
asthma, gonorrhea, cough, syphilis, and leprosy (6). Amongst these species, Artemisia
capillaris Thunberg (AC) has long been used in Asian countries
as a therapeutic drug (7,8). According to previous studies on its
medicinal properties, AC exhibits various pharmacological effects,
such as antimicrobial (8),
anti-obesitic (9,10), and antitumor (11) effects. In particular, there have
been many studies on the therapeutic and pharmacological effects of
AC against liver diseases, including hepatoprotective effects in
obese mice (12) or
ethanol-administered mice (13),
antiviral effects by suppressing the replication and secretion of
the hepatitis B virus (14–16), and anti-fibrotic effects in animal
models (7,17,18).
HCC, the most common primary liver cancer, demands
more effective remedies because of a low 5-year survival rate,
about 10% (19), and poor reactions
refractory to available treatments (20). Increasing evidence indicates the
efficacy of AC in suppressing the proliferation of human hepatoma
cells (21–23). In a recent study, capillarisin
derived from AC was found to have anticancer effects by suppressing
STAT3 phosphorylation in human multiple myeloma cells (24). However, there have been no previous
studies evaluating the anticancer effects of AC via inhibition of
the STAT3 signaling pathway in vitro models of HCC.
Therefore, in this study, we examined the
possibility of AC exerting its antitumor activity against HCC by
modulating IL-6-induced STAT3 activation.
Materials and methods
Preparation of AC extract (ACE)
AC was purchased from Kyung Hee Herb Pharm (Wonju,
Korea), a licensed herb company. AC was pulverized and extracted
with 70% EtOH under reflux three times, and the extract was
filtered and evaporated on a rotary evaporator under reduced
pressure and then lyophilized. ACE was subjected to LC-MS/MS
analysis and Scopoletin and liquiritin were found to be present at
concentrations of 393.3±45.7 µg/g and 39.33±6.3 µg/g,
respectively.
Cells
Huh7 and HepG2 human hepatocellular carcinoma cells
were maintained in Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (FBS) and penicillin/streptomycin
(100 U/ml). NIH3T3-W4P cell line expressing W4P mutant LHB was
established and maintained as previously described (4).
Cell viability assay
Anti-proliferative effects of ACE on HCC cells were
analyzed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Huh7 and HepG2 cells were treated with increasing
concentrations of ACE for 72 h followed by further incubation with
the MTT-containing medium for 4 h. After lysis with dimethyl
sulfoxide, the absorbance of lysates was determined at 570 nm by
using a microplate reader.
Colony forming assay
Six-hundred Huh7 cells and 200 cells of NIH3T3-W4P
were seeded onto 6-well plates and incubated for 24 h. The NIH3T3
and Huh7 cells were then treated with different concentrations of
ACE for 3 and 8 days, respectively. Colonies were fixed with 100%
methanol for 10 min and then stained with 0.1% crystal violet for
an hour. After washing with PBS, the number of colonies was
counted.
Immunoblotting
The effect of ACE on the phosphorylation status of
STAT3 was analyzed by immunoblotting. HepG2 cells were treated with
ACE for 3 h prior to treatment with IL-6 (25 ng/ml). After
incubation for 25 min, the cells were lysed and the cell lysates
were subjected to immunoblotting using anti-STAT3 (Santa Cruz
Biotechnology, sc-482), anti-phosphoSTAT3 (Cell Signaling, #9131),
and anti-β-actin antibodies (Santa Cruz Biotechnology, sc-47778).
To analyze the effect of ACE on the expression of cyclin D2 and
P21, HepG2 cells were treated with ACE for 48 h and subjected to
immunoblotting using anti-cyclin D2 (Santa Cruz Biotechnology,
sc-593) and anti-P21 (Santa Cruz Biotechnology, I2807)
antibodies.
RNA preparation and
reverse-transcription quantitative PCR (RT-qPCR)
To determine the effect of ACE on the mRNA levels of
cyclin D1, which are dependent on STAT3 transcriptional activity,
RT-qPCR assays were performed. HepG2 cells and NIH3T3-W4P cells
were treated with the increasing concentrations of ACE for 4 and 8
h, respectively. Total RNA was extracted using an RNeasy RNA
extraction kit (Qiagen) according to the manufacturer's
instructions. cDNA was synthesized from RNA using Superscript III
reverse transcriptase (Invitrogen) with oligo20(dT) primers and
analyzed by real-time PCR.
Scratch wound assay
Effect of ACE on Huh7 cell migration was
investigated by performing a scratch wound assay. The confluent
monolayer of Huh7 cells in a 6-well plate was wounded with a
sterile pipette tip and incubated with increasing concentrations of
ACE for 48 h. The cells were observed and images were obtained at
24 and 48 h after the treatment.
Transwell cell migration assay
Cell migration assay was performed as previously
described (25). Briefly, after
being treated with ACE (0, 25, 50, 100, and 200 µg/ml) for 24 h,
Huh7 cells were harvested and seeded on a Boyden chamber (Neuro
Probe, Cabin John, MD, USA) at a density of 105 cells
per well in a serum-free medium and then incubated for 6 h at 37°C.
The migrated cells were fixed with 100% methanol and stained with
5% Giemsa. Cells were counted under a light microscope.
