Introduction
The Warburg effect is a well-documented metabolic
hallmark of cancer cells (1).
Cancer cells are thought to exclusively activate glycolysis, even
in the presence of adequate oxygen. One technique in clinical
practice that takes advantage of glycolysis is fluorodeoxyglucose
positron emission tomography/computed tomography (FDG-PET/CT)
(2,3). Normally, in cancer cells, glucose is
transported into the cytoplasm via glucose transporters (GLUTs),
especially GLUT1 (4). Unlike
glucose, FDG is a glucose-like substance that is also transported
into tissues but is not further metabolized, resulting in its
accumulation in tissues with active glucose metabolism (5). By detecting the accumulation of FDG,
FDG-PET/CT is used as a diagnostic aid to distinguish cancer cells
from normal cells. One of the quantitative values that reflect the
degree of FDG accumulation is the maximum standardized uptake value
(SUVmax). Indeed, recent studies have suggested its
diagnostic value. In addition, some studies have demonstrated a
correlation between SUVmax and patient prognosis
(6–8).
Moreover, it is also well-known that cancer cells
not only uptake the most glucose (glycolysis), but they also uptake
the most glutamine (glutaminolysis) (9), which is the most abundant amino acid
in serum, indicating that cancer cells do not always show
activation of glycolysis (or a high SUVmax). Then we
became interested in the characteristics of cancer cells with a low
activation of glycolysis (low SUVmax).
First, using medical records, we identified cases
with a low SUVmax among patients with ovarian cancer. We
found that most of the cases were limited to patients with ovarian
clear cell carcinoma (CCC). Indeed, some studies previously
indicated that ovarian CCC had a lower SUVmax or lower
uptake of FDG compared to adenocarcinoma of other histological
types (5,10). Then we became interested in the
biology of CCC which causes low activation of glycolysis, and we
speculated that CCC had the properties which were likely to utilize
glutaminolysis compared to glycolysis. To investigate this
hypothesis, we conducted an in vitro experiment to clarify
the biology, which could explain the low SUVmax of
CCC.
We obtained cells with cancer stem cell (CSC)-like
properties by forming spheroids as previously described (11). We then used western blotting to
compare the expression levels of transporters, which correlate with
cancer metabolism. Whereas the expression level of alanine, serine,
cysteine-preferring transporter 2 (ASCT2, a glutamine influx
transporter) was nearly unchanged between non-CSCs and CSCs, the
expression levels of system L-type amino acid transporter 1 (LAT1,
a glutamine efflux transporter) and GLUT1, a glucose influx
transporter were decreased in CSCs compared to non-CSCs. These
results indicated that glutamine metabolism was essential in cells
with CSC-like properties and that the low SUVmax of CCC
may reflect the metabolism of glutamine in CSCs.
In the present study, we used FDG-PET analysis and
showed that ovarian CCC may rely on glutaminolysis, and we
confirmed the significance of glutamine metabolism in ovarian CCC
by an in vitro experiment. We suggest that ovarian CCC has a
low uptake of FDG in FDG-PET/CT, and this may reflect
glutaminolysis of its CSC-like properties.
Materials and methods
Patients
This study was approved by the Institutional Ethics
Committee. All procedures involving human participants were in
accordance with the Ethical Standards of the Institutional and/or
National Research Committee and with the 1964 Helsinki Declaration
and its later amendments or comparable ethical standards.
Medical records and data from the gynecological
oncology database were retrospectively reviewed. Only epithelial
tumors, which were confirmed by pathological examinations after
surgery, were included.
First, patients with ovarian tumors who had
undergone FDG-PET within one month prior to their primary surgery
at the University of Tokyo Hospital between 2010 and 2012 were
enrolled (13 cases of benign tumors, 11 cases of borderline tumors
and 26 cases of malignant tumors). The SUVmax of
non-malignant tumors (including benign and borderline tumors) and
malignant tumors were compared. A cut-off value was chosen based on
a receiver operating characteristic (ROC) curve.
Second, patients who had undergone primary surgery
at the University of Tokyo Hospital between 2012 and 2015 and who
were thought to have a low SUVmax were also enrolled.
The definition of low or high SUVmax is discussed in the
Results.
FDG-PET/CT
Patients underwent PET/CT not only at the University
of Tokyo Hospital but also at other imaging centers. A
representative protocol is as follows. Patients fasted for at least
4 h before being intravenously injected with 185–370 MBq of FDG,
and then rested for 50 min before evaluation. Patients were scanned
when their plasma glucose levels were below 200 mg/dl. Patients
were assessed using an integrated Discovery ST Elite Performance
PET/CT (GE Healthcare Japan Corp., Tokyo, Japan) scanner.
Unenhanced CT images of 3.75-mm thick sections that matched the PET
images were acquired from the head to the pelvic floor of each
patient using a standard protocol. The PET images were
reconstructed using 3D-OSEM VUE point HD (iteration no. 2, subset
no. 21), with a 6 mm Full-Width Half-Maximum (FWHM) Gaussian
in-plane post filter (GE Healthcare).
