Introduction
As a leading cause of cancer-related death, gastric
cancer has a high rate of mortality and incidence, particularly in
the Eastern world (1,2). Despite the recent progress in cancer
therapy, including biological immune therapy, radiotherapy and
chemotherapy, a majority of malignancies are still not curable
today (3). Therefore, it is
important to conduct studies to discover and develop advanced
therapeutics.
Programmed cell death has been considered as a
defense mechanism whereby defective and harmful cells are
eliminated (4). Disruption of
programmed cell death signaling pathways may result in elevated
cell proliferation and eventually cancer. As a cationic amphipathic
peptide which disrupts the mitochondrial membrane, KLA (sequence,
KLAKLAKKLAKLAK) may lead to mitochondrial swelling and
permeabilization (5–7), and ultimately programmed cell death by
causing the release of cytochrome c (8–10).
However, this peptide fails to effectively permeate the eukaryotic
plasma membrane and in the end shows low cytotoxicity in mammalian
cells (11,12). Accordingly, KLA requires the
assistance of other cell penetrating peptides for effective
translocation in micromolar concentrations. As KLA can pass through
the plasma membrane, it preferentially interferes with the charged
mitochondrial membrane to release cytochrome c, causes
disruption in mitochondrial membrane potential and eventually
induces cell death (13,14).
We undertook the present study to fully understand
the action mechanism of KLA, and explore its use as an antitumor
drug. In the present study, to circumvent the difficulties of low
drug penetration into solid tumors and to efficiently deliver KLA
into the tumor, CRGDKGPDC, a C-end rule peptide iRGD, was
introduced into KLA. Similar to regular peptides of the RGD
(Arg-Asp-Gly) type, iRGD mediates tumor-homing by first binding to
αv integrins, which are selectively expressed in various cell types
in tumors and the endothelium of tumor vessels, but not in normal
tissues (15). When proteolytically
cleaved in tumors, iRGD generates CRGDK/R which has an affinity for
NRP-1, a receptor which becomes activated and mediates its
penetration into tissue (16).
KLA-iRGD was shown to exhibit all the characteristics of a
tumor-specific homing peptide with tissue penetration activity.
Additionally, the penetration of KLA-iRGD was assessed using in
vivo approaches. Finally, the anticancer activity of the
KLA-iRGD recombinant protein was examined.
Materials and methods
Reagents, cell lines and tumors
Fluorescein isothiocyanate (FITC) was obtained from
Sigma Chemical Co. (St. Louis, MO, USA). DAPI was purchased from
Beyotime Biotechnology (Wuhan, China). To assay for cell viability,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma Chemical Co. The Annexin V-FLUOS staining
kit was purchased from Roche Applied Science (Indianapolis, IN,
USA). The primary antibodies mouse monoclonal anti-human CD31
(GM082301 1 ml RTU) were purchased from Gene-Tech Co. Ltd.
(Shanghai, China). The secondary antibody rat monoclonal anti-mouse
CD31 (553370; 0.5 mg/ml) was purchased from BD Pharmingen (San
Jose, CA, USA). The Cell Death Detection kit for terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) was acquired from Roche Applied Science. The αvβ3 antibody
was purchased from Cell Signaling Technology (Danvers, MA, USA).
The human NRP-1 antibody was purchased from Abcam (Cambridge, MA,
USA).
The human gastric carcinoma cell lines MKN45 and
KATO III were purchased from the Cell Bank of the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). All
human cell lines were cultured in RPMI-1640 medium with 10% fetal
bovine serum (FBS) (both from Invitrogen, Carlsbad, CA, USA), with
glutamine, streptomycin and penicillin at 37°C and 5%
CO2.
BALB/c mice (male, 18–22 g and 5- to 6-weeks old)
were purchased from the BK Lab Animal Ltd. (Shanghai, China), and
were maintained at 25±1°C, under specific pathogen-free (SPF)
condition, and were given access to food and water ad
libitum. All animal procedures were conducted according to the
regulations of the Animal Care Committee at Drum Tower Hospital
(Nanjing, China). MKN45 gastric cancer cells (5×106) in
0.1 ml phosphate-buffered saline (PBS) were subcutaneously injected
into the lower right flank of athymic nude BALB/c mice, so as to
generate tumor xenografts. The volume of the tumors was computed
with a digital Vernier caliper and using the following formula:
Tumor volume = (length × width2)/2, where the width has
the widest dimension and the length has the longest dimension.
Peptide synthesis
High purity (>90%) peptides were obtained from
Bankpeptide Biological Technology Co. Ltd. (Hefei, China). An
integrated product of iRGD and KLA was generated for delivery into
the mitochondria. Two unstructured glycine residues, as a spacer,
were inserted. All the peptide products were analyzed by analytic
high-pressure liquid chromatography. Additionally, the predicted
molecular weight (MW) of the resulting conjugates are listed in
Table I. Freeze-dried peptides were
reconstituted in high-purity dimethyl sulfoxide (DMSO) (10 mM) and
kept at −70°C until use.
 | Table I.Peptide sequences and molecular weight
of the peptides. |
Table I.
Peptide sequences and molecular weight
of the peptides.
|
| Sequences |
|
|---|
|
|
|
|
|---|
| Peptides | Protein transduction
domain | Linker | Pro-apoptotic
domain | Expected molecular
weight (Da) |
|---|
| KLA |
|
| KLAKLAKKLAKLAK | 1,524.01 |
| KLA-iRGD | CRGDKGPDC | GGGGS GGGGS | KLAKLAKKLAKLAK | 3,086.62 |
| KLA-RGD | ACDCRGDCFC | GGGGS GGGGS | KLAKLAKKLAKLAK | 3,228.81 |
Western blotting to assess αvβ3 and
NRP-1 expression
Protein extracts were prepared from MKN45 and KATO
III cells (2×106), cultured for 30 min, using the
following protein lysis buffer: 150 mM NaCl, 1 mM dithiothreitol
(Merck, Cefoperazone, Germany), 50 mM Tris-HCl (pH 7.5), 0.1%
sodium dodecyl sulfate (SDS), and protease inhibitors (Roche,
Basel, Switzerland). The extracts were separated using SDS
polyacrylamide gel electrophoresis and electrophoretically
transferred to nitrocellulose membrane (Millipore, Waltham, MA,
USA). Standard western blot analyses were conducted using the
antibodies against αvβ3 and NRP-1. The blots were incubated at room
temperature for 1 h with the antibody conjugated to horseradish
peroxidase (HRP) and detected with an enhanced chemiluminescence
reagent (Cell Signaling Technology Inc., USA). The autoradiographic
intensity of each band was scanned and quantified using BandScan
software (Glyko, Inc., Novato, CA, USA). Values were normalized to
GAPDH and the ratio to the control values was calculated.
