Introduction
Breast cancer is the second leading cause of
cancer-related death among women after lung cancer, accounting for
~23% of all cases of cancer in women (1). According to the American Cancer
Society's estimates for 2016, ~246,660 new cases of invasive breast
cancer are expected to be diagnosed and ~40,450 women may die from
breast cancer in the US (2). In
China, the incidence and mortality of breast cancer have
progressively increased during the past few decades (3). There are several treatment strategies
including surgery, chemotherapy, radiation, hormone and targeted
therapies, for breast cancer, which are dependent on various
factors, such as the type of breast cancer, its stage and other
special situations.
Breast cancer is a molecular heterogeneous
malignancy, which can be divided into three basic types based on
their immunohistochemical properties (receptor status):
HR-positive, human epidermal growth factor receptor 2-positive
(HER-2+), and triple-negative breast cancers (4). The receptor status of a breast cancer
is a vital factor in treatment decisions, since it determines
whether the tumor can be treated with hormone or targeted therapy;
this has significantly improved the prognosis of breast cancer
patients (5,6). Approximately 70% of breast cancers are
ERα-positive. ERα-positivity is a crucial factor for deciding
whether breast cancer patients can benefit from anti-estrogen
therapy, which leads to a significant reduction in the mortality of
breast cancer patients (7).
However, despite advances in first-line endocrine therapy of
advanced HR-positive breast cancer, cancer recurrence and
subsequent drug resistance appear to be inevitable and present
serious obstacles to successful treatment (8,9).
Therefore, it is essential to explore new molecular markers to
identify the subgroup of breast tumors and to identify prognostic
markers to improve the outcome of breast cancer patients.
TMPRSS3 encodes a protein that belongs to the serine
protease family, which participate in various biological processes,
and whose dysregulation often leads to human diseases and
disorders. TMPRSS3 has been reported as a tumor-associated gene
that is overexpressed in pancreatic cancer (10) and ovarian tumors. It promotes the
proliferation, invasion and migration of ovarian cancer cells
(11). As an important
tumor-associated gene, TMPRSS3 expression and its prognostic value
in breast cancer has been reported, but the results are
controversial (12,13).
TNFRSF11B, also called osteoprotegerin (OPG) or
osteoclastogenesis inhibitory factor, is a cytokine receptor
belonging to the tumor necrosis factor (TNF) receptor superfamily.
OPG is a decoy receptor for the receptor activator of nuclear
factor-κB ligand (RANKL), which blocks RANKL-RANK interaction by
binding to RANKL (14). Receptor
activator of nuclear factor-κB and its ligand (RANK/RANKL) and OPG
are key molecules for bone metabolism. The RANK/RANKL/OPG pathway
plays an important role in the development and progression of bone
metastasis in various types of cancers (15). However, little is known concerning
the role of OPG in breast cancer prognosis. Therefore, we aimed to
evaluate OPG expression and the associated clinical significance in
breast cancer.
Although, TMPRSS3 and TNFRSF11B have been found to
play an important role in breast cancer, the expression levels and
the clinical significance of TMPRSS3 and TNFRSF11B in breast cancer
remain unclear. In the present study, we analyzed TMPRSS3 and
TNFRSF11B expression and explored their prognostic value to provide
useful insight into the development of more effective targeted
therapies for breast cancer.
Materials and methods
Statement of ethics
The study protocol and acquisition of tissue
specimens were approved by the Ethics Committee of Ganzhou City
People's Hospital (2015-RES-15). Each participant provided written
informed consent before participating in the present study.
Patients and tissue samples
The paraffin specimens used for immunohistochemistry
were collected from 86 breast cancer patients undergoing surgical
resection and were classified according to the most recent World
Health Organization (WHO) classification which was confirmed by two
experienced pathologists independently at the Shanghai 10th
People's Hospital, Tongji University School of Medicine between
2007 and 2015. Clinical data were collected from patient operative
and pathological records, and follow-up data were gathered by
telephone or direct correspondence. The time of tumor relapse or
death was verified by the patient or their relatives, by medical
recording, or by the social security record. Overall survival (OS)
was calculated in months from the date of diagnosis to the time of
death, regardless of cause. Disease-free survival (DFS) was defined
as the period from the initial date of diagnosis to the time of
tumor progression, or to the time of death due to the disease.
Bioinformatic analysis
The expression levels of TMPRSS3 and TNFRSF11B were
investigated in a gene microarray from the GEO database.
