Introduction
Phospholipase C (PLC) is a type of
membrane-associated enzymes consisting of thirteen mammalian PLC
isozymes that are classified into five isotype groups (β, γ, δ, ε,
ζ) based on their different structures (1). PLC-α also known as protein disulfide
isomerase family A member 3 (PDIA3) interacts with calnexin and
calreticulin to coordinate the folding of newly synthesised
glycoprotein (2). According to gene
sequence analysis studies, the major homology shared by different
isozymes is presented in N-terminus with around 250 amino acid
residues (3). The core enzyme of
PLC is composed of a triosephosphate isomerase (TIM) barrel, a
pleckstrin homology (PH) domain, four tandem EF hand domains, and a
C2 domain (4). Specifically, an
insert in the TIM barrel named the X-Y linker interrupts its
function by occluding the active site. This can be remedied with
the removal of the X-Y linker (5).
Activated by all types of cell surface membrane receptors, PLC
cleaves the phospholipid, phosphatidylinositol 4, 5-bisphosphate
(PIP2), into diacyl glycerol (DAG) and inositol 1, 4,
5-trisphosphate (IP3). These two products of the PLC-catalyzed
reaction are important second messengers that control diverse
cellular processes and are substrates for synthesis of other
important signalling molecules (6).
As a phospholipid-hydrolyzing enzyme, PLC plays a
significant role in the metabolism of inositol lipids. The
bioactive lipid mediators generated by PLC families are involved in
a variety of cellular processes, which are implicated in promoting
tumourigenesis through intercellular and extracellular signalling
pathways (7). Several studies have
been carried out to detect the function of PLC subfamilies and the
result demonstrated their essential role in regulating
proliferation, invasion, and migration in cancer development
(8–11). PLC-γ and PLC-ε work as oncogenes in
regulating cell proliferation which is associated with Ras
activities (12–14). Conversely, the deletion or
downregulation of PLC-β and PLC-δ isoforms has been shown in human
leukaemia (15,16). PLC-γ-mediated cell spreading and
motility is achieved by its binding with the complex GPCR kinase
interacting ARF-GAP 1 (GIT1) and the RAC1 and CDC42 GEF β-Pix, and
subsequently leads to the activation of the RHO family GTPases
CDC42 and RAC1. Downregulation of PLC-γ1 expression severely
impairs the activation of RAC, as well as cell invasion in breast
cancer, glioblastoma and head and neck cancer cell lines (17).
The occurrence and development of breast cancer is
related to aberrant signalling pathways in cell proliferation,
invasion and migration, but there is limited relevant therapy
targeting these signalling pathways. The evidence indicating the
relationship between PLC isoforms and breast cancer has suggested a
possibility to develop target therapy for breast cancer. Some of
the previous studies highlighted the alteration of PLC expression
levels in breast tumour cells. For example, the expression level of
PLC-γ1 is seen to be changed in breast cancer cells, with PLC-γ1
having a role in the regulation of cell migration by targeting EGFR
(18,19). Furthermore, PLC-δ4 is upregulated in
breast tumour cells, and its overexpression enhances cell
proliferation in those breast cancer cells with lower oncogenicity
(20). It has been reported that a
poor clinical outcome of breast tumour is associated with an
increased expression level of PLC-β2, which appears to be a
molecular marker indicating the severity of breast cancer. In
addition, PLC-β2 provokes the transition from G0/G1 to S/G2/M cell
cycle phase, which is important in cancer progression and inositol
lipid-related modifications of the cytoskeleton architecture
occurring during tumour cell division, motility and invasion
(10). In vivo,
dominant-negative PLC-γ1 fragment reduced the metastatic potential
of breast cancer in transgenic mouse (21). Metastasis assays also demonstrated
that nude mice with knockdown of PLC-γ1 presented inhibition of
breast cancer-derived lung metastasis (8).
Based on a previous study, the aim of this work was
to establish a correlation for the pattern of expression of 5 PLC
isoforms (α, β, δ, ε, and γ) in human breast cancer tissues with
clinical information (differentiation, tumour staging, histology
type and clinical outcome). We demonstrated that mammary tissues
widely expressed PLC-α, -β1, -δ, -ε, and -γ1. No significant
difference in the levels of the different isozymes was seen between
node positive and node negative tumours. Poorly differentiated
breast tumours (grade 2 and grade 3) had significantly higher
levels of PLC-γ1. Over a 10-year period, high levels of PLC-δ were
significantly correlated with a shorter disease-free survival.
Materials and methods
Materials and reagents
A polyclonal antibody to human PLC-γ1 was purchased
from Santa Cruz Biotechnology Ltd. (Santa Cruz, CA, USA). RNA
extraction kit and the first strand cDNA synthesis kit were from
AbGene Laboratories (Surrey, UK). The master mix for conventional
PCR and a customer quantitation PCR master mix for quantitative PCR
were also purchased from AbGene Laboratories.
Breast cancer cell lines MCF-7 and MDA MB 231 were
purchased from the European Collection of Animal Cell Cultures
(ECACC, Salisbury, UK). Human umbilical vein endothelial cells
(HUVEC) were purchased from TCS Biologicals Ltd. (Oxford, UK).
Breast cancer tissues (n=120) and normal background tissues (n=32)
were collected immediately after surgery and stored at −20°C until
use. Patients were routinely followed clinically after surgery. The
median follow-up period was 72 months. The presence of tumour cells
in the collected tissues was verified by examination of frozen
sections using H&E staining by a consultant pathologist.
Tissue processing and extraction of
RNA and generation of cDNA
Over 20 frozen sections from the each tissue sample
were homogenised in an RNA extraction solution using a hand held
homogeniser to extract total RNA. The concentration of RNA was
quantified using a UV spectrophotometer. RNA (1 µg) was used to
generate cDNA using a commercially available RT kit (AbGene
Laboratories).
Detection of PLC isoforms using
RT-PCR
Routine RT-PCR was carried out using a PCR master
mix that was commercially available (AbGene). Primers were designed
using the Beacon Designer software (version 2, CA, USA) (17), to amplify regions of human PLC-γ1
that have no significant overlap with other known sequences and to
ensure that the amplified products span over at least one intron.
