Introduction
Nasopharyngeal cancer (NPC) is prevalent in
Southeast Asia, especially in the Cantonese region (1). Radiotherapy is the primary treatment
and outcomes have improved considerably in the last decade. The
5-year overall survival rate is presently at least 85.8% for
early-stage NPC (2,3). However, most patients are diagnosed
with advanced NPC, which has a local control rate of only 50% and
5-year overall survival rate of 40–70% (4). Radioresistance is a key factor that
contributes to local recurrence and distant metastasis in NPC.
However, increasing the radiotherapy dose alone would affect
anatomically-close crucial structures and reduce the quality of
life (5). Moreover, present
standard chemoradiation strategies have already reached the upper
toxicity limits in terms of side effects and complications
(6). Therefore, a novel, effective,
less toxic radiosensitizer is urgently needed to improve the
therapeutic outcomes of patients with NPC.
Resveratrol is a polyphenol present in a wide
variety of dietary sources including grapes, peanuts and red wine,
as well as plant species such as Polygonum cuspidatum and
Yucca schidigera (7–9). Resveratrol exhibits many
health-beneficial properties such as antioxidant and
anti-inflammatory effects, and has been used as healthcare product
for cardiac protection, anti-aging and lifespan extension (10–12).
Apart from these traditional applications of resveratrol, studies
in a wide range of cancer cell lines suggest resveratrol may also
exert anticancer effects by promoting G1 or G2 phase cell cycle
arrest and subsequently inducing apoptosis (13–15). A
recent study showed resveratrol retarded cell cycle progression and
eventually arrested NPC cells in the G0/G1 phase.
As G1 or G2 phase cells are more sensitive to
irradiation than S phase cells (16), we hypothesized that resveratrol may
enhance radiosensitivity of NPC cells. Here, we assessed the
ability of resveratrol to sensitize CNE-1 cells to irradiation both
in vitro and in vivo and explored the associated
molecular mechanisms.
Materials and methods
Cell culture and treatment
Human NPC CNE-1 cells were obtained from Sun Yat-Sen
University Cell Collection and cultured in RPMI-1640 (Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Hyclone, Logan, UT, USA) and 100 U/ml penicillin
plus streptomycin (Life Technologies, Gaithersburg, MD, USA). Cells
were irradiated at 12.7 Gy/min at room temperature using an RS 2000
X-ray Biological Irradiator (Rad Source Technologies, Inc.,
Suwanee, GA, USA). Cells were treated as follows: control [dimethyl
sulfoxide (DMSO)]; resveratrol (0–150 µM resveratrol dissolved in
DMSO, no. R5010; Sigma-Aldrich, St. Louis, MO, USA); irradiation
(0–8 Gy irradiation); and resveratrol + irradiation (pretreatment
with resveratrol for 24 h followed by irradiation).
Cell viability assay
CNE-1 cells were seeded into 96-well plates in
triplicate (3,000 cells/well), cultured for 24 h, then treated with
resveratrol for 24, 48 or 72 h. Cell viability was measured using
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT; Sigma-Aldrich) assay. Absorbance values were measured at 490
nm using a microplate reader (Bio-Tek ELx800; Bio-Tek Instruments,
Inc., Winooski, VT, USA).
Radiation cell survival assay
CNE-1 cells were seeded into 6-well plates
(5х102 cells/well), incubated overnight, treated with
resveratrol and/or irradiation as described above, then the media
was removed and replaced with fresh resveratrol-free culture
medium. After 15 days, the cells were fixed in ice-cold methanol,
stained with Giemsa solution, photographed and the numbers of
colonies containing at least 50 cells were scored. Cell survival
curves were fitted using the linear-quadratic formula: surviving
fraction = e(−αD - βD2) (17); D represents the dose of irradiation;
α and β are radibiological cell survival parameters.
Cell cycle and apoptosis analysis
CNE-1 cells (5×104 cells/well) in 6-well
plates were treated as described above, trypsinized, fixed and
stained according to the instructions of the Cell Cycle and
Apoptosis kit (no. 559763; BD Biosciences, Franklin Lakes, NJ,
USA). Cell cycle distribution and apoptosis were analyzed using a
Cytomics™ FC500 flow cytometer and CXP analysis software (both from
Beckman-Coulter, Anacortes, WA, USA).
Immunoblotting analysis
CNE-1 cells were treated in 6-well plates, lysed in
RIPA buffer (0.25 M Tris-HCl, pH 6.8, 8% SDS, 1 mM
phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin and 1.0 mg/ml
leupeptin). The cell extracts were separated by 10% SDS-PAGE,
transferred onto PVDF membranes (Millipore, Bedford, MA, USA) and
immunoblotted using anti-E2F transcription factor 1 (anti-E2F1)
(no. ab179445), anti-pan-AKT (phospho T308, no. ab38449),
anti-pan-AKT (no. ab64148), anti-ERK1 (pT202/pY204) + ERK2
(pT185/pY187, no. ab50011), anti-ERK1 + ERK2 (no. ab17942) and
anti-GAPDH (no. ab181602) (all from Abcam, Cambridge, UK).
Recombinant lentivirus construction
and transduction
We inserted the sequence: 5-GTCACGCTATGAGACCTCA-3
into the LV008 lentiviral silencing vector (Forevergen, Guangzhou,
China) to express a short hairpin RNA (shRNA) in order to
effectively knock down human E2F1. To overexpress E2F1, we
subcloned the E2F1 gene (GenBank accession no. GI: 168480109) into
the LV003 lentiviral expression vector (Forevergen). The
recombinant lentiviruses (and corresponding negative controls) were
packaged in 293T cells and used to create stable CNE-1 cells. The
expression of E2F1 in the stable CNE-1 cell lines was validated by
immunoblotting.
Animals
Forty specific pathogen-free BALB/c nude mice (male,
6-weeks-old) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Peking, China). All protocols were approved
by the Animal Experimentation Ethics Committee of Guangzhou
University of Chinese Medicine and in compliance with recommended
NIH guidelines for Care and Use of Animals for scientific
purposes.
