Introduction
Ewing sarcoma (EWS) is a malignant primitive bone
tumor, that mainly affects children and young adults, with a high
tendency to metastasize to lung and/or bone (1,2).
Unlike carcinomas, sarcomas arise abruptly and diffuse
micrometastases are thought to be present at the time of diagnosis
as indicated by a survival rate as low as 5% in patients treated
with surgery alone (3). Although
significant improvements in prognosis have been reported in
patients with localized tumors at diagnosis, prognosis for patients
with metastasis remains very disappointing (4,5) and
less than few treatment options can be offered to metastatic
patients, indicating that new drugs are required. Thus, the primary
need in the field is a deeper understanding of the biology of the
metastasic process in EWS in order to facilitate the development of
therapeutic agents that may specifically counteract cell
dissemination and optimize systemic-disease control.
The key cellular processes underlying metastasis
include the ability of cancer cells to migrate toward new
environments outside of the primary tumor. The two major types of
cancer migration (mesenchymal or amoeboid) require different
intracellular molecular signaling but both rely on driving forces
generated by actin cytoskeleton dynamics. The actin status is in
fact used as a signaling intermediate by several pathways,
including the Rho/Rac GTPases and the Hippo-pathway, which
culminate in the transcriptional regulation of cytoskeletal and
growth-promoting genes, respectively. In particular, the main
downstream effectors of the Rho family GTPases, ROCK1 and ROCK2,
are serine-threonine kinases that, through the phosphorylation of
several target substrates linked to cytoskeleton organization,
control multiple events in cell migration, such as regulation of
actomyosin contractility, formation of stress fibers, rear
retraction and turnover of focal adhesions (6). The implication of ROCK1 and ROCK2 in
cancer cell dissemination and metastasis is therefore not
surprising. Although the effects of ROCK activity on migration and
invasion have been found to be dependent on the cellular context,
ROCK overexpression has been associated with greater invasion and
poor survival in many tumors, including hepatocellular carcinoma
(7), osteosarcoma (8), breast (9), testicular (10), colon (11) and bladder cancer (12). Conversely, ROCK inhibition or
depletion frequently produces suppression of invasion and
metastasis (7,13–15),
thus supporting the view of ROCK inhibitors as a novel therapeutic
strategy to block the migration and invasion of metastatic cancers.
Most of the anti-ROCK agents currently available are inhibitors
that simultaneously target both the two kinases ROCK1 and ROCK2, in
the hypothesis that the two isoforms drive similar intracellular
signaling pathways and biological processes. However, despite their
high degree of sequence homology and substrate promiscuity, several
studies have clearly reported how the two isoforms may exhibit
individual physiological roles (16,17)
and give distinct contribution to cancer progression (18).
In this study, we analyzed the role of ROCK isoforms
in EWS malignancy and evaluated the in vitro efficacy of
pan- vs. specific ROCK inhibitors. Our results indicate that
targeting of ROCK2 could represent an effective approach to
counteract EWS malignancy in favor of cell differentiation.
Materials and methods
Cell lines and treatments
SK-ES-1, SK-N-MC, and RD-ES EWS cell lines were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA); TC-71 and 6647 cell lines were a generous gift from T.J.
Triche (Children's Hospital, Los Angeles, CA, USA); WE-68 was
established and kindly provided by F. van Valen (University
Hospital Muenster, Muenster, Germany); A673, STA-ET 2.1 and STA-ET
2.2 EWS cell lines were a kind gift from H. Kovar (St. Anna
Kinderkrebsforshung, Vienna, Austria); the latter two cell lines
were established from the primary tumor and a bone marrow
infiltrate of the same patient (19). LAP-35 was previously established and
characterized at the Istituto Ortopedico Rizzoli, Bologna, Italy
(20). IOR/CAR was established and
characterized at the Experimental Oncology Laboratory of the
Istituto Ortopedico Rizzoli, Bologna, Italy, from an EWS patient.
All cell lines were tested for the absence of mycoplasma
contamination by MycoAlert™ Mycoplasma Detection kit (Lonza,
Allendale, NJ, USA), last control March 2015, and authenticated by
STR analysis using genRESVR MPX-2 and genRESVR MPX-3 kits (Serac,
Bad Homburg, Germany). The following loci were verified: D16S539,
D18S51, D19S433, D21S11, D2S1338, D3S1358, D5S818, D8S1179, FGA,
SE33, TH01 and TPOX VWA. Last control was performed in November
2012. Cells were cultured in a humidified 5% CO2
atmosphere at 37°C in Iscove Modified Dulbeccos medium (IMDM;
Lonza) supplemented with 10% fetal bovine serum (FBS; EuroClone
S.p.A, Milan, Italy), and 1% penicillin-streptomycin.
