Introduction
Endoscopic ultrasound-guided fine needle aspiration
(EUS-FNA) is reported to be a safe and useful method for obtaining
diagnostic tissue samples in cases of suspicious pancreatic
adenocarcinoma (PCA) (1). However,
specimens obtained with EUS-FNA are often tiny and fragmented
leading to an inconclusive and doubtful diagnosis in up to 20% of
patients with PCA (2–4). A variety of factors, such as
desmoplastic reaction in PCA associated with poor cellularity
(4–8), difficulty in discriminating between
well-differentiated PCA and reactive atypia (9,10), and
technical aspects of EUS-FNA (11–15),
may lead to inconclusive or doubtful diagnoses in small EUS-FNA
samples. In addition, the sensitivity of EUS-FNA for PCA ranges
widely from 44 to 100% (1,16), and the negative predictive value
(NPV) also shows an enormous spread (16–92%) (17). Thus, negative results cannot
completely exclude malignancy, and diagnosing PCA using EUS-FNA is
still challenging.
PCA is characterized by a variety of molecular
alterations, and molecular and biological markers for diagnosing
PCA have been developed. Among these markers, S100P has been noted
as a detection marker for PCA (18,19).
S100P is specifically expressed in PCA cells with a high frequency
(10,19–27).
Moreover, a meta-analysis investigating whether S100P can detect
PCA using EUS-FNA or surgical specimens showed a pooled
sensitivity, specificity, and area under the receiver operating
characteristic (ROC) curve (AUC) of 87% [95% confidence interval
(CI), 83–90%], 88% (95% CI, 82–93%) and 0.927, respectively
(28).
However, these studies assessing S100P in EUS-FNA
samples used mRNA quantification or immunohistochemical (IHC)
analysis (10,19,21–27,29).
When samples are collected from pancreatic lesions by EUS-FNA,
there is a potential risk that mRNA is exposed to RNase digestion,
resulting in the fragmentation of mRNA (4,22,30).
In addition, IHC analysis can only be achieved, when an adequate
amount of EUS-FNA sample is obtained (4,29).
Thus, detection of S100P using mRNA quantification or IHC analysis
from EUS-FNA samples has limitations, and hence, a detection method
for S100P in small EUS-FNA samples is needed.
There have been no studies on the assessment of the
S100P protein itself in any pathological samples, including
EUS-FNA, surgical and serological samples. Thus, the quantification
of the S100P protein itself may overcome the limitations observed
with mRNA and IHC analysis in small pancreatic samples obtained by
EUS-FNA, and provide promising results for the diagnosis of
PCA.
Therefore, the aim of the present study was to
establish a novel, simple system of quantifying the S100P protein
even in small EUS-FNA samples and evaluate whether quantitative
analysis of the S100P protein combined with EUS-FNA cytology can
provide a reliable diagnosis for PCA.
Materials and methods
The present study consisted of 3 parts: i) the ex
vivo development of a high sensitivity sandwich ELISA for the
S100P protein as a quantitative analysis for detecting small
amounts of PCA cells in tiny EUS-FNA samples, ii) the in
vivo evaluation of this newly developed assay using cell lines
and mouse xenograft tumors, iii) a pilot clinical trial to
investigate the efficacy of S100P protein assessment combined with
EUS-FNA cytology for the diagnosis of PCA.
Ethics statement
All mice used in the present study were cared for
according to animal care regulations of national and international
guidelines (The National Institutes of Health Guide for the Care
and Use of Laboratory Animals and the ARRIVE Guidelines). Animal
protocols were approved by the Animal Care Committee of the Jikei
University School of Medicine (identification no. 24–044). All
efforts were made to minimize suffering.
The clinical trial was approved by the Human
Subjects Committee at the Jikei University School of Medicine
[identification no. 26–109 (7614)], and was subsequently registered
with the University Hospital Medical Information Network (UMIN)
Clinical Trials Registry (identification no. UMIN000015871). The
clinical trial was conducted in accordance with the Declaration of
Helsinki. All participants provided written informed consent to
participate in the study.
