Introduction
Pancreatic cancer is the most intractable disease
among human cancers, with a low 5-year survival rate ranging from 5
to 6% (1,2). Pancreatic cancer patients are
asymptomatic so that nearly 85% cases are diagnosed at a late stage
accompanied by blood vessel invasion or distant metastasis, thus
are not available for surgical resection (3). Even for the few patients who underwent
a potentially curative resection, the long-term survival remains
disappointing due to early recurrence or metastatic disease
(4). Hence, a better understanding
of the molecular mechanisms underlying pancreatic cancer
progression is needed to investigate and design more effective
therapies.
N-myc downstream-regulated gene 1 (NDRG1) is a
member of the N-myc downregulated gene family which belongs to the
α/β hydrolase superfamily. Previous studies found that NDRG1 was
downregulated in colorectal (5),
gastric (6), cervical (7), ovarian (8) and prostate (9) cancer. Moreover, it has been reported
that NDRG1 played a tumor suppressive role in a variety of
tumorigenic signaling pathways. For example, Chen et al
found that NDRG1 inhibits the TGF-β-induced EMT by maintaining
E-cadherin and β-catenin at the cell membrane that cause decreased
vimentin and ability of cancer cell migration and invasion
(10). NDRG1 inhibits
phosphorylation and nuclear translocation of β-catenin which leads
to increased cell-cell adhesion and inhibition of the WNT pathway
(9). Overexpression of NDRG1 in
cancer cells not only induces differentiation but also reduces
invasion and metastasis (10).
Several studies further reported that a positive correlation
between NDRG1 expression and patient prognosis, indicating NDRG1
may be a prognostic biomarker in prostate (11) and colorectal cancer (12). Although NDRG1 was largely regarded
as a metastasis suppressor, there are also some studies revealing
that NDRG1 is positively associated with cancer cell proliferation,
differentiation and metastasis. In hepatocellular carcinoma, NDRG1
is significantly associated with advanced tumor stages and poor
survival of patients by promoting cancer cell growth and preventing
β-catenin degradation (13). In
lung cancer, NDRG1 is upregulated by HIF-1α and its overexpression
results in more proliferation and less apoptosis of cancer cells
(14). These above results may
implicate the tissue-specific effects of NDRG1, which suggests that
this protein plays pleiotropic roles in cancer progression. While
in pancreatic cancer, the biological functions of NDRG1 in the
pathogenesis of pancreatic cancer has not been investigated in
detail. In this study we explored the expression of NDRG1 in
pancreatic cancer and elucidate the effects of NDRG1 on the
invasion and migration capability of pancreatic cancer cell lines.
In addition, we examined whether change of those tumor related
capability could be influenced by NDRG1 mediated STAT3, MMP2, MMP9
expression.
Materials and methods
Cell lines and culture
The human pancreatic cancer cells, including
MIA-PACA-2, ASPC-1, BXPC-3, CAPAN-1, CAPAN-2, CFPAC-1, HPAF-II,
HS766T, PANC1 and SW1990 were obtained from the Americacn Type
culture Collection (ATCC, Manassas, VA, USA). PANC1, SW1990,
CAPAN-2, HS766T and MIA-PACA-2 cell lines were maintained in DMEM
with 10% FBS (Gibco, Carlsbad, CA, USA). ASPC-1, BXPC-3, CFPAC-1,
HPAF-II cell lines were maintained in 1640 with 10% FBS. CAPAN-1
cell line was maintained in IMDM with 10% FBS. The cells were
incubated in a humidified atmosphere of 5% CO2 and 95%
air.
RNA extraction and qRT-PCR
Total RNA was extracted from pancreatic tissues,
adjacent normal tissues and cell lines using TRIzol reagent
(Invitrogen, USA) according to the manufacturer's instructions.
cDNA was synthesized using the Prime-Script RT reagent kit
(Tiangen, Beijing, China). qRT-PCR was performed using SYBR Green
PCR Master Mix (Takara, Dalian, China) on an ABI 7500 fast
real-time PCR system (Applied Biosystems, Foster City, CA, USA).
Expression data were uniformly normalized to the internal control
U6 and the relative expression levels were evaluated using the ∆∆Ct
method. The primers for NDRG1 were 5′-CTCTGTTCACGTCACGCTGT-3′
(forward) and 5′-CTCCACCATCTCAGGGTTGT-3′ (reverse) and for GAPDH
were 5′-GGACCTGACCTGCCGTCTAG-3′ (forward) and
5′-GTAGCCCAGGATGCCCTTGA-3′ (reverse) according to the human NDRG1
and GAPDH cDNA sequences in GenBank. The GAPDH mRNA level was used
for normalization.
Western blotting
Cell lysates were prepared by SDS lysis solution.
Protein concentration was measured using a BCA protein assay kit.
Equal amount of protein was separated by electrophoresis on a 10%
SDS-polyacrylamide gel. The proteins were electrotransferred from
the gel to nitrocellulose membrane. The membrane was blocked with
5% non-fat milk solution for 1 h, and then was incubated with
primary monoclonal antibody against NDRG1 (Abcam), β-actin
(Calbiochem) at 4°C overnight. β-actin was used as an internal
control. After washing with TBS-T, the membrane was incubated with
secondary antibodies against rabbit immunoglobulin G. The membrane
was washed and detected by the enhanced chemiluminescence (ECL)
detection system (Thermo Fisher Scientific) according to the
manufacturer's instructions.