To inhibit ROCK kinases the pan-ROCK inhibitor
(R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide
dihydrochloride (Y27632) (Calbiochem, San Diego, CA, USA) as well
as the ROCK2 inhibitor
N-(2-(2-(dimethylamino)ethoxy)-4-(1H-pyrazol-4-yl)phenyl)-2,3dihydrobenzo[b][1,4]dioxine-2-carboxamide
(Stemolecule™ ROCKII Inhibitor; Stemgent, Cambridge, MA, USA) were
used.
Analysis of growth features in
monolayer conditions
The effects of ROCK inhibition on cell growth was
determined by daily harvesting of cells after the seeding of 25,000
cells/cm2 (for the 6647 cell line) or 50,000
cells/cm2 (for the SKES-1 cell line) in IMDM and 10%
FBS. ROCK inhibitors (10 µM) were added to the culture medium 24 h
after seeding. Cell viability was determined by trypan blue vital
cell count.
Motility assay
Motility assay was performed using Transwell
chambers (Costar, Cambridge, MA, USA). Cells (1×105)
were seeded in the upper compartment in the presence or not of the
ROCK inhibitors (10 µM), and incubated for 18 h in a humidified 5%
CO2 atmosphere at 37°C. The migrated cells were fixed in
absolute methanol, counterstained with Giemsa (CARLO ERBA Reagents
S.A.S., Milan, Italy) and counted. All the experiments were
performed in triplicate.
Soft agar assay
Anchorage-independent growth was determined in 0.33%
agarose (SeaPlaque™ Agarose; Lonza) with a 0.5% agarose underlay.
Cell suspensions (1×103) were plated in semisolid medium
(IMDM 10% FBS plus agar 0.33%) in the presence or not of the ROCK
inhibitors (10 µM), and incubated at 37°C in a humidified 5%
CO2 atmosphere. Colonies were counted after 10 to 14
days. All of the experiments were performed in triplicate.
Western blotting
Cells were lysed with phospho-protein extraction
buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40,
0.25% Na-deoxycholate, 1 mM EGTA, 1 mM NaF, supplemented with
protease-phosphatase cocktail inhibitor (Sigma-Aldrich, St. Louis,
MO, USA). Equivalent amounts of total cell lysates were separated
by 7.5% SDS-PAGE under denaturating conditions and transferred onto
nitrocellulose membrane. Membranes were incubated overnight with
the following primary antibodies: rabbit polyclonal anti-ROCK1
(H-85, sc-5560; Santa Cruz Biotechnology, Inc., San Diego, CA, USA)
(1:1,000), goat polyclonal anti-ROCK2 (C-20, sc-1851; Santa Cruz
Biotechnology) (1:1,000), mouse monoclonal anti-actin (C-4,
MAB1501; Chemicon International, Inc., Temecula, CA, USA)
(1:100,000). Donkey anti-rabbit (NA934; GE Healthcare, Piscataway,
NJ, USA), donkey anti-goat (sc-2020; Santa Cruz Biotechnology) or
sheep anti-mouse (NA931; GE Healthcare) horseradish
peroxidase-linked secondary antibodies were employed and the signal
was revealed by ECL western blotting detection reagents
(EuroClone). Densitometric analysis was performed using GS-800
Imaging Densitometer and Quantity One 4.6.9 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence
EWS cells were seeded at low density on
fibronectin-coated coverslips in standard medium. After 48 h, cells
were exposed to ROCK inhibitors (2 µM or 10 µM). Twenty-four hours
later, the cells were fixed in 4% paraformaldehyde, permeabilized
with 0.15% Triton X-100 in phosphate-buffered saline (PBS), and
incubated overnight with the mouse monoclonal anti-βIII-tubulin
antibody (SDL.3D10, T5076; Sigma-Aldrich) (1:50). Goat polyclonal
anti-mouse FITC (31569; Pierce Biotechnology, Inc., Rockford, IL,
USA) (1:100) was used as a secondary antibody. Nuclei were
counterstained with Hoechst 33256 (Sigma-Aldrich). For neurite
outgrowth assay, the cells were classed as differentiated if they
exhibited an outgrowth extending from the cell which was at least
1.5 times the diameter of the cell. At least 200 cells from five
randomly selected fields were counted from each slide.
Analysis of apoptosis
Detection and quantification of apoptotic cells was
obtained by flow cytometric analysis (FACSCalibur; Becton
Dickinson, San Jose, CA, USA) of Annexin V-FITC-labeled cells. This
test was carried out according to the manufacturer's instructions
(code no. 4700, Mebcyto® apoptosis kit; Medical &
Biological Laboratories, Naka-ku Nagoya, Japan).
Statistical analysis
Correlation analysis was performed using Spearman's
rank correlation coefficient. Differences among means were analyzed
using the 2-tailed Students t-test.
