Introduction
Liver cancer (or hepatocarcinoma) currently accounts
for 7% of all cancer cases diagnosed worldwide, resulting in
>600,000 deaths each year (1,2).
Treatments for early-stage hepatocellular carcinoma (HCC) include
tumor resection, liver transplantation and local ablation. However,
only 30–40% of early-stage liver cancer patients are eligible to
receive these therapies, and half of them may suffer from tumor
recurrence within 3 years of treatment (1–3). New
therapeutic strategies that improve the prognosis of liver cancer
are warranted, and immunotherapy is one alternative approach that
is showing therapeutic promise.
Anticancer vaccination has been applied as an active
immunotherapeutic strategy to stimulate specific and durable
antitumor immunity, and vaccines have been used in the treatment of
liver cancer or even prevention of cancer recurrence (4,5). The
rationale for vaccination against cancer is based on the presence
of circulating T cells that are reactive towards specific
cancer-associated antigens expressed by tumor cells (6,7). In
this respect, immunization with univalent cancer antigen such as
α-fetoprotein (AFP), carcinoembryonic antigen and glypican-3 (GPC3)
peptide has been proved to boost the antitumor immune response,
leading to the decreased tumor burden and increased overall
survival in patients with HCC (3,4,8,9).
However, the rapid genesis of mutant with decreased expression of
targeted antigen restrains the efficacy of univalent cancer
vaccine. Until now, it is difficult to identify all these targeted
antigens and select the most critical one for developing cancer
vaccine. Thus, whole cell vaccine that contain all these targeted
antigens was thought to induce more robust antitumor immune
responses than the traditional univalent cancer vaccine.
Recent studies have shown that embryonic stem cells
(ESCs) can boost the immune system when used as a whole-cell cancer
vaccine, and this stimulation leads to a reduction in tumor burden
(10–14). ESCs and certain cancer cells express
a specific subset of antigens called oncofetal antigens, and ESCs
are effective at presenting these antigens to the immune system and
eliciting an anticancer response (10–18).
Murine models of lung, colon and ovarian cancer have confirmed the
antitumor efficacy of ESC vaccination, but it is not known if
immunization with ESCs will have a measurable impact on liver
cancer. Some evidence suggests that liver cancer may arise from the
aberrant activation of hepatic stem cells (HSCs)/hepatic progenitor
cells (HPCs), as well as share cellular and molecular phenotypes
with HSC/HPCs (19,20). Theoretically, HSC/HPCs may serve as
attractive vaccine candidates that trigger T-cell responses as
effectively as ESCs and drive an antitumor response that is
specific, for liver cancer.
Collectively, both ESC and HSC/HPCs share cell
surface antigens with liver cancer cells (16–20),
and both cell types are potential cancer vaccine candidates that
may generate antitumor immunity against liver cancer. For this
reason, we compared the effectiveness of ESC and HSC whole-cell
cancer vaccines in the prophylaxis and treatment of subcutaneous
hepatic tumors transplanted into adult mice. Our results show that
immunization with the HSC vaccine prevented the development of
liver cancer for up to four weeks after vaccination. In addition,
established subcutaneous tumors significantly shrank after HSC
vaccination. Interestingly, ESC vaccine is less effective than HSC
vaccine in prophylaxis and treatment of liver. These data support
that HSC is a superior vaccine candidate for durable antitumor
protection in this hepatocarcinoma model.
Materials and methods
Mice
Wild-type C57BL/6j mice were obtained from the Wushi
Laboratory (http://www.fzzmsoft.com/xieli/index.asp) and
maintained at the Laboratory Animal Center in Fujian Medical
University according to standard guidelines. To induce development
of hepatic oval cells (HOCs) in the liver, 4-week-old C57BL/6j mice
were fed a choline-deficient, ethionine-supplemented (CDE) diet for
three weeks as previously described (21). This diet was comprised of
choline-deficient chow (Medicience Ltd.) and 0.15% (w/v)
DL-ethionine (Sigma-Aldrich) added to the drinking water. Wild-type
C57BL/6j mice were immunized at 10–12 weeks of age. All animal
protocols were approved by the Animal Care Committee of Fujian
Medical University.
Cell lines
HOCs were selected as the source of HSCs in our
vaccination strategy. HOCs were isolated from C57BL/6j mice that
were fed a three-week CDE diet, and the cells were cultured under
standard conditions (37°C, 5% CO2) as previously
described with some modifications (22–24).
Briefly, mice were anaesthetized, the abdominal cavity was opened,
and the liver was perfused via the hepatic portal vein with two
buffers: 50 ml of EGTA buffer [0.5 mM EGTA (Sigma-Aldrich), 137 mM
NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 0.65 mM
MgSO4, 10.07 mM HEPES (Sigma-Aldrich), pH 7.4], and 55
ml of collagenase buffer [67 mM NaCl, 6.7 mM KCl, 4.76 mM
CaCl2, 100.7 mM HEPES, 0.035% collagenase type I
(Sigma-Aldrich), pH 7.6] at a flow rate of 5 ml/min. Next, the
liver was removed, dissected, and digested in liver digestion
buffer [0.1% collagenase type VIII (Sigma-Aldrich), 0.09% Pronase
(Roche Diagnostics), 0.025% trypsin/0.01% EDTA, 0.004% DNase
(Sigma-Aldrich)] at 37°C for 50 min. After incubation, an equal
volume of cold Williams' E medium containing 2% FBS was added to
the digestion buffer, and the mixtures were filtered through a
40-µm cell strainer (BD Biosciences). Cells were pelleted,
re-suspended in phosphate-buffered saline (PBS), and separated by
density gradient centrifugation at 1400 × g for 20 min. Cells were
collected, washed and plated at a density of 1×106
viable cells/ml onto collagen-coated dishes in Williams' E medium
with 10% FBS (Gibco), 10 mM nicotinamide (Sigma-Aldrich), 2 mM
glutamine (Solarbio), 10−7 M dexamethasone, 1X
ITS+ (Sigma-Aldrich), 0.2 mM ascorbic acid
(Sigma-Aldrich), 20 mM HEPES, 1 mM Na pyruvate (Sigma-Aldrich),
0.15% NaHCO3, 14 mM glucose, 20 ng/ml EGF (BD
Biosciences), 1.0% (v/v) fungizone (Solarbio), and 1.0% (v/v)
penicillin/streptomycin (Solarbio). Cells were sub-cultured for 10
weeks (30–35 passages), until the HOCs became stable and displayed
consistent morphology.
The C57BL/6 mouse embryonic stem cells (mESCs) were
purchased from a commercial supplier (Cyagen Bioscience Inc.), and
co-cultured with C57BL/6 mouse embryonic fibroblasts (MEFs) as
feeder cells in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 15% fetal bovine serum (FBS), 2 mM L-glutamine,
0.1 mM non-essential amino acids, 0.1 mM 2-mercaptoethanol, and
1000 U/ml leukemia inhibitory factor. Preparation of MEF feeders
was performed as previously described, with some modifications
(25). Briefly, the MEFs were
derived from C57BL/6 mouse embryos (around day 13 of gestation) and
cultured in DMEM supplemented with 10% FBS. After cell propagation
(2–3 passages), MEFs were mitotically inactivated by treatment with
10 µg/ml mitomycin C in MEF culture medium for 3 h, plated onto
untreated culture vessels at density of 3.0–4.0
cells/cm2, and used as feeder cells.
The hepatoma cell line of C57BL/6 origin (Hepa 1–6)
was purchased from the Cell Bank of the Chinese Academy of Sciences
(http://www.cellbank.org.cn/mulu.asp)
and cultured under standard conditions in DMEM supplemented with
10% FBS as described above. At the time of inoculation, Hepa 1–6
and mESCs were in passages 5–10 and 10–15, respectively.
