Introduction
Apoptosis, or programmed cell death, occurs through
both extrinsic and intrinsic pathways, which are modulated by
apoptotic proteins including cytochrome c, caspase-8 and −3
(1). Morphological and biochemical
hallmarks of apoptosis include cell shrinkage, chromatin
condensation, DNA fragmentation and formation of apoptotic bodies
(2). Anticancer agents and
radiation are able to induce apoptotic protein-mediated signal
transduction pathways and consequent inhibition of tumor growth
(1,3). Anticancer agents and radiation-induced
apoptosis can be blocked by overexpression of anti-apoptotic
proteins in cancer cells leading to treatment failure (4). Human hepatocellular carcinoma (HCC) is
endemic in Asia and among the deadliest types of cancers (5). Overexpression of anti-apoptotic
proteins such as cellular FLICE-like inhibitory protein (c-FLIP),
myeloid cell leukemia-1 (Mcl-1), and X-linked inhibitor of
apoptosis protein (XIAP) has been identified in HCC and is
associated with the poor prognosis of HCC patients (6–8).
Nuclear factor-κB (NF-κB) is a transcription factor
of a number of oncogenes which modulate tumorigenesis (9). Cancer hallmarks that include
self-sufficiency in proliferative growth signals, insensitivity to
anti-growth signals, evasion of apoptosis, limitless replicative
potential, tissue invasion and metastasis, and sustained
angiogenesis have been related to NF-κB-modulated expression of
downstream effector proteins (10).
Various anticancer agents and radiation not only trigger apoptosis,
but also activate expression of NF-κB-induced anti-apoptotic
proteins resulting in the reduction of therapeutic efficacy in HCC
both in vitro and in vivo (11,12).
Constitutive NF-κB activation is observed in patients with advanced
HCC and may be used as a negative prognostic biomarker (13). Therefore, development of NF-κB
signal inhibitors may facilitate the treatment of HCC patients.
Regorafenib (Stivarga®) is a multi-kinase
inhibitor with a similar chemical structure to sorafenib
(Nexavar®), but has an additional functional group,
which produces more potent activity to inhibit oncogenic receptor
tyrosine and cytoplasmic signaling kinases (14). Regorafenib has been approved to
treat colorectal cancer and gastrointestinal stromal tumors. A
recent update of an ongoing phase III clinical trial reported that
regorafenib was effective in patients with sorafenib-resistant HCC
(15). In our previous study,
sorafenib, as an inhibitor of NF-κB signaling, was found to reduce
the expression of NF-κB-modulated anti-apoptotic proteins in HCC
both in vitro and in vivo (12). However, whether regorafenib, an
analogue of sorafenib, can induce apoptosis through blockage of
NF-κB activation in HCC cells remains obscure. The aim of the
present study was to investigate the role of NF-κB inactivation on
regorafenib-induced apoptosis in SK-HEP-1 cells using MTT assay,
flow cytometry, DNA gel electrophoresis, western blotting and NF-κB
reporter gene assay. ERK, AKT, JNK and P38 inhibitors were used to
determine the mechanism of regorafenib-induced NF-κB inactivation
in HCC.
Materials and methods
Agents and antibodies
Regorafenib was provided by Bayer Health Care
Pharmaceuticals (Whippany, NJ, USA). Dulbecco's modified Eagles
medium (DMEM), fetal bovine serum (FBS), L-glutamine and
penicillin-streptomycin (PS) were purchased from Gibco/Life
Technologies (Carlsbad, CA, USA). Propidium iodide (PI) and
DiOC6 were purchased from BioVision (Mountain View, CA,
USA) and Enzo Life Sciences (Farmingdale, NY, USA), respectively.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and RNase were purchased from Sigma-Aldrich (St. Louis, MO, USA)
and Fermentas (St. Leon-Rot, Baden-Württemberg, Germany),
respectively. Primary antibodies of cleaved-caspase-3, cellular
FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein
(c-FLIP) and pAKT (Ser473) were purchased from Cell Signaling
Technology (Beverly, MA, USA). Primary antibodies of caspase-8 and
X-linked inhibitor of apoptosis protein (XIAP) were obtained from
Thermo Fisher Scientific (Fremont, CA, USA). Primary antibodies of
ERK, AKT, NF-κB p65, β-actin and cytochrome c were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary
antibodies of MCL-1 and pERK were purchased from BioVision
(Milpitas, CA, USA) and Merck Millipore (Billerica, MA, USA),
respectively. Secondary antibodies were purchased from Jackson
ImmunoResearch (West Grove, PA, USA). Nuclear and Cytoplasmic
Extraction and Genomic DNA Miniprep kits were obtained from
Chemicon (Temecula, CA, USA) and Axygen Biosciences (Union City,
CA, USA), respectively. NF-κB inhibitor
4-N-[2-(4-phenoxyphenyl)ethyl]quinazoline-4,6-diamine (QNZ), AKT
inhibitor LY294002, c-Jun N-terminal kinase (JNK) inhibitor
SP600125, P38 inhibitor SB203580 and extracellular signal-regulated
kinase (ERK) inhibitor PD98059 were purchased from Selleckchem
(Houston, TX, USA). NF-κB-luciferrase2 vector (pNF-κB/luc2)
and D-luciferin were obtained from Promega (Madison, WI, USA) and
Caliper Life Science (Hopkinton, MA, USA), respectively. Hygromycin
B was purchased from Santa Cruz Biotechnology.
