Introduction
Scirrhous gastric cancer is unique and tends to
spread over the peritoneum with rapid growth and early metastasis
(1–4). The prognosis for patients with
scirrhous gastric carcinoma remains very poor, with the 5-year
survival rate being low. However chemotherapy for other types of
gastric cancer has improved, with good results being obtained in
Japan (5–9).
HSC-39 cells are a cell line established from
pleural effusion of a Japanese male scirrhous gastric cancer
patient (10), and can be used to
test therapies for scirrhous gastric cancer in vitro. HSC-39
cells are round, freely floating and tend to aggregate loosely in
tissue culture medium. In addition, they have characteristics
similar to those of the original ascitic tumor cell phenotypes of
signet ring cell carcinoma (11).
HSC-39 cells have a mutation in exon7 of the p53 gene, which
provides a possible selective advantage for tumor cell
proliferation (12). Since these
cells were derived from a human scirrhous gastric carcinoma
patient, they possess the appropriate phenotypes, including
histological characteristics and metastatic ability in
vitro, on which to test new therapies for scirrhous gastric
cancer.
The chemotherapeutic agents 5-fluorouracil (5-FU),
adriamycin (ADR) and irinotecan (CPT-11), as well as reactive
oxygen species (ROS), have all been reported to be cytotoxic
towards tumor cells. Among anticancer drugs for gastric cancer,
S-1, a 5-FU analog, has recently become the standard first-line
chemotherapeutic drug in Japan, while several other new drugs,
including the topoisomerase I inhibitors CPT-11 and ADR, are less
frequently used (13,14). These drugs provide an improved
prognosis for advanced gastric cancer (6,15).
ROS, including non-radical hydrogen peroxide
(H2O2), organic hydroperoxide (ROOH), and
hypochlorous acid (HClO), are generated from inflammatory immune
cells such as activated macrophages and neutrophils, which
accumulate at sites of inflammation. ROS are important for the
induction of apoptosis not only in inflammatory cells, but also in
neighboring cells (16,17). However, it is still largely unclear
how anticancer drugs and ROS induce apoptosis of scirrhous gastric
cancer cells.
In the present study, we aimed to develop new
therapeutic approaches based on the characteristic biological
features of scirrhous cancer cells by investigating the mechanisms
underlying the cytotoxicity of 5-FU, ADR, CPT-11 and ROS towards
the scirrhous cancer cell line, HSC-39, in vitro.
Materials and methods
Reagents
5-FU, ADR and CPT-11 were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Cisplatin (CDDP) was obtained
from Nichi-Iko (Toyama, Japan), and peroxynitrite and NOC-18 were
obtained from Dojindo (Kumamoto, Japan). H2O2
and HClO were purchased from Wako Pure Chemicals (Osaka, Japan).
Primary antibodies, including rabbit anti-caspase-3, anti-cleaved
caspase-3, anti-caspase-7 and anti-cleaved caspase-7, and secondary
anti-rabbit immunoglobulin G (IgG) antibody conjugated to
horseradish peroxidase (HRP), were purchased from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture
The human scirrhous gastric cancer cell line HSC-39
was derived from the peritoneal ascites of a 54-year-old male
patient with scirrhous gastric cancer (10). The cells were routinely maintained
in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island,
NY, USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS), 50 µg/ml streptomycin sulfate and 50 U/ml penicillin G
sodium (Nacalai Tesque, Kyoto, Japan). Media and sera were obtained
from Gibco. The cells were seeded at low density in 100-mm diameter
dishes (Iwaki, Tokyo, Japan) or Falcon T-25 tissue culture flasks
in standard medium containing 10% fetal calf serum (FCS), and
incubated in 5% CO2-95% humidified air at 37̊C.
Morphological observations
Cells were observed under a phase-contrast
microscope (Diamat; Nikon, Tokyo, Japan), and images were captured
from random fields at a magnification of ×400.
LDH assay for estimation of cellular
cytotoxicity
The amount of lactate dehydrogenase (LDH) released
into the culture medium from injured cells was used to estimate the
extent of cell damage. Briefly, HSC-39 cells were seeded at
4×105 cells/ml into a 48-well multiplate (Coaster), and
treated with 5-FU, ADR or CPT-11 for 48 h or incubated with
peroxynitrite, NOC-18, H2O2 or HClO for 18 h.
The supernatants were collected and assayed for LDH using an
LDH-Cytotoxic Test wako (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan), according to the manufacturer's protocol. Results
are expressed as percentage release according to the formula below.
Total activity was obtained by treatment of the same number of
cells with 0.1% Triton X-100 (Sigma-Aldrich) only, and background
release of LDH was determined by collecting the culture supernatant
at time 0 of incubation.
% of Release = {(Experimental release) - (0 time
release)/{(Total release) - (0 time release)} × 100
MTT assay for estimation of cell
viability
An MTT assay was performed using a CellTiter 96 kit
(Promega, Madison, WI, USA) to evaluate the number of viable cells
and the cellular metabolic activity. Briefly, 2×104
cells/0.1 ml/well were seeded onto 96-well clustered plates
(96-well plates), and incubated at 37̊C for 72 h in the presence or
absence of various concentrations of 5-FU, ADR or CPT-11. Then, 100
µl of WST-1 solution (Cell Proliferation Reagent WST-1; Roche
Diagnostics, Indianapolis, IN, USA) was added to each well, and the
cells were incubated at 37̊C for 1 h. The absorbance at 450/630 nm
was measured with a micro-ELISA reader (Bio-Rad, Hercules, CA,
USA). Background activity, containing culture medium only, was also
measured for each plate for subtraction of the background signal.
Cell viability was estimated by the following formula:
% of Viability = {(Experimental activity) -
(Background activity)/ (Control activity without drug) -
(Background activity)} × 100
Flow cytometry
For flow cytometric analysis, HSC-39 cells were
seeded at 1×106 cells/ml/dish (Corning Inc., Corning,
NY, USA), and treated with 5-FU, ADR or CPT-11 for 20–24 h, or with
peroxynitite, NOC-18, H2O2 or HClO for 18 h
at 37°C. The cells were collected into a 5-ml tube (Corning Falcon)
and washed once with phosphate-buffered saline [PBS(−)] before the
reagents of an apoptosis kit Annexin V-FITC kit
(MEBCYTO®; MBL, Nagoya, Japan) were added to detect
early-stage apoptotic cells. Cells were suspended in Annexin V-FITC
and propidium iodide (PI), and incubated at room temperature for 15
min in the dark. The cells were then mixed well with
fluorescence-activated cell sorting (FACS) buffer (PBS containing
0.1% bovine serum albumin and 0.1% sodium azide), filtered through
a 200-mesh nylon cloth, and analyzed by a cell sorter (FACSAria
III; BD Biosciences, San Jose, CA, USA). Signals from Annexin
V-FITC were detected by the FITC channel (BP530/30 filter), and
that from PI by the PerCP-Cy5.5 channel (BP695/40 filter); data
were processed with BD FACSDiva software (BD Biosciences).
SDS-PAGE and western blot
analysis
HSC-39 cells were seeded at 1×106
cells/60-mm dish (#3000-035; Iwaki) and treated with either CPT-11
and incubated for 4 h or 5-FU and ADR for 24 h at 37°C. The cells
were chilled on ice and washed twice with PBS by centrifugation at
4°C at 200 × g for 10 min. The final cell pellets were suspended in
100 µl of lysis buffer comprised of 10 mM EDTA (pH 8.0), 0.5%
Triton X-100 and 10 mM Tris-HCl buffer (pH 7.4). After standing on
ice for 10 min, the cells were centrifuged at 4°C at 11,000 × g for
5 min and the resultant supernatants were used as cell
extracts.
SDS-PAGE/western blotting was performed as
previously described (18).