Preparation of HOC and mESC whole-cell
vaccine
HOCs were removed from the dish by treatment with
0.25% trypsin-EDTA whereas mESCs were detached and separated from
MEFs using the ‘differential adhesion method’ as previously
described (26). The HOC and mESC
whole-cell vaccines were generated by fixing HOCs and mESCs in a
solution of 4% paraformaldehyde for 1 h, followed by three washes
with sterile PBS. Finally the cells were re-suspended in PBS at a
concentration of 1×106 cells/ml, stored at 4°C, and used
for vaccination within one week.
Immunization protocol and tumor
challenge
Paraformaldehyde-fixed, whole-cell vaccines
(1×106 HOCs or mESCs) and live hepatoma cells
(4×106 Hepa 1–6 cells/mouse) were administered by
subcutaneous inoculation in the mid-left and mid-right abdominal
region of mice, respectively. The scheme of immunization and tumor
inoculation was described elsewhere, with some modifications
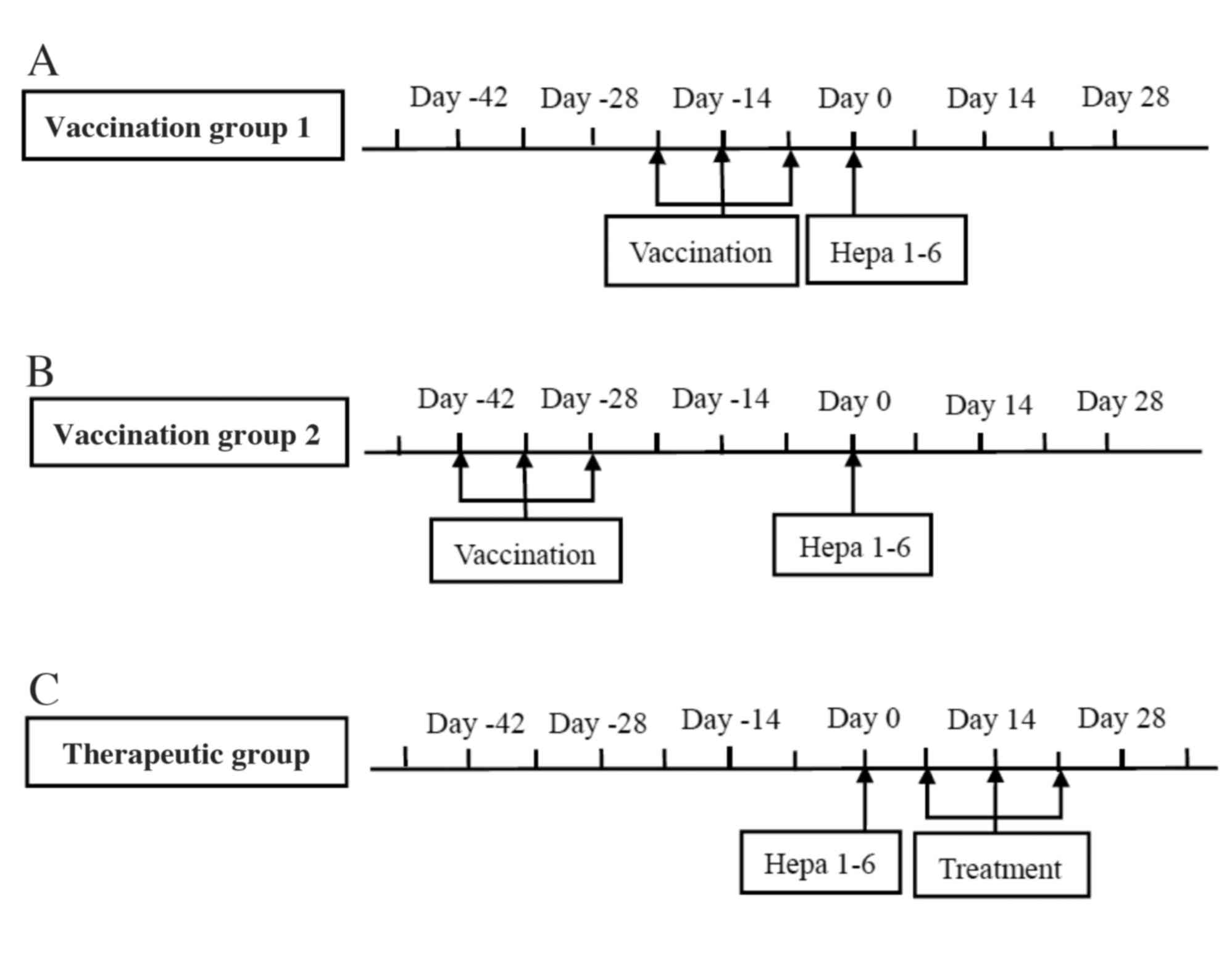
(Fig. 1) (11,12).
To determine and compare the efficacy of HSC or mESC as a
whole-cell vaccine, naive C57BL/6j mice were vaccinated with either
HOCs or mESCs three times in one-week intervals, followed by
subcutaneous inoculation with Hepa 1–6 cells at one week after the
last HOC/mESC boost (Fig. 1A). For
long-term memory responses, mice were challenged with Hepa 1–6
cells at four weeks after the last vaccination (Fig. 1B).
To determine and compare the therapeutic efficacy of
HSC or mESC as a whole-cell vaccine (Fig. 1C), C57BL/6 mice received
immunization three times (at one-week interval) 7 days after tumor
inoculation. As a control, mice were inoculated with Hepa 1–6
without vaccination or treatment. Tumor growth was monitored every
other day using digital calipers to measure both the longitudinal
(L, mm) and transverse diameters (T, mm). Tumor area (LxT,
mm2) was calculated as previously described (10). Mice were also monitored for general
health indicators after immunization, such as overall behavior,
feeding, body weight and ruffled fur. If the tumor diameter
exceeded 15 mm, or if tumor ulceration was observed, the mice were
euthanized. Per our approved protocol that was primarily designed
to evaluate the tumor formation rate of mice and response rate and
to ease the pain and suffering of tumor-bearing mice, no survival
experiments were conducted in this study.
Indirect immunofluorescence
cytochemistry
Isolated HOCs were confirmed by immunofluorescence
analysis and testing for the expression of two commonly associated
HOC markers: cytokeratin 19 (CK19) and muscle pyruvate kinase 2
(M2PK). Briefly, cells were harvested from culture
dishes and grown on poly-L-lysine-coated coverslips. Cells were
fixed with 4% paraformaldehyde at room temperature for 20–30 min
and then permeabilized with 0.5% Triton X-100 for 5 min. After
permeabilization, cells were blocked with 1% bovine serum albumin
(BSA) and incubated with the following primary antibodies at 4°C
overnight: goat anti-CK19 (1 µg/ml, Santa Cruz Biotechnology), or
rabbit anti-M2PK (1 µg/ml, Abcam). Next, cells were
washed three times in PBS and incubated with a secondary donkey
anti-goat CruzFluor™ 594 antibody (1 µg/ml, Santa Cruz
Biotechnology), or donkey anti-rabbit Alexa Fluor® 488
antibody (2 µg/ml, Abcam), for 30 min at 37°C. Finally cells were
washed three more times, mounted in antifade mounting medium
(Beyotime) with 4′,6- diamidino-2-phenylindole (DAPI), and examined
under a fluorescence microscope (Carl-Zeiss Axiovert 200).
Statistical analysis
The statistical analysis was performed using SPSS
statistical software 13.0 and GraphPad Prism 5.0 software.