Construction of the NDRG1 expression
vector pcDNA3.1(+)/NDRG1 and shRNA
The primer sequences used for NDRG1 expression were:
NDRG1 forward, 5′-TTAGATCATGTCTCGGGAGATGCAGGAT-3′; and reverse
5′-TTGAATTCCTAGCAGGAGACCTCCATGG-3. The PCR product was then cloned
into pcDNA3.1(+) (Invitrogen) using standard techniques. Either the
NDRG1 expression vector pcDNA3.1(+)/NDRG1 or empty vector
pcDNA3.1(+) was transiently transfected into MIA-PACA2 and PANC1
cells using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions. For the cell proliferation assay,
cells were seeded into a 96-well plate (2×104
cells/well), and positive colonies were selected with G418
(Invitrogen) supplemented with growth medium. NDRG1 short hairpin
RNA (shRNA) target sequence was: 5′-GCATTATTGGCATGGGAAC-3′.
Double-stranded DNA coding NDRG1 shRNA was cloned into pRNATH1.1
Adeno shuttle vector containing a cGFP marker (Genscript).
Adenovirus was packaged in Ad-293 cells (Agilent) and purified by
CsCl2 gradient ultracentrifugation. Viral particle titer
was determined by plague assay. For adenoviral transduction,
MIA-PACA-2 and PANC1 cell was transduced with 100 multiplicity of
infection of adenoviral control or shRNA for 24–48 h. Stable clones
were confirmed by real-time RT-PCR and western blotting.
Wound healing assay
Cells from each group (NDRG1 overexpression and
control, NDRG1 shRNA and control) were seeded into 6-well plates
(5×105 cells/well). The confluent monolayer was starved
overnight, and then a single, linear scratch was created using a
20-µl pipette tip. After wounding, the cells were washed gently
with PBS to remove cell debris and placed in fresh DMEM
supplemented with 0.1% FBS to block cell proliferation. Images were
captured using a phase contrast microscope at 0 and 48 h. Wound
closure was expressed as a percentage of the wound area at 0 h.
Cell migration and invasion
assays
Invasion assays were performed using the BD BioCoat™
Matrigel chamber in 24-well plates (BD, USA). Resuspension solution
(100 µl) containing 1×105 cells in DMEM with 1% FBS was
added to the upper chamber, and 600 µl DMEM supplemented with 10%
FBS was added to the bottom chamber. After a 48-h incubation at
37°C in a 5% CO2 incubator, cells in the upper well were
wiped off using a cotton swab. Cells in the lower chamber were
fixed, stained with H&E, and counted under a light microscope.
The migration assay was performed in a similar manner except that
the chambers were covered without Matrigel.
Cell proliferation assay
Cell proliferation assay was performed with Cell
Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the
manufacturer's instructions. Briefly, indicated pancreatic cancer
cells were seeded in 96-well plates (1×104 cells/well)
24 h post-transfection and cultured in the growth medium. Cells
were examined at 0, 24, 48, 72, 96 and 120 h. CCK-8 (10 µl) was
added to each well at different time-points. After an incubation of
2 h at 37°C, absorbance was measured at 450 nm.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 4.02 software (GraphPad Software Inc., La Jolla, CA,
USA) or SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Values
are expressed as the mean ± standard deviation. Comparisons between
multiple groups were made using a one-way analysis of variance,
followed by t-test. P<0.05 was considered to indicate a
statistically significant difference between values. All
experiments were conducted at least in triplicate.