Briefly, 15-µg aliquots of cell extract were electrophoresed
through a 5–20% gradient polyacrylamide gel (ATTO, Tokyo, Japan),
and the proteins were transferred to Immobilon®-P
polyvinylidene difluoride (PVDF) membranes (Merck Millipore,
Billerica, MA, USA) for western blotting. The membranes were
blocked with 30 mg/ml milk casein (MEGMILK SNOW BRAND Co., Ltd.,
Tokyo, Japan) in a rinse buffer comprised of 0.1% Triton X-100, 0.1
mM EDTA and 0.8% NaCl in 10 mM Tris-HCl buffer, pH 7.4, and then
incubated with rabbit anti-caspase-3 or anti-caspase-7 antibodies
(Cell Signaling Technology) at 4̊C overnight. The blots were
reacted with primary antibodies: anti-caspase-3 (1:1,000),
anti-cleaved caspase-3 (1:1,000), anti-caspase-7 (1:1,000) or
anti-cleaved caspase-7 (1:1,000), followed by reaction with a
secondary anti-rabbit IgG antibody conjugated to HRP (1:1,000).
Chemiluminescence was generated using Pierce ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
detected using a LAS 3000 Mini Image Analyzer (FujiFilm, Tokyo,
Japan). The intensity of each band was analyzed and quantitated
using ImageJ software (ver. 1.48V).
Statistical analysis
Results are shown as the mean ± standard error of
the mean (SEM) for at least 3 experiments performed independently
using separate cell preparations. Significant differences between
two groups were analyzed using Student's t-test and were considered
statistically significant at P<0.05.
Results
Morphological changes in HSC-39 cells
treated with chemotherapeutic reagents
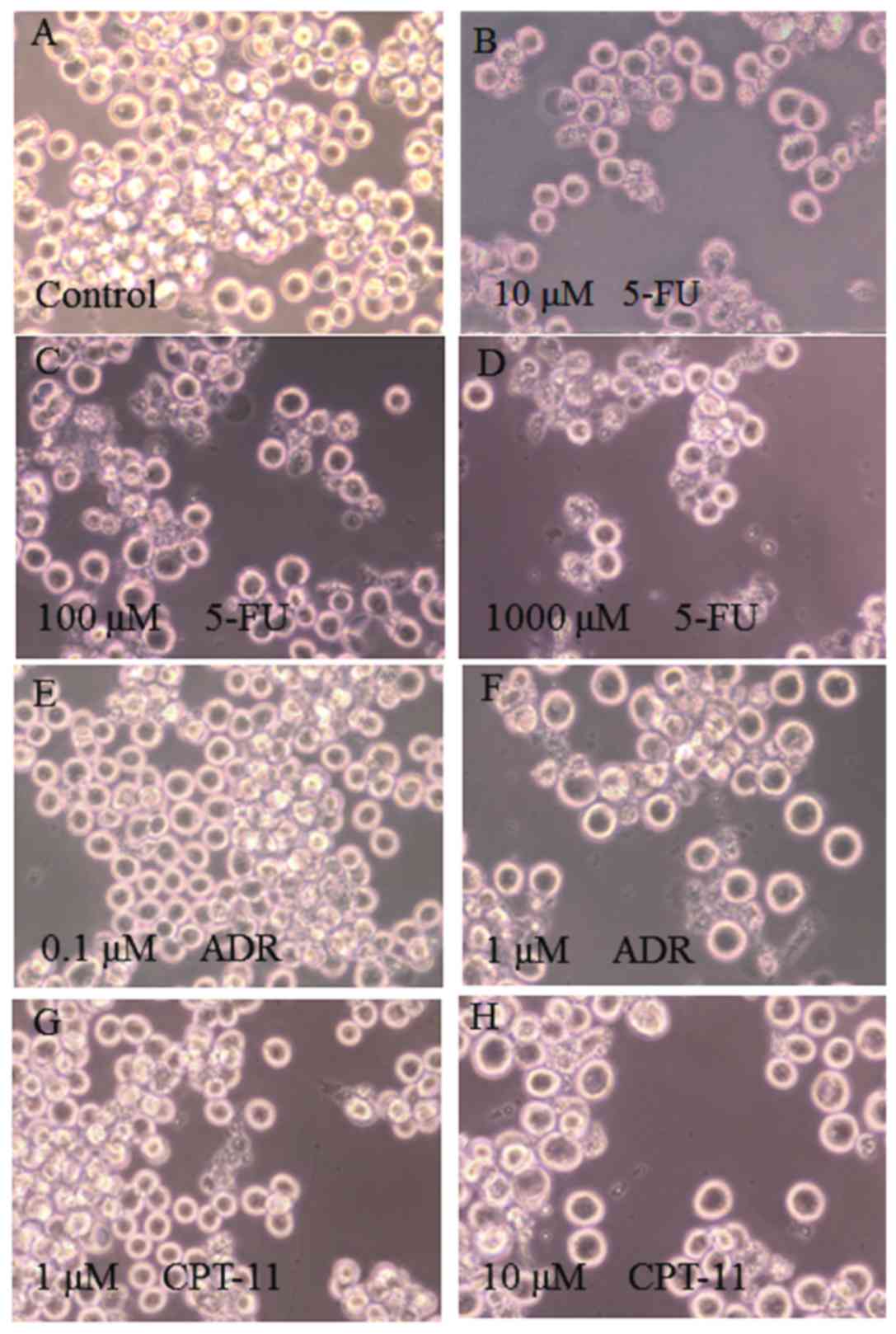
The morphology of HSC-39 cells was observed under a
phase-contrast microscope after treatment with the anticancer
reagents. The addition of 100 µM of 5-FU (Fig. 1C) induced the most marked changes in
the cells, such as agglutination and cell membrane rupture, while
treatment with 10 µM of 5-FU (Fig.
1B) induced no marked changes. When treated with 1 µM of ADR
(Fig. 1F) or 10 µM of CPT-11
(Fig. 1H), the number of intact and
apoptotic cells were both decreased. Fragmentation of cells was
also frequently observed after treatment with 100 µM of CPT-11
(data not shown). These results suggest that treatment of HSC-39
cells with the anticancer reagents led to a decrease in the number
of living cells with a concomitant increase in either apoptotic
cells when treated with 5-FU or aponecrotic cells when treated with
ADR and CPT-11.
