Introduction
Numerous post-translational modifications (PTMs)
exist to further specify protein function; some examples include
histone phosphorylation, acetylation, methylation and
ubiquitination (1). Histone PTMs
are the points of convergence in signaling pathways and these nodal
events are crucial for gene expression (2). Different histone PTMs have three major
distinct outcomes. Firstly, cooperative interactions: two or more
signals collaborate to promote protein stabilization or
recruitment. Secondly, mutually exclusive PTMs: PTMs can be
mutually exclusive, however, these different PTMs cannot occur
simultaneously. Thirdly, antagonistic PTMs: a PTM that is attached
to one amino acid can, for example, antagonize the ability of an
adjacent modified residue to recruit a binding partner (3). Histone H3 is a substrate of signal
kinase and its phosphorylation specificity involves cell cycle
progression and gene expression regulation (4). An increasing number of histone
modifying complexes are found to have more than one distinct
enzymatic activity. These enzymes can act in concert to determine
the functional status of chromatin by coordinating multiple histone
modifications. It is now well established that there is intense
crosstalk between histone modifications to drive distinct
downstream functions as well. Cross-regulation can occur in
different ways; one modification can promote/block the addition of
another modification, while another modification can
stimulate/block the removal of another (5).
Akt plays an important role in the growth factor
signaling pathway and can regulate cell growth, transcription and
nutrition metabolism (6). If the
Akt signaling pathway is inhibited, it may therefore lead to tumor
cell death and growth inhibition (7). Zinc finger proteins (ZACs) are
transcription factors that have transactivation and DNA-binding
functions which can induce apoptosis and cell cycle arrest
(8). P300 is a transcription
cofactor with histone acetyltransferase (HAT) activity which is
capable of acetylating all four core histones as well as over 70
other proteins. Its histone HAT domain is the core domain by which
it acetylates substrate proteins and promotes transcription
(9–11). ZAC also acts as a transcriptional
cofactor that works with HATs and histone deacetylases to regulate
gene expression. Previous research has shown that the ZAC
gene exhibits hypermethylation in gastric adenocarcinomas (12).
When somatostatin (SST) binds to the SST receptor
(SSTR) it can regulate cell proliferation in normal tissues and
tumors. The SSTR is a G protein-coupled receptor and Akt is one of
the downstream molecules in the SSTR pathway (13,14).
In gastric carcinomas, the expression of SST and ZAC are both
downregulated implying that a positive correlation between SST and
ZAC expression exists (15).
Octreotide (OCT), an SST analogue, is able to upregulate the
expression of the ZAC gene and inhibit the proliferation of
gastric cancer cells (16). The
inhibitory effect of OCT on the proliferation of gastric cancer
cells was significantly reduced after siRNA knockdown of the
ZAC gene. This suggests that ZAC plays an important role in
OCT signaling to inhibit the proliferation of gastric cancer cells
(16).
Simultaneous induction of histone phosphorylation
and acetylation is crucial for the activation of specific mammalian
genes. For instance, the phosphorylation and acetylation of histone
H3 can influence the regulation of cell proliferation gene
expression (17). Histone H3 serine
residue phosphorylation is a highly dynamic process and the
phosphorylation of serine 10 on histone H3 (pS10-H3) plays a
significant role in the NF-κB pathway of gene transactivation
(18). S10-H3 phosphorylation is
closely related to the acetylation of lysine 14 on histone H3
(acK14-H3) and acK9-H3 (19); in
fact, pS10-H3 has been shown to stimulate acK14-H3 via GCN5, a
prototypical HAT that has a 10-fold preference for pS10-H3 over
non-phosphorylated H3 as a substrate in vitro (20–22).
Although effort has been made to elucidate how OCT
works, the exact mechanism is yet to be determined. The purpose of
the present study was to determine the inhibitory mechanism of OCT
in gastric cancer cells. More specifically, OCT was proposed to
increase the activity of P300-histone acetyltransferase (P300-HAT)
in gastric cancer cells by downregulating p-Akt and upregulating
ZAC. We anticipated that acK14-H3 and pS10-H3 are target sites of
P300; therefore, OCT may be able to regulate pS10-H3 and acK14-H3
to inhibit gastric cancer cell proliferation.
Materials and methods
Cell culture
A human gastric carcinoma cell line (SGC-7901) was
provided by the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China), and routinely cultured in RPMI-1640 medium
(Gibco, Grand Island, NY, USA). The medium was supplemented with
10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml
streptomycin (both from Sigma-Aldrich, St. Louis, MO, USA) at 37°C
in a humidified atmosphere of 5% CO2. OCT, which was
used to treat cells, was obtained from Beijing Dingguo Co.
(Beijing, China) formulated as OCT acetate injection.
Cell proliferation assay
Cell proliferation was assessed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
viability assay. Briefly, cells (5×103 cells/well) were
plated in 96-well plates. The cells were treated with different
concentrations of OCT (0, 1, 10, 100 and 1,000 nmol/l) for 24 h. At
the end of each treatment, 10 µl of MTT stock solution (5 mg/ml)
was then added to each well, and the cells were incubated for an
additional 4 h. The blue formazan salts produced from the cells
were dissolved by adding 100 µl of dimethyl sulfoxide (DMSO) and
the absorbance of the blue solution at 490 nm was measured using a
Microplate Reader Model 550 (Bio-Rad, Hercules, CA, USA). The
cytotoxicity of OCT was determined by plotting the inhibitory
efficiency: IE% = [1 - (optical density of sample/optical density
of control)] × 100%.
Western blot analysis
The SGC-7901 cells that underwent different
treatments (10 nmol/l of OCT treatment for 12, 24 and 48 h, or no
OCT) were collected after various time periods. Cells were then
washed with cold phosphate-buffered saline (PBS) and lysed in
ice-cold lysis buffer (10 mmol/l Tris-HC1, pH 8.0, 20% SDS, 1
mmol/l EDTA, 5 mmol/l DTT, 10 mmol/l PMSF) for 30 min at 4̊C. Cell
lysates were centrifuged at 13,400 × g for 10 min at 4̊C and
protein concentrations in the supernatants were determined using a
bicinchoninic acid (BCA) protein assay (BCA assay kit; Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's instructions. A total of 100 mg of protein was
separated by 15% SDS-polyacrylamide gel electrophoresis (PAGE).
After electrophoresis, gels were transferred to
nitrocellulose/polyvinylidene fluoride (PVDF) membranes by
electroblotting. The membranes were blocked with 5% non-fat dry
milk in TBST (20 mmol/l Tris-HCl, pH 7.6, 137 mmol/l NaCl, 0.05%
Tween-20) for 2 h at room temperature and incubated with primary
antibodies (1:500) against Akt, p-Akt, ZAC, P300, pS10-H3 and
acK14-H3 (Santa Cruz Biotechnology, Inc., Santa Cruz, USA), and
total β-actin overnight at 4̊C. After being washed three times for
20 min in TBST, the membranes were incubated for 1.5 h at room
temperature with an appropriately diluted horseradish
peroxidase-labeled secondary antibody (1:2,000; Zhongshan Jinqiao
Beijing Biotechnology Co., Ltd., Beijing, China) in blotting
buffer. The membranes underwent three 30-min washes in TBST with
gentle shaking. The Akt, p-Akt, ZAC, P300, pS10-H3, acK14-H3 and
total β-actin protein bands were visualized using the enhanced
chemiluminescence (ECL) method. All proteins of interest were run
alongside a housekeeping gene (β-actin, 1:1,000). The relative
level of protein in the SGC-7901 cells was expressed as the gray
value/β-tubulin.