Cell culture
SK-HEP-1 cells were gifted by Professor Jing-Gung
Chung of the Department of Biological Science and Technology, China
Medical University, (Taichung, Taiwan). Cells were cultured in DMEM
and supplemented with 10% FBS, 2 mM L-glutamine and PS (100 U/ml
and 100 µg/ml) at 37°C in an atmosphere of 5% CO2
(16).
Plasmid transfection and stable clone
selection
SK-HEP-1 cells were transfected with
pNF-κB/luc2 using JetPEI™. Cells (1×106) were
seeded into 10-cm dish and incubated overnight. DNA solution (10 µg
NF-κB/luc2 plasmid dissolved in 250 µl of 150 mM NaCl) was mixed
with 250 µl JetPEI solution (20 µl of JetPEI reagent diluted in 230
µl of 150 mM NaCl), and then incubated for 30 min at room
temperature to make 500 µl DNA/JetPEI mixture. The DNA/JetPEI
mixture was added to the SK-HEP-1 cells in a 10-cm diameter dish
and incubated for 24 h. After transfection, the cells were cultured
in medium containing 200 µg/ml of hygromycin B for two weeks. The
surviving clones were subsequently subcultured into 96-well plates.
Function of the NF-κB reporter gene in each clone was assayed using
the IVIS 200 Imaging System (Xenogen, Alameda, CA, USA). Cells with
functional NF-κB reporter gene product were renamed as
SK-HEP-1/NF-κB-luc2 cells (12).
MTT assay
SK-HEP-1 cells were seeded into 96-well plates at a
density of 3×104 cells/well and incubated overnight.
Cells were treated with different concentrations of QNZ (0–0.4 µM
in 0.1% DMSO) or regorafenib (0–50 µM in 0.1% DMSO) for different
periods, and then the change in cell viability was determined with
the MTT assay as previously described (17).
Detection of mitochondrial membrane
potential (MMP)
SK-HEP-1 cells were seeded into 12-well plates at a
density of 2×105 cells/well and incubated overnight.
Cells were treated with 0.4 µM QNZ or 20 µM regorafenib for
different periods. Cells were harvested by centrifugation and
washed twice with phosphate-buffered saline (PBS), and then stained
with DiOC6 solution (4 µM DiOC6 in 500 µl
PBS) for 30 min at 37°C. Detection of MMP was performed using flow
cytometry (FACS101; FACScan; Becton-Dickinson, Franklin Lakes, NJ,
USA) as described by Wang et al (18).
Analysis of the sub-G1
population
SK-HEP-1 cells were seeded into 12-well plates at a
density of 2×105 cells/well and incubated overnight.
Cells were treated with 0.4 µM QNZ or 20 µM regorafenib for
different periods. The cells were harvested by centrifugation and
fixed with 70% ethanol and incubated overnight at −20°C. Cells were
washed twice with PBS and then stained with 500 µl of PI buffer (40
µg/ml PI, 100 µg/ml RNase and 1% Triton X-100 in PBS) for 1 h in
darkness at room temperature. Detection of the sub-G1
cell population was performed using flow cytometry (FACS101;
FACScan) as described by Huang et al (19).