Cytotoxic effects of chemotherapeutic
agents on HSC-39 cells
Apoptotic stimuli induce necrosis of cells through
depletion of cellular ATP. The type of cell death varies depending
on the mechanisms and processes that lead to these responses in
cells and tissues (19). LDH
release from cells principally results from rupture of the cell
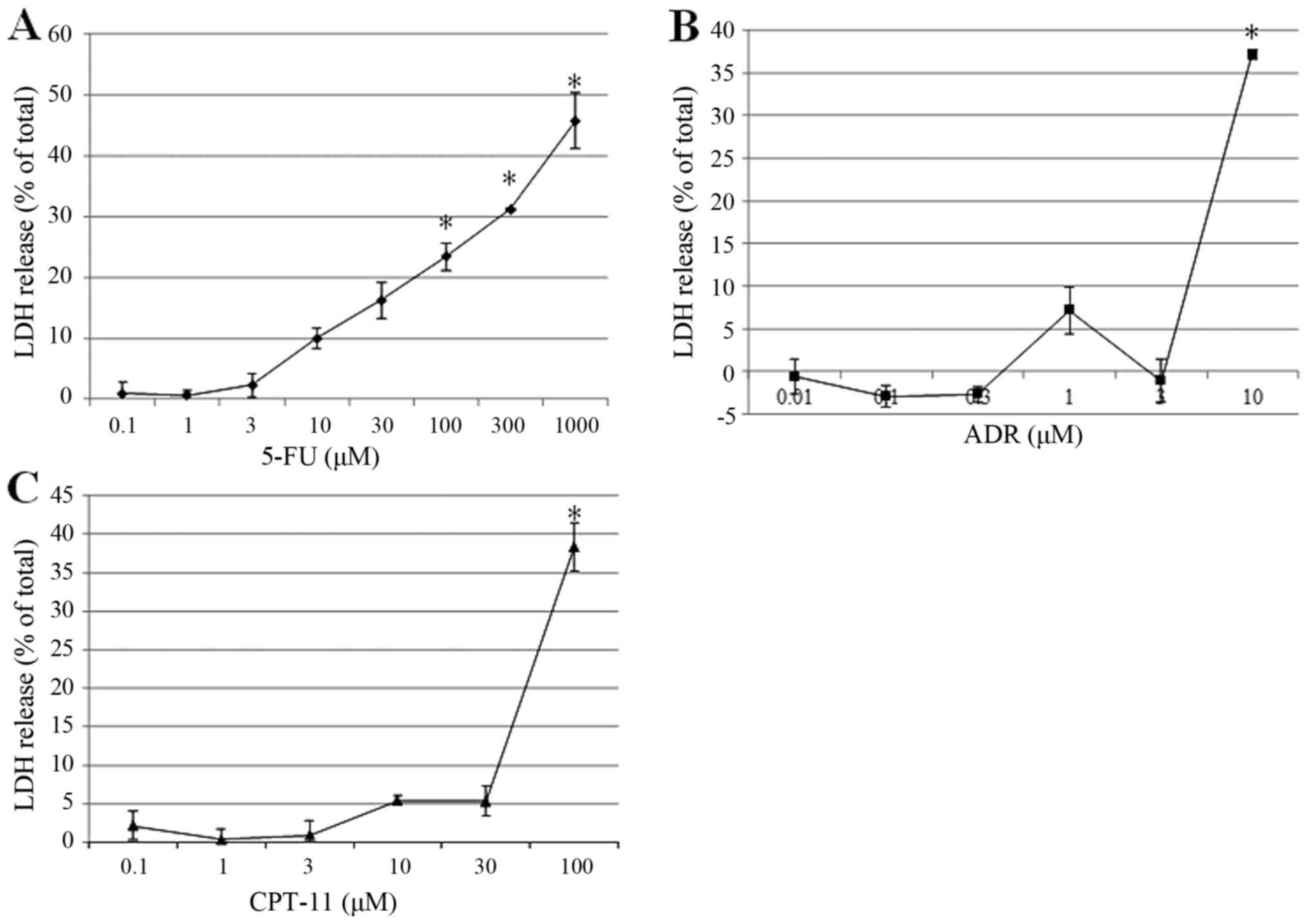
membrane. Fig. 2, LDH was
significantly released from the cells after treatment with 5-FU at
10 mM or higher in a dose-dependent manner (Fig. 2A). In contrast, significant release
of LDH was predominantly observed with treatment at high
concentrations of ADR at (10 µM; Fig.
2B) and CPT-11 (100 µM; Fig.
2C).
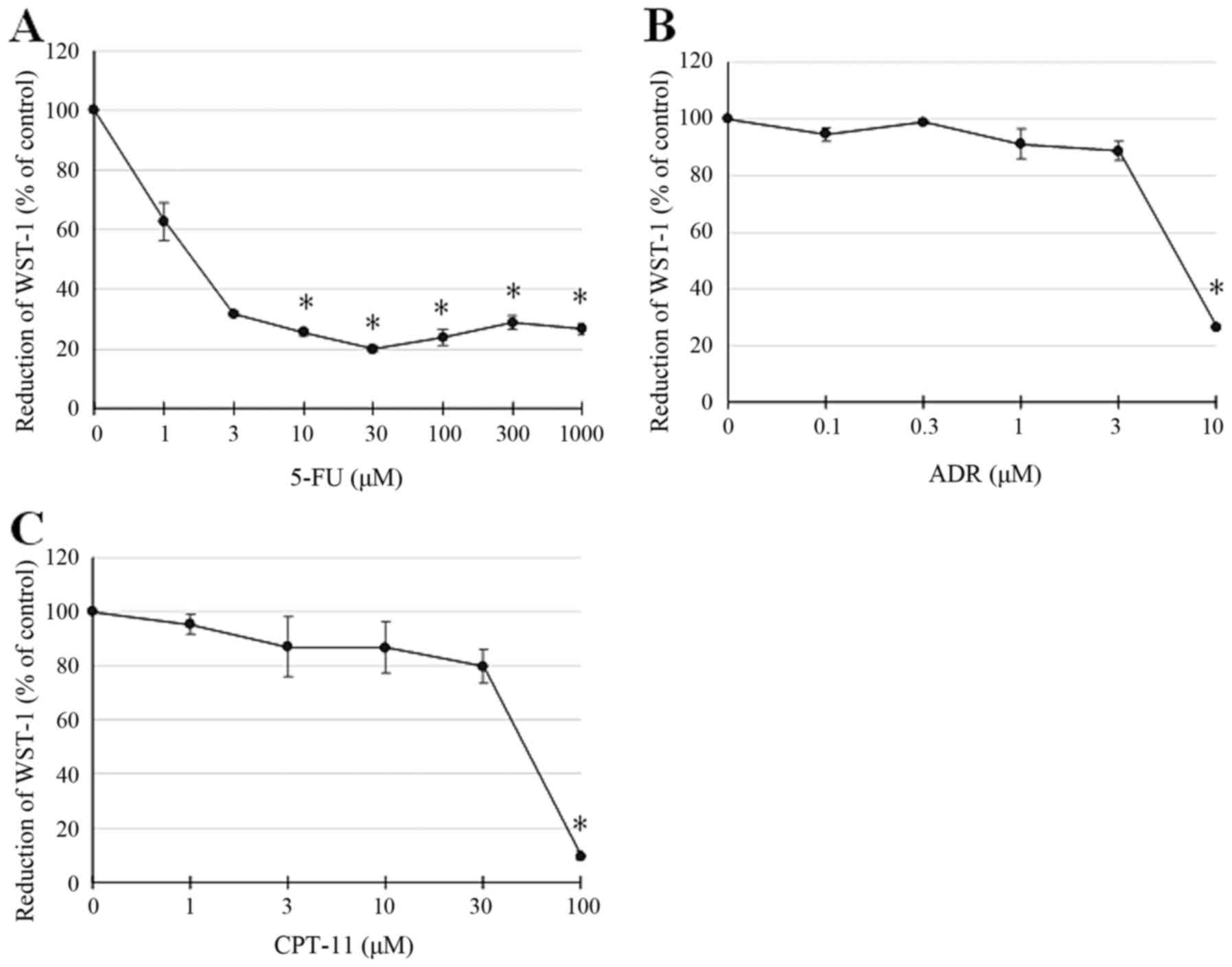
Similarly, viability, as estimated by the MTT assay
using WST-1 as a substrate, differed between treatment groups. 5-FU
at 1 mM attenuated WST-1 reduction, which was more pronounced and
significant at higher doses (Fig.
3A). In contrast, ADR and CPT-11, which exerted similar effects
on cell viability, only significantly attenuated WST-1 reduction at
10 and 100 µM, respectively (Fig. 3B
and C). These results showed that the effect of 5-FU treatment
on cell damage and decrease in cell viability as measured by LDH
release and MTT assay was different compared to that for ADR or
CPT-11. This suggests that 5-FU may induce cell damage via a
different mechanism than cell membrane disruption for LDH release,
indicating different functions in apoptotic cell death.