Results
Cell models for NDRG1 cellular
functions
In order to select suitable cell lines for
subsequent upregulation or downregulation of NDRG1 and to observe
these effects on proliferation, invasion, migration of cancer
cells, we assessed NDRG1 expression in pancreatic cancer cell
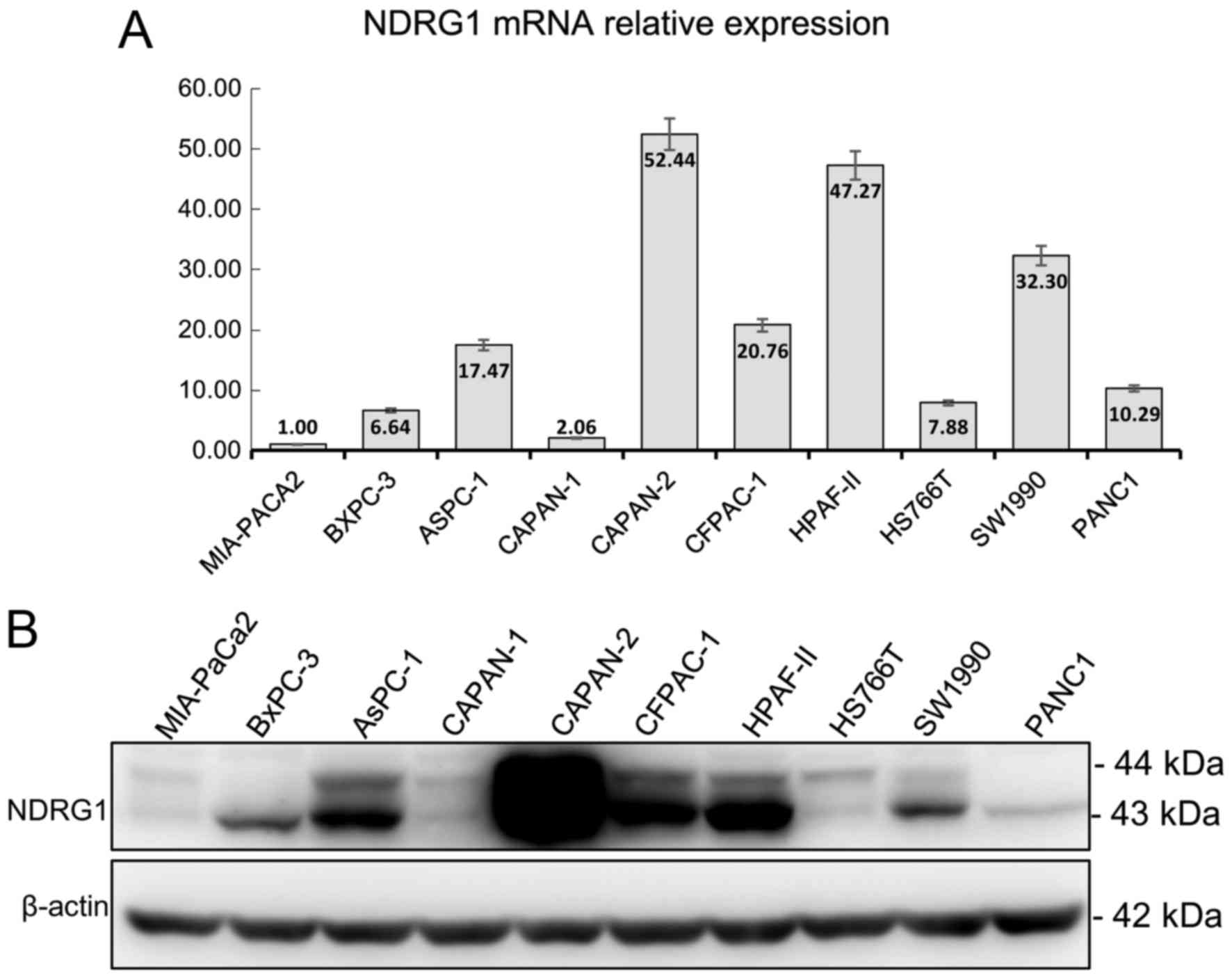
lines. As shown in Fig. 1, the
expression of NDRG1 mRNA and protein were slightly mismatched. The
top three highest mRNA expressions were CAPAN-2, HPAF-II, SW1990
but in NDRG1 protein expression, CAPAN-2, HPAF-II, PANC1 is the
highest. Whereas, the lowest expression of mRNA and protein were
MIA-PaCa2, BxPC-3, CAPAN-1 and MIA-PaCa2, CAPAN-1, HS766T,
respectively (Fig. 1).
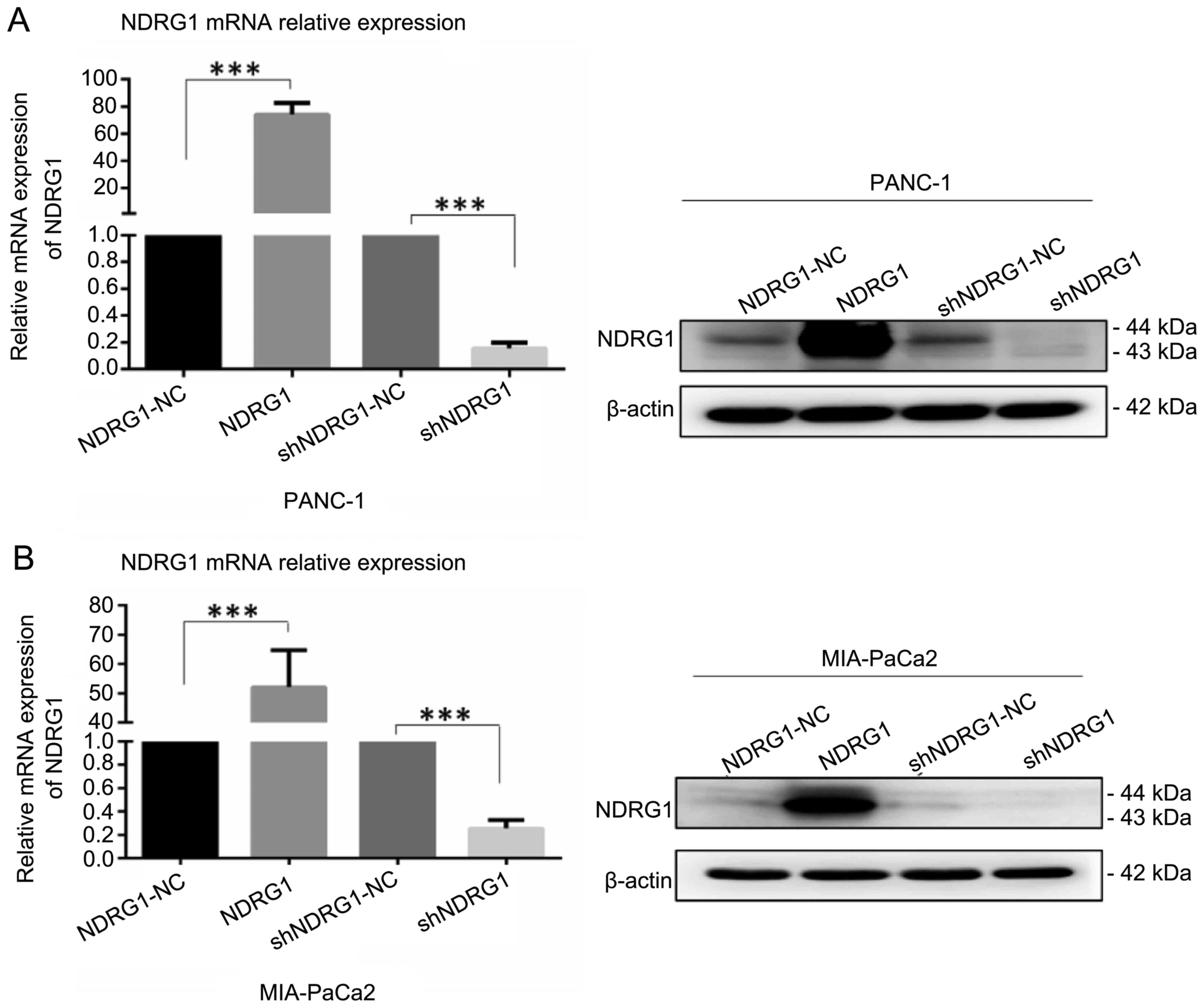
We employed PANC-1 and MIA-PaCa2 transfected with
NDRG1 expressing plasmid and shRNA as cell models for the following
in vitro study. After transfection with 48 h, cells were