The primer sequences are given in Table
I. β-actin was used as a housekeeping control. Reactions were
carried out using the following conditions: 94°C for 5 min, 36
cycles of 94°C for 15 sec, 55°C for 25 sec and 72°C for 15 sec. PCR
products were separated on a 2% agarose gel and photographed using
a digital camera mounted over a UV transluminator.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| PLC-α |
tagaactcacggacgacaa |
actgaacctgaccgtacacatactcaggtgcaagtctct |
| PLC-β1 |
gcaaaaagcaaaccatatct |
actgaacctgaccgtacagacttgctcttgttttggag |
| PLC-δ |
gttctccatcgaggacatt |
actgaacctgaccgtacacttgaagacaatggagaagc |
| PLC-ε |
ttgccaaagattctctcaat |
actgaacctgaccgtacacatgattttctcccaacatt |
| PLC-γ1 |
agaccttccaggtcaagc |
actgaacctgaccgtacagcggatctccttaatttca |
| β-actin |
atgatatcgccgcgctcg |
cgctcgtgtaggatcttca |
Quantitative analysis of the
transcript of PLC isoforms
The level of PLC-γ1 transcripts from the prepared
cDNA described above was determined using real-time quantitative
PCR, based on Amplifluor™ technology that was modified from
previous studies (18,19). In brief, pairs of PCR primers were
designed using the Beacon Designer software (version 2), with one
of the primers having an additional sequence, known as the Z
sequence (5′-actgaacctgaccgtaca) which is complementary to the
universal Z probe (Intergen Inc., Oxford, UK). A Taqman detection
kit for β-actin was purchased from Perkin-Elmer. The reaction was
carried out using the following: Hot-start Q-master mix (Abgene),
10 pmol of the specific forward primer, 1 pmol of the reverse
primer (which has the Z sequence), 10 pmol of FAM-tagged probe
(Intergen Inc.), and cDNA transcribed from approximately 50 ng of
RNA. The reaction was carried out using an iCycler iQ™ (Bio-Rad)
which equipped with an optic unit that allows real-time detection
of 96 reactions, using the following condition: 94°C for 12 min,
100 cycles of 94°C for 15 sec, 55°C for 40 sec and 72°C for 20 sec.
The levels of the transcripts were generated from a standard that
was simultaneously amplified with the samples.
Immunohistochemical staining of PLC-γ1
protein
The procedure was carried out as previously reported
with minor modifications (19,20).
Frozen sections of breast tumour and background tissue were cut at
a thickness of 6 µm using a cryostat. The sections were mounted on
Superfrost Plus (Gerhard Menzel, Germany), microscope slides,
air-dried and fixed in a mixture of 50% acetone and 50% methanol.
The sections were then placed in ‘Optimax’ wash buffer for 5–10 min
to rehydrate before being incubated for 20 min in a 0.6% BSA
blocking solution and probed with the primary antibody. Following
extensive washings, sections were incubated for 30 min in the
secondary biotinylated antibody (Multilink Swine
anti-goat/mouse/rabbit immunoglobulin, Dako Inc.). Avidin Biotin
Complex (Vector Laboratories, Nottingham, UK) was then applied to
the sections followed by more extensive washings. Diaminobenzidine
chromogen (Vector Laboratories) was to the sections which were
incubated in the dark for 5 min. Sections were then counter-stained
in Gill's haematoxylin and dehydrated in ascending grades of
methanol before clearing in xylene and mounting under a cover slip.
Images were obtained using a digital camera.
Statistical analysis
Statistical analysis was carried out using
Mann-Whitney U test and Kruskal-Wallis test. Survival analysis was
performed using Kaplan-Meier survival and Cox proportional
analysis.
Results
Expression of PLC isoforms in human
breast cancer tissues
We first studied the levels of expression of the 5
PLC isoforms using quantitative analysis of the respective
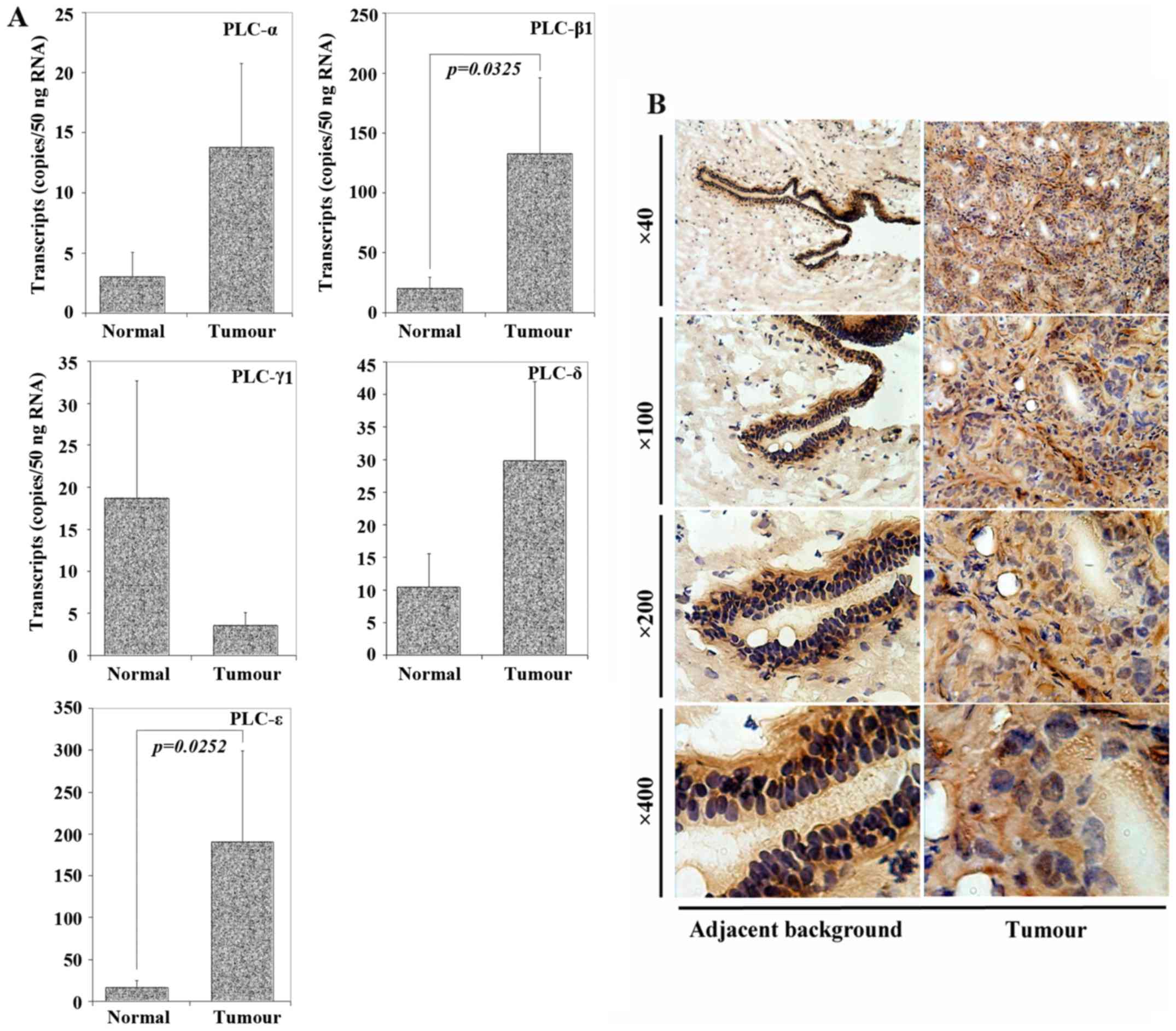
transcripts. As shown in Fig. 1A,
PLC-α, -β1, -δ and -ε showed increased levels in tumour tissues
compared with normal tissues. On the contrary, the expression level
of PLC-γ1 in tumour tissues was lower than in normal tissues.