Induction of apoptosis and/or necrosis
in HSC-39 cells
To examine apoptotic cell death, we examined
cell-surface binding of Annexin V, a phosphatidylserine
(PS)-binding protein, and staining of cells with PI by flow
cytometric analysis. Translocation of PS to the external cell
surface is not unique to apoptosis; it also occurs during cell
necrosis. The difference between apoptosis and necrosis is in cell
membrane integrity: during the initial stages of apoptosis the cell
membrane remains intact, but for necrosis, the cell membrane
becomes leaky, allowing access to PI to stain nucleic acids. The
Annexin V assay allows for the detection of the early phase of
apoptosis, before the loss of cell membrane integrity, and permits
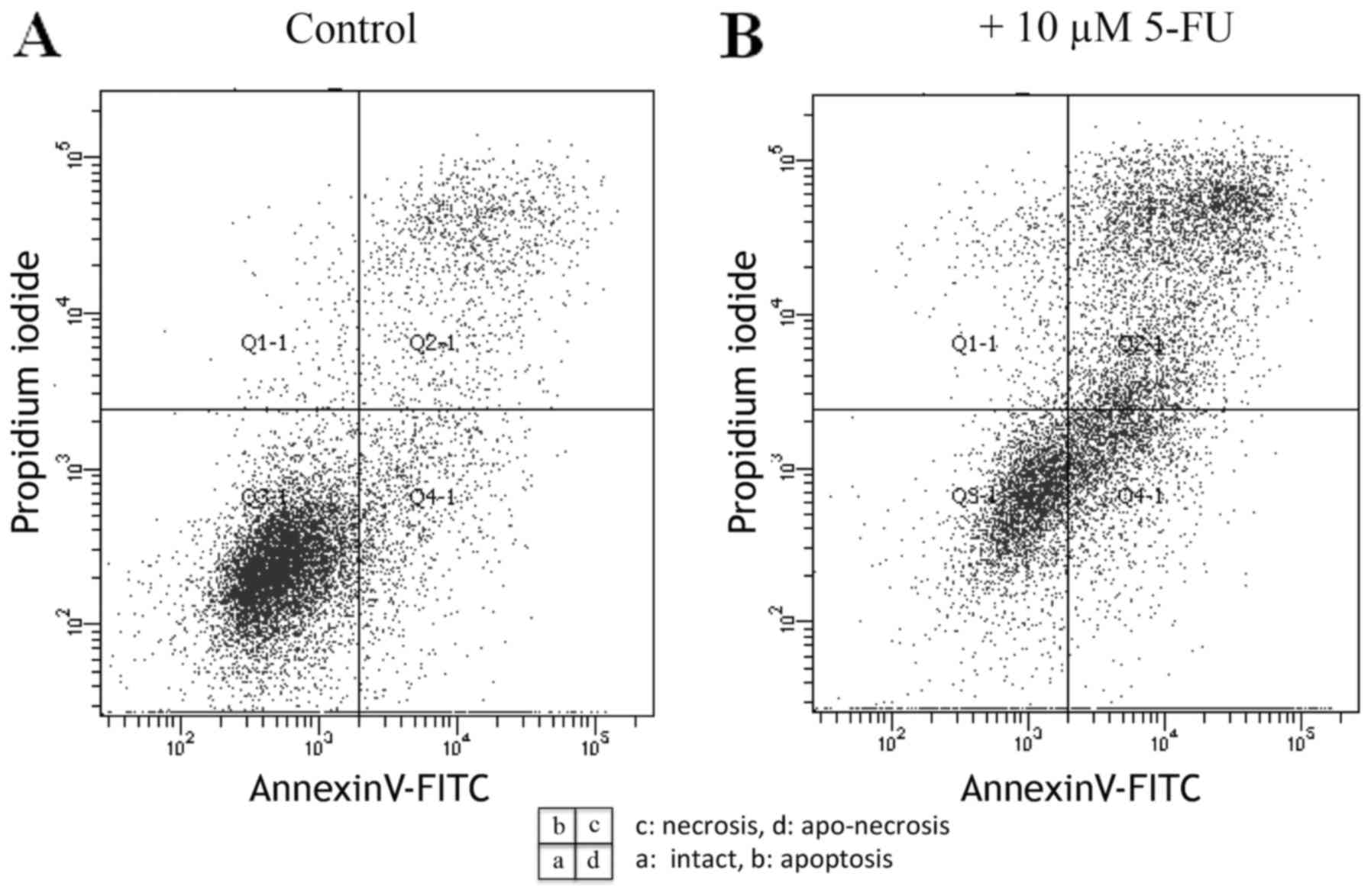
assessment of apoptotic death. As shown in Fig. 4A, the control cells showed no
fluorescein staining. In contrast, cells treated with 10 µM of 5-FU
for 48 h displayed significant binding of Annexin V-fluorescein to
the membrane surface (Fig. 4B),
indicating apoptosis. To distinguish between apoptotic and
potential necrotic or lysed cells that may also have been exposed
to PS due to loss of membrane integrity, we concomitantly examined
Annexin V+ and PI+ cells, which correspond to
aponecrotic HSC-39 cells (Fig. 4B).
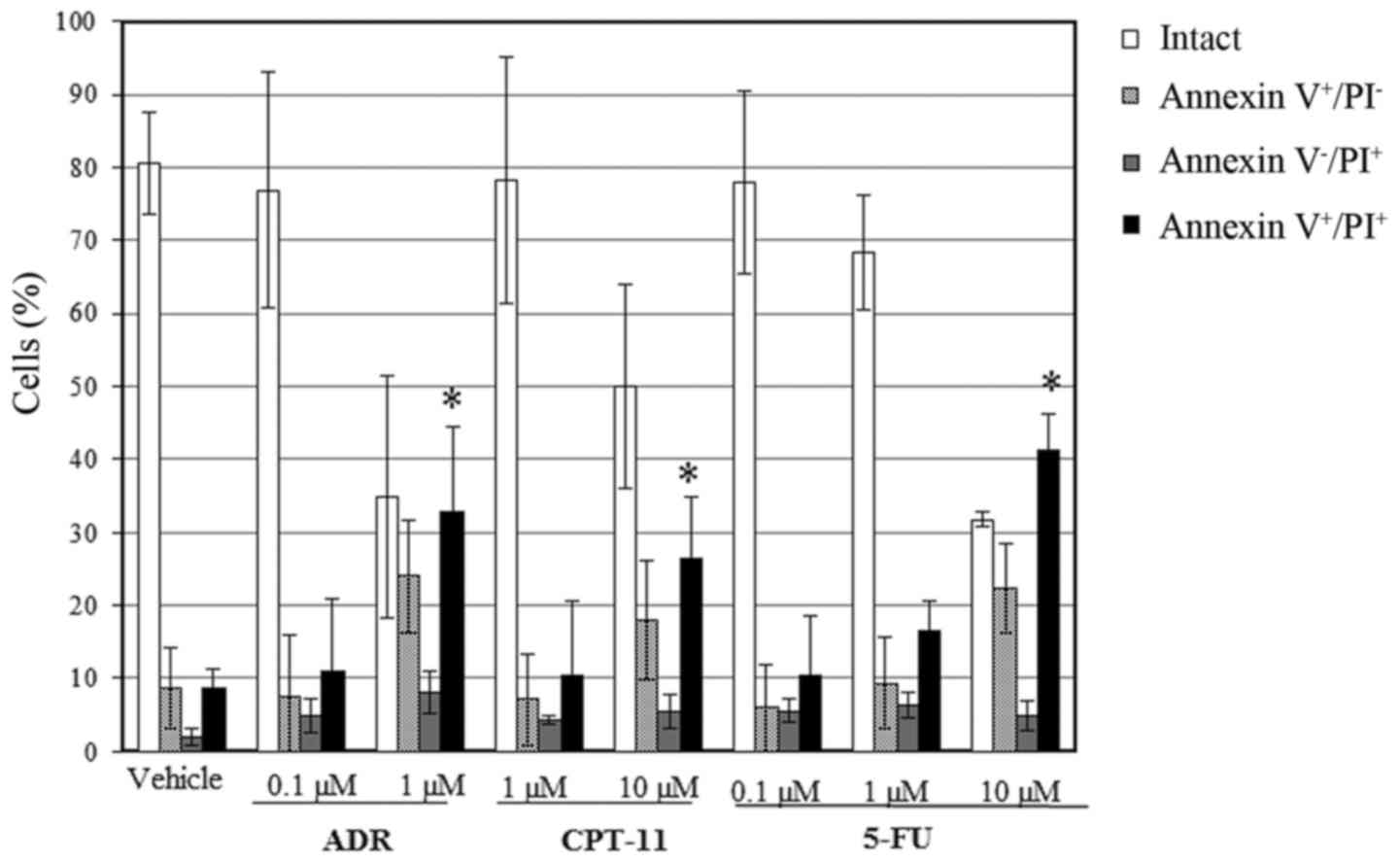
Annexin V+ and PI+ cells were also observed
after treatment with 1 µM of ADR and 10 µM of CPT-11, suggesting
that these drugs induced apoptosis and aponecrosis (Fig. 5).