Spectrophotometric activity
SGC-7901 cells received either 10 nmol/l of OCT
treatment for 12, 24 and 48 h (experimental group), or no OCT
treatment (control group). The nuclear proteins from all cells were
extracted using the Nc-cell nucleus/plasma extraction kit (Beijing
Dingguo Co.). The sample total activity and sample non-specific
activity assays were performed using the kit following the
manufacturer's instructions. The sample total activity and sample
non-specific activity were evaluated via a UV-9000
spectrophotometer (Shanghai Yuanxi Instrument Co., Ltd., Shanghai,
China).
The sample activity was calculated with the
following equation:
[(SR-BR)×0.1(SC:ml)SDM][0.01(SV:ml)×13.6(MAC×15(min))]=unit/mlSPC(mg/ml)=unit/mg
Here, SR signifies the sample reading, BR the
background reading, SC the system capacity, SDM the sample dilution
multiple, SV the sample volume, MAC the molar absorption
coefficient and SPC the sample protein concentration. Unit = mMol
Con A/min.
Samplespecificactivity=sampletotalactivity–samplenon–specificactivity
Co-immunoprecipitation assays
Total proteins were extracted using the Nc-cell
nucleus/plasma extraction kit obtained from Beijing Dingguo Co.,
and protein concentrations were determined using a BCA assay. The
supernatant was then collected and incubated with an
immunoprecipitating antibody [2 g (100 µl) P300 antibody; Santa
Cruz Biotechnology, Inc.] overnight at 4̊C on a rotating wheel.
Then, 40 µl protein A agarose (Beijing Dingguo Co.) was added and
incubation was carried out at 4̊C overnight on a rotating wheel.
Samples were then washed five times with lysis buffer and the final
pellets were resuspended in 25 µl of loading buffer (100 mmol/l
Tris-HCl, pH 6.8, 200 mmol/l dithiothreitol, 4% SDS, 0.2%
bromophenol blue, 20% glycerol, 8% urea). Protein complexes were
examined by western blotting with anti-ZAC (1:800; Santa Cruz
Biotechnology, Inc.) to detect P300-ZAC interactions.
Semi-quantitative analysis was carried out using ImageJ software
and the band density indicated the relative amount of the P300-ZAC
complex.
Immunocytochemistry
Immunocytochemistry was performed on slides with
SGC-7901 cells after treatment with 10 nmol/l OCT for 12, 24 and 48
h and on those without OCT treatment. The slides were rinsed three
times with 0.01 M PBS, fixed with 4% paraformaldehyde, incubated
with 0.3% Triton X-100 in PBS for 1 h at room temperature, and
placed in 1% H2O2 in PBS for 30 min to quench
endogenous peroxidases. The slides were then blocked with 5% bovine
serum albumin (BSA) and incubated with primary polyclonal antibody
[anti-pS10-H3 (1:200); anti-acK14-H3 (1:100); Santa Cruz
Biotechnology, Inc.], and after washes with PBS a secondary
antibody [biotinylated goat anti-rabbit (1:500)] was added. Next,
the slides were incubated with a strepavidin-biotin complex (SABC)
and processed with 3,3-diaminobenzidine solution. A 0.1% methyl
green staining solution was used to stain the cell nuclei.
All cells were scored based on immunoreactivity (IR)
under a microscope. Cells with no IR were considered negative (0
points); cells with light brown granules were considered weakly
positive (1 point); cells that had brown granules were considered
positive (2 points).
Statistical analysis
The Student's t-test and one-way ANOVA were used to
statistically analyze the data using SPSS 20.0 statistical
software. The data from triplicate experiments are expressed as the
mean ± SD and a p<0.05 was considered to indicate a
statistically significant result.
Results
In vitro anticancer effects of
OCT
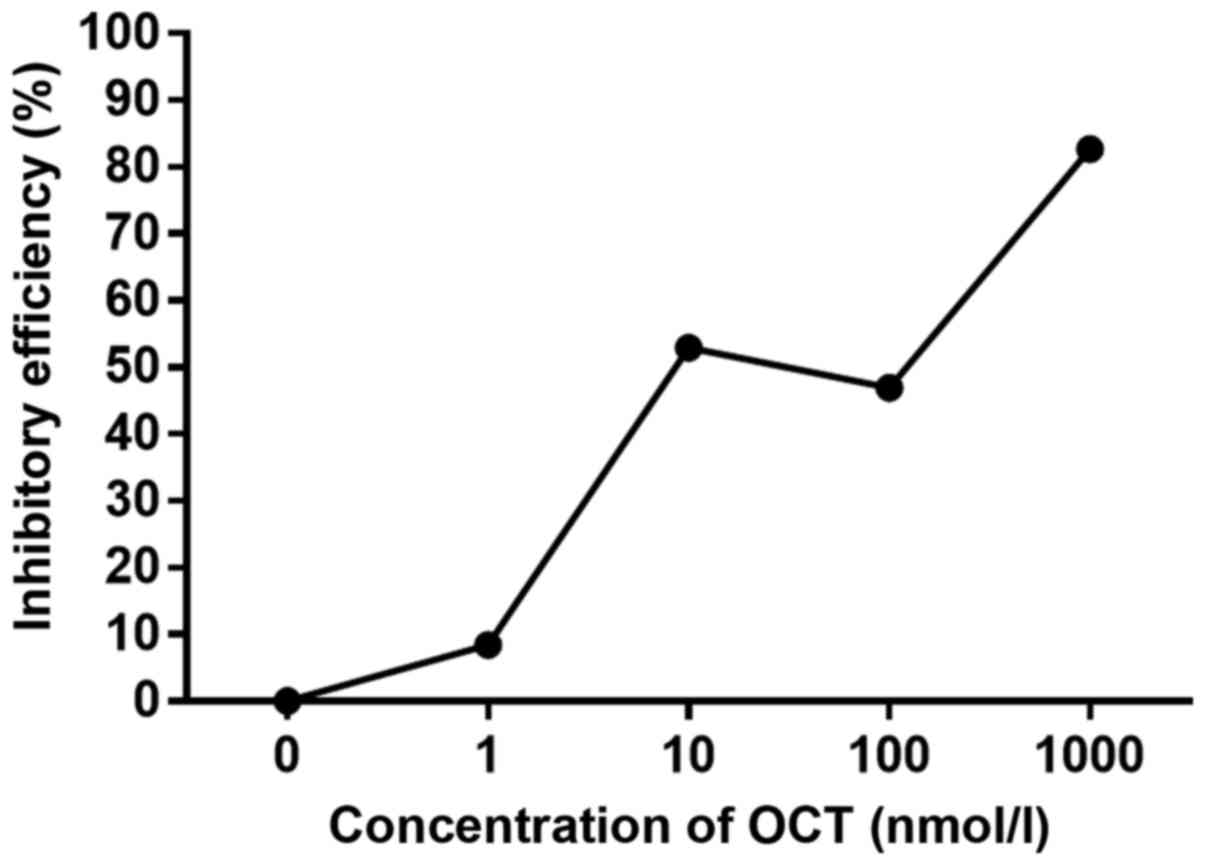
SGC-7901 cells were incubated with increasing
concentrations of OCT for 24 h and the viability was evaluated by
MTT assay (Fig. 1). Four different
concentrations of OCT (1, 10, 100 and 1,000 nmol/l) inhibited the
growth of SGC-7901 cells to varying extents. Considering that the
efficiency of inhibition for 10 and 100 nmol/l were comparable, 10
nmol/l of OCT was chosen as the effective concentration for the
present study.