Western blot assay
SK-HEP-1 cells (3×106) were seeded into
10-cm diameter dishes and incubated overnight. Then, the cells were
treated with 0.4 µM QNZ or 20 µM regorafenib for different periods.
Lysis buffer (50 mM Tris-HCl pH 8.0, 120 mM NaCl, 0.5% NP-40 and 1
mM phenylmethanesulfonyl fluoride) was used for total protein
extraction from the cells in the different treatment groups.
Cytosolic proteins from cells in each group were extracted using a
cytosol extraction kit following the instructions provided by the
manufacturer. Protein expression of NF-κB p65, NF-κB p65 (Ser536),
XIAP, Mcl-1, c-FLIP, cleaved-caspase-3, caspase-8, cytochorme
c, ERK, pERK, AKT and pAKT were evaluated with western blot
assay as described by Ting et al (20). Quantification of protein bands was
performed using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Detection of DNA fragmentation
SK-HEP-1 cells were seeded into 6-well plates at a
density of 1×106 cells/well and incubated overnight, and
then treated with 0.4 µM QNZ or 20 µM regorafenib for different
periods. Genomic DNA from the cells was purified using the
GenElute™ Mammalian Genomic DNA Miniprep kit (Sigma-Aldrich)
following the instructions provided by the manufacturer. Analysis
of DNA fragmentation was performed using 1.5% agarose gel
electrophoresis (12).
NF-κB reporter gene assay
SK-HEP-1 cells were seeded into 96-well plates at a
density of 3×104 cells/well and incubated overnight. The
detailed conditions for the different treatment groups are provided
in detail in the figure legends. D-luciferin solution (500 µM
D-luciferin in 100 µl PBS) was added to each well, and photon
signal was acquired for 1 min using the IVIS 200 Imaging System.
Relative NF-κB activity was corrected by cell viability which was
evaluated by the MTT assay as previously described (17).
Statistical analysis
Results are all representative of at least three
independent experiments. Statistical significance was determined