Notably, statistical significance was shown in PLC-β1 and PLC-ε
transcript levels, with p=0.0325 and p=0.0252, respectively, which
demonstrated the considerable differences in the expression levels
of these two isoforms between breast cancer and normal tissues.
In addition to the quantitative analysis of
transcript levels, we used an anti-PLC-γ1 as a case study to assess
the protein expression and distribution in breast cancer.
Immunochemical staining was performed for PLC-γ1 in a small number
of frozen sections of the breast tumour tissue samples together
with some adjacent background mammary tissues. As seen in Fig. 1B, normal mammary epithelial cells
stained very strongly for the PLC, but little staining was present
in normal stromal cells. However, in breast cancer, the pattern of
staining varied from that of normal epithelial cells. As shown in
Fig. 1B (right panel), breast
cancer cells stained rather weakly for the protein, whereas stromal
cells stained strongly.
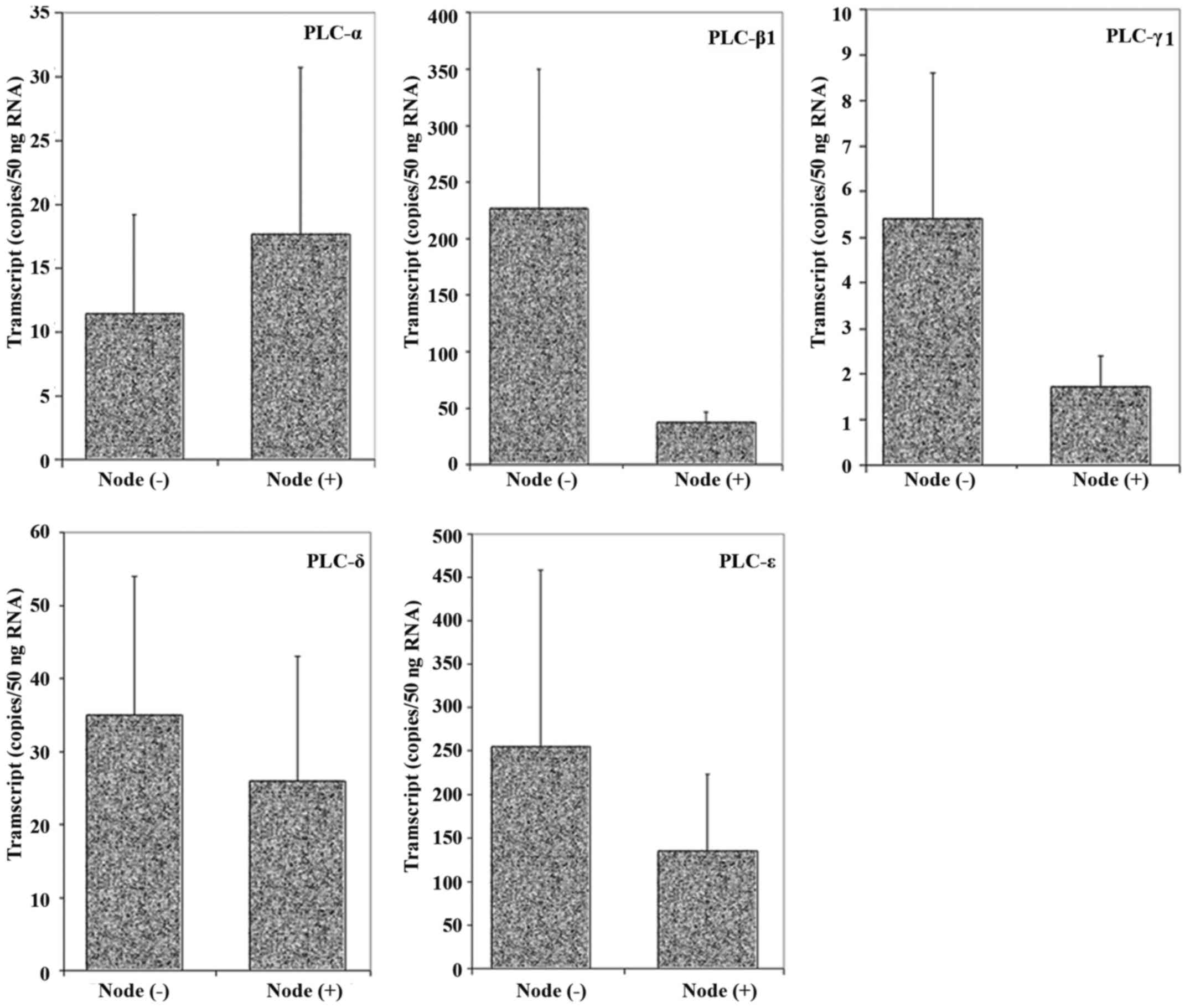
Levels of PLCs and nodal status
With the aim to investigate whether the expression
level of PLC is associated with lymph node status, cDNA samples
from node-positive and node- negative breast tumour tissues were
tested using real-time quantitative PCR. As shown in Fig. 2, node-positive tissues had lower
PLC-β1, -δ, -γ1, and -ε levels, whilst higher PLC-α expression was
shown. However, no statistically significant differences in the
isoform levels were detected between the node-positive and
node-negative breast tumour tissues.