lysed and subjected to qRT-PCR and western blotting for detecting
NDRG1 expression. As indicated in Fig.
2, NDRG1 mRNA and protein were significantly elevated after
transfection with NDRG1 expressing plasmid in PANC-1 and MIA-PaCa2
cell lines in comparison with the vector control cells, while the
levels of NDRG1 were reduced remarkably with NDRG1 shRNA
transfection as compared with controls. These data suggest that
overexpression and knockdown of DRG1 in pancreatic cancer cell
lines were established and ready for in vitro
experiments.
NDRG1 inhibits cell proliferation,
migration and invasion in pancreatic cancer cell lines
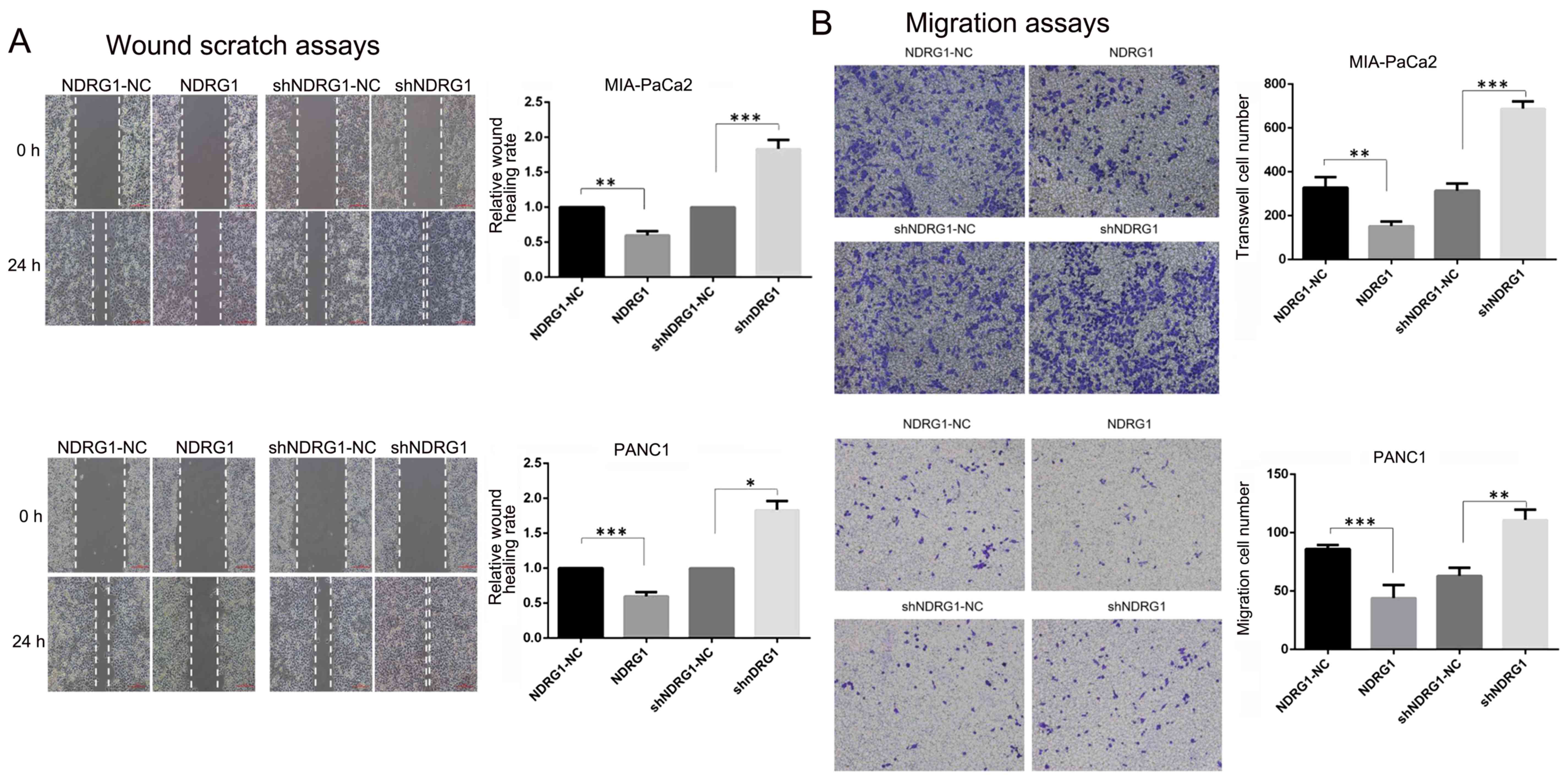
Wound scratch assays and Transwell assays were
performed to evaluate the influence of NDRG1 on pancreatic cancer
migration and invasion. As shown in Fig. 3, the distance changes were
calculated by defining the change of control cells as 100%.