using the Student's t-test. p-values of <0.05 were considered
statistically significant.
Results
NF-κB inhibitor diminishes the
expression of anti-apoptotic proteins and induces both extrinsic
and intrinsic apoptosis in the SK-HEP-1 cells
In order to verify the effects of NF-κB inactivation
on pro-apoptotic and anti-apoptotic signal transduction, SK-HEP-1
cells were initially treated with different concentrations of QNZ
for different periods. Subsequently, cell viability, expression of
NF-κB p65 (Ser536), anti-apoptotic and pro-apoptotic proteins, and
the effects of apoptosis were evaluated with MTT assay, western
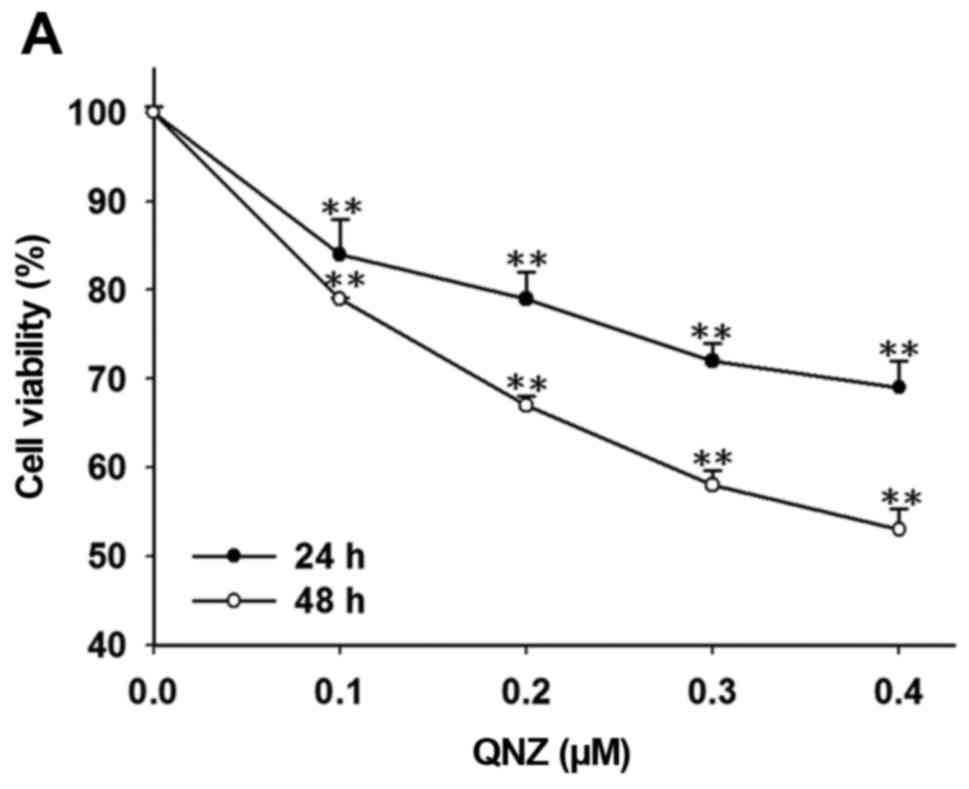
blotting, DNA gel electrophoresis and flow cytometry. Fig. 1A indicates that QNZ significantly
reduced cell viability in a dose- and time-dependent manner as
compared to that noted in the control cells (vehicle treatment with
0.1% DMSO). Fig. 1B shows that QNZ
not only inhibited expression of NF-κB p65 (Ser536) and
anti-apoptotic proteins (XIAP, MCL-1 and c-FLIP), but also
increased levels of pro-apoptotic proteins (cleaved-caspase-3 and
−8, and cytochrome c). DNA fragmentation is one of the
apoptotic hallmarks and QNZ-induced DNA fragmentation is
demonstrated in Fig. 1C. Apoptosis
also can be measured by flow cytometry to detect the
sub-G1 cell population and MMP. The sub-G1
cell population and loss of MMP were significantly enhanced by
regorafenib treatment in a time-dependent manner as compared to the
control (Fig. 1D and E).
Regorafenib inhibits expression of
NF-κB-modulated anti-apoptotic proteins and induces both extrinsic
and intrinsic apoptosis in the SK-HEP-1 cells
The SK-HEP-1 cells were treated with different
concentrations of regorafenib for different periods. Cell
viability, expression of NF-κB p65 (Ser536), expression of
anti-apoptotic and pro-apoptotic proteins, and regorafenib effects
on apoptosis were evaluated with MTT assay, western blotting, DNA