Cell internalization of the
recombinant proteins
MKN45 and KATO III cells, in the logarithmic growth
phase, were seeded at 1.0×106/well in 24-well chamber
slides (Nalge Nunc International Corp., Rochester, NY, USA).
Subconfluent tumor cells on the chamber slides were incubated for 1
h with 10 µg/ml FITC-labelled protein. Afterwards, the cells were
rinsed 3 times with PBS (pH 7.4) and fixed with ice-cold methanol
for 10 min. The cells were then rinsed 3 times with PBS (pH 7.4)
and the nuclei were stained with DAPI. Subsequently, the cells were
observed with a laser scanning confocal microscope (LSCM; Zeiss LSM
710).
Antiproliferation assay
MKN45 and KATO III cells, in the logarithmic growth
phase, were seeded at 5×103 cells/well in 96-well plates
and cultured in a humid environment at 37°C in 5% CO2
for 12 h. Thereafter, the medium was removed, and the cells were
exposed to serum-free medium containing KLA, KLA-iRGD and KLA-RGD.
After incubation for 24 h, the viability of the cells was evaluated
using an MTT assay following the manufacturer's instructions. The
cell viability values were calculated by a nonlinear regression
study utilizing GraphPad Prism v5.0 software (San Diego, CA,
USA).
Flow cytometric analysis
The induction of apoptosis was detected with the
Annexin V-FLUOS staining kit following the manufacturer's
instructions. Briefly, the cells were cultured at a density of
1.0×106 cells/ml at 37°C in 5% CO2, treated
with 100 ng/ml fusion protein (KLA, KLA-iRGD and KLA-RGD), and
continued to culture for 4 h. Afterwards, the cells were washed 2
times with cold PBS and a volume of 100 µl of Annexin V-FLUOS was
added to the cells. Then, all cell samples were cultured in the
dark for 15 min at room temperature and gently vortexed, before
flow cytometric analysis on a FACSort flow cytometer
(Becton-Dickinson, San Jose, CA, USA).
Tumor tissue penetration in vivo
An animal model was used to study the recombinant
protein penetration and to trace the location of the protein.
BALB/c mice were subcutaneously inoculated with MKN45 cells. The
MKN45 tumor-bearing mice were inoculated via a tail vein with
FITC-labeled KLA-iRGD when tumors of ~200 mm3 were
formed. One hour after administration of the peptide, the mice were
sacrificed and tumors were harvested. As mentioned previously,
immunofluorescence analysis was carried out with frozen tumor
tissues. For immunostaining, the slides were first blocked at room
temperature with 20% goat serum for 1 h, and then incubated with an
anti-mouse antibody against CD31 (1×300 dilution) at 4°C overnight.
Finally, the slides were subjected to DAPI staining for nuclear
counterstaining and visualized with a LSCM laser scanning confocal
microscope.
Tumor treatment in nude mice
Animal handling was performed in accordance with the
protocols of the Institutional Animal Care and Use Committee
(IACUC) of Ohio State University. Five-week-old BALB/c nude mice
were subcutaneously implanted with 2×106 MKN45 cells in
the right flank. Tumor-bearing mice were assigned to 3 treatment
groups ~2 weeks after the inoculation of the MKN45 cells. The
assignment was based on tumor size to ensure that there was no
statistical difference in tumor volume among the groups when the
treatment began. The peptide molecules, at 1 mg/ml in PBS, were
intraperitoneally injected. A total of 4 injections were
administered into the mice of the MKN45 model every 3 days. The
volume of the tumor was evaluated with an electronic caliper and
the tumor volume was computed from measurements of 2 parameters
utilizing the following formula: Tumor volume = (length ×
width2)/2. Based on the guideline of IACUC, the
experiments were halted when the diameter of the tumors reached 1.5
cm. Finally, xenografts were weighed and removed and mice were
sacrificed. Regarding the toxicological assessment of the peptide
treatments, organs, including the heart, kidney, liver, spleen and
lung, were fixed in neutral buffered formalin, embedded in
paraffin, and stained with hematoxylin and eosin (H&E) for
pathological study after the animals were euthanized. TUNEL assay
was used for evaluation of tumor cell apoptosis. The collected
slices were evaluated using optical microscopy.
Statistical analysis
All the in vitro experiments were repeated at
least 3 times. Statistical analysis was conducted using GraphPad
Prism version 5.0 (GraphPad Software). Statistical values are
expressed as means ± SD. The statistical analysis was performed
using the two-tailed Student's t-test, followed by the Bonferroni
test. Different group comparison was conducted by one-way analysis
of variance (ANOVA). Two-way ANOVA was used to examine whether the
difference between the data from the various groups was
significantly different regarding peptide and the effects of the
fused peptides for every parameter. A p-value of <0.05 was
regarded as significantly different.