Statistics
Statistical analysis was performed by SigmaPlot
10.0. Data are shown as means ± SD, and group differences were
analyzed using paired Student's t-test. p<0.05 was considered as
statistically significant.
Results
Anticancer effects of ACE on HCC cell
lines
AC has been well known for its therapeutic effect in
liver diseases and several previous studies suggested its antitumor
activity. Here, we evaluated the anticancer activity of ACE on HCC
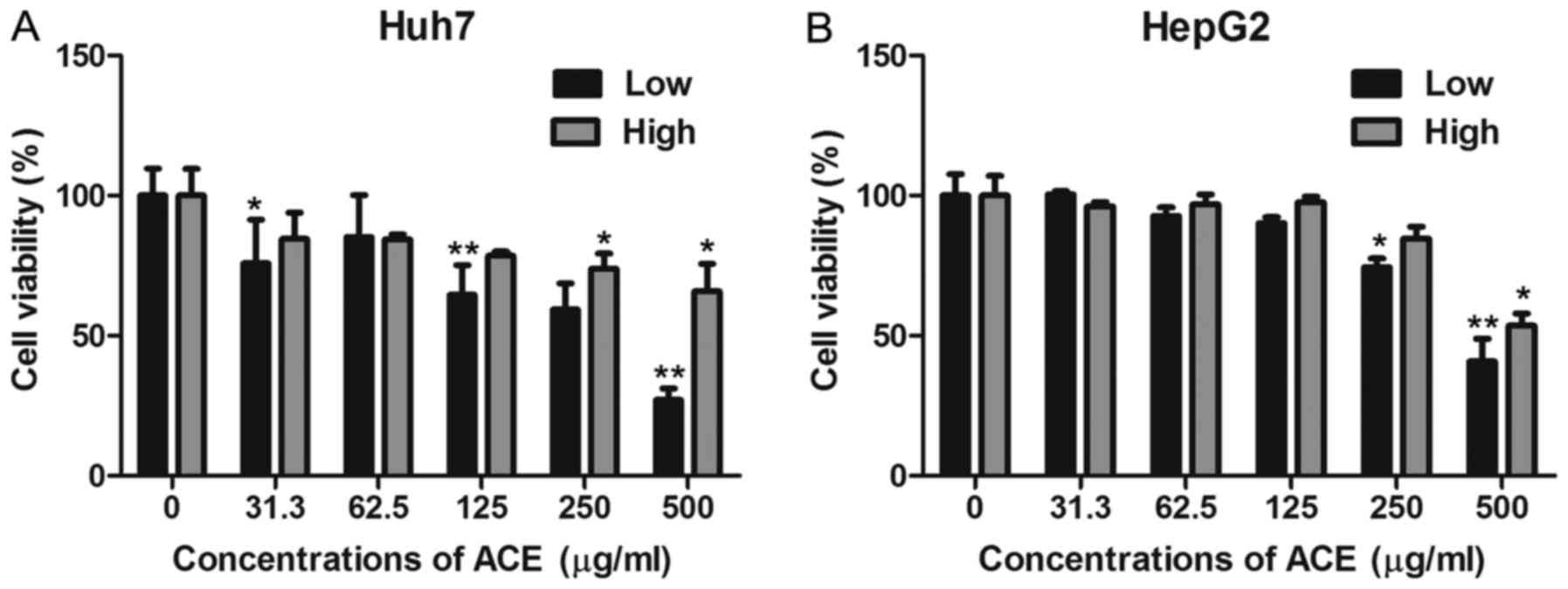
by examining its effect on HCC cell proliferation by MTT assay. ACE
displayed significant anti-proliferative effects against Huh7 and
HepG2 human hepatoma cell lines in a dose-dependent manner
(Fig. 1A and B). To further confirm
the anticancer effect of ACE, its effect on the colony-forming
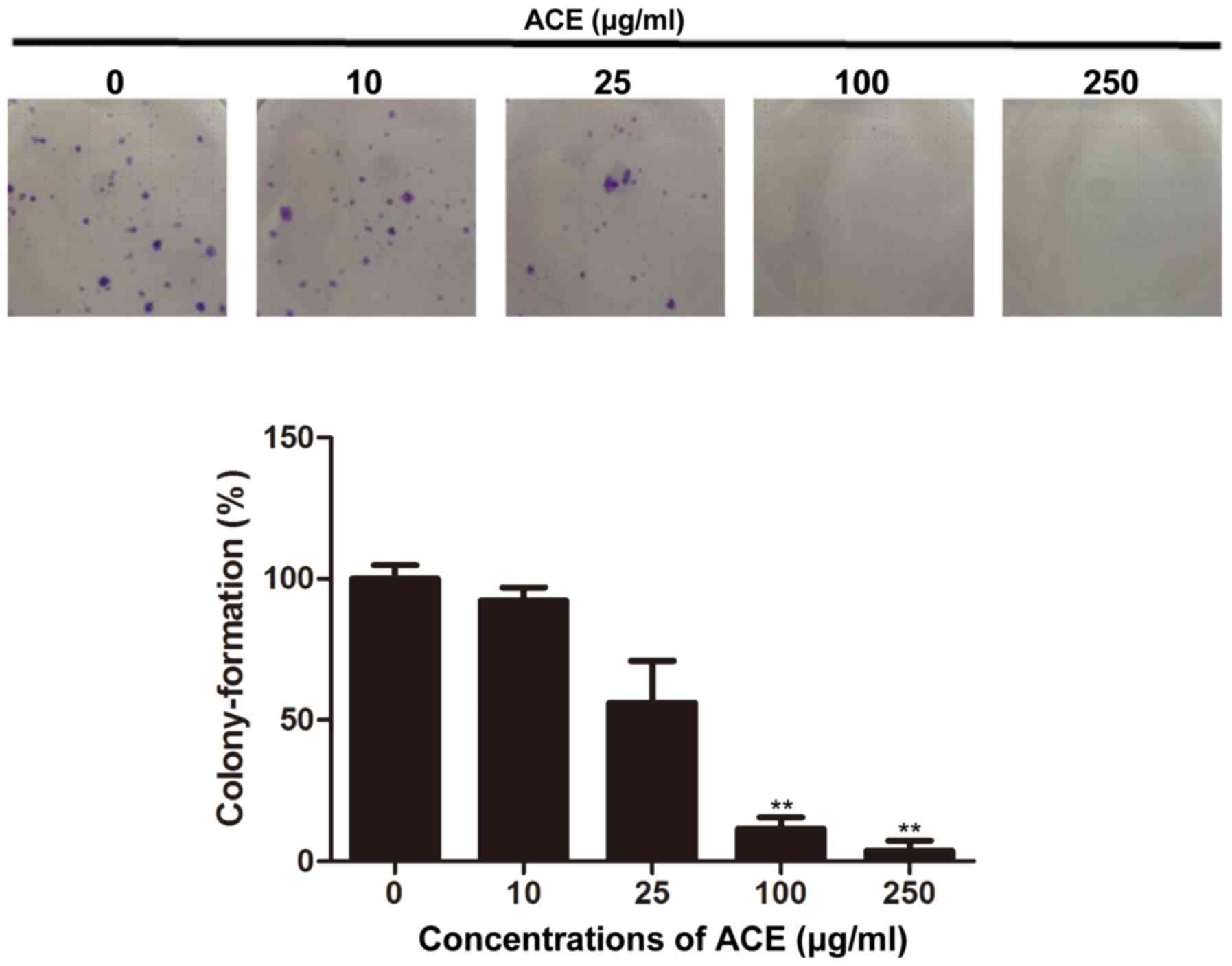