gel electrophoresis, and flow cytometry. Regorafenib significantly
decreased cell viability in a dose- and time-dependent manner as
compared to that noted in the control cells (Fig. 2A). Regorafenib also significantly
inhibited expression of NF-κB p65 (Ser536) and anti-apoptotic
proteins (XIAP, MCL-1 and c-FLIP) while increased the levels of
pro-apoptotic proteins (cleaved-caspase-3 and −8, and cytochrome
c) in a time-dependent manner as compared to the control
group (Fig. 2B). Fig. 2C shows that regorafenib induced DNA
fragmentation and significantly induced the sub-G1 cell
population and loss of MMP in a time-dependent manner as compared
to the control (Fig. 2D and E).
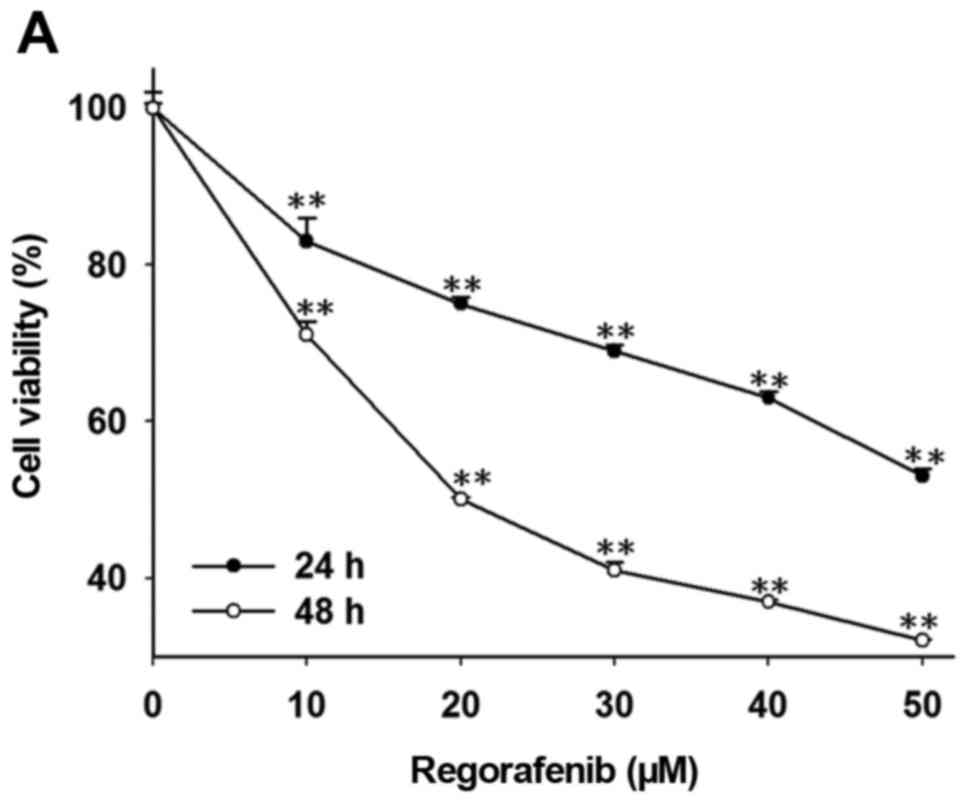 | Figure 2.Effects of regorafenib on cell
viability, expression of NF-κB-modulated anti-apoptotic proteins
and apoptosis pathways in SK-Hep1 cells. Cells were treated with
different concentration (0, 10, 20, 30, 40 and 50 µM in 0.1% DMSO)
of regorafenib for 24 and 48 h. (A) Change in cell viability was
determined with MTT assay. **p<0.01. (B) Protein levels of NF-κB
p65 (Ser536), XIAP, c-FLIP, MCL-1, cleaved-caspase-3 and −8, and
cytochrome c were evaluated by western blot assay.
ap<0.05 and bp<0.01 as compared with
the control. (C) Detection of DNA fragmentation was performed using
gel electrophoresis. Determination of Sub G1 population
was performed using flow cytometry. **p<0.01 as compared with
the control. (E) Change of MMP was investigated using flow
cytometry. *p<0.05 and **p<0.01 as compared with the
control. |
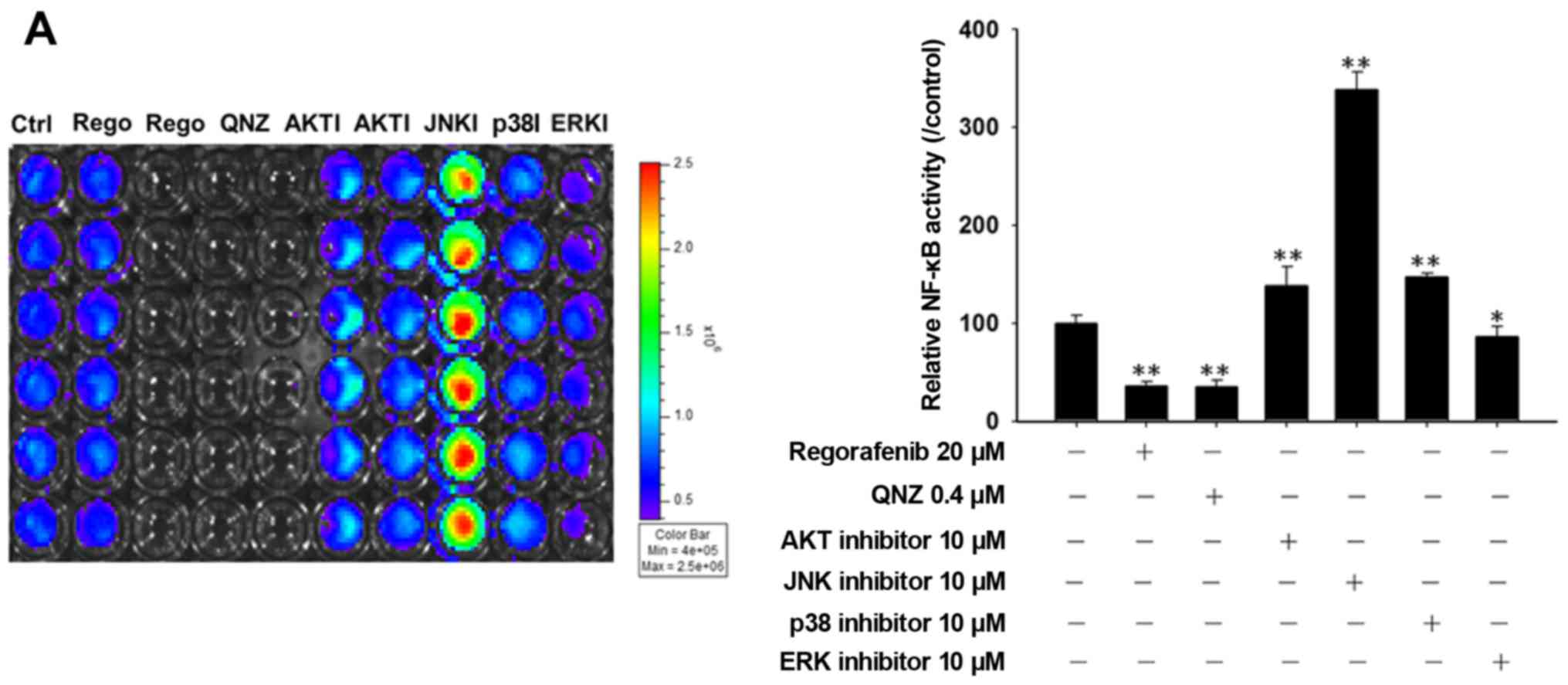
Regorafenib inhibits NF-κB activation
through ERK dephosphorylation in the SK-HEP-1 cells
We found that regorafenib reduced NF-κB activation
and this turns regorafenib into an inhibitor of NF-κB signaling. In
the next step, we used different kinase (AKT, JNK, P38 and ERK)
inhibitors to investigate the mechanism of regorafenib-induced