Hierarchical clustering was performed using the multiple experiment
viewer (MeV) 4.9.0 software (http://www.tm4.org/mev/).
Immunohistochemistry and H&E
staining
Immunohistochemical and hematoxylin and eosin
(H&E) staining were used to evaluate TMPRSS3 and TNFRSF11B
expression levels in 86 breast cancer samples. Tissue samples
stained for TMPRSS3 and TNFRSF11B expression were classified into
five categories and given a score from 0 to 5 according to the
percentage of positively stained cells in each sample: ‘0’ (0%),
‘1’ (1–25%), ‘2’ (>25–50%), ‘3’ (>50–75%) and ‘4’
(>75–100%). Additionally, the staining intensity of tissue
samples was used to divide them into four categories and assign
them a score between 0 and 3: 0, negative; 1, weak; 2, moderate;
and 3, strong. Then, the product of the first and second score was
used to determine TMPRSS3 and TNFRSF11B expression levels.
Statistical analysis
The results are expressed as mean ± SD (standard
deviation). Statistical significance between the groups was
assessed using one-way analysis of variance (ANOVA). Univariate
survival and multivariate analyses were carried out using the
Kaplan-Meier method. All calculations were performed using the SPSS
20.0 software program (SPSS, Inc., Chicago, IL, USA). The level of
significance was chosen as P<0.05.
Results
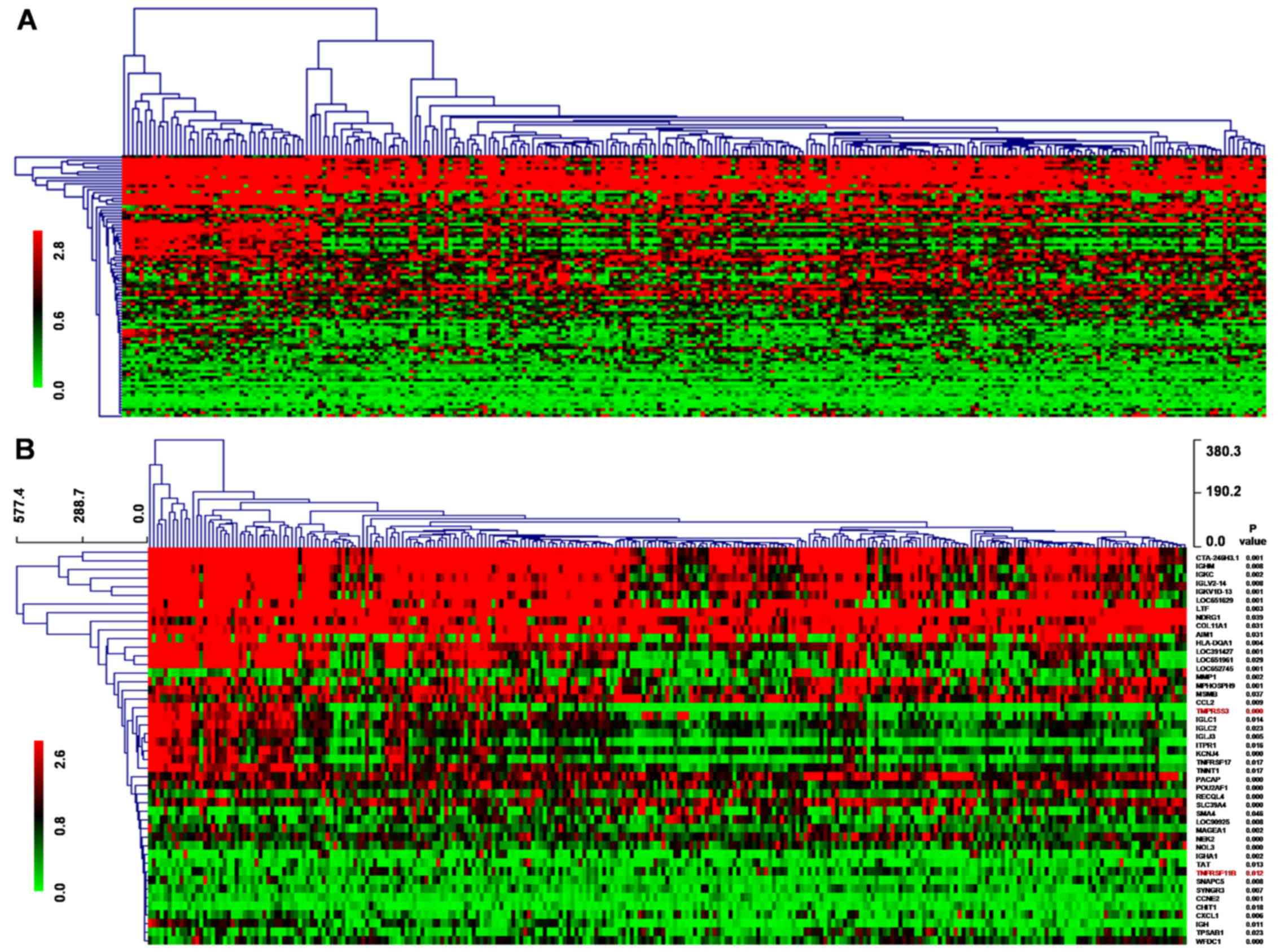
Gene microarray analysis
We downloaded a 286 breast cancer gene-expression
profile dataset of Wang et al (16) from the GEO database (GSE2034), among
which 180 cases were lymph node-negative relapse-free patients and
106 were lymph node-negative patients that developed distant
metastasis. Wang et al mainly discussed genes associated
with distant metastasis, but we aimed to study prognosis-associated
genes based on this dataset.