Results
ROCK2, rather than ROCK1, affects EWS
malignancy
Taking into account the pivotal role of ROCK-kinases
in regulating actin cytoskeleton and cell movement, ROCK1 and ROCK2
expression levels were evaluated by western blotting on a panel of
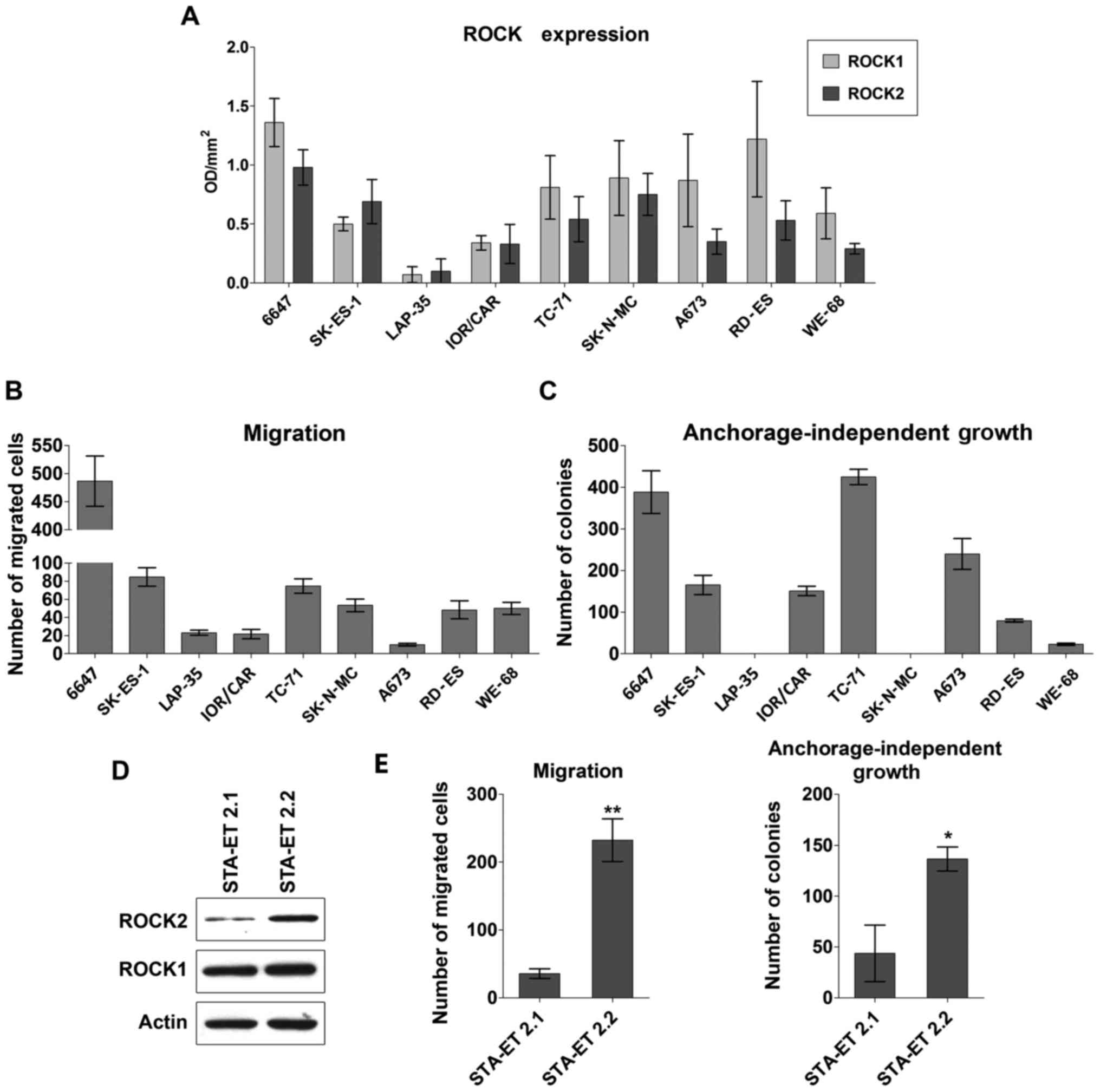
representative, patient-derived EWS cell lines (Fig. 1A). With the only exception of
LAP-35, which barely expressed both the kinases, ROCK1 and ROCK2
were expressed in all EWS cell lines, with a generally higher
expression of ROCK1 with respect to ROCK2. However, only the
expression of ROCK2 appeared to be important for EWS aggressive
behavior. Indeed, whenever ROCK expression levels were analyzed in
relation to migration capabilities and anchorage-independent growth
properties of EWS cells (Fig. 1B and
C), a direct correlation was observed for ROCK2 (Spearman
correlation test: r=0.791; p=0.002 and r=0.661; p=0.033
respectively), but not for ROCK1 (Spearman correlation test:
r=0.400; p=0.210 and r=0.273; p=0.425 respectively). This
association was confirmed when the expression of the ROCKs was
analyzed in STA-ET 2.1 and STA-ET 2.2, two cell lines that were
generated from the primary tumor and a bone marrow infiltrate of
the same EWS patient (19).
