ACC is a very rare tumor accounting for 0.7–2.0
cases/million people per year with an increased incidence in the
first and fourth-fifth decades of life. By gender, females are the
most affected (55–60%) (1). ACC is
burdened by a poor prognosis with a mean 5-year survival rate
between 16 and 47%, falling to 5–10% in the advanced stages
(2). The cornerstones in the
pathogenesis of ACC are considered to be the genetic alterations of
the IGF-2, p53 and β-catenin molecular pathways (2,3).
Additionally, other genes, such as ZNFR3, identified by a
genome-wide study, appear potentially involved in the tumorigenesis
of ACC (4). Comparative genomic
hybridization (CGH) demonstrated several complex mutations in ACC
with chromosomal gains at 4q, 4p16, 5p15, 5q12-13, 9q34, 12q13,
12q24, 19p and losses at 1p, 2q, 11q, 17p, 22p and 22q. Genes
within these regions that are potentially involved in neoplastic
transformation include fibroblast growth factor 4 (FGF4),
cyclin-dependent kinase 4 (CDK4), and cyclin E1
(CCNE1) (1). A recent
epigenetic study performed on 51 ACCs identified a promoter
hypermethylation of the H19, GOS2, PLAGLI and NDRG2
genes (1). However, it has been
recently observed that the dysregulation of some miRNAs, such as
the upregulation of miR-483 and the downregulation of miR-195 and
miR-335, could play a substantial role in the ACC tumorigenesis
(5).
When ACC manifests as a condition of steroid hormone
excess, the clinical picture is dominated mainly by
hypercortisolism and/or hyperandrogenism, whereas symptoms of
estrogen hypersecretion such as gynaecomastia and testicular
atrophy are pathognomonic in male patients (6). DHEA-S represents a possible hormonal
marker of ACC, conversely a decreased serum DHEA-S concentration
likely indicates an adrenal adenoma (7). Mineralocorticoid excess is a rare
event, that occurs with severe hypertension and hypokalemia.
Notably however, an excess of glucocorticoids could produce a
similar effect (6). Although few
ACC appear non-secreting, they may produce an excessive amount of
adrenal precursors (8). The
diagnostic employement of chromatography/mass spectrometry methods
revealed that >95% of ACC patients are able to autonomously
secrete steroids or steroid precursors (9). Imaging plays a key role in the
diagnosis of primary ACC, in the involvement of surrounding tissues
and in its spread to distance sites. Either computerized tomography
(CT) or magnetic resonance imaging (MRI) exploiting particular
features such as Hounsfield unit (HU) values or chemical shift
imaging, respectively allow adequate diagnostic accuracy to be
achieve (10,11) as suggested by a recent analysis of
the German ACC registry showing that the value of 13 HU may be
considered as the threshold for discriminating benign from
malignant adrenal masses (12).
More recently, the fluorine 18 fluorodeoxyglucose
(18F-FDG) positron emission tomography (PET) or PET/CT
was introduced as a diagnostic tool for ACC (13). 11C-metomidate, due to its
particular ability to bind 11 β-hydroxylase, has been proposed for
the identification of tumors of adrenocortical origin (14). The introduction of
[123I]IMTO for single photon emission computed
tomography (SPECT) and planar scintigraphy has provided a
diagnostic alternative to PET for the discrimination of adrenal
masses from non-adrenal tissues (15).
The first official TNM classification for ACC was
established only in 2004 by the International Union Against Cancer
(UICC) and the World Health Organization (WHO) (16). It was based on the criteria
described by MacFarlane (17) and
later modified by Sullivan et al (18). A significant improvement in the
prognostic assessment was due to the adoption of the ENSAT
(European Network for the Study of Adrenal Tumors) ACC staging
system, which proposes a careful prognostic differentiation among
the stages (19) (Table I). The use of this system in recent
years has greatly improved the diagnostic accuracy and the
prediction of survival for stage compared to the criteria
previously adopted (20).
ACC is a neoplastic disease with a poor prognosis.
Current studies in this field have indicated the need for a
multidisciplinary approach in the management of this tumor
(21,22). Surgery remains the most effective
treatment choice for the primary tumor or in for the removal of
isolated metastases (1,23). The experience that at least
one-third of patients show loco-regional recurrence or distant
metastases even after a radical surgical excision introduced the
concept of adjuvant therapy in these patients (24). Despite an extensive surgical
resection, the survival rate of these patients is estimated as ~50%
after 5 years (25). Although these
data support the need for an adjuvant cancer therapy, the
therapeutic options in ACC currently remain under debate. At
present, mitotane represents the only drug approved in Europe and
in the United States for ACC treatment; however opinions regarding
its use in adjuvant settings are still highly discordant (26). Currently, chemotherapy is reserved
for those cases of advanced disease with evidence of distant
metastases unresponsive to mitotane treatment. Many efforts are
directed to the development of targeted therapy in ACC. Several
strategies have been developed in vitro and some clinical
trials have been conducted with small molecules, such as inhibitors
of tyrosine kinase receptors or serine/threonine kinase receptors
and monoclonal antibodies.
Surgery is the only truly effective therapy in the
treatment of ACC. A complete surgical resection (R0) is the
treatment of choice, avoiding tumor spread that is considered an
adverse prognostic factor. The achievement of R0 resection status
often requires a radical surgery with a wide dissection of the
neighboring organs. It represents a predictor of long-term survival
(27). The choice of an open
approach vs laparoscopic approach is debated. Open adrenalectomy is
classically the more secure treatment recommended in patients with
localized (stage I–II) and locally advanced (stage III) ACC.
Comparative data concerning the two surgical techniques are lacking
and originate from retrospective data that involved selection bias
(28,29). It is likely that laparoscopic
surgery might be reserved only for selected cases with masses of
small size. However, these statements must be confirmed by
prospective trials. Regardless of the surgical option chosen, the
surgical team must have proven experience in the oncologic ACC
surgery.
Although lymphadenectomy has never been considered
as a standard procedure in the adrenalectomy, recent studies show
that lymph nodes dissection is significantly associated with a
reduction of the relapse rate in patients with localized disease
(30,31). However, confirmatory data are needed
in order to standardize the surgical procedure.
The therapeutic option of removing metastases is
founded on the observation that their excision is associated with
long-term survival (32,33) and the consideration that many ACC
are metastatic at the onset (34).
Encouraging results from several retrospective studies show that
the metastasectomy correlates with an improvement of
progression-free and overall survival (35,36).
Finally, although the objective of debulking surgery is to reduce
either the compressive effect exerted by a large size mass, on
surrounding organs or the hormonal excess secreted by the tumor,
lacking in this surgical approach is an oncological rationale
(23).
Currently, mitotane represents the only therapeutic
option approved by the US Food and Drug Administration and European
Medicine Executive Agency for the treatment of ACC (37). Mitotane is a derivative of the
insecticide dichlorodiphenyldichloroethane (DDT) with adrenolytic
and cytotoxic activity toward the fasciculata and reticularis
adrenal areas. It inhibits steroidogenic pathways acting mainly at
the level of the cholesterol side chain cleavage enzymes CYP11A1
and CYP11B1 (38,39). Mitotane metabolites (o',p-DDA and
o',p-DDE, respectively) are the products of a hydroxylation that
occurs in the liver and of which o',p-DDA represents the active
form (40). It has indeed been
shown, that o',p-DDA measurements reflect the mitotane response in
treated patients (41). Drug
administration is oral, with the aim of reaching the therapeutic
target between 14 and 20 mg/dl (41,42)
above which side effects involving the gastrointestinal tract and
central nervous system frequently manifest (43). A recent study showed that blood
mitotane concentrations ≥14 mg/l were associated with a prolonged
recurrence-free survival (RFS) in patients following
macroscopically radical surgery (44). Furthermore, the measurement of
plasma mitotane levels in the management of patients with ACC is
mandatory. Different treatment strategies have been proposed to
achieve the therapeutic dose even if the high-dose regimen appears
to be the most effective for reaching the target concentration more
rapidly (43,45). Mitotane has been shown to be an
inducer of hepatic cytochrome CYP3A4 and thus able to interfere
with the metabolism of other drugs including chemotherapeutic
agents (46). This drug property
complicates the management of other codition-related treatments,
such as antihypertensives, statins, antibiotics and others, that
are also being used in ACC patients (47). Furthermore, future protocols
involving mitotane and antineoplastic drugs in combination should
consider this particular drug feature.