ability of HCC cells was examined. As shown in Fig. 2, the colony formation of Huh7 cells
was significantly inhibited in the presence of ACE; 100 µg/ml of
ACE treatment resulted in 90% reduction of the colony formation.
Compared to its direct cell cytotoxicity and the suppression of
cell proliferation, ACE exerted potent inhibitory effect on colony
forming.
Inhibition of STAT3 activation in HCC
cell lines by ACE
Inhibition of proliferation and colony formation of
HCC cells by ACE indicate its anticancer effect against HCC. Given
that IL-6-mediated STAT3 activation is implicated in HCC
development and progression, the role of ACE in STAT3 activation
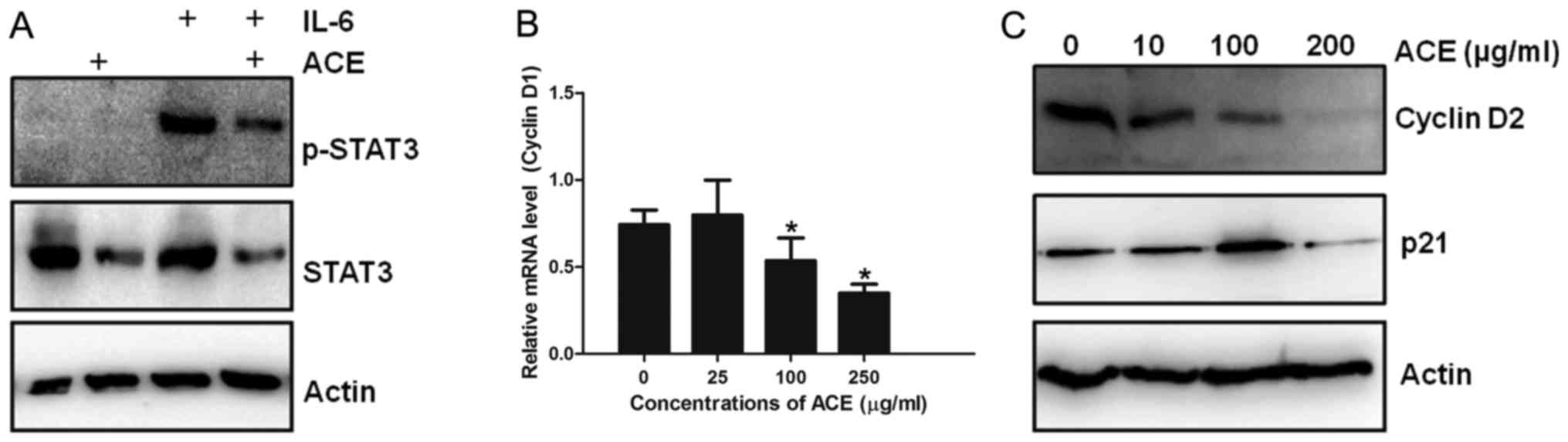
was examined. IL-6 treatment induced strong phosphorylation of
STAT3, which is a hallmark of STAT3 activation in HepG2 cells.