In vivo experiments
CNE-1 cells (5×106) in the exponential
phase of growth were subcutaneously injected into the right flank
of BALB/c mice (day 0). The volumes of the xenograft tumors were
measured every 4 days. When the tumors reached 100 mm3
(day 8), the mice were randomly assigned to four groups (n=10 mice
each) and treated as follows: vehicle (5% ethanol and 25%
polyethylene glycol 400 in distilled water); vehicle and 4 Gy
irradiation; resveratrol (50 mg/kg/day); and resveratrol (50
mg/kg/day) plus 4 Gy irradiation. On day 8, mice were treated
intraperitoneally with resveratrol or vehicle until the completion
of the experiment. On day 12, mice were irradiated once a day for
consecutive 3 days. Mice were sacrificed on day 28 to measure tumor
volume and tumor weight.
Statistical analysis
All data are mean ± standard deviation. SPSS 13.0
software was used for statistical analyses (SPSS, Inc., Chicago,
IL, USA). Each in vitro experiment was repeated
independently three times. Differences between groups were compared
using the Students t-test; one-way analysis of variance (ANOVA) was
used for multiple comparisons. Two-tailed P-values <0.05 were
considered statistically significant.
Results
Resveratrol reduces the viability of
CNE-1 NPC cells
To investigate the effect of resveratrol on the
radiosensitivity of NPC cells, we first optimized the resveratrol
dose and treatment time. The MTT assay showed exposure to <100
µM resveratrol for 24 h had no significant effect on CNE-1 cell
viability (Fig. 1A). Next, we
treated CNE-1 cells with 25, 50 or 100 µM resveratrol for 24 h and
assessed cell proliferation using the colony formation assay and
cell cycle analysis. Resveratrol dose-dependently decreased colony
formation and induced cell cycle G1 arrest (Fig. 1B and C). Notably, 50 µM resveratrol
significantly inhibited the proliferation of CNE-1 cells.
Therefore, we pretreated CNE-1 cells with 50 µM resveratrol for 24
h in all subsequent experiments.
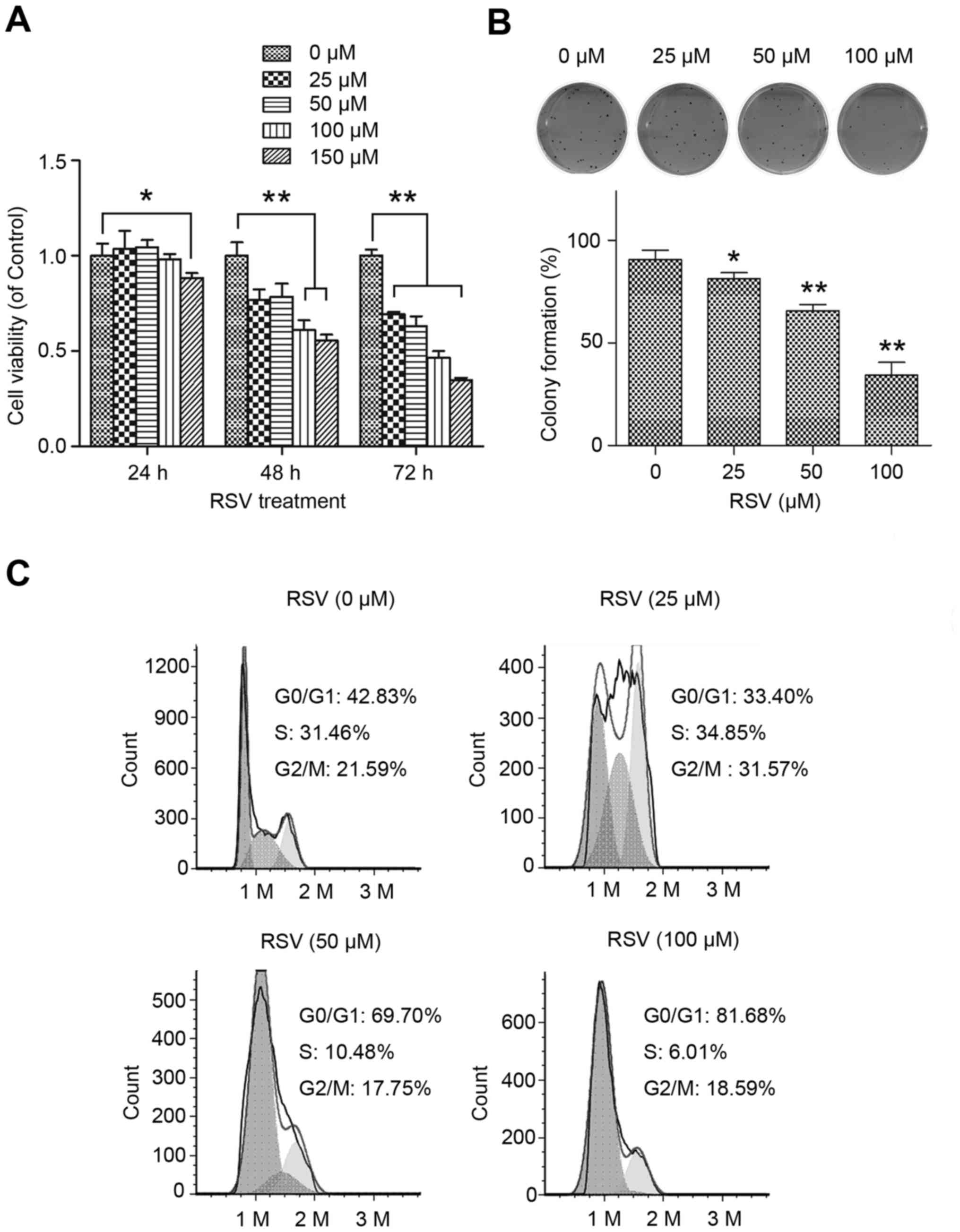 | Figure 1.RSV reduces the viability and
proliferation of NPC cells. (A) Effect of RSV on the viability of