Results
Internalization of the recombinant
proteins into cells
To deliver the KLA molecule into cells and
potentially into the cytoplasm, we fused KLA to a tumor-penetrating
iRGD peptide. For comparison, we also used RGD (sequence:
ACDCRGDCFC), a peptide in which the CendR motif is constitutively
active. The internalization of the conjugate by MKN45 and KATO III
cells was studied by LSCM using FITC-labeled KLA, KLA-iRGD and
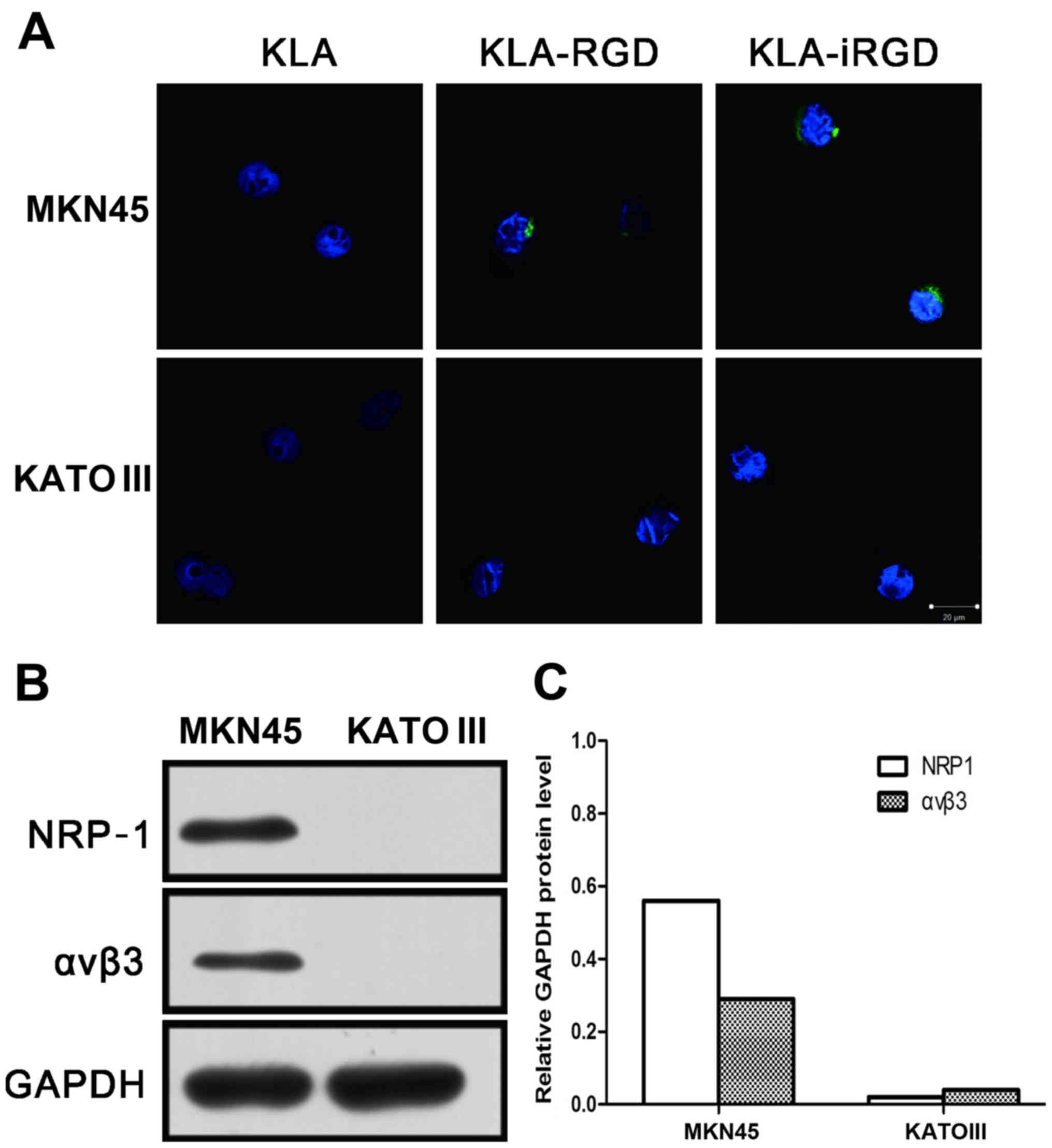
KLA-RGD. KLA with no CendR peptide showed negligible binding to the
MKN45 cells (Fig. 1A), a cell line
that expresses high levels of NRP-1 and αvβ3 integrin (Fig. 1B and C). Additionally, KLA also
showed negligible binding to the KATO III cells, a cell line that
expresses low levels of NRP-1 and αvβ3 integrin. In contrast, both
KLA-iRGD and KLA-RGD effectively bound to the MKN45 cells, but
KLA-RGD was only weakly internalized. As expected, KLA-iRGD and
KLA-RGD did not bind to or were internalized into the
NRP-1-deficient KATO III cells. The entry of KLA-iRGD into the
cells was rapid, as indicated by the finding that after a 30-min
incubation, the protein was detectable in the MKN45 cells.
Reduction in cell viability by
recombinant proteins
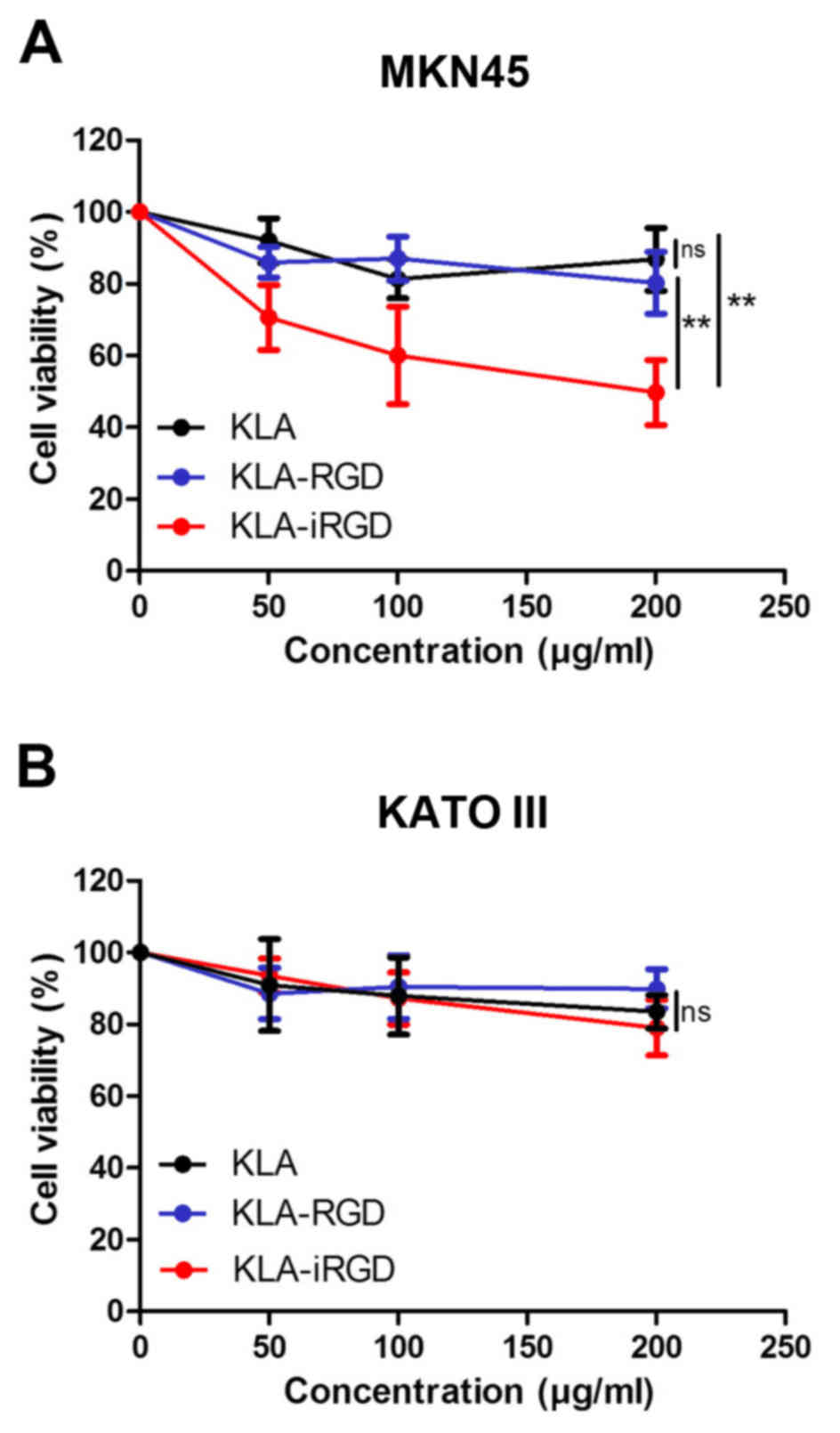
In order to confirm the pharmacological activity of
KLA-iRGD, in vitro cytotoxicity analyses were performed
using the MTT assay to monitor the viability of the MKM45 and KATO
III cells. The results revealed that KLA-RGD had no
antiproliferative activity against the MKN45 cells at the highest
concentration (200 µg/ml) tested compared with KLA (p=0.136)
(Fig. 2A). In contrast, a
dose-dependent cytotoxicity was detected for KLA-iRGD (p=0.002),
indicating that the extra iRGD motif enhanced the antiproliferative
activity of KLA-iRGD in the human gastric cancer cell line MKN45.