Treatment of IL-6-treated HepG2 cells with ACE resulted in marked
decrease of both STAT3 and phosphorylated STAT3, indicating that
ACE is capable of suppressing the action of STAT3 (Fig. 3A). Since STAT3 upregulates the
transcription of cell cycle related genes including cyclin D1 and
p21 and enhances cell cycle progression, the effect of ACE on the
amounts of cyclin D1 and p21 was examined. Consistent with the
suppression of STAT3 phosphorylation by ACE, synthesis of cyclin D1
mRNA as well as protein levels of cyclin D2 and p21 were also
decreased by ACE treatment (Fig. 3B and
C).
Inhibition of HBV W4P mutant large
surface protein-induced STAT3 activation by ACE
In our previous study, we showed that male-specific
W4P mutant HBV LHB drives HCC tumorigenesis by activating the
IL-6/STAT3 signaling pathway (4).
Thus, we evaluated whether ACE suppresses the STAT3 activation
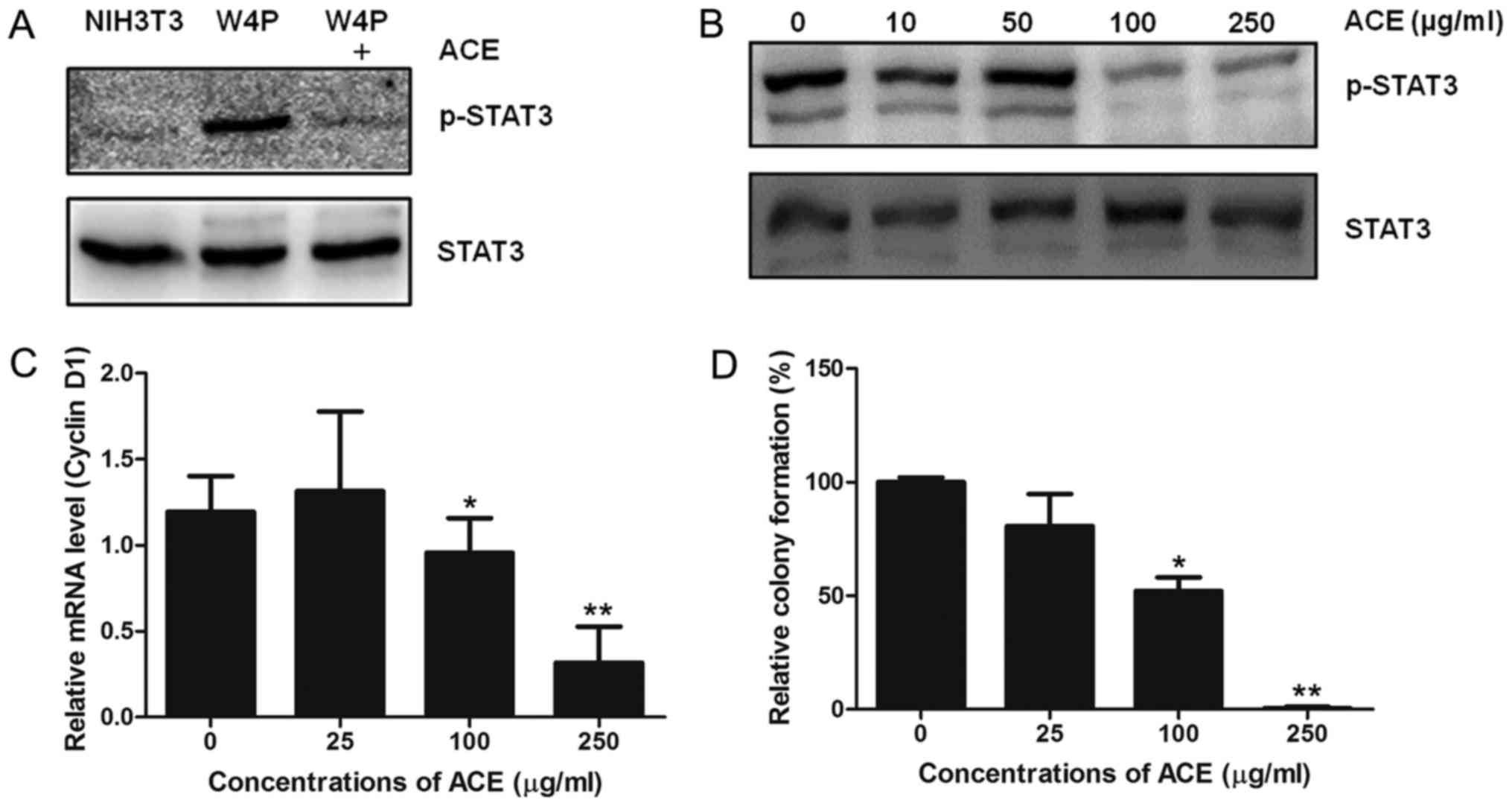
induced by W4P LHB. NIH3T3 cells stably expressing W4P LHB
(NIH3T3-W4P) showed strong STAT3 phosphorylation compared to
control NIH3T3 cells and the phosphorylation was decreased by ACE
(Fig. 4A). The activity of ACE was
dose-dependent and 100 µg/ml of ACE was sufficient to lower the
phosphorylation to significant extent (Fig. 4B). In line with the results, cyclin
D1 mRNA level of NIH3T3-W4P was also decreased in a dose-dependent
manner by ACE treatment, further confirming that ACE suppresses the
transcriptional activity of STAT3 (Fig.