CNE-1 cells. CNE-1 cells were exposed to 0, 25, 50, 100 or 150 µM
RSV for 24, 48 or 72 h, then cell viability was assessed using the
MTT assay. (B and C) Effect of RSV on the proliferation of CNE-1
cells. CNE-1 cells were treated with 0, 25, 50 or 100 µM RSV for 24
h, then analyzed using (B) the colony formation assay and (C) flow
cytometric cell cycle analysis. *P<0.05; **P<0.01. RSV,
resveratrol; NPC, nasopharyngeal cancer. |
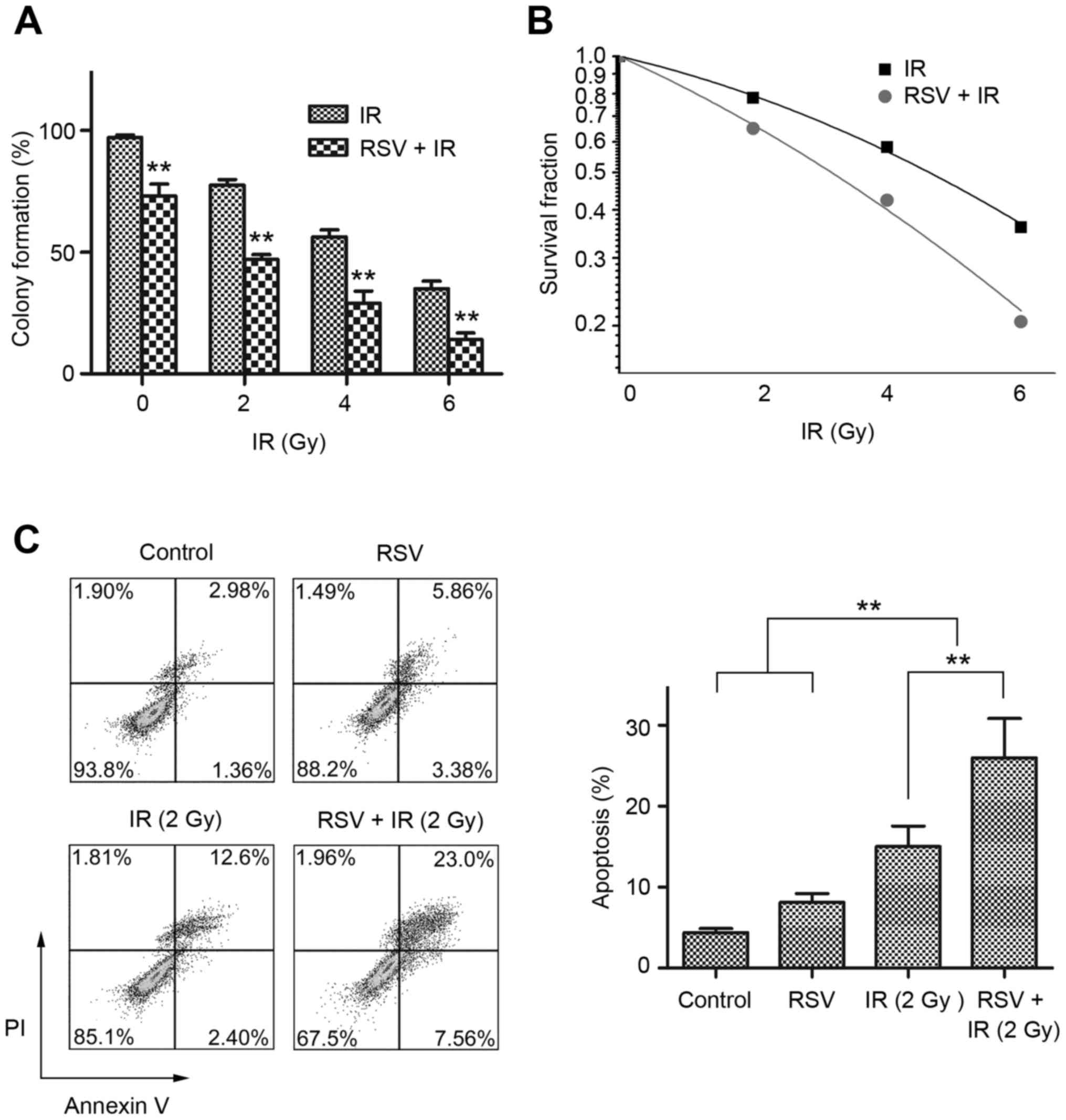
Resveratrol enhances the
radiosensitivity of CNE-1 NPC cells
CNE-1 cells seeded in 6-well plates were treated
with resveratrol for 24 h, then switched to media without
resveratrol, irradiated at 2, 4 or 6 Gy and cultured for 15 days.
Colony formation assays showed the survival rates of the cells
treated with resveratrol and irradiation decreased significantly as
the dose of radiation increased, compared to cells treated with
irradiation alone (P<0.05; Fig.
2A). We applied the linear-quadratic model to fit
radiosensitivity curves for each group; resveratrol-treated cells
were more sensitive to irradiation than untreated cells [survival
rate after irradiation with 2 Gy (SF2): 65.01±6.78% vs.
78.01±4.89%, P=0.03; Fig. 2B].
Radiosensitivity can also be evaluated using the radiobiological
parameters α derived from the linear-quadratic formula. The α-value
indicates radiosensitivity. When resveratrol-treated and untreated
cells were compared, the α-value increased (0.09±0.01 vs.
0.18±0.02, P=0.004), demonstrating pretreatment with resveratrol
enhanced the radiosensitivity of NPC cells.
To further assess the effects of resveratrol and/or
irradiation on NPC cells, CNE-1 cells treated as described above
were stained using Annexin V/PI. Flow cytometric analysis showed
(Fig. 2C) resveratrol did not
significantly increase the apoptotic rate compared to control
cells. Although irradiation alone induced an apoptotic rate of 15%,
the apoptotic rate of CNE-1 irradiated cells pretreated with
resveratrol was ~30% (P<0.01), indicating that pretreatment with
resveratrol enhances the cell-killing effect of irradiation in NPC
cells.