Several studies regarding mitotane use in the
clinical setting of ACC have reported conflicting results. However,
the retrospective nature of these studies exposes them to numerous
biases, mainly related to the different concentrations used and the
lack of mitotane plasma level measurements. In fact, the response
rate, as evaluated in series in which mitotane treatment was given
without considering plasma concentration and those with patients in
whose the drug concentration had been assessed, ranged from 25 to
55% respectively (40,41,48,49).
An extensive retrospective case-control study performed on two
independent cohorts showed significantly improved RFS compared to
untreated control patients (p<0.0001). Overall survival (OS) was
110 months in the mitotane group vs 52 and 67 months in the control
group (p=0.01) (50).
These data have allowed the introduction of the
concept of using mitotane in the adjuvant therapy of patients
affected by ACC. Currently, an international, multicentric,
prospective, randomized trial, called ADIUVO (http://www.adiuvo-trial.org/), designed to evaluate
the effectiveness of mitotane in adjuvant therapy is ongoing.
The recent ESMO guidelines recommend adjuvant
mitotane treatment in stage III patients with potential residual
disease (R1 or Rx resection status) and Ki-67 >10%. For patients
in stages I or II, R0 resection and Ki-67 <10%, adjuvant
mitotane therapy is not considered mandatory. Mitotane should be
administered progressively to reach the dose of 6 g/day over 4–6
days, adjusting the dosage according to the patient's tolerance and
the plasma drug level (51).
Among the different available treatment protocols
for advanced ACC, chemotherapy is offered in combination with
mitotane. The rationale of this combination is related to the
ability of mitotane to overcome the drug-resistance induced by
P-glycoprotein which is widely expressed in ACC (52). Several chemotherapeutic agents, such
as adriamycin, cisplatin, doxorubicin, and others have been used
alone or in combination with mitotane in the treatment of advanced
ACC (53–56). Although variable percentages have
been reported, the results from these studies demonstrate that
cisplatin alone or in combination with etoposide have a higher
effectiveness in advanced ACC (55,57–59).
The First International Randomized Trial in Locally Advanced and
Metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT) clearly
confirmed the advantage of the regimen of
etoposide-doxorubicin-cisplatin (EDP) in combination with mitotane
(Berruti et al protocol) (53) compared to streptozotocin plus
mitotane (Khan et al protocol) (54) (Table
II). In Berruti et al study, an overall response rate of
48.6% was achieved (53), whereas
in the Khan et al regimen, the response rate was 36.4%
(54). According to these results,
the International Consensus Conference on Adrenal Cancer of Ann
Arbor recommended the use of these protocols as first-line regimens
against metastatic ACC in 2003 (37). Despite the expectations, no
significant differences were found in OS (median 14.8 vs 12 months,
respectively). Similarly, quality of life and adverse events were
comparable in patients receiving the two treatments, thus
confirming the poor prognosis of patients with advanced ACC
(60). Gemcitabine alone or in
combination with mitotane demonstrated a good efficacy in
vitro and its effectiveness was dependent on the sensitivity of
the ACC cells to mitotane (61).
The effectiveness of radiotherapy in ACC has been
extensively debated. A retrospective analysis from the German ACC
Registry, demonstrated that adjuvant radiotherapy resulted in a
significantly better 5-year RFS, but did not affect OS and
disease-free survival (DFS) (62).
In a recent retrospective study from the United States,
radiotherapy was reported to decrease the risk of local failure 4.7
times compared with surgery alone (63). In contrast another retrospective
study did not find a difference between adjuvant radiotherapy and
surgery alone (64). Some in
vitro studies support the potential combination of mitotane and
radiotherapy. In fact, these studies reported an inhibitory effect
of mitotane in association with ionizing radiations on ACC cell
lines (65,66). Considering the current data,
radiotherapy is intended for patients with R1 or Rx resection
status with a high risk for local recurrence (67). However, the potential use of this
method alone or in combination with other therapy should be
investigated in future prospective clinical trials.
The failure of conventional therapies in advanced
ACC and recent knowledge regarding the molecular pathways,
involving oncosuppressor genes, such as TP53, CDKN1C, CDKN2A
and MEN1, and oncogenes such as IGF2, CTNNB1 and
RAS involved in this malignancy have encouraged many efforts
in developing new strategies against ACC (2).
Overexpression of insulin-like growth factor-2
(IGF-2) is the most important molecular event occurring in >90%
of ACCs (3). Its hypersecretion
induces an uncontrolled activation of the PI3K/Akt/mTOR pathway by
IGF-1R (68). Preclinical in
vitro and in vivo studies on xenograft models showed
that NVP-AEW541, a small molecule inhibitor, and IMC-A12, a human
monoclonal antibody, were able to reduce cell proliferation,
inhibiting the IGF-2 downstream pathway. The association of both
molecules with mitotane sinergistically inhibited tumor growth
(69,70). Two phase I studies have demonstrated
the effectiveness of figitumumab, a monoclonal anti-IGF-1R antibody
and linsitinib (OSI-906), a tyrosine kinase inhibitor binding
IGF-1R inducing a clinical response in 57 and 33% of patients,
respectively (71,72). Recently, the results of a phase III
study to evaluate the therapeutic potential of OSI-906 were
published, which were disappointing (73). An association was found between
IGF2 overexpression, mTOR hyper-activation and reduced
expression of miR-99a and miR-100 (74). A role of mTOR in normal and adrenal
tumors has been demonstrated by several studies (74,75)
and its inhibition by everolimus (RAD-001) leads to cell growth
reduction both in vitro and in vivo, confirming the
importance of microRNA regulation of the IGF-2/mTOR signalling
cascade (74). Based on these data,
a phase I trial tested the effects of temsirolimus (CCI-779),
another inhibitor of mTOR in combination with cixutumumab, an
anti-IGF-R1 monoclonal antibody, demonstrating a positive effect on
tumor growth in 4 of 10 patients treated (76). A recent trial from the United States
investigating the combination of cixutumumab and mitotane as first
line treatment in patients with metastatic ACC reported
effectiveness in 8/20 patients enrolled (77).
Most solid tumors display marked angiogenesis and
substantial data highlight the vascular endothelial growth factor
(VEGF) overexpression in ACC (70).
Despite expectations for the inhibitors of this pathway, the
results of clinical trials have been quite disappointing. A
monoclonal VEGF antibody in combination with capecitabine
administered in a series of 10 patients affected by advanced ACC
did not show any positive results (78). A partial response with capecitabine
at a dose of 200 mg/die has been described only in one case, a
40-year-old patient with chemoresistant ACC (79). Both sorafenib and sunitinib,
tyrosine kinase inhibitors able to target VEGF, produced poor
results also (70). A phase II
trial consisting of the administration of sunitinib in 38 patients
with unresponsive ACC recorded a progression-free survival ranging
from 5.6 to 12.2 months (80). A
phase I trial described a positive response in two patients
affected by advanced ACC who received sorafenib in combination with
tipifarnib, a farnesyltransferase inhibitor (81). Moreover, only in a single case
report, a regression of metastatic ACC with sorafenib
administration has been observed (82). Recently, a phase II study
investigating the combined effect of sorafenib with metronomic
paclitaxel did not show any clinical improvement, contradicting the
obtained in vitro results (83). A partial response to sunitinib in a
patients with metastatic ACC, after chemotherapy treatment, has
been described (84). Moreover,
Gangadhar et al reported a partial response, in a patient
with advanced ACC, who received combination treatment with
sirolimus, an mTOR inhibitor, and sunitinib (85). Finally, a role for heparanase-1 in
ACC angiogenesis has been hypothesized thus representing a new
selective therapeutic target in ACC (86).
Microarray transcriptome analyses have provided new
knowledge regarding the hyperactivated molecular pathways involved
in ACC, thereby suggesting new potential target molecules for
treatment strategies to address (3,87).