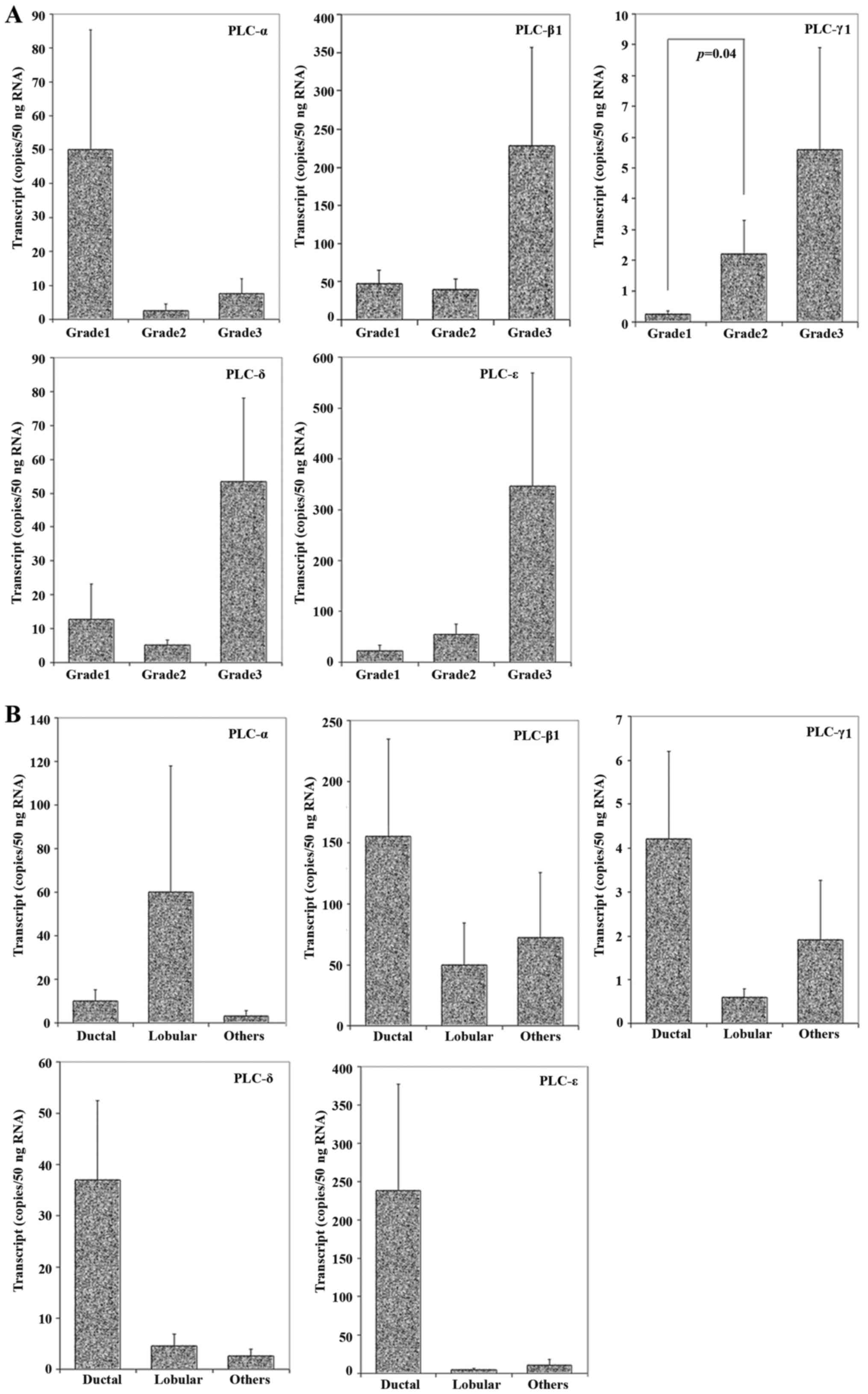
Correlation of PLC levels with tumour
grade and histology types
We examined PLC levels in breast tumours of
different grades. As shown in Fig.
3A, grade 3 tumours had higher levels of PLC-β1, -γ1, -δ and
-ε. Higher levels of PLC-α were detected in grade 1 tumours. A
statistical difference was seen in PLC-γ1 expression between grade
1 and grade 2 (p=0.04), with the patients in grade 2 showing higher
levels compared to those in grade 1.
When histology types were considered, there was a
trend in ductal tumours to have higher levels of PLC-β1, -γ1, -δ
and -ε, but lower levels of PLC-α (Fig.
3B). However, no statistical significance was found between
these groups.
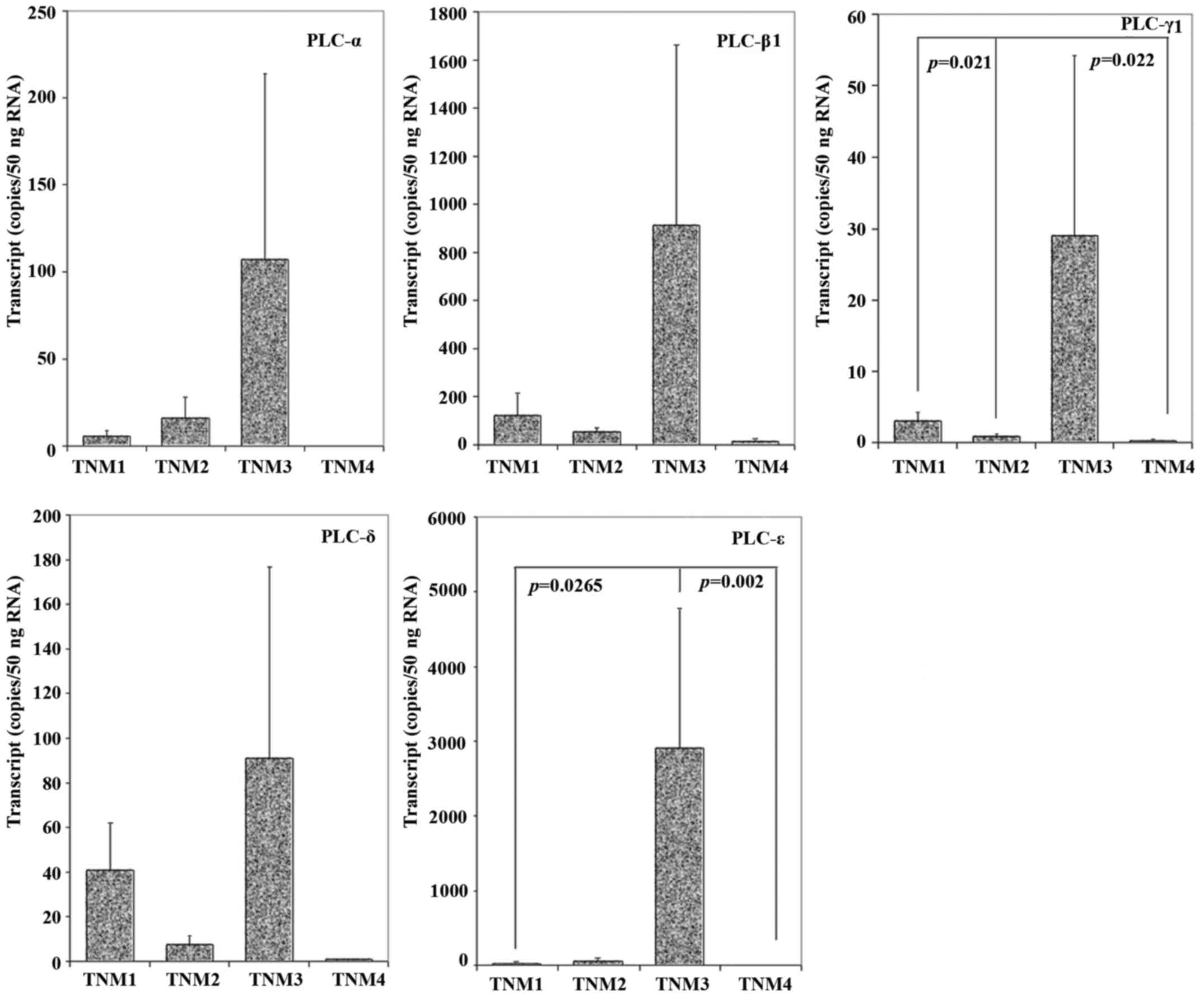
Levels of PLC in TNM stages
To evaluate the correlation between PLC levels and
TNM stages, we quantified the levels of the different isoforms in
breast tumours from TNM1 to TNM4. In particular, all the isoforms
we examined presented the highest expression levels in TNM3 stage.