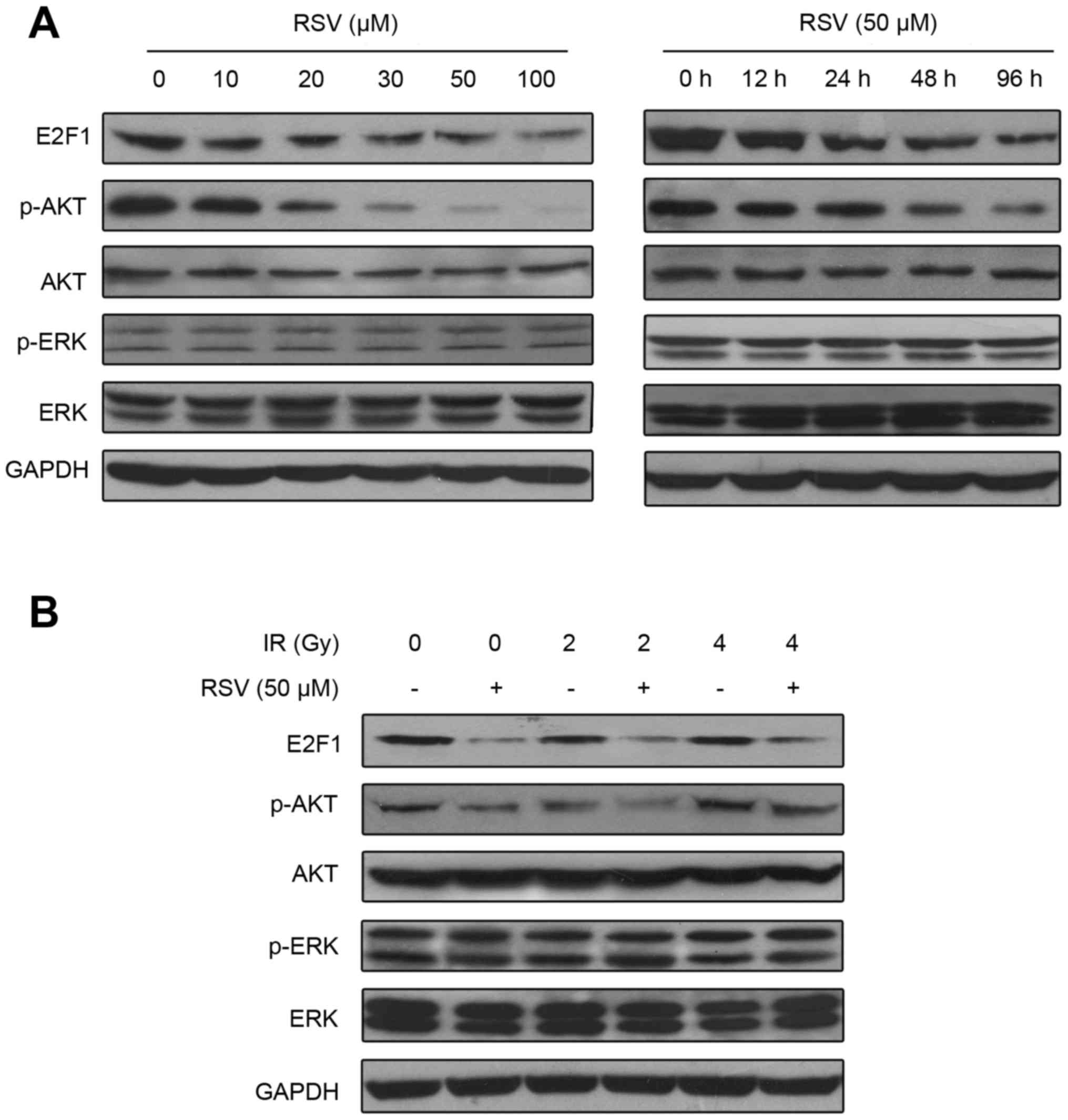
Resveratrol downregulates E2F1 in
CNE-1 NPC cells
As resveratrol dose-dependently induced G0/G1 cell
cycle arrest, we next examined the expression of E2F1, a major
protein that regulates G0/G1 phase (18). CNE-1 cells were pretreated with
resveratrol for 24 h then irradiated at 0, 2 or 4 Gy.
Immunoblotting revealed resveratrol downregulated E2F1 in a dose-
and time-dependent manner (Fig. 3A)
in both non-irradiated and irradiated cells (Fig. 3B). Notably, p-AKT was simultaneously
downregulated by resveratrol, though the expression of p-ERK did
not change. These results suggest resveratrol may inhibit p-AKT by
downregulating E2F1.
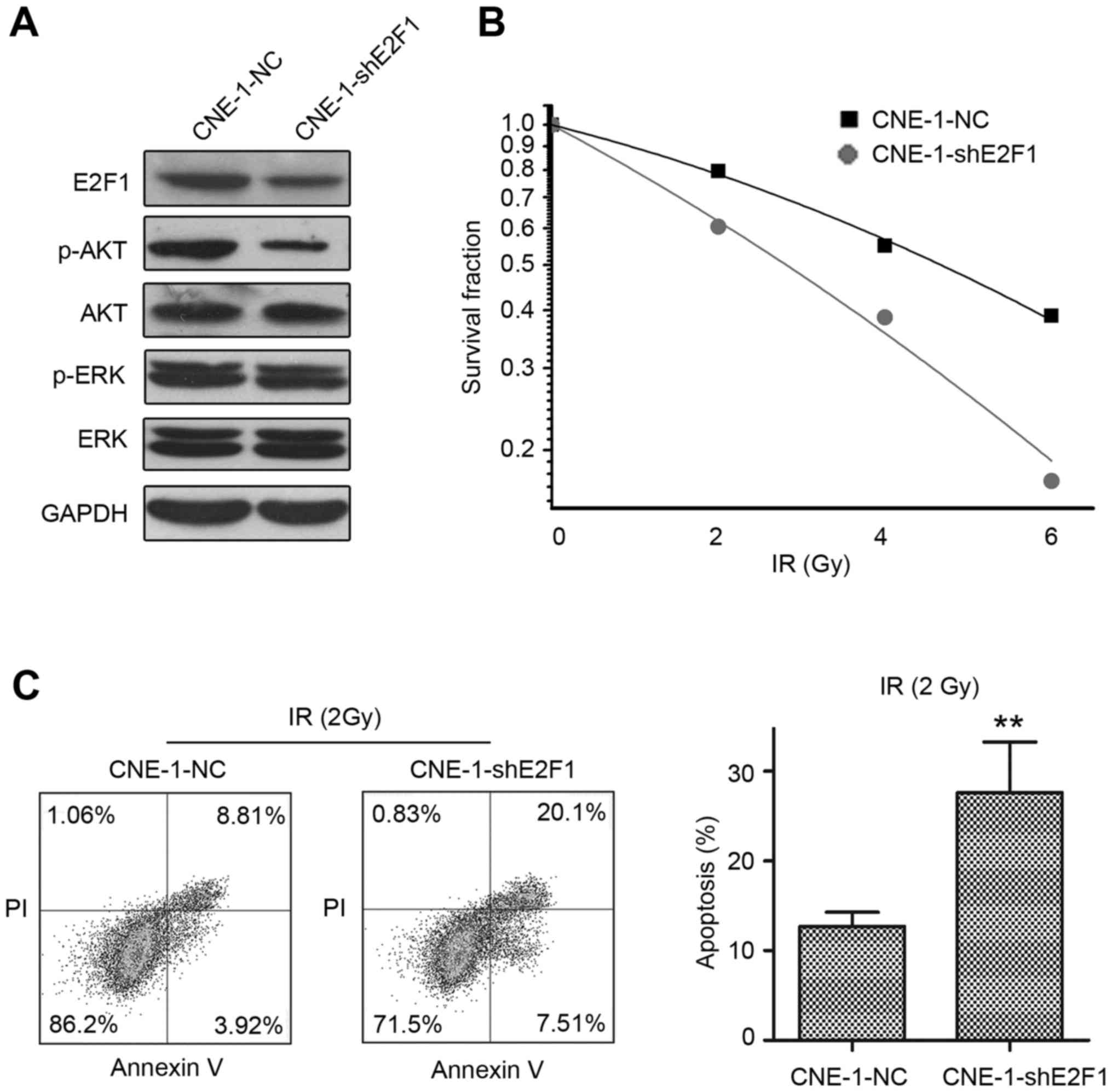
Knockdown of E2F1 enhances the
radiosensitivity of CNE-1 NPC cells
To elucidate whether E2F1 regulates the
radiosensitivity of NPC cells, we knocked down E2F1 using a
lentiviral system; immunoblotting confirmed E2F1 was downregulated
and furthermore, p-AKT was concomitantly reduced (Fig. 4A). As E2F1 regulates the G0/G1
progression of the cell cycle (18), we performed cell cycle analysis by
PI staining and flow cytometry. Stable knockdown of E2F1
(CNE-1-shE2F1) increased the percentage of cells in the G0/G1 phase
compared to control CNE-1 cells (CNE-1-NC). Furthermore, the colony
formation assay indicated that the depletion of E2F1 reduced the
colony formation rate (data not shown), indicating E2F1 plays an
important role in the proliferation of NPC cells (Fig. 4B).
Next, CNE-1-NC and CNE-1-shE2F1 cells were seeded in
6-well plates, treated with or without resveratrol, irradiated at