Antitumor responses to immunotherapeutic vaccination were evaluated
using a modification of the WHO criteria (Table I). Comparison of the cancer
formation rate between the prophylaxis groups, and the disease
control rate [DCR: complete response (CR) + partial response (PR) +
stable disease (SD)] between treatment groups, was made by the
χ2 method. The P-value was adjusted to 0.0125. The
dynamic tumor growth size was analyzed using a multivariate linear
model. After confirming the inequality of variance with the Levene
test, the post-therapeutic change in tumor size was analyzed with
the Wilcoxon test. Differences in tumor growth size and weight
between groups were detected by the Nemenyi-test. Most data were
presented as mean ± SEM, and a P-value <0.05 was considered
statistically significant.
 | Table I.The modified WHO criteria. |
Table I.
The modified WHO criteria.
|
| Response
criteria |
|---|
| CR | Complete
disappearance of all lesions |
| PR | At least 50% decrease
in tumor burden compared with baseline |
| SD | Neither sufficient
shrinkage to qualify for PR nor sufficient increase to qualify for
PD |
| PD | At least 25% increase
in tumor burden compared with baseline |
Results
Characterization of cultured HOCs and
ESCs for vaccine preparation
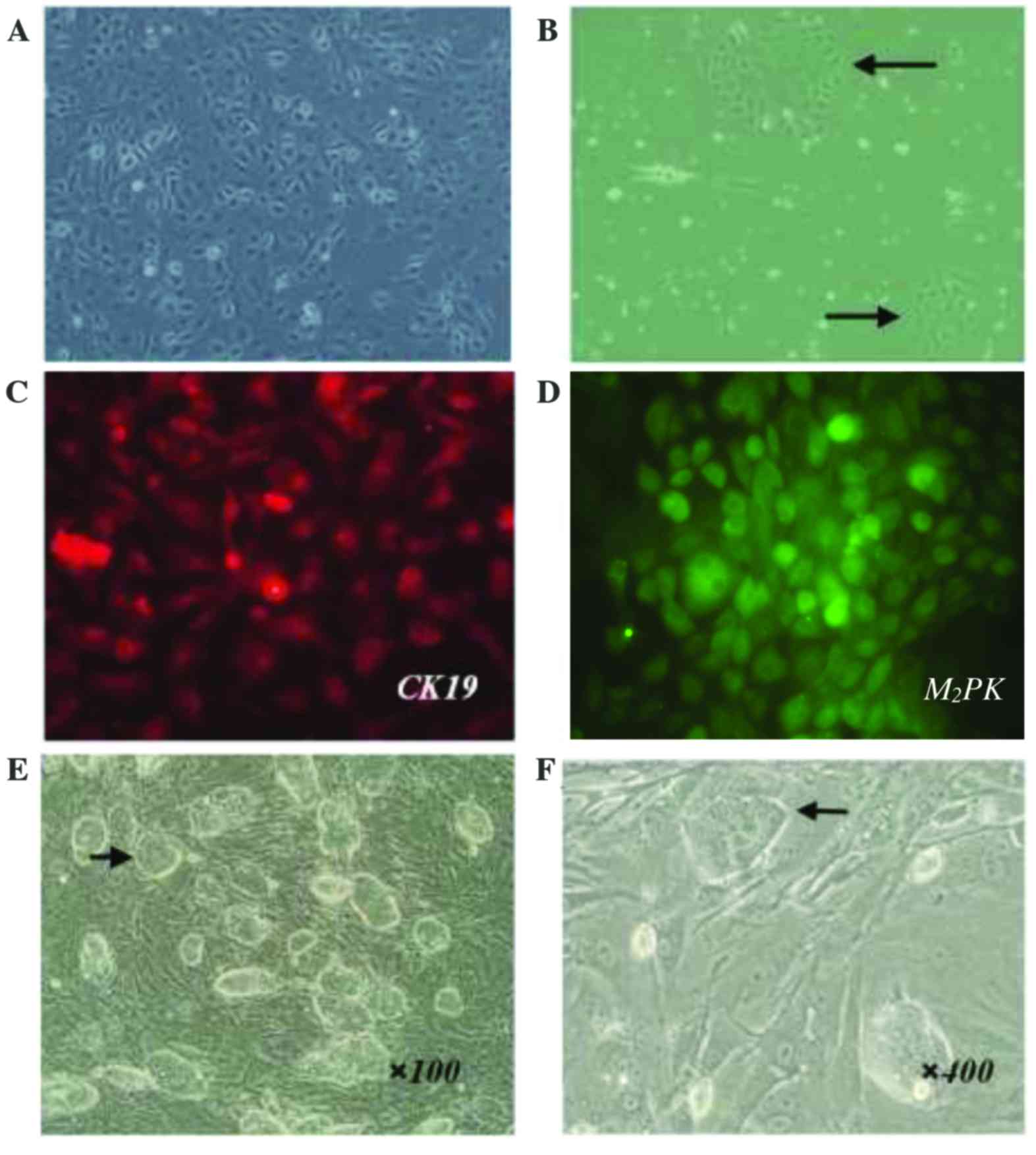
In this study, HOCs were readily isolated from
C57BL/6j mice by a two-step collagenase digestion and density
gradient centrifugation. Following serial passage in vitro,
HOC cell lines displayed consistent morphology (Fig. 2). HOCs were adherent,
cobblestone-like cells with ovoid nuclei and scant cytoplasm. The
majority of cells formed uniform monolayers (Fig. 2A), and a small number of cells
produced identifiable clones (Fig.
2B). Vaccine preparation was performed after HOCs had been
continuously cultured for >3 months. Following serial passaging
(30–35 times), HOCs maintained viability, proliferative capacity,
and typical cellular morphology. These results suggest that the
HOCs were immortalized.
HOC cell lines derived from primary HOCs were
further characterized by immunofluorescence staining for two
commonly known murine oval cell markers: the hepatic progenitor and
cholangiocyte marker (CK19), as well as the hepatic progenitor and
early hepatocyte marker M2PK. HOC cell lines were
positive for expression of both CK19 and M2PK, further
suggesting that these HOCs were hepatic progenitor cells and ideal
candidates for HSCs (Fig. 2C and
D). Together these data show the establishment of a stable HOC
cell line that can be used as a source of HSCs in a cancer
vaccine.
To establish an ESC vaccine, we purchased mESCs
(C57BL/6 origin) from a commercial supplier. The cells were
maintained in an undifferentiated state by co-culturing them with
MEFs (see Materials and methods). The undifferentiated mESC clones
typically had clear borders, were round in appearance, and
demonstrated a high nuclear-cytoplasmic ratio and prominent
nucleoli (Fig. 2E and F).