Significant wound closure inhibition was observed in the NDRG1
overexpression PANC-1 (1.00 vs 0.60±0.05, P=0.0035) and MIA-PaCa2
(1.00 vs 0.58±0.055, P=0.0001) cells. However, those with NDRG1
siRNA transfection migrated faster than control cells (PANC-1: 1.00
vs 1.83±0.1, P=0.0003; MIA-PaCa2: 1.00 vs 1.48±0.18, P=0.01).
Furthermore, Transwell assays with or without Matrigel were used to
examine PANC-1 and MIA-PaCa2 cell properties of invasion and
migration. In Transwell assays, the numbers of migrated and invaded
PANC-1 (migration: 302.67±23.03 vs 688.33±33.5, P=0.00008;
invasion: 225.67±26.10 vs 400±43.86, P=0.004) and MIA-PaCa2
(migration: 63±5.71 vs 111±7.12, P=0.0017; invasion: 72.67±12.50 vs
173.33±7.77, P=0.0002) cells significantly increased after NDRG1
knockdown compared to controls. While the numbers of migrated and
invaded PANC-1 (migration: 328.33±47.06 vs 152.33±21.55, P=0.004;
invasion: 691.3±66.03 vs 356±21.63, P=0.001) and MIA-PaCa2
(migration: 86±2.94 vs 44±9.09, P=0.003; invasion: 229.7±23.71 vs
143.7±10.21, P=0.004) cells were less than control after NDRG1
overexpression. Taken together, these findings demonstrated NDRG1
prevented pancreatic cancer cell migration and invasion. In CCK-8
proliferation assay, OD450 values were significantly higher than
control cells at day 5 after NDRG1 knockdown in PANC-1 and
MIA-PaCa2 cell lines. In contrast, the readouts of OD450 declined
significantly after NDRG1 overexpression in both cell lines
(Fig. 3). These results indicated
that NDRG1 had the potential of inhibiting pancreatic cancer cell
proliferation, migration and invasion.
Modulation of p-STAT3, MMP2, MMP9,
PTEN, p-AKT and PI3K expression by NDRG1 in pancreatic cancer
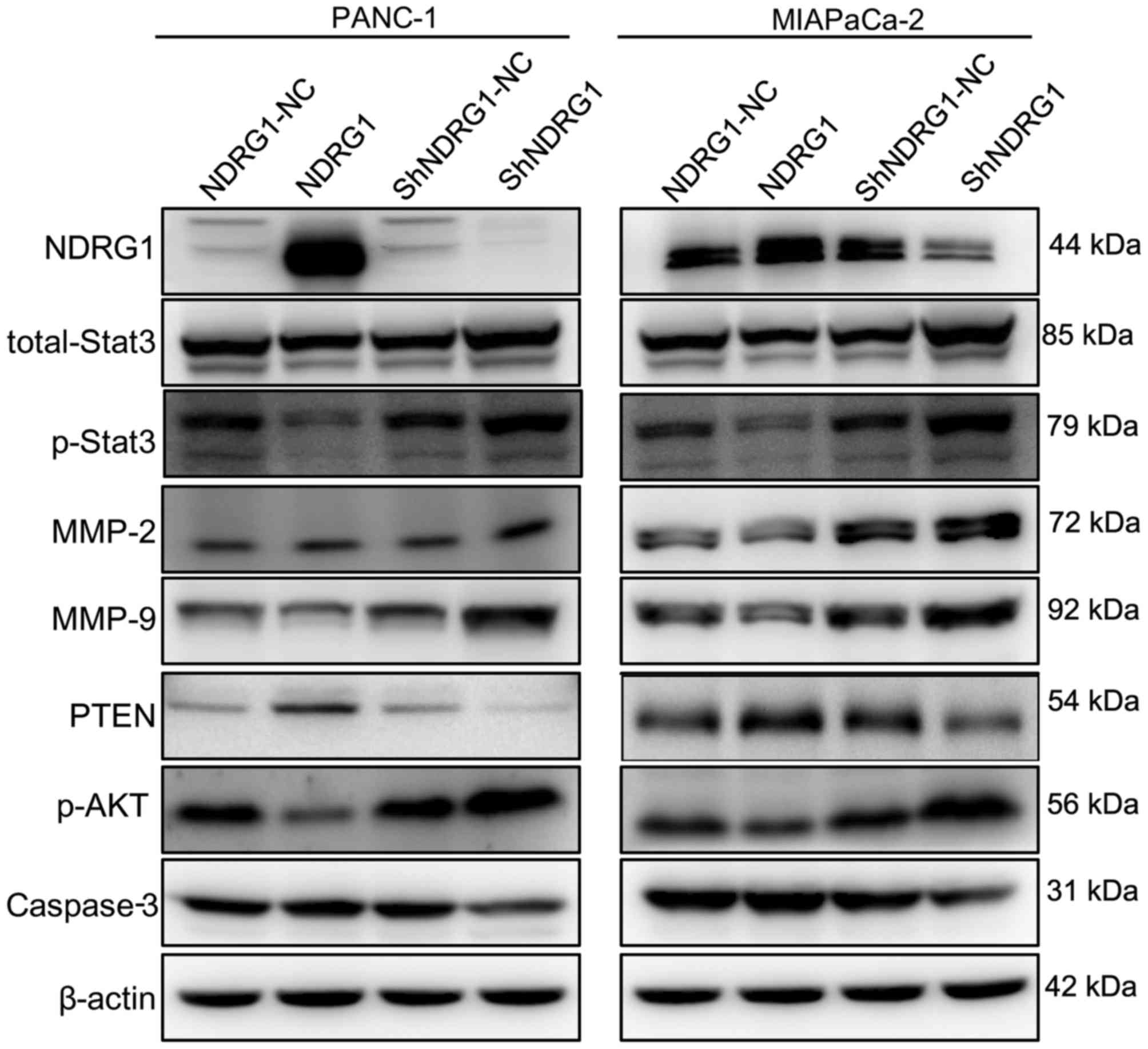
Next, we determined the p-STAT3, MMP2, MMP9, PTEN,
PI3K and p-AKT after NDRG1 regulation. By western blot assay,
p-STAT3, MMP2, MMP9, PTEN, PI3K and p-AKT level were tested after
NDRG1 plasmid or shRNA transfection. In both PANC-1 and MIA-PaCa2
cell lines, we observed that p-STAT3, MMP2, MMP9, PI3K and p-AKT
expression were negatively correlated to NDRG1 level while PTEN was
positively correlated to NDRG1 overexpression. Specifically,
Fig. 4 shows stronger p-STAT3,
MMP2, MMP9, PI3K and p-AKT band and much fainter PTEN band in NDRG1