For semi-quantitative analysis, a region of interest
was drawn for each lesion in the transverse section where the
lesion seemed to have the largest uptake according to size and
intensity. Maximal SUV was calculated using the formula: SUV =
Cdc/(di/w), where Cdc is the
decay-corrected tracer tissue concentration (in becquerels per
gram), di is the injected dose (in becquerels), and w is
the patient's body weight (in grams) (12,13).
Cell lines and cell culture
The cancer cell lines OVTOKO and OVISE were obtained
both from JCRB Cell Bank (Osaka, Japan), and JHOC5 was obtained
from RIKEN Cell Bank (Tsukuba, Japan) (14). These cell lines all derived from
ovarian CCC and verified in writing as being ovarian in origin.
They were cultured in RPMI-1640 medium or Dulbecco's modified
Eagle's medium (DMEM) (both from Wako Pure Chemical Industries,
Ltd., Osaka, Japan) supplemented with 10% fetal bovine serum (FBS;
Invitrogen Life Technologies, Carlsbad, CA, USA), 100 U/ml
penicillin, 100 µg/ml streptomycin and subcultured by 0.25%
trypsin/EDTA (all from Wako Pure Chemical Industries, Ltd.)
detachment. All of the cells were grown in a humidified atmosphere
at 37°C and 5% CO2.
Suspension (spheroid-forming)
culture
Dissociated single cells (2×105 cells/ml)
were seeded into ultra-low attachment plates (Corning Inc.,
Corning, NY, USA) and were cultured for 2 days. For collecting
spheroids, the medium was centrifuged at 100 × g for 2 min, and the
supernatants were carefully aspirated.
Western blotting
The same amounts of protein from whole cell lysates
were subjected to SDS-polyacrylamide gel electrophoresis (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and electrotransferred onto
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA).
The membranes were blocked with 5% (w/v) skim milk in TBS-Tween-20
for 1 h at room temperature. Then, the blots were probed with
primary antibodies at 1:500 dilutions overnight at 4°C, followed by
incubation with appropriate secondary antibodies conjugated to
horseradish peroxidase (GE Healthcare) for 1 h at room temperature.
The secondary antibodies were detected using Immobilon Western
Chemiluminescent HRP Substrate (Millipore) according to the
manufacturer's instructions.
Antibodies
Anti-CD44v6, anti-ASCT2 and anti-GLUT1 antibodies
were purchased all from Abcam (Cambridge, MA, USA) (cat. nos.
78960, 84903 and 40084, respectively). Anti-aldehyde dehydrogenase
1 (ALDH1) antibody was purchased from BD Biosciences (Franklin
Lakes, NJ, USA) (cat. no. 611195). Anti-LAT1, anti-p-AMP-activated
protein kinase (p-AMPK) and anti-AMPK antibodies were purchased all
from Cell Signaling Technology, Inc. (Danvers, MA, USA) (cat. nos.
5347, 2535 and 2532, respectively). Anti-α-tublin antibody was
purchased from Millipore (cat. no. CP06).
Statistical analysis
The Wilcoxon singed-rank test was used to compare
the medians. Logistic regression analysis was used to assess the
association between max and malignant/non-malignant tumors. ROC
curves were used to assess the criterion value. P-values <0.05
were considered statistically significant. JMP/SAS Institute was
used for statistical analysis.
Results
Patient characteristics
To define the cut-off value, a total of 50 patients
with ovarian tumors who underwent FDG-PET/CT before primary surgery
were enrolled. A total of 24 cases were pathologically confirmed as
non-malignant (benign and borderline) tumors, and 26 cases were
confirmed as malignant tumors. The median age of the patients with
non-malignant tumors was 43.5 years (range, 24–79 years). The
median age of the patients with malignant tumors was 48 years
(range, 22–79 years). The malignant tumors included serous
carcinoma (19%), mucinous carcinoma (8%), endometrioid carcinoma
(27%) and CCC (35%) (Table I).
 | Table I.Clinical FIGO stage and histologic
type of malignant cases. |
Table I.
Clinical FIGO stage and histologic
type of malignant cases.