OCT decreases p-Akt levels and
increases ZAC expression
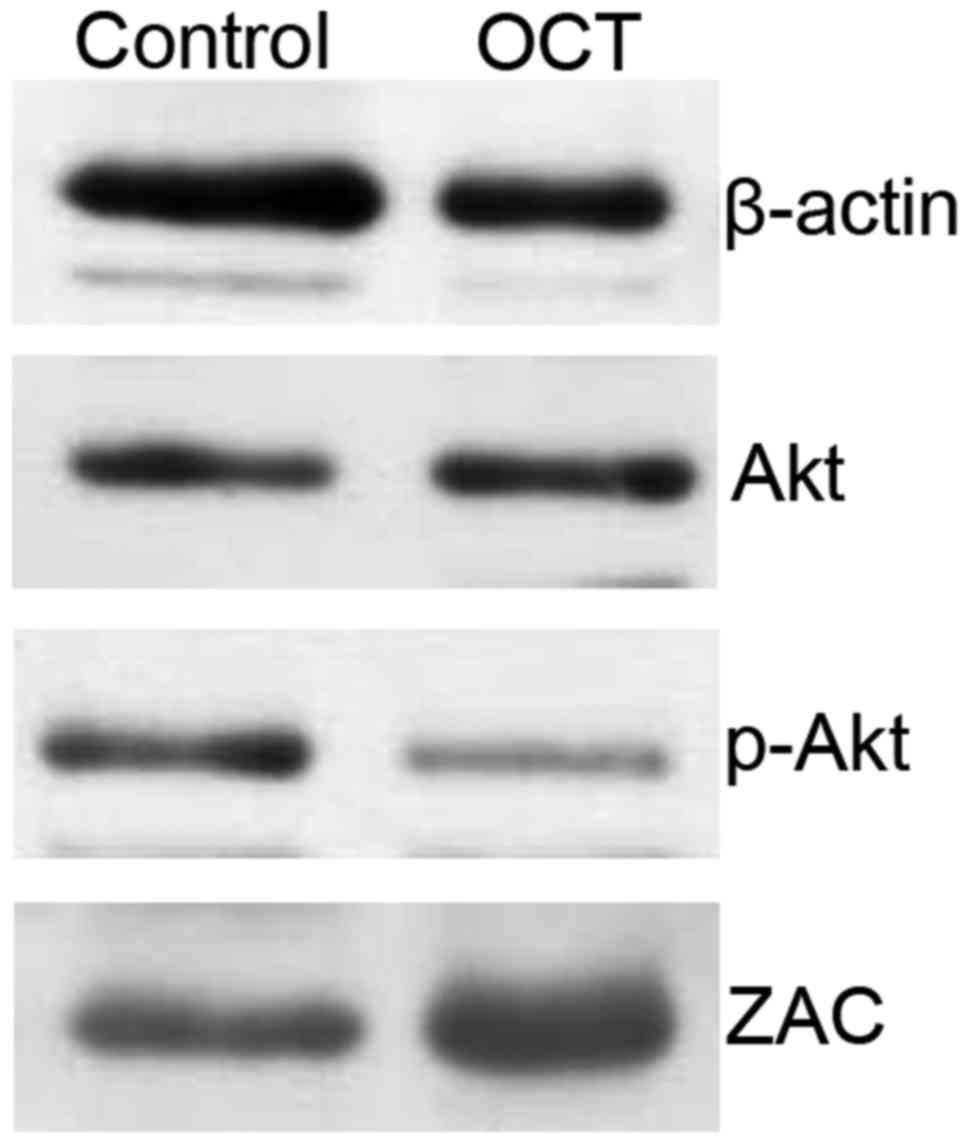
SGC-7901 cells were treated with 10 nmol/l of OCT
for 24 h. Compared to the control group, no significant change in
the expression of Akt was detected (p>0.05); however, the level
of p-Akt was significantly decreased (p<0.05). In addition, the
expression of ZAC was significantly increased in the OCT-treated
cells when compared to that noted in the control group (p<0.05;
Fig. 2). Overall, Akt levels were
not affected by OCT, yet OCT decreased the amount of p-Akt and
increased the levels of ZAC in the SGC-7901 cells.
OCT increases P300-HAT activity in the
gastric cancer cells
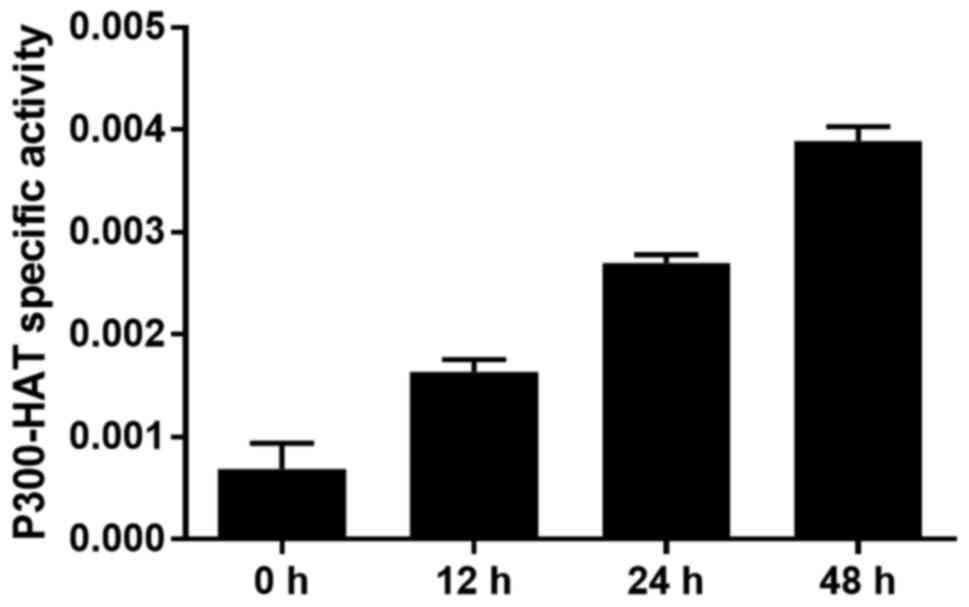
The P300-HAT activity in the nucleoprotein of
SGC-7901 cells was detected spectrophotometrically after incubation
with OCT for 12, 24 and 48 h. OCT (10 nmol/l) significantly
enhanced P300-HAT activity in the SGC-7901 cells in a
time-dependent manner (p<0.05; Fig.
3). This outcome revealed that OCT increased the overall
activity of P300-HAT in the SGC-7901 cells.
OCT does not alter P300 gene
expression in the gastric cancer cells
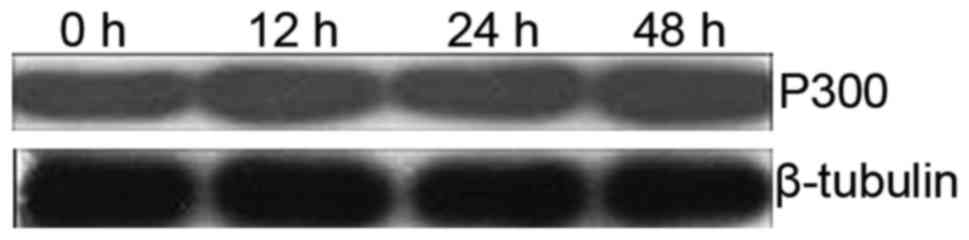
To observe the effects of OCT on P300 gene
expression, the amount of P300 in SGC-7901 cells was determined
using western blotting. There was no significant difference in
P300 gene expression between the control and the
experimental groups when SGC-7901 cells were incubated with OCT for
12, 24 and 48 h (p>0.05; Fig.
4). This result demonstrated that the P300 gene
expression was not altered by OCT treatment in the SGC-7901
cells.
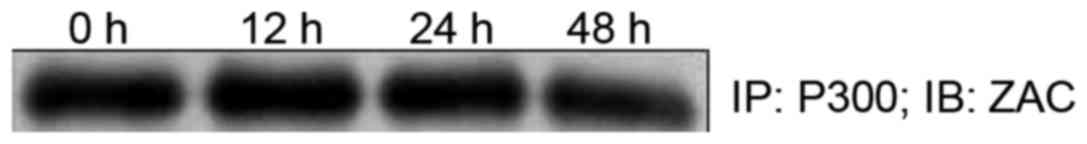
Interaction between ZAC and P300 in
the gastric cancer cells
The co-immunoprecipitation assay showed that ZAC
interacted with P300 in the SGC7901 cells. There was no significant
difference in the amount of ZAC-P300 complex after 12 and 24 h of
treatment with 10 nmol/l of OCT when compared to the control group
(p>0.05); however, the amount of ZAC-P300 at 48 h was
significantly decreased (p<0.05; Fig. 5). Overall, the interaction between
ZAC and P300 remained constant in the SGC-7901 cells without OCT
treatment and in those treated with 10 nmol/l of OCT for 12 and 24
h.