There was also a significant difference in PLC-γ1 levels when
comparing tumours of the different TNM stages (TNM1 versus TNM2
p=0.021; TNM1 versus TNM4 p=0.022), with a decreased level in TNM2
and TNM4 in comparison with TNM1. The quantity of PLC-ε also
reached statistical significance (TNM1 versus TNM3 p=0.0265; TNM3
versus TNM4 p=0.002), and the result showed that patients in TNM1
and TNM4 had a markedly reduced level of PLC-ε expression compared
with the patients in TNM3 stages (Fig.
4).
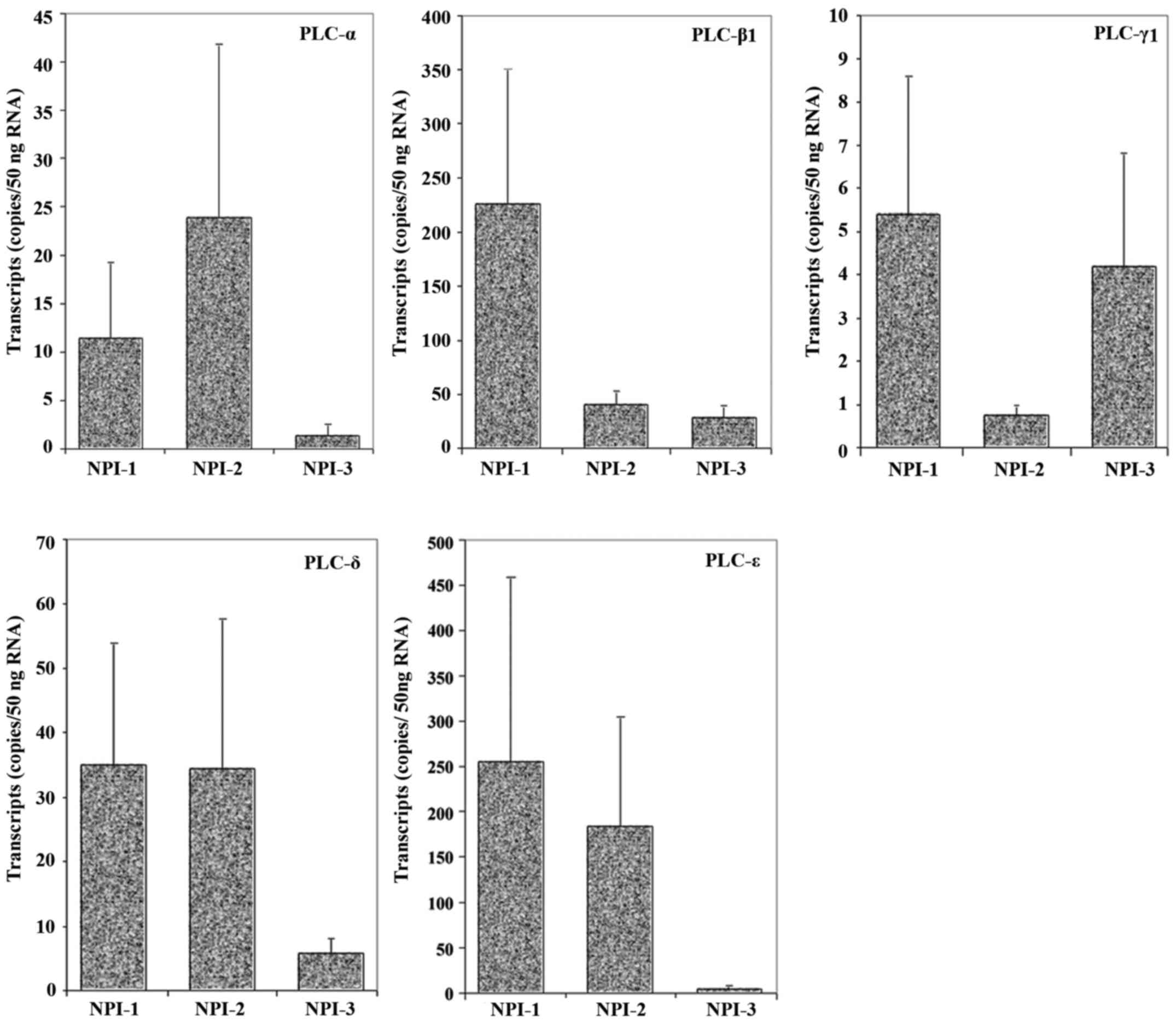
PLC expression and predicted
prognosis
In order to establish if a link exists between the
isoforms of PLC and the predicted clinical outcome at the time of
operation, the Nottingham Prognostic Index was used as an
indicator, where NPI <3.4 was designated as NPI1, and comprised
of patients who had a good prognosis. NPI2 and NPI3, with the value
3.4–5.4 and >5.4, respectively, had moderate and poor prognosis.
As shown in Fig. 5, all the
isoforms appeared to be at lower levels in NPI3 tumours compared
with NPI1 tumours. However, the results were not statistically
significant.