HSC vaccination is superior to ESC
vaccination in generating long-lasting antitumor protection against
subcutaneously injected hepatic tumor cells
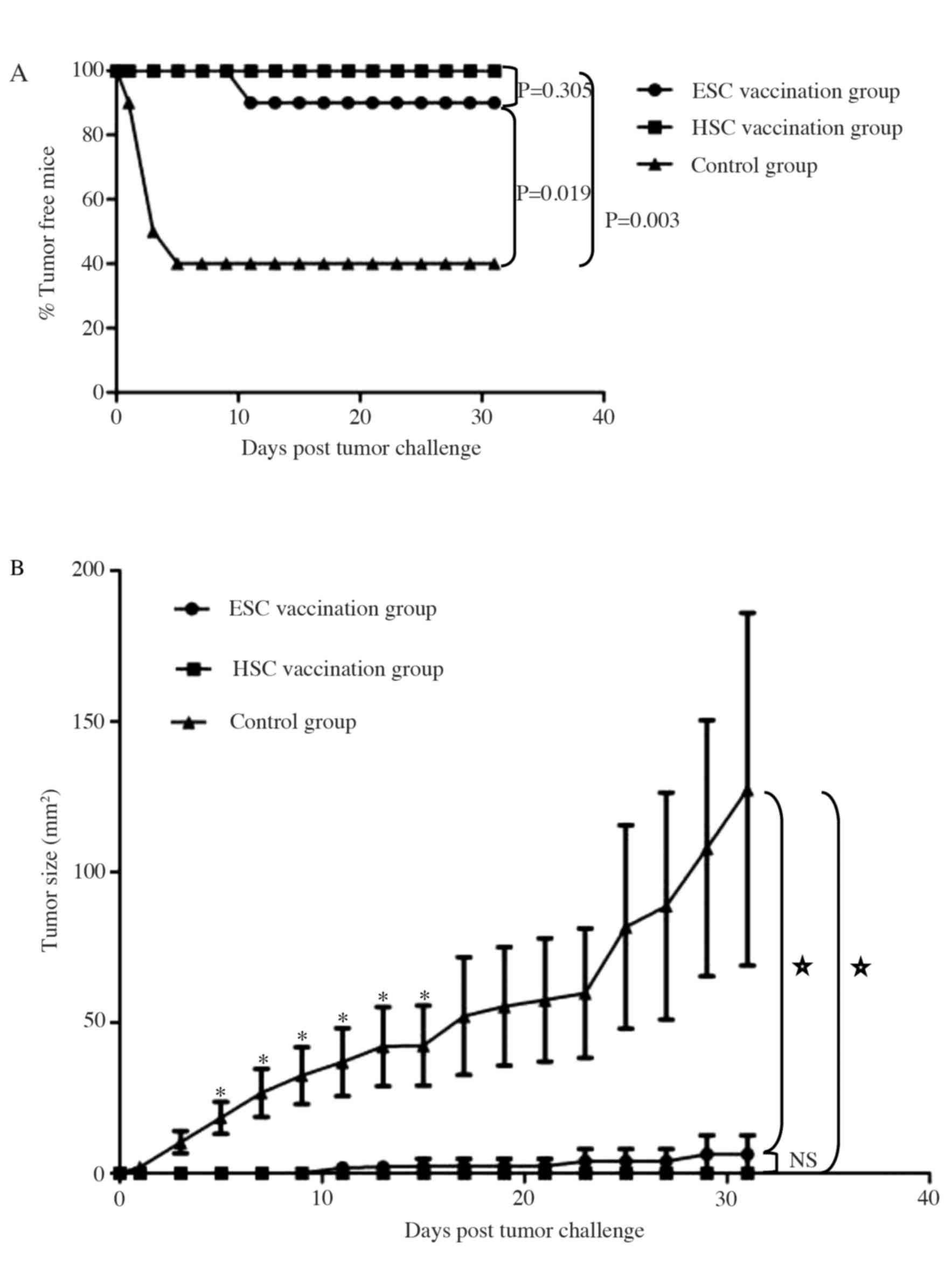
We compared the effectiveness of HSC and ESC
whole-cell vaccination in the prophylaxis of subcutaneous hepatic
tumor cells transplanted into adult mice (Fig. 3). Animals were challenged with live
Hepa 1–6 cells shortly after receiving a series of immunizations,
and tumor formation was monitored. At 31 days post-tumor challenge,
the rate of tumor formation in the HSC vaccination group was 0% (0
out of 10 mice), 10% in the ESC vaccination group (1 out of 10
mice), and 60% in the control group (6 out of 10 mice; Fig. 3A). Tumor incidence was significantly
increased in the control group compared to either the HSC or ESC
vaccination group (P<0.05), but the rate of tumor incidence was
similar between the HSC and ESC vaccination groups (P>0.05).
Preventative vaccination significantly reduced tumor growth after
day 5 (Fig. 3B; P<0.05). Hence
both HSC and ESC vaccination effectively prevented the
establishment of implantable hepatocarcinomas especially when the
tumor challenge was administered within one week of the last
immunization.
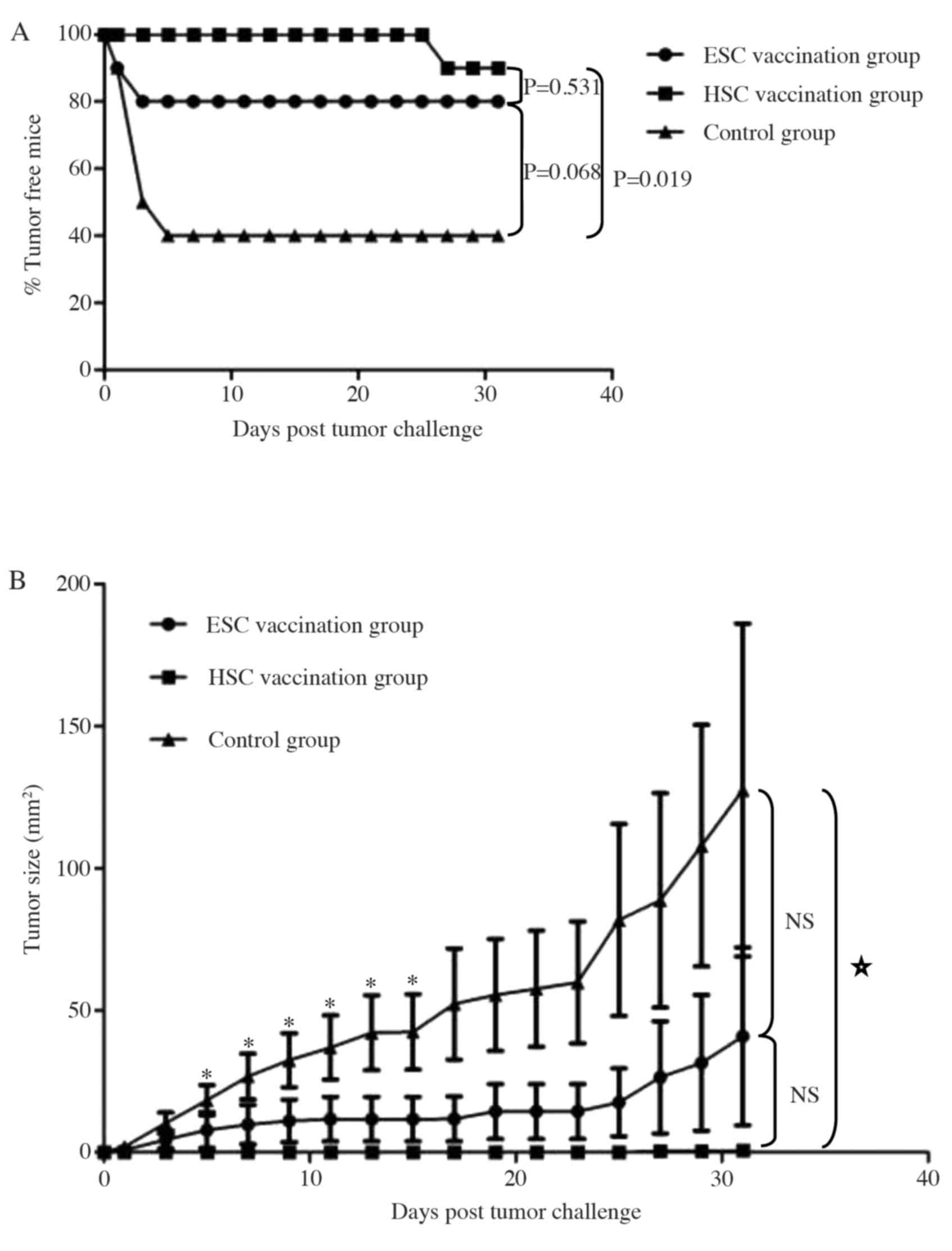
We further investigated if preventative immunization
with HSC or ESC vaccines conferred long-lasting protection against
a subsequent challenge with hepatocarcinoma cells (Fig. 4). A second challenge was
administered by inoculating immunized mice with live Hepa 1–6 cells
at four weeks after the last immunization. As shown in Fig. 4A, 90% of mice immunized with the HSC
whole-cell vaccine and 80% of mice that received the ESC whole-cell
vaccine did not develop subcutaneous hepatocarcinomas for up to 31
days after the tumor challenge. Both the rate of tumor formation
and the average tumor size were significantly reduced in the HSC
vaccination group (Fig. 4B;
P<0.05), but the ESC immunization group demonstrated a less
robust decrease in tumor and tumor size (P>0.05). These results
suggest that HSC vaccination was superior to ESC vaccination in
generating long-lasting antitumor protection.