4C). It has been shown that W4P LHB confers in vitro
colony forming ability and in vivo tumor forming capability
in nude mice to NIH3T3 cells by activating the STAT3 signaling
pathway (4). Since ACE suppresses
STAT3 activation in NIH3T3-W4P cells, we evaluated whether ACE
suppresses the colony-forming capability of NIH3T3-W4P cells. As
shown in Fig. 4D, ACE treatment
decreased the number of colonies in a dose-dependent manner, and
250 µg/ml of ACE almost completely abolished the colony-forming
ability of NIH3T3-W4P cells. These results suggest that ACE is
capable of suppressing tumorigenesis induced by HBV mutations and
subsequent inflammatory responses including STAT3 activation in
addition to HCC cell proliferation.
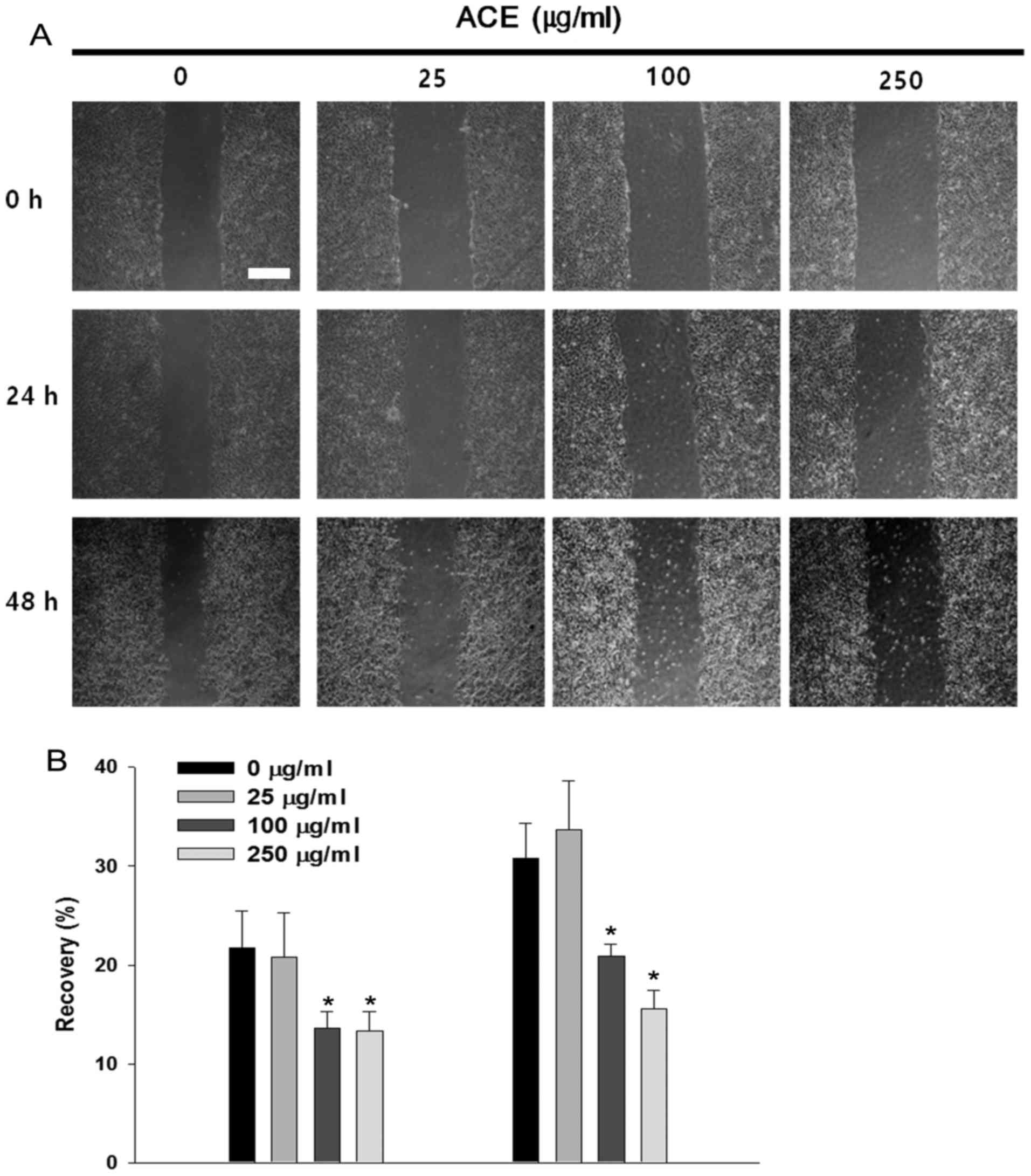
Suppressive effects of ACE on HCC cell
migration
STAT3 has been implicated in cancer cell migration
and invasion through the upregulation of pro-migratory genes
including MMP-2 (26). In the
absence of ACE, Huh7 cells migrated along the edge and repaired
approximately 30% of the wound after 48 h. Significant suppression
of cell migration and wound recovery were observed in the presence
of ACE and the inhibitory activity was concentration-dependent
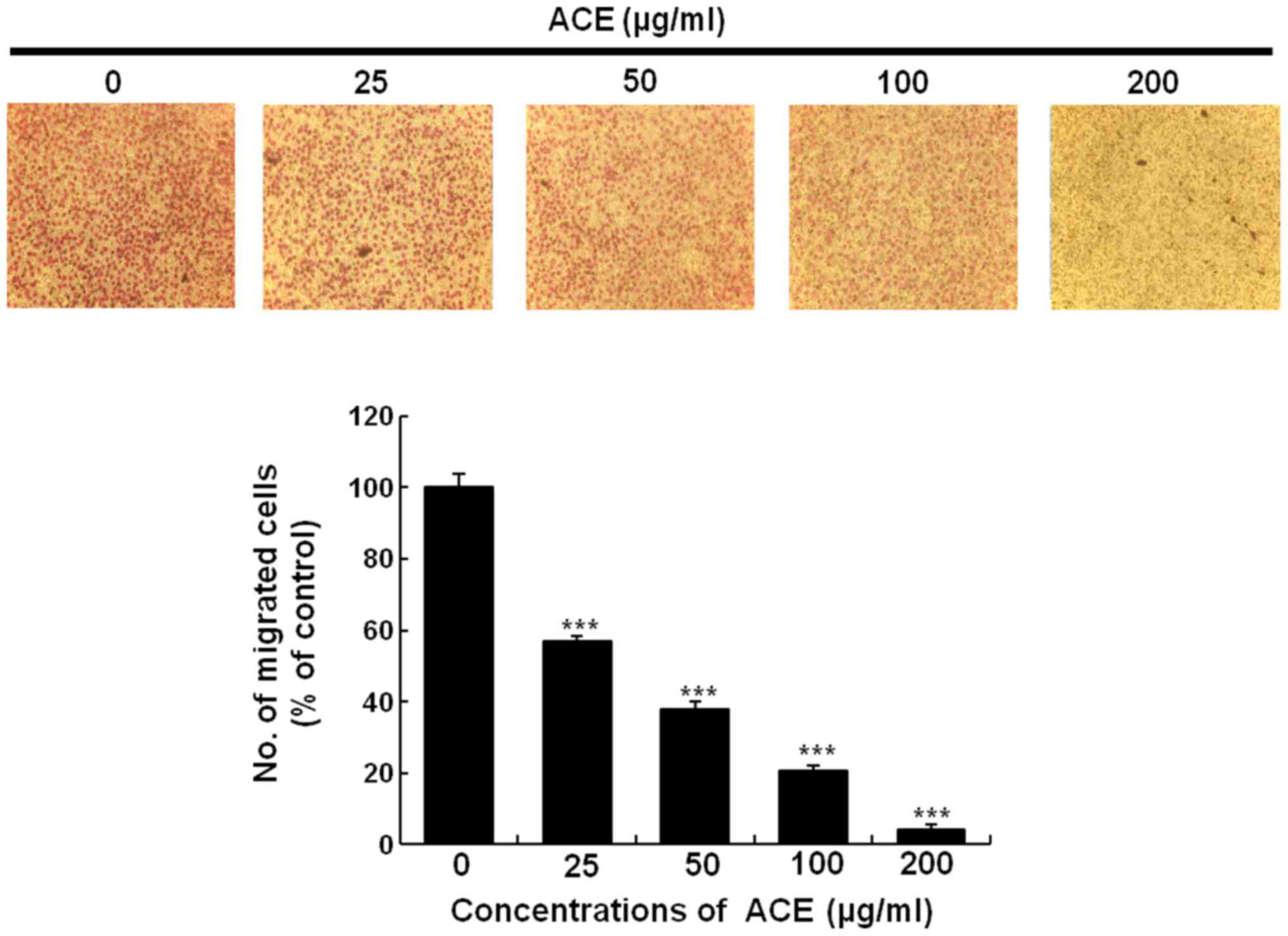
(Fig. 5A and B). Cell migration
assay using Boyden chamber showed similar result. Treatment with
200 µg/ml of ACE suppressed over 95% of cell migration further
confirming the suppressive activity of ACE on tumor cell migration
(Fig. 6).
Discussion
AC has been widely used as a traditional remedy for
various liver diseases in Asian countries. In the present study, we
showed that ACE moderately suppressed the proliferation of HCC
cells and it exerted stronger effect on colony-forming activity of
HCC. In addition, we have shown that ACE treatment effectively
suppressed STAT3 activation resulting in decreased cell
proliferation and migration of HCC cells. Since its discovery,
various tumor-promoting effects of STAT3, including cell cycle
progression, anti-apoptosis, angiogenesis, and invasion/migration,
have been studied and considered as key factors in oncogenesis and
cancer progression (27). In
particular, long-term IL-6-mediated inflammatory responses and
subsequent STAT3 activation have been implicated in various liver
pathogenic conditions including hepatitis, liver fibrosis,
cirrhosis, and HCC carcinogenesis (28), suggesting that inhibition of STAT3
activation can be an effective strategy to treat various liver
diseases. Given that IL-6/STAT3 signaling plays a crucial role in
HCC development and progression, it is conceivable that the
anticancer effect of ACE on HCC might be attributable to its
inhibitory activity on the IL-6/STAT3 signaling pathway and
subsequent cell cycle-related gene expression.