Development of a quantitative
determination method for the S100P protein
Microplates, standard antigens, antibodies and
reagents for the S100P sandwich ELISA
For the ex vivo analysis, we utilized 96-well
Optimiser™ microplates and their accompanying reagents, which were
purchased from Siloam Biosciences, Inc. (Cincinnati, OH, USA).
His-tagged human recombinant S100P (1–95) (no. ATGP 0565) was used as the
standard antigen and was purchased from ATGen Co., Ltd.
(Seongnam-si, Gyeonggi-do, Korea). S100P rabbit monoclonal antibody
(no. GTX63569) was used as the capture antibody (GeneTex Inc.,
Irvine, CA, USA), and human S100P monoclonal antibody was used as
the detection antibody (no. MAB2957; R&D Systems Inc.,
Minneapolis, MN, USA). Biotin, for labeling the detection antibody
and Sample Diluent Concentrate 2™ (no. DYC002) were also purchased
from R&D Systems. Leupeptin (no. L8511) was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and aprotinin from bovine lung
(no. 018-18111) was purchased from Wako Pure Chemical Industries
Ltd. (Osaka, Japan). To make solubilized samples for the S100P
ELISA, we originally produced a new buffer named ‘Lysis Buffer No.
9’ which consisted of 1% NP-40 alternative, 20 mM Tris, 137 mM
NaCl, 10% glycerol, 2 mM EDTA, 1 mM activated sodium orthovanadate,
10 µg/ml aprotinin and 10 µg/ml leupeptin.
Assessment of total protein concentration in each
sample by bicinchoninic acid (BCA) protein assay prior to S100P
protein analysis
To determine the effective dilute concentration of
the solubilized samples for S100P protein analysis, and to evaluate
whether human EUS-FNA samples contained cell components and could
be useful as a rapid on-site test, total protein concentrations in
the solubilized samples were analyzed by the BCA protein assay,
using the Micro BCA™ Protein Assay kit (no. 23235) purchased from
Thermo Fisher Scientific Inc. (Waltham, MA, USA). The total protein
concentration of each sample was determined by an absorbance plate
reader (XR680TM; Bio-Rad Laboratories Inc., Hercules, CA, USA),
according to the manufacturer's instructions.
S100P protein assessment protocol by sandwich
ELISA
According to the total protein concentration
results, each solubilized sample was diluted to an effective
assessment range by adding sandwich ELISA blocking buffer (no.
OM-055; Siloam Biosciences, Inc.); for cell lines and xenograft
tumors, dilutions were to 500, 100 and 50 µg/ml of protein, and for
human samples to 50, 25, 12.5, 6.25, 3.13, 1.56 and 0.78 µg/ml of
protein.
Using the same conditions described in the
manufacturer's users manual for human vascular endothelial growth
factor (Document ID: ETS-1-MS-0011-A1; Siloam Biosciences, Inc.), a
96-well Optimiser™ microplate was coated with GTX63569 as a capture
antibody and blocked. S100P standard antigen or solubilized samples
were dispensed to each well, then biotinylated MAB2957 was added as
the detection antibody. After the accompanying horseradish
peroxidase and fluorescent substrate were added, fluorescence
intensity was assessed using a fluorescence plate reader (2300
EnSpire™; PerkinElmer, Inc., Waltham, MA, USA) with an excitation
wavelength of 570 nm and an emission wavelength of 585 nm. Finally,
the concentration of S100P protein in the samples was determined by
comparison to the standard curve. This protocol was performed
twice, and the concentrations of S100P protein were averaged.