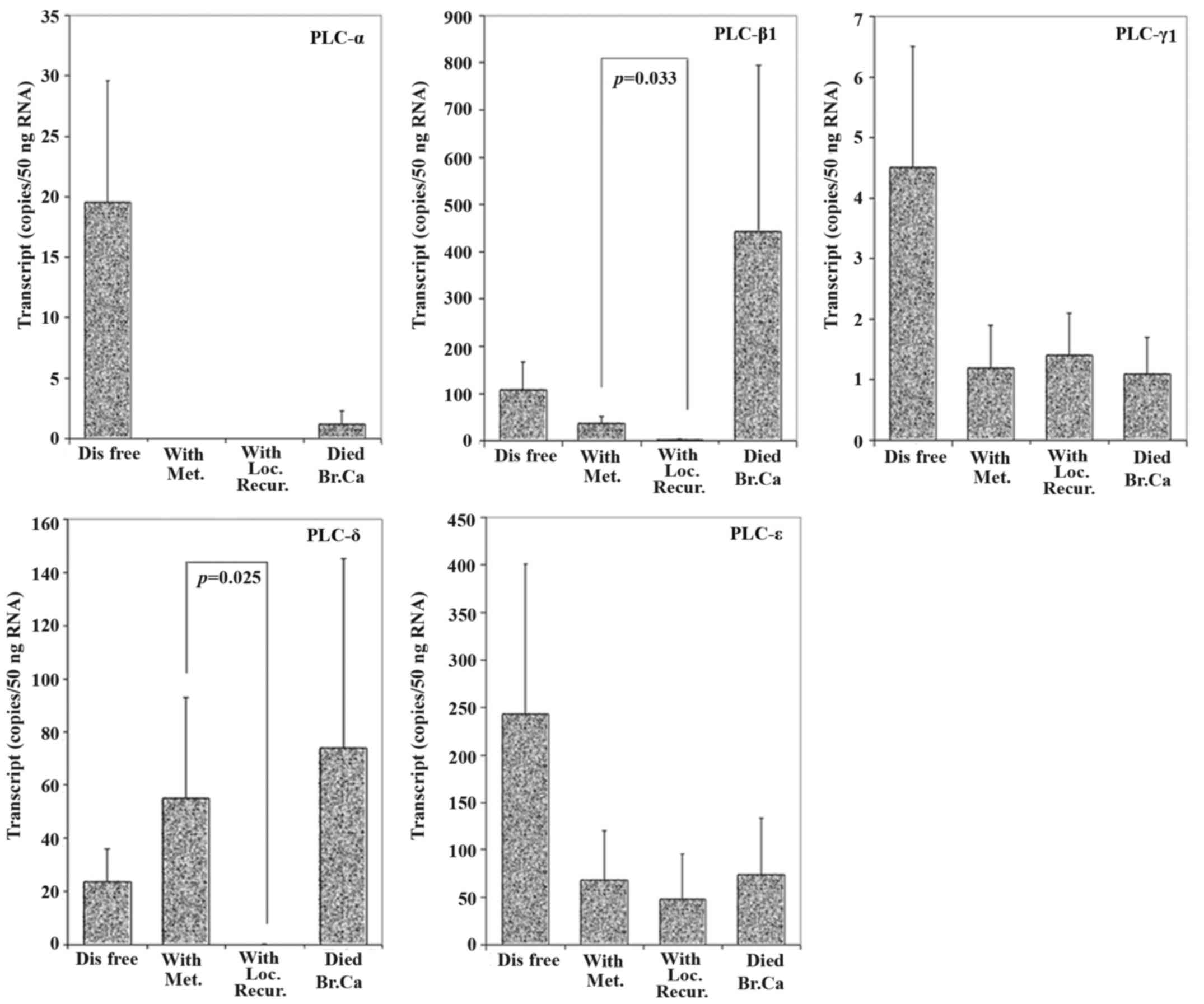
PLC expression and clinical
outcomes
To determine the correlation between PLC and
clinical outcome, patients were divided into four groups: patients
who remained disease-free, patients with metastasis, patients with
local recurrence and patients who died from breast cancer. As shown
in Fig. 6, after a 10-year follow
up, patients who suffered from complications had low expression
levels of PLC-α, -γ1 and -ε, but high levels of PLC-β1 and -δ. This
was particularly evident when all the patients with complications
were considered together. Moreover, the differences of expression
between metastasis and recurrence tumour tissues in PLC-β1 level
and PLC-δ level were statistically different (p=0.033 and p=0.025,
respectively).
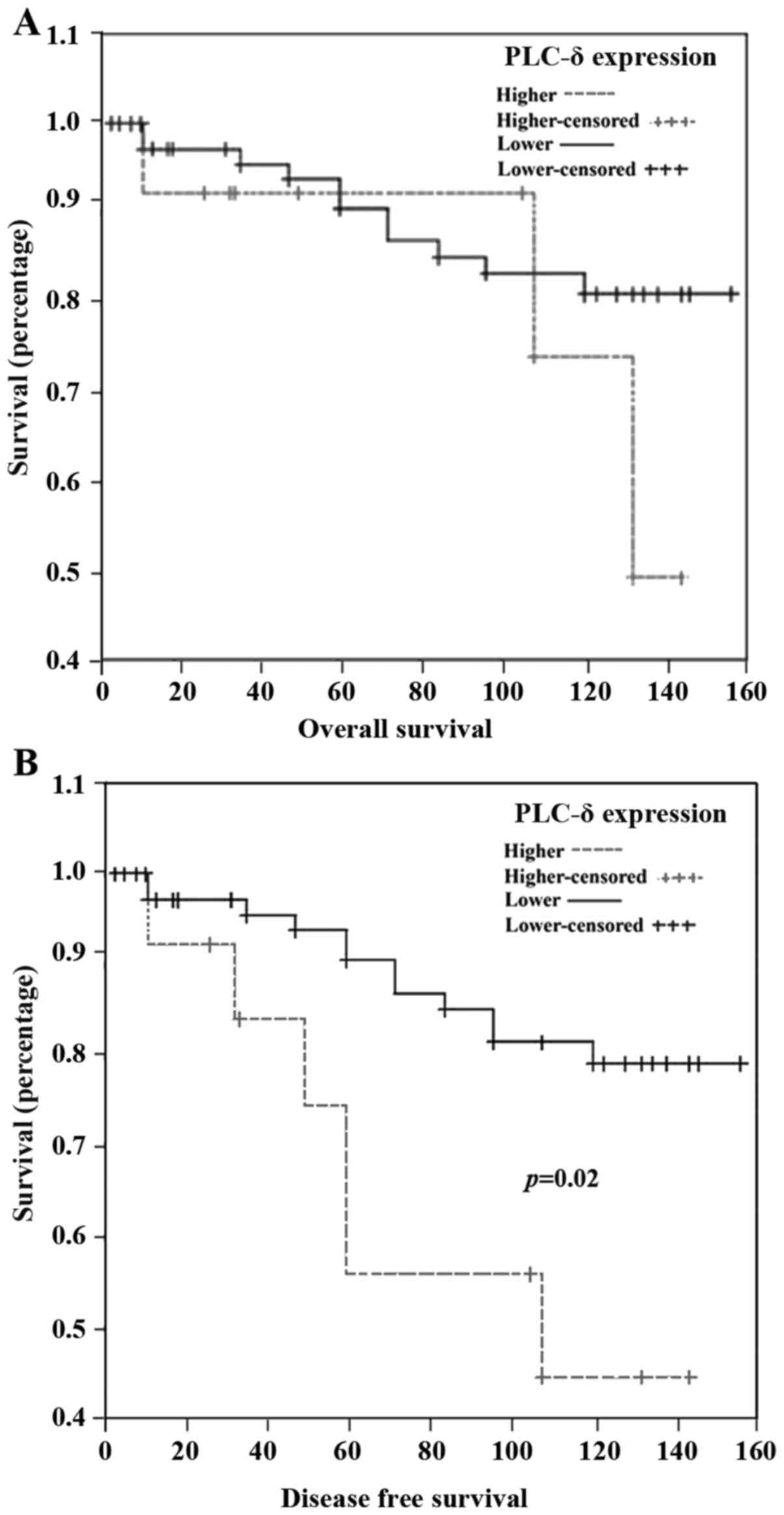
Levels of PLC and long-term
survival
We assessed the association between PLC levels and
breast cancer-related death using Kaplan-Meier survival analysis.
