Introduction
As one of the most common human malignancies,
hepatocellular carcinoma (HCC) possesses the third highest
mortality rate (1). The therapies
for HCC treatment have been notably promoted. Whereas, the overall
rate of 5-year survival in HCC patients is only 12%, which remains
dismal (2). Surgical resection,
radiofrequency ablation and liver transplantation may benefit some
patients with early-stage HCC. However, advanced-stage HCC is
diagnosed in most patients (3).
Therefore, the development of new strategies for better
understanding of HCC and improving efficiency of HCC therapy are
anticipated.
Recently, the dysregulated microRNAs (miRNAs or
miRs) in HCC have been highlighted (4). miRNAs are a family of non-coding RNAs,
which comprise 20~25 nucleotides and are endogenous and conserved.
Messenger RNA (mRNA) transcription can be suppressed by miRNAs,
which directly target the 3′-untranslated regions (3′-UTRs) of
complementary mRNAs in eukaryotes (5,6).
Accumulating research suggests that a diversity of biologic
processes involve miRNAs, such as carcinogenesis, differentiation,
apoptosis, infection, and immunity (7–9).
Furthermore, it has been reported that the dysregulation of certain
miRNAs are related to the progression and clinical outcomes of
diverse cancers (10–12). By targeting the complementary genes,
miRNAs are able to regulate cancer cell proliferation, migration,
invasion and apoptosis (13),
thereby suggesting that miRNAs play critical roles in cancers and
probably provide a promising new way to treat cancer. In HCC, some
miRNAs such as miR-145, miR-133a (1), miR-144 (14) and miR-506 (15) have been reported with aberrant
expression. Nevertheless, the roles of the dysregulated miRNAs and
more specific miRNAs in HCC are still not well understood. miR-217
has been acknowledged as an inhibitor in various cancers including
osteosarcoma (16,17), lung cancer (18), pancreatic cancer (19) and clear cell renal cell carcinoma
(20). Moreover, it was reported
that miR-217 inhibited invasion of HCC (21). However, the detailed regulation
mechanism of miR-217 in HCC is still under investigation.
Metadherin (MTDH), a 582-amino acid single pass
transmembrane protein, is also known as lysine-rich CEACAM1
co-isolated (LYRIC) and astrocyte elevated gene-1 (AEG-1) (22). Since its initial cloning in 2002
(23), plenty of studies have
demonstrated that MTDH expression is elevated in a great diversity
of cancers (24), such as
hepatocellular renal cell and gallbladder carcinomas, colorectal,
gastric, prostate, lung, breast, ovarian, esophageal cancers and
melanoma, glioma, neuroblastoma and osteosarcoma. With further
studies, the expression of MTDH is associated with the development
of cancers and the high expression is especially found in the
aggressive metastatic stage (25).
It was also identified that the overall survival rate and prognosis
were poorer in HCC patients with MTDH overexpression (22). MTDH is overexpressed in 90% of HCC
patients and plays a pivotal role in HCC (26), which suggests that MTDH may serve as
an ideal target for anti-HCC therapy. However, the underlying
molecular mechanisms of miR-217 and MTDH in HCC are poorly studied.
The current research aimed to explore the roles of miR-217 and MTDH
and the potential mechanisms regulating MTDH expression via miR-217
in HCC.
Materials and methods
Tissue specimen collection
HCC tissues and the normal adjacent liver tissues
were collected from 20 patients in Nanfang Hospital, Southern
Medical University (Guangzhou, China). All the tissues were
collected before any therapy. The Ethics Committee of Nanfang
Hospital, Southern Medical University approved the research. Each
patient signed the informed consent.
Cell cultures
The human hepatocellular carcinoma cell line (HepG2)
was purchased from the American Type Culture Collection (ATCC)
(Rockville, MD, USA). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 100 µg/ml
streptomycin and 100 U/ml penicillin (Gibco, Carlsbad, CA, USA).
The cells were incubated at 37°C in a moist atmosphere containing
5% CO2.
Quantitative real-time RT-PCR
(qRT-PCR)
RNA extraction from the tissues or cells was by
TRIzol reagent obtained from Invitrogen (Carlsbad, CA, USA). The
TaqMan MicroRNA Assays (Invitrogen) were applied to quantitate the
relative expression of miR-217 and the standard SYBR Green RT-PCR
kit (Takara Shuzo, Kyoto, Japan) was used to detect the mRNA
expression of MTDH via qRT-PCR. Primers assigned for miR-217 and
MTDH were obtained from GeneCopoeia (Rockville, MD, USA). Values
were normalized to either small nucleolar RNA U6 or GAPDH.
Cell transfection
GenePharma (Jiangsu, China) provided the miR-217
mimics and negative control (NC) RNA oligonucleo-tides. Cells were
transfected with miR-217 or NC mimics (100 nM) by Lipofectamine
2000 (Invitrogen) when 70–80% cell confluence in 6-well plates.
After the transfection for 36 h, cells were collected for further
tests.
Dual luciferase reporter assay
The expression plasmid for MTDH 3′-UTR wild-type or
mutation and miR-217 were transfected into HepG2 cells. The firefly
and renilla (the internal control) luciferase activities were
examined by the dual-luciferase reporter assay system (Promega,
Madison, WI, USA).