Quantification of the S100P protein in
cell lines and xenograft tumors
Cell line preparation for the assessment of the
S100P protein
AsPC-1, PANC-1 and MIA PaCa-2 were used as human PCA
cell lines. MCF-7 (a human breast cancer cell line), known as an
S100P-expressing tumor, was also used as a positive control for
S100P. Fibroblasts obtained from the primary culture of PCA,
hTERT-HPNE E6/E7/K-RasG12D/st human normal pancreatic duct
epithelium [hTERT1, no. CRL4039; American Type Culture Collection
(ATCC), Manassas, VA, USA] and human umbilical vein endothelial
cells (HUVECs, no. C12208; PromoCell GmbH, Heidelberg, Germany)
were used as non-cancerous cell lines.
FNA sampling of xenograft tumors from mice to
quantify the S100P protein
PANC-1 (2×106 cells/mouse), MIA PaCa-2
(2×106 cells/mouse) and AsPC-1 (1×106
cells/mouse) were subcutaneously inoculated into the back of two 6-
to 8-week-old female NOD/ShiJic-scid mice (Clea Japan Inc., Tokyo,
Japan). All mice were maintained under controlled conditions
(specific pathogen-free conditions, 22̊C, 55% humidity, 12-h
light/dark cycle), with food and water ad libitum, in groups
of 2/cage. Tumor growth was monitored weekly by caliper
measurements. Animal husbandry and daily care were provided by
veterinary technicians. When a tumor grew to 15 mm, the maximum
diameter, the mice were sacrificed by occipital dislocation under
general anesthesia. Xenograft tumor tissues were then sampled with
an FNA using a 22-gauge needle (Expect™; Boston Scientific, Boston,
MA, USA). Each FNA sample was solubilized by adding ×2 the volume
of Lysis Buffer No. 9, and incubated on ice for 15 min. After
centrifugation at 2,000 × g for 5 min, the supernatants were
transferred to clean test tubes and stored at −80̊C until
analysis.
Clinical pilot trial in patients with
suspicious pancreatic carcinoma
Patients
After the successful development of the quantitative
assay for the S100P protein, consecutive patients with suspicious
pancreatic carcinoma who were referred for EUS-FNA sampling at
Jikei University Hospital and Jikei University-affiliated
institutions were prospectively recruited in this pilot study
between October 12, 2014 and September 1, 2016. The inclusion
criteria were as follows: i) patients >20 years old, ii)
provision of written informed consent to participate in the present
study, and iii) presence of a suspicious pancreatic carcinoma which
was detected by at least a single investigational modality such as
computed tomography, magnetic resonance imaging or EUS. The
exclusion criteria were as follows: i) internal use of an
antiplatelet or anticoagulant agent, ii) bleeding disorders, acute
pancreatitis, treatment history of radiology or chemotherapy, iii)
inability to sample the lesion due to the presence of intervening
blood vessels, and iv) pancreatic lesions such as cystic neoplasms
with the risk of peritoneal dissemination by puncture.
Before the trial began, we empirically anticipated
that the number of participants required was 30 cases at least to
evaluate the feasibility of this novel technology in the phase I
trial (31).
Tissue sampling method for EUS-FNA
EUS was performed using a curvilinear echoendoscope
(GF-UCT260; Olympus Medical Systems, Tokyo, Japan) under conscious
sedation using intravenous midazolam and pethidine with color
Doppler ultrasound assistance. EUS-FNA was performed with a 19-,
22- or 25-gauge needle (Expect™) by 5 endosonographers (3 experts
and 2 trainees). During a puncture, the needle traversed the lesion
to and fro >10 times with negative aspiration using a 20 ml
syringe. Without on-site evaluation, 2 or 3 EUS-FNA punctures are
routinely performed for pancreatic lesions to obtain an adequate
specimen for cytological diagnosis at our institution (32). The specimen obtained with one of the
punctures was transferred to a clean test tube and immediately
stored at −80̊C for later quantitative analysis of the S100P
protein, while the remaining specimen obtained with 1 or 2
punctures was used for cytological diagnosis. Thus, for the purpose
of evaluating the utility of the S100P protein assessment combined
with EUS-FNA cytology, we never performed an additional puncture to
obtain the specimen for the S100P protein analysis.