0, 2, 4 or 6 Gy, cultured for 15 days, then subjected to the colony
formation assay (Fig. 4A). Based on
the clone formation rate, we applied the linear-quadratic model to
fit radiosensitivity curves for each group (Fig. 4B). CNE-1-shE2F1 cells had a higher
α-value (0.22±0.03 vs. 0.10±0.01, P=0.002) and lower SF2
(60.45±3.24% vs. 79.51±5.42%, P=0.03) than control cells,
demonstrating knockdown of E2F1 enhanced the radiosensitivity of
CNE-1 cells.
CNE-1-NC and CNE-1-shE2F1 were seeded in 6-well
plates, irradiated at 2 Gy and cultured for 72 h. Annexin V/PI
double staining and flow cytometric analysis (Fig. 4C) revealed CNE-1-shE2F1 cells had a
significantly higher apoptotic rate than CNE-1-NC cells.
Collectively, the data indicate that knockdown of E2F1 enhanced the
cell-killing effect of irradiation in NPC cells.
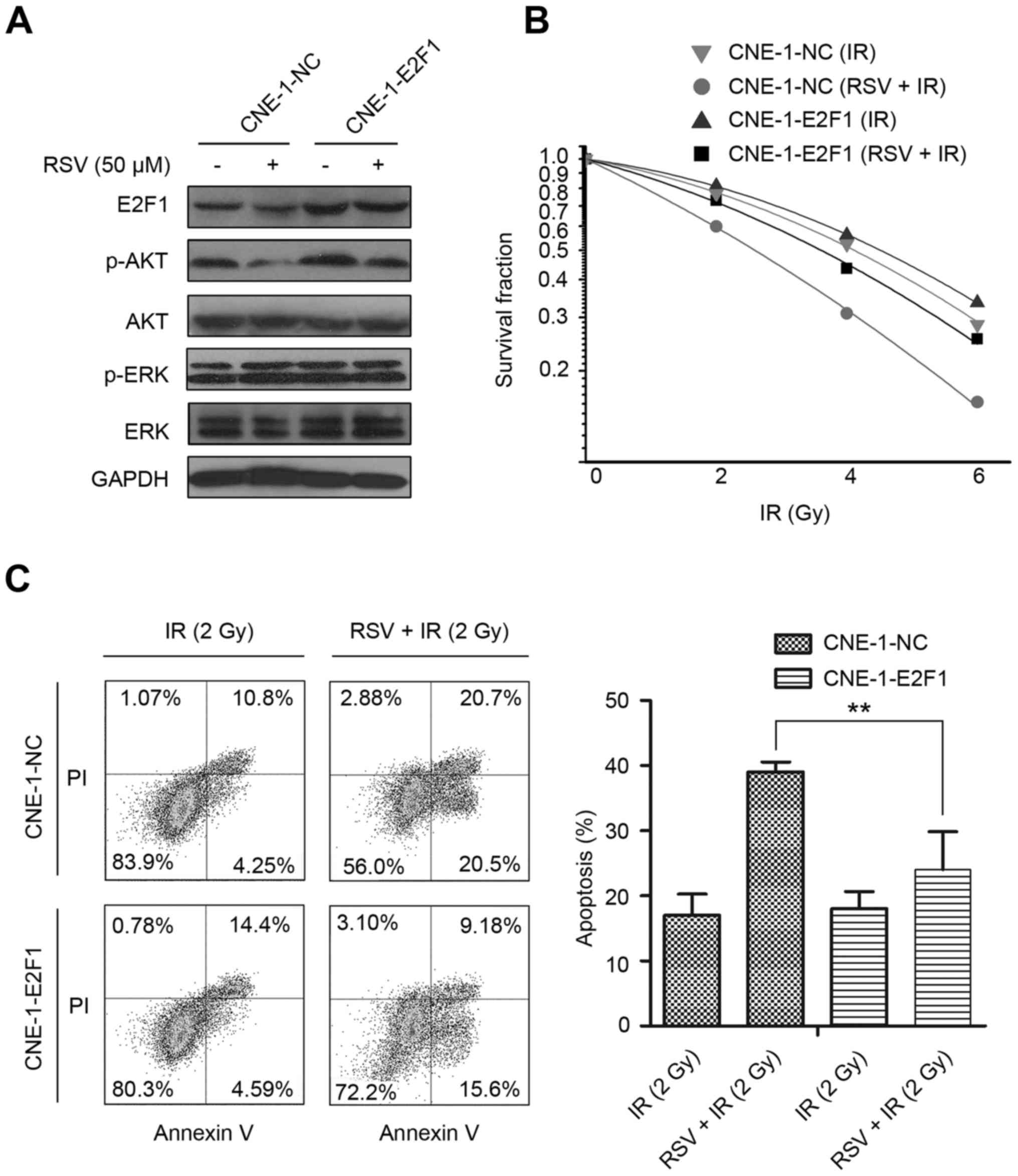
Overexpression of E2F1 reverses
resveratrol-induced radiosensitivity in CNE-1 NPC cells
To confirm if E2F1 is involved in the
resveratrol-induced radiosensitivity of CNE-1 cells, we established
an E2F1-overexpressing stable CNE-1 cell line (CNE-1-E2F1) and
control cells (CNE-1-NC) using a lentiviral system. Immunoblotting
revealed E2F1 was overexpressed in CNE-1-E2F1 cells cultured in
resveratrol-free media and resveratrol for 24 h compared to the
respective CNE-1-NC cells (Fig.
5A). The colony formation assay and cell cycle analysis
demonstrated resveratrol decreased the colony formation rate and
induced G0/G1 cell cycle arrest in CNE-1-NC cells, whereas
overexpression of E2F1 reversed the ability of resveratrol to
inhibit colony formation and induce cell cycle arrest in CNE-1-E2F1