| Clinical
stages | Clear | Endometrioid | Mucinous | Serous | Other |
|---|
| I (n=17) | 6 | 7 | 1 | 1 | 2 |
| II (n=1) | 0 | 0 | 0 | 1 | 0 |
| III (n=5) | 2 | 0 | 0 | 2 | 1 |
| IV (n=3) | 1 | 0 | 1 | 1 | 0 |
Diagnostic value of SUVmax
and cut-off point
The median SUVmax for non-malignant
tumors was 2.0 (range, 0–7.5). The median SUVmax for
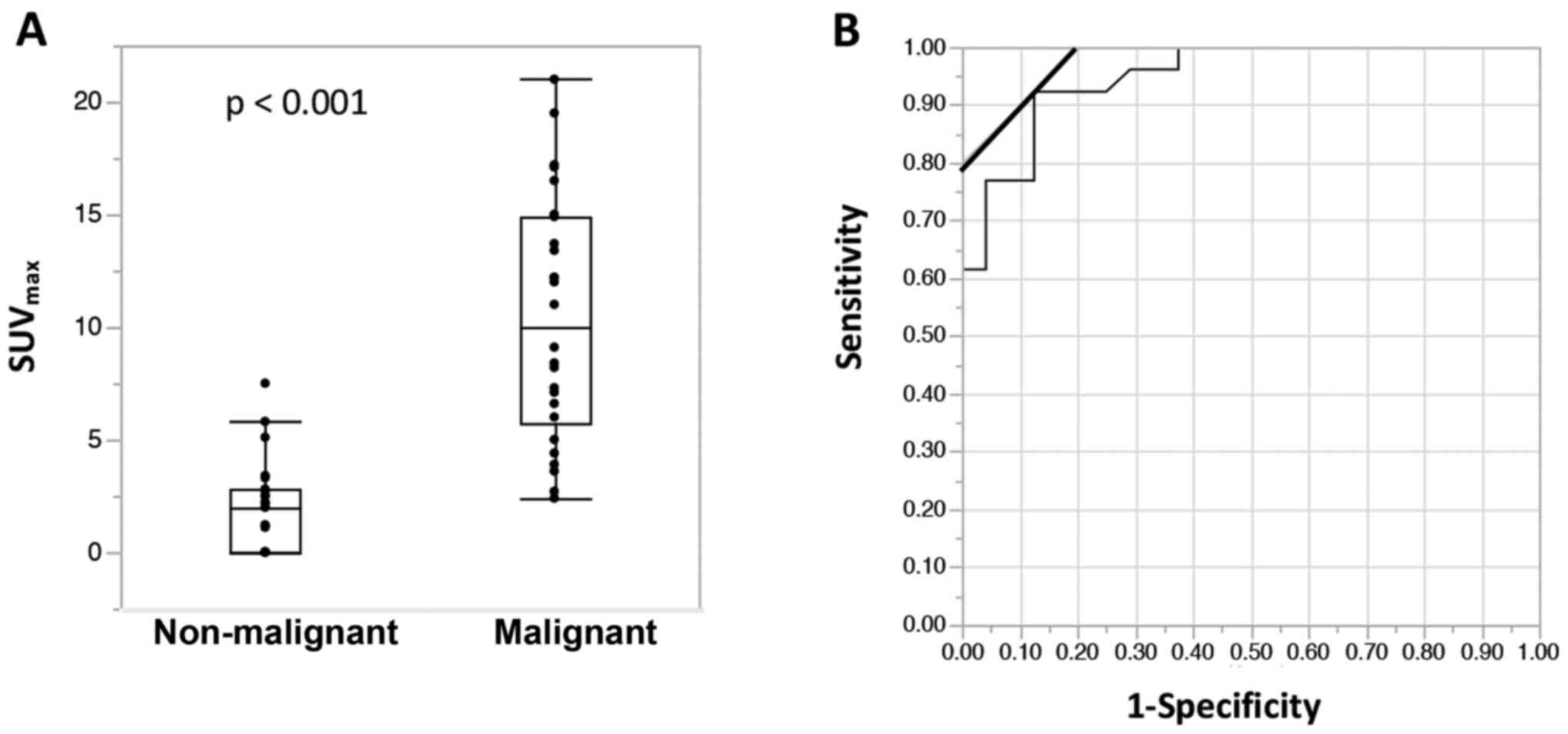
malignant tumors was 10.1 (range, 2.4–21.0). Malignant cases had a
significantly higher SUVmax as shown in Fig. 1A (p<0.001). The cut-off value for
predicting malignant tumors was 3.6 with a sensitivity/specificity
of 0.92/0.88, which was determined by ROC curves (Fig. 1B).
Cancers with a low
SUVmax
In the literature, cut-off values for predicting
ovarian malignant tumors ranged from 2.5 to 3.7 (4,8,10).
These differences could come from the histological differences and
the numbers of borderline tumors included. It is also known that
SUV varies according to the facility's protocol for conducting
FDG/PET (15). Although there was
no definition for ‘low SUVmax’, we considered ‘below
4.0’ as ‘low SUVmax’. We identified ovarian cancer
patients with a low SUVmax as described in the Materials
and methods section. Eight patients were found to have a low
SUVmax. The clinicopathological characteristics of these
patients are shown in Table II. We
found that most of the cases were CCC. Patients with ovarian CCC
were often identified in the early stages (FIGO stage I/II).