During the 5-year follow-up of these patients, 168
were alive, 95 were deceased and 23 were lost to follow-up.
Compared to the surviving patient group, 89 genes differentially
expressed in the deceased patient group displayed a 2-fold change
in expression at the P<0.01 level with a false discovery rate,
among which 59 were upregulated while 30 were downregulated. CCNE2
and EEF1A2 were included in the 76-gene signature of this study to
predict tumor metastasis while another 87 genes were newly
discovered differential genes associated with prognosis. Then,
hierarchical clustering analysis was used to detect the expression
profile of 89 differentially expressed genes by MEV4.9.0 software
(Fig. 1A). Kaplan-Meier survival
analysis of the 89 genes showed that 46 genes displayed a log-rank
P<0.05 level in expression, among which 29 genes were
upregulated while 17 genes were downregulated. The 46 genes
underwent cluster analysis with MEV4.9.0 software (Fig. 1B).
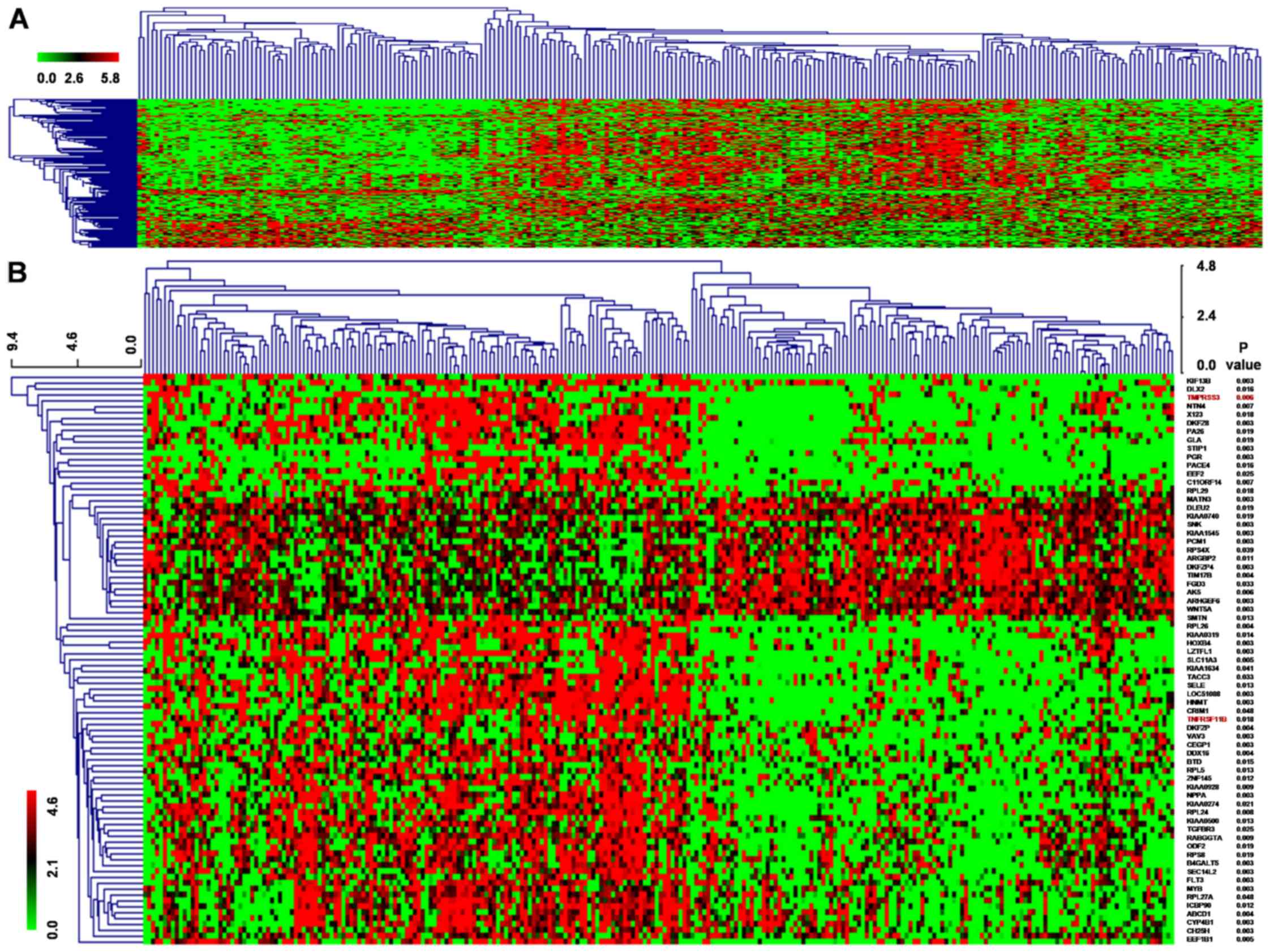
We also downloaded a 260 breast cancer
gene-expression profile dataset of van de Vijver et al
(17) which included clinical
prognostic data, for which 195 patients were alive while 65 were
deceased according to the 5-year follow-up. Compared to the
surviving patient group, 540 genes of the deceased patient group
displayed a 2-fold change in expression at the P<0.01 level,
