Introduction
Prostatic carcinoma is a metabolically active tumor
which mostly occurs among elderly men. More than 80% of metastatic
tumors achieve remission with androgen deprivation therapy, but
develop hormone refractory prostate cancer leading to high
mortality rates after 14–30 months of the median overall survival
(1). Warburg effect is an important
characteristic of tumor cell metabolism including prostate cancer
cells, demonstrating that a tumor cell produces energy through
glycolysis followed by pyruvic acid or lactic acid fermentation,
rather than by tricarboxylic acid cycle or oxidative
phosphorylation (2,3). Hexokinase (HK), phosphofructokinase
(PFK) and pyruvate kinase (PK) are three major rate-limiting
enzymes involved in Warburg effect (4–6), in
which HK is the first enzyme in the regulation of glycolysis,
promoting the production of fructose 6-phosphate from glucose.
There are 4 subtypes (HK I–IV) in the HKs, which are relevant in
tumor metastasis. HK2 protein is expressed in a high percentage of
oncologists in human, such as lung, liver and prostate cancer
(7–10). HK2 coordinates with voltage
dependent anion channel (VDAC) on the mitochondrial outer membrane
through hydrophobic N-terminal domain, which allows HK2 to utilize
ATP produced by mitochondria and protect cells from being avoid of
apoptosis (11). Likewise,
EGFR/PI3K/Akt activation constitutes a hallmark of most cancer
cells and plays an important role in tumor genesis and progression.
The serine/threonine kinase (Akt) is a major downstream effector of
phosphatidylinositol 3-kinase (PI3K) signaling pathway and a
transducer of EGFR effects on cell survival (12), which constitutes a characteristic of
most cancer cells and as such presumably plays important roles in
Warburg effect of cancer cells. Akt-induced aerobic glycolysis may
be mediated by multiple non-exclusive mechanisms, including the
expression and membrane translocation of glucose transporters
(GLUT) and effects on HK expression, activity and mitochondrial
interaction (13–15).
The lectin PCL extracted from the Polygonatum
sibiricum Red, is a galanthus nivalis agglutinin (GNA)-related
lectin. Its molecular weight was estimated to be 4.2 kDa by
SDS-PAGE. The lectin could agglutinate rabbit erythrocytes to
different degrees (13). Several
studies have reported that PCL has antitumor activity, which is
associated with multiple signal cascades (14,15),
but the precise mechanism of the lectin in inducing apoptosis
remains to be clarified.
In the present study, we demonstrated that PCL could
inhibit glucose consumption, lactate production and HK2 expression
of human prostate cancer PC3 cells in vitro, thereby
inducing PC3 cell metabolism. Because PCL has larger molecular
structures that could target the outside of the cell, we
hypothesized that PCL competed with epidermal growth factor (EGF)
for binding to EGFR, suppressing activation of PI3K/Akt downstream
pathways. Therefore, subsequent in vitro experiments were
performed to investigate the interaction of PCL with EGFR by
western blot analysis, molecular docking and molecular dynamics
simulations. Taken together, these studies explored the intricate
apoptosis-inducing mechanisms of PCL with various approaches.
Materials and methods
Materials and chemicals
The rhizome of Polygonatum sibiricum was
collected from the Red River Valley located in the Taibai Mountain
of China. Human prostate cancer cell lines (PC3) were obtained from
the Institute of Biochemistry and Cell Biology (Shanghai, China).
Anti-Hexokinase type 2 (HK2), pyruvate kinase
(PKM2) and phosphofructokinase (PFK) antibody were
supplied by the Cell Signaling Technology, Inc. (Danvers, MA, USA).
EGF was obtained from the Cell Signaling Technology. The
X-tremeGENE transfection reagent was purchased from Roche
Diagnostics Corp. (Indianapolis, IN, USA). Real-time PCR reagents
and primer were purchased from Takara Shuzo Co., Ltd. (Kyoto,
Japan). Chromatograph was supplied by General Electric Co. (Boston,
MA, USA). HPLC instrument was purchased from Waters Co. (Milford,
MA, USA). Real-time PCR was purchased from (Life Technologies
(Carlsbad, CA, USA). HK2 interference fragment and reference were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). The
crystal structures of the extracellular domain of epidermal growth
factor receptor (EGFR), the natural ligand epidermal growth factor
(EGF) and the polygonatum cyrtonema lectin (PCL) obtained from RCSB
Protein Data Bank were used as templates, PDB ID: 1NQL_A (16), 1NQL_B (16) and 3A0D_A (17). Protein-protein docking of the EGFR
and PCL were carried out by using the online ClusPro 2.0 server
(18–21). The binding structure of the EGFR
with the natural ligand EGF was the crystal structure, PDB ID: 1NQL
(16).
Extraction and purification of
PCL
The fresh rhizome of Polygonatum sibiricum
Red (100 g) was ground into small pieces and extracted with
phosphate-buffered saline (PBS) at −4°C for 12 h followed by
centrifugation at 5120 × g for 15 min. The supernatant was
precipitated by saturated ammonium sulfate with a saturation degree
of 80%. After centrifugation, the precipitate was dissolved with 50
ml PBS and dialyzed against PBS. The dialysate was subjected to
chromatography on an S-100 molecular sieve column and eluted with
Tris-HCI buffer of pH 7.0, the eluent was loaded on a cation
exchange column and eluted with PBS of pH 7.2. The final eluent was
freeze-dried to give the PCL (22).
Glucose uptake assay
To measure glucose content, PC3 cells were plated
into 6-well plates (1×105 cells/well) and treated with
50 µg/ml PCL for 24 h, then the cells were grown in Dulbeccos
modidied Eagles medium (DMEM) containing 10% fetal bovine serum
(FBS) for 48 h at 37°C in an atmosphere of 5% CO2. The
supernatants were collected, the glucose contents were assayed by
ELISA (23). The assay was repeated
3 times.