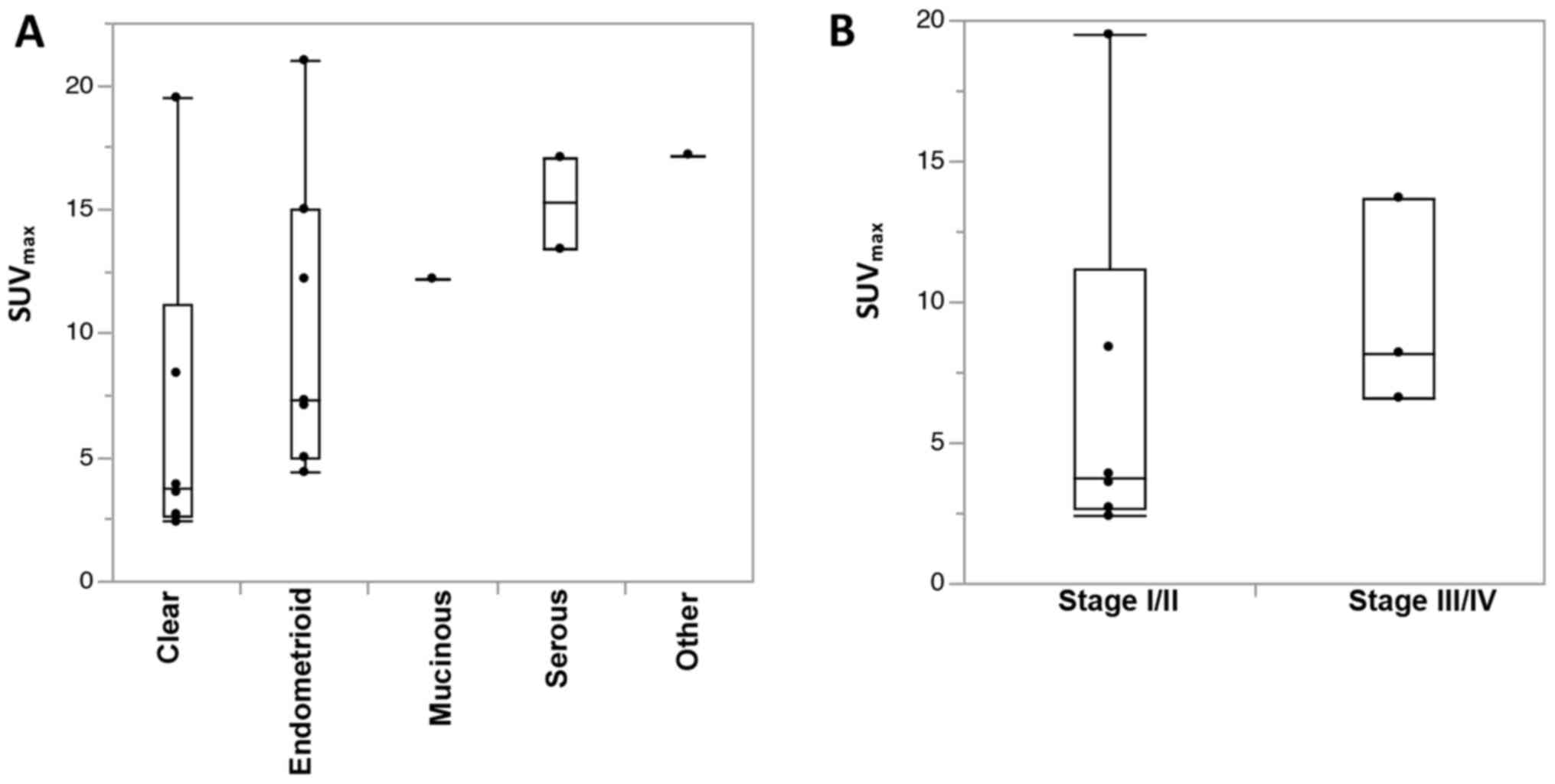
However, as shown in Fig. 2A, the
population of cases in the early stages could not necessarily
explain the low SUVmax of CCC, suggesting that a
specific biology of CCC should cause the low SUVmax.
Furthermore, among patients with CCC, the characteristic of a low
SUVmax is thought to apply in particular to early-stage
cases (Fig. 2B).
 | Table II.Characteristics of patients with a
low SUVmax. |
Table II.
Characteristics of patients with a
low SUVmax.
| Patients | Age (years) | Stage | Histology |
SUVmax |
|---|
| 1 | 70 | I | clear | 2.7 |
| 2 | 43 | I | clear | 3.6 |
| 3 | 43 | I | clear | 3.9 |
| 4 | 64 | II | clear | 3.9 |
| 5 | 51 | I | other | 3.3 |
| 6 | 65 | I | clear | 3.1 |
| 7 | 54 | III | other | 3.5 |
| 8 | 36 | I | clear | 2.3 |
Metabolic features of CCC in
vitro
To investigate and gain insight into the metabolic
features of CCC, we then proceeded to conduct an in vitro
experiment. We previously reported that OVTOKO, a CCC cell line,
had different metabolic features between its cells with CSC-like
properties and its non-CSCs (11).