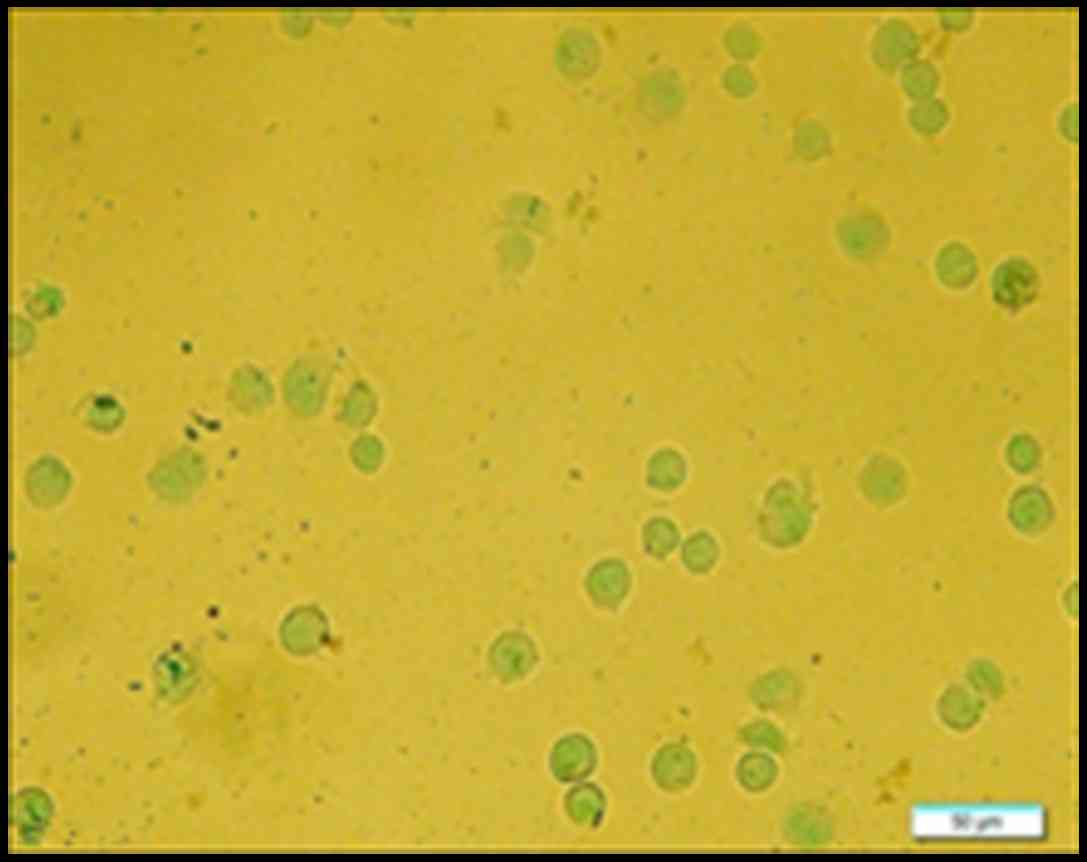
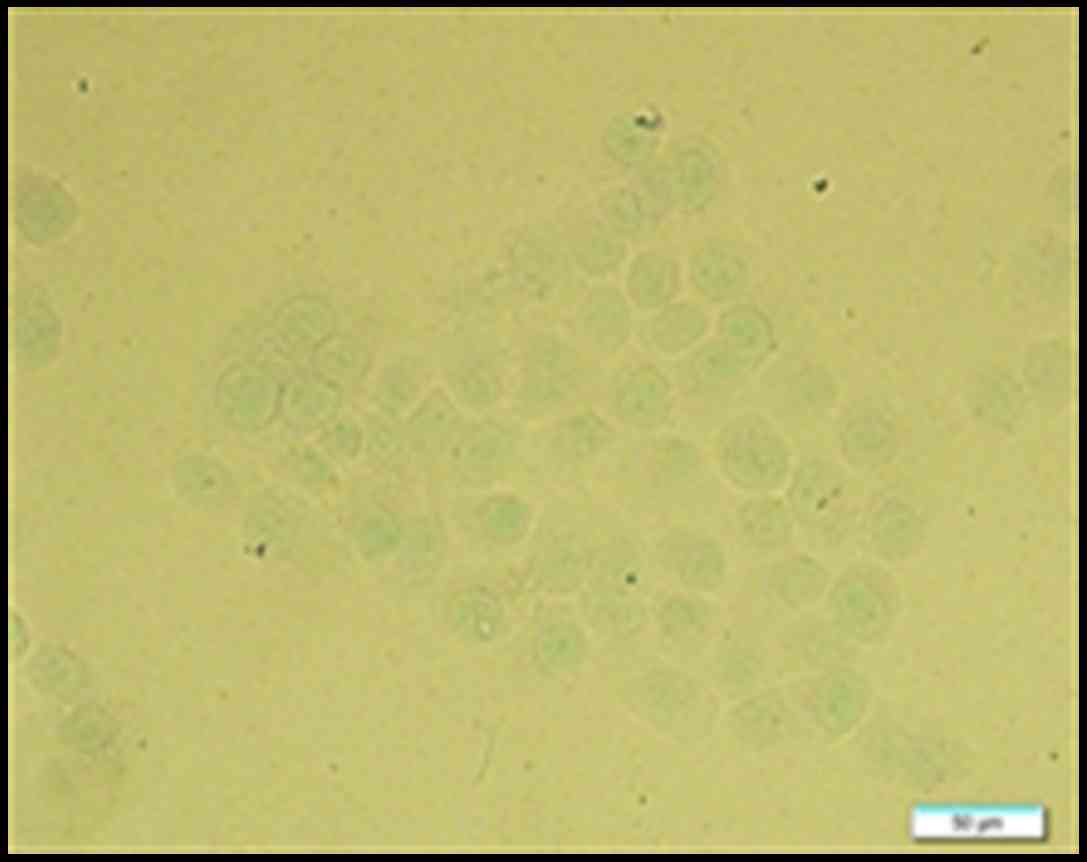
OCT decreases pS10-H3 IR in positive
gastric cancer cells
The IR of pS10-H3 in SGC-7901 cells was determined
using immunocytochemistry. pS10-H3 IR appeared as brown granules
and was mainly distributed in the periphery of the nucleus
(Fig. 6). The average percentage of
weakly positive and positive cells in each group was calculated.
The average IR of pS10-H3-positive cells decreased in a
time-dependent manner. However, there was no significant difference
(p>0.05) in the average IR of weakly positive cells between the
control and experimental groups (Fig.
7). Thus, OCT decreased pS10-H3 IR levels in the
SGC-7901-positive cells.