Lactate production assay
Lactate content was analyzed by ELISA according to
the manufacturer's instructions (23). In brief, PC3 cells were added onto
6-well plates at a density of 1×105 cells/well and
treated with 50 µg/ml PCL for 24 h. The cultures were incubated at
37°C in an atmosphere of 5% CO2 for 48 h. The
supernatants were collected and quantified by a microplate reader.
The assay was repeated 3 times.
Western blot analysis
Western blot analysis was carried out as previously
described (23). Cells were fully
lysed in a cell lysis buffer to prepare protein extracts. The
protein concentrations were determined using the BCA assay.
Briefly, the proteins (60 µg) extracted from cultured PC3 cells by
immunoprecipitation and immunoblotting with corresponding
antibodies were loaded and separated by SDS polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred with 120 V constant
voltage for 2 h, then blocked in 5% bovine albumic (BSA) for 30 min
at room temperature. The nitrocellulose membranes were incubated
with primary antibody for 1 h. The blots were washed with PBST
buffer for 3 times (10 min/each time). Addition of a secondary
antibody was conjugated to HRP and incubated for 30 min. The cells
were examined using ECL glow kits. The assay was repeated 3
times.
siRNA interference
The PC3 cells were grown in DMEM supplemented with
10% FBS and penicillin-streptomycin (1%) and transfected after cell
density to 80% according to the manusfacturers instructions
(24). siRNA-HK2 and siRNA-EGFR
were synthetized by Guangzhou Ruibo Biotechnology, Co. (Guangzhou,
China).
Quantitative real-time PCR assay
The miRNA expression of HK2 in human prostate cancer
PC3 cells was analyzed by real-time PCR using TaqMan MicroRNA
(24). The cDNA was reverse
transcribed from the total RNA extract and isolated from the PC3
cells treated with PCL using gene-specific primers according to the
TaqMan MicroRNA assay protocol. Real-time PCR was performed using
cDNA template. The reactions were incubated at 95°C for 2 min, at
93°C for 40 sec, at 60°C for 35 sec and at 72°C for 30 sec,
respectively and cycled 40 times. The assay was run in
intriplicate.
MTT assay
Cell viability was measured by the MTT assay
according to the providers instructions (Invitrogen, Carlsbad CA,
USA) (24). In brief, PC3 cells
were plated into 96-plates (5,000 cells/well). The PCL solution was
added into each well. Cell viability was detected at 24 h. Then,
100 µl fresh medium and 10 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were added to each well, and then the plate was incubated for an
additional 4 h at 37°C in an atmosphere of 5% CO2. The
medium was then removed and the crystals were solubilized with 100
µl of dimethyl sulfoxide (DMSO) for 10 min. The absorbance was
measured at a wavelength of 490 nm. Cell viability was normalized
as relative percentages in comparison with the untreated control
and the assay was repeated 3 times.
Molecular docking and molecular
dynamics simulations
We introduced the well-tempered metadynamics
simulation (25,26) to NAMD (27) in a portable plugin PLUMED (28) for exploring the binding mechanism
between the PCL and EGFR through free-energy calculations. The
temperature is 310 K, and the value of bias-factor equal to 6.0 to
allow for the system to escape from the initial local minimum in
the time scale of this simulation. The metadynamic simulation
starts as a standard MD simulation, but after 1 ps an artificial
potential hill is added by using the PLUMED (28). After another 1 ps a new hill is
added and so forth. These potential hills accumulate and gradually
disfavor the free energy minima having been already visited and
thus facilitate an exploration of the whole free energy space. At
the end of the simulation, the free energy surface is completely
flooded and the system can walk on the free energy surface without
barriers.
All the simulations were carried out under a
constant pressure of 1 bar and a constant temperature of 310 K. The
minimization was based on the steepest descent method. The
electrostatics potential and the van der Waals interactions were
calculated with a non-bonded cutoff of 12 Å based on the Particle
Mesh Ewald (PME) method (29). The
constant temperature and pressure were calculated by the langevin
thermostat (30,31) and langevin barostat method (32), respectively. All the MD simulations
were performed with a time step of 1 fs. The trajectories of MD
simulations were stored every 5 ps for the analysis of MD simulated
data, and 10 ns MD simulations were performed on the systems. All
the MD simulations were carried out using NAMD (27) (version 2.9) with the AMBER force
field (33) and explicit TIP3P
water.
Statistical analysis
The results are expressed as the means ± standard
deviation, and analyzed by using SPSS 17.0 software. Statistical
significance was compared with other groups for significance by
one-way ANOVA followed by Bonferroni post hoc test (multiple
comparison tests) using GraphPad Prism version 5.01. P<0.05 was
considered statistically significant.