We also found that CSCs had significantly higher concentrations of
glutamine than non-CSCs. Taken together, we hypothesized that the
CSC-like properties should explain the low SUVmax of
CCC. We added the cancer cell lines of CCC, OVISE and JHOC5 and
obtained cells with CSC-like properties by forming spheroid
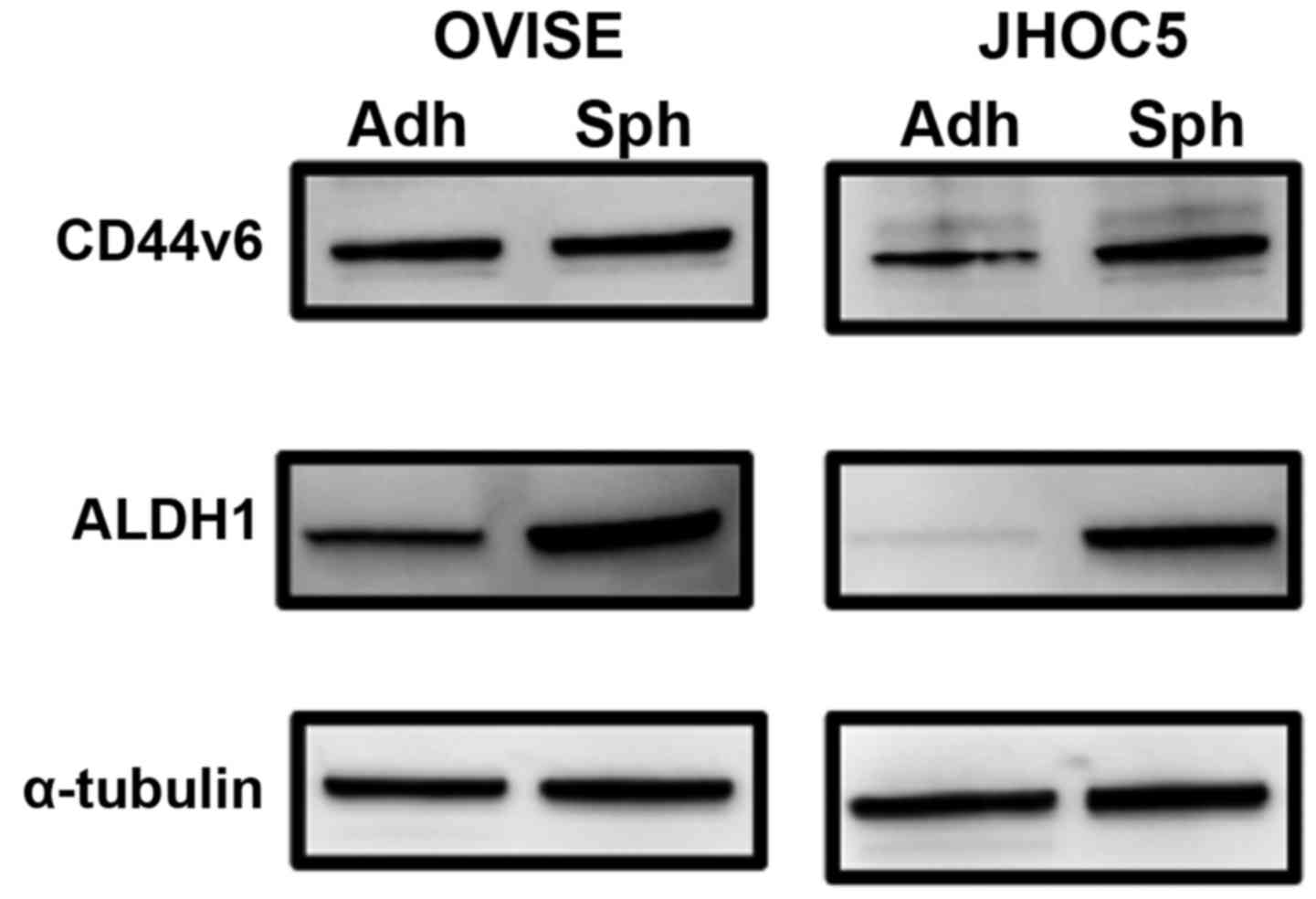
(described later, CSCs). Although a CSC marker for ovarian CCC has
yet to be identified, we applied CD44v6 and ALDH1 as CSC markers
(16,17). The expression of CD44v6 was not
increased in CSCs from OVISE but was increased in CSCs from JHOC5
compared with respective adherent non-CSCs (Fig. 3). The expression of ALDH1 was
increased in CSCs from both OVISE and JHOC5 (Fig. 3). Next, the expression levels of
representative transporters that have significant roles in
metabolism were investigated by western blotting. Whereas the
expression level of ASCT2, a glutamine influx transporter, was
nearly unchanged between non-CSCs and CSCs, the expression levels
of LAT1, a glutamine efflux transporter, and GLUT1, a glucose
influx transporter, were decreased in CSCs compared to non-CSCs,
except for in JHOC5 (Fig. 4A).
These changes indicated that there was metabolic reprograming
between non-CSCs and CSCs. Indeed, the phosphorylation levels of
AMPK, which is thought to be one of the metabolic sensors and
switches (18), were reduced in
CSCs compared to non-CSCs (Fig.
4B).