among which 274 were upregulated and 266 were downregulated. Then,
the 540 genes underwent cluster analysis with MEV4.9.0 software
(Fig. 2A). Kaplan-Meier survival
analysis of the 89 genes showed that 67 genes displayed a log-rank
P<0.05 level in expression, among which 45 genes were
upregulated while 22 genes were downregulated. The 67 genes
underwent cluster analysis with MEV4.9.0 software (Fig. 2B). Moreover, TMPRSS3 and TNFRSF11B
were identified as common genes among the 46 genes from Wang et
al results and the 67 genes from van de Vijver et al
data.
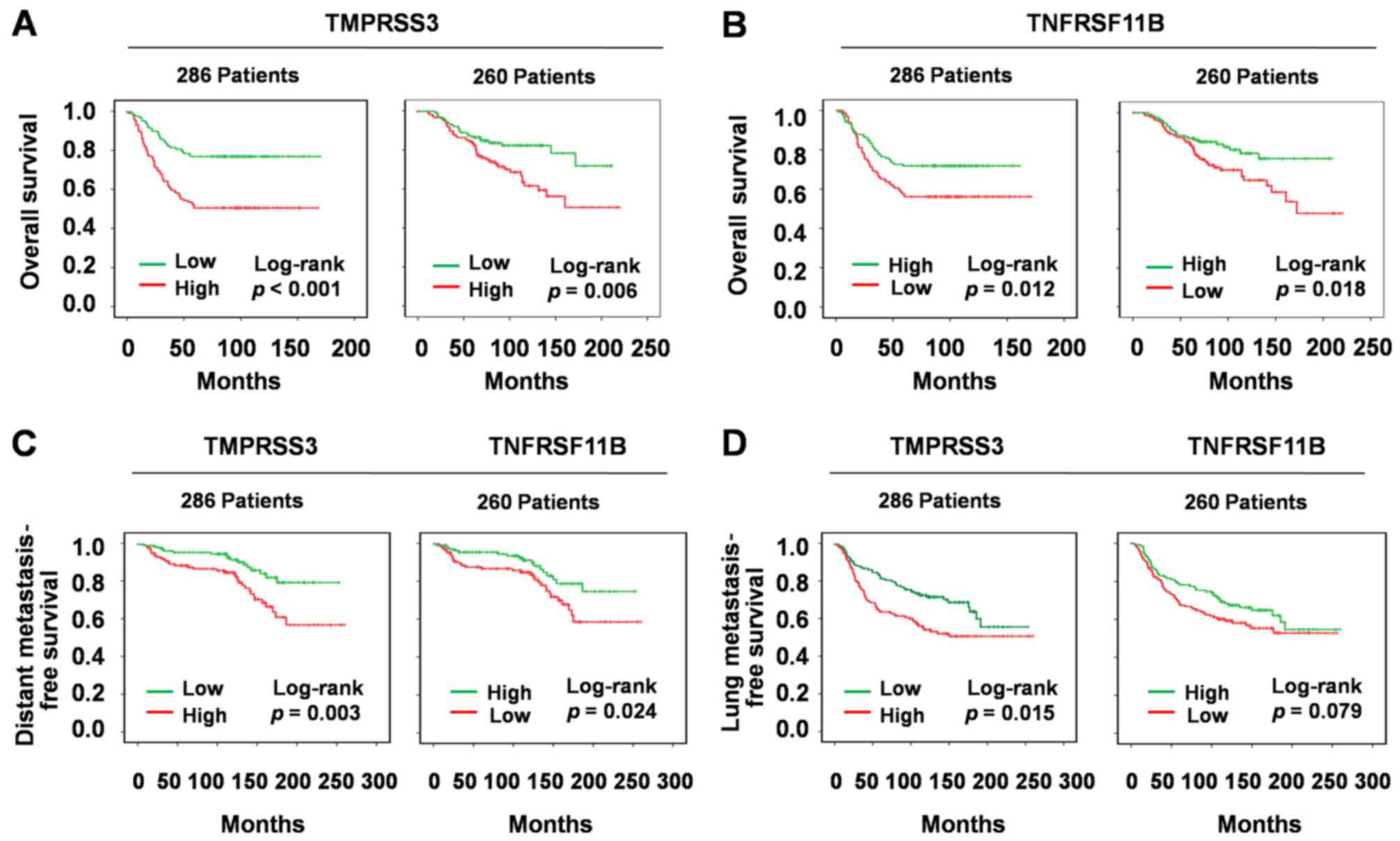
Survival analysis of TMPRSS3 and
TNFRSF11B in breast cancer
Subsequently, Kaplan-Meier survival curves were
plotted to estimate the prognostic value of TMPRSS3 and TNFRSF11B
in breast cancer. Firstly, we recorded TMPRSS3 and TNFRSF11B gene
expression levels as high vs. low using a cut-off point according
to the median. Univariate analysis of OS by Kaplan-Meier survival
analysis indicated that high TMPRSS3 expression was associated with
shorter OS both in the 286 breast cancer patients in Wang et
al group (P<0.001; Fig. 3A)
and 260 breast cancer patients in van de Vijver et al group
(P=0.006; Fig. 3A). However, high
TNFRSF11B expression was associated with longer OS both in the 286
breast cancer patients in Wang et al group (P=0.012;