Results
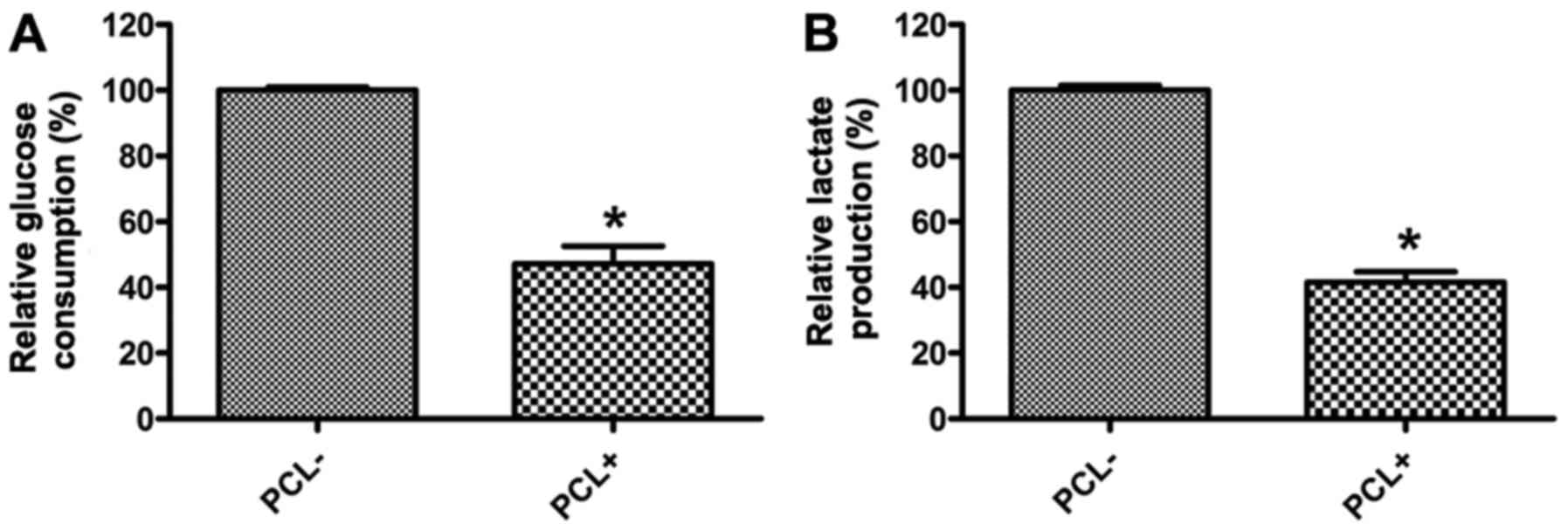
PCL inhibits the Warburg effect of
prostate cancer PC3 cells
A previous study revealed that it is common in tumor
that glucose uptake and lactate production increase through
non-exclusive dependent signaling cascade (34). Therefore, to study whether PCL is
involved in the Warburg effect, we analyzed the effect of PCL on
PC3 metabolism. The results indicated that PCL inhibited PC3 cells
glucose absorption obviously (Fig.
1A), and it also reduced the lactate production (Fig. 1B).
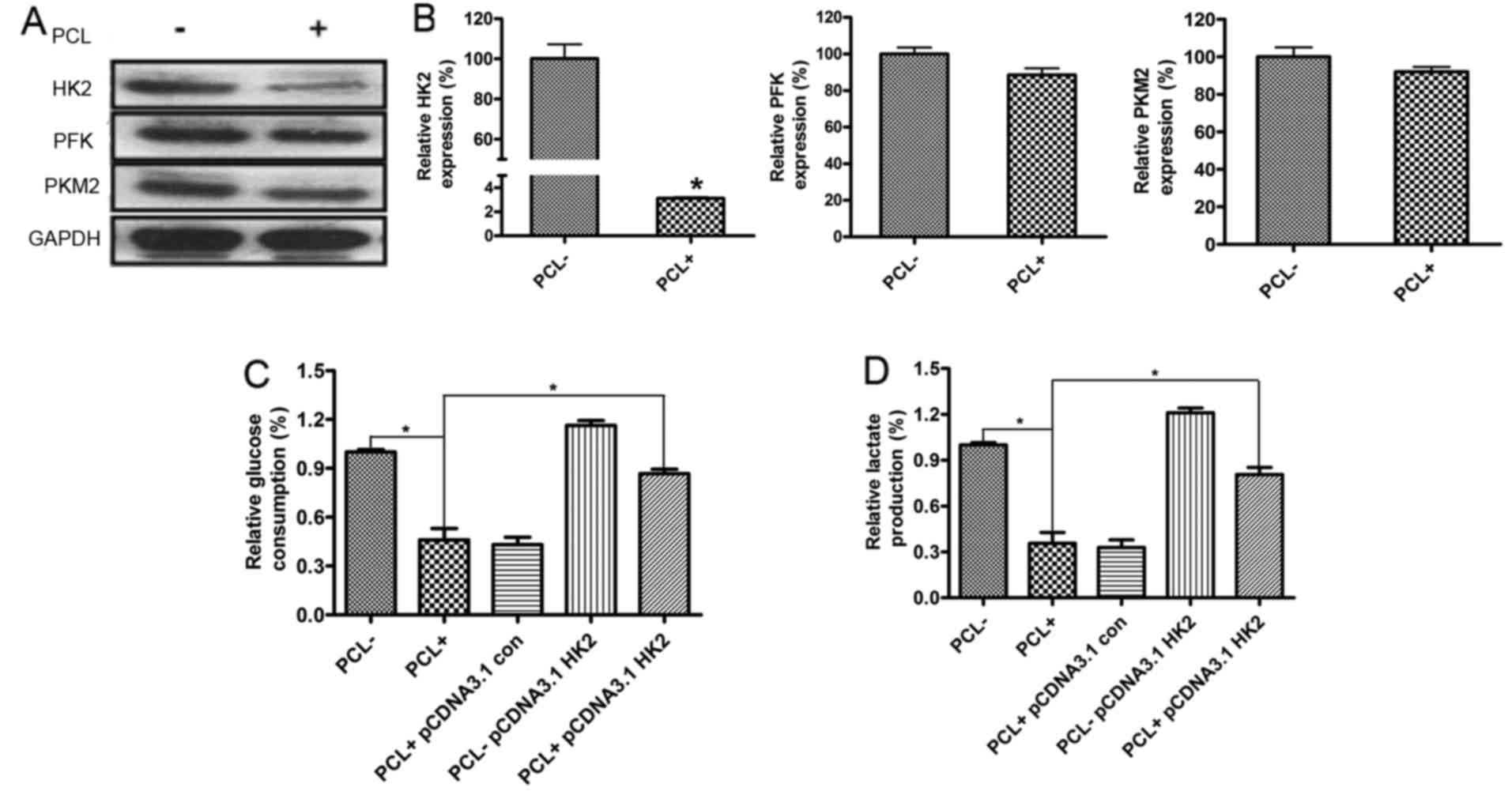
The effect of PCL on expression of
HK2, PFK or PKM2 in PC3 cells
It was earlier identified that HK2, PFK and PKM2 are
expressed in a high percentage of many tumors (3–5). In
the present study, we explored the expression of the three key
rate-limiting enzymes in PC3 cells treated with PCL. The results of
western blot analysis and real-time PCR indicated that PCL enhanced
the expression of HK2 and had no effects on the expression of PFK
or PKM2 in PC3 cells (Fig. 2A and
B). However, with siRNA-HK2 interference, the effect of PCL on
glucose absorption and lactate production in PC3 cells was reversed
(Fig. 2C and D). The results showed
that PC3 cells exhibited a comparable respose to PCL treatment, PCL
could inhibit tumor cell glycolysis through reducing HK2
expression.