Western blot analysis
Proteins were extracted from HCC tissues or cells
with RIPA lysis buffer and quantitated by the BCA protein assay kit
(Bio-Rad, Hercules, CA, USA). Proteins were subjected to 12%
SDS-PAGE gels and electrophoretically transfered onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Immunoblots were exposed to primary antibodies against MTDH
or GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C
overnight. Then HRP-conjugated secondary antibodies were added to
analyze the immunoreactive bands with the chemiluminescence reagent
(Western Lightning, Perkin Elmer Life Sciences, Boston, MA,
USA).
Cell proliferation assay
Cells were seeded in a 96-well plate and cultured
for 24, 48, 72 or 96 h at 37°C with 5% CO2. The cell
counting kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) was
used to assess the cell proliferation. The numerical values
obtained on an enzyme-labeled instrument (Thermo Fisher Scientific,
Bonn, German) with 450 nm wavelength were used to evaluate the cell
viability.
Apoptosis assay
Cells were trypsinised, collected in PBS and then
fixed in 70% ethanol (4°C, overnight), then washed with PBS and
collected by centrifugation (1000 rpm, 5 min). The Annexin V-FITC
apoptosis detection kit (Beyotime, Jiangsu, China) was used to
analyze apoptotic cells via flow cytometry (BD FACSCalibur).
Immunofluorescence
Sells were seeded on coverslips (Nalge Nunc
International, Penfield, NY, USA) in a 24-well plate and allowed to
adhere overnight. then fixed with 4% paraformaldehyde and incubated
with primary anti-MTDH antibody (Invitrogen) overnight at 4°C.
Cy3-conjugated secondary antibody was added and maintained at room