Fig. 3B) and 260 breast cancer
patients in van de Vijver et al group (P=0.018; Fig. 3B). However, these two genes were not
in the list of the 93 protein-coding cancer genes which carry
probable driver mutations (18). We
inferred that their dysfunction may not arise by gene mutation, but
by change of gene expression level.
Since TMPRSS3 and TNFRSF11B were associated with OS
of the breast cancer patients, we further aimed to ascertain
whether they were associated with tumor metastasis or tumor
occurrence. Thus, we downloaded gene microarray dataset of Minn
et al (19) including
clinical information of breast cancer metastasis to lung. Distant
metastasis-free survival by Kaplan-Meier survival analysis
demonstrated that both TMPRSS3 and TNFRSF11B were positively
correlated with breast cancer metastasis (log-rank P=0.003;
log-rank P=0.024; Fig. 3C). Lung
metastasis-free survival by Kaplan-Meier survival analysis
indicated that TMPRSS3 was positively correlated with breast cancer
metastasis to the lung (log-rank P=0.015; Fig. 3D), but there was no evidence to
demonstrate that TNFRSF11B was correlated with breast cancer
metastasis to the lung (log-rank P=0.079; Fig. 3D). However, a previous study showed
that TNFRSF11B was inversely correlated with breast cancer
metastasis to the bone as it can inhibit osteoclast activity and
bone metastasis (20).