PCL inhibits the cell viability of
prostate cancer PC3 cells
Reduced levels of HK2 in PCL-treated PC3 cells
prompted us to further study the effect of PCL on the cell
viability of prostate cancer PC3 cells. Our western blot analysis
conducted on HK2 expression showed that PCL inhibited the activity
of PC3 cells, after siRNA-HK2 interference, and had no effect on
the activity of PC3 cells (Fig.
3).
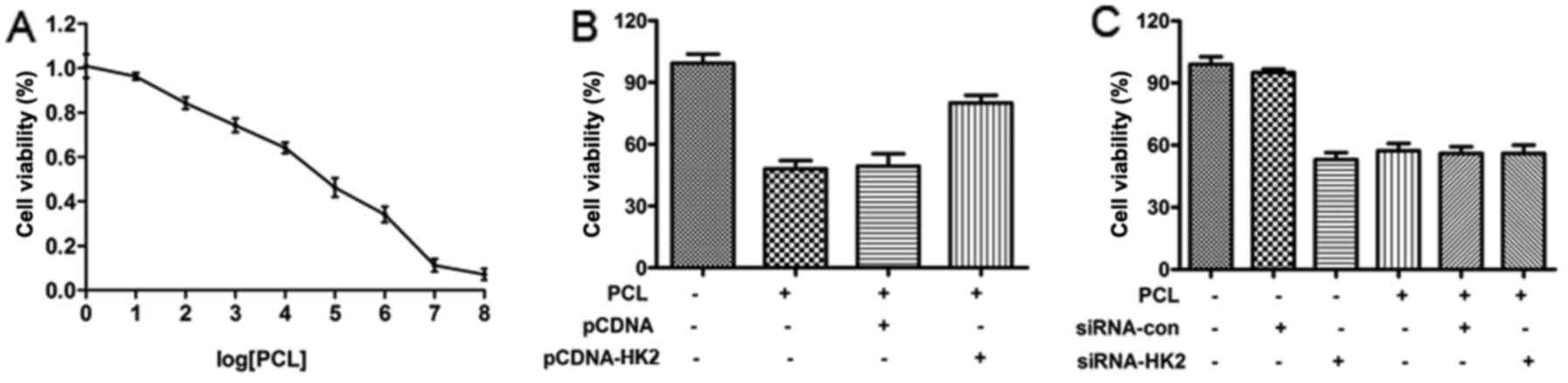 | Figure 3.PCL inhibits the cell viability of
prostate cancer PC3 cells. (A) PC3 cells were plated at 80%
confluence and 0, 6.25, 12.5, 25, 50, 100, 200, 400 and 800 µg/ml
PCL was added into each well, respectively. At 24 h, the cell
viability was detected by the MTT method. (B) PC3 cells were plated
at 80% confluence and 50 µg/ml PCL was added into each well. At 24
h, the cell viability was detected by the MTT method. (C) Cells
were plated at 80% confluence and transfected with siRNAs for HK2.
After post-transfection, the cells were treated with 50 µg/ml PCL.
At 24 h, the cell viability was detected by the MTT method. All
experiments were repeated 3 times. |
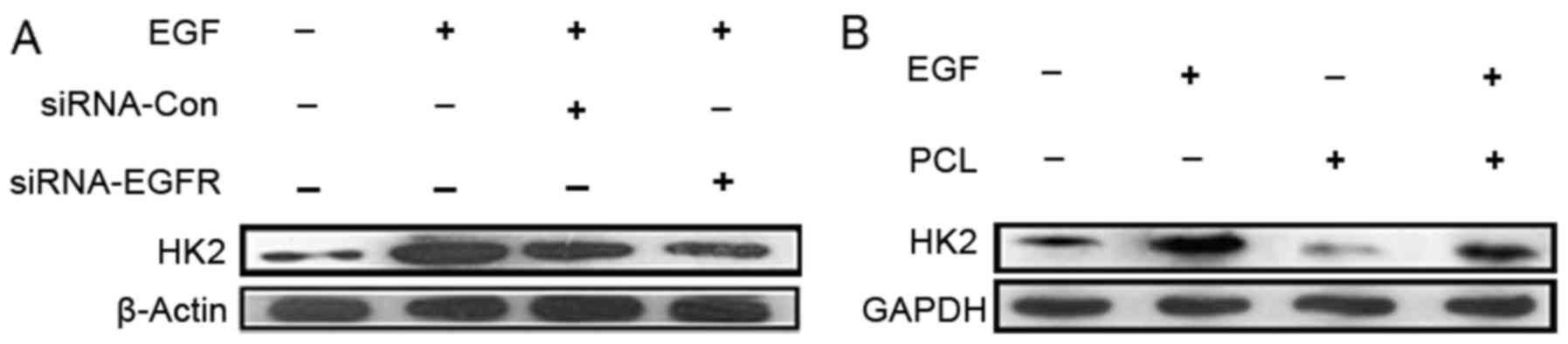
PCL reduces HK2 expression by
competitively binding to EGFR binding pocket with EGF and causes
accelerated apoptosis of the tumor cells
EGFR/PI3K/Akt signaling pathway activation
upregulated the expression of HK2. PCL has larger molecular
structures that could target the outside of the cell, we
hypothesized that PCL could compete with EGF for binding to EGFR,
suppress activation of PI3K/Akt downstream pathways and HK2
expression. As shown in Fig. 4,
introduction of the EGF significantly increased the level of HK2 in
PC3 cells. With PCL treatment, the expression of HK2 was
suppressed. These data supported the hypothesis that PCL could
suppress the aerobic glycolysis of PC3 cells by combining with
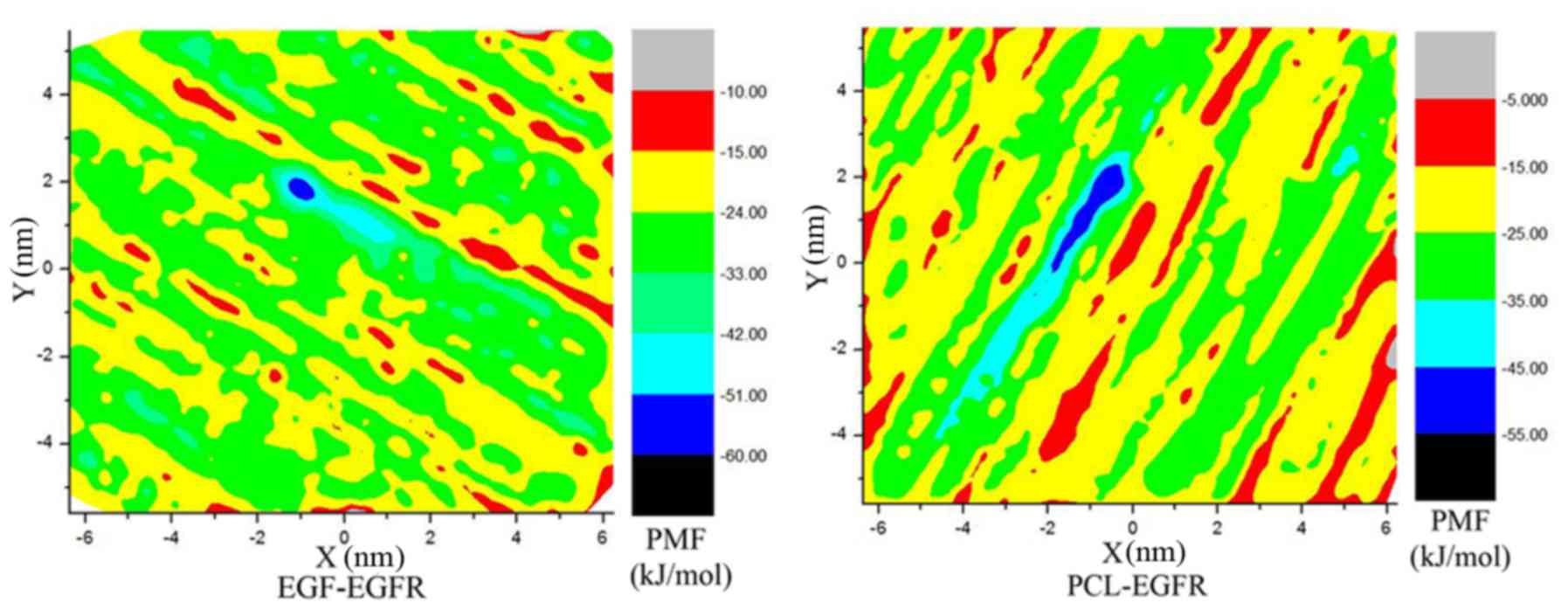
EGFR. To further verify the mechanism, we explored the binding
mechanism between the PCL and EGFR through molecular dynamics
simulations. The calculation of the free energy of binding from a
two-dimensional potential of mean force (PMF) (35) along a reaction coordinate was