cells (data not shown).
Next, we pretreated CNE-1-NC and CNE-1-E2F1 cells
seeded in 6-well plates with resveratrol for 24 h, irradiated the
cells at 0, 2, 4 and 6 Gy, and then returned to the incubator for
15 days in resveratrol-free culture media. Based on the colony
formation rate, we applied the linear-quadratic model to plot
radiosensitivity curves (Fig. 5B).
Compared to CNE-1-NC cells, CNE-1-E2F1 cells had a lower α-value
(0.13±0.02 vs. 0.23±0.01, P=0.0006) and higher SF2 value
(73.01±3.48% vs. 59.94±6.05%, P=0.01), demonstrating overexpression
of E2F1 reversed resveratrol-induced radiosensitivity.
Finally, CNE-1-NC and CNE-1-E2F1 were seeded into
6-well plates, pretreated with resveratrol for 24 h, irradiated at
2 Gy, and then cultured for 72 h. Annexin V/PI double staining and
flow cytometry showed CNE-1-E2F1 cells had a significantly higher
rate of apoptosis than CNE-1-NC cells (Fig. 5C), indicating that overexpression of
E2F1 impaired the cell-killing effect of resveratrol plus
irradiation in CNE-1 cells.
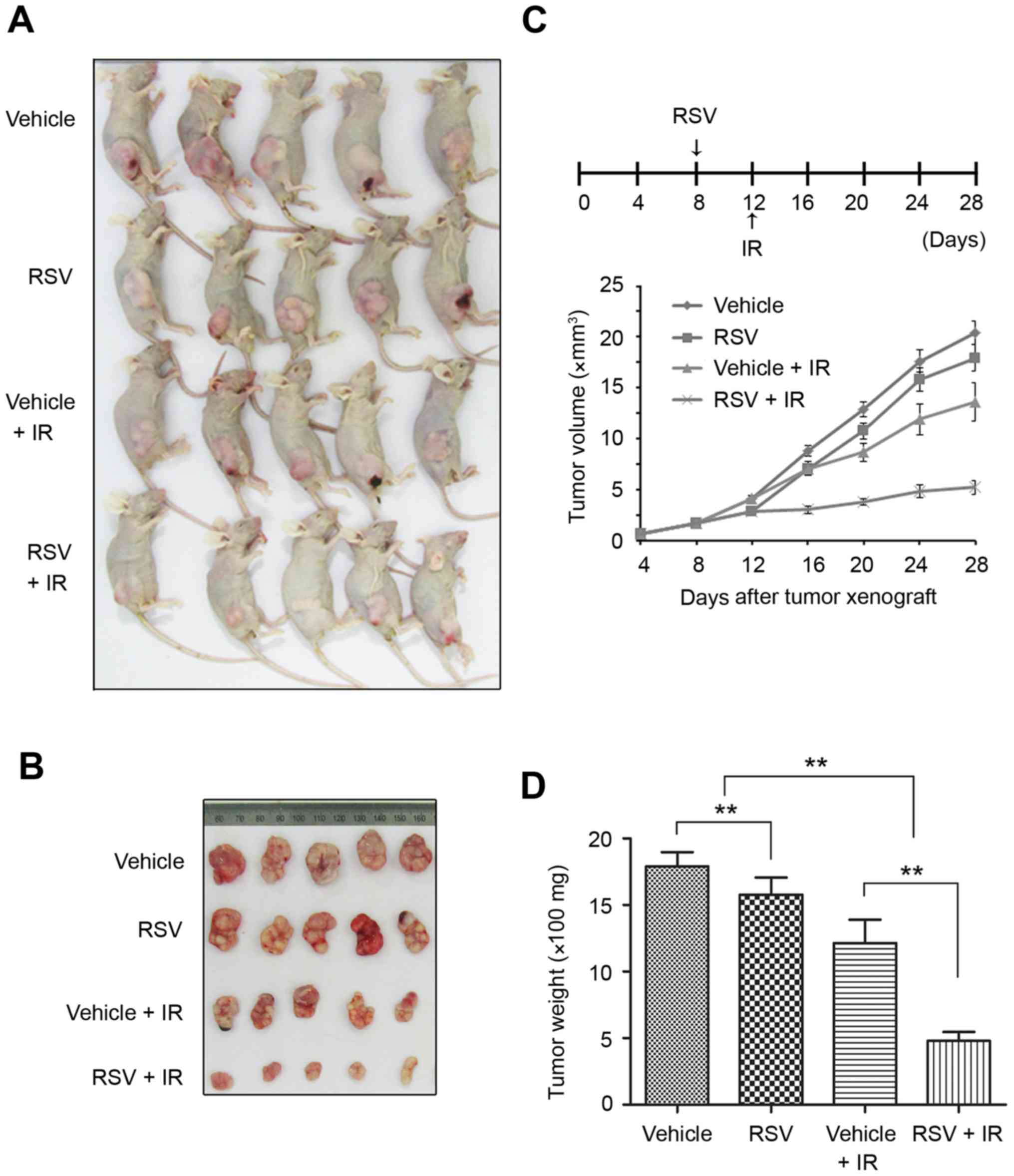
Resveratrol and irradiation
synergistically inhibit NPC xenograft tumor growth in vivo
To assess resveratrol-induced radiosensitivity in
vivo, we established a xenograft model of NPC in BALB/c nude
mice. When the volume of tumors reached ~0.1 cm3 (day
8), the mice were intraperitoneally injected with resveratrol (50
mg/kg/day) or vehicle until the completion of the experiment. On
day 12, tumor-bearing mice were irradiated (4 Gy/day) for
consecutive 3 days. On sacrifice (day 28), the weight of the mice
was not significantly different between groups (data not
shown).