temperature for 1 h. After washing the slides were counterstained
with DAPI (Sigma-Aldrich). A confocal microscope (Olympus Corp.,
Tokyo, Japan) was used to obtain the fluorescence images.
Cell migration and invasion
assays
Boyden chamber Transwells (8 µm, Millipore) was used
to assess cell migration and invasion. The upper chamber with
uncoated membrane (migration assay) or the membrane pre-coated with
100 µg Matrigel (invasion assay) was added with 200 µl cell
suspension (1×105 cells/ml). The lower chamber was added
with 600 µl DMEM and 10% FBS as a chemoattractant. The nonfiltered
cells were gently removed and fixed with 4% paraformaldehyde after
incubation for 24 h at 37°C. Crystal violet (0.1%) (Sigma-Aldrich)
was applied to stain the lower chamber with filtered cells.
Quantitation was performed with a microscope (Olympus Corp.).
Statistical analysis
The data are presented as mean ± SD. Student's
t-test was used to evaluate differences between the stimulated
sample and the respective control. For multiple comparisons,
statistically significant differences were assessed via one-way
ANOVA. P-value <0.05 was deemed statistically significant.
Results
miR-217 expression decreases and MTDH
expression increases in HCC tissues
As depicted in Fig.
1A, compared with the matched normal tissues, miR-217
expression level in HCC tissues significantly decreased
(P<0.0001). Moreover, the expression of MTDH was examined via
qRT-PCR and western blotting in HCC tissues. Compared with the
normal tissues, the mRNA expression of MTDH was significantly
upregulated in HCC tissues (Fig.