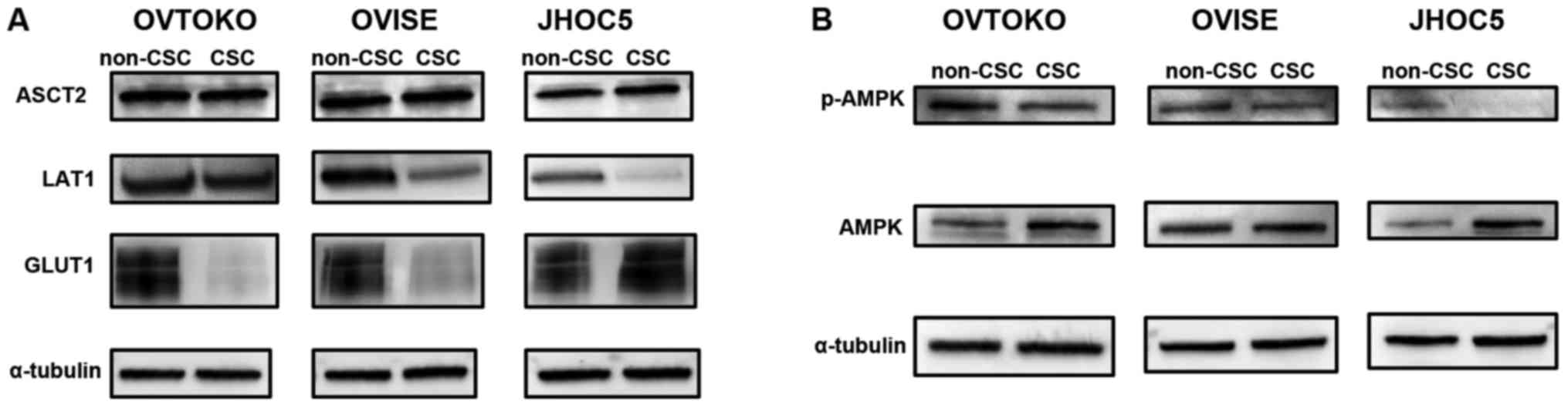 | Figure 4.Expression level of transporters and
metabolic sensors between non-CSCs and CSCs. (A) Expression level
of transporters relating to glycolysis and glutaminolysis. Whereas
the expression level of ASCT2, glutamine influx transporter, was
nearly unchanged from non-CSCs to CSCs, the expression levels of
LAT1, glutamine efflux transporter, and GLUT1, glucose influx
transporter, were decreased in CSCs compared to non-CSCs, except
for in JHOC5. (B) Phosphorylation level of AMPK. The
phosphorylation levels of AMPK, which is thought to be one of the
metabolic sensors and switches, were reduced in CSCs compared to
non-CSCs. CSC, cancer stem cell; ASCT2, alanine, serine,
cysteine-preferring transporter 2; LAT1, system L-type amino acid
transporter 1; GLUT1, glucose transporter 1; AMPK, AMP-activated
protein kinase. |
Discussion
Here, we demonstrated that ovarian CCC had a low
uptake of FDG in FDG-PET/CT, and this may reflect glutaminolysis of
its CSC-like properties.
Cancers have two major pathways of metabolism. One
is glycolysis, and the other is glutaminolysis (1,9).
One technique in clinical practice that takes
advantage of glycolysis is the FDG-PET/CT (2,3).
Glucose is transported into the cytoplasm via GLUTs, especially
GLUT1. FDG is a glucose analog that is also transported into
tissues, like glucose, but is not further metabolized, unlike
glucose, resulting in its accumulation in high-glucose-using
tissues, which is how FDG-PET/CT is used as a diagnostic aid to
distinguish cancer cells from normal cells (5). One of the measurements that reflect
the activation of glycolysis is the SUVmax. Indeed, many
studies have suggested that this tool not only has diagnostic
value, but it also has prognosis predicting value (6–8).
As mentioned above, however, cancer cells do not
always show activation of glycolysis (or high SUVmax)
because they can also consume glutamine. Accordingly, we became
interested in the characteristics of cancer cells with a low
activation of glycolysis (low SUVmax).
In the first half of this study in which we
identified cases with a low SUVmax among patients with
ovarian cancer, we showed that most of the cases were limited to
patients with ovarian CCC (especially cases in early stages).
It is important to consider that a low uptake of
SUVmax does not always indicate a tumor biology of
glycolysis but can indicate technical limitations of the
FDG-PET/CT. For instance, ovarian mucinous carcinoma tends to have
a low SUVmax because of its low tumor cellularity and
high amount of mucin (5).
Hepatocellular adenocarcinoma expresses GLUT1 and uptakes glucose
but has a low SUVmax because it has
glucose-6-phosphatase, which de-phosphorylates and effluxes FDG
(19). Conditions such as high
concentrations of serum glucose and inflammation make it difficult
to detect the expected accumulation of FDG (15,20).
However, it is empirically known that biologically, ovarian CCC has
a lower uptake of glucose compared with carcinoma of other
histological types (5,10). Indeed, one study demonstrated that
ovarian CCC cell lines had a lower uptake of FDG than cell lines
from other histological types in vitro (21).
In the latter half of this study, we performed an
in vitro experiment to investigate the metabolic biology of
CCC, which could explain its low SUVmax. In particular,
we focused on the metabolic differences between CSCs and
non-CSCs.