downregulation cell lysates, whereas the opposite were revealed in
NDRG1 upregulated pancreatic cancer cells.
Discussion
Metastasis is the process by which tumor cells move
from a primary tumor site to a distal secondary site (15). Plenty of reports have underscored
the involvement of oncogenes, tumor suppressor genes and metastasis
genes in the occurrence, development and progression of cancer
(16,17). NDRG1, a paradoxical gene reported by
many studies, plays a metastasis suppressor or a facilitator
depending on the type of the tumor.
We found that NDRG1 was involved in proliferation,
migration, invasion of pancreatic cancer cell lines in experiments
in vitro. Moreover, knockdown of NDRG1 reduced colony
formation of pancreatic cancer cells but not significantly. In
addition, the effects of NDRG1 on above mentioned cancer behavior
might be achieved by activating PTEN and deactivating p-STAT3,
MMP2, MMP9, PI3K and p-AKT. Our data indicated that NDRG1 has the
propensity to inhibit progression of pancreatic cancer by
repressing proliferation and impeding invasion and migration,
acting as an antitumor function in pancreatic cancer.
In other pancreas studies on NDRG1, Angst et
al found that this protein was highly expressed in
well-differentiated cells of pancreatic cancer, whereas the poorly
differentiated tumor cells were negative. These data suggested that
NDRG1 may serve as a marker of differentiation and functioned as a
tumor suppressor in the invasive ability and metastasis of cancer
cells by inducing differentiation or reversing a metastatic
phenotype, which was in agreement with studies of colorectal and
prostatic cancer (18). Maruyama
et al (19) reported that
tumor growth in vivo decreased remarkably by NDRG1
overexpression. Moreover, NDRG1 overproduction could reduce MMP9, a
cellular production of angiogenesis-related factors so as to
restrict cellular locomotion and invasion ability, those result are
similar to our data. They further observed a close association
between low NDRG1 expression and poor prognosis in pancreatic
cancer patients. MMPs are a family of zinc endopeptidases that
cleave ECM molecules and are classified according to their
substrate specificity. High levels of MMPs have also been
implicated in multiple stages of cancer progression including
invasion and migration (20).