Compared to the control group, tumor-bearing nude
mice exposed to irradiation alone exhibited a 33% reduction in
tumor volume (1360.69±188.45 vs. 2034.86±112.96 mm3,
P<0.01) and 32% decrease in tumor weight (1213.75±163.87 vs.
1789.44±101.85 mg, P<0.01; Fig.
6). Although resveratrol alone had a certain antitumor effect,
the combinatorial therapy of resveratrol and irradiation resulted
in a 74% reduction in tumor volume (520.13±68.51 vs. 2034.86±112.96
mm3, P<0.01) and 73% reduction in tumor weight
(479.22±62.84 vs. 1789.44±101.85 mg, P<0.01; Fig. 6) compared to the control group.
These results indicate that administration of resveratrol enhanced
the radiosensitivity of the NPC xenograft tumors in
vivo.
Discussion
Radioresistance can lead to local recurrence and
distant metastasis and is associated with a poor prognosis,
especially in advanced stage NPC (19). Due to its antioxidant and anti-aging
properties, resveratrol has become a popular healthcare product for
cardiovascular protection (20,21).
This study demonstrated that resveratrol enhances the
radiosensitivity of NPC cells in vitro and in vivo
via a mechanism involving E2F1 and its downstream target p-AKT.
Therefore, resveratrol combined with irradiation may represent a
promising strategy to further improve the outcomes of patients with
NPC.
Resveratrol is reported to exert a diverse range of
effects, low concentrations (0.1–2.5 µM) promoted the viability and
proliferation of human umbilical cord mesenchymal stem cells while
higher concentrations had the opposite effects (22). High-dose resveratrol also exerts
cytotoxic effects in a variety of cancer cell lines (23,24).
However, to achieve effective and desirable outcomes with minimum
toxicity to normal cells, a clinical resveratrol and irradiation
regime would need to be designed very carefully. Although different
types of cancer cells respond to resveratrol and irradiation in a
varied manner, the existing studies all suggested that the highest
concentration of 100 mM resveratrol and highest dose of 5 Gy
irradiation are sufficient to kill cells by induction of apoptosis
(25). In this study, we pretreated
CNE-1 NPC cells with 50 µM resveratrol for 24 h before irradiation.
This moderate dose of resveratrol did not affect the cell viability
of CNE-1 cells, but did significantly reduce the proportion of S
phase cells. Therefore, this non-toxic dose of resveratrol may have
the potential to increase the radiosensitivity of NPC cells, as S
phase cells normally exhibit resistance to irradiation therapy
(16).
Resveratrol and irradiation have been reported to
modulate target molecules that inhibit proliferation and induce
apoptosis. A number of proliferation or apoptosis-related molecules
such as cyclins, p53, p21, NF-κB and Bcl-2 have been reported to be
involved in the resveratrol-mediated radiosensitivity of cancer
cells (26–28).
This study showed resveratrol could also arrest NPC
cells in the G0/G1 phase. Therefore, we examined the expression of
E2F1, a key molecule that regulates the G0/G1 progression (18). Resveratrol downregulated E2F1 in
CNE-1 cells in a dose- and time-dependent manner. Furthermore,
knocking down E2F1 using RNA interference inhibited proliferation
and increased the sensitivity of CNE-1 cells to irradiation
(Fig. 4). Conversely,
overexpression of E2F1 reversed resveratrol-induced
radiosensitivity (Fig. 5),
demonstrating E2F1 is required to mediate resveratrol-induced
radiosensitivity in CNE-1 cells. E2F1 regulates AKT and ERK
(29,30). Immunoblotting revealed resveratrol
upregulated p-AKT, but not p-ERK, indicating E2F1 modulates
resveratrol-induced radiosensitivity in NPC cells by activating the
AKT pathway. Further investigation is required to confirm this
observation.
In conclusion, we demonstrated that a moderate dose
of resveratrol sensitizes NPC cells to irradiation both in
vitro and in vivo. Furthermore, E2F1 and its downstream
target p-AKT are major regulators of resveratrol-induced
radiosensitivity. This fundamental research provides a starting
point for further studies to assess the clinical application of
resveratrol as a radiosensitizer in order to improve the treatment
outcomes of radiotherapy in patients with NPC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81274145) and the Ministry
of Education, Science Technology Development Center (no.
20124425120012).