We obtained cells with CSC-like properties by
forming spheroids as previously described (11). Then, we compared the expression
levels of transporters between non-CSCs and CSCs, which correlate
with cancer metabolism (western blotting). Whereas the expression
level of ASCT2, a glutamine influx transporter, was nearly
unchanged between non-CSCs and CSCs, the expression levels of
system LAT1, a glutamine efflux transporter, and GLUT1, a glucose
influx transporter, were decreased in CSCs compared to non-CSCs,
except for in JHOC5. These patterns of changes in transporter
expression could mean that glutamine metabolism had a prominent
part in CSC metabolism. Although the expression of GLUT1 was
increased in CSCs compared to non-CSCs in JHOC5, the concomitant
decrease in LAT1 expression could still indicate that glutamine
accumulation was important in the CSCs of JHOC5. Recent studies
have demonstrated that the high expression levels of LAT1 and the
high uptake of glucose were correlated with poor prognosis in
ovarian (clear cell) carcinoma (4,22–24).
These facts may reflect the status in which CSCs differentiate and
propagate into non-CSC, indicated by our in vitro data.
Some studies referred to the low SUVmax
of CCC, and they often attributed it to lower expression levels of
GLUT1 or lower cell proliferation compared with other histological
types of cancers (5,10). In this study, we propose a new
possible explanation, by comparing the metabolic features not
between CCC and other histological types of cancers but between
CSCs and non-CSCs in ovarian CCC. It is that the low
SUVmax of CCC may represent its reliance on
glutaminolysis compared to glycolysis, and that it may be CSCs that
account for the activation of glutaminolysis.
The limitation of our study is that we only obtained
indirect evidence that glutaminolysis was significant in the CSCs
of CCC. Although we confirmed that the phosphorylation level of
AMPK, which is one of the metabolic sensors and has been recently
indicated to be related to glycolysis (18,25),
was reduced in CSCs compared to non-CSCs, this remains indirect
evidence of metabolic reprograming between non-CSCs and CSCs.
Ideally, performing glutamine-based PET in patients with ovarian
cancer should provide direct biological information about glutamine
metabolism. Indeed, fluoroglutamine (FGln)-PET was recently
introduced as a diagnostic tool for gliomas (26). We expect that this technology would
not only be a tool for diagnosis, but combined with FDG-PET, it
could also help investigate tumor metabolic behavior. Studies from
this perspective are expected in the future.
In conclusion, we suggest that ovarian CCC has a low
uptake of FDG-PET, and this may reflect glutaminolysis of its
CSC-like properties.
References
|
1
|
Dang CV: Rethinking the Warburg effect
with Myc micromanaging glutamine metabolism. Cancer Res.
70:859–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarker A, Im HJ, Cheon GJ, Chung HH, Kang
KW, Chung JK, Kim EE and Lee DS: Prognostic implications of the
SUVmax of primary tumors and metastatic lymph node measured by
18F-FDG PET in patients with uterine cervical cancer: a
meta-analysis. Clin Nucl Med. 41:34–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto M, Tsujikawa T, Fujita Y, Chino
Y, Kurokawa T, Kiyono Y, Okazawa H and Yoshida Y: Metabolic tumor
burden predicts prognosis of ovarian cancer patients who receive
platinum-based adjuvant chemotherapy. Cancer Sci. 107:478–485.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim C, Chung HH, Oh SW, Kang KW, Chung JK
and Lee DS: Differential diagnosis of borderline ovarian tumors
from stage I malignant ovarian tumors using FDG PET/CT. Nucl Med
Mol Imaging. 47:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Konishi H, Takehara K, Kojima A, Okame S,
Yamamoto Y, Shiroyama Y, Yokoyama T, Nogawa T and Sugawara Y:
Maximum standardized uptake value of fluorodeoxyglucose positron
emission tomography/computed tomography is a prognostic factor in
ovarian clear cell adenocarcinoma. Int J Gynecol Cancer.
24:1190–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung HH, Kwon HW, Kang KW, Park NH, Song
YS, Chung JK, Kang SB and Kim JW: Prognostic value of preoperative
metabolic tumor volume and total lesion glycolysis in patients with
epithelial ovarian cancer. Ann Surg Oncol. 19:1966–1972. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Risum S, Loft A, Engelholm SA, Høgdall E,
Berthelsen AK, Nedergaard L, Lundvall L and Høgdall C: Positron
emission tomography/computed tomography predictors of overall
survival in stage IIIC/IV ovarian cancer. Int J Gynecol Cancer.