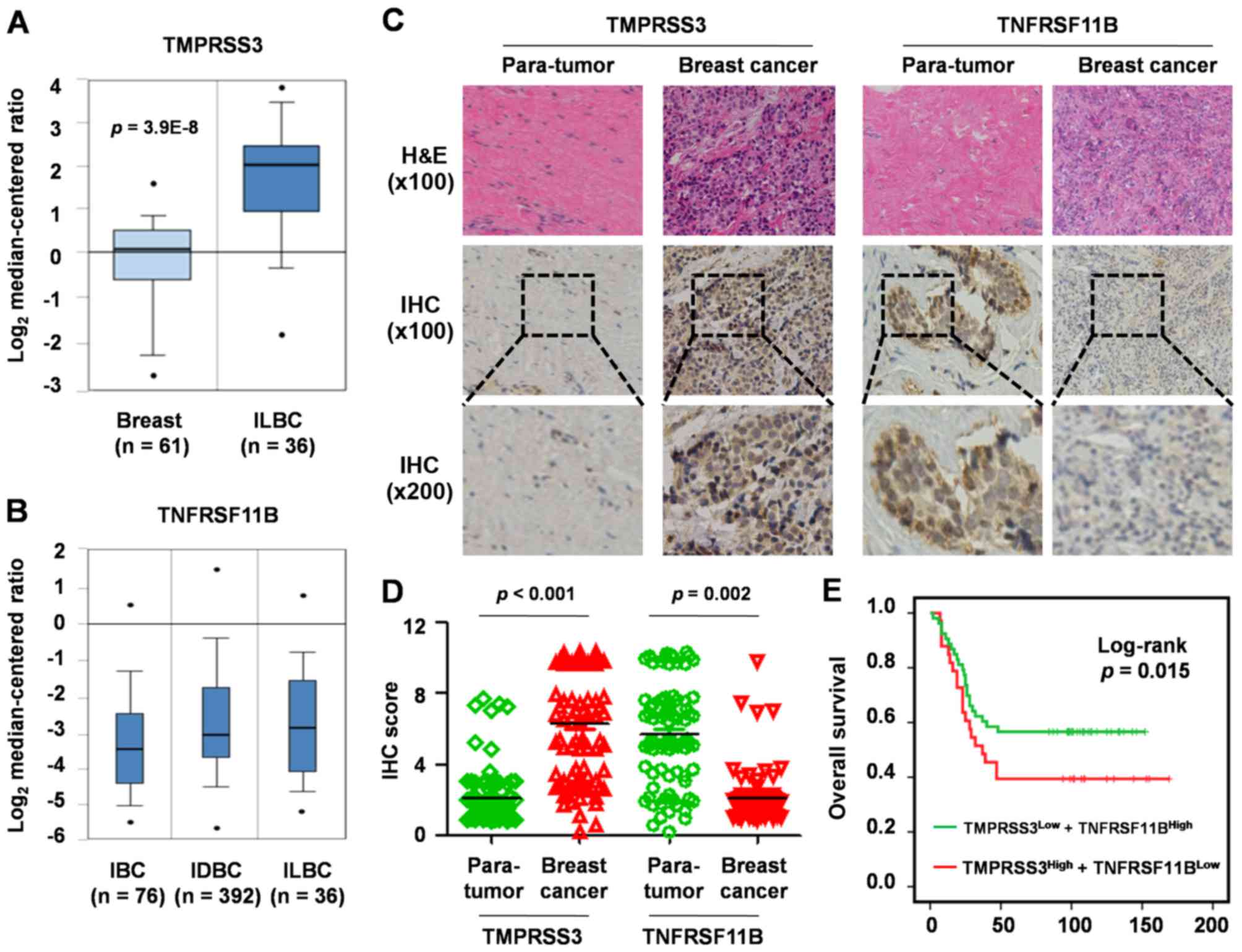
Oncomine on line analysis
To further elucidate the TMPRSS3 and TNFRSF11B
expression signature in breast cancer, we analyzed their expression
levels in 593 breast cancer patients in the TCGA database by
Oncomine. The results showed that TMPRSS3 was significantly
upregulated in invasive lobular breast carcinoma compared with that
in normal breast tissue (P=3.9E-8; fold-change, 3.8; Fig. 4A) and TNFRSF11B was significantly
downregulated in different types of breast cancer, such as invasive
breast carcinoma (IBC; n=76), invasive ductal breast carcinoma
(IDBC; n=392) and invasive lobular breast carcinoma (ILBC; n=36)
(Fig. 4B).
H&E and IHC analysis
Moreover, H&E staining and immunohistochemistry
technique were used to analyze TMPRSS3 and TNFRSF11B expression in
86 breast cancer tissues vs. para-tumor tissues (Fig. 4C). When compared with the normal
breast tissues, TMPRSS3 expression was significantly upregulated
(fold-change, 3.6; P<0.001; Fig.