STAT3, a central cytoplasmic transcription factor
that is activated by phosphorylation in response to extracellular
signals and oncogenes, is activated in many human cancers (21), including pancreatic cancer (22). STAT3 activity is positively
associated with MMP2 and MMP9 (23), both of which are considered to be
downstream of STAT3 pathway (24).
They could promote cancer cell migration by degrading the
extracellular matrix, and thus regarded as the invasive and
metastatic promoter in pancreatic cancer (25,26).
In our previous study, we found that activation and blocking of the
STAT3 signaling pathway can affect invasion ability and expression
of the MMP2 genes in pancreatic cancer (27,28).
While in this study, NDRG1 inhibited pancreatic cancer invasion and
migration, as well as p-STAT3, MMP2, MMP9 expression. So we assume
that the migration and invasion suppressive role of NDRG1 might be
attributed to downregulation of p-STAT3, MMP2, MMP9.
PTEN, a phosphatase with opposing function to PI3K,
functions as a tumor suppressor to increase PI3K pathway signaling
(29) and thus leads to activation
of AKT, mTOR signaling pathway (30). The dysregulation of the signaling
pathway has been implicated in tumor initiation, cell growth and
survival (31). PTEN appears to be
a mediator of NDRG1 expression, as silencing of PTEN expression has
been shown to downregulate NDRG1 levels, whereas overexpression of
PTEN upregulated NDRG1 in a dose- and time-dependent manner
(32). However, we found NDRG1 is
able to induce PTEN expression. These results indicate that NDRG1
and PTEN may constitute a positive feedloop. This inference was
supported by the report that when PI3K signaling overwhelms PTEN
signaling, upregulation of NDRG1 restores the coupling of the PI3K
and PTEN pathways via upregulation of PTEN and downregulation of
PI3K (33). Hence, NDRG1 may
synergize with PTEN to decrease pancreatic cancer proliferation
through deactivating the PI3K and AKT signaling pathway.
Intriguingly, differing from its tumor suppressive
role in pancreatic cancer and some other carcinoma, NDRG1 promotes
lung cancer, colon cancer, and liver cancer development. One
possible explanation for these conflicting phenomena is that NDRG1
may regulate tumor cell proliferation, migration and invasion
through a variety of signaling pathways (34), such as nuclear factor-κB (NF-κB),
mammalian target of rapamycin (mTOR), and MAPK signaling pathways
(35). Future studies in depth are
required to identify other signaling pathways that NDRG1 is
involved in affecting pancreatic cancer progression.
In conclusion, we have identified NDRG1 as a novel
factor that inhibits the growth, invasion and migration of
pancreatic cancer. Moreover, NDRG1 appears to play a tumor
suppressive role by deactivating STAT3 and PI3K/AKT signaling
pathways.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China [no. 81372640 (to Q.Z.J.)].
References
|
1
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: An overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han H and Von Hoff DD: SnapShot:
Pancreatic cancer. Cancer Cell. 23:424–424 e421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto J, Grossbard ML and Kozuch P:
Metastatic pancreatic cancer 2008: Is the glass less empty?
Oncologist. 13:562–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Y, Lv L, Du J, Yue L and Cao L:
Correlation of N-myc downstream-regulated gene 1 subcellular
localization and lymph node metastases of colorectal neoplasms.
Biochem Biophys Res Commun. 439:241–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang X, Xu X, Ma J, Xue X, Li Z, Deng P,
Zhang S, Zhi Y, Chen J and Dai D: NDRG1 expression is related to
the progression and prognosis of gastric cancer patients through
modulating proliferation, invasion and cell cycle of gastric cancer
cells. Mol Biol Rep. 41:6215–6223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Cai J, Li Z, Hu S, Yu L, Xiao L
and Wang Z: Expression and biological function of N-myc
down-regulated gene 1 in human cervical cancer. J Huazhong Univ Sci
Technolog Med Sci. 30:771–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Li J, Ye Z, Li Z and Wu X: N-myc
downstream regulated gene 1 acts as a tumor suppressor in ovarian
cancer. Oncol Rep. 31:2279–2285. 2014.PubMed/NCBI
|
|
9
|
Jin R, Liu W, Menezes S, Yue F, Zheng M,
Kovacevic Z and Richardson DR: The metastasis suppressor NDRG1
modulates the phosphorylation and nuclear translocation of
β-catenin through mechanisms involving FRAT1 and PAK4. J Cell Sci.
127:3116–3130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic
Z and Richardson DR: The iron chelators Dp44mT and DFO inhibit
TGF-β-induced epithelial-mesenchymal transition via up-regulation
of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem.
287:17016–17028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Iiizumi-Gairani M, Okuda H,
Kobayashi A, Watabe M, Pai SK, Pandey PR, Xing F, Fukuda K, Modur
V, et al: KAI1 gene is engaged in NDRG1 gene-mediated metastasis
suppression through the ATF3-NFkappaB complex in human prostate
cancer. J Biol Chem. 286:18949–18959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM and
Pardee AB: Drg-1 as a differentiation-related, putative metastatic
suppressor gene in human colon cancer. Cancer Res. 60:749–755.
2000.PubMed/NCBI
|
|
13
|
Lu WJ, Chua MS, Wei W and So SK: NDRG1
promotes growth of hepatocellular carcinoma cells by directly
interacting with GSK-3β and Nur77 to prevent β-catenin degradation.