NF-κB inactivation in the SK-HEP-1 cells. Fig. 3A and B shows that regorafenib, QNZ
(NF-κB inhibitor) and the ERK inhibitor (PD98059) significantly
reduced NF-κB activation. Fig. 3C
indicates that regorafenib also inhibited ERK and AKT
phosphorylation in a time-dependent manner in the SK-HEP-1
cells.
Discussion
Regorafenib, a sorafenib analogue, has been approved
for the treatment of metastatic colorectal cancer and advanced
gastrointestinal stromal tumors (15). Sorafenib, as an inhibitor of NF-κB
signaling, was indicated in our previous study to reduce the
expression of NF-κB-modulated anti-apoptotic proteins and trigger
the apoptotic pathway in HCC both in vitro and in
vivo (12). However, whether
regorafenib induces apoptosis through inhibition of NF-κB
activation in HCC cells requires elucidation. Therefore, we
evaluated the effects of regorafenib on NF-κB inhibition-related
apoptosis and the mechanism in HCC SK-HEP-1 cells in vitro.
First, we found that the NF-κB inhibitor QNZ reduced NF-κB
activation and anti-apoptotic protein levels (XIAP, c-FLIP and
MCL-1) while triggered extrinsic and intrinsic apoptotic pathways
(Fig. 1A-E). Secondly, regorafenib
as inhibitor of NF-κB signaling also suppressed NF-κB activation
and anti-apoptotic protein levels, while induced extrinsic and
intrinsic apoptotic pathways (Fig.
2A-E). Finally, we found that the ERK inhibitor reduced NF-κB
activation and regorafenib diminished ERK phosphorylation (Fig. 3A and B).
Expression of anti-apoptotic proteins such as XIAP,
c-FLIP and MCL-1 is linked to constitutive NF-κB activation in
cancer cells (12,21). XIAP can interact with the active
site of caspase-3 resulting in inhibition of caspase-3-mediated
apoptosis (22). c-FLIP, a
caspase-8 inhibitor, disrupts caspase-8 and prevents initiation of
the extrinsic apoptotic pathway (23). MCL-1 suppresses loss of MMP and
cytochrome c release from mitochondria that subsequently
leads to inhibition of the intrinsic apoptotic pathway (24,25).