1B), as well as the protein expression of MTDH (Fig. 1C).
miR-217 targets MTDH in HCC cells
As miR-217 and MTDH expressed negatively in HCC, we
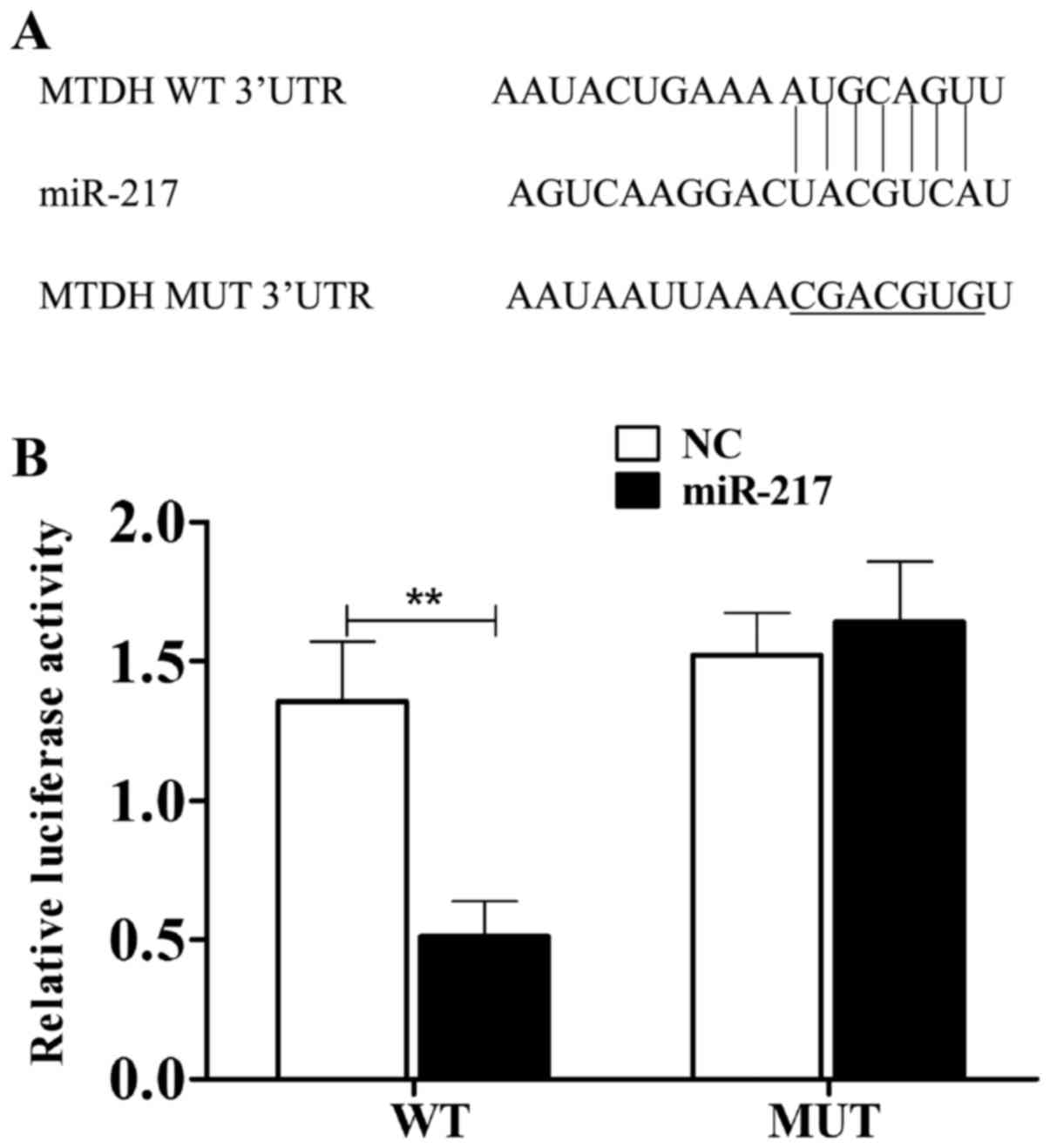
investigated whether miR-217 can target MTDH. As shown in Fig. 2A, miR-217 bound to the 3′-UTR of
MTDH gene. The dual luciferase reporter assay was further performed
to confirm their relationship. As displayed in Fig. 2B, after cotransfection with the
3′-UTR of wild-type MTDH and miR-217, the relative luciferase
activity was remarkably decreased in HepG2 cells (P<0.05). While
the luciferase activity of the mutant construct showed little
change, indicating that miR-217 inhibited MTDH transcription via
directly targeting the 3′-UTR of MTDH.
miR-217 negatively regulates MTDH
expression in HCC cells
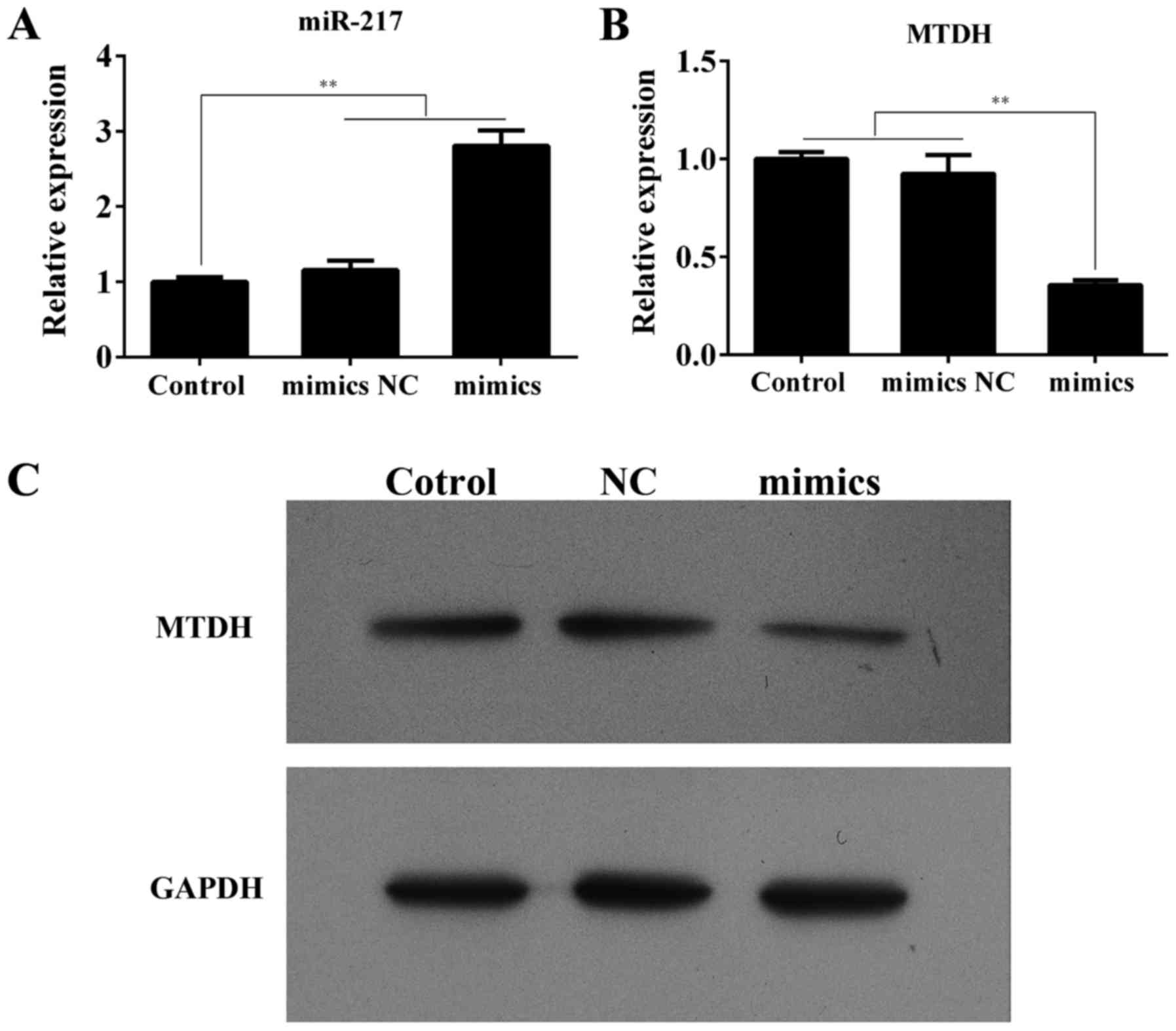
To further validate the prediction of the
suppression effect of miR-217 on MTDH gene, we explored whether
miR-217 could regulate the expression of endogenous MTDH in HepG2
cells. As depicted in Fig. 3A and
B, compared with the control or mimics NC, the miR-217
expression in the cells transfected with miR-217 mimics was
notablely upregulated (P<0.01), indicating that the transfection
efficiency was satisfactory. We next examined the MTDH mRNA
expression and observed a remarkably downregulation after
transfection with miR-217 mimics (P<0.01). Furthermore, we found
by western blotting that the MTDH expression in the HepG2 cells
with miR-217 overexpressed was markedly reduced compared with the
control or mimics NC (Fig. 3C).
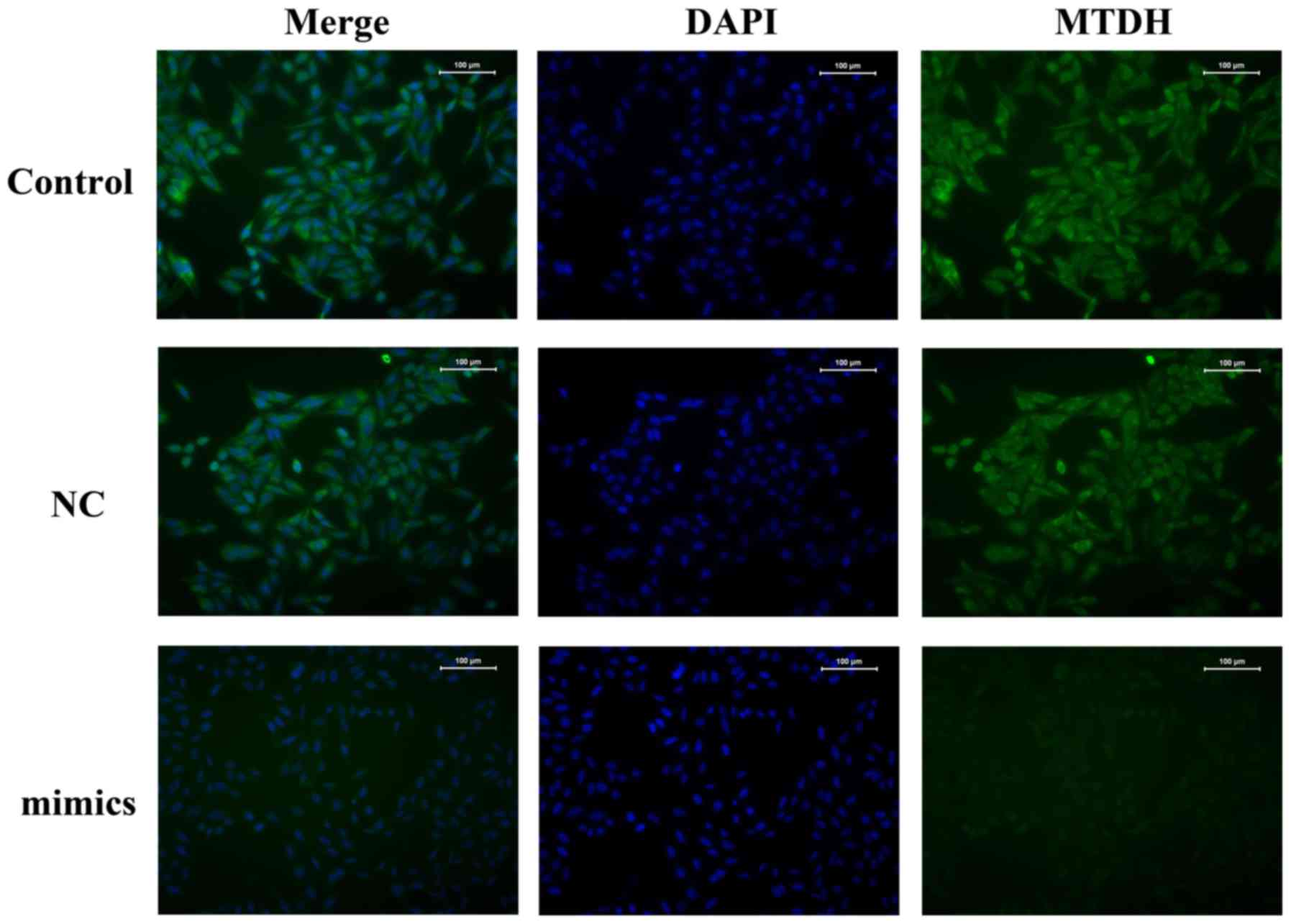
Similar results were obtained by immunofluorescence assays
(Fig. 4).