Frequently, these targets are represented by growth factors and
therefore the therapeutic concept is based on the inhibition of
protein kinases involved in signal transduction, often tyrosine
kinase receptors (70). A clinical
study performed on 10 patients with advanced disease treated with
erlotinib, an EGFR inhibitor, in combination with gemcitabine
demonstrated very limited effectiveness (88). Similarly, in another study, that
investigated treatment with gefitinib as a second-line monotherapy
in a series of 19 patients with unresectable ACC, no response was
obtained (89). In a phase II
study, treatment with imatinib mesilate, a PDGFR inhibitor, was
associated with disease progression in 75% of patients with severe
side effects (90). It is likely
that the failure of these therapies is related to the low presence
of these receptors in ACC. Interestingly, no mutation of the EGFR
gene has been detected (91).
The chemoresistant properties of ACC have been
classically related to the overexpression of the multidrug
resistance protein MDR-1 (P-glycoprotein, Pgp), a debated drug
efflux pump (52). Even with
results from an in vitro study suggesting that mitotane
enhances doxorubicin activity by interfering with Pgp (92), the exact role of MDR-1 protein in
ACC needs to be elucidated. Furthermore, a clinical trial using
doxorubicin, vincristine, and etoposide in combination with
mitotane failed to demonstrated therapeutic effectiveness (93).
Steroidogenic factor-1 (SF-1) is a nuclear
transcription factor involved in the steroidogenic tissue
development (104). It is
frequently overexpressed in pediatric ACCs, whereas in the adult
population some abnormalities on chromosome 9 have been described.
However, a higher nuclear SF-1 expression level has been associated
with a worse prognosis in ACC (104) and is positively correlated with
advanced ENSAT stages, and a higher mitotic index and Weiss score
(105). An increased SF-1 dosage
was observed to stimulate proliferation, decrease apoptosis in
adrenocortical cells, and induce tumorigenesis in transgenic mice
(106). SF-1 silencing affected
TGF-β and Wnt/β-catenin signaling, suggesting crosstalk between
these pathways in a study performed on the H295R adrenocortical
cell line. Moreover, SF-1 knockdown showed a significant reduction
of cell proliferation be interference with S-phase of the cell
cycle (106,107). Two members of the alkyloxyphenol
class, AC-45594 and OOP, the synthetic SF-1 inverse agonists have
been shown to inhibit proliferation in both H295R and SW13
adrenocortical cell lines through an SF-1 non-selective mechanism.
In contrast, SID7969543 (IsoQ A) and the compounds numbered 31 and
32, members of the IsoQ class, induced a selective inhibition of
cell proliferation when SF-1 was increased strongly suggesting that
the IsoQ molecules targeted SF-1-related genes (108).
The rationale of gene therapy lies in correcting the
gene regulation, reactivating oncosuppressor genes and/or
inhibiting oncogenes during tumorigenesis. Systemic therapy with
antisense oligonucleotides represents an innovative approach for
ACC treatment. A construct, composed of the herpes simplex virus
thymidine kinase (HSV-TK) gene driven by the CYP11B1 promoter with
a P450scc enhancer element, increased the chemosensitivity in a Y1
mouse ACC cell line (109).
Immunotherapy represents another therapeutic approach that relies
on the stimulation of the immune system against specific target
proteins of neoplastic cells. This approach, using dendritic cells,
was effective in stimulating the immune response (110), inducing antigen-specific Th1
immunity in a study performed on two patients with advanced
secreting ACC. However, no clinical benefit has been shown
(111).
Recent biological advances concerning microRNA
dysregulation in all cancers including ACC highlights the
hypothetical consideration of these small non-coding RNAs as
potential target molecules for anti-cancer treatment (114). Because miRNAs may function as
tumor suppressors, the assumption of replacement miRNA cancer
therapy must not disregard the identification of an miRNA
deficiency. In a previous analysis of miRNA expression in
adrenocortical tumors, miR-7 was the most significantly
under-expressed miRNA when compared to normal adrenal tissue
(115). Glover et al
provided the first demonstration of the effectiveness of the
nanoparticle systemic delivery of miR-7 in the reduction of cell
growth in both cell lines and in an ACC xenograft model,
respectively. Furthermore, they demonstrated that miR-7 functions
as a tumor suppressor in ACC leading to the repression of several
genes involved in the pathogenesis of ACC, including RAF-1,
mTOR and CDK1 (116).
Recent advance confirm an estrogenic pathway in
normal adrenal tissue and in adrenal tumors. A differential
expression of estrogen receptors (ER) α and β has been demonstrated
in ACCs (117). Moreover, Barzon
et al showed an increased aromatase activity in ACC,
hypothesizing a paracrine estrogenic effect at the tumor level
(118). An in vitro study
demonstrated that hydroxytamoxifen, increasing the pro-apoptotic
factor FasL expression, reduced H295R cell proliferation by ERα
downregulation and ERβ upregulation, respectively (119). ERα activation may occur by an 17-β
estradiol (E2)-dependent mechanism or alternatively by IGF-II/IGF1R
in a ligand-independent manner, activating proliferative pathways
in vitro, such as IGF1R/AKT signaling, in H295R cell lines.
Furthermore, in the same study, hydroxytamoxifen, an active
metabolite of the estrogen antagonist tamoxifen, reduced IGF1R
protein levels and cell proliferation induced by E2 and IGF-II both
in vitro and in an ACC xenograft model (120). These data indicate a crucial role
of the estrogenic pathway in ACC and support the possibility of
using anti-estrogens in the treatment of ACC. A recent interesting
study elucidated the ability of a non-steroidal G-protein-coupled
estrogen receptor (GPER) agonist to exert a growth inhibitory
effect, mediated by activation of the ERK1/2 pathway, both in the
H295R cell line and in xenograft ACC (121). Finally, the compound XCT790, an
inverse agonist of the transcription factor estrogen-related
receptor α (ERRα) (122), an
orphan member of the nuclear hormone receptor superfamily with a
similar sequence to ERα involved in cellular metabolism and
mitochondrial biogenesis (123),
was able to reduce cell growth in both the H295R cell line and in
an ACC xenograft model, with impaired mitochondrial functioning
leading progressively to cell death (124).
Minimally invasive procedures such as radiofrequency
thermal ablation (RFA), or transarterial chemoembolization (TACE)
represent an alternative to surgery in advanced metastatic
malignancies. The same approach was adopted also in the treatment
of lesions in the liver, kidney, lymph nodes and lung for stage IV
ACC patients (126). Wood et
al observed that RFA induced a growth arrest in 8 of 15 lesions
after 6 months of follow-up. The procedure was safe and was not
associated with any particular side effects (126). TACE allows the selective infusion
of high doses of cytotoxic drugs in the metastatic lesion reducing
the systemic toxicity. In a French study, this technique was
associated with a median survival of 11 months in 21 patients with
liver metastatic disease (127).
These procedures provide palliative benefits, are safe and
inexpensive while implying minimal morbidity and a short recovery,
however, none of these methods have been supported by a clinical
trial.