The present study results demonstrated that both the NF-κB
inhibitor and regorafenib inhibited NF-κB activation, reduced
anti-apoptotic protein (XIAP, c-FLIP and MCL-1) expression, and
activated extrinsic and intrinsic apoptotic pathways. Chen et
al suggested that regorafenib activates NF-κB-regulated
expression of p53-upregulated modulator of apoptosis (PUMA) and
inhibits colorectal tumor growth (26). RAF/mitogen-activated protein kinase
kinase (MEK)/ERK and phosphoinositide 3-kinase (PI3K)/AKT signaling
transduction are the most critical pathways in the development and
progression of HCC. Activation of ERK and AKT can be used as
biomarkers to predict poor prognosis in HCC (27). Sorafenib induces apoptosis and
inhibits angiogenesis in HCC via blockage of the RAF/MEK/ERK
pathway. However, AKT activation is not inhibited by sorafenib
(28). Fig. 3C shows that regorafenib
significantly reduced both ERK and AKT phosphorylation. NF-κB can
be activated through different kinases, such as AKT, JNK, P38 or
ERK in different types of cancer cells (12,29–30).
We used inhibitors of AKT, JNK, P38 and ERK to verify the mechanism
of regorafenib-induced NF-κB inactivation in the SK-HEP-1 cells. We
found that the ERK inhibitor revealed similar effects in the
inhibition of NF-κB activation as regorafenib or QNZ (Fig. 3A and B). Therefore, we suggest that
regorafenib inhibits NF-κB activation via dephosphorylation of ERK.
In previous studies, we also found that sorafenib inhibited
NF-κB-modulated tumor progression through suppression of ERK
activation in HCC Huh7 cells (12,17).
In conclusion, the present study demonstrated that
regorafenib triggered extrinsic and intrinsic apoptotic pathways
through blockage of ERK/NF-κB activation in SK-HEP-1 cells in
vitro. We propose that regorafenib may be a potential
anticancer agent for the treatment of advanced HCC.
Acknowledgements
The present study was supported by a grant to
J.-J.T. (RD2016-020) from the National Yang-Ming University
Hospital (Yilan, Taiwan). We acknowledge the technical services
provided by the Clinical Medicine Research Laboratory of National
Yang-Ming University Hospital.
Glossary
Abbreviations
Abbreviations:
|
MMP
|
mitochondrial membrane potential
|
|
C-FLIP
|
cellular FLICE-like inhibitory
protein
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
|
Mcl-1
|
myeloid leukemia cell differentiation
protein
|
|
NF-κB
|
nuclear factor-κB
|
|
AKT
|
protein kinase B
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
QNZ
|
NF-κB inhibitor
|
|
JNK
|
Jun amino-terminal kinases
|
|
P38
|
P38 mitogen-activated protein
kinase
|
References
|
1
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fulda S and Debatin KM: Apoptosis
signaling in tumor therapy. Ann NY Acad Sci. 1028:150–156. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verheij M and Bartelink H:
Radiation-induced apoptosis. Cell Tissue Res. 301:133–142. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pommier Y, Sordet O, Antony S, Hayward RL
and Kohn KW: Apoptosis defects and chemotherapy resistance:
Molecular interaction maps and networks. Oncogene. 23:2934–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du X, Bao G, He X, Zhao H, Yu F, Qiao Q,
Lu J and Ma Q: Expression and biological significance of c-FLIP in
human hepatocellular carcinomas. J Exp Clin Cancer Res. 28:24–31.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fleischer B, Schulze-Bergkamen H,
Schuchmann M, Weber A, Biesterfeld S, Müller M, Krammer PH and
Galle PR: Mcl-1 is an anti-apoptotic factor for human
hepatocellular carcinoma. Int J Oncol. 28:25–32. 2006.PubMed/NCBI
|
|
8
|
Augello C, Caruso L, Maggioni M, Donadon
M, Montorsi M, Santambrogio R, Torzilli G, Vaira V, Pellegrini C,
Roncalli M, et al: Inhibitors of apoptosis proteins (IAPs)
expression and their prognostic significance in hepatocellular
carcinoma. BMC Cancer. 9:125–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen JH, Chen WL and Liu YC: Amentoflavone
induces anti-angiogenic and anti-metastatic effects through
suppression of NF-κB activation in MCF-7 cells. Anticancer Res.
35:6685–6693. 2015.PubMed/NCBI
|
|
10
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu FT, Liu YC, Chiang IT, Liu RS, Wang
HE, Lin WJ and Hwang JJ: Sorafenib increases efficacy of vorinostat
against human hepatocellular carcinoma through transduction
inhibition of vorinostat-induced ERK/NF-κB signaling. Int J Oncol.