An average transcript level of NPI2 group was set as a threshold.
Higher levels of PLC-δ transcripts were associated with a shorter
disease-free survival, but not for overall survival (Fig. 7). Patients with higher PLC-δ
transcript level had an average disease-free survival of 96.7
months (95% confidence interval 67.7–124.4 months) compared to
137.4 months (95% confidence interval 127.6–147.2 months) of
patients who had lower expression of PLC-δ. No correlation with
either overall survival or disease-free survival was observed for
other PLC isozymes (data not shown).
Discussion
Globally, breast cancer is the most frequently
diagnosed cancer and the leading cause of cancer death in women.
Considerable evidence has suggested that diverse molecules can
induce aberrant signalling events in breast cancer and lead to
breast tumour proliferation, invasion, and migration. The recent
identification of phospholipase C (PLC), a phospholipid-hydrolyzing
enzyme, has a role in the regulation of a number of cellular
behaviours and promotes tumourigenesis by regulating cell motility,
transformation and cell growth, partly by acting as signalling
intermediates for cytokines such as EGF and interleukins in cancer
cells. A number of PLC isoforms have been investigated with studies
focusing on their functions and expression profiles in human
cancers, however, the importance of PLC in clinical diagnosis is
largely unknown. Our study aimed to examine the pattern of
expression of 5 PLC isoforms, namely, α, β, γ, δ and ε in human
breast cancer, and to predict their correlation with clinical
significance.
Oestrogen exposure is one of the risk factors for
the development of breast cancer (22). Ovarian hormones induce cell
proliferation, and the uncorrected signal molecules result in the
generation of malignant phenotypes (23). Up to now, there have been limited
studies focusing on the functions of PLC-α in breast cancer, but it
has been reported that PLC-α is able to mediate the
phosphatidylinositol (PI) second messenger pathway and the
components in this pathway can increase some effects of estrogen
(24). However, there needs to be
further studies to investigate if there is a direct correlation
between PLC-α and oestrogen.
The important factors for the prediction of clinical
outcomes in breast cancer patients are tumour size, lymph node
status and tumour grade, moreover, the involvement of molecular
markers is beneficial to develop a treatment plan for patients with
different tumour stages. Previous studies presented PLC-β2, an
isoform of PLC family, as a potential marker to predict the
malignancy of breast cancer, with upregulated expression levels
being detected in different types of breast tumours (25). In addition, breast cancer cell lines
with high invasive potential show higher expression levels of
PLC-β2 in comparison with less-tumourigenic breast cancer cell
lines (11).
In the present study, we detected isoform of the
PLC-β subfamily and showed that there is a significantly increased
level of PLC-β1 in breast tumour tissues. Tissue microarray (TMA)
analysis demonstrated a poor expression of PLC-β2 in normal tissues
and significantly higher expression levels in most tumour tissue
specimens. Furthermore, there is no relationship between node
status and PLC-β1 level in breast tumour tissues. This result was
also verified in our study focused on PLC-β1. PLC-β2 expression
closely correlated with tumour grading, with an increase of
staining intensity from grade 1 to grade 3. Similarly, our study
demonstrated that grade 3 tumours showed the highest expression
levels of PLC-β1 (11). Lower
overall survival has been demonstrated in patients whose primary
tumours expressed high levels of PLC-β2. The above findings
therefore indicate a strong correlation between PLC-β levels and
poor prognosis of breast cancer. Moreover, our present study
demonstrated a significant difference in PLC-β1 expression between
metastasis and recurrence tumour tissues (p=0.033), which may
indicate its role in promoting migration in breast cancer.
The expression of PLC, primarily PLC-γ, has been
studied in a number of cancer types, although most of these studies
have concentrated on established cell lines. In terms of clinical
cancers, studies are available on squamous cell carcinoma, lung
cancer, thyroid cancer, and limited on breast cancer (26–28).
In a small-scale study in human breast cancer, PLC-γ was shown to
be overexpressed in the majority of tumours in comparison with
normal mammary tissues. The role of PLC in cells and indeed in
cancer cells has been long studied. As already discussed, it seems
that the prime role of the enzyme is the regulation of cell
migration and adhesion by acting downstream of a number of cellular
signalling pathways (29).
The current study was unable to provide further
details of the link between PLC-γ and its associated signalling
complexes. Future studies would need to address these points. The
relationship between the PLC isoforms and long-term survival for
patients with breast tumours was examined here. Our observations
are interesting and suggest that these PLC isoforms may be
potential therapeutic targets. Therapeutic applications of PLC-γ
have been studied. PLC-γ inhibitors have been shown to inhibit
PLC-γ mediated cell function in cancer cells. For example, the
PLC-γ inhibitor, U73122, is able to inhibit the activation of
PLC-γ, and hence PLC-γ mediated cell migration and invasion in a
number of cancer cell types. The inhibitor has also the potential
of reducing the growth of mammary tumours in vivo (30).
The loss of PLC-δ expression is highly associated
with its role as a tumour suppressor in esophageal squamous cell
carcinoma (ESCC). The tumourigenic ability of ESCC cells is
suppressed both in vitro and in vivo assays. PLC-δ
has been suggested to have a significant role in ESCC metastasis
since its downregulation has been shown to have a reduction in cell
mobility and an increase in cell adhesion (16). In addition, decreased PLC-δ
expression correlated with poor clinical outcome in patients with
acute or chronic myeloid leukaemia (16). In our study, patients with tumour
metastasis expressed higher levels of PLC-δ than those with local
recurrence. Significantly, breast cancer patients with higher
expression levels of PLC-δ experience a shorter disease-free
survival period, and this result might indicate a correlation
between PLC-δ and recurrence for breast cancer patients.
Taken together, the findings of the current study
demonstrate for the first time that isoforms of PLC are aberrantly
expressed in human breast cancer. The abnormalities are linked to
both prognosis and to some degree to clinical outcomes. The study
strongly indicated the potential therapeutic value of these
signalling intermediates in human breast cancer and therefore
warrants further investigation.
Acknowledgements
We thank Breast Cancer Campaign, the Emery Jane
Bequest Fund and Cancer Research Wales for supporting this
study.