Caspase-7 mediates apoptosis in HSC-39
cells
Caspase family members play important roles in the
progression of apoptosis in various cells. Among them, caspase-3
and −7 are the effector caspases activated by apical caspase and
cleavage of cellular death substrates. Therefore, we examined which
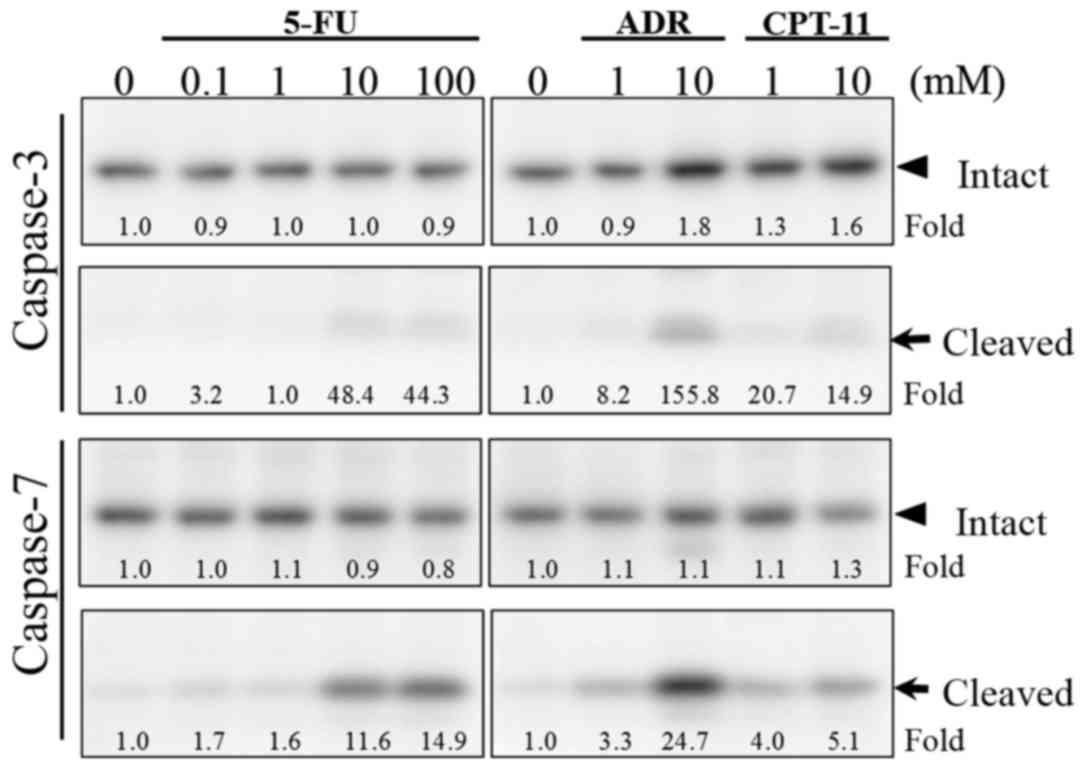
caspase was involved in the induction of cytotoxicity. The level of
cleaved caspase-7, an activated form, was much higher than that of
the cleaved caspase-3 after treatment with 10–100 µM of 5-FU for 48
h compared to the control (Fig. 6).
Similar results were obtained with treatment at 1–10 µM of ADR or
CPT-11. These results suggest that caspase-7 is responsible for the
progression of apoptosis of HSC-39 cells induced by these
chemotherapeutic drugs.
Cytotoxic effects and induction of
apoptosis of HSC-39 cells by ROS
ROS have been shown to induce apoptosis in many
different cell systems (20). In
the present study, we examined the cytotoxic effects of ROS on
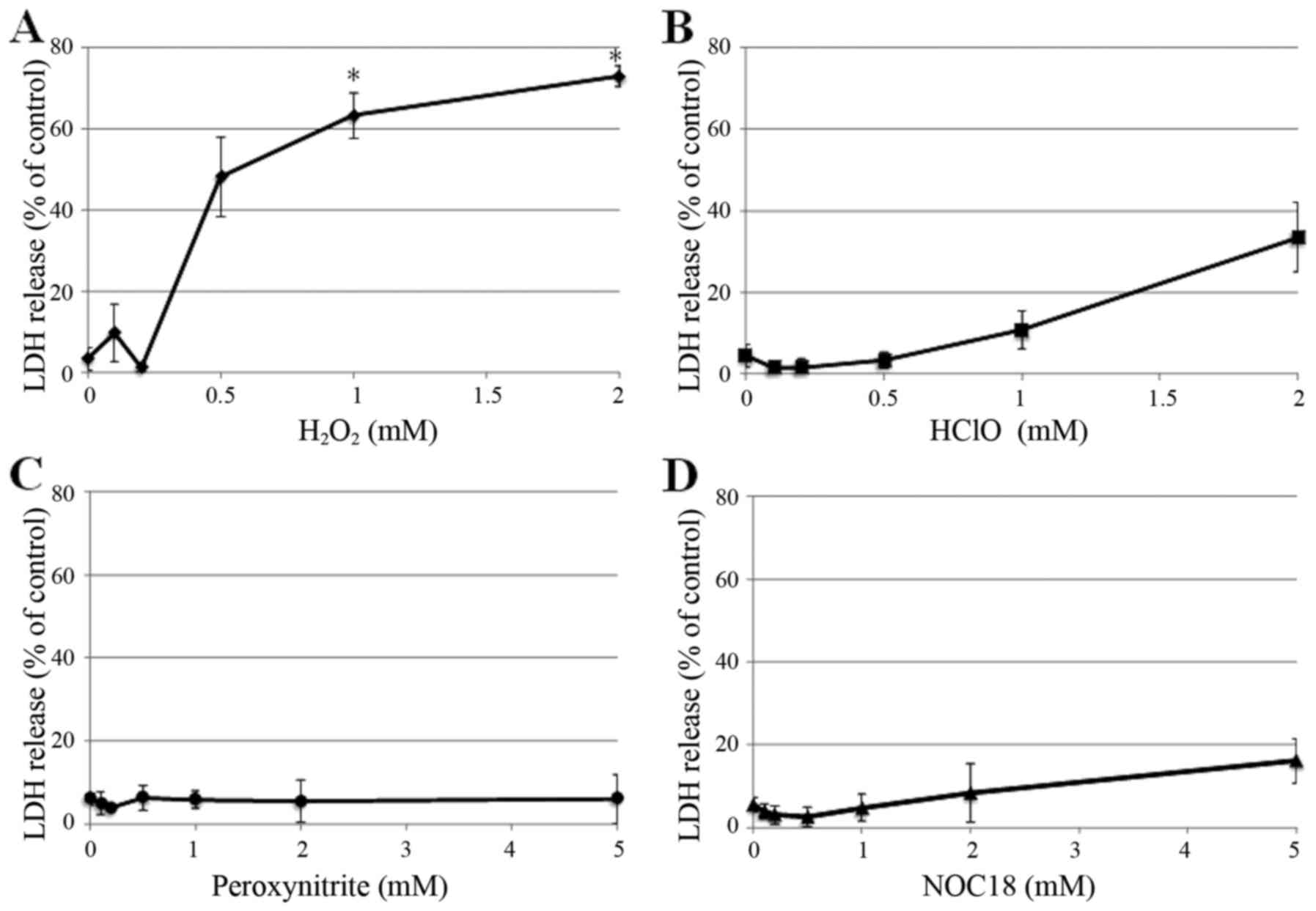
HSC-39 cells. HSC-39 cells released amounts of LDH upon treatment
with 0.5 mM or higher of H2O2, with >60%
release significantly occurring at 1 mM and greater (Fig. 7A). In contrast, HSC-39 cells showed
less sensitivity to HClO (Fig. 7B)
and NOC-18 (Fig. 7D). Little to no
LDH release was induced by peroxynitrite (Fig. 7C).