ACC in the past was considered an orphan disease for
which surgery represented the only feasible therapy. Over the years
the focus on this aggressive endocrine malignancy has gradually
grown, capturing the interest of many investigators. Despite the
enormous progress achieved in the biological knowledge of this
tumor, the ACC remains an oncological disease burdened by a high
mortality. Surgery is still the first therapeutic option and the
only potentially curative treatment. Mitotane has represented the
first drug in the treatment of ACC since 1959. Subsequently,
knowledge regarding its mechanism of action has increased
substantially while its clinical use has become much more
controlled and appropriate. Currently, mitotane is the only drug
approved by international pharmaceutical agencies for ACC
treatment. Although mitotane treatment in the adjuvant setting is
still debated, the recent ESMO guidelines recommend its use in
adjuvant setting after surgery in patients with incomplete
resection status. The optimal chemotherapy regimen is considered to
be etoposide-doxorubicin-cisplatin (EDP) in combination with
mitotane in patients with advanced metastatic disease, although
this regimen is burdened by substantial side effects. The current
-OMICs approach has permitted the discover of different molecules
belonging to pathways potentially involved in the pathogenesis of
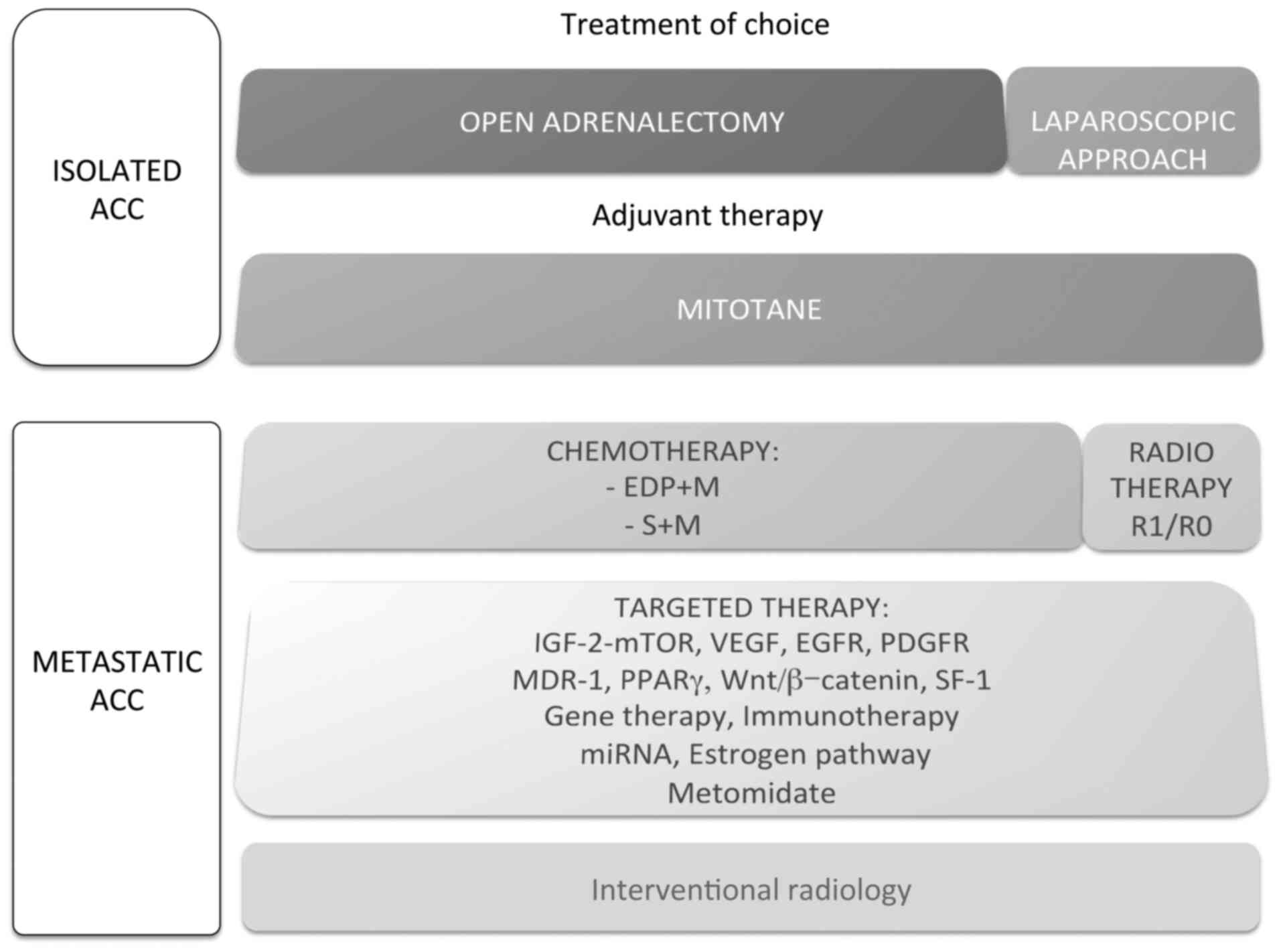
the ACC. The therapeutic option described in isolated and
metastatic ACC are summarized in Fig.
1. Future efforts should be made not only to explore new
frontiers but also to investigate innovative therapies in the
clinical field.
ACC urgently requires new therapeutic strategies.
The clinical translation of new research products in vitro
and in preclinical studies may help improve the standard of care in
these patients. Achieving this objective in a rare disease such as
ACC will require the carefully selection of the clinical series to
be devoted to experimentation and an increasead collaborative
network of research centers involved in the study of this
malignancy.
Recent contributions made by applying -OMICs to
large-scale analyses such as the genomics, transcriptomics,
proteomics and epigenetics of tumor samples had steadily increased
the scientific knowledge concerning the heterogeneity of ACC.
However, despite recent progress achieved in this field, the
prognosis of this cancer remains poor. Beyond the surgery that is
considered the standard of care, current therapeutic options
include mitotane in adjuvant therapy and the use of different
chemotherapeutic agents in combination, among which EDP plus
mitotane is the prevailing combination, in the treatment of
advanced ACC. In the coming years, the working agenda includes
defining the current therapeutic protocols by prospective trials
to: i) optimize the surgical techniques and procedural strategies;
ii) validate mitotane use in the adjuvant setting by ADIUVO trial;
and iii) evaluate post-surgery radiotherapy effectiveness.
Additional efforts to manage and treat this aggressive tumor must
be pursued by clinical, basic and genetic research studies. The
main purpose of these studies should be aimed at testing new
therapeutic targets. The final hope is to prompt multicenter
clinical trials for the investigation of the most promising
molecules to fight ACC.
• Open surgery represents the first therapeutic
choice in ACC.
• Mitotane treatment is recommended in patients at
high risk for recurrence, whereas low-risk patients can be
recruited into the ADIUVO trial or followed according to an
individual management plan.
• Monotherapy with mitotane is useful in patients
after incomplete surgical excision or in metastatic disease with
limited metastatic spread.
• The ‘therapeutic window’ of 14–20 mg/l should be
considered in monitoring blood mitotane concentration.
• Post-surgery radiotherapy of the tumor bed is
suggested in patients with R1/RX resection status.
• The combination chemotherapeutic treatment of
etoposide, doxorubicin and cisplatin plus mitotane is currently
considered the most suitable regimen for ACC metastatic
disease.
• Several target molecules have been identified;
currently however, none have shown established effectiveness due to
the lack of confirmatory clinical data.
|
1
|
Else T, Kim AC, Sabolch A, Raymond VM,
Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ and Hammer
GD: Adrenocortical carcinoma. Endocr Rev. 35:282–326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barlaskar FM and Hammer GD: The molecular
genetics of adrenocortical carcinoma. Rev Endocr Metab Disord.
8:343–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ragazzon B, Assié G and Bertherat J:
Transcriptome analysis of adrenocortical cancers: From molecular
classification to the identification of new treatments. Endocr
Relat Cancer. 18:R15–R27. 2011.PubMed/NCBI
|
|
4
|
Assié G, Letouzé E, Fassnacht M, Jouinot
A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K,
René-Corail F, et al: Integrated genomic characterization of
adrenocortical carcinoma. Nat Genet. 46:607–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assié G, Jouinot A and Bertherat J: The
‘omics’ of adrenocortical tumours for personalized medicine. Nat
Rev Endocrinol. 10:215–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fassnacht M, Libé R, Kroiss M and Allolio
B: Adrenocortical carcinoma: A clinician's update. Nat Rev
Endocrinol. 7:323–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fassnacht M, Kenn W and Allolio B: Adrenal
tumors: How to establish malignancy? J Endocrinol Invest.
27:387–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tauchmanovà L, Colao A, Marzano LA,
Sparano L, Camera L, Rossi A, Palmieri G, Marzano E, Salvatore M,
Pettinato G, et al: Andrenocortical carcinomas: Twelve-year
prospective experience. World J Surg. 28:896–903. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arlt W, Biehl M, Taylor AE, Hahner S, Libé
R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, et al:
Urine steroid metabolomics as a biomarker tool for detecting
malignancy in adrenal tumors. J Clin Endocrinol Metab.