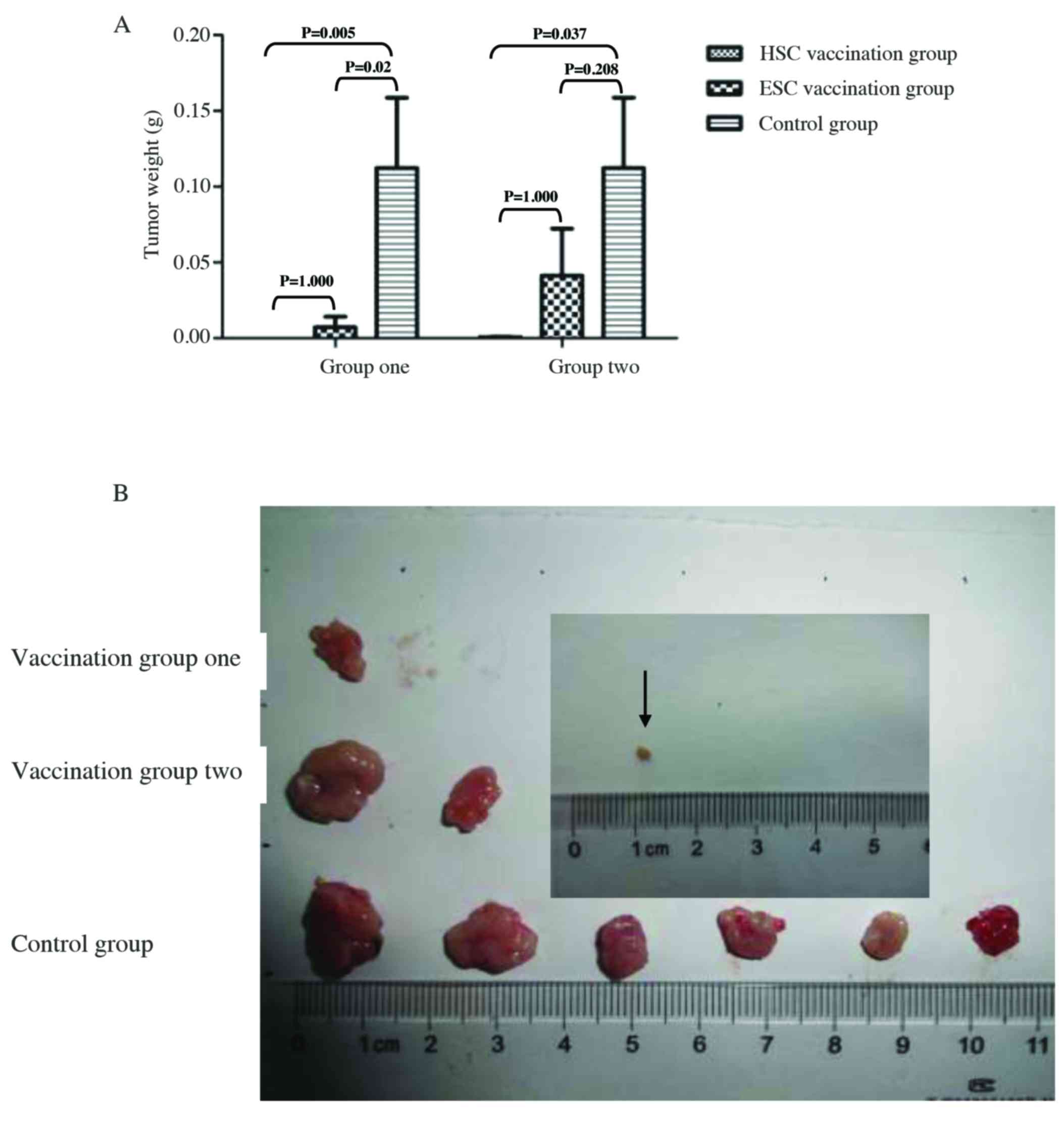
We confirmed these results by comparing the weight
of subcutaneous hepatomas harvested from different groups at 31
days post-tumor challenge when most of subcutaneous tumor lesion
became ulcerated. The average weight of the subcutaneous hepatomas
was significantly reduced in the HSC vaccination group compared to
the control group (P<0.05), but modestly decreased in both ESC
vaccination groups compared with the control group (P>0.05;
Fig. 5A). Macroscopic examination
of subcutaneous hepatomas indicated that no mass was found in HSC
vaccination group 1 while one mass was found in a mouse in ESC
vaccination group 1, although the number of mice in each group was
equal (10 mice per group) in this study. Furthermore, two of ten
mice in ESC vaccination group 2 and one of ten mice in HSC
vaccination group 2 developed subcutaneous hepatoma at the end of
the study. Obviously, the size and weight of hepatoma developed in
a mouse HSC vaccination group was significantly reduced compared
with either ESC vaccination group or control group (Fig. 5). Collectively, our results indicate
that HSC vaccination is better than ESC vaccination in the
prophylaxis of implantable hepatocarcinomas in mice.