Regarding the cell line KATO III (Fig.
2B), KLA-iRGD and KLA-RGD exhibited no obvious
antiproliferative activity compared with KLA (p=0.144 and p=0.287).
Although KLA-iRGD exhibited a stronger inhibitory effect on KATO
III proliferation, the differences between KLA, KLA-iRGD and
KLA-RGD were not evident.
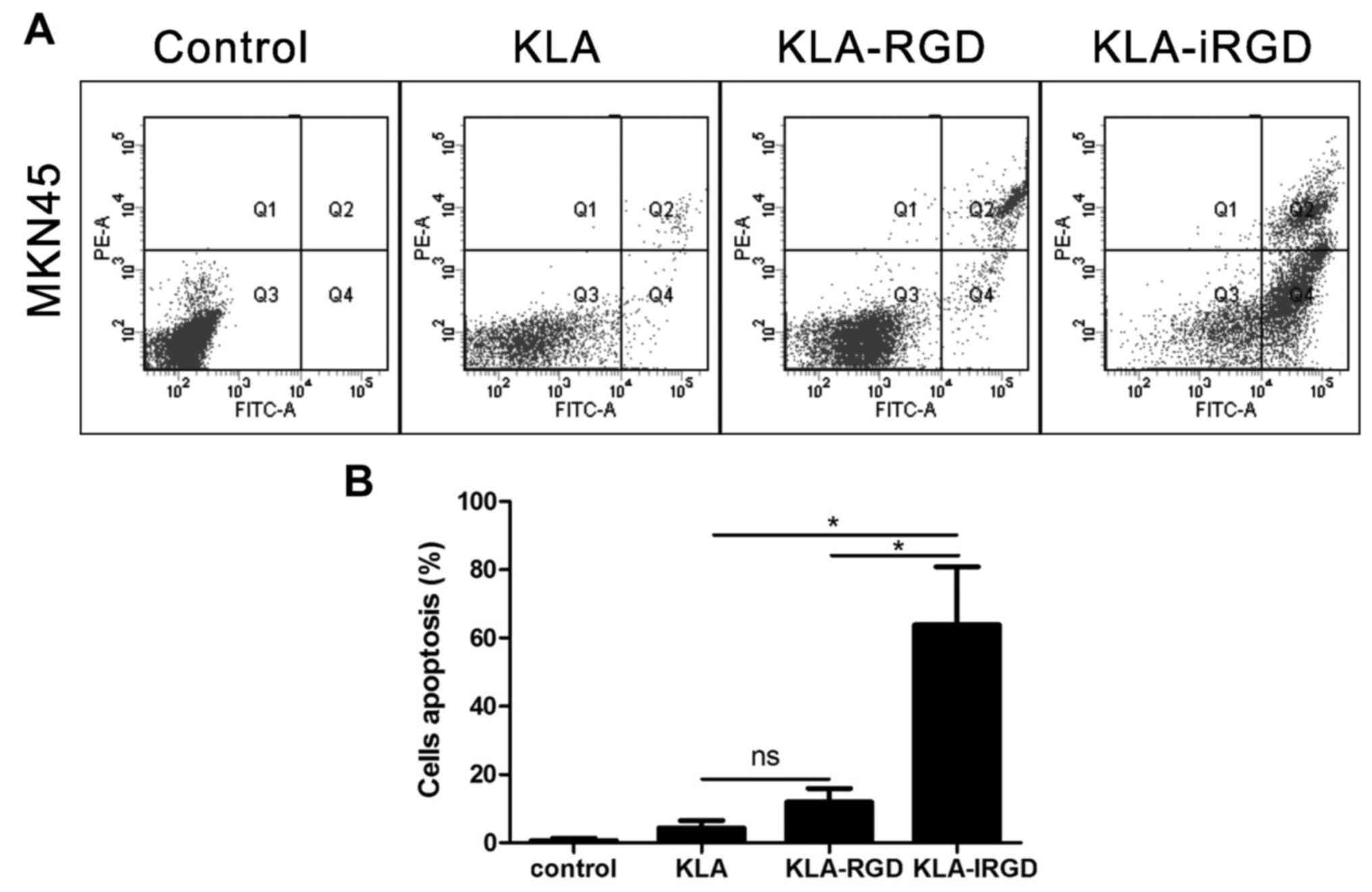
To ascertain whether these fusion peptides induce
apoptosis, we analyzed the apoptotic rates by flow cytometric
analysis. The overlapping effects in the MKN45 cells were measured
by an Annexin V-FLUOS staining apoptosis assay. The finding of
differential staining can be expressed as the percentage of
positive cells compared to the total number of cells. Our results
revealed that both KLA and KLA-RGD induced low levels of apoptosis
in the MKN45 cells (Fig. 3A), as
indicated by the percentages of early and late apoptotic MKN45
cells following treatment with KLA and KLA-RGD (4.3±2.2 and
11.9±3.9%, respectively; p=0.115). In comparison, KLA-iRGD induced
not only apoptosis, but also necrosis as shown in Fig. 3A, and the percentage of early and
late apoptosis was much higher (63.9±17.0%) compared with KLA and
KLA-RGD (p=0.023 and p=0.024). According to these findings,
KLA-iRGD increased apoptosis in vitro and effectively
entered into cancer cells (Fig.
3B).