completed using metadynamic method by NAMD (28) and PLUMED (28). It is because both EGF and PCL only
go through the dual (X and Y) directions out of the receptor
control, the distance between the mass center of ligands and that
of EGFR along these two directions was defined as the relevant
reaction coordinate. Fig. 5 showed
the PMF as a function of the distances between different ligands
and receptors along the reaction coordinates within 10 ns
metadynamics simulations. From the figure, it is clear that the
lowest energy well is located in the pockets of the acceptor
protein. The PMFs of different ligands have only one domain region,
and the domain of PCL is larger than that of EGF. The hydrogen bond
(HB) analysis provided evidence of benefits for this conclusion
(Fig. 6). The numbers of HB between
the PCL and protein are more than any other. There are only four HB
between the EGF and EGFR, but fourteen for PCL. Due to the
influence of HB, the binding of PCL to the receptor is more stable.
Outside the combination region, the values of PMF present a trend
of fluctuation. This may be the effect of the reservoir, to be more
exact, ligands also can produce HB with the aquatic, and all that
instantaneously and unsterdily.
Discussion
In the core high necrotic areas in any given tumor,
the amount of oxygen observed is extremely low, thereby
accelerating the cells to synthesize and obtain energy by
glycolysis, which is termed the Warburg effect (1,2). PCL
is a mannose binding lectin. Previous studies have reported that
PCL could induce tumor cell apoptosis and autophagy. Zhang and
colleagues (13) found that PCL
could induce apoptosis of L929 cell to murine fibrosarcoma in a
caspase-dependent manner, as well as amplify TNF-induced L929 cell
apoptosis. Liu and colleagues (14,15)
found that PCL treatment abrogated glutathione antioxidant system
and induced mitochondria to generate ROS accumulation, resulting in
p38-p53 activation. They also confirmed that the ROS-p38-p53
pathway was involved in PCL-induced autophagy. In view of these
findings, we hypothesized that PCL might interact energy metabolism
in cancer cells. Our findings therefore emphasized the importance
of studying the effect of PCL on the Warburg effect of PC3 cells.