Preparation of EUS-FNA samples prior to the
quantification of S100P protein
On the day of analysis, the volume of each EUS-FNA
sample was first assessed. Then, the samples were solubilized by
adding ×2 the volume of Lysis Buffer No. 9, and incubated on ice
for 15 min. When the sample volume was <10 µl, 10 µl of Lysis
Buffer No. 9 was added. Following the incubation on ice, the
samples were centrifuged at 2,000 × g for 5 min and the supernatant
was transferred to a clean test tube. Total protein concentrations
were then assessed by BCA protein assay prior to quantification of
the S100P protein.
Test methods (index test, cut-off value,
reference standard)
We complied with the Transparent Reporting of
Evaluations with Nonrandomized Designs (TREND) statement and the
Standards for Reporting Diagnostic Accuracy Studies 2015 (STARD
2015) (33,34). The definition of a positive
cytological test was ‘V. Suspicious (for malignancy) and VI.
Positive/malignant’ according to the Papanicolaou Society of
Cytopathology Guidelines for pancreatobiliary cytology (35–37).
For the S100P protein analysis, the index test represented the
concentration of the S100P protein in the human FNA samples. The
definition of the index test ‘positivity’ and the ‘cut-off value’
were determined from ROC curves. An ‘inconclusive’ index test
represented the inability to quantify the S100P protein due to a
low concentration of total protein (<10 mg/ml). The reference
standard represented the final diagnosis of the pancreatic mass, on
the basis of a pathological diagnosis from the surgical specimen or
an overall determination from the clinical course with an
observation period of >6 months.
Outcome measures
In this human trial, the primary end point was the
evaluation of the accuracy of the S100P protein quantification
combined with EUS-FNA cytology to detect PCA.
Statistical analysis
The Kruskal-Wallis and Mann-Whitney tests were used
to evaluate comparisons of S100P protein concentrations among cell
lines, xenograft tumors and human EUS-FNA samples. The Mann-Whitney
test was also used to evaluate the difference in S100P protein
concentrations between patients with PCA and benign pancreatic
lesions (BPL). The linear regression test was used to evaluate the
correlation between S100P protein concentration and the clinical
stage, or the concentration of total protein in human
EUS-FNA-samples. ROC curves and AUC were used to assess the
diagnostic performance of the quantitative S100P protein analysis
alone, and in combination with EUS-FNA cytology. The cut-off value
was determined by ROC curve, which revealed the highest sum of
sensitivity and specificity for the diagnosis of PCA. A P-value of
<0.05 was considered significant. All analyses were performed
using Stata version 13 (StataCorp LP, College Station, TX,
USA).
Results
Quantitative analysis specific for the
S100P protein in an ex vivo experiment
The antibodies (GTX63569 and MAB2957 with biotin
labeling) used in the sandwich ELISA exhibited high specificity for
the S100P antigen, and cross reactivity with other relevant
proteins was not observed. A standard curve was constructed using
the S100P standard antigen, and a linear approximation equation (y
= 157x + 347.38) was calculated from the standard curve plot,
allowing S100P protein concentrations to be determined in each
sample. The effective assessment range of the assay was from 1.5625
to 50.0000 pg/ml S100P protein.