miR-217 suppresses proliferation and
promotes apoptosis in HCC cells
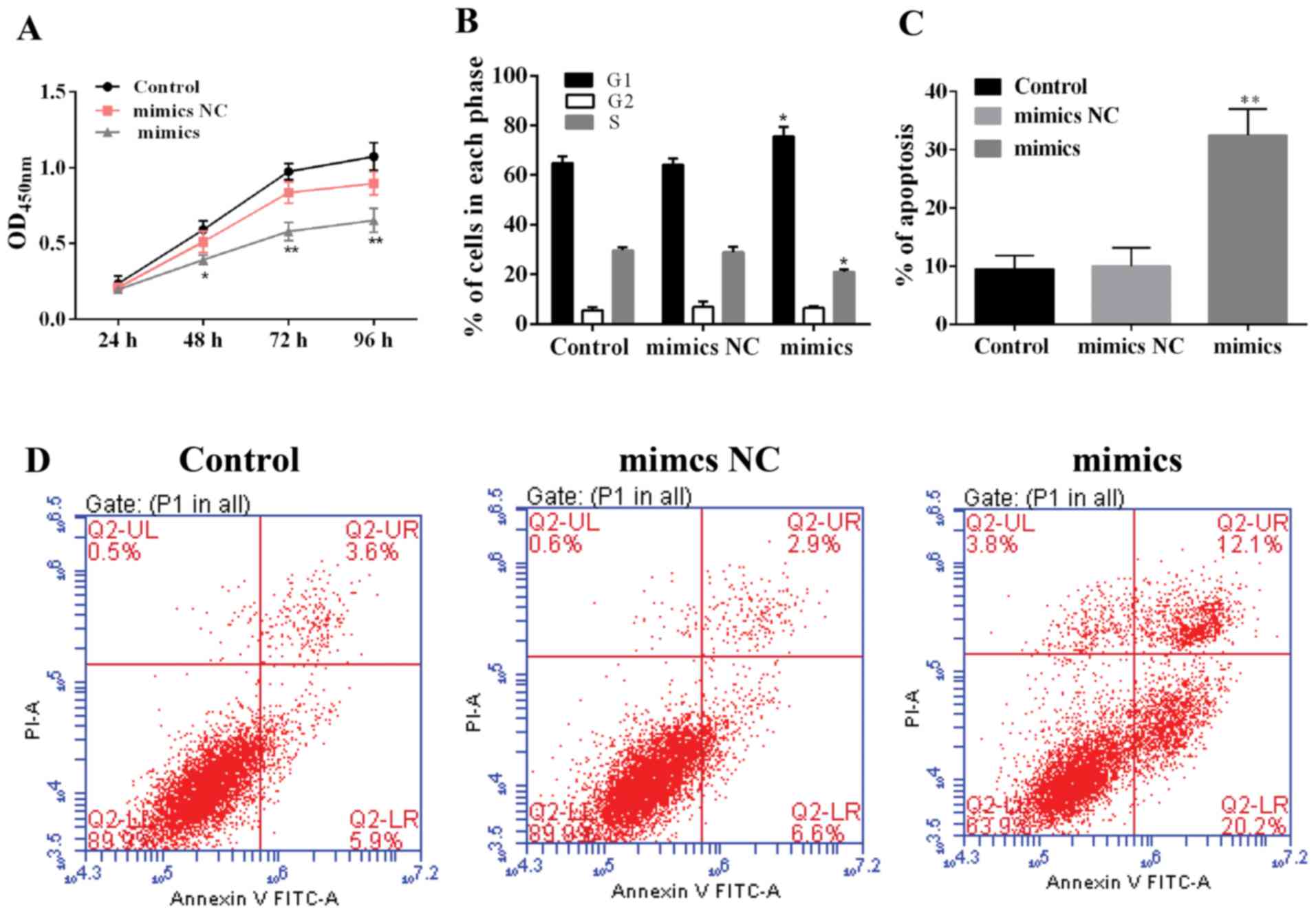
miR-217 or NC mimics were transfected into HepG2
cells to explore the role of miR-217 in HCC cells. As shown in
Fig. 5A, the proliferation of
miR-217-overexpressed cells was inhibited compared with the control
or mimics NC. Moreover, the cell cycle showed similar results
(Fig. 5B). These data suggest that
miR-217 played a proliferation suppressing role in HCC. In
addition, we explored the effect of miR-217 upregulation on
apoptosis in HCC. Compared with the control groups, the cell
apoptosis notably increased in miR-217-upregulated HepG2 cells
(Fig. 5C and D), indicating that
miR-217 expression promoted apoptosis of HCC cells.