To begin addressing this question, we investigated the effect of
PCL on glucose consumption of PC3 cells, a characteristic of the
Warburg effect and found that the level of glucose upstake was
strongly correlated to PCL treatment. Another characteristic of the
Warburg effect is the reduction of pyruvate, the end product of
glycolysis, to lactic acid, which is accompanied by the oxidation
of NADH to NAD+. This reaction takes the place of the
conversion of pyruvate to acetyl-CoA, which may enter the
tricarboxylic acid (TCA) cycle (25). We next examined whether PCL
treatment suppressed lactate production in PC3 cells and found that
PCL could inhibit PC3 cells from producing lactate acid. These data
indicated that PCL could suppress the Warburg effect of PC3
cells.
The hallmark of the Warburg effect is the
overexpression of several important rate-limiting enzymes, for
example HK, PFK and PK. HK, involved in the first regulatory step,
appears predominantly in an HK2 isoenzyme form in tumors which is
bound to the mitochondrial outer membrane facing the cytosol.
Microlocation of this enzyme enables preferential access to newly
synthesized ATP for phosphorylating glucose, and it is resistant to
product inhibition (36).
Accumulated evidence suggests that codeletion of the tumor
suppressor genes PTEN and p53 plays a crucial role in the
development of castration-resistant prostate cancer in vivo.
Wang and colleagues (37)
demonstrated that HK2 is selectively upregulated by the combined
loss of Pten and p53 in prostate cancer cells. Mechanistically,
PTEN deletion increases HK2 mRNA translation through the activation
of the AKT-mTORC1-4EBP1 axis, and p53 loss enhances HK2 mRNA
stability through the inhibition of miR143 biogenesis. HK2-mediated
aerobic glycolysis is required for Pten-/p53-deficiency-driven
tumor growth in xenograft mouse models of prostate cancer. PFK, the
‘gatekeeper’ of glycolysis, catalyses the committed step of the
glycolytic pathway by converting fructose-6-phosphate to
ructose-1,6-bisphosphate, which is proposed to have important roles
in metabolic reprogramming in cancer (36). PKM2 is a limiting
glycolytic enzyme that catalyzes the final step in glycolysis,
which is the key in tumor metabolism and growth (38). We therefore explored the expression
of the three key rate-limiting enzymes in PC3 cells treated with
PCL and found that PCL enhanced the expression of HK2 in PC3 cells,
whereas, PCL has no effect on the expression of PFK or
PKM2. The results showed that PC3 cells exhibited a
comparable respose to PCL treatment, PCL inhibited tumor cell
glycolysis through reducing HK2 expression. Futhermore, we examined
the effect of suppressing the HK2 activity on the glucose upstake
and lacate production in the PC3 cells. The results showed that the
upstake of glucose and the production of lactate were dramatically
increased when the dominant negative HK2 mutants were introduced.
In addition, we examined the effect of PCL on the PC3 cell
viability. The data showed that PCL inhibited the viability of PC3
cells, but after transfection with siRNA-HK2, it has no effect on
the activity of PC3 cells. The results showed that PC3 cells
exhibited a comparable respose to PCL treatment, PCL was able to
inhibit tumor cell glycolysis through reducing HK2 expression.
PCL has larger molecular structures (4.2 kDa) that
may target the outside of the cell (13). While EGFR is a widespread oncogenic
signature in prostatic carcinoma, Akt (the serine/threonine kinase)
is a major downstream effector of EGFR/PI3K that may induce aerobic
glycolysis and increase HK2 expression in cancer cells through
multiple non-exclusive mechanisms (15–17).
Therefore, we hypothesized that PCL may combine with EGFR, suppress
activation of PI3K/Akt downstream pathways and expression of HK2.
Western blot analysis showed that the activation of EGFR