Expression of ROCK2, but not of ROCK1, was increased in the cell
line that was derived from metastasis compared to that derived from
the primary tumor (Fig. 1D), in
agreement with the more aggressive phenotype of the STA-ET 2.2
cells (Fig. 1E).
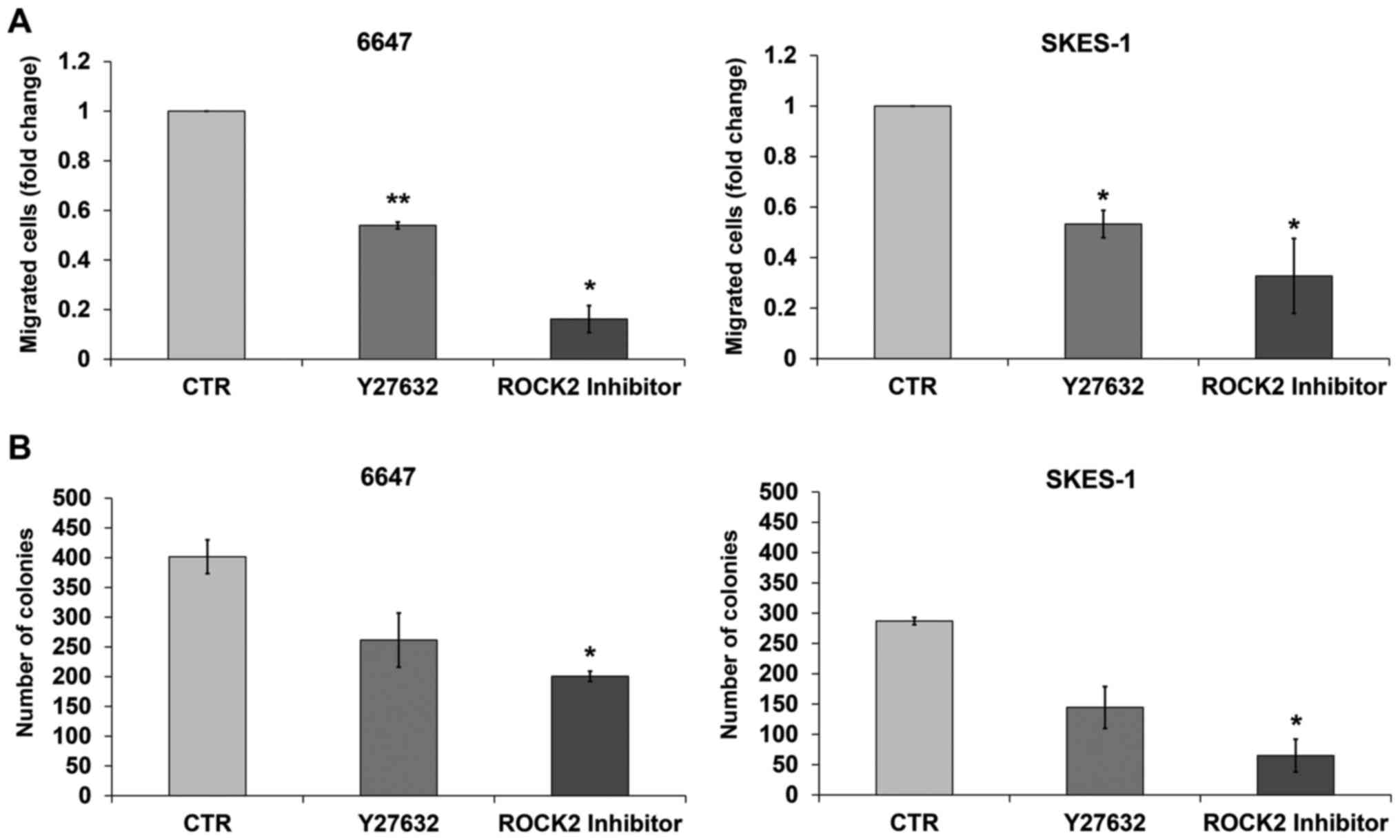
To further confirm the prevalent role of the isoform
ROCK2 in the malignancy of EWS cells, we compared the in
vitro efficacy of Stemolecule™ ROCKII Inhibitor, a specific
ROCK2 inhibitor (21) with that of
Y27632, which blocks both ROCK1 and ROCK2 activity. Activity of the
two compounds was analyzed in the 6647 and SKES-1 cell lines, as
representative of EWS cells with a high or intermediate expression
of ROCK2. Both inhibitors significantly reduced the migration of
the EWS cells in vitro. The specific ROCK2 inhibitor
appeared, however, to be more effective, particularly in the cells
showing the highest expression of ROCK2 (Fig. 2A). The higher efficacy of the ROCK2
inhibitor was also confirmed with respect to cell growth in an
anchorage-independent condition. The number of colonies in soft
agar, an in vitro assay closely suggestive of tumor
malignancy level (22), was
significantly lower after cell exposure to the ROCK2 inhibitor than
to Y27632 (Fig. 2B), further
confirming the prevalent role of ROCK2 in regulating EWS
aggressiveness.