Oncotarget. 6:29847–29859. 2015.PubMed/NCBI
|
|
14
|
Wang Q, Li LH, Gao GD, Wang G, Qu L, Li JG
and Wang CM: HIF-1α up-regulates NDRG1 expression through binding
to NDRG1 promoter, leading to proliferation of lung cancer A549
cells. Mol Biol Rep. 40:3723–3729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stafford LJ, Vaidya KS and Welch DR:
Metastasis suppressors genes in cancer. Int J Biochem Cell Biol.
40:874–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokota J: Tumor progression and
metastasis. Carcinogenesis. 21:497–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Angst E, Sibold S, Tiffon C, Weimann R,
Gloor B, Candinas D and Stroka D: Cellular differentiation
determines the expression of the hypoxia-inducible protein NDRG1 in
pancreatic cancer. Br J Cancer. 95:307–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maruyama Y, Ono M, Kawahara A, Yokoyama T,
Basaki Y, Kage M, Aoyagi S, Kinoshita H and Kuwano M: Tumor growth
suppression in pancreatic cancer by a putative metastasis
suppressor gene Cap43/NDRG1/Drg-1 through modulation of
angiogenesis. Cancer Res. 66:6233–6242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Luo J and He S: Induction of MMP-9
release from human dermal fibroblasts by thrombin: Involvement of
JAK/STAT3 signaling pathway in MMP-9 release. BMC Cell Biol.
8:142007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Huang C, Huang K, Wu W, Jiang T, Cao
J, Feng Z and Qiu Z: STAT3 knockdown reduces pancreatic cancer cell
invasiveness and matrix metalloproteinase-7 expression in nude
mice. PLoS One. 6:e259412011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang X, Dai S, Dai J, Xiao Y, Bai Y, Chen
B and Zhou M: Luteolin decreases invasiveness, deactivates STAT3
signaling, and reverses interleukin-6 induced
epithelial-mesenchymal transition and matrix metalloproteinase
secretion of pancreatic cancer cells. Onco Targets Ther.
8:2989–3001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xuan X, Li S, Lou X, Zheng X, Li Y, Wang
F, Gao Y, Zhang H, He H and Zeng Q: Stat3 promotes invasion of
esophageal squamous cell carcinoma through up-regulation of MMP2.
Mol Biol Rep. 42:907–915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian LW, Mizumoto K, Urashima T, Nagai E,
Maehara N, Sato N, Nakajima M and Tanaka M: Radiation-induced
increase in invasive potential of human pancreatic cancer cells and
its blockade by a matrix metalloproteinase inhibitor, CGS27023.
Clin Cancer Res. 8:1223–1227. 2002.PubMed/NCBI
|
|
26
|
Fukuda A, Wang SC, Morris JP IV, Folias
AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, et
al: Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma
initiation and progression. Cancer Cell. 19:441–455. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang C, Jiang T, Zhu L, Liu J, Cao J,
Huang KJ and Qiu ZJ: STAT3-targeting RNA interference inhibits
pancreatic cancer angiogenesis in vitro and in vivo. Int J Oncol.
38:1637–1644. 2011.PubMed/NCBI
|
|
28
|
Huang C, Yang G, Jiang T, Huang K, Cao J
and Qiu Z: Effects of IL-6 and AG490 on regulation of Stat3
signaling pathway and invasion of human pancreatic cancer cells in
vitro. J Exp Clin Cancer Res. 29:512010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S and Yu D: PI(3)king apart PTEN's
role in cancer. Clin Cancer Res. 16:4325–4330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mukherjee A, Samanta S and Karmakar P:
Inactivation of PTEN is responsible for the survival of Hep G2
cells in response to etoposide-induced damage. Mutat Res.
715:42–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wallin JJ, Edgar KA, Guan J, Berry M,
Prior WW, Lee L, Lesnick JD, Lewis C, Nonomiya J, Pang J, et al:
GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust
activity in cancer models driven by the PI3K pathway. Mol Cancer
Ther. 10:2426–2436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bandyopadhyay S, Pai SK, Hirota S, Hosobe
S, Tsukada T, Miura K, Takano Y, Saito K, Commes T, Piquemal D, et
al: PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in
prostate and breast cancer. Cancer Res. 64:7655–7660. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kovacevic Z, Chikhani S, Lui GY,
Sivagurunathan S and Richardson DR: The iron-regulated metastasis
suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the
PI3K and Ras signaling pathways. Antioxid Redox Signal. 18:874–887.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu ZY, Xie WB, Yang F, Xiao LW, Wang XY,
Chen SY and Li ZG: NDRG1 attenuates epithelial-mesenchymal
transition of nasopharyngeal cancer cells via blocking Smad2
signaling. Biochim Biophys Acta. 1852:1876–1886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun J, Zhang D, Bae DH, Sahni S, Jansson
P, Zheng Y, Zhao Q, Yue F, Zheng M, Kovacevic Z, et al: Metastasis
suppressor, NDRG1, mediates its activity through signaling pathways
and molecular motors. Carcinogenesis. 34:1943–1954. 2013.
View Article : Google Scholar : PubMed/NCBI
|