We next examined the induction of
apoptosis and/or necrosis of HSC-39 cells by Annexin V and PI
staining and flow cytometric analysis
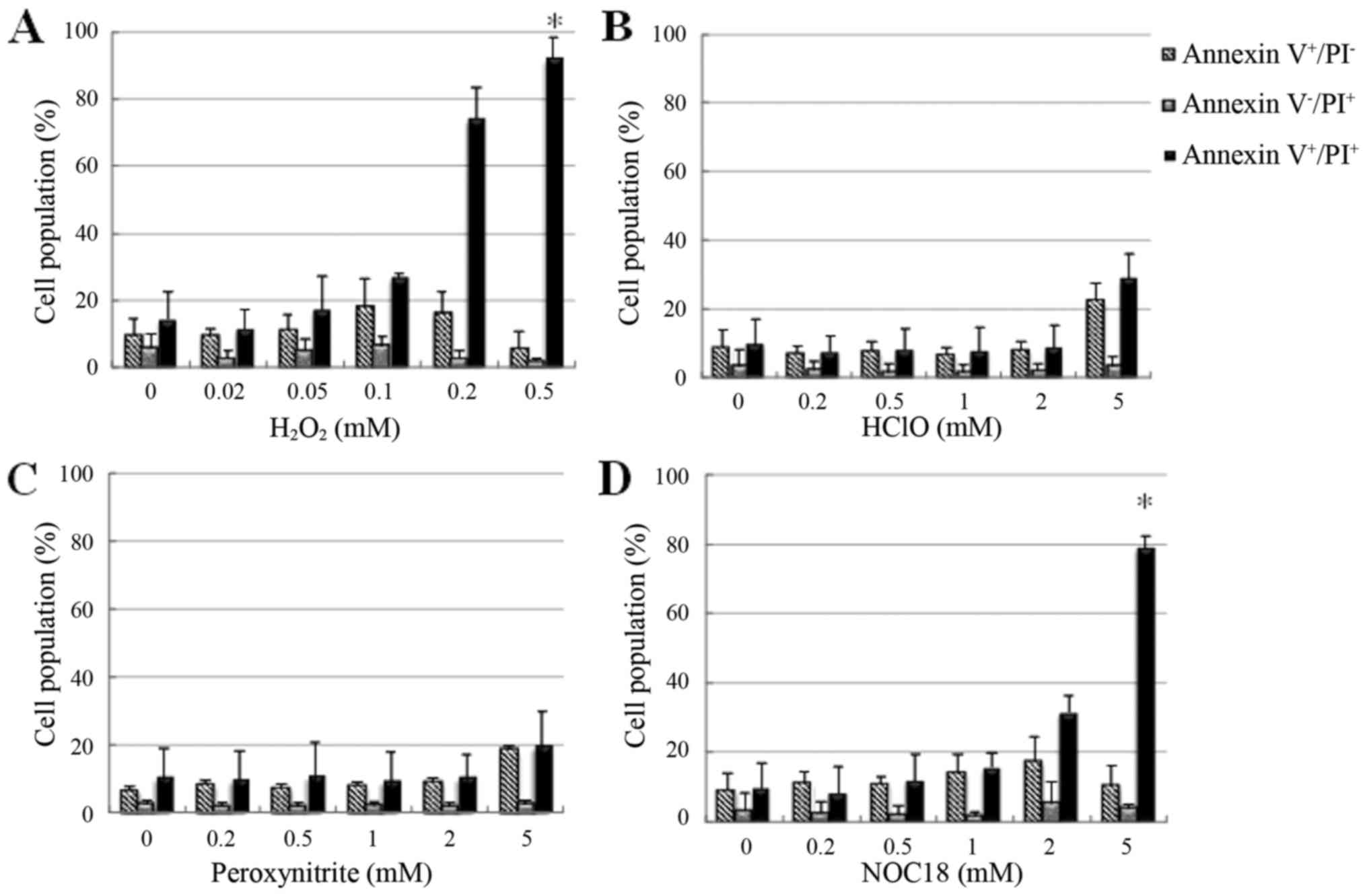
Treatment with 0.1 mM H2O2
induced Annexin V+ and PI− cells, indicating
early stage apoptosis, while treatment with 0.2–0.5 mM
H2O2 or 2–5 mM NOC-18 induced Annexin
V+ and PI+ cells, indicating necrosis or
aponecrosis, in a dose-dependent manner. HSC-39 cells showed high
sensitivity to H2O2 and NOC-18. These were
significant differences compared with the controls. At low doses,
HClO had no effect on the cell membrane, but at high doses (5 mM)
induced Annexin V+ and PI− cells and Annexin
V+ and PI+ cells, indicating the induction of
apoptosis and aponecrosis (Fig. 8A and
B). Similarly, the cells were induced after treatment with
peroxynitrite (Fig. 8C). In
addition, the pattern of cytotoxicity appeared to change over time
in all treatment groups, suggesting that induction of apoptosis
occurs first, followed by necrosis. However, treatment with
H2O2 or HClO did not result in typical
apoptosis (data not shown).
These results suggest that HSC-39 cells showed high
dose-dependent sensitivity to H2O2 and
NOC-18, but low sensitivity to HClO and peroxynitrite, suggesting
that the cell damage may be linked to the degree of membrane
permeability induced by the various ROS.
Discussion
In the present study, we examined the mechanisms
underlying the cytotoxicity of anticancer drugs and reactive oxygen
species (ROS) toward a human scirrhous cancer cell line, HSC-39,
in vitro. We demonstrated that 5-FU induced apoptosis and
ADR and CPT-11 induced necrosis and/or aponecrosis of the HSC-39
cells. 5-FU inhibited cell viability and induced little LDH release
at low doses, while ADR and CPT-11 inhibited cell viability and
induced LDH release at high doses. Our findings suggest that 5-FU
is a reasonable chemotherapeutic drug for scirrhous gastric cancer.
In phase II trials, the 5-FU analog S-1 showed a 33% response rate
against scirrhous gastric cancer. Due to the reported promising
effects of S-1 for neoadjuvant chemotherapy against scirrhous
gastric cancer in a pilot study, a new phase II trial was planned
to determine the survival benefit of S-1 treatment (21).