HSC vaccination is superior to ESC
vaccination in the treatment of established hepatocarcinoma tumors
in mice
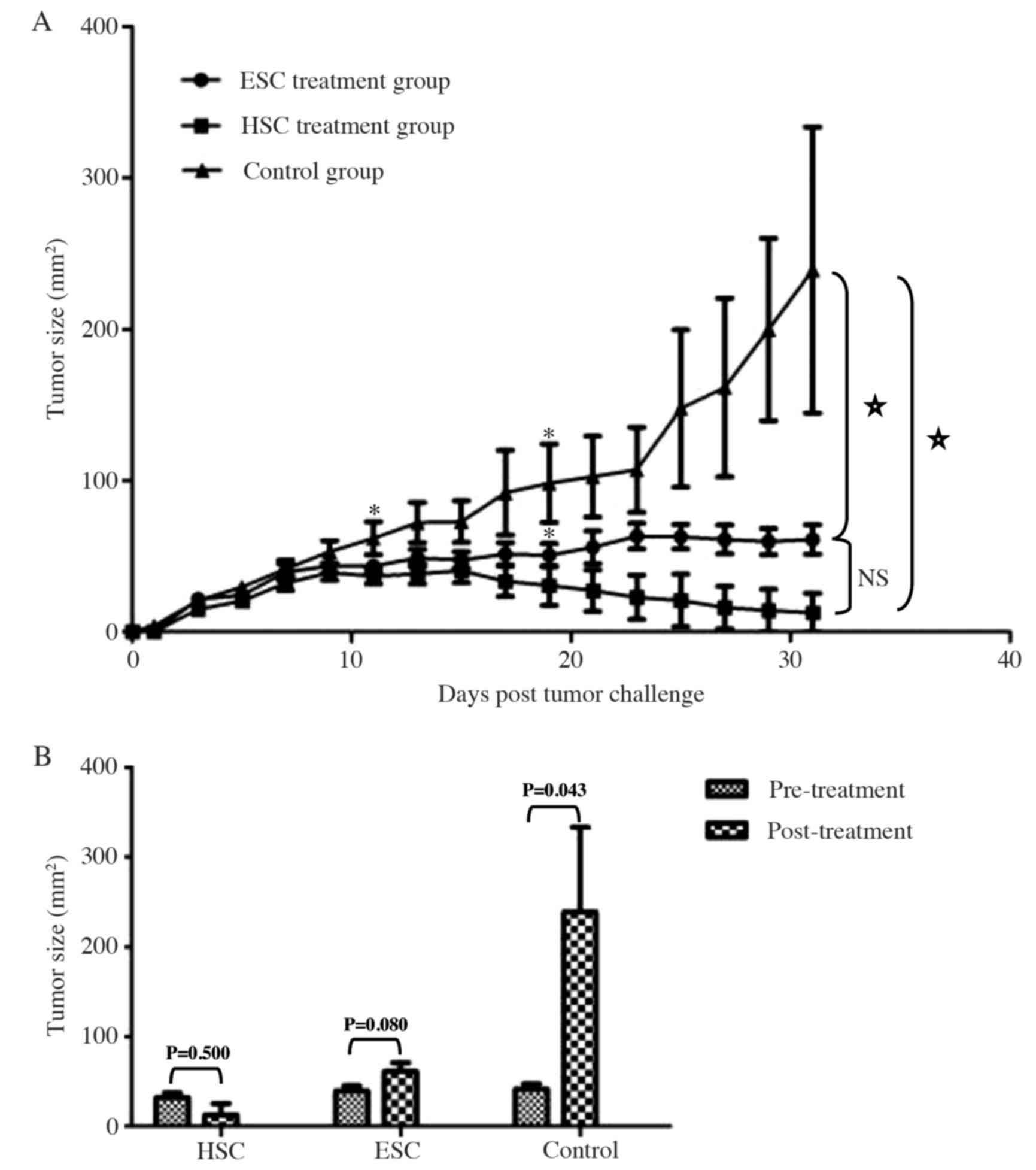
Next, we investigated whether HSC or ESC whole-cell
vaccination was effective at reducing tumor load in established
hepatocarcinomas. Mice were inoculated with Hepa 1–6 cells, and
solid tumors were established (day 5 post-inoculation). Mice were
randomly divided into a control group (no vaccine), or into HSC and
ESC vaccine treatment groups. No significant differences in the
initial tumor burden or size were found between the three groups at
the start of the treatment, as confirmed by the Wilcoxon
signed-rank test (P>0.05). When tumors were examined at day six
after the first vaccination, the average size of the subcutaneous
hepatomas was significantly reduced in the HSC treatment group
(P<0.05; Fig. 6A). Immunization
with the ESC vaccine significantly reduced tumor growth at 14 days
post-vaccination (P<0.05).
The subcutaneous hepatocarcinomas grew progressively
in the control group, and most of the tumor lesions became
ulcerated by the end of study (day 31). However, none of the mice
developed obvious tumor ulceration in the vaccine treatment groups
during this study (data not shown). Strikingly, the average size of
the subcutaneous hepatocarcinomas continued to decline modestly
following HSC treatment (P>0.05), although a slight increase in
tumor volume was observed in mice receiving ESC treatment
(P>0.05; Fig. 6).
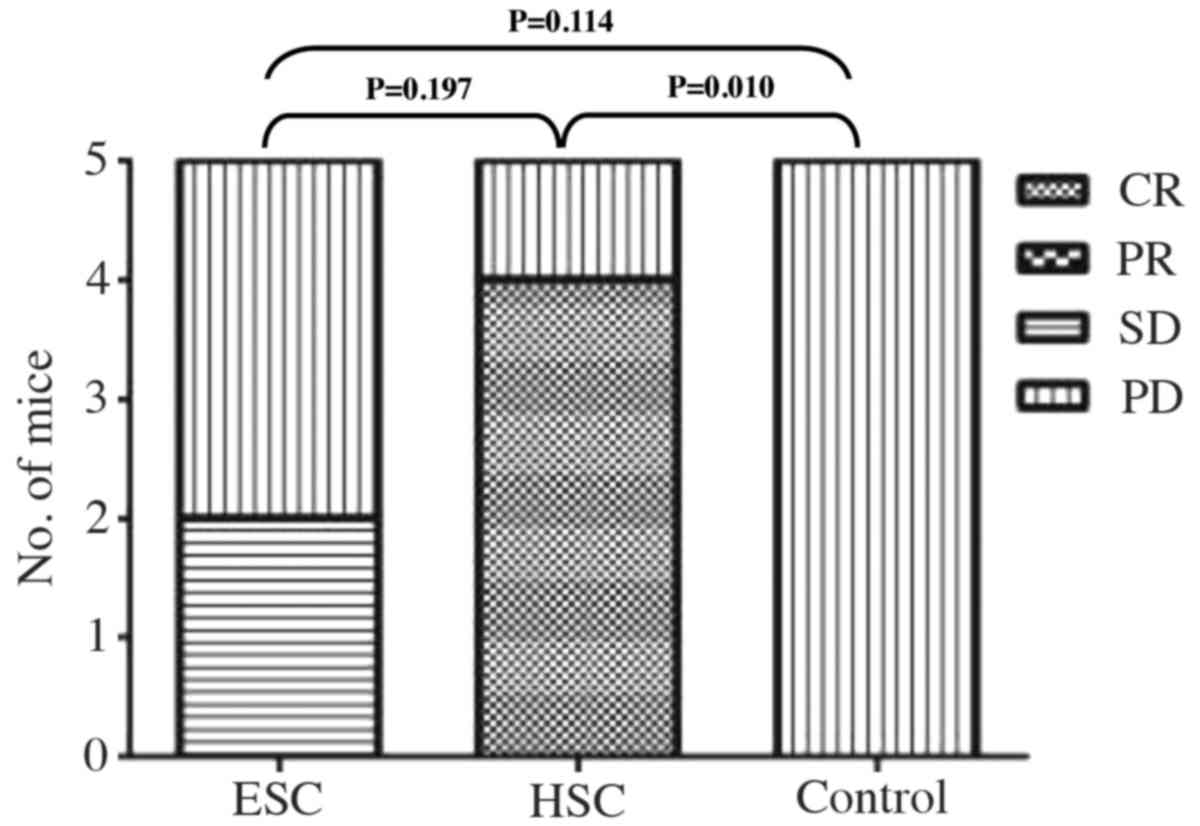
We also evaluated the antitumor responses to
immunotherapeutic vaccination using a modification of the WHO
criteria (Table I). Although 1 out
of 5 mice still suffered from progressive disease during HSC
treatment, 4 out of 5 mice showed complete clearance of tumor
burden following the three-week treatment (Fig. 7). In the ESC treatment group, 2 out
of 5 mice presented with stable tumor loads, and 3 out of 5 mice
succumbed to progressive disease by the end of study. In contrast,
all of the mice developed progressive disease in the control group
(Fig. 7).