96:3775–3784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Terzolo M, Stigliano A, Chiodini I, Loli
P, Furlani L, Arnaldi G, Reimondo G, Pia A, Toscano V, Zini M, et
al: Italian Association of Clinical Endocrinologists: AME position
statement on adrenal incidentaloma. Eur J Endocrinol. 164:851–870.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blake MA, Kalra MK, Sweeney AT, Lucey BC,
Maher MM, Sahani DV, Halpern EF, Mueller PR, Hahn PF and Boland GW:
Distinguishing benign from malignant adrenal masses: Multi-detector
row CT protocol with 10-minute delay. Radiology. 238:578–585. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petersenn S, Richter PA, Broemel T, Ritter
CO, Deutschbein T, Beil FU, Allolio B and Fassnacht M: German ACC
Study Group: Computed tomography criteria for discrimination of
adrenal adenomas and adrenocortical carcinomas: Analysis of the
German ACC registry. Eur J Endocrinol. 172:415–422. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong KK, Arabi M, Zerizer I, Al-Nahhas A,
Rubello D and Gross MD: Role of positron emission
tomography/computed tomography in adrenal and neuroendocrine
tumors: Fluorodeoxyglucose and nonfluorodeoxyglucose tracers. Nucl
Med Commun. 32:764–781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hennings J, Lindhe O, Bergström M,
Långström B, Sundin A and Hellman P: [11C]metomidate positron
emission tomography of adrenocortical tumors in correlation with
histopathological findings. J Clin Endocrinol Metab. 91:1410–1414.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hahner S, Kreissl MC, Fassnacht M,
Haenscheid H, Bock S, Verburg FA, Knoedler P, Lang K, Reiners C,
Buck AK, et al: Functional characterization of adrenal lesions
using [123I]IMTO-SPECT/CT. J Clin Endocrinol Metab. 98:1508–1518.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Delellis RA, Lloyd RV and Heitz PU:
Pathology and Genetics of Tumours of Endocrine OrgansEble JN,
Sauter G, Epstein JE and Sesterhenn IA: IARC; Lyon: 2004
|
|
17
|
MacFarlane DA: Cancer of the adrenal
cortex; the natural history, prognosis and treatment in a study of
fifty-five cases. Ann R Coll Surg Engl. 23:155–186. 1958.PubMed/NCBI
|
|
18
|
Sullivan M, Boileau M and Hodges CV:
Adrenal cortical carcinoma. J Urol. 120:660–665. 1978.PubMed/NCBI
|
|
19
|
Fassnacht M, Johanssen S, Quinkler M,
Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH,
Hahner S and Allolio B: German Adrenocortical Carcinoma Registry
Group; European Network for the Study of Adrenal Tumors: Limited
prognostic value of the 2004 International Union Against Cancer
staging classification for adrenocortical carcinoma: Proposal for a
Revised TNM Classification. Cancer. 115:243–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lughezzani G, Sun M, Perrotte P, Jeldres
C, Alasker A, Isbarn H, Budäus L, Shariat SF, Guazzoni G, Montorsi
F, et al: The European Network for the Study of Adrenal Tumors
staging system is prognostically superior to the international
union against cancer-staging system: A North American validation.
Eur J Cancer. 46:713–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Creemers SG, Hofland LJ, Korpershoek E,
Franssen GJ, van Kemenade FJ, de Herder WW and Feelders RA: Future
directions in the diagnosis and medical treatment of adrenocortical
carcinoma. Endocr Relat Cancer. 23:R43–R69. 2016.PubMed/NCBI
|
|
22
|
Stigliano A, Chiodini I, Giordano R,
Faggiano A, Canu L, Casa S Della, Loli P, Luconi M, Mantero F and
Terzolo M: Management of adrenocortical carcinoma: A consensus
statement of the Italian Society of Endocrinology (SIE). J
Endocrinol Invest. 39:103–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crucitti F, Bellantone R, Ferrante A,
Boscherini M and Crucitti P: The ACC Italian Registry Study Group:
The Italian Registry for Adrenal Cortical Carcinoma: Analysis of a
multiinstitutional series of 129 patients. Surgery. 119:161–170.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donatini G, Caiazzo R, Do Cao C, Aubert S,
Zerrweck C, El-Kathib Z, Gauthier T, Leteurtre E, Wemeau JL,
Vantyghem MC, et al: Long-term survival after adrenalectomy for
stage I/II adrenocortical carcinoma (ACC): A retrospective
comparative cohort study of laparoscopic versus open approach. Ann
Surg Oncol. 21:284–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaughan ED Jr.: Diseases of the adrenal
gland. Med Clin North Am. 88:443–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang H and Fojo T: Adjuvant mitotane for
adrenocortical cancer - a recurring controversy. J Clin Endocrinol
Metab. 93:3730–3732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Icard P, Goudet P, Charpenay C,
Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF and
Proye C: Adrenocortical carcinomas: Surgical trends and results of
a 253-patient series from the French Association of Endocrine
Surgeons study group. World J Surg. 25:891–897. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lombardi CP, Raffaelli M, De Crea C,
Boniardi M, De Toma G, Marzano LA, Miccoli P, Minni F, Morino M,
Pelizzo MR, et al: Open versus endoscopic adrenalectomy in the
treatment of localized (stage I/II) adrenocortical carcinoma:
Results of a multiinstitutional Italian survey. Surgery.
152:1158–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porpiglia F, Fiori C, Daffara F, Zaggia B,
Bollito E, Volante M, Berruti A and Terzolo M: Retrospective
evaluation of the outcome of open versus laparoscopic adrenalectomy
for stage I and II adrenocortical cancer. Eur Urol. 57:873–878.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reibetanz J, Jurowich C, Erdogan I, Nies
C, Rayes N, Dralle H, Behrend M, Allolio B and Fassnacht M: German
ACC study group: Impact of lymphadenectomy on the oncologic outcome
of patients with adrenocortical carcinoma. Ann Surg. 255:363–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gaujoux S and Brennan MF: Recommendation
for standardized surgical management of primary adrenocortical
carcinoma. Surgery. 152:123–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kemp CD, Ripley RT, Mathur A, Steinberg
SM, Nguyen DM, Fojo T and Schrump DS: Pulmonary resection for
metastatic adrenocortical carcinoma: The National Cancer Institute
experience. Ann Thorac Surg. 92:1195–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ripley RT, Kemp CD, Davis JL, Langan RC,
Royal RE, Libutti SK, Steinberg SM, Wood BJ, Kammula US, Fojo T, et
al: Liver resection and ablation for metastatic adrenocortical
carcinoma. Ann Surg Oncol. 18:1972–1979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stojadinovic A, Ghossein RA, Hoos A,
Nissan A, Marshall D, Dudas M, Cordon-Cardo C, Jaques DP and
Brennan MF: Adrenocortical carcinoma: Clinical, morphologic, and
molecular characterization. J Clin Oncol. 20:941–950. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Datrice NM, Langan RC, Ripley RT, Kemp CD,
Steinberg SM, Wood BJ, Libutti SK, Fojo T, Schrump DS and Avital I:
Operative management for recurrent and metastatic adrenocortical
carcinoma. J Surg Oncol. 105:709–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Erdogan I, Deutschbein T, Jurowich C,
Kroiss M, Ronchi C, Quinkler M, Waldmann J, Willenberg HS,
Beuschlein F, Fottner C, et al: German Adrenocortical Carcinoma
Study Group: The role of surgery in the management of recurrent
adrenocortical carcinoma. J Clin Endocrinol Metab. 98:181–191.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schteingart DE, Doherty GM, Gauger PG,
Giordano TJ, Hammer GD, Korobkin M and Worden FP: Management of
patients with adrenal cancer: Recommendations of an international
consensus conference. Endocr Relat Cancer. 12:667–680. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin CW, Chang YH and Pu HF: Mitotane
exhibits dual effects on steroidogenic enzymes gene transcription
under basal and cAMP-stimulating microenvironments in NCI-H295
cells. Toxicology. 298:14–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lehmann TP, Wrzesiński T and Jagodziński
PP: The effect of mitotane on viability, steroidogenesis and gene
expression in NCI-H295R adrenocortical cells. Mol Med Rep.
7:893–900. 2013.PubMed/NCBI
|
|
40
|
van Slooten H, Moolenaar AJ, van Seters AP
and Smeenk D: The treatment of adrenocortical carcinoma with
o,p'-DDD: Prognostic implications of serum level monitoring. Eur J
Cancer Clin Oncol. 20:47–53. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hermsen IG, Fassnacht M, Terzolo M,
Houterman S, den Hartigh J, Leboulleux S, Daffara F, Berruti A,
Chadarevian R, Schlumberger M, et al: Plasma concentrations of
o,p'DDD, o,p'DDA, and o,p'DDE as predictors of tumor response to
mitotane in adrenocortical carcinoma: Results of a retrospective
ENS@T multicenter study. J Clin Endocrinol Metab. 96:1844–1851.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kerkhofs TM, Baudin E, Terzolo M, Allolio
B, Chadarevian R, Mueller HH, Skogseid B, Leboulleux S, Mantero F,
Haak HR, et al: Comparison of two mitotane starting dose regimens
in patients with advanced adrenocortical carcinoma. J Clin
Endocrinol Metab. 98:4759–4767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fassnacht M and Allolio B: Clinical
management of adrenocortical carcinoma. Best Pract Res Clin
Endocrinol Metab. 23:273–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Terzolo M, Baudin AE, Ardito A, Kroiss M,
Leboulleux S, Daffara F, Perotti P, Feelders RA, deVries JH, Zaggia
B, et al: Mitotane levels predict the outcome of patients with
adrenocortical carcinoma treated adjuvantly following radical
resection. Eur J Endocrinol. 169:263–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mauclère-Denost S, Leboulleux S, Borget I,
Paci A, Young J, Al Ghuzlan A, Deandreis D, Drouard L, Tabarin A,
Chanson P, et al: High-dose mitotane strategy in adrenocortical
carcinoma: Prospective analysis of plasma mitotane measurement
during the first 3 months of follow-up. Eur J Endocrinol.