Blockage of ROCK2 activity inhibits
cell proliferation and favors cell differentiation of EWS
cells
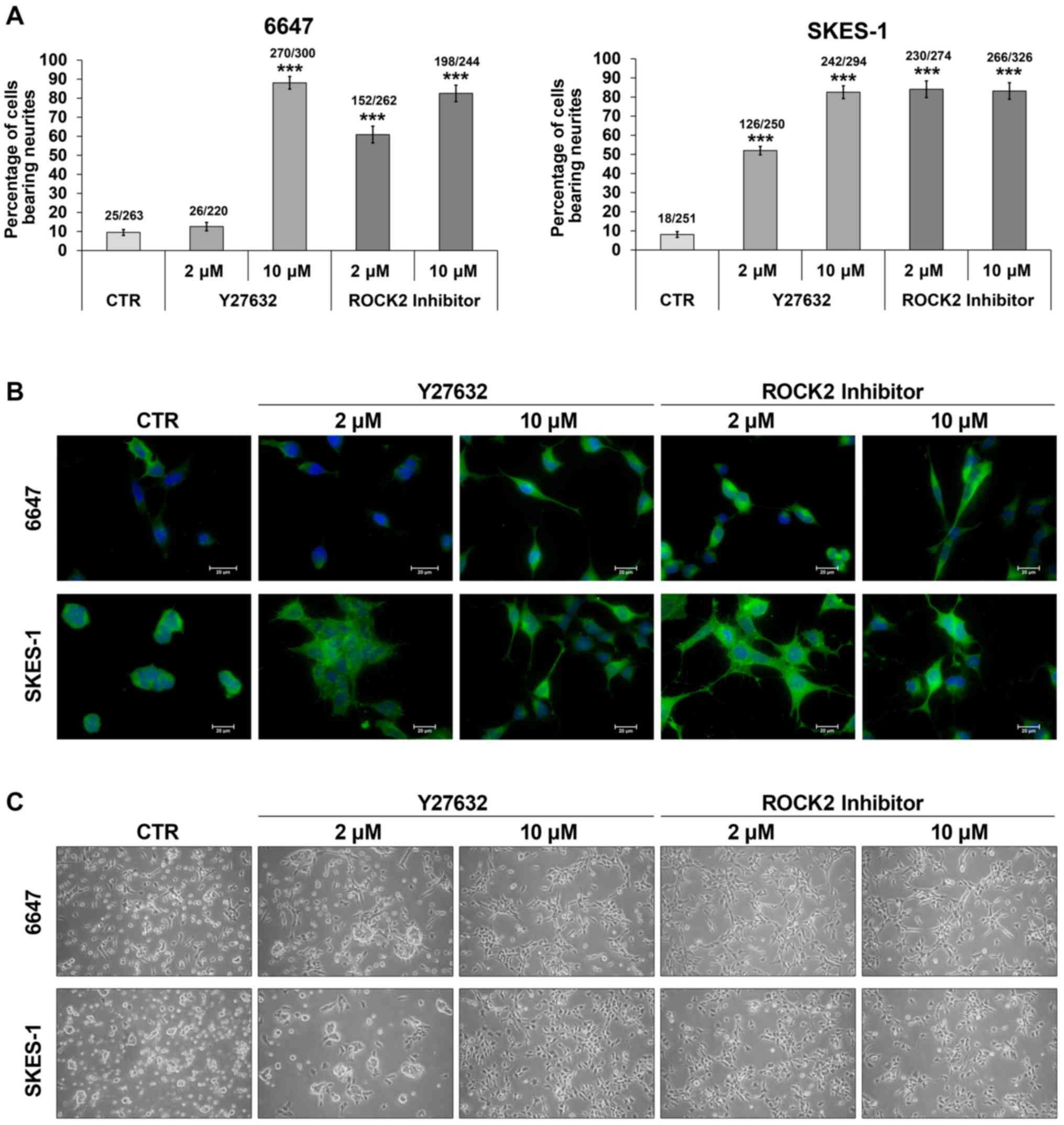
EWS, 6647 and SKES-1 cells were treated with the
ROCK2 or Y27632 inhibitor in monolayer conditions to explore the
additional effects of these agents on cell proliferation, survival
and differentiation. Recent reports have shown that the RhoA-ROCK
pathway is pivotal in the control of neurite outgrowth and its
inhibition (23). We showed here
that inhibition of ROCK2 improved neuronal differentiation of EWS
cells. Both Y27632 and the specific ROCK2 inhibitor were able to
promote neurite outgrowth and to induce expression of β-III-tubulin
(Fig. 3A and B). This was
accomplished with marked changes in EWS cell shape (Fig. 3C), in line with the role of ROCK as