Penetration of KLA-iRGD into tumor
tissue
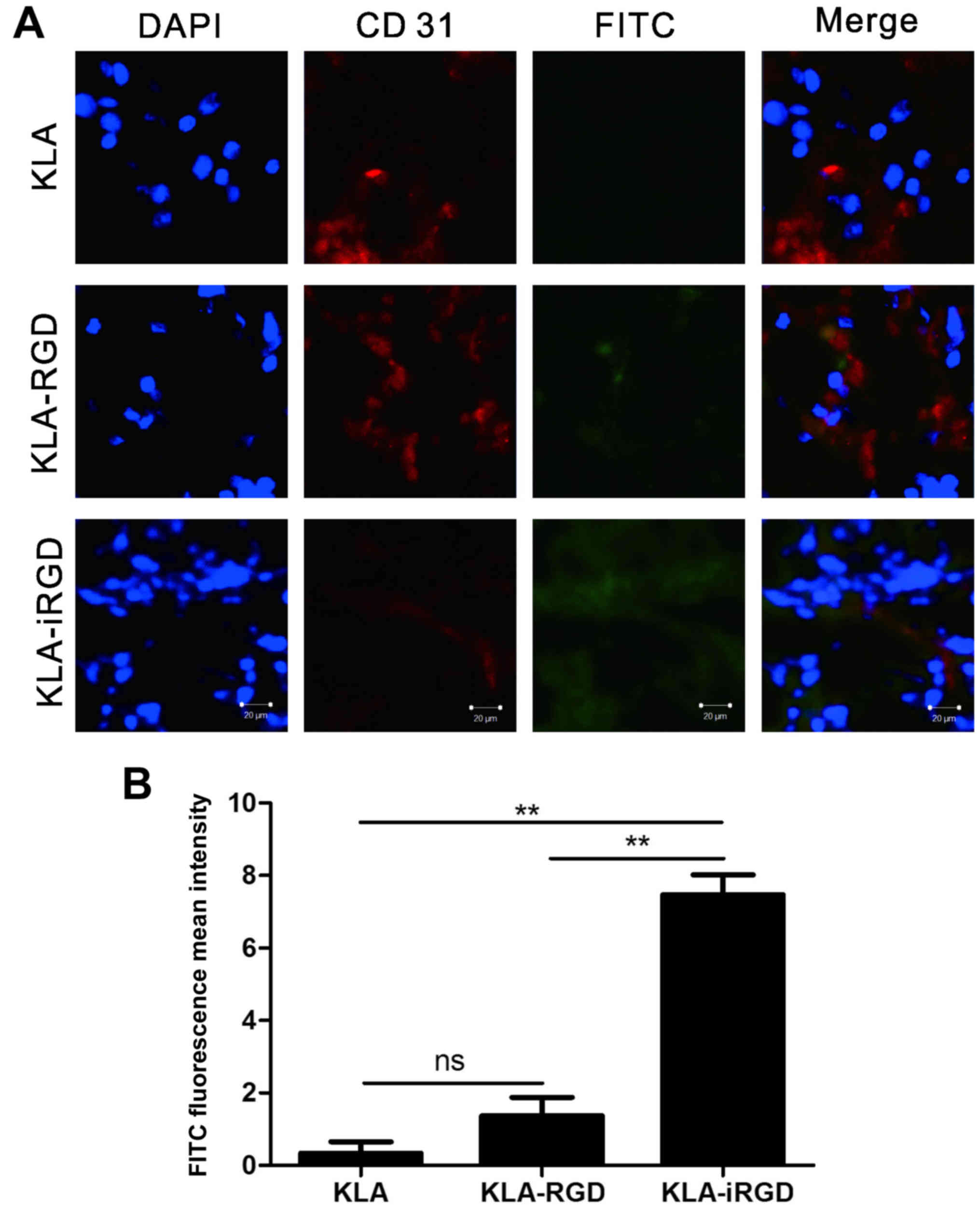
Through the use of tumor tissue derived from the
MKN45 tumor-bearing mice, we studied the penetration capability of
KLA-iRGD (Fig. 4A). According to
the fluorescence signal on the LSCM images, the locations of the
recombinant protein were displayed as FITC-labelled KLA, KLA-iRGD
and KLA-RGD. An anti-CD31 antibody and a Cy3-conjugated secondary
antibody bound to blood vessels. Therefore, red fluorescence was
detected in blood vessels. Actually, KLA-RGD was localized in the
vicinity of blood vessels 1 h post-injection, and it was
additionally found that it could be targeted to the tumor tissue.
In contrast, the KLA-iRGD peptide was found to penetrate into
extravascular tumor tissue, with a tumor penetration activity that
was readily apparent. In fact, a strong KLA-iRGD signal was
detectable in all sections from the entire tumor. KLA-iRGD traveled
further from the tumorous vessels and was found on a greater field
in the tumor tissues in comparison with KLA-RGD. Specifically, the
FITC fluorescence mean intensity of KLA-RGD was 5 times weaker than
that of KLA-iRGD (Fig. 4B). All the
statistical analysis results support the notion that as a
functional group, iRGD mediates the active tumor penetration of
KLA-iRGD protein.