166:261–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van Erp NP, Guchelaar HJ, Ploeger BA,
Romijn JA, Hartigh J and Gelderblom H: Mitotane has a strong and a
durable inducing effect on CYP3A4 activity. Eur J Endocrinol.
164:621–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kroiss M, Quinkler M, Lutz WK, Allolio B
and Fassnacht M: Drug interactions with mitotane by induction of
CYP3A4 metabolism in the clinical management of adrenocortical
carcinoma. Clin Endocrinol (Oxf). 75:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Haak HR, Hermans J, van De Velde CJ,
Lentjes EG, Goslings BM, Fleuren GJ and Krans HM: Optimal treatment
of adrenocortical carcinoma with mitotane: Results in a consecutive
series of 96 patients. Br J Cancer. 69:947–951. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Baudin E, Pellegriti G, Bonnay M,
Penfornis A, Laplanche A, Vassal G and Schlumberger M: Impact of
monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p'DDD)
levels on the treatment of patients with adrenocortical carcinoma.
Cancer. 92:1385–1392. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Terzolo M, Angeli A, Fassnacht M, Daffara
F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P,
Grossrubatscher E, et al: Adjuvant mitotane treatment for
adrenocortical carcinoma. N Engl J Med. 356:2372–2380. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Berruti A, Baudin E, Gelderblom H, Haak
HR, Porpiglia F, Fassnacht M and Pentheroudakis G: ESMO Guidelines
Working Group: Adrenal cancer: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23 Suppl
7:vii131–vii138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bates SE, Shieh CY, Mickley LA, Dichek HL,
Gazdar A, Loriaux DL and Fojo AT: Mitotane enhances cytotoxicity of
chemotherapy in cell lines expressing a multidrug resistance gene
(mdr-1/P-glycoprotein) which is also expressed by adrenocortical
carcinomas. J Clin Endocrinol Metab. 73:18–29. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Berruti A, Terzolo M, Sperone P, Pia A,
Casa S Della, Gross DJ, Carnaghi C, Casali P, Porpiglia F, Mantero
F, et al: Etoposide, doxorubicin and cisplatin plus mitotane in the
treatment of advanced adrenocortical carcinoma: A large prospective
phase II trial. Endocr Relat Cancer. 12:657–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Khan TS, Imam H, Juhlin C, Skogseid B,
Gröndal S, Tibblin S, Wilander E, Oberg K and Eriksson B:
Streptozocin and o,p'DDD in the treatment of adrenocortical cancer
patients: Long-term survival in its adjuvant use. Ann Oncol.
11:1281–1287. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bukowski RM, Wolfe M, Levine HS, Crawford
DE, Stephens RL, Gaynor E and Harker WG: Phase II trial of mitotane
and cisplatin in patients with adrenal carcinoma: A Southwest
Oncology Group study. J Clin Oncol. 11:161–165. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schteingart DE: Conventional and novel
strategies in the treatment of adrenocortical cancer. Braz J Med
Biol Res. 33:1197–1200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bonacci R, Gigliotti A, Baudin E,
Wion-Barbot N, Emy P, Bonnay M, Cailleux AF, Nakib I and
Schlumberger M: Réseau Comète: Cytotoxic therapy with etoposide and
cisplatin in advanced adrenocortical carcinoma. Br J Cancer.
78:546–549. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Burgess MA, Legha SS and Sellin RV:
Chemotherapy with cisplatinum and etoposide (UP16) for patients
with advanced adrenal cortical carcinoma (ACC). Proc ASCO.
12:1881993.
|
|
59
|
Williamson SK, Lew D, Miller GJ, Balcerzak
SP, Baker LH and Crawford ED: Phase II evaluation of cisplatin and
etoposide followed by mitotane at disease progression in patients
with locally advanced or metastatic adrenocortical carcinoma: A
Southwest Oncology Group Study. Cancer. 88:1159–1165. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fassnacht M, Terzolo M, Allolio B, Baudin
E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A,
Jarzab B, et al: FIRM-ACT Study Group: Combination chemotherapy in
advanced adrenocortical carcinoma. N Engl J Med. 366:2189–2197.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Germano A, Rapa I, Volante M, Lo Buono N,
Carturan S, Berruti A, Terzolo M and Papotti M: Cytotoxic activity
of gemcitabine, alone or in combination with mitotane, in
adrenocortical carcinoma cell lines. Mol Cell Endocrinol. 382:1–7.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fassnacht M, Hahner S, Polat B, Koschker
AC, Kenn W, Flentje M and Allolio B: Efficacy of adjuvant
radiotherapy of the tumor bed on local recurrence of adrenocortical
carcinoma. J Clin Endocrinol Metab. 91:4501–4504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sabolch A, Feng M, Griffith K, Hammer G,
Doherty G and Ben-Josef E: Adjuvant and definitive radiotherapy for
adrenocortical carcinoma. Int J Radiat Oncol Biol Phys.
80:1477–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Habra MA, Ejaz S, Feng L, Das P, Deniz F,
Grubbs EG, Phan A, Waguespack SG, Ayala-Ramirez M, Jimenez C, et
al: A retrospective cohort analysis of the efficacy of adjuvant
radiotherapy after primary surgical resection in patients with
adrenocortical carcinoma. J Clin Endocrinol Metab. 98:192–197.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cerquetti L, Bucci B, Marchese R, Misiti
S, De Paula U, Miceli R, Muleti A, Amendola D, Piergrossi P,
Brunetti E, et al: Mitotane increases the radiotherapy inhibitory
effect and induces G2-arrest in combined treatment on both H295R
and SW13 adrenocortical cell lines. Endocr Relat Cancer.
15:623–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cerquetti L, Sampaoli C, Amendola D, Bucci
B, Misiti S, Raza G, De Paula U, Marchese R, Brunetti E, Toscano V,
et al: Mitotane sensitizes adrenocortical cancer cells to ionizing
radiations by involvement of the cyclin B1/CDK complex in G2 arrest
and mismatch repair enzymes modulation. Int J Oncol. 37:493–501.
2010.PubMed/NCBI
|
|
67
|
Polat B, Fassnacht M, Pfreundner L,
Guckenberger M, Bratengeier K, Johanssen S, Kenn W, Hahner S,
Allolio B and Flentje M: Radiotherapy in adrenocortical carcinoma.
Cancer. 115:2816–2823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
De Reyniès A, Assié G, Rickman DS, Tissier
F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E and
Bertherat J: Gene expression profiling reveals a new classification
of adrenocortical tumors and identifies molecular predictors of
malignancy and survival. J Clin Oncol. 27:1108–1115. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Barlaskar FM, Spalding AC, Heaton JH,
Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E and Hammer GD:
Preclinical targeting of the type I insulin-like growth factor
receptor in adrenocortical carcinoma. J Clin Endocrinol Metab.
94:204–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tacon LJ, Prichard RS, Soon PSH, Robinson
BG, Clifton-Bligh RJ and Sidhu SB: Current and emerging therapies
for advanced adrenocortical carcinoma. Oncologist. 16:36–48. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Haluska P, Worden F, Olmos D, Yin D,
Schteingart D, Batzel GN, Paccagnella ML, De Bono JS, Gualberto A
and Hammer GD: Safety, tolerability, and pharmacokinetics of the
anti-IGF-1R monoclonal antibody figitumumab in patients with
refractory adrenocortical carcinoma. Cancer Chemother Pharmacol.