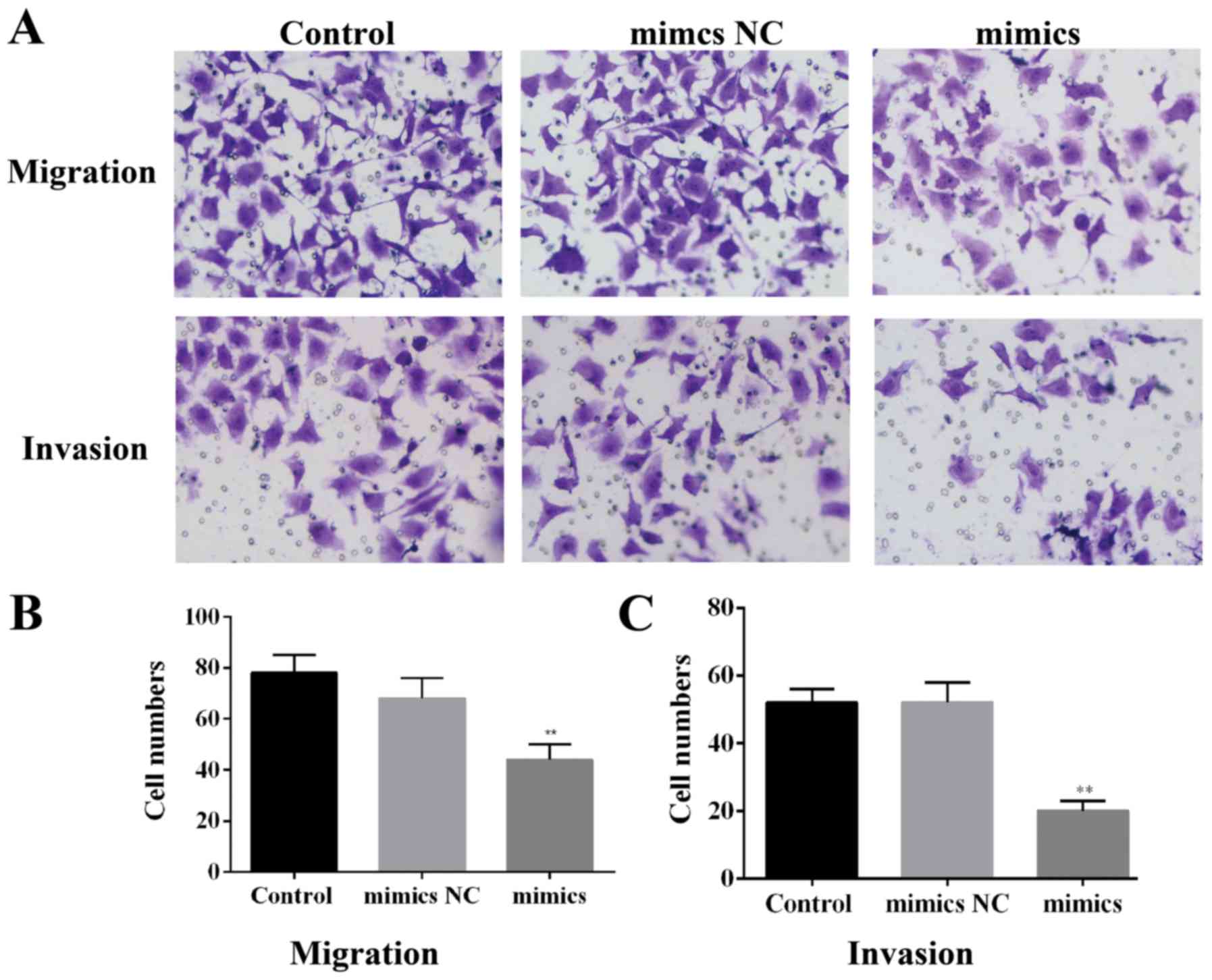
miR-217 restraines migration and
invasion in HCC cells
As displayed in Fig.
6A, the HepG2 cells overexpressing miR-217 had lower migration
and invasion compared with the control groups. Moreover, the
statistical results demonstrated that miR-217 overexpression
markedly suppressed migration and invasion in HCC cells (Fig. 6B and C).
Discussion
HCC is well known as one of the most lethal cancers
globally for its low cure rate, high recurrence rate and high
mortality (15). The development of
miRNAs provides a potential novel choice for HCC diagnosis and
therapy (27). miR-217 expression
has been reported to be lower in cancer cells, however the role of
miR-217 in HCC has not been well investigated (28). To further identify the potential
function and molecular mechanism of miR-217 in HCC, we used human
HCC tissues or normal liver tissues to test miR-217 expression. As
expected, the miR-217 expression in HCC tissues was remarkably
decreased. These data suggest that miR-217 possibly serves as a new
marker for HCC diagnosis or a novel target for HCC therapy.