Chronic infection of HBV is a leading cause of HCC
development and approximately 15–40% of chronic hepatitis B
patients may develop progressed liver diseases including cirrhosis
and HCC. In our previous study, we showed that a male-specific
mutation of HBV is closely related to HCC development through the
activation of STAT3. Ectopic expression of W4P LHB strongly induces
STAT3 activation in NIH3T3 cells and transforming of the cells
(4). In the current study, ACE
suppressed not only STAT3 activation in IL-6-treated HCC cells but
also STAT3 activation by W4P HBV LHB expression in fibroblast
cells, suggesting that ACE may suppress tumorigenesis induced by
HBV-mediated STAT3 activation. Thus, this study provides
theoretical basis for the use of ACE in the treatment of HCC and
prevention of HCC in chronic hepatitis patients.
The exact mechanisms of STAT3 inhibition by ACE and
active ingredients inhibiting STAT3 activity remain elusive.
Several compounds in ACE including capillarisin, scoparone,
scopoletin and cholorogenic acid have been reported to suppress the
activity of STAT3 (24,29–31).
Thus, it can be postulated that several compounds in ACE are
responsible for STAT3 inhibition. ACE treatment on HepG2 cells
resulted in decrease of STAT3 protein levels, indicating that the
suppression of STAT3 activity is partly due to the lowered level of
STAT3 (Fig. 3A). However, STAT3
phosphorylation of NIH3T3 cells induced by W4P LHB expression was
markedly reduced despite the similar protein level of STAT3,
indicating that ACE is also capable of suppressing the activation
of STAT3 (Fig. 4A and B).
Regulation of IL-6/STAT3 signaling by ACE suggests
its potential as a therapeutic agent against various liver diseases
related to IL-6/STAT3 inflammatory responses. Identification of the
constituents responsible for the STAT3 inhibition and unveiling of
underlying molecular mechanism remain to be elucidated.
Acknowledgements
This study was supported by a grant of the Korea
Health Technology R&D Project through the Korea Health Industry
Development Institute (KHIDI), funded by the Ministry of Health and
Welfare, Republic of Korea (grant number: HI14C0955).
References
|
1
|
Abiru S, Migita K, Maeda Y, Daikoku M, Ito
M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, et al:
Serum cytokine and soluble cytokine receptor levels in patients
with non-alcoholic steatohepatitis. Liver Int. 26:39–45. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Li P-K, Li C and Lin J: Inhibition
of STAT3 signaling blocks the anti-apoptotic activity of IL-6 in
human liver cancer cells. J Biol Chem. 285:27429–27439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Svinka J, Mikulits W and Eferl R: STAT3 in
hepatocellular carcinoma: New perspectives. Hepat Oncol. 1:107–120.
2014. View
Article : Google Scholar
|
|
4
|
Lee SA, Kim H, Won YS, Seok SH, Na Y, Shin
HB, Inn KS and Kim BJ: Male-specific hepatitis B virus large
surface protein variant W4P potentiates tumorigenicity and induces
gender disparity. Mol Cancer. 14:232015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cronquist A: Vascular Flora of the
Southeastern United States: Asteraceae. UNC Press Books. 2001.
|
|
6
|
Nibret E and Wink M: Volatile components
of four Ethiopian Artemisia species extracts and their in vitro
antitrypanosomal and cytotoxic activities. Phytomedicine.
17:369–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han JM, Kim HG, Choi MK, Lee JS, Lee JS,
Wang JH, Park HJ, Son SW, Hwang SY and Son CG: Artemisia capillaris
extract protects against bile duct ligation-induced liver fibrosis
in rats. Exp Toxicol Pathol. 65:837–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha J-D, Jeong M-R, Jeong S-I, Moon SE,
Kim JY, Kil BS and Song YH: Chemical composition and antimicrobial
activity of the essential oils of Artemisia scoparia and A.
capillaris. Planta Med. 71:186–190. 2005. View Article : Google Scholar
|
|
9
|
Hong J-H, Hwang E-Y, Kim H-J, Jeong Y-J
and Lee IS: Artemisia capillaris inhibits lipid accumulation in
3T3-L1 adipocytes and obesity in C57BL/6J mice fed a high fat diet.