References
|
1
|
Jia WH, Huang QH, Liao J, Ye W, Shugart
YY, Liu Q, Chen LZ, Li YH, Lin X, Wen FL, et al: Trends in
incidence and mortality of nasopharyngeal carcinoma over a 20–25
year period (1978/1983-2002) in Sihui and Cangwu counties in
southern China. BMC Cancer. 6:1782006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su SF, Han F, Zhao C, Chen CY, Xiao WW, Li
JX and Lu TX: Long-term outcomes of early-stage nasopharyngeal
carcinoma patients treated with intensity-modulated radiotherapy
alone. Int J Radiat Oncol Biol Phys. 82:327–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao WW, Han F, Lu TX, Chen CY, Huang Y
and Zhao C: Treatment outcomes after radiotherapy alone for
patients with early-stage nasopharyngeal carcinoma. Int J Radiat
Oncol Biol Phys. 74:1070–1076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou J, Wang L, Xu X, Tu Y, Qin S and Yin
Y: Antitumor activity of Endostar combined with radiation against
human nasopharyngeal carcinoma in mouse xenograft models. Oncol
Lett. 4:976–980. 2012.PubMed/NCBI
|
|
5
|
Lee AW, Ng WT, Hung WM, Choi CW, Tung R,
Ling YH, Cheng PT, Yau TK, Chang AT, Leung SK, et al: Major late
toxicities after conformal radiotherapy for nasopharyngeal
carcinoma-patient- and treatment-related risk factors. Int J Radiat
Oncol Biol Phys. 73:1121–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akervall J, Nandalur S, Zhang J, Qian CN,
Goldstein N, Gyllerup P, Gardinger Y, Alm J, Lorenc K, Nilsson K,
et al: A novel panel of biomarkers predicts radioresistance in
patients with squamous cell carcinoma of the head and neck. Eur J
Cancer. 50:570–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arichi H, Kimura Y, Okuda H, Baba K,
Kozawa M and Arichi S: Effects of stilbene components of the roots
of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem
Pharm Bull (Tokyo). 30:1766–1770. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soleas GJ, Diamandis EP and Goldberg DM:
Resveratrol: a molecule whose time has come? and gone? Clin
Biochem. 30:91–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bradamante S, Barenghi L and Villa A:
Cardiovascular protective effects of resveratrol. Cardiovasc Drug
Rev. 22:169–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das S and Das DK: Anti-inflammatory
responses of resveratrol. Inflamm Allergy Drug Targets. 6:168–173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Lastra CA and Villegas I:
Resveratrol as an anti-inflammatory and anti-aging agent:
mechanisms and clinical implications. Mol Nutr Food Res.
49:405–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Z, Liu B, E C, Liu J, Zhang Q, Liu J,
Chen N, Chen R and Zhu R: Resveratrol inhibits the proliferation of
human melanoma cells by inducing G1/S cell cycle arrest and
apoptosis. Mol Med Rep. 11:400–404. 2015.PubMed/NCBI
|
|
14
|
Yu XD, Yang JL, Zhang WL and Liu DX:
Resveratrol inhibits oral squamous cell carcinoma through induction
of apoptosis and G2/M phase cell cycle arrest. Tumour Biol.
37:2871–2877. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trung L Quoc, Espinoza JL, Takami A and
Nakao S: Resveratrol induces cell cycle arrest and apoptosis in
malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS One.
8:e551832013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fowler JF: The linear-quadratic formula
and progress in fractionated radiotherapy. Br J Radiol. 62:679–694.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong JV, Dong P, Nevins JR, Mathey-Prevot
B and You L: Network calisthenics: control of E2F dynamics in cell
cycle entry. Cell Cycle. 10:3086–3094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee AW, Sze WM, Au JS, Leung SF, Leung TW,
Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al: Treatment results
for nasopharyngeal carcinoma in the modern era: the Hong Kong
experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das DK and Maulik N: Resveratrol in
cardioprotection: a therapeutic promise of alternative medicine.
Mol Interv. 6:36–47. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pervaiz S and Holme AL: Resveratrol: its
biologic targets and functional activity. Antioxid Redox Signal.
11:2851–2897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Ma S, Meng N, Yao N, Zhang K, Li
Q, Zhang Y, Xing Q, Han K, Song J, et al: Resveratrol exerts
dosage-dependent effects on the self-renewal and neural
differentiation of hUC-MSCs. Mol Cells. 39:418–425. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
García-Zepeda SP, García-Villa E,
Díaz-Chávez J, Hernández-Pando R and Gariglio P: Resveratrol
induces cell death in cervical cancer cells through apoptosis and
autophagy. Eur J Cancer Prev. 22:577–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong W, Yin A, Mao X, Zhang W, Huang H
and Zhang X: Resveratrol suppresses human glioblastoma cell
migration and invasion via activation of RhoA/ROCK signaling
pathway. Oncol Lett. 11:484–490. 2016.PubMed/NCBI
|
|
25
|
Kma L: Synergistic effect of resveratrol
and radiotherapy in control of cancers. Asian Pac J Cancer Prev.
14:6197–6208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang Y, DeMarco VG and Nicholl MB:
Resveratrol enhances radiation sensitivity in prostate cancer by
inhibiting cell proliferation and promoting cell senescence and
apoptosis. Cancer Sci. 103:1090–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao HF, Kuo CD, Yang YC, Lin CP, Tai HC,
Chen YY and Chen YJ: Resveratrol enhances radiosensitivity of human
non-small cell lung cancer NCI-H838 cells accompanied by inhibition
of nuclear factor-kappa B activation. J Radiat Res (Tokyo).
46:387–393. 2005. View Article : Google Scholar
|
|
28
|
Lu KH, Chen YW, Tsai PH, Tsai ML, Lee YY,
Chiang CY, Kao CL, Chiou SH, Ku HH, Lin CH, et al: Evaluation of
radiotherapy effect in resveratrol-treated medulloblastoma cancer
stem-like cells. Childs Nerv Syst. 25:543–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaussepied M and Ginsberg D:
Transcriptional regulation of AKT activation by E2F. Mol Cell.
16:831–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korotayev K, Chaussepied M and Ginsberg D:
ERK activation is regulated by E2F1 and is essential for
E2F1-induced S phase entry. Cell Signal. 20:1221–1226. 2008.
View Article : Google Scholar : PubMed/NCBI
|