a regulator of cytoskeletal dynamics: EWS cells lost the capability
to grow in suspension, acquired increased adherence to the culture
matrix and developed long neurite-like extensions, acquiring a
cellular phenotype consistent with neural differentiation. Although
these effects were observed after exposure of the cells to the two
inhibitors, ROCK2 inhibitor appeared to be more effective in
inducing cell shape variations and neural differentiation already
at the lower dose of 2 µM (Fig. 3B and
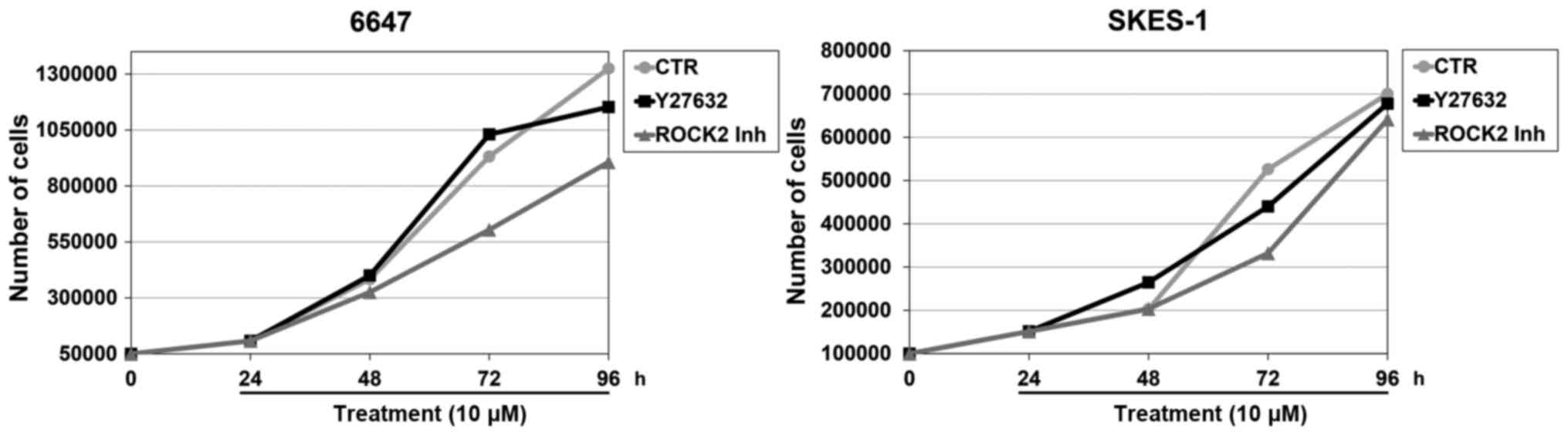
C). When tumor growth was examined in parallel with
differentiation, we observed reduction in the EWS cell growth rate
only after treatment with the ROCK2 inhibitor but not with the
pan-ROCK inhibitor Y27632 (Fig. 4).
Neither ROCK2 inhibitor nor Y27632 induced apoptotic cell death
(data not shown).