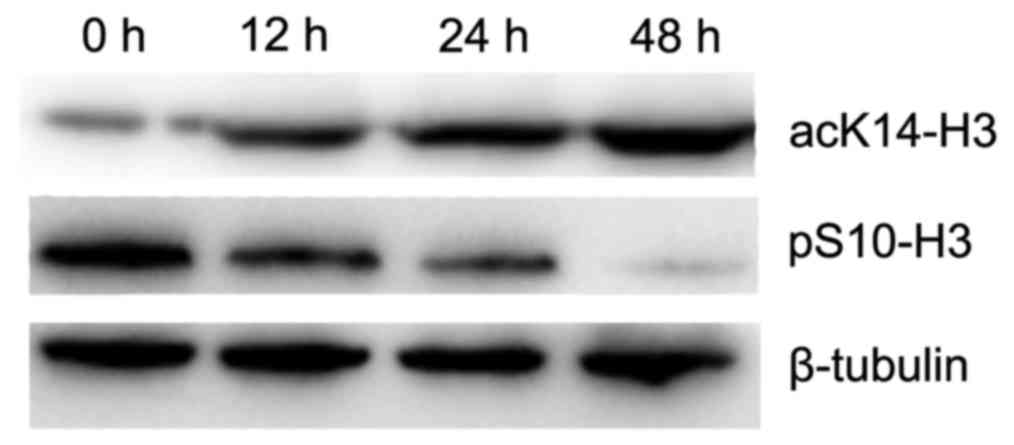
OCT decreases the level of pS10-H3 in
the gastric cancer cells
To determine the effect of OCT on pS10-H3, the
amount of pS10-H3 in SGC-7901 cells was quantified using western
blotting. The relative level of pS10-H3 was significantly decreased
in a time-dependent manner in cells treated with 10 nmol/l of OCT
(p<0.01). Therefore, OCT significantly decreased the level of
pS10-H3 in the SGC-7901 cells (Fig.
8).
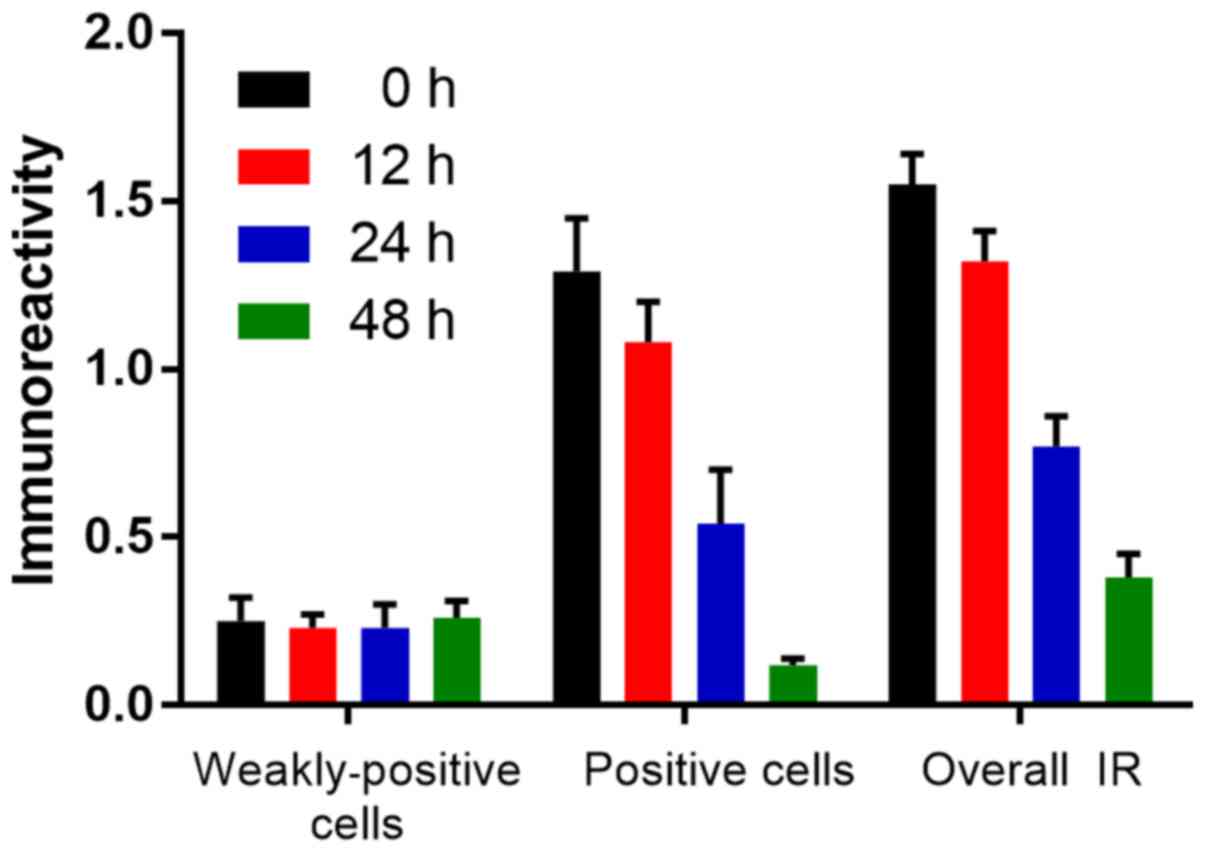
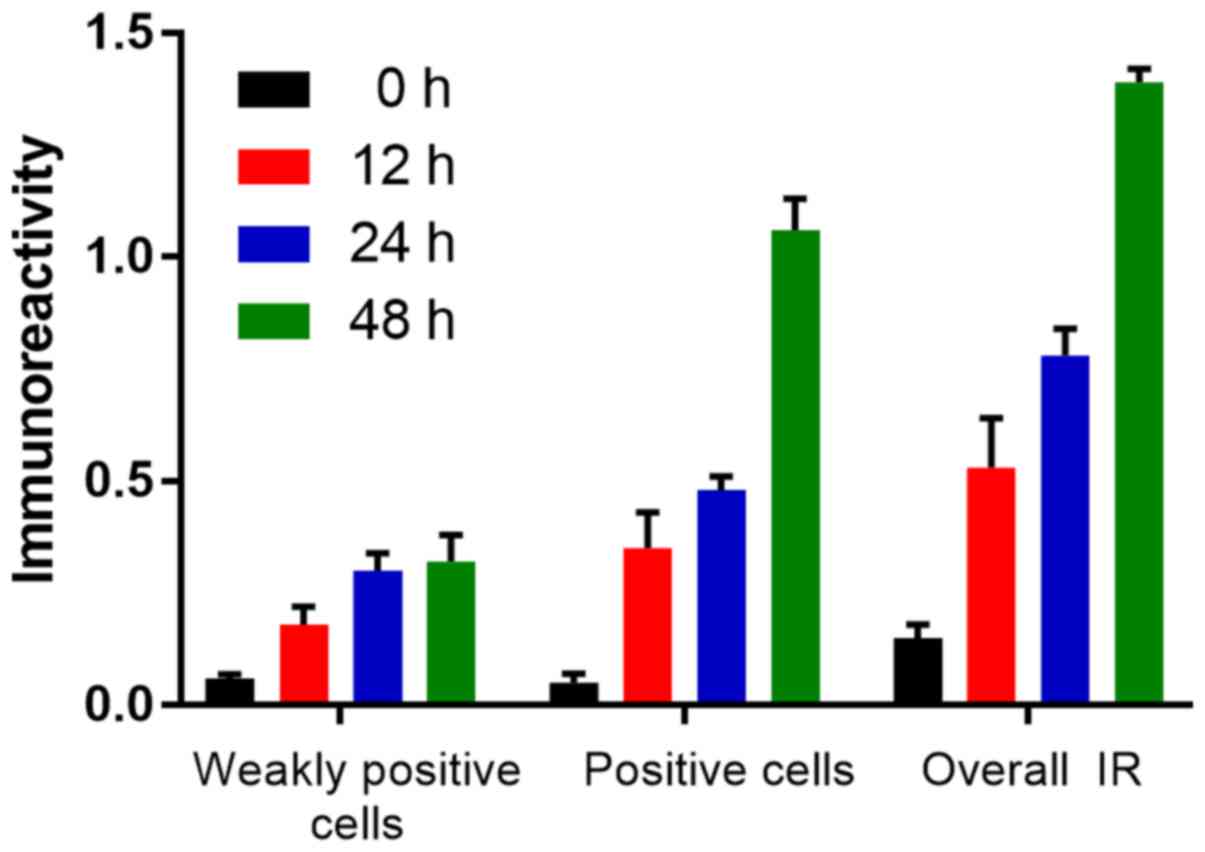
OCT increases acK14-H3 IR in gastric
cancer cells
Similar to the pS10-H3 IR, the acK14-H3 IR appeared
as brown granules and was mainly distributed in the periphery of
the nucleus (Fig. 9). The average
level of acK14-H3 IR for weakly positive and positive cells in the
control group was significantly lower than that in the experimental
groups (p<0.01; Fig. 10). OCT
was therefore able to significantly increase acK14-H3 IR in the
SGC-7901 cells.