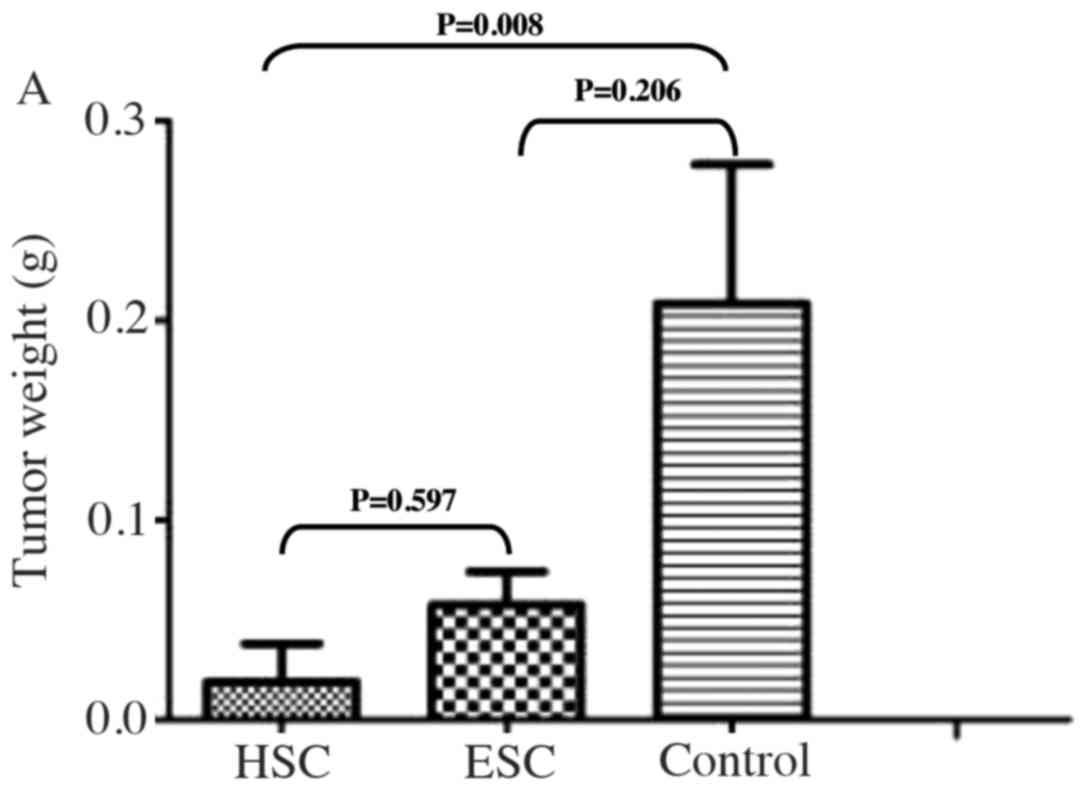
All mice were euthanized at the end of this study,
and the subcutaneous hepatocarcinomas were harvested and weighed.
The average tumor weight decreased modestly in the HSC treatment
group and was slightly reduced in the ESC treatment group, but any
decline in tumor weight was not statistically significant
(P>0.05; Fig. 8A). Since most of
subcutaneous tumor lesions disappeared following the HSC
vaccination, only one mass that was visible in one of the five mice
in HSC treatment group could be harvested for further comparison.
However, the tumor masses were all detectable in the five mice in
ESC treatment group following the ESC vaccination. Macroscopic
examination of the existing tumors revealed that the size of
subcutaneous hepatomas reduced significantly in HSC vaccination
group and decreased modestly in ESC vaccination group compared to
the control group (Fig. 8B).
Collectively, our results suggest that therapeutic immunization
with HSCs is superior to ESCs in the treatment of established
hepatocarcinomas in mice.
Discussion
The goal of activating the immune system for the
prophylaxis and treatment of liver cancer has led to the
investigation of several immunotherapeutic approaches, including
cancer vaccines (3,4,7). Since
the antigens expressed by cancer cells are usually heterogeneous
and plastic, traditional cancer vaccines that target single or
multiple cancer-associated antigens may not be sufficient enough to
eradicate all cancer cells (27).
As knowledge about the nature of these targeted antigens expands,
and as new antigenic oncoproteins are identified, the promise of
developing an effective vaccine for the prevention and treatment of
liver cancer is growing. In this respect, development of a stem
cell-based vaccine is a good therapeutic strategy due to the shared
expression of specific oncofetal antigens with liver cancer cells,
as well as the ability of stem cells to generate a robust immune
response against other types of cancer.
To investigate if stem cell-based vaccines are
effective against hepatic tumors, we compared both HSC and
ESC-derived whole-cell vaccines regards to the prevention of tumor
establishment and shrinkage of existing tumors in a mouse model of
implantable liver cancer. To minimize the impact of individual
difference on the interpretation of results, we used the method of
randomization and confirmed that there was no significant
difference in either group before vaccination. We found that both
HSC and ESC whole-cell vaccination was effective in preventing the
outgrowth of implanted tumor cells in 100 and 90% of mice that were
challenged one week after the last immunization. The benefits of
prophylactic immunization appeared to wane over time, because the
tumor burden in mice increased when the tumor challenge was
administered four weeks after the last vaccination. It indicated
that the immune memory was relatively weak in this study and thus
the antitumor effect of prophylactic vaccination wanes overtime.
Even so, both the rate of tumor formation and the average size of
the subcutaneous tumors were still decreased significantly in the
HSC vaccination group compared with the control group. In contrast,
there were no statistically significant differences in the rate of
tumor formation or in the average tumor size between the ESC
vaccination group and the control group.
Although HSCs and ESCs share expression of several
antigens, there was a question of whether HSCs would be better than
ESCs at generating durable protection against established hepatoma.
We discovered that immunization with an HSC-based vaccine in the
absence of an adjuvant could result in the dynamic retardation or
shrinkage of established liver cancer. In contrast, an ESC-based
vaccine treatment led to a delayed progression of subcutaneous
liver cancer but did not eliminate the tumor burden in this model.
Thus we concluded that HSCs were the better option for a stem
cell-based vaccine targeting liver cancer.
Although our results point to HSCs as being the
better vaccine candidate, the precise mechanisms underlying the
antitumor protection conferred by HSCs versus ESCs remain obscure.
Recent evidence suggests that liver cancer cells express a subset
of embryonic antigens that are downregulated during fetal
development, and this downregulation occurs before the mammalian
immune system determines ‘self’ versus ‘non-self’ (11). Embryonic antigens that are shared by
both ESC and liver cancer cell might be included in the ‘non-self’
repertoire and remain immunogenic, leading to a robust antitumor
immunity. ESC vaccination has been shown to increase the intratumor
infiltration of CD8+ effector T cells, as well as
decrease both T regulatory cells (Treg) and myeloid derived
suppressor cells (MDSCs) within the tumor microenvironment
(10–14). Further study indicated that the
antitumor immunity conferred by ESC vaccination was primarily
mediated by CD8+ T cells because depletion of this
subset completely abrogated the protective effect in vivo.
In addition, ESC vaccines can modulate the Th1/Th2 balance and tip
the immune response towards Th1-driven antitumor immunity (10–12).
Less is known regarding the effectiveness of HSCs as
whole-cell vaccine candidates in the prophylaxis and treatment of
liver cancer, or which antitumor mechanisms that HSCs induce are
superior to ESCs. One possible explanation could be that hepatic
tumor cells arise from the malignant transformation of normal HSCs
and would thereby share the expression of stem cell-related
pathways and antigens associated with immune escape mechanisms
(19,20). Vaccination with HSCs could elicit
robust antitumor immunity against these malignantly transformed
stem cells, since HSCs would present the identical immune targets
that are expressed by the hepatic tumors (27). In this respect, antigens that were
highly expressed in both HSC and liver cancer cells, such as
α-fetoprotein (AFP) and telomerase reverse transcriptase (TERT),
have been shown to be frequently recognized by T cell and were
capable of generating peptide-specific CTLs (6). However, HSC-derived whole-cell
vaccines is not without potential risks and warrants a careful
consideration for possible adverse side effects, although no mice
showed signs of discomfort (such as the change of behavior,
feeding, neuromuscular tone or appearance of fur) or died in this
study. The most obvious risk would be cross-reactivity with normal
HSCs in the liver, which could potentially lead to liver damage.