Discussion
In the present study, we provide evidence that the
specific blockage of ROCK2 activity significantly affects cell
migration and growth, and induces marked modification in cell
shape, driving EWS cells toward a neurally differentiated
phenotype. The role of morphology in tumor progression has become
evident in recent years and the shape of a cell has been shown to
regulate cancer cell differentiation, proliferation, survival, and
motility through the activation of mechanosensitive transcriptional
regulators, which are able to link shape information with gene
expression (23–30). Despite the existing evidence on the
role of the transcriptional coactivators Yes-associated protein
(YAP) and transcriptional coactivator with PDZ-binding motif (TAZ)
in mechanotransduction, the signaling mediators linking cell shape
to modulation of cell differentiation and migration are still
poorly understood. ROCK kinases have been shown to participate in a
wide range of cellular functions, including control of cell
morphology, proliferation and differentiation in addition to their
well-known role as regulators of cell migration and invasion
(31). These effects are likely due
to ROCK activity in the regulation of cytoskeletal structures and
the formation of actin stress fibers. Noteworthy, YAP/TAZ response
to cytoskeletal tensions requires Rho/ROCK signaling, and
inhibition of ROCK2 significantly inhibits nuclear localization of
YAP/TAZ (25). In this study, we
highlighted the privileged role of ROCK2 with respect to ROCK1 in
the regulation of EWS malignancy. It is the specific inhibition of
ROCK2, rather than the use of a pan-ROCK inhibitor, that
significantly reduced cell migration and growth in vitro,
while inducing cell differentiation. This evidence is in line with
our previous study concerning the role of ROCK2 in osteosarcoma
(21). The effects of ROCK2
inhibition on cell differentiation may be particularly relevant in
mesenchymal tumors, which are highly malignant and poorly
differentiated cancers. Differently from other solid tumors,
sarcomas are thought to derive from molecular aberrations occurring
during the differentiation process of mesenchymal stem cells and
they could be reprogrammed to resume normal differentiation
(32). The specific inhibition of
ROCK2 thus offers an intriguing approach for the design of new
options for the treatment of these tumors, also considering that
any effects on cell differentiation in sarcomas may also affect
stem cell pluripotency and cell fate. We found that, in EWS cells,
ROCK2 inhibition induced the acquisition of a neuron-like
morphology, increased expression of neuronal markers and,
concurrently, slowed down proliferation. This is in line with
studies that highlight the pivotal role of the RhoA-ROCK pathway in
the control of neurite outgrowth (23), and the pro-differentiation activity
of ROCK inhibitors in mesenchymal stem cells towards the neuronal
lineage (33). Achieving a greater
understanding of the pathways involved in neuritogenesis may help
the identification of novel therapeutic targets for the treatment
of neurodegenerative diseases. By indicating for the first time
that ROCK2, besides its well-described anti-migratory activity, is
also crucial for re-directing a tumor cell toward neural
differentiation, the present study opens new avenues for the
therapeutic use of ROCK inhibitors also in oncology.
Overall, our observations indicate that the specific
inhibition of ROCK2 can facilitate EWS cell differentiation toward
a neural phenotype, in addition to decreasing cell proliferation
and migration. Although all of these effects were also observed by
using the pan-ROCK inhibitor Y27632, specific deprivation of ROCK2
activity provided an advantage in terms of efficacy. Further
investigations in term of toxicity, dosage and side effects are
warranted. However our findings render ROCK2 inhibition a promising
candidate for novel treatment against EWS.
Acknowledgements
The authors would like to thank C. Ghinelli and E.E.
Pinca for their help in editing the manuscript. This study was
supported by grants from the Associazione Italiana per la Ricerca
sul Cancro (AIRC; IG14049 to K.S.), the Liddy Shriver Sarcoma
Initiative (international grant to K.S.) and Ricerca Fondamentale
Orientata (RFO 2012 to C.Z.). R.S.P. received a fellowship from the
Associazione Onlus ‘il Pensatore: Matteo Amitrano’ and ‘Liberi di
Vivere Luca Righi’.
References
|
1
|
Bernstein M, Kovar H, Paulussen M, Randall
RL, Schuck A, Teot LA and Juergens H: Ewings sarcoma family of
tumors: Current management. Oncologist. 11:503–519. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riggi N and Stamenkovic I: The biology of
Ewing sarcoma. Cancer Lett. 254:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campanacci M: Ewings sarcoma, primitive
neuroectodermal tumor (PNET)Bone and Soft Tissue Tumors. 2nd
edition. Springer-Verlag; Wien, Vienna: pp. 653–682. 1999,
View Article : Google Scholar
|
|
4
|
Paulussen M, Craft AW, Lewis I, Hackshaw
A, Douglas C, Dunst J, Schuck A, Winkelmann W, Köhler G, Poremba C,
et al: European Intergroup Cooperative Ewings Sarcoma Study-92:
Results of the EICESS-92 Study: Two randomized trials of Ewings
sarcoma treatment - cyclophosphamide compared with ifosfamide in
standard-risk patients and assessment of benefit of etoposide added
to standard treatment in high-risk patients. J Clin Oncol.
26:4385–4393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luksch R, Tienghi A, Hall KS, Fagioli F,
Picci P, Barbieri E, Gandola L, Eriksson M, Ruggieri P, Daolio P,
et al: Primary metastatic Ewings family tumors: Results of the
Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV
Study including myeloablative chemotherapy and total-lung
irradiation. Ann Oncol. 23:2970–2976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton. 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong CCL, Wong CM, Tung EKK, Man K and Ng
IOL: Rho-kinase 2 is frequently overexpressed in hepatocellular
carcinoma and involved in tumor invasion. Hepatology. 49:1583–1594.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lane J, Martin TA, Watkins G, Mansel RE
and Jiang WG: The expression and prognostic value of ROCK I and
ROCK II and their role in human breast cancer. Int J Oncol.