OCT increases the level of acK14-H3 in
the gastric cancer cells
To determine the effect of OCT on acK14-H3, the
amount of acK14-H3 in the SGC-7901 cells was determined using
western blotting. The level of acK14-H3 was significantly increased
in a time-dependent manner in cells treated with 10 nmol/l of OCT
(p<0.01). OCT therefore significantly increased the level of
acK14-H3 in the SGC-7901 cells (Fig.
8).
Discussion
The present study examined the mechanism of OCT in
the inhibition of SGC-7901 cell proliferation. Since the inhibitory
efficiency of 10 and 100 nmol/l of OCT were similar, 10 nmol/l of
OCT was selected as the effective concentration (Fig. 1).
Gao et al suggested that Akt/PKB and
telomerase activity in SGC-7901 cells was significantly inhibited
when the cells were exposed to 1 µg/ml of OCT for 12, 24 and 48 h
compared to that of the control counterparts (23). The present study demonstrated that
there was no difference in the level of Akt between the OCT-treated
group and that of the control group in SGC-7901 cells. However,
there was a decrease in the p-Akt level in the SGC-7901 cells
treated with 10 nmol/l of OCT for 24 h compared to the control
group (Fig. 2); this suggested that
OCT inhibited Akt activity rather than Akt expression. This
decrease in Akt activity may shift SSTR signaling from pro-survival
to promoting the inhibition of SGC-7901 cell growth.
In pituitary tumor cells, OCT produces its
antiproliferative action by acting on the PI3K/Akt signaling
pathway and by increasing ZAC1 gene expression (24). It has been previously shown that
there is a positive correlation between the expression of ZAC and
SST in gastric cancer tissue. Through the combination of SST and
the SSTR3, ZAC expression was promoted and resulted in the
inhibition of gastric tumor cell growth (15). Our present study revealed that OCT
upregulated the expression of ZAC in SGC-7901 cells (Fig. 2) which supports the notion that OCT
inhibits the proliferation of gastric cancer cells by reducing the
level of Akt phosphorylation and upregulating ZAC expression.
Our research demonstrated that the P300-HAT activity
in the SGC-7901 cells was increased in a time-dependent manner
after being incubated with 10 nmol/l of OCT (Fig. 3); this indicated that OCT
upregulated the activity of P300-HAT. The mechanism by which OCT
upregulates P300-HAT activity in SGC-7901 cells is potentially
multi-faceted. Firstly, OCT increases the activity of P300-HAT by
upregulating the expression of P300 in SGC-7901 cells. Secondly,
since OCT upregulates the expression of ZAC in SGC-7901 cells, ZAC
and P300 proteins can interact to increase P300-HAT activity.
Thirdly, increased P300 and ZAC have a synergistic action on the
activity of P300-HAT. Our results revealed that there was no
difference in the P300 gene expression between the control
and the experimental groups (Fig.
4), therefore, the first and the third hypotheses can be
excluded. In light of this, we applied co-immunoprecipitation to
observe the interaction of ZAC and P300 protein in SGC-7901
cells.
Coordinated binding of ZAC zinc fingers and C
terminus to P300 regulates HAT function by increasing histone and
acetyl coenzyme A affinities and catalytic activity. This concerted
regulation of HAT function is mediated via the KIX and CH3 domains
of P300 in an interdependent manner. Notably, ZAC zinc fingers 6
and 7 simultaneously play key roles in DNA binding and P300
regulation (25). We suggested that
P300-ZAC was present in the control and experimental groups by way
of co-immunoprecipitation within SGC-7901 cells. Notably, there was
no significant difference in the amount of P300-ZAC between the
control and the 12 and 24-h groups. However, the amount of P300-ZAC
in the 48-h group was significantly less than that in the other
three groups (Fig. 5).
There are many potential reasons as to why the
amount of P300-ZAC decreased at 48 h of OCT treatment. ZAC
regulates the activities of the nuclear receptor, different members
of the p53 family and all of the proteins that are crucial
regulatory factors for cell growth, differentiation, equilibrium
and development (26). The
formation of a complex between P300 and p53 can, in turn, activate
p53 (27,28). The activity of P300-HAT was found to
be significantly increased when it interacted with special
transcription factors (29).
P300/CBP not only catalyzes the acetylation of all of the four core
histones, but it also acetylates 70 other proteins and itself
(10). Therefore, we speculated
that OCT would upregulate the expression of the ZAC gene in
SGC-7901 cells and that the interaction of ZAC and P300 not only
would upregulate the activity of P300-HAT, but also influence the
expression and activity of other transcription factors which can
competitively inhibit the formation of ZAC-P300. This may thereby
create a negative feedback loop, causing the amount of ZAC-P300 in
SGC-7901 cells to decrease after OCT treatment for 48 h. However,
the combination of these transcription factors and P300 may also
increase the activity of P300-HAT, which can explain why ZAC plays
a key role in inhibiting the pathway of gastric cancer cell
proliferation. Our previous research demonstrated that 10 nmol/l of
OCT, in the time period of 12–48 h, induced the expression of the
ZAC gene in a time-dependent manner in gastric cancer cells
(16). The result of the present
study also showed that, in the time perios of 12–48 h, 10 nmol/l of
OCT increased the activity of P300-HAT in the gastric cancer cells
in a time-dependent manner (Fig.
3). Since OCT upregulates the activity of P300-HAT, it most
likely affects the histone acetylation of ZAC and other
tumor-suppressor gene promoters in SGC-7901 cells. ZAC may then
affect histone acetylation of other tumor-suppressor gene promoters
by the upregulation of P300-HAT activity, in addition to inducing
apoptosis and cell cycle arrest. The increased expression of these
genes may likely inhibit the proliferation of SGC-7901 cells.
Since tumor cells are heterogeneous, we observed the
effect of OCT on pS10-H3 IR in SGC-7901 cells using an
immunocytochemistry method. The levels of pS10-H3 IR in each cell
were not uniform which indicates the functional status of
heterogeneity in SGC-7901 cells. The results showed that pS10-H3 IR
was mainly distributed in the periphery of the nucleus (Fig. 6), which may relate to the
translocation of p-Akt from the cytoplasm to the nucleus. Our
results revealed that the average IR of pS10-H3-positive cells was
decreased compared to that of the control group, however, there was
no significant difference between all groups for the average IR of
weakly positive pS10-H3 cells (Fig.
7). This indicates that OCT mainly decreased the expression of
highly active proliferationpromoting genes (positive cells). There
was, however, little effect on proliferation-inhibiting genes that
were being subtly expressed (weakly positive cells) which further
describes the heterogeneity of pS10-H3 expression in SGC-7901
cells.
In mammalian cells, pS10-H3 effects and causes
stress of the stimulation of certain genes, such as c-fos
and c-jun, via the mitogen stimulation signaling pathway
(30,31). Our current results showed that OCT
significantly decreased pS10-H3 (Fig.
8), which indicates that it may downregulate the mitogen
stimulation signaling pathway. The expression of some
proliferation-promoting genes may therefore be decreased by the
observed decrease in pS10-H3.