However, previous studies have clearly demonstrated that the use of
ESC and HSC did not result in significant autoimmune response in
the context of immunization and transplantation (28), respectively. Since the potential
side effect of autoimmunity of HSC vaccine has not been fully
studied in the context of immunization, further studies are needed
to investigate whether the antitumor immune responses elicited by
HSC vaccination are vigorous enough to target the subset of HSCs
present in the canals of Hering within the liver.
There were a few limitations in our study. First, we
challenged immunized mice with a single hepatic cancer cell line
(Hepa 1–6), so it is not known if HSC or ESC-based vaccines would
produce the same antitumor protection against other liver cancer
cell lines (especially cell lines derived from orthotopic liver
cancer). Second, the size of the subcutaneous hepatic tumors was
evaluated in our study, but less is known if reducing tumor size is
associated with prolonged survival in vaccinated mice. Finally, the
underlying antitumor mechanisms of HSC and ESC-derived whole-cell
vaccines, as well as the potential toxicity of each type of vaccine
are not fully illustrated in this study but it will be revealed in
subsequent research.
In conclusion, recent developments in cancer vaccine
design have explored the use of ESCs and HSCs as potential vaccine
candidates, due to the expression of similar cell surface antigens
by stem cells and cancer cells. Because effective treatments for
patients with advanced-stage liver cancer are not currently
available, a stem cell-based vaccine is one therapeutic strategy
that may stimulate durable antitumor immunity and prolong patients
survival. Our study supports the hypothesis that HSCs are better
than ESCs as a vaccine candidate for durable antitumor protection
in a murine hepatocarcinoma model. We propose that HSCs are more
effective than ESCs at generating long-lasting protection against
hepatic tumors and could be used as both a prophylactic and
therapeutic vaccine in the prevention and treatment of liver
cancer.
Acknowledgements
The authors would like to thank the staff of the
laboratory animal center in Fujian Medical University for providing
excellent animal care. This study was supported by the Fujian
Province Natural Science Fund (grant no. 2015J0105).
References
|
1
|
European Association for The Study of The
Liver European Organisation for Research and Treatment of Cancer, .
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Agency for Research on
Cancer (IARC), . About CANCERMondial. http://www-dep.iarc.fr/January 11–2013.
|
|
3
|
Breous E and Thimme R: Potential of
immunotherapy for hepatocellular carcinoma. J Hepatol. 54:830–834.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greten TF, Manns MP and Korangy F:
Immunotherapy of hepatocellular carcinoma. J Hepatol. 45:868–878.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palmer DH, Midgley RS, Mirza N, Torr EE,
Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS and Adams DH: A
phase II study of adoptive immunotherapy using dendritic cells
pulsed with tumor lysate in patients with hepatocellular carcinoma.
Hepatology. 49:124–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizukoshi E, Nakamoto Y, Arai K, Yamashita
T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M and Kaneko S:
Comparative analysis of various tumor-associated antigen-specific
T-cell responses in patients with hepatocellular carcinoma.
Hepatology. 53:1206–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Butterfield LH, Ribas A, Dissette VB, Lee
Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter
DM, et al: A phase I/II trial testing immunization of
hepatocellular carcinoma patients with dendritic cells pulsed with
four alpha-fetoprotein peptides. Clin Cancer Res. 12:2817–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sawada Y, Yoshikawa T, Nobuoka D,
Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K,
Konishi M, et al: Phase I trial of a glypican-3-derived peptide
vaccine for advanced hepatocellular carcinoma: Immunologic evidence
and potential for improving overall survival. Clin Cancer Res.
18:3686–3696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Zeng H, Xu RH, Liu B and Li Z:
Vaccination with human pluripotent stem cells generates a broad
spectrum of immunological and clinical responses against colon
cancer. Stem Cells. 27:3103–3111. 2009.PubMed/NCBI
|
|
11
|
Yaddanapudi K, Mitchell RA, Putty K,
Willer S, Sharma RK, Yan J, Bodduluri H and Eaton JW: Vaccination
with embryonic stem cells protects against lung cancer: Is a
broad-spectrum prophylactic vaccine against cancer possible? PLoS
One. 7:e422892012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong W, Du J, Shen H, Gao D, Li Z, Wang G,
Mu X and Liu Q: Administration of embryonic stem cells generates
effective antitumor immunity in mice with minor and heavy tumor
load. Cancer Immunol Immunother. 59:1697–1705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mocan T and Iancu C: Effective colon
cancer prophylaxis in mice using embryonic stem cells and carbon
nanotubes. Int J Nanomed. 6:1945–1954. 2011. View Article : Google Scholar
|
|
14
|
Zhang ZJ, Chen XH, Chang XH, Ye X, Li Y
and Cui H: Human embryonic stem cells - a potential vaccine for
ovarian cancer. Asian Pac J Cancer Prev. 13:4295–4300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schoenhals M, Kassambara A, De Vos J, Hose
D, Moreaux J and Klein B: Embryonic stem cell markers expression in
cancers. Biochem Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brewer BG, Mitchell RA, Harandi A and
Eaton JW: Embryonic vaccines against cancer: An early history. Exp
Mol Pathol. 86:192–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ben-David U and Benvenisty N: The
tumorigenicity of human embryonic and induced pluripotent stem
cells. Nat Rev Cancer. 11:268–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao W, Ji X, Zhang F, Li L and Ma L:
Embryonic stem cell markers. Molecules. 17:6196–6236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishra L, Banker T, Murray J, Byers S,
Thenappan A, He AR, Shetty K, Johnson L and Reddy EP: Liver stem
cells and hepatocellular carcinoma. Hepatology. 49:318–329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oishi N and Wang XW: Novel therapeutic
strategies for targeting liver cancer stem cells. Int J Biol Sci.
7:517–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhurst B, Croager EJ, Farley-Roche CA,
Ong JK, Dumble ML, Knight B and Yeoh GC: A modified
choline-deficient, ethionine-supplemented diet protocol effectively
induces oval cells in mouse liver. Hepatology. 34:519–522. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tirnitz-Parker JE, Tonkin JN, Knight B,
Olynyk JK and Yeoh GC: Isolation, culture and immortalisation of
hepatic oval cells from adult mice fed a choline-deficient,
ethionine-supplemented diet. Int J Biochem Cell Biol. 39:2226–2239.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dumble ML, Croager EJ, Yeoh GC and Quail
EA: Generation and characterization of p53 null transformed hepatic
progenitor cells: Oval cells give rise to hepatocellular carcinoma.
Carcinogenesis. 23:435–445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang P, Liu T, Cong M, Wu X, Bai Y, Yin C,
An W, Wang B, Jia J and You H: Expression of extracellular matrix
genes in cultured hepatic oval cells: An origin of hepatic stellate
cells through transforming growth factor beta? Liver Int.
29:575–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conner DA: Mouse embryo fibroblast (MEF)
feeder cell preparation. Curr Protoc Mol Biol. 2001.Chapter 23:
Unit 23.2: May 1, 2001 (Epub ahead of primt). doi:
10.1002/0471142727.mb2302s51. View Article : Google Scholar :
|
|
26
|
Xu C, Huang HR, Yu H, Zhao XL, Lu YL, Dai
LC and Yao X: The study of separating murine embryonic fibroblast
from embryonic stem cells by the differential adhesion method. Fen
Zi Xi Bao Sheng Wu Xue Bao. 39:477–481. 2006.(In Chinese).
PubMed/NCBI
|
|
27
|
Dhodapkar MV and Dhodapkar KM: Vaccines
targeting cancer stem cells: Are they within reach? Cancer J.
17:397–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oertel M: Fetal liver cell transplantation
as a potential alternative to whole liver transplantation? J
Gastroenterol. 46:953–965. 2011. View Article : Google Scholar : PubMed/NCBI
|