33:585–593. 2008.PubMed/NCBI
|
|
10
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vishnubhotla R, Sun S, Huq J, Bulic M,
Ramesh A, Guzman G, Cho M and Glover SC: ROCK-II mediates colon
cancer invasion via regulation of MMP-2 and MMP-13 at the site of
invadopodia as revealed by multiphoton imaging. Lab Invest.
87:1149–1158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–26412003.
|
|
13
|
Zhang C, Zhang S, Zhang Z, He J, Xu Y and
Liu S: ROCK has a crucial role in regulating prostate tumor growth
through interaction with c-Myc. Oncogene. 33:5582–5591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang N, Feng Y, Lau EP, Tsang C, Ching Y,
Man K, Tong Y, Nagamatsu T, Su W and Tsao S: F-Actin reorganization
and inactivation of Rho signaling pathway involved in the
inhibitory effect of Coptidis Rhizoma on hepatoma cell migration.
Integr Cancer Ther. 9:354–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patel RA, Liu Y, Wang B, Li R and Sebti
SM: Identification of novel ROCK inhibitors with anti-migratory and
anti-invasive activities. Oncogene. 33:550–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoneda A, Multhaupt HA and Couchman JR:
The Rho kinases I and II regulate different aspects of myosin II
activity. J Cell Biol. 170:443–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoneda A, Ushakov D, Multhaupt HAB and
Couchman JR: Fibronectin matrix assembly requires distinct
contributions from Rho kinases I and -II. Mol Biol Cell. 18:66–75.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mertsch S and Thanos S: Opposing signaling
of ROCK1 and ROCK2 determines the switching of substrate
specificity and the mode of migration of glioblastoma cells. Mol
Neurobiol. 49:900–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kovar H, Pospisilova S, Jug G, Printz D
and Gadner H: Response of Ewing tumor cells to forced and activated
p53 expression. Oncogene. 22:3193–3204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bagnara GP, Serra M, Giovannini M, Badiali
M, Stella M, Montaldi A, Granchi D, Paolucci P, Rocchi P, Pession
A, et al: Establishment and characterization of a primitive
neuroectodermal tumor of bone continuous cell line (LAP-35). Int J
Cell Cloning. 8:409–424. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zucchini C, Manara MC, Pinca RS, De
Sanctis P, Guerzoni C, Sciandra M, Lollini PL, Cenacchi G, Picci P,
Valvassori L, et al: CD99 suppresses osteosarcoma cell migration
through inhibition of ROCK2 activity. Oncogene. 33:1912–1921. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manara MC, Bernard G, Lollini PL, Nanni P,
Zuntini M, Landuzzi L, Benini S, Lattanzi G, Sciandra M, Serra M,
et al: CD99 acts as an oncosuppressor in osteosarcoma. Mol Biol
Cell. 17:1910–1921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang P, Wen HZ and Zhang JH: Expression of
a dominant-negative Rho-kinase promotes neurite outgrowth in a
microenvironment mimicking injured central nervous system. Acta
Pharmacol Sin. 31:531–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aragona M, Panciera T, Manfrin A, Giulitti
S, Michielin F, Elvassore N, Dupont S and Piccolo S: A mechanical
checkpoint controls multicellular growth through YAP/TAZ regulation
by actin-processing factors. Cell. 154:1047–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CS, Mrksich M, Huang S, Whitesides GM
and Ingber DE: Geometric control of cell life and death. Science.
276:1425–1428. 2008. View Article : Google Scholar
|
|
28
|
Sero JE, Thodeti CK, Mammoto A, Bakal C,
Thomas S and Ingber DE: Paxillin mediates sensing of physical cues
and regulates directional cell motility by controlling lamellipodia
positioning. PLoS One. 6:e283032011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mcbeath R, Pirone DM, Nelson CM,
Bhadriraju K and Chen CS: Cell shape, cytoskeletal tension, and
RhoA regulate stem cell lineage commitment. Dev Cell. 6:483–495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Discher DE, Mooney DJ and Zandstra PW:
Growth factors, matrices, and forces combine and control stem
cells. Science. 324:1673–1677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Charytonowicz E, Terry M, Coakley K, Telis
L, Remotti F, Cordon-Cardo C, Taub RN and Matushansky I: PPARγ
agonists enhance ET-743-induced adipogenic differentiation in a
transgenic mouse model of myxoid round cell liposarcoma. J Clin
Invest. 122:886–898. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Zhang Z, Yan X, Liu H, Zhang L, Yao
A, Guo C, Liu X and Xu T: The Rho kinase inhibitor Y-27632
facilitates the differentiation of bone marrow mesenchymal stem
cells. J Mol Histol. 45:707–714. 2014. View Article : Google Scholar : PubMed/NCBI
|