4D) and TNFRSF11B expression was significantly downregulated
(fold-change, 2.4; P=0.002; Fig.
4D) in the breast cancer tissues. Next, we analyzed the
correlation between TMPRSS3 and TNFRSF11B and found that the
relative expression levels were inversely correlated
(R2=0.87; Fig. 4D).
Clinical significance of TMPRSS3 and
TNFRSF11B in 86 paired breast cancer tissues
Univariate analysis of OS showed that upregulation
of TMPRSS3 expression was associated with shorter OS (P=0.033)
while downregulation of TNFRSF11B expression was associated with
shorter OS (P=0.042). Multivariate analysis of OS with a Cox
proportional hazards model in breast cancer based on TMPRSS3 and
TNFRSF11B expression demonstrated that upregulation of TMPRSS3
accompanied by downregulation of TNFRSF11B was associated with a
shorter median OS and is an indicator for poor prognosis (P=0.015;
Fig. 4E).
Discussion
Breast cancer is the most commonly diagnosed cancer
in women, and bone is one of the most common metastatic sites, with
~75% of all women with breast cancer diagnosed with bone metastases
upon autopsy examination (21,22).
Receptor activator of nuclear factor-κB and its ligand (RANK/RANKL)
play an important role in the development and maintenance of
osteoclastic activity in bone. TNFRSF11B or osteoprotegerin (OPG)
is a decoy of RANKL which blocks RANKL-RANK interaction by binding
to RANKL. In this way, it acts as a natural modulator of
osteoclastic activity. The RANK/RANKL/OPG pathway plays an
important role in the development and progression of bone
metastasis in various types of cancers. However, little is known
concerning the role of OPG in breast cancer prognosis.
Type II transmembrane serine protease (TTSP) family
consisting of 17 members is a class of membrane-bound proteolytic
enzymes, whose activities on the surface of tumor cells can break
down surrounding extracellular matrix components, thereby promoting
metastasis. In this way, they contribute to tumor growth, invasion
and metastasis. A range of proteolytic activities occurs in
malignant cells with TTSPs Thus, TTSPs are suitable candidates as
tumor markers (23). TMPRSS3 is a
member of the TTSP family which has been found to be highly
expressed in pancreatic cancer and ovarian tumors. TMPRSS3 promotes
the proliferation, invasion and migration of ovarian cancer cells
(10,11), and TMPRSS3 expression was found to
be significantly associated with breast cancer (24). Although, it has been reported that
TMPRSS3 expression is associated with the outcome of breast cancer
patients, the pattern of TMPRSS3 expression in human breast cancer
remains controversial.
In the present study, survival analysis of OS in two
groups of gene-expression profile datasets showed that TMPRSS and
TNFRSF11B were common prognostic-associated genes in breast cancer.
Further univariate analysis of OS, distant metastasis-free and lung
metastasis-free survival based on TMPRSS3 and TNFRSF11B expression
in breast cancer demonstrated that high TMPRSS3 expression and low
TNFRSF11B expression were associated with shorter OS, and they were
positively correlated with breast cancer metastasis. While TMPRSS3
was positively correlated with breast cancer metastasis to the
lung, there was no evidence to demonstrate that TNFRSF11B was
correlated with breast cancer metastasis to the lung. Next, we
explored their expression levels in breast cancer vs. normal breast
tissues in the TGCA database using Oncomine and verified the
results with H&E and IHC techniques. The results showed that
TMPRSS3 was upregulated and TNFRSF11B was downregulated in breast
cancer. Moreover, TMPRSS3 and TNFRSF11B expression levels presented
an inverse correlation.
Finally, multivariate analysis of OS based on
TMPRSS3 and TNFRSF11B expression demonstrated that upregulated
TMPRSS3 accompanied with downregulated TNFRSF11B was associated
with a shorter median OS and was indicative of a poor prognosis.
Therefore, high TMPRSS3 and low TNFRSF11B expressions are
independent prognostic factors for poor breast cancer survival.
However, the molecular mechanisms of TMPRSS3 and TNFRSF11B in
breast cancer and bone metastasis remain unclear.
Taken together, TMPRSS3 and TNFRSF11B are
independent prognostic factors that may be used as potential
biomarkers to identify subgroups of breast cancer tumors with
different degrees of aggressiveness. They could also serve as
prognostic markers and even potential therapeutic targets for
breast cancer patients in the future.
Acknowledgements
The present study was supported partly by grants
from the National Natural Science Foundation of China (nos.