Acetylated DNA is usually labeled as active
chromatin. Lieberman-Aiden et al showed that the genome is
divided into open (active) and closed (inert) states (32). The present study, revealed that
acK14-H3 IR was mainly distributed in the periphery of the nucleus
(Fig. 9) which seems contrary to
the view of inert chromatin being distributed in the periphery of
the nucleus (33). However, it has
been shown that epigenetic modification can occur in chromatin
located in the peripheral area of the nucleus in tumor cells to
alter the three-dimensional structure of chromatin, rendering it
active (34,35). The abrogation of the ability of the
tumor cells to alter between open and closed states of chromatin
may be related to the finding that acK14-H3 IR was mainly
distributed in the periphery of the nucleus. The level of acK14-H3
IR between weakly positive and positive cells was not uniform which
indicated that the state of chromatin in the SGC-7901 cells could
be both open or closed. Both acK14-H3 IR (Fig. 10) and acK14-H3 (Fig. 8) increased in a time-dependent
manner in the SGC-7901 cells treated with 10 nmol/l of OCT.
Moreover, the IR results indicated that OCT may increase acK14-H3
in cells with a high expression of proliferation-inhibiting genes
(positive cells), and have a smaller effect in cells with a low
expression of proliferation-promoting genes (weakly positive
cells), thereby inhibiting the growth of SGC-7901 cells.
All the results indicate that OCT may affect pS10-H3
and acK14-H3 in SGC-7901 cells to balance the expression of genes
involved in proliferation and inhibition. OCT can significantly
decrease pS10-H3 and significantly increase acK14-H3, which appears
to be contrary to the view of mutual promotion between pS10-H3 and
acK14-H3. This may be due to the fact that histone modifications
have a high degree of background dependence. Histone PTMs may play
a role in a gene-signal-specificity manner and do not have a
universal code, instead, they are annotated against various
regulatory signals within the genome (36). The biological outcomes of certain
PTMs are usually dependent on the modification of chromatin and the
cell background (37). Histone PTMs
behave with a less strict ‘code’ and more with a complex
‘language’, which better illustrates that it is more reliant on the
importance of the context, rather than convention. Only the
‘language’ deciphered in a certain context can produce a specific
functional outcome (38).
There are, however, some limitations to the double
modification of pS10-H3 and acK14-H3 as they may not be suitable
for the expression of some genes. P300 is a cofactor of
oncoproteins (such as fos, jun and myb) and transforming virus
proteins (such as E1A), in addition to being a cofactor for
tumor-suppressor proteins (such as p53, E2F, Rb or BRCA1 (39). By upregulating P300-HAT activity,
OCT may also affect the expression of oncogenes in SGC-7901 cells.
In addition, cancer cells express multiple SSTR subtypes which
indicate that these receptors are coupled with other intracellular
receptor systems and extracellular signal cascades (40). There is still a great deal of
information on SSTR subtypes pertaining to their involvement in
physiological functions and different kinase and phosphatase
activities that are still not fully understood.
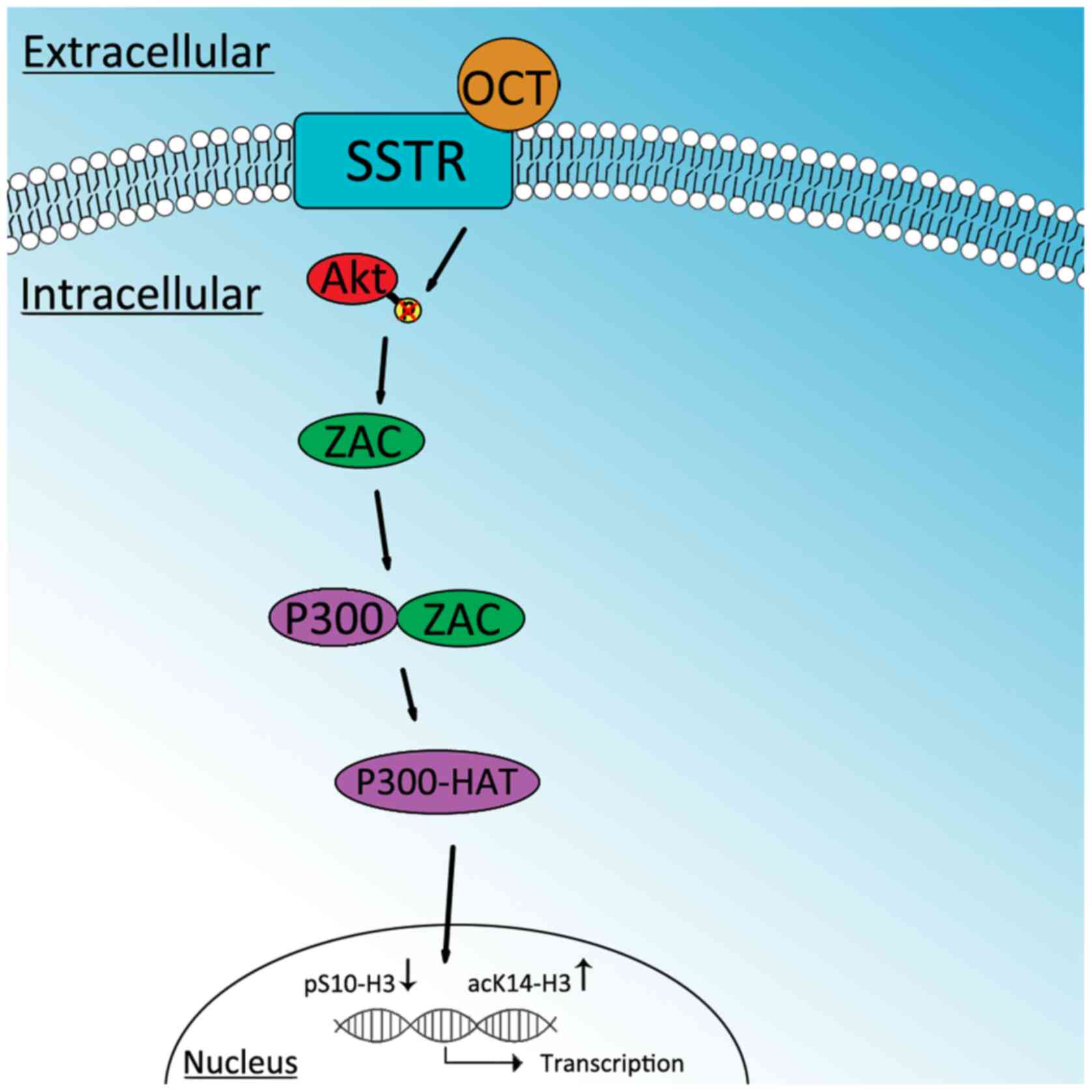
In conclusion, the present study demonstrated that
OCT may inhibit the proliferation of SGC-7901 cells by decreasing
p-Akt, which in turn, increases ZAC. ZAC then interacts with P300
to increase P300-HAT activity. This ultimately decreases pS10-H3
and increases acK14-H3. Therefore, a decrease in pS10-H3 and an
increase in acK14-H3 may be involved in the antiproliferative
effects of OCT in SGC-7901 cells (Fig.
11).
Acknowledgements
The present study was funded by the Department of
Science and Technology of Henan Province (project no.
102102310111).
References
|
1
|
Proietto M, Bianchi MM, Ballario P and
Brenna A: Epigenetic and posttranslational modifications in light
signal transduction and the circadian clock in Neurospora
crassa. Int J Mol Sci. 16:15347–15383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banerjee T and Chakravarti D: A peek into
the complex realm of histone phosphorylation. Mol Cell Biol.