References
|
1
|
Danielsen SA, Cekaite L, Ågesen TH, Sveen
A, Nesbakken A, Thiis-Evensen E, Skotheim RI, Lind GE and Lothe RA:
Phospholipase C isozymes are deregulated in colorectal cancer -
insights gained from gene set enrichment analysis of the
transcriptome. PLoS One. 6:e244192011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helenius A and Aebi M: Roles of N-linked
glycans in the endoplasmic reticulum. Annu Rev Biochem.
73:1019–1049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakurai J, Nagahama M and Oda M:
Clostridium perfringens alpha-toxin: Characterization and mode of
action. J Biochem. 136:569–574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadamur G and Ross EM: Mammalian
phospholipase C. Annu Rev Physiol. 75:127–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hicks SN, Jezyk MR, Gershburg S, Seifert
JP, Harden TK and Sondek J: General and versatile autoinhibition of
PLC isozymes. Mol Cell. 31:383–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rebecchi MJ and Pentyala SN: Structure,
function, and control of phosphoinositide-specific phospholipase C.
Physiol Rev. 80:1291–1335. 2000.PubMed/NCBI
|
|
7
|
Park JB, Lee CS, Jang JH, Ghim J, Kim YJ,
You S, Hwang D, Suh PG and Ryu SH: Phospholipase signalling
networks in cancer. Nat Rev Cancer. 12:782–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sala G, Dituri F, Raimondi C, Previdi S,
Maffucci T, Mazzoletti M, Rossi C, Iezzi M, Lattanzio R, Piantelli
M, et al: Phospholipase Cgamma1 is required for metastasis
development and progression. Cancer Res. 68:10187–10196. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas SM, Coppelli FM, Wells A, Gooding
WE, Song J, Kassis J, Drenning SD and Grandis JR: Epidermal growth
factor receptor-stimulated activation of phospholipase Cgamma-1
promotes invasion of head and neck squamous cell carcinoma. Cancer
Res. 63:5629–5635. 2003.PubMed/NCBI
|
|
10
|
Bertagnolo V, Benedusi M, Brugnoli F,
Lanuti P, Marchisio M, Querzoli P and Capitani S: Phospholipase
C-beta 2 promotes mitosis and migration of human breast
cancer-derived cells. Carcinogenesis. 28:1638–1645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bertagnolo V, Benedusi M, Querzoli P,
Pedriali M, Magri E, Brugnoli F and Capitani S: PLC-β2 is highly
expressed in breast cancer and is associated with a poor outcome: A
study on tissue microarrays. Int J Oncol. 28:863–872.
2006.PubMed/NCBI
|
|
12
|
Kim MJ, Chang JS, Park SK, Hwang JI, Ryu
SH and Suh PG: Direct interaction of SOS1 Ras exchange protein with
the SH3 domain of phospholipase C-gamma1. Biochemistry.
39:8674–8682. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelley GG, Reks SE, Ondrako JM and Smrcka
AV: Phospholipase C(epsilon): A novel Ras effector. EMBO J.
20:743–754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bunney TD, Harris R, Gandarillas NL,
Josephs MB, Roe SM, Sorli SC, Paterson HF, Rodrigues-Lima F,
Esposito D, Ponting CP, et al: Structural and mechanistic insights
into ras association domains of phospholipase C epsilon. Mol Cell.
21:495–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Follo MY, Finelli C, Clissa C, Mongiorgi
S, Bosi C, Martinelli G, Baccarani M, Manzoli L, Martelli AM and
Cocco L: Phosphoinositide-phospholipase C β1 mono-allelic deletion
is associated with myelodysplastic syndromes evolution into acute
myeloid leukemia. J Clin Oncol. 27:782–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu L, Qin YR, Xie D, Hu L, Kwong DL,
Srivastava G, Tsao SW and Guan XY: Characterization of a novel
tumor-suppressor gene PLC delta 1 at 3p22 in esophageal squamous
cell carcinoma. Cancer Res. 67:10720–10726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones NP and Katan M: Role of
phospholipase Cgamma1 in cell spreading requires association with a
beta-Pix/GIT1-containing complex, leading to activation of Cdc42
and Rac1. Mol Cell Biol. 27:5790–5805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piccolo E, Innominato PF, Mariggio MA,
Maffucci T, Iacobelli S and Falasca M: The mechanism involved in
the regulation of phospholipase Cgamma1 activity in cell migration.
Oncogene. 21:6520–6529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katterle Y, Brandt BH, Dowdy SF, Niggemann
B, Zänker KS and Dittmar T: Antitumour effects of
PLC-gamma1-(SH2)2-TAT fusion proteins on EGFR/c-erbB-2-positive
breast cancer cells. Br J Cancer. 90:230–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung DW, Tompkins C, Brewer J, Ball A,
Coon M, Morris V, Waggoner D and Singer JW: Phospholipase C delta-4
overexpression upregulates ErbB1/2 expression, Erk signaling
pathway, and proliferation in MCF-7 cells. Mol Cancer. 3:152004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lattanzio R, Piantelli M and Falasca M:
Role of phospholipase C in cell invasion and metastasis. Adv Biol
Regul. 53:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Preston-Martin S, Pike MC, Ross RK, Jones
PA and Henderson BE: Increased cell division as a cause of human
cancer. Cancer Res. 50:7415–7421. 1990.PubMed/NCBI
|
|
23
|
Wiseman BS and Werb Z: Stromal effects on
mammary gland development and breast cancer. Science.
296:1046–1049. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mobbs CV, Kaplitt M, Kow LM and Pfaff DW:
PLC-alpha: A common mediator of the action of estrogen and other
hormones? Mol Cell Endocrinol. 80:C187–C191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmid P, Wischnewsky MB, Sezer O, Böhm R
and Possinger K: Prediction of response to hormonal treatment in
metastatic breast cancer. Oncology. 63:309–316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu D, Tan Y, Yang X, Qiao J, Yu C, Wang
L, Li J, Zhang Z and Zhong L: Phospholipase C gamma 1 is a
potential prognostic biomarker for patients with locally advanced
and resectable oral squamous cell carcinoma. Int J Oral Maxillofac
Surg. 43:1418–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salgia R: Role of c-Met in cancer:
Emphasis on lung cancer. Semin Oncol. 36 Suppl 1:S52–S58. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuccuru G, Lanzi C, Cassinelli G, Pratesi
G, Tortoreto M, Petrangolini G, Seregni E, Martinetti A, Laccabue
D, Zanchi C, et al: Cellular effects and antitumor activity of RET
inhibitor RPI-1 on MEN2A-associated medullary thyroid carcinoma. J
Natl Cancer Inst. 96:1006–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kassis J, Moellinger J, Lo H, Greenberg
NM, Kim HG and Wells A: A role for phospholipase C-gamma-mediated
signaling in tumor cell invasion. Clin Cancer Res. 5:2251–2260.
1999.PubMed/NCBI
|