Accumulating convincing studies have pointed out
that MTDH may act as a pivotal element to cancer onset and
progression (29). It has been
reported that MTDH is markedly upregulated in HCC patients and
could be a novel serum biomarker for HCC (30). MTDH has been demonstrated to be a
potential critical gene regulating various biochemical phenotypes
of cancer progression, such as metastasis, chemoresistance,
transformation and evasion of apoptosis (31). This study concurs that the
expression levels of both mRNA and protein MTDH were significantly
upregulated in the 20 HCC tissues compared to the matched normal
adjacent tissues. MTDH contributes to HCC initiation and
progression by a variety of mechanisms, such as interference with
thyroid hormone (T3) function, downregulation of type I
5′-deiodinase (DIO1) level, a local hypothyroid state creation in
the liver, activation of nuclear factor κB (NF-κB) and other
protumorigenic signaling pathways (32). Knockdown of MTDH inhibits
proliferation, invasion and notably abolishes cancer onset, growth
and metastasis (33,34). Thus, MTDH inhibition might be an
effective way to counteract this fatal malady for which there is no
effective treatment. MTDH inhibition will not only eliminate HCC
but also ameliorate detrimental effects associated with the disease
such as non-thyroidal illness syndrome (NTIS). Efforts need to be
spent on the development of MTDH inhibitors in the clinic.
miRNAs perform as an essential component in cancer
initiation and development via targeting and then decreasing the
expression of key regulator (15).
The present investigation was carried out to determine the
relationship between miR-217 and MTDH. The findings show that
miR-217 directly targeted 3′-UTR of MTDH, inhibiting both the mRNA
and protein expression levels of MTDH. Moreover, miR-217 acts as an
MTDH inhibitor in HCC. Thus, the cellular function of miR-217 was
further examined. The miR-217 overexpression notablely restrained
proliferation and promoted apoptosis of HCC cells. Furthermore,
migration and invasion strongly decreased in miR-217-transfected
cells. Taken together, this study indicates that miR-217 inhibits
proliferation, migration, invasion inducing apoptosis possibly by
targeting MTDH in HCC. The current study also revealed that miR-217
shows little effect on the cell cycle, suggesting the tumor
suppression effect of miR-217 is not associated with cell cycle in
HCC. As each miRNA may regulate several genes and each gene
possibly is regulated by a variety of miRNAs, the detailed
mechanism and upstream regulators of MTDH in HCC still need more
exploration. In addition, we must point out that this study was
executed in a single cell line and thus, more confirmation is
needed.
In conclusion, this study indicates that miR-217
downregulates the expression of MTDH, a key regulator of tumor
proliferation, migration and invasion in HCC cells. The findings in
this study also encourage us to develop miR-217 as a new potential
target for diagnosis and gene therapy of HCC.
References
|
1
|
Wang G, Zhu S, Gu Y, Chen Q, Liu X and Fu
H: MicroRNA-145 and microRNA-133a inhibited proliferation,
migration, and invasion, while promoted apoptosis in hepatocellular
carcinoma cells via targeting FSCN1. Dig Dis Sci. 60:3044–3052.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng Q, Xie L and Li H: MiR-506 suppresses
cell proliferation and tumor growth by targeting Rho-associated
protein kinase 1 in hepatocellular carcinoma. Biochem Biophys Res
Commun. 467:921–927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singal AG, Nehra M, Adams-Huet B, Yopp AC,
Tiro JA, Marrero JA, Lok AS and Lee WM: Detection of hepatocellular
carcinoma at advanced stages among patients in the HALT-C trial:
Where did surveillance fail? Am J Gastroenterol. 108:425–432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Callegari E, Gramantieri L, Domenicali M,
D'Abundo L, Sabbioni S and Negrini M: MicroRNAs in liver cancer: A
model for investigating pathogenesis and novel therapeutic
approaches. Cell Death Differ. 22:46–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu AM, Xu Z, Shek FH, Wong KF, Lee NP,
Poon RT, Chen J and Luk JM: miR-122 targets pyruvate kinase M2 and
affects metabolism of hepatocellular carcinoma. PLoS One.
9:e868722014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang N, Ekanem NR, Sakyi CA and Ray SD:
Hepatocellular carcinoma and microRNA: New perspectives on
therapeutics and diagnostics. Adv Drug Deliv Rev. 81:62–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan K, Cunningham D, Peckitt C, Barton S,
Tait D, Hawkins M, Watkins D, Starling N, Rao S, Begum R, et al:
miR-21 expression and clinical outcome in locally advanced
pancreatic cancer: Exploratory analysis of the pancreatic cancer
Erbitux, radiotherapy and UFT (PERU) trial. Oncotarget.