65:765–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Carden CP, Frentzas S and Langham M:
Preliminary activity in adrenocortical tumor (ACC) in phase I dose
escalation study of intermittent oral dosing of OSI-906, a
small-molecule insulin-like growth factor-1 receptor (IGF-1R)
tyrosine kinase inhibitor in patients with advanced solid tumors. J
Clin Oncol. 27:35442009.
|
|
73
|
Fassnacht M, Berruti A, Baudin E, Demeure
MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL,
et al: Linsitinib (OSI-906) versus placebo for patients with
locally advanced or metastatic adrenocortical carcinoma: A
double-blind, randomised, phase 3 study. Lancet Oncol. 16:426–435.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Doghman M, El Wakil A, Cardinaudet B,
Thomas E, Wang J, Zhao W, Peralta-Del Valle MH, Figueiredo BC,
Zambetti GP and Lalli E: Regulation of IGF-mTOR signalling by miRNA
in childhood adrenocortical tumors. Cancer Res. 70:4666–4675. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
De Martino MC, van Koetsveld PM, Pivonello
R and Hofland LJ: Role of the mTOR pathway in normal and tumoral
adrenal cells. Neuroendocrinology. 92 Suppl 1:28–34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Naing A, Kurzrock R, Burger A, Gupta S,
Lei X, Busaidy N, Hong D, Chen HX, Doyle LA, Heilbrun LK, et al:
Phase I trial of cixutumumab combined with temsirolimus in patients
with advanced cancer. Clin Cancer Res. 17:6052–6060. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lerario AM, Worden FP, Ramm CA, Hesseltine
EA, Stadler WM, Else T, Shah MH, Agamah E, Rao K and Hammer GD: The
combination of insulin-like growth factor receptor 1 (IGF1R)
antibody cixutumumab and mitotane as a first-line therapy for
patients with recurrent/metastatic adrenocortical carcinoma: A
multi-institutional NCI-sponsored trial. Horm Cancer. 5:232–239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wortmann S, Quinkler M, Ritter C, Kroiss
M, Johanssen S, Hahner S, Allolio B and Fassnacht M: Bevacizumab
plus capecitabine as a salvage therapy in advanced adrenocortical
carcinoma. Eur J Endocrinol. 162:349–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chacón R, Tossen G, Loria FS and Chacón M:
CASE 2. Response in a patient with metastatic adrenal cortical
carcinoma with thalidomide. J Clin Oncol. 23:1579–1580. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kroiss M, Quinkler M, Johanssen S, van Erp
NP, Lankheet N, Pöllinger A, Laubner K, Strasburger CJ, Hahner S,
Müller HH, et al: Sunitinib in refractory adrenocortical carcinoma:
A phase II, single-arm, open-label trial. J Clin Endocrinol Metab.
97:3495–3503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hong DS, Sebti SM, Newman RA, Blaskovich
MA, Ye L, Gagel RF, Moulder S, Wheler JJ, Naing A, Tannir NM, et
al: Phase I trial of a combination of the multikinase inhibitor
sorafenib and the farnesyltransferase inhibitor tipifarnib in
advanced malignancies. Clin Cancer Res. 15:7061–7068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Butler C, Butler WM and Rizvi AA:
Sustained remission with the kinase inhibitor sorafenib in stage IV
metastatic adrenocortical carcinoma. Endocr Pract. 16:441–445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Berruti A, Sperone P, Ferrero A, Germano
A, Ardito A, Priola AM, De Francia S, Volante M, Daffara F,
Generali D, et al: Phase II study of weekly paclitaxel and
sorafenib as second/third-line therapy in patients with
adrenocortical carcinoma. Eur J Endocrinol. 166:451–458. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lee JO, Lee KW, Kim CJ, Kim YJ, Lee HE,
Kim H, Kim JH, Bang SM, Kim JS and Lee JS: Metastatic
adrenocortical carcinoma treated with sunitinib: A case report. Jpn
J Clin Oncol. 39:183–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gangadhar TC, Cohen EEW, Wu K, Janisch L,
Geary D, Kocherginsky M, House LK, Ramirez J, Undevia SD, Maitland
ML, et al: Two drug interaction studies of sirolimus in combination
with sorafenib or sunitinib in patients with advanced malignancies.
Clin Cancer Res. 17:1956–1963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu YZ, Zhu Y, Shen ZJ, Sheng JY, He HC, Ma
G, Qi YC, Zhao JP, Wu YX, Rui WB, et al: Significance of
heparanase-1 and vascular endothelial growth factor in
adrenocortical carcinoma angiogenesis: Potential for therapy.
Endocrine. 40:445–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Assié G, Guillaud-Bataille M, Ragazzon B,
Bertagna X, Bertherat J and Clauser E: The pathophysiology,
diagnosis and prognosis of adrenocortical tumors revisited by
transcriptome analyses. Trends Endocrinol Metab. 21:325–334. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Quinkler M, Hahner S, Wortmann S,
Johanssen S, Adam P, Ritter C, Strasburger C, Allolio B and
Fassnacht M: Treatment of advanced adrenocortical carcinoma with
erlotinib plus gemcitabine. J Clin Endocrinol Metab. 93:2057–2062.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Samnotra V, Vassilopoulou-Sellin R and
Fojo AT: A phase II trial of gefitinib monotherapy in patients with
unresectable adrenocortical carcinoma (ACC). J Clin Oncol.
27:155272007.
|
|
90
|
Gross DJ, Munter G, Bitan M, Siegal T,
Gabizon A, Weitzen R, Merimsky O, Ackerstein A, Salmon A, Sella A,
et al: Israel Glivec in Solid Tumors Study Group: The role of
imatinib mesylate (Glivec) for treatment of patients with malignant
endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer.
13:535–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Adam P, Hahner S, Hartmann M, Heinrich B,
Quinkler M, Willenberg HS, Saeger W, Sbiera S, Schmull S, Voelker
HU, et al: Epidermal growth factor receptor in adrenocortical
tumors: Analysis of gene sequence, protein expression and
correlation with clinical outcome. Mod Pathol. 23:1596–1604. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gagliano T, Gentilin E, Benfini K, Di
Pasquale C, Tassinari M, Falletta S, Feo C, Tagliati F, Uberti ED
and Zatelli MC: Mitotane enhances doxorubicin cytotoxic activity by
inhibiting P-gp in human adrenocortical carcinoma cells. Endocrine.
47:943–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Abraham J, Bakke S, Rutt A, Meadows B,
Merino M, Alexander R, Schrump D, Bartlett D, Choyke P, Robey R, et
al: A phase II trial of combination chemotherapy and surgical
resection for the treatment of metastatic adrenocortical carcinoma:
Continuous infusion doxorubicin, vincristine, and etoposide with
daily mitotane as a P-glycoprotein antagonist. Cancer.
94:2333–2343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kahn CR, Chen L and Cohen SE: Unraveling
the mechanism of action of thiazolidinediones. J Clin Invest.
106:1305–1307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Betz MJ, Shapiro I, Fassnacht M, Hahner S,
Reincke M and Beuschlein F: German and Austrian Adrenal Network:
Peroxisome proliferator-activated receptor-γ agonists suppress
adrenocortical tumor cell proliferation and induce differentiation.
J Clin Endocrinol Metab. 90:3886–3896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ferruzzi P, Ceni E, Tarocchi M, Grappone
C, Milani S, Galli A, Fiorelli G, Serio M and Mannelli M:
Thiazolidinediones inhibit growth and invasiveness of the human
adrenocortical cancer cell line H295R. J Clin Endocrinol Metab.
90:1332–1339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Luconi M, Mangoni M, Gelmini S, Poli G,
Nesi G, Francalanci M, Pratesi N, Cantini G, Lombardi A, Pepi M, et
al: Rosiglitazone impairs proliferation of human adrenocortical
cancer: Preclinical study in a xenograft mouse model. Endocr Relat
Cancer. 17:169–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cerquetti L, Sampaoli C, Amendola D, Bucci
B, Masuelli L, Marchese R, Misiti S, De Venanzi A, Poggi M, Toscano
V, et al: Rosiglitazone induces autophagy in H295R and cell cycle
deregulation in SW13 adrenocortical cancer cells. Exp Cell Res.