Inhibitory effect of KLA-iRGD in the
xenograft model
In order to examine the in vivo antitumor
effects of KLA-iRGD, we systemically administered the peptide into
nude mice with established MKN45 tumors. We initially injected KLA
and KLA-iRGD intraperitoneally at 10 mg/kg of mouse body weight
every 3 days. The tumor size was measured during the different
treatment times. The size of the MKN45 tumors increased rapidly in
mice intraperitoneally injected with PBS. In comparison, in the
KLA-iRGD-treated mice, the inhibition of tumor growth was evident
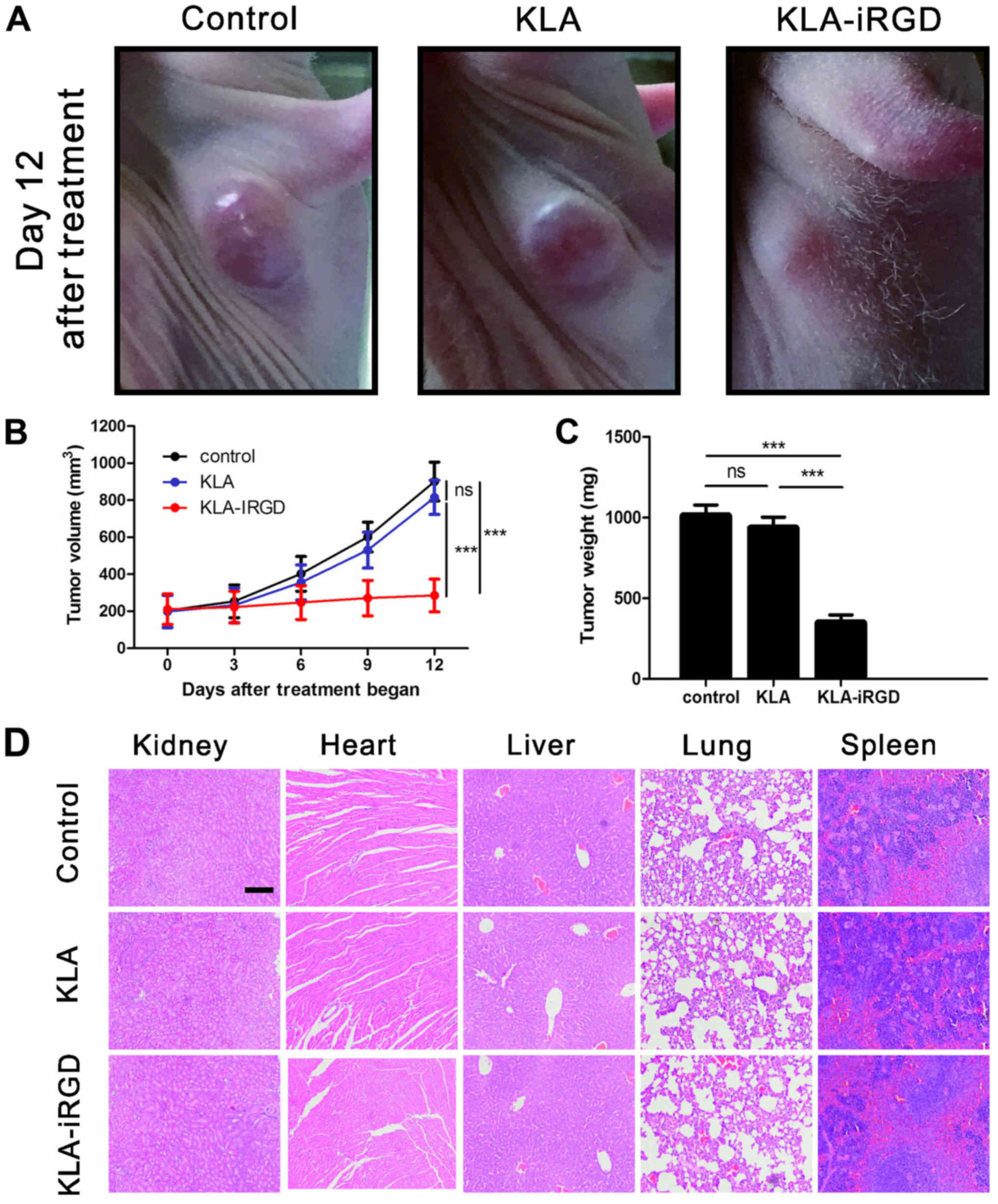
as early as 3 days after treatment. Representative images of nude
mice with established MKN45 tumors after a 12-day post treatment
period with KLA-iRGD, KLA or PBS as control are presented in
Fig. 5A. The time-dependent changes
in MKN45 tumor growth in the nude mice are shown in Fig. 5B. Clearly, systemic delivery of
KLA-iRGD led to the effective suppression of MKN45 tumor growth in
the xenograft model. After completion of the xenograft experiment,
we dissected and weighed the MKN45 tumor xenografts. The results
shown in Fig. 5C, revealed that
KLA-iRGD significantly suppressed MKN45 tumor growth. To test the
potential toxicity of KLA-iRGD in the experimental mice,
H&E-stained section of the kidney, lung, heart, liver and
spleen were examined after peptide treatment. As shown in Fig. 5D, there were no obvious changes in
these organs following treatment with KLA-iRGD compared with the
control. Additionally, no skin abnormality around the injection
sites was observed, suggesting the tumor-specific penetration of
KLA-iRGD.
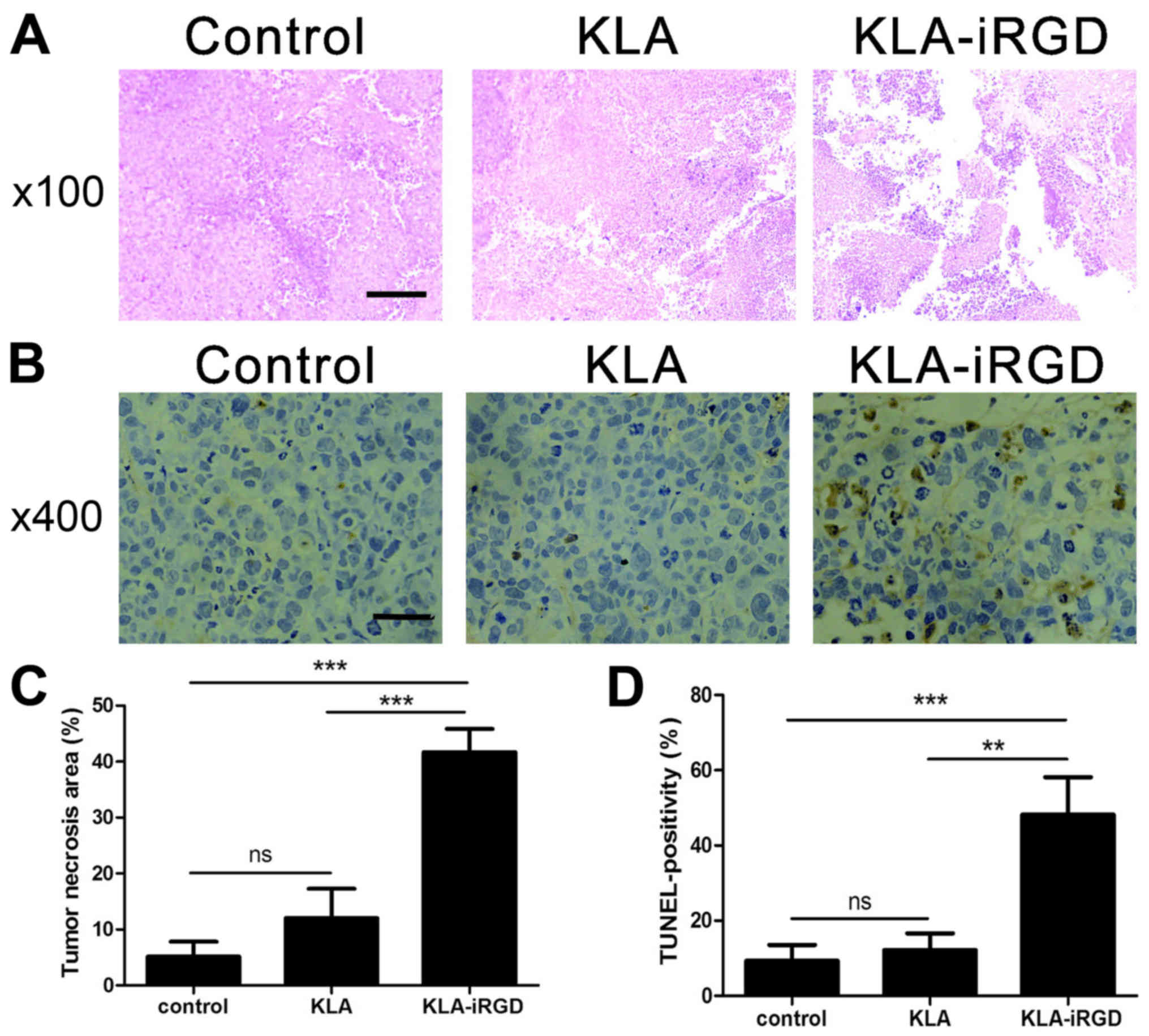
H&E-stained tumor sections showed differences
among tumors treated with KLA-iRGD, KLA or PBS (Fig. 6A). In the control groups treated
with either KLA or PBS, small amounts of necrotic regions were
present. In contrast, the KLA-iRGD-treated group displayed larger
necrotic regions, which explains the smaller volumes of the tumors
in the group at the pathological level. Furthermore, tumors from
the KLA-iRGD group showed significantly stronger TUNEL staining
than tumors treated with PBS or KLA, indicating substantial cell
death in the KLA-iRGD-treated tumors (Fig. 6B).