significantly increased the expression of HK2 in PC3 cells, but PCL
treatment might inhibit this effect. These data support the
hypothesis that PCL could suppress the aerobic glycolysis of PC3
cells by combining with EGFR. The EGFR consists of an extracellular
ligand-binding domain, a single hydrophobic transmembrane region,
and the intracellular part harbouring the highly conserved tyrosine
kinase domain. Ligand binding induces the formation of homo- or
heterodimers which subsequently trigger autophosphorylation of
cytoplasmic tyrosine residues. These phosphorylated amino acids
represent docking sites for a variety of signal transducers which
regulate membrane-proximal steps of a complex signaling network
ultimately defining the biological response to a given signal. The
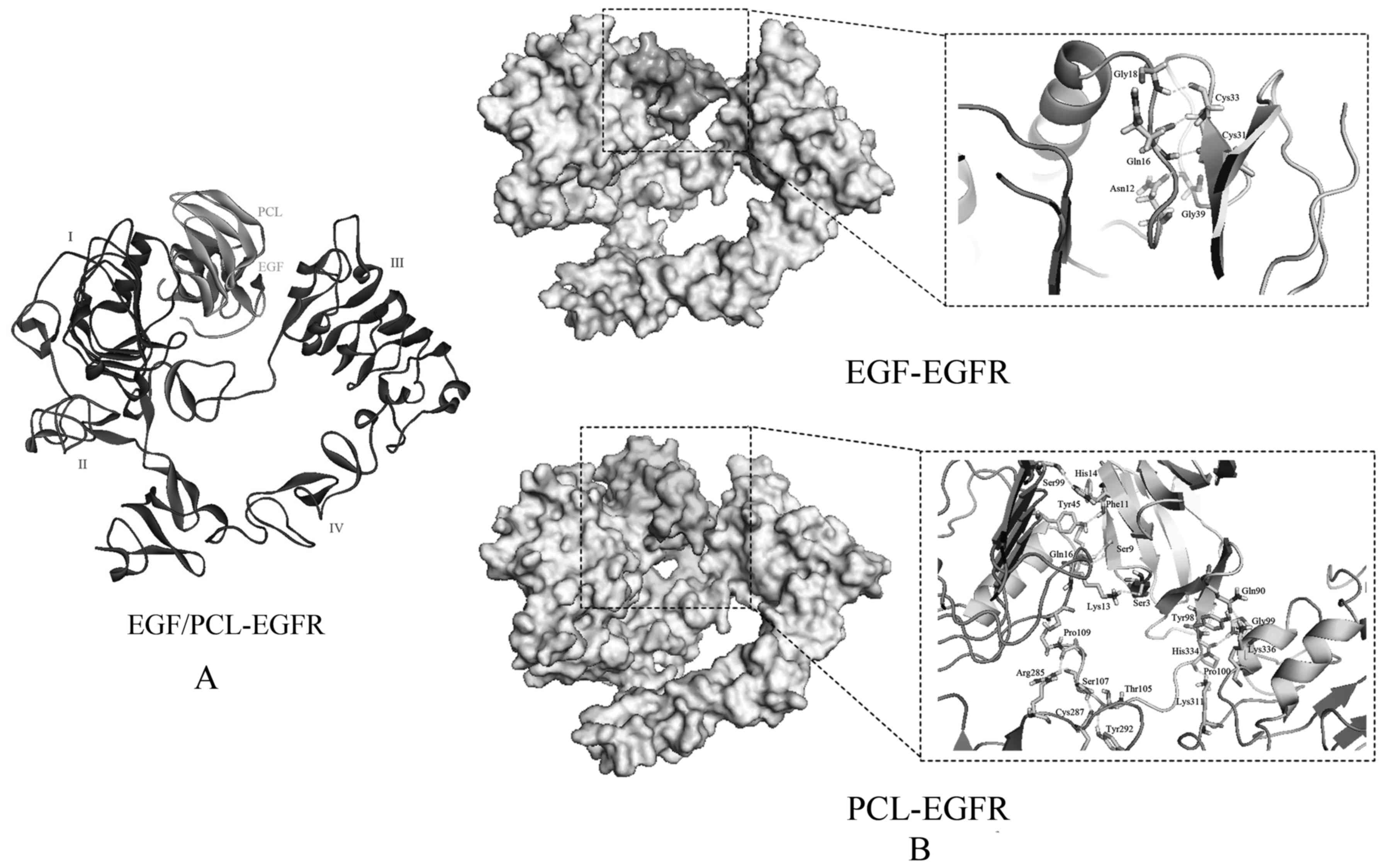
extracellular ligand-binding region has four domains (I–IV;
Fig. 6). By the protein-protein
docking and MD, we found that PCL can bind to the pocket between
domain I and III, which is also the binding site of the nature
ligand EGF. In short, PCL could competitively bind to EGFR binding
pocket and then prevent EGF from binding to EGFR, block the
autophosphorylation of the EGFR tyrosine kinase, after that the
EGFR activation is inhibited. As a result, the PCL could inhibit
the cell cycle progression, and cause accelerated apoptosis of the
tumor cells.
Our current findings revealed that the plant lectin
PCL treatment reduced PC3 cell growth, highlighting the distinct
functions of PCL relative to tumor development. The illustrated
mitochondrial function of PCL and its interaction with EGFR
provided important insights into prostate tumor progression and its
role in regulating mechanisms for tumor glycolysis. In summary,
this study suggests that PCL may serve as a novel therapeutic drug
in treating prostate cancer with EGFR-mediated energy mechanisms.
In future, the mechanisms of PCL and the downregulation of HK2
expression by EGFR mediation need to be addressed.
Acknowledgements
The present study was supported by the Foundation of
Science and Technology Resource of Shaanxi Province (no.
2016KTCL03-14).
References
|
1
|
Mahjoubi M, Azab M, Ghosn M, Theodore C
and Droz JP: Phase II trial of ifosfamide in the treatment of
metastatic hormone-refractory patients with prostatic cancer.
Cancer Invest. 8:477–481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: cancer ‘Achilles’ heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warburg O, Posener K and Negelein E: Ueber
den stoffwechsel dercarcinomzelle. Biochem Z. 152:309–344.
1924.
|
|
4
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayevsky A: Mitochondrial function and
energy metabolism in cancer cells: past overview and future
perspectives. Mitochondrion. 9:165–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang XZ, Yan LN, Sun GY, Zhao SY, Gu JM,
Lei KJ, Zhu Y and Wang CS: Expression of hypoxia-inducible factor-1
alpha, hexokinase-II and lactate dehydrogenase-V in hepatocellular
carcinoma and their biological significance. Chin J Cancer Prev
Treat. 1:41–44. 2013.
|
|
8
|
Fan YX, Shi WM, Li J, Yin JL, Yang CH,
Huang KL, Liu QZ, Li KB and Wu JZ: Relationship between
fluorodeoxyglucose uptake and overexpression of glucose transport
protein 1 and hexokinase-II in early-stage nasopharyngeal
carcinoma. Chin J Nucl Medi. 30:166–169. 2010.
|
|
9
|
Herrala AM, Porvari KS, Kyllönen AP and
Vihko PT: Comparison of human prostate specific glandular
kallikrein 2 and prostate specific antigen gene expression in
prostate with gene amplification and overexpression of prostate
specific glandular kallikrein 2 in tumor tissue. Cancer.
92:2975–2984. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He HC, Bi XC, Zheng ZW, Dai QS, Han ZD,
Liang YX, Ye YK, Zeng GH, Zhu G and Zhong WD: Real-time
quantitative RT-PCR assessment of PIM-1 and HK2 mRNA expression in
benign prostate hyper-plasia and prostate cancer. Med Oncol.