22:1163–1169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto Y, Oguri H, Yamada R, Maeda N,
Kohsaki S and Fukaya T: Preoperative evaluation of pelvic masses
with combined 18F-fluorodeoxyglucose positron emission tomography
and computed tomography. Int J Gynaecol Obstet. 102:124–127. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin L, Alesi GN and Kang S: Glutaminolysis
as a target for cancer therapy. Oncogene. 35:3619–3625. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanizaki Y, Kobayashi A, Shiro M, Ota N,
Takano R, Mabuchi Y, Yagi S, Minami S, Terada M and Ino K:
Diagnostic value of preoperative SUVmax on FDG-PET/CT for the
detection of ovarian cancer. Int J Gynecol Cancer. 24:454–460.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato M, Kawana K, Adachi K, Fujimoto A,
Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Takahashi J,
et al: Spheroid cancer stem cells display reprogrammed metabolism
and obtain energy by actively running the tricarboxylic acid (TCA)
cycle. Oncotarget. 7:33297–33305. 2016.PubMed/NCBI
|
|
12
|
Kadoya T, Aogi K, Kiyoto S, Masumoto N,
Sugawara Y and Okada M: Role of maximum standardized uptake value
in fluorodeoxyglucose positron emission tomography/computed
tomography predicts malignancy grade and prognosis of operable
breast cancer: a multi-institute study. Breast Cancer Res Treat.
141:269–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JC, Lee JH, Cheoi K, Chung H, Yun MJ,
Lee H, Shin SK, Lee SK and Lee YC: Predictive value of pretreatment
metabolic activity measured by fluorodeoxyglucose positron emission
tomography in patients with metastatic advanced gastric cancer: the
maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med
Mol Imaging. 39:1107–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashiyama T, Oda K, Ikeda Y, Shiose Y,
Hirota Y, Inaba K, Makii C, Kurikawa R, Miyasaka A, Koso T, et al:
Antitumor activity and induction of TP53-dependent apoptosis toward
ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor
DS-7423. PLoS One. 9:e872202014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keyes JW Jr: SUV: standard uptake or silly
useless value? J Nucl Med. 36:1836–1839. 1995.PubMed/NCBI
|
|
16
|
Mizuno T, Suzuki N, Makino H, Furui T,
Morii E, Aoki H, Kunisada T, Yano M, Kuji S, Hirashima Y, et al:
Cancer stem-like cells of ovarian clear cell carcinoma are enriched
in the ALDH-high population associated with an accelerated
scavenging system in reactive oxygen species. Gynecol Oncol.
137:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tjhay F, Motohara T, Tayama S, Narantuya
D, Fujimoto K, Guo J, Sakaguchi I, Honda R, Tashiro H and Katabuchi
H: CD44 variant 6 is correlated with peritoneal dissemination and
poor prognosis in patients with advanced epithelial ovarian cancer.
Cancer Sci. 106:1421–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kishton RJ, Barnes CE, Nichols AG, Cohen
S, Gerriets VA, Siska PJ, Macintyre AN, Goraksha-Hicks P, De Cubas
AA, Liu T, et al: AMPK is essential to balance glycolysis and
mitochondrial metabolism to control T-ALL cell stress and survival.
Cell Metab. 23:649–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horsager J, Bak-Fredslund K, Larsen LP,
Villadsen GE, Bogsrud TV and Sørensen M: Optimal
2-[(18)F]fluoro-2-deoxy-D-galactose PET/CT protocol for detection
of hepatocellular carcinoma. EJNMMI Res. 6:562016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shida M, Murakami M, Tsukada H, Ishiguro
Y, Kikuchi K, Yamashita E, Kajiwara H, Yasuda M and Ide M: F-18
fluorodeoxyglucose uptake in leiomyomatous uterus. Int J Gynecol
Cancer. 17:285–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lutz AM, Ray P, Willmann JK, Drescher C
and Gambhir SS: 2-Deoxy-2-[F-18]fluoro-D-glucose accumulation in
ovarian carcinoma cell lines. Mol Imaging Biol. 9:260–266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan X, Ross DD, Arakawa H, Ganapathy V,
Tamai I and Nakanishi T: Impact of system L amino acid transporter
1 (LAT1) on proliferation of human ovarian cancer cells: a possible
target for combination therapy with anti-proliferative
aminopeptidase inhibitors. Biochem Pharmacol. 80:811–818. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaira K, Nakamura K, Hirakawa T, Imai H,
Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Tsukamoto N, Oyama T,
et al: Prognostic significance of L-type amino acid transporter 1
(LAT1) expression in patients with ovarian tumors. Am J Transl Res.
7:1161–1171. 2015.PubMed/NCBI
|
|
24
|
Lamkin DM, Spitz DR, Shahzad MM, Zimmerman
B, Lenihan DJ, Degeest K, Lubaroff DM, Shinn EH, Sood AK and
Lutgendorf SK: Glucose as a prognostic factor in ovarian carcinoma.
Cancer. 115:1021–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito Y, Chapple RH, Lin A, Kitano A and
Nakada D: AMPK protects leukemia-initiating cells in myeloid
leukemias from metabolic stress in the bone marrow. Cell Stem Cell.
17:585–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venneti S, Dunphy MP, Zhang H, Pitter KL,
Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S,
Ploessl K, et al: Glutamine-based PET imaging facilitates enhanced
metabolic evaluation of gliomas in vivo. Sci Transl Med.
7:274ra172015. View Article : Google Scholar : PubMed/NCBI
|