J Med Food. 12:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee J, Chae K, Ha J, Park BY, Lee HS,
Jeong S, Kim MY and Yoon M: Regulation of obesity and lipid
disorders by herbal extracts from Morus alba, Melissa officinalis,
and Artemisia capillaris in high-fat diet-induced obese mice. J
Ethnopharmacol. 115:263–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori H, Xu Q, Sakamoto O, Uesugi Y, Koda A
and Nishioka I: Mechanisms of antitumor activity of aqueous
extracts from Chinese herbs: Their immunopharmacological
properties. Jpn J Pharmacol. 49:423–431. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong JH and Lee IS: Effects of Artemisia
capillaris ethyl acetate fraction on oxidative stress and
antioxidant enzyme in high-fat diet induced obese mice. Chem Biol
Interact. 179:88–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HI, Seo KO, Yun KW, Kim MJ and Lee MK:
Comparative study of the hepatoprotective efficacy of Artemisia
iwayomogi and Artemisia capillaris on ethanol-administered mice. J
Food Sci. 76:T207–T211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Geng CA, Ma YB, Huang XY, Chen H,
Cao TW, He K, Wang H, Zhang XM and Chen JJ: UFLC/MS-IT-TOF guided
isolation of anti-HBV active chlorogenic acid analogues from
Artemisia capillaris as a traditional Chinese herb for the
treatment of hepatitis. J Ethnopharmacol. 156:147–154. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Geng CA, Chen H, Ma YB, Huang XY,
Cao TW, He K, Wang H, Zhang XM and Chen JJ: Isolation, synthesis
and anti-hepatitis B virus evaluation of p-hydroxyacetophenone
derivatives from Artemisia capillaris. Bioorg Med Chem Lett.
25:1509–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Geng CA, Sun CL, Ma YB, Huang XY,
Cao TW, He K, Wang H, Zhang XM and Chen JJ: Polyacetylenes and
anti-hepatitis B virus active constituents from Artemisia
capillaris. Fitoterapia. 95:187–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim KS, Yang HJ, Lee JY, Na YC, Kwon SY,
Kim YC, Lee JH and Jang HJ: Effects of β-sitosterol derived from
Artemisia capillaris on the activated human hepatic stellate cells
and dimethylnitrosamine-induced mouse liver fibrosis. BMC
Complement Altern Med. 14:3632014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J-H, Choi M-K, Shin J-W, Hwang S-Y
and Son C-G: Antifibrotic effects of Artemisia capillaris and
Artemisia iwayomogi in a carbon tetrachloride-induced chronic
hepatic fibrosis animal model. J Ethnopharmacol. 140:179–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: The need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang C-C, Lee M-R, Hsu S-L and Chang C-MJ:
Supercritical fluids extraction of capillarisin from Artemisia
capillaris and its inhibition of in vitro growth of hepatoma cells.
J Supercrit Fluids. 42:96–103. 2007. View Article : Google Scholar
|
|
22
|
Hu YQ, Tan RX, Chu MY and Zhou J:
Apoptosis in human hepatoma cell line SMMC-7721 induced by
water-soluble macromolecular components of Artemisia capillaris
Thunberg. Jpn J Cancer Res. 91:113–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong SH, Seo SH, Lee JH and Choi BT: The
aqueous extract from Artemisia capillaris Thunb. inhibits
lipopolysaccharide-induced inflammatory response through preventing
NF-kappaB activation in human hepatoma cell line and rat liver. Int
J Mol Med. 13:717–720. 2004.
|
|
24
|
Lee JH, Chiang SY, Nam D, Chung WS, Lee J,
Na YS, Sethi G and Ahn KS: Capillarisin inhibits constitutive and
inducible STAT3 activation through induction of SHP-1 and SHP-2
tyrosine phosphatases. Cancer Lett. 345:140–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang SF, Chen MK, Hsieh YS, Yang JS,
Zavras AI, Hsieh YH, Su SC, Kao TY, Chen PN and Chu SC:
Antimetastatic effects of Terminalia catappa L. on oral cancer via
a down-regulation of metastasis-associated proteases. Food Chem
Toxicol. 48:1052–1058. 2010.
|
|
26
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann NY Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furtek SL, Backos DS, Matheson CJ and
Reigan P: Strategies and approaches of targeting STAT3 for cancer
treatment. ACS Chem Biol. 11:308–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Subramaniam A, Shanmugam MK, Perumal E, Li
F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, et al:
Potential role of signal transducer and activator of transcription
(STAT)3 signaling pathway in inflammation, survival, proliferation
and invasion of hepatocellular carcinoma. Biochim Biophys Acta.
1835:46–60. 2013.PubMed/NCBI
|
|
29
|
Kim JK, Kim JY, Kim HJ, Park KG, Harris
RA, Cho WJ, Lee JT and Lee IK: Scoparone exerts anti-tumor activity
against DU145 prostate cancer cells via inhibition of STAT3
activity. PLoS One. 8:e803912013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhattacharyya SS, Paul S, Dutta S,
Boujedaini N and Khuda-Bukhsh AR: Anti-oncogenic potentials of a
plant coumarin (7-hydroxy-6-methoxy coumarin) against
7,12-dimethylbenz [a] anthracene-induced skin papilloma in mice:
The possible role of several key signal proteins. Zhong Xi Yi Jie
He Xue Bao. 8:645–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan Z, Luo M, Yang J, Cheng Y, Huang J, Lu
C, Song D, Ye M, Dai M, Gonzalez FJ, et al: Chlorogenic acid
inhibits cholestatic liver injury induced by
α-naphthylisothiocyanate: Involvement of STAT3 and NFκB signalling
regulation. J Pharm Pharmacol. 68:1203–1213. 2016. View Article : Google Scholar : PubMed/NCBI
|