Discussion
As one of the main cause of cancer-related death
worldwide, gastric cancer has a high rate of incidence and
mortality (2). Accordingly, it is
important to develop therapeutic agents with reduced side-effects
and high specificity for gastric cancer (17). Several available cancer chemotherapy
agents are limited by their serious side-effects and acquired
resistance (18). Thus, there is an
urgent need for a drug delivery system which delivers drugs at the
target sites of tumor tissues. Phage display peptide libraries
improve the therapeutic efficacy of anticancer drugs by providing
peptides with high affinity and specificity, and in addition they
typically have low interaction with the immune system and good
tumor and tissue penetration (19).
In the targeted delivery of anticancer drugs into cancer tissue the
ability to penetrate deep into the solid tumor tissue can
potentially be a great challenge for the targeted therapeutic
(3). In solid cancer, the
homeostatic regulation of tissue fails, cancer cells are in a
hypoxic state, the pressure of interstitial fluid increases
(20–23), and the extracellular matrix (ECM)
impedes the motion of molecules and drugs into the tumor tissue
(24–26).
As a pro-apoptotic peptide, KLA leads to cellular
apoptosis by disrupting mitochondrial homeostasis and activating
caspases. Caspase-activating cytochrome c release from
damaged mitochondria induces activation of caspases-9 and −3, and
mediates both early and late apoptosis (17,27).
Originally developed as an antibacterial peptide, KLA is non-toxic
in eukaryotic cells as it cannot be actively internalized (6,28).
Indeed, since KLA fails to efficiently pass through eukaryotic
plasma membranes (29), an
alternative method has been developed that involves the integration
of KLA with other drugs, which are more efficient than the
individual therapy. In this regard, the pro-apoptotic activity of
the targeted fusion protein KLA-iRGD was reported. The iRGD peptide
was shown to follow a multistep tumor-targeting mechanism. The
intact peptide binds to αv integrins expressed on the cell surface,
where it is proteolytically cleaved to generate the fragment of
CRGDK (15). Such fragmenst bind to
neuropilin-1 and are internalized into tumor cells, thus
penetrating tumor tissues (16).
The cell-penetrating activity of iRGD is greater than that of
common RGD peptides (15). It is
actually greater than what can be accomplished with regular RGD
peptides and mimics, which only deliver payloads to the tumor
vessels.
In the present study, we coupled iRGD to KLA to
study their antiproliferation and penetration activities on gastric
cancer. We also showed that the KLA peptide is highly active when
introduced into tumor cells using protein transduction mediated by
the cell-internalizing peptide iRGD. Moreover, the present study
showed that the fusion protein is an excellent antitumor agent
which can be released and spread in tumor tissues through
intravenous injection. In addition, in the present study, we
demonstrated that these peptides provide a protein with anticancer
activity with the ability to penetrate readily into tumor tissue.
Furthermore, the in vitro cytotoxicity of KLA-iRGD to MKN45
cells was effectively enhanced. The results from the MTT assay
indicated that KLA-iRGD clearly decreased cell viability. Flow
cytometric analysis of cell apoptosis also demonstrated that
KLA-iRGD increased the induction of both early and late apoptosis
in the MKN45 cells. We also showed that KLA-iRGD is effective in
suppressing the growth of gastric cancer cell lines in vivo.
The improved antitumor activity of KLA-iRGD can be attributed to
the iRGD peptide, which facilitates the internalization of the
formulation into MKN45 cells.
A recombinant protein iRGD-CDD was defined and
constructed by Chen et al (30) who confirmed the extensive
distribution of iRGD-CDD in tumors and showed that the iRGD peptide
can be active in protein transduction. Similarly, in the present
study, after intraperitoneal injection, the fusion protein KLA-iRGD
was found to be a promising antitumor agent to spread in tumor
tissue. We made use of the tumor penetration activity of KLA-iRGD
in the development of a new anticancer treatment approach. The
treatment of tumor-bearing mice with KLA-iRGD effectively inhibited
tumor growth in vivo, resulting in marked reduction in MKN45
tumor volume. Furthermore, KLA-iRGD did not cause detectable
toxicity to important organs, such as the lung, liver, heart,
spleen and kidney, in the tumor-bearing mice. KLA together with
iRGD led to an improved pro-apoptotic effect, likely due to the
high concentration of KLA in the targeted tumor cells.