26:303–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pedersen PL: Warburg, me and Hexokinase 2:
Multiple discoveries of key molecular events underlying one of
cancers most common phenotypes, the ‘Warburg effect’, i.e.,
elevated glycolysis in the presence of oxygen. J Bioenerg
Biomember. 39:211–222. 2007. View Article : Google Scholar
|
|
12
|
Bhaskar PT and Hay N: The two TORCs and
Akt. Dev Cell. 12:487–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang ZT, Peng H, Li CY, Liu JJ, Zhou TT,
Yan YF, Li Y and Bao JK: Polygonatum cyrtonema lectin induces
murine fibrosarcoma L929 cell apoptosis via a caspasedependent
pathway as compared to ophiopogon japonicus lectin. Phytomedicine.
18:25–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Wu JM, Li J, Liu JJ, Li WW, Li CY,
Xu HL and Bao JK: Polygonatum cyrtonema lectin induces murine
fibrosarcoma L929 cell apoptosis and autophagy via blocking Ras-Raf
and PI3K-Akt signaling pathways. Biochimie. 92:1934–1938. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Cheng Y, Zhang B, Bian HJ and Bao
JK: Polygonatum cyrtonema lectin induces apoptosis and autophagy in
human melanoma A375 cells through a mitochondria-mediated
ROS-p38-p53 pathway. Cancer Lett. 275:54–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferguson KM, Berger MB, Mendrola JM, Cho
HS, Leahy DJ and Lemmon MA: EGF activates its receptor by removing
interactions that auto-inhibit ectodomain dimerization. Mol Cell.
11:507–517. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding JJ, Bao JK, Zhu DY, Zhang Y and Wang
DC: Crystal structures of a novel anti-HIV mannose-binding lectin
from Polygonatum cyrtonema Hua with unique ligand-binding property
and super-structure. J Struct Biol. 171:309–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozakov D, Beglov D, Bohnuud T, Mottarella
SE, Xia B, Hall DR and Vajda S: How good is automated protein
docking? Proteins. 81:2159–2166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozakov D, Brenke R, Comeau SR and Vajda
S: PIPER: An FFT-based protein docking program with pairwise
potentials. Proteins. 65:392–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Comeau SR, Gatchell DW, Vajda S and
Camacho CJ: ClusPro: an automated docking and discrimination method
for the prediction of protein complexes. Bioinformatics. 20:45–50.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Comeau SR, Gatchell DW, Vajda S and
Camacho CJ: ClusPro: a fully automated algorithm for
protein-protein docking. Nucl Acids Res. 32:W96–W99. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao JK, Zeng ZK and Zhou H: Purification
and characterization of the polygonatum cyrtonema Hua. Lectin II.
Chin Biochem J. 12:165–170. 1996.
|
|
23
|
Velpula KK, Bhasin A, Asuthkar S and Tsung
AJ: Combined targeting of PDKi and EGFR triggers regression of
glioblastoma by reversing the Warburg effect. Cancer Res.
73:7277–7289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toschi A, Lee E, Thompson S, Gadir N,
Yellen P, drain C Michael, Ohh M and Foster DA: Phospholipase
D-mTOR requirement for the Warburg effect in human cancer cells.
Cancer Lett. 299:72–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barducci A, Bussi G and Parrinello M:
Well-tempered metadynamics: A smoothly converging and tunable
free-energy method. Phys Rev Lett. 100:0206032008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ilott AJ, Palucha S, Hodgkinson P and
Wilson MR: Well-tempered metadynamics as a tool for characterizing
multi-component, crystalline molecular machines. J Phys Chem B.
117:12286–12295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phillips JC, Braun R, Wang W, Gumbart J,
Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L and Schulten K:
Scalable molecular dynamics with NAMD. J Comput Chem. 26:1781–1802.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonomi M, Branduardi D, Bussi G, Camilloni
C, Provasi D, Raiteri P, Donadio D, Marinelli F, Pietrucci F,
Broglia RA and Parrinello M: Plumed: A portable plugin for
free-energy calculations with molecular dynamics. Comput Phys
Commun. 180:1961–1972. 2009. View Article : Google Scholar
|
|
29
|
Darden T, York D and Pedersen L: Particle
mesh Ewald: An N•log(N) method for Ewald sums in large systems. J
Chem Phys. 98:10089–10092. 1993. View
Article : Google Scholar
|
|
30
|
Adelman SA and Doll JD: Generalized
langevin equation approach for atom/solid-surface scattering:
general formulation for classical scattering off harmonic solids. J
Chem Phys. 64:2375–2388. 1976. View
Article : Google Scholar
|
|
31
|
Davidchack RL, Handel R and Tretyakov MV:
Langevin thermostat for rigid body dynamics. J Chem Phys.
130:2341012009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feller SE, Zhang Y, Pastor RW and Brooks
BR: Constant pressure molecular dynamics simulation: the Langevin
piston method. J Chem Phys. 103:4613–4621. 1995. View Article : Google Scholar
|
|
33
|
Cornell WD, Cleplak P and Bayly CI: A
second generation force field for the simulation of proteins,
nucleic acids, and organic molecules. J Am Chem Soc. 117:5179–5197.
1995. View Article : Google Scholar
|
|
34
|
Liu B, Cheng Y, Bian HJ and Bao JK:
Molecular mechanisms of Polygonatum cyrtonema lectin-induced
apoptosis and autophagy in cancer cells. Autophagy. 5:253–255.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kirkwood JG: Statistical mechanics of
fluid mixtures. J Chem Phys. 3:300–313. 1935. View Article : Google Scholar
|
|
36
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: cancer's stygian link to the
‘Warburg Effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Xiong H, Wu F, Zhang Y, Wang J,
Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, et al: Hexokinase
2-mediated Warburg effect is required for PTEN- and
p53-deficiency-driven prostate cancer growth. Cell Rep.
8:1461–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L
and Jiang F: PKM2 and cancer: The function of PKM2 beyond
glycolysis. Oncol Lett. 11:1980–1986. 2016.PubMed/NCBI
|