81201535, 81472202 and 81302065), the Shanghai Natural Science
Foundation (12ZR1436000 and 16ZR1428900) and the Shanghai Municipal
Commission of Health and Family Planning (201440398 and
201540228).
References
|
1
|
Nattinger AB and Mitchell JL: Breast
cancer screening and prevention. Ann Intern Med. 164:ITC81–ITC96.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prat A, Cheang MC, Galván P, Nuciforo P,
Paré L, Adamo B, Muñoz M, Viladot M, Press MF, Gagnon R, et al:
Prognostic value of intrinsic subtypes in hormone receptor-positive
metastatic breast cancer treated with letrozole with or without
lapatinib. JAMA Oncol. 2:1287–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maciejczyk A: New prognostic factors in
breast cancer. Adv Clin Exp Med. 22:5–15. 2013.PubMed/NCBI
|
|
6
|
Recondo G Jr, de la Vega M, Galanternik F,
Díaz-Cantón E, Leone BA and Leone JP: Novel approaches to target
HER2-positive breast cancer: Trastuzumab emtansine. Cancer Manag
Res. 8:57–65. 2016.PubMed/NCBI
|
|
7
|
Bianco S and Gévry N: Endocrine resistance
in breast cancer: From cellular signaling pathways to epigenetic
mechanisms. Transcription. 3:165–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giessrigl B, Schmidt WM, Kalipciyan M,
Jeitler M, Bilban M, Gollinger M, Krieger S, Jäger W, Mader RM and
Krupitza G: Fulvestrant induces resistance by modulating GPER and
CDK6 expression: Implication of methyltransferases, deacetylases
and the hSWI/SNF chromatin remodelling complex. Br J Cancer.
109:2751–2762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marquette C and Nabell L:
Chemotherapy-resistant metastatic breast cancer. Curr Treat Options
Oncol. 13:263–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wallrapp C, Hähnel S, Müller-Pillasch F,
Burghardt B, Iwamura T, Ruthenbürger M, Lerch MM, Adler G and Gress
TM: A novel transmembrane serine protease (TMPRSS3) overexpressed
in pancreatic cancer. Cancer Res. 60:2602–2606. 2000.PubMed/NCBI
|
|
11
|
Zhang D, Qiu S, Wang Q and Zheng J:
TMPRSS3 modulates ovarian cancer cell proliferation, invasion and
metastasis. Oncol Rep. 35:81–88. 2016.PubMed/NCBI
|
|
12
|
Rui X, Li Y, Jin F and Li F: TMPRSS3 is a
novel poor prognostic factor for breast cancer. Int J Clin Exp
Pathol. 8:5435–5442. 2015.PubMed/NCBI
|
|
13
|
Pelkonen M, Luostari K, Tengström M,
Ahonen H, Berdel B, Kataja V, Soini Y, Kosma VM and Mannermaa A:
Low expression levels of hepsin and TMPRSS3 are associated with
poor breast cancer survival. BMC Cancer. 15:4312015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dougall WC and Chaisson M: The
RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer
Metastasis Rev. 25:541–549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM,
Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME and
Yu J: Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van de Vijver MJ, He YD, van't Veer LJ,
Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C,
Marton MJ, et al: A gene-expression signature as a predictor of
survival in breast cancer. N Engl J Med. 347:1999–2009. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nik-Zainal S, Davies H, Staaf J,
Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB,
Martin S, Wedge DC, et al: Landscape of somatic mutations in 560
breast cancer whole-genome sequences. Nature. 534:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park HR, Min SK, Cho HD, Kim DH, Shin HS
and Park YE: Expression of osteoprotegerin and RANK ligand in
breast cancer bone metastasis. J Korean Med Sci. 18:541–546. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suva LJ, Washam C, Nicholas RW and Griffin
RJ: Bone metastasis: Mechanisms and therapeutic opportunities. Nat
Rev Endocrinol. 7:208–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Lu J, Ji Y, Hong A and Xie Q:
Cytokines in osteoblast-conditioned medium promote the migration of
breast cancer cells. Tumour Biol. 35:791–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hooper JD, Clements JA, Quigley JP and
Antalis TM: Type II transmembrane serine proteases. Insights into
an emerging class of cell surface proteolytic enzymes. J Biol Chem.
276:857–860. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luostari K, Hartikainen JM, Tengström M,
Palvimo JJ, Kataja V, Mannermaa A and Kosma VM: Type II
transmembrane serine protease gene variants associate with breast
cancer. PLoS One. 9:e1025192014. View Article : Google Scholar : PubMed/NCBI
|