Apoptosis is a crucial mechanism for many biological
processes. The apoptotic process is characterized by distinct
morphological features. In HSC-39 cells, typical structural changes
and related alterations in cell function of the apoptotic pathway
were observed, including cell rupture, translocation of PS to the
outer layer of the plasma membrane, and altered mitochondrial
metabolic activity.
Several chemotherapeutic drugs have been evaluated
for their antitumor function. Compared with the best supportive
care, the survival benefit of 5-FU has been reported based on
chemotherapy for metastatic gastric cancer (22). In the present study, 5-FU, ADR and
CPT-11 induced apoptosis and/or aponecrosis in HSC-39 cells in a
dose-dependent manner, as assessed by FACScan analysis (Figs. 4 and 5). 5-FU effectively inhibited WST-1
decrease even at low doses where little LDH release was observed,
whereas ADR and CPT-11 inhibited WST-1 decrease only at high doses
where LDH release occurred (Figs.
2A and 3A). A previous study
indicated that administration of 5-FU results in an increase in the
S phase fraction in human gastric carcinoma, which is coincidental
with the appearance of apoptosis-positive cells (23). Moreover, thymidylate synthase (TS)
activity is immediately markedly suppressed. These findings suggest
that induction of apoptosis and inhibition of DNA synthesis, both
induced by 5-FU, may be closely associated with its antitumor
effects. At a low dose, 5-FU inhibits energy metabolism, resulting
in decreased ATP levels to impair membrane barrier function, rather
than inducing direct damage to cells (24). In contrast, ADR induces an increase
of c-jun and ATF3 mRNA levels in the
mitogen-activated protein kinase (MAPK) pathway, followed by
apoptosis (25). This may explain
why ADR predominantly induced apoptosis and LDH release in HSC-39
cells at high doses. The mechanism underlying the similar actions
of CPT-11 remains unclear.
We also demonstrated that the progression of
apoptosis upon treatment with 5-FU, ADR and CPT-11 was accompanied
by cleavage of caspase-7, but not caspase-3 (Fig. 6). Caspase-3 normally exists in the
cytosol as an inactive precursor that becomes activated through
cleavage in apoptotic cells (26).
Caspase-7, but not caspase-3, undergoes proteolytic activation
during lovastatin-induced apoptosis, an effect prevented by
mevalonate, and was identified as a possible mediator of
lovastatin-induced apoptosis (27).
The activation of caspase-7 during the apoptosis of HSC-39 cells
(Fig. 6) demonstrated in the
present study, may indicate a new pathway in the apoptotic cascade
in scirrhous gastric cancer cells.
HSC-39 cells also showed high sensitivity to
H2O2 (Fig.
7A), as indicated by LDH release and Annexin V and PI staining.
H2O2 has strong intracellular cytotoxic
effects due to its high membrane permeability. Furthermore,
H2O2 can induce apoptosis in neutrophils;
this can be prevented by catalase, an enzyme that also prevents
spontaneous neutrophil apoptosis. This suggests that
H2O2 may be an important triggering mechanism
responsible for the short life-span of mature neutrophils.
Caspase-3, but not other caspases, is required for commitment to
ROS-induced apoptosis (28).
The susceptibility of HSC-39 cells to 5-FU and
H2O2 suggests that there may be common
mechanisms underlying the cytotoxicity of these reagents. Manganese
superoxide dismutase (Mn-SOD) negatively regulates 5-FU-mediated
apoptosis induction in squamous carcinoma cells (29), and 5-FU increases cellular
accumulation of H2O2 in CT26 colon cancer
cells (30). These studies suggest
that 5-FU induces increases in cellular H2O2
levels, which may lead to decreased metabolic activity, as
indicated by the WST-1 assay (Fig.
3A), and increased apoptotic and subsequent necrotic changes
(Fig. 5), as well as increased LDH
release (Fig. 2A) in HSC-39 cells
as observed in the present study.
HSC-39 cells undergo apoptosis when treated with
TGF-β under serum-free culture conditions, which is mediated by
activation of an apoptosis signal transduction pathway (31,32).
Recent findings showed that 5-FU treatment activated the TGF-β
pathway in drug resistant colorectal carcinoma cells in an in
vivo and in vitro model (33). Liu and Desai reported that TGF-β1
increased ROS production and suppressed antioxidant enzymes,
leading to a redox imbalance. Therapeutic targeting of
TGF-β-induced and ROS-dependent cellular signaling represents a
novel approach in the treatment of fibrotic disorders (34). Therefore, it may be of interest to
examine the interaction between HSC-39 and TGF-β-producing cells
such as activated fibroblasts or macrophages for peritoneal
metastasis. Furthermore, it may be beneficial to examine whether
various cytokines act as transcriptional regulators in
TGF-β-mediated apoptosis. We are now in the process of
investigating this point. Several studies provided evidence
supporting the involvement of ROS in the induction of apoptosis and
demonstrated the importance of ROS in the release of cytochrome
c from the mitochondria. 5-FU-induced autophagy may function
as a resistance mechanism against apoptotic cell death (35). It may provide a novel strategy to
overcome therapy resistance.
Cisplatin (cis-diamminedichloroplatinum) was
developed by Rosenberg (36) in the
1960s and was initially used in the treatment of head and neck,
uterine and bladder cancers (37).
Cisplatin is one of the most important drugs for the treatment of
gastric cancer; it has demonstrated a high positive-response rate
in the treatment of gastric cancer. Although, combination treatment
of 5-FU and cisplatin has demonstrated a significantly increased
cancer-free survival compared to treatment with 5-FU alone, no
significant differences have been observed in overall survival rate
between the two treatments (38).
This survival difference, however, may result from chance in
conducting the subgroup analysis and may have limited influence on
the interpretation of the primary conclusion of the study.
In conclusion, we demonstrated that 5-FU induces
apoptosis of HSC-39 cells, inhibits cell viability and induces
little LDH release at low doses. In contrast, ADR and CPT-11 induce
necrosis and/or aponecrosis of HSC-39 cells, inhibit cell viability
and induce LDH release at high doses. The present study provides
important insights into the underlying mechanisms of apoptosis
behind the cytotoxicity for scirrhous gastric cancer. Furthermore,
consistent with the widening acceptance of combination
chemotherapies in clinical practice, such as with fluoropyrimidine
agents, cisplatin, irinotecan and taxanes, our findings suggest
that 5-FU has potential efficacy and that the use of 5-FU in
combination with other chemotherapeutic agents that attack the
membrane barrier may be a successful chemotherapy regimen for
scirrhous gastric cancer.
References
|
1
|
Ikeguchi M, Miyake T, Matsunaga T,
Yamamoto M, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S and
Tsujitani S: Recent results of therapy for scirrhous gastric
cancer. Surg Today. 39:290–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hippo Y, Yashiro M, Ishii M, Taniguchi H,
Tsutsumi S, Hirakawa K, Kodama T and Aburatani H: Differential gene
expression profiles of scirrhous gastric cancer cells with high
metastatic potential to peritoneum or lymph nodes. Cancer Res.
61:889–895. 2001.PubMed/NCBI
|
|
3
|
Kitamura K, Beppu R, Anai H, Ikejiri K,
Yakabe S, Sugimachi K and Saku M: Clinicopathologic study of
patients with Borrmann type IV gastric carcinoma. J Surg Oncol.