To conclude, the present study provides an approach
to construct a fusion protein of peptide KLA and iRGD to target
tumors. The construct facilitated the entry of KLA into human MKN45
gastric carcinoma cells, where it decreased the growth of tumors in
mice and exerted an apoptotic effect. There are still many issues
associated with this approach that need to be addressed in order to
ensure the enhanced clinical application, including pharmacokinetic
properties and stability of the recombinant proteins, and the
mechanisms underlying the antitumor effect of KLA-iRGD. We believe
that KLA-iRGD could be utilized as an anticancer agent. However,
further research is warranted to confirm this suggestion.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81370064, 81502037
and 81572329).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sha H, Zou Z, Xin K, Bian X, Cai X, Lu W,
Chen J, Chen G, Huang L, Blair AM, et al: Tumor-penetrating peptide
fused EGFR single-domain antibody enhances cancer drug penetration
into 3D multicellular spheroids and facilitates effective gastric
cancer therapy. J Control Release. 200:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Refaat A, Abd-Rabou A and Reda A: TRAIL
combinations: The new ‘trail’ for cancer therapy (Review). Oncol
Lett. 7:1327–1332. 2014.PubMed/NCBI
|
|
5
|
Foillard S, Jin ZH, Garanger E, Boturyn D,
Favrot MC, Coll JL and Dumy P: Synthesis and biological
characterisation of targeted pro-apoptotic peptide. Chembiochem.
9:2326–2332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellerby HM, Arap W, Ellerby LM, Kain R,
Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E,
et al: Anti-cancer activity of targeted pro-apoptotic peptides. Nat
Med. 5:1032–1038. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marks AJ, Cooper MS, Anderson RJ, Orchard
KH, Hale G, North JM, Ganeshaguru K, Steele AJ, Mehta AB, Lowdell
MW, et al: Selective apoptotic killing of malignant hemopoietic
cells by antibody-targeted delivery of an amphipathic peptide.
Cancer Res. 65:2373–2377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thornberry NA, Rosen A and Nicholson DW:
Control of apoptosis by proteases. Adv Pharmacol. 41:155–177. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu DS, Bocek MJ, Shi J, Ta A,
Ngambenjawong C, Rostomily RC and Pun SH: Multivalent display of
pendant pro-apoptotic peptides increases cytotoxic activity. J
Control Release. 205:155–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Javadpour MM, Juban MM, Lo WC, Bishop SM,
Alberty JB, Cowell SM, Becker CL and McLaughlin ML: De novo
antimicrobial peptides with low mammalian cell toxicity. J Med
Chem. 39:3107–3113. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hyun S, Lee S, Kim S, Jang S, Yu J and Lee
Y: Apoptosis inducing, conformationally constrained, dimeric
peptide analogs of KLA with submicromolar cell penetrating
abilities. Biomacromolecules. 15:3746–3752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mai JC, Mi Z, Kim SH, Ng B and Robbins PD:
A proapoptotic peptide for the treatment of solid tumors. Cancer
Res. 61:7709–7712. 2001.PubMed/NCBI
|
|
14
|
Ma JL, Wang H, Wang YL, Luo YH and Liu CB:
Enhanced Peptide delivery into cells by using the synergistic
effects of a cell-penetrating Peptide and a chemical drug to alter
cell permeability. Mol Pharm. 12:2040–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugahara KN, Teesalu T, Karmali PP,
Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF and
Ruoslahti E: Tissue-penetrating delivery of compounds and
nanoparticles into tumors. Cancer Cell. 16:510–520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugahara KN, Teesalu T, Karmali PP,
Kotamraju VR, Agemy L, Greenwald DR and Ruoslahti E:
Coadministration of a tumor-penetrating peptide enhances the
efficacy of cancer drugs. Science. 328:1031–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu B, Long W, Zhang Y, Zhang A, Miao F,
Shen Y, Pan N, Gan G, Nie F, He Y, et al: Enhanced antitumor
effects of the BRBP1 compound peptide BRBP1-TAT-KLA on human brain
metastatic breast cancer. Sci Rep. 5:80292015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bryde S and de Kroon AI: Nanocapsules of
platinum anticancer drugs: Development towards therapeutic use.
Future Med Chem. 1:1467–1480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Xiao W, Zhang Y, Meza L, Tseng H,
Takada Y, Ames JB and Lam KS: Optimization of RGD-containing cyclic
peptides against αvβ3 integrin. Mol Cancer Ther. 15:232–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jain RK: The Eugene M. Landis Award
Lecture 1996. Delivery of molecular and cellular medicine to solid
tumors. Microcirculation. 4:1–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Milosevic MF, Fyles AW, Wong R, Pintilie
M, Kavanagh MC, Levin W, Manchul LA, Keane TJ and Hill RP:
Interstitial fluid pressure in cervical carcinoma: Within tumor
heterogeneity, and relation to oxygen tension. Cancer.
82:2418–2426. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heldin CH, Rubin K, Pietras K and Ostman
A: High interstitial fluid pressure - an obstacle in cancer
therapy. Nat Rev Cancer. 4:806–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li R, Xie L, Zhu Z, Liu Q, Hu Y, Jiang X,
Yu L, Qian X, Guo W, Ding Y, et al: Reversion of pH-induced
physiological drug resistance: A novel function of copolymeric
nanoparticles. PLoS One. 6:e241722011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Netti PA, Berk DA, Swartz MA, Grodzinsky
AJ and Jain RK: Role of extracellular matrix assembly in
interstitial transport in solid tumors. Cancer Res. 60:2497–2503.
2000.PubMed/NCBI
|
|
25
|
Davies CL, Berk DA, Pluen A and Jain RK:
Comparison of IgG diffusion and extracellular matrix composition in
rhabdomyosarcomas grown in mice versus in vitro as spheroids
reveals the role of host stromal cells. Br J Cancer. 86:1639–1644.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown E, McKee T, diTomaso E, Pluen A,
Seed B, Boucher Y and Jain RK: Dynamic imaging of collagen and its
modulation in tumors in vivo using second-harmonic generation. Nat
Med. 9:796–800. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma X, Jia J, Cao R, Wang X and Fei H:
Histidine-iridium (III) coordination-based peptide luminogenic
cyclization and cyclo-RGD peptides for cancer-cell targeting. J Am
Chem Soc. 136:17734–17737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agemy L, Friedmann-Morvinski D, Kotamraju
VR, Roth L, Sugahara KN, Girard OM, Mattrey RF, Verma IM and
Ruoslahti E: Targeted nanoparticle enhanced proapoptotic peptide as
potential therapy for glioblastoma. Proc Natl Acad Sci USA.
108:17450–17455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alves ID, Carré M, Montero MP, Castano S,
Lecomte S, Marquant R, Lecorché P, Burlina F, Schatz C, Sagan S, et
al: A proapoptotic peptide conjugated to penetratin selectively
inhibits tumor cell growth. Biochim Biophys Acta. 1838:2087–2098.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen R, Braun GB, Luo X, Sugahara KN,
Teesalu T and Ruoslahti E: Application of a proapoptotic peptide to
intratumorally spreading cancer therapy. Cancer Res. 73:1352–1361.
2013. View Article : Google Scholar : PubMed/NCBI
|