317:1397–1410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tissier F, Cavard C, Groussin L,
Perlemoine K, Fumey G, Hagneré AM, René-Corail F, Jullian E,
Gicquel C, Bertagna X, et al: Mutations of β-catenin in
adrenocortical tumors: Activation of the Wnt signaling pathway is a
frequent event in both benign and malignant adrenocortical tumors.
Cancer Res. 65:7622–7627. 2005.PubMed/NCBI
|
|
100
|
Gaujoux S, Grabar S, Fassnacht M, Ragazzon
B, Launay P, Libé R, Chokri I, Audebourg A, Royer B, Sbiera S, et
al: β-catenin activation is associated with specific clinical and
pathologic characteristics and a poor outcome in adrenocortical
carcinoma. Clin Cancer Res. 17:328–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Doghman M, Cazareth J and Lalli E: The T
cell factor/β-catenin antagonist PKF115-584 inhibits proliferation
of adrenocortical carcinoma cells. J Clin Endocrinol Metab.
93:3222–3225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gaujoux S, Hantel C, Launay P, Bonnet S,
Perlemoine K, Lefèvre L, Guillaud-Bataille M, Beuschlein F, Tissier
F, Bertherat J, et al: Silencing mutated β-catenin inhibits cell
proliferation and stimulates apoptosis in the adrenocortical cancer
cell line H295R. PLoS One. 8:e557432013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Demeure MJ, Bussey KJ and Kirschner LS:
Targeted therapies for adrenocortical carcinoma: IGF and beyond.
Horm Cancer. 2:385–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sbiera S, Schmull S, Assie G, Voelker HU,
Kraus L, Beyer M, Ragazzon B, Beuschlein F, Willenberg HS, Hahner
S, et al: High diagnostic and prognostic value of steroidogenic
factor-1 expression in adrenal tumors. J Clin Endocrinol Metab.
95:E161–E171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Duregon E, Volante M, Giorcelli J, Terzolo
M, Lalli E and Papotti M: Diagnostic and prognostic role of
steroidogenic factor 1 in adrenocortical carcinoma: A validation
study focusing on clinical and pathologic correlates. Hum Pathol.
44:822–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Doghman M, Karpova T, Rodrigues GA,
Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti
GP, Figueiredo BC, et al: Increased steroidogenic factor-1 dosage
triggers adrenocortical cell proliferation and cancer. Mol
Endocrinol. 21:2968–2987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ehrlund A, Jonsson P, Vedin LL, Williams
C, Gustafsson JÅ and Treuter E: Knockdown of SF-1 and RNF31 affects
components of steroidogenesis, TGFβ, and Wnt/β-catenin signaling in
adrenocortical carcinoma cells. PLoS One. 7:e320802012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Doghman M, Cazareth J, Douguet D, Madoux
F, Hodder P and Lalli E: Inhibition of adrenocortical carcinoma
cell proliferation by steroidogenic factor-1 inverse agonists. J
Clin Endocrinol Metab. 94:2178–2183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chuman Y, Zhan Z and Fojo T: Construction
of gene therapy vectors targeting adrenocortical cells: Enhancement
of activity and specificity with agents modulating the cyclic
adenosine 3′,5′-monophosphate pathway. J Clin Endocrinol Metab.
85:253–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sbiera S, Wortmann S and Fassnacht M:
Dendritic cell based immunotherapy - a promising therapeutic
approach for endocrine malignancies. Horm Metab Res. 40:89–98.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Papewalis C, Fassnacht M, Willenberg HS,
Domberg J, Fenk R, Rohr UP, Schinner S, Bornstein SR, Scherbaum WA
and Schott M: Dendritic cells as potential adjuvant for
immunotherapy in adrenocortical carcinoma. Clin Endocrinol (Oxf).
65:215–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
van Koetsveld PM, Vitale G, Feelders RA,
Waaijers M, Sprij-Mooij DM, De Krijger RR, Speel EJ, Hofland J,
Lamberts SW, De Herder WW, et al: Interferon-β is a potent
inhibitor of cell growth and cortisol production in vitro and
sensitizes human adrenocortical carcinoma cells to mitotane. Endocr
Relat Cancer. 20:443–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu-Chittenden Y, Jain M, Kumar P, Patel
D, Aufforth R, Neychev V, Sadowski S, Gara SK, Joshi BH,
Cottle-Delisle C, et al: Phase I trial of systemic intravenous
infusion of interleukin-13-Pseudomonas exotoxin in patients with
metastatic adrenocortical carcinoma. Cancer Med. 4:1060–1068. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Soon PS, Tacon LJ, Gill AJ, Bambach CP,
Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson
BG, et al: miR-195 and miR-483-5p identified as predictors of poor
prognosis in adrenocortical cancer. Clin Cancer Res. 15:7684–7692.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Glover AR, Zhao JT, Gill AJ, Weiss J,
Mugridge N, Kim E, Feeney AL, Ip JC, Reid G, Clarke S, et al:
MicroRNA-7 as a tumor suppressor and novel therapeutic for
adrenocortical carcinoma. Oncotarget. 6:36675–36688.
2015.PubMed/NCBI
|
|
117
|
De Cremoux P, Rosenberg D, Goussard J,
Brémont-Weil C, Tissier F, Tran-Perennou C, Groussin L, Bertagna X,
Bertherat J and Raffin-Sanson ML: Expression of progesterone and
estradiol receptors in normal adrenal cortex, adrenocortical
tumors, and primary pigmented nodular adrenocortical disease.
Endocr Relat Cancer. 15:465–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Barzon L, Masi G, Pacenti M, Trevisan M,
Fallo F, Remo A, Martignoni G, Montanaro D, Pezzi V and Palù G:
Expression of aromatase and estrogen receptors in human
adrenocortical tumors. Virchows Arch. 452:181–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Montanaro D, Maggiolini M, Recchia AG,
Sirianni R, Aquila S, Barzon L, Fallo F, Andò S and Pezzi V:
Antiestrogens upregulate estrogen receptor β expression and inhibit
adrenocortical H295R cell proliferation. J Mol Endocrinol.
35:245–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sirianni R, Zolea F, Chimento A, Ruggiero
C, Cerquetti L, Fallo F, Pilon C, Arnaldi G, Carpinelli G,
Stigliano A, et al: Targeting estrogen receptor-α reduces
adrenocortical cancer (ACC) cell growth in vitro and in vivo:
Potential therapeutic role of Selective Estrogen Receptor
modulators (SERMs) for ACC treatment. J Clin End Metab.
97:E2238–E2250. 2012. View Article : Google Scholar
|
|
121
|
Chimento A, Sirianni R, Casaburi I, Zolea
F, Rizza P, Avena P, Malivindi R, De Luca A, Campana C, Martire E,
et al: GPER agonist G-1 decreases adrenocortical carcinoma (ACC)
cell growth in vitro and in vivo. Oncotarget. 6:19190–19203. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Giguère V, Yang N, Segui P and Evans RM:
Identification of a new class of steroid hormone receptors. Nature.
331:91–94. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Deblois G and Giguère V: Functional and
physiological genomics of estrogen-related receptors (ERRs) in
health and disease. Biochim Biophys Acta. 1812:1032–1040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Casaburi I, Avena P, De Luca A, Chimento
A, Sirianni R, Malivindi R, Rago V, Fiorillo M, Domanico F, Campana
C, et al: Estrogen related receptor α (ERRα) a promising target for
the therapy of adrenocortical carcinoma (ACC). Oncotarget.
6:25135–25148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hahner S, Kreissl MC, Fassnacht M,
Haenscheid H, Knoedler P, Lang K, Buck AK, Reiners C, Allolio B and
Schirbel A: [131I]iodometomidate for targeted radionuclide therapy
of advanced adrenocortical carcinoma. J Clin Endocrinol Metab.
97:914–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wood BJ, Abraham J, Hvizda JL, Alexander
HR and Fojo T: Radiofrequency ablation of adrenal tumors and
adrenocortical carcinoma metastases. Cancer. 97:554–560. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Cazejust J, De B, aère T, Auperin A,
Deschamps F, Hechelhammer L, Abdel-Rehim M, Schlumberger M,
Leboulleux S and Baudin E: Transcatheter arterial chemoembolization
for liver metastases in patients with adrenocortical carcinoma. J
Vasc Interv Radiol. 21:1527–1532. 2010. View Article : Google Scholar : PubMed/NCBI
|