31:4858–4873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seet BT, Dikic I, Zhou MM and Pawson T:
Reading protein modifications with interaction domains. Nat Rev Mol
Cell Biol. 7:473–483. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edmunds JW and Mahadevan LC: MAP kinases
as structural adaptors and enzymatic activators in transcription
complexes. J Cell Sci. 117:3715–3723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Izzo A and Schneider R: Chatting histone
modifications in mammals. Brief Funct Genomics. 9:429–443. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: A hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tokunaga E, Oki E, Egashira A, Sadanaga N,
Morita M, Kakeji Y and Maehara Y: Deregulation of the Akt pathway
in human cancer. Curr Cancer Drug Targets. 8:27–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varrault A, Ciani E, Apiou F, Bilanges B,
Hoffmann A, Pantaloni C, Bockaert J, Spengler D and Journot L:
hZAC encodes a zinc finger protein with antiproliferative
properties and maps to a chromosomal region frequently lost in
cancer. Proc Natl Acad Sci USA. 95:8835–8840. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J and Li Q: Use of histone
deacetylase inhibitors to examine the roles of bromodomain and
histone acetylation in p300-dependent gene expression. Methods Mol
Biol. 977:353–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Lf, Fischle W, Verdin E and Greene
WC: Duration of nuclear NF-kappaB action regulated by reversible
acetylation. Science. 293:1653–1657. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Ouyang S, Kong X, Liang Z, Lu J,
Zhu K, Zhao D, Zheng M, Jiang H, Liu X, et al: Catalytic mechanism
of histone acetyltransferase p300: From the proton transfer to
acetylation reaction. J Phys Chem B. 118:2009–2019. 2014.PubMed/NCBI
|
|
12
|
Li Z, Ding Y, Zhu Y, Yin M, Le X, Wang L,
Yang Y and Zhang Q: Both gene deletion and promoter
hyper-methylation contribute to the down-regulation of ZAC/PLAGL1
gene in gastric adenocarcinomas: A case control study. Clin Res
Hepatol Gastroenterol. 38:744–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Florio T: Molecular mechanisms of the
antiproliferative activity of somatostatin receptors (SSTRs) in
neuroendocrine tumors. Front Biosci. 13:822–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gatto F and Hofland LJ: The role of
somatostatin and dopamine D2 receptors in endocrine
tumors. Endocr Relat Cancer. 18:R233–R251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang GZDY, Liu J, Le XP and Zhang QX:
Correlation of ZAC with somatostatin and its receptor expression in
gastric cancer tissues. Acta Anat Sinica. 39:703–707. 2008.
|
|
16
|
Dai W, Ding Y and Zhang QX: Role of ZAC
gene in the pathway of octreotide inhibiting proliferation of
gastric cancer cells in vitro. Acta Anat Sinica. 44:492–497.
2013.
|
|
17
|
Simboeck E, Sawicka A, Zupkovitz G, Senese
S, Winter S, Dequiedt F, Ogris E, Di Croce L, Chiocca S and Seiser
C: A phosphorylation switch regulates the transcriptional
activation of cell cycle regulator p21 by histone deacetylase
inhibitors. J Biol Chem. 285:41062–41073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winter S, Simboeck E, Fischle W, Zupkovitz
G, Dohnal I, Mechtler K, Ammerer G and Seiser C: 14-3-3 proteins
recognize a histone code at histone H3 and are required for
transcriptional activation. EMBO J. 27:88–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawicka A and Seiser C: Histone H3
phosphorylation - a versatile chromatin modification for different
occasions. Biochimie. 94:2193–2201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung P, Tanner KG, Cheung WL,
Sassone-Corsi P, Denu JM and Allis CD: Synergistic coupling of
histone H3 phosphorylation and acetylation in response to epidermal
growth factor stimulation. Mol Cell. 5:905–915. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clements A, Poux AN, Lo WS, Pillus L,
Berger SL and Marmorstein R: Structural basis for histone and
phosphohistone binding by the GCN5 histone acetyltransferase. Mol
Cell. 12:461–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu
JY, Allis CD, Marmorstein R and Berger SL: Phosphorylation of
serine 10 in histone H3 is functionally linked in vitro and in vivo
to Gcn5-mediated acetylation at lysine 14. Mol Cell. 5:917–926.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao S, Yu BP, Li Y, Dong WG and Luo HS:
Antiproliferative effect of octreotide on gastric cancer cells
mediated by inhibition of Akt/PKB and telomerase. World J
Gastroenterol. 9:2362–2365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Theodoropoulou M, Zhang J, Laupheimer S,
Paez-Pereda M, Erneux C, Florio T, Pagotto U and Stalla GK:
Octreotide, a somatostatin analogue, mediates its antiproliferative
action in pituitary tumor cells by altering phosphatidylinositol
3-kinase signaling and inducing Zac1 expression. Cancer Res.
66:1576–1582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoffmann A, Barz T and Spengler D:
Multitasking C2H2 zinc fingers link Zac DNA
binding to coordinated regulation of p300-histone acetyltransferase
activity. Mol Cell Biol. 26:5544–5557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Theodoropoulou M, Stalla GK and Spengler
D: ZAC1 target genes and pituitary tumorigenesis. Mol Cell
Endocrinol. 326:60–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arora A, Gera S, Maheshwari T, Raghav D,
Alam MJ, Singh RK and Agarwal SM: The dynamics of stress p53-Mdm2
network regulated by p300 and HDAC1. PLoS One. 8:e527362013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang F, Marshall CB and Ikura M:
Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis:
Structural and functional versatility in target recognition. Cell
Mol Life Sci. 70:3989–4008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soutoglou E, Viollet B, Vaxillaire M,
Yaniv M, Pontoglio M and Talianidis I: Transcription
factor-dependent regulation of CBP and P/CAF histone
acetyltransferase activity. EMBO J. 20:1984–1992. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dyson MH, Thomson S and Mahadevan LC: Heat
shock, histone H3 phosphorylation and the cell cycle. Cell Cycle.
4:13–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ray PD, Huang BW and Tsuji Y: Coordinated
regulation of Nrf2 and histone H3 serine 10 phosphorylation in
arsenite-activated transcription of the human heme oxygenase-1
gene. Biochim Biophys Acta. 1849:1277–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lieberman-Aiden E, van Berkum NL, Williams
L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ,
Dorschner MO, et al: Comprehensive mapping of long-range
interactions reveals folding principles of the human genome.
Science. 326:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrulis ED, Neiman AM, Zappulla DC and
Sternglanz R: Perinuclear localization of chromatin facilitates
transcriptional silencing. Nature. 394:592–595. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berman BP, Weisenberger DJ, Aman JF,
Hinoue T, Ramjan Z, Liu Y, Noushmehr H, Lange CP, van Dijk CM,
Tollenaar RA, et al: Regions of focal DNA hypermethylation and
long-range hypomethylation in colorectal cancer coincide with
nuclear lamina-associated domains. Nat Genet. 44:40–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hansen KD, Timp W, Bravo HC, Sabunciyan S,
Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, et al:
Increased methylation variation in epigenetic domains across cancer
types. Nat Genet. 43:768–775. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paska AV and Hudler P: Aberrant
methylation patterns in cancer: A clinical view. Biochem Med.
25:161–176. 2015. View Article : Google Scholar
|
|
37
|
Berger SL: The complex language of
chromatin regulation during transcription. Nature. 447:407–412.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oliver SS and Denu JM: Dynamic interplay
between histone H3 modifications and protein interpreters: Emerging
evidence for a ‘histone language’. ChemBioChem. 12:299–307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Winter S, Fischle W and Seiser C:
Modulation of 14-3-3 interaction with phosphorylated histone H3 by
combinatorial modification patterns. Cell Cycle. 7:1336–1342. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ferjoux G, Bousquet C, Cordelier P, Benali
N, Lopez F, Rochaix P, Buscail L and Susini C: Signal transduction
of somatostatin receptors negatively controlling cell
proliferation. J Physiol Paris. 94:205–210. 2000. View Article : Google Scholar : PubMed/NCBI
|