45:177–188. 2014.PubMed/NCBI
|
|
12
|
Hsu FT, Liu YC, Liu TT and Hwang JJ:
Curcumin sensitizes hepatocellular carcinoma cells to radiation via
suppression of radiation-induced NF-κB activity. Biomed Res Int.
2015:3636712015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin Y, Chen J, Feng Z, Fan W, Wang Y, Li J
and Tong D: The expression of Survivin and NF-κB associated with
prognostically worse clinicopathologic variables in hepatocellular
carcinoma. Tumour Biol. 35:9905–9910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ravi S and Singal AK: Regorafenib: An
evidence-based review of its potential in patients with advanced
liver cancer. Core Evid. 9:81–87. 2014.PubMed/NCBI
|
|
15
|
Tai WT, Chu PY, Shiau CW, Chen YL, Li YS,
Hung MH, Chen LJ, Chen PL, Su JC, Lin PY, et al: STAT3 mediates
regorafenib-induced apoptosis in hepatocellular carcinoma. Clin
Cancer Res. 20:5768–5776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma CY, Ji WT, Chueh FS, Yang JS, Chen PY,
Yu CC and Chung JG: Butein inhibits the migration and invasion of
SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK,
JNK, p38, and uPA signaling multiple pathways. J Agric Food Chem.
59:9032–9038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang IT, Liu YC, Wang WH, Hsu FT, Chen
HW, Lin WJ, Chang WY and Hwang JJ: Sorafenib inhibits TPA-induced
MMP-9 and VEGF expression via suppression of ERK/NF-κB pathway in
hepatocellular carcinoma cells. In Vivo. 26:671–681.
2012.PubMed/NCBI
|
|
18
|
Wang WH, Chiang IT, Ding K, Chung JG, Lin
WJ, Lin SS and Hwang JJ: Curcumin-induced apoptosis in human
hepatocellular carcinoma j5 cells: Critical role of
Ca+2-dependent pathway. Evid Based Complement Alternat
Med. 2012:5129072012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang SH, Wu LW, Huang AC, Yu CC, Lien JC,
Huang YP, Yang JS, Yang JH, Hsiao YP, Wood WG, et al: Benzyl
isothiocyanate (BITC) induces G2/M phase arrest and
apoptosis in human melanoma A375.S2 cells through reactive oxygen
species (ROS) and both mitochondria-dependent and death
receptor-mediated multiple signaling pathways. J Agric Food Chem.
60:665–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ting CY, Wang HE, Yu CC, Liu HC, Liu YC
and Chiang IT: Curcumin triggers DNA damage and inhibits expression
of DNA repair proteins in human lung cancer cells. Anticancer Res.
35:3867–3873. 2015.PubMed/NCBI
|
|
21
|
Liu H, Yang J, Yuan Y, Xia Z, Chen M, Xie
L, Ma X, Wang J, Ouyang S, Wu Q, et al: Regulation of Mcl-1 by
constitutive activation of NF-κB contributes to cell viability in
human esophageal squamous cell carcinoma cells. BMC Cancer.
14:98–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scott FL, Denault JB, Riedl SJ, Shin H,
Renatus M and Salvesen GS: XIAP inhibits caspase-3 and −7 using two
binding sites: Evolutionarily conserved mechanism of IAPs. EMBO J.
24:645–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perciavalle RM and Opferman JT: Delving
deeper: MCL-1's contributions to normal and cancer biology. Trends
Cell Biol. 23:22–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morciano G, Giorgi C, Balestra D, Marchi
S, Perrone D, Pinotti M and Pinton P: Mcl-1 involvement in
mitochondrial dynamics is associated with apoptotic cell death. Mol
Biol Cell. 27:20–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen D, Wei L, Yu J and Zhang L:
Regorafenib inhibits colorectal tumor growth through PUMA-mediated
apoptosis. Clin Cancer Res. 20:3472–3484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH,
Kim WK and Kim HS: Curcumin suppresses phorbol ester-induced matrix
metalloproteinase-9 expression by inhibiting the PKC to MAPK
signaling pathways in human astroglioma cells. Biochem Biophys Res
Commun. 335:1017–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|