7:12672–12681. 2016.PubMed/NCBI
|
|
12
|
van Rooij E and Kauppinen S: Development
of microRNA therapeutics is coming of age. EMBO Mol Med. 6:851–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borel F, Konstantinova P and Jansen PL:
Diagnostic and therapeutic potential of miRNA signatures in
patients with hepatocellular carcinoma. J Hepatol. 56:1371–1383.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao T, Li H, Hu Y, Ma D and Cai X: miR-144
suppresses the proliferation and metastasis of hepatocellular
carcinoma by targeting E2F3. Tumour Biol. 35:10759–10764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai W, Huang HL, Hu M, Wang SJ, He HJ,
Chen NP and Li MY: microRNA-506 regulates proliferation, migration
and invasion in hepatocellular carcinoma by targeting F-spondin 1
(SPON1). Am J Cancer Res. 5:2697–2707. 2015.PubMed/NCBI
|
|
16
|
Sun B, Yang M, Li M and Wang F: The
microRNA-217 functions as a tumor suppressor and is frequently
downregulated in human osteosarcoma. Biomed Pharmacother. 71:58–63.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen L, Wang P, Yang J and Li X:
MicroRNA-217 regulates WASF3 expression and suppresses tumor growth
and metastasis in osteosarcoma. PLoS One. 9:e1091382014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo J, Feng Z, Huang Z, Wang H and Lu W:
MicroRNA-217 functions as a tumour suppressor gene and correlates
with cell resistance to cisplatin in lung cancer. Mol Cells.
37:664–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM and
Chen J: The miR-217 microRNA functions as a potential tumor
suppressor in pancreatic ductal adenocarcinoma by targeting KRAS.
Carcinogenesis. 31:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Zhao J, Zhang JW, Huang QY, Huang
JZ, Chi LS, Tang HJ, Liu GQ, Zhu DJ and Ma WM: MicroRNA-217,
down-regulated in clear cell renal cell carcinoma and associated
with lower survival, suppresses cell proliferation and migration.
Neoplasma. 60:511–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su J, Wang Q, Liu Y and Zhong M: miR-217
inhibits invasion of hepatocellular carcinoma cells through direct
suppression of E2F3. Mol Cell Biochem. 392:289–296. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li WF, Ou Q, Dai H and Liu CA:
Lentiviral-mediated short hairpin RNA knockdown of MTDH inhibits
cell growth and induces apoptosis by regulating the PTEN/AKT
pathway in hepatocellular carcinoma. Int J Mol Sci. 16:19419–19432.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi X and Wang X: The role of MTDH/AEG-1
in the progression of cancer. Int J Clin Exp Med. 8:4795–4807.
2015.PubMed/NCBI
|
|
25
|
Sarkar D and Fisher PB: AEG-1/MTDH/LYRIC:
Clinical significance. Adv Cancer Res. 120:39–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoo BK, Emdad L, Su ZZ, Villanueva A,
Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet
JM, et al: Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression. J Clin Invest. 119:465–477.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei R, Deng Z and Su J: miR-217 targeting
Wnt5a in osteosarcoma functions as a potential tumor suppressor.
Biomed Pharmacother. 72:158–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Shen ZL, Jiang KW, Zhao G, Wang
CY, Yan YC, Yang Y, Zhang JZ, Shen C, Gao ZD, et al: MicroRNA-217
functions as a prognosis predictor and inhibits colorectal cancer
cell proliferation and invasion via an AEG-1 dependent mechanism.
BMC Cancer. 15:4372015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robertson CL, Srivastava J, Rajasekaran D,
Gredler R, Akiel MA, Jariwala N, Siddiq A, Emdad L, Fisher PB and
Sarkar D: The role of AEG-1 in the development of liver cancer.
Hepat Oncol. 2:303–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang G, Yang J, Cao S, Li J and Liu B:
AEG-1 acts as a novel bio-marker in the diagnosis of patients with
hepatocellular carcinoma. Int J Clin Exp Pathol. 9:1940–1946.
2016.
|
|
31
|
Zhou Z, Deng H, Yan W, Huang H, Deng Y, Li
Y and Tian D: Expression of metadherin/AEG-1 gene is positively
related to orientation chemotaxis and adhesion of human
hepatocellular carcinoma cell lines of different metastatic
potentials. J Huazhong Univ Sci Technolog Med Sci. 32:353–357.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Srivastava J, Robertson CL, Gredler R,
Siddiq A, Rajasekaran D, Akiel MA, Emdad L, Mas V, Mukhopadhyay ND,
Fisher PB, et al: Astrocyte elevated gene-1 (AEG-1) contributes to
non-thyroidal illness syndrome (NTIS) associated with
hepatocellular carcinoma (HCC). J Biol Chem. 290:15549–15558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robertson CL, Srivastava J, Siddiq A,
Gredler R, Emdad L, Rajasekaran D, Akiel M, Shen XN, Guo C,
Giashuddin S, et al: Genetic deletion of AEG-1 prevents
hepatocarcinogenesis. Cancer Res. 74:6184–6193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wan L, Hu G, Wei Y, Yuan M, Bronson RT,
Yang Q, Siddiqui J, Pienta KJ and Kang Y: Genetic ablation of
metadherin inhibits autochthonous prostate cancer progression and
metastasis. Cancer Res. 74:5336–5347. 2014. View Article : Google Scholar : PubMed/NCBI
|