58:112–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura R, Saikawa Y, Wada N, Yoshida M,
Kubota T, Kumai K and Kitajima M: Retrospective analysis of
prognosis for scirrhous-type gastric cancer: One institution's
experience. Int J Clin Oncol. 12:291–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohtsu A, Boku N, Yoshida S, Miyata Y,
Shirao K, Shimada Y and Kurihara M: Japan Clinical Oncology Group:
Response of the primary lesion in gastric cancer to
chemotherapeutic trials. Int J Clin Onco1. 3:3–6. 1998. View Article : Google Scholar
|
|
6
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: RAINBOW Study Group: Ramucirumab plus paclitaxel versus placebo
plus paclitaxel in patients with previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry
DR, et al: REGARD Trial Investigators: Ramucirumab monotherapy for
previously treated advanced gastric or gastro-oesophageal junction
adenocarcinoma (REGARD): An international, randomised, multicentre,
placebo-controlled, phase 3 trial. Lancet. 383:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nashimoto A, Akazawa K, Isobe Y, Miyashiro
I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, et
al: Gastric cancer treated in 2002 in Japan: 2009 annual report of
the JGCA nationwide registry. Gastric Cancer. 16:1–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanagihara K, Seyama T, Tsumuraya M,
Kamada N and Yokoro K: Establishment and characterization of human
signet ring cell gastric carcinoma cell lines with amplification of
the c-myc oncogene. Cancer Res. 51:381–386. 1991.PubMed/NCBI
|
|
11
|
Semba S, Kodama Y, Ohnuma K, Mizuuchi E,
Masuda R, Yashiro M, Hirakawa K and Yokozaki H: Direct
cancer-stromal interaction increases fibroblast proliferation and
enhances invasive properties of scirrhous-type gastric carcinoma
cells. Br J Cancer. 101:1365–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishida M, Gomyo Y, Ohfuji S, Ikeda M,
Kawasaki H and Ito H: Evidence that expression of a mutated p53
gene attenuates apoptotic cell death in human gastric
intestinal-type carcinomas in vivo. Jpn J Cancer Res. 88:468–475.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boku N, Yamamoto S, Shirao K, Doi T,
Sawaki A, Koizumi W, Saito H, Yamaguchi K, Kimura A, et al:
Randomized phase III study of 5-fluorouracil (5-FU) alone versus
combination of irinotecan and cisplatin (CP) versus S-1 alone in
advanced gastric cancer (JCOG9912). J Clin Oncol 2007 ASCO Annual
Meeting Proceedings. 25:LBA45132007.
|
|
14
|
Ichikawa W and Sasaki Y: Challenges in
predicting the clinical outcome in S-1-based chemotherapy for
gastric cancer patients. Int J Clin Oncol. 13:206–211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shitara K, Ishiguro A, Munakata M, Wada R
and Sakata Y: Retrospective analysis of stage IV advanced gastric
cancer treated with S-1 or other chemotherapy. Int J Clin Oncol.
11:367–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato K, Akaike T, Kojima Y, Ando M, Nagao
M and Maeda H: Evidence of direct generation of oxygen free
radicals from heterocyclic amines by NADPH/cytochrome P-450
reductase in vitro. Jpn J Cancer Res. 83:1204–1209. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanks KW, Gao JP and Sharma T:
Photodynamic enhancement of doxorubicin cytotoxicity. Cancer
Chemother Pharmacol. 35:17–20. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawakami T, Kawamura K, Fujimori K, Koike
A and Amano F: Influence of the culture medium on the production of
nitric oxide and expression of inducible nitric oxide synthase by
activated macrophages in vitro. Biochem Biophys Rep. 5:328–334.
2016.
|
|
19
|
Leist M, Single B, Castoldi AF, Kühnle S
and Nicotera P: Intracellular adenosine triphosphate (ATP)
concentration: A switch in the decision between apoptosis and
necrosis. J Exp Med. 185:1481–1486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinoshita T, Konishi M, Nakagohri T, Inoue
K, Oda T, Takahashi S, Boku N, Ohtsu A and Yoshida S: Neoadjuvant
chemotherapy with S-1 for scirrhous gastric cancer: A pilot study.
Gastric Cancer. 6:(Suppl 1). S40–S44. 2003. View Article : Google Scholar
|
|
22
|
Ohtsu A: Current status and future
prospects of chemotherapy for metastatic gastric cancer: A review.
Gastric Cancer. 8:95–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnston PG, Lenz HJ, Leichman CG,
Danenberg KD, Allegra CJ, Danenberg PV and Leichman L: Thymidylate
synthase gene and protein expression correlate and are associated
with response to 5-fluorouracil in human colorectal and gastric
tumors. Cancer Res. 55:1407–1412. 1995.PubMed/NCBI
|
|
25
|
Yu R, Shtil AA, Tan TH, Roninson IB and
Kong AN: Adriamycin activates c-jun N-terminal kinase in
human leukemia cells: A relevance to apoptosis. Cancer Lett.
107:73–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Henkels KM and Turchi JJ:
Cisplatin-induced apoptosis proceeds by caspase-3-dependent and
-independent pathways in cisplatin-resistant and -sensitive human
ovarian cancer cell lines. Cancer Res. 59:3077–3083.
1999.PubMed/NCBI
|
|
27
|
Marcelli M, Cunningham GR, Walkup M, He Z,
Sturgis L, Kagan C, Mannucci R, Nicoletti I, Teng B and Denner L:
Signaling pathway activated during apoptosis of the prostate cancer
cell line LNCaP: Overexpression of caspase-7 as a new gene therapy
strategy for prostate cancer. Cancer Res. 59:382–390.
1999.PubMed/NCBI
|
|
28
|
Matsura T, Kai M, Fujii Y, Ito H and
Yamada K: Hydrogen peroxide-induced apoptosis in HL-60 cells
requires caspase-3 activation. Free Radic Res. 30:73–83. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ueta E, Yoneda K, Yamamoto T and Osaki T:
Manganese superoxide dismutase negatively regulates the induction
of apoptosis by 5-fluorouracil, peplomycin and gamma-rays in
squamous cell carcinoma cells. Jpn J Cancer Res. 90:555–564. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alexandre J, Nicco C, Chéreau C, Laurent
A, Weill B, Goldwasser F and Batteux F: Improvement of the
therapeutic index of anticancer drugs by the superoxide dismutase
mimic mangafodipir. J Natl Cancer Inst. 98:236–244. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yanagihara K and Tsumuraya M: Transforming
growth factor β1 induces apoptotic cell death in
cultured human gastric carcinoma cells. Cancer Res. 52:4042–4045.
1992.PubMed/NCBI
|
|
32
|
Ohta S, Yanagihara K and Nagata K:
Mechanism of apoptotic cell death of human gastric carcinoma cells
mediated by transforming growth factor β. Biochem J. 324:777–782.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Romano G, Santi L, Bianco MR, Giuffrè MR,
Pettinato M, Bugarin C, Garanzini C, Savarese L, Leoni S, Cerrito
MG, et al: The TGF-β pathway is activated by 5-fluorouracil
treatment in drug resistant colorectal carcinoma cells. Oncotarget.
7:22077–22091. 2016.PubMed/NCBI
|
|
34
|
Liu RM and Desai LP: Reciprocal regulation
of TGF-β and reactive oxygen species: A perverse cycle for
fibrosis. Redox Biol. 6:565–577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosenberg B: Fundamental studies with
cisplatin. Cancer. 55:2303–l6. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hecquet B, Vennin P, Fournier C, Lefebvre
JL, Caty A, Bonneterre J, Adenis L and Demaille A: Platinum
concentration in human tumors of head and neck, uterine cervix, and
breast following treatment with cisplatin. Cancer Chemother
Pharmacol. 15:310–312. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohtsu A, Shimada Y, Shirao K, Boku N,
Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S, et
al: Japan Clinical Oncology Group Study (JCOG9205): Randomized
phase III trial of fluorouracil alone versus fluorouracil plus
cisplatin versus uracil and tegafur plus mitomycin in patients with
unresectable, advanced gastric cancer: The Japan Clinical Oncology
Group Study (JCOG9205). J Clin Oncol. 21:54–59. 2003. View Article : Google Scholar : PubMed/NCBI
|