S100P protein concentrations in cell
lines and xenograft tumors
The amount of S100P protein in the cultured cells
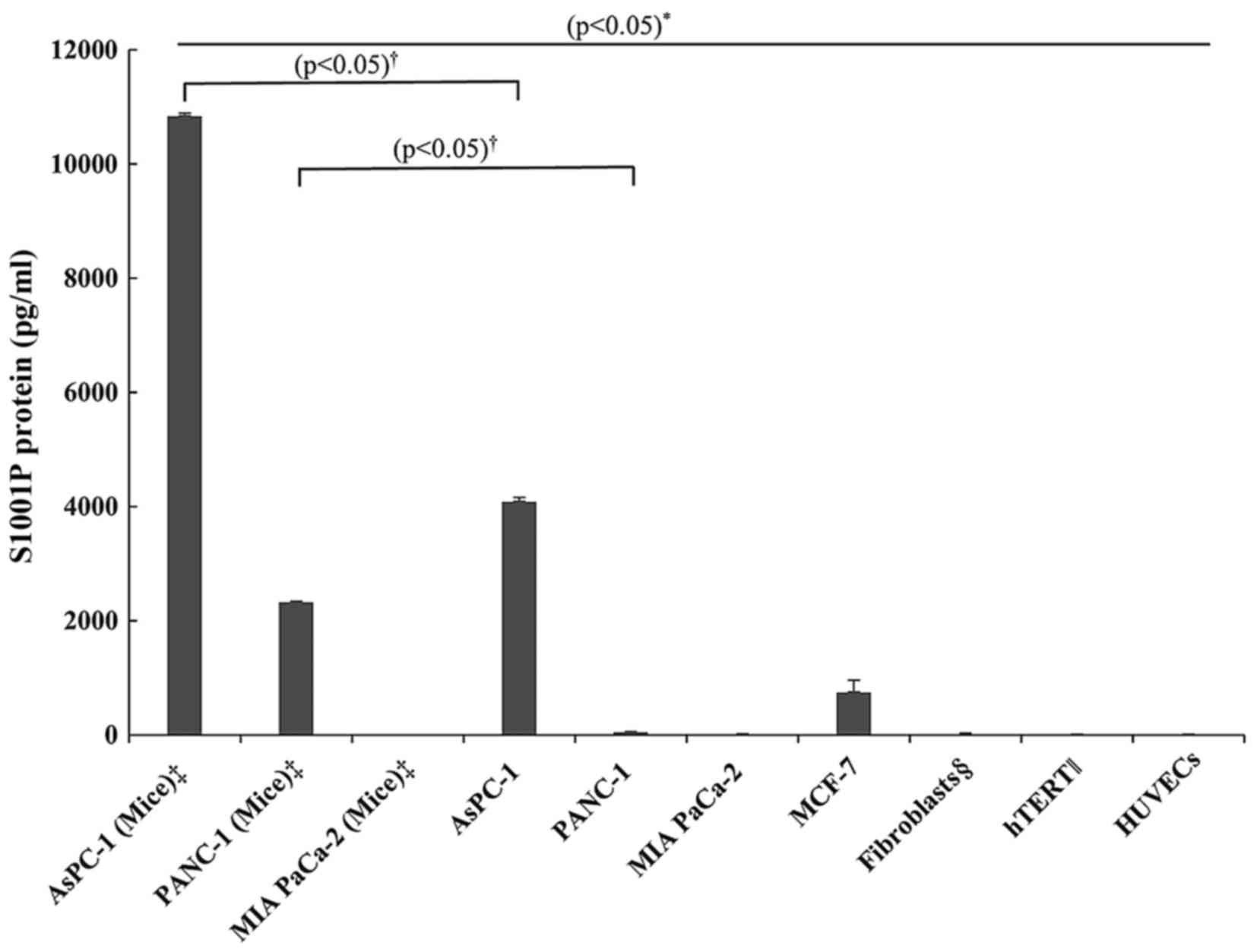
and xenograft tumors was determined using ELISA (Fig. 1). The S100P protein concentration in
AsPC-1 xenograft tumors was higher than that in the AsPC-1 cultured
cells, and the concentration in the cultured AsPC-1 cells was
higher than that in the MCF-7 cells, the positive control for the
S100P protein. Although, the S100P protein was not detected in the
cultured PANC-1 cells, the PANC-1 xenograft tumors showed
significant S100P protein concentrations, while the MIA PaCa2 cells
had no detectable S100P protein in either the cultured cells or the
xenograft tumors. The non-cancerous cells (fibroblasts derived from
the PCA primary culture, hTERT1 and HUVECs) also had no detectable
S100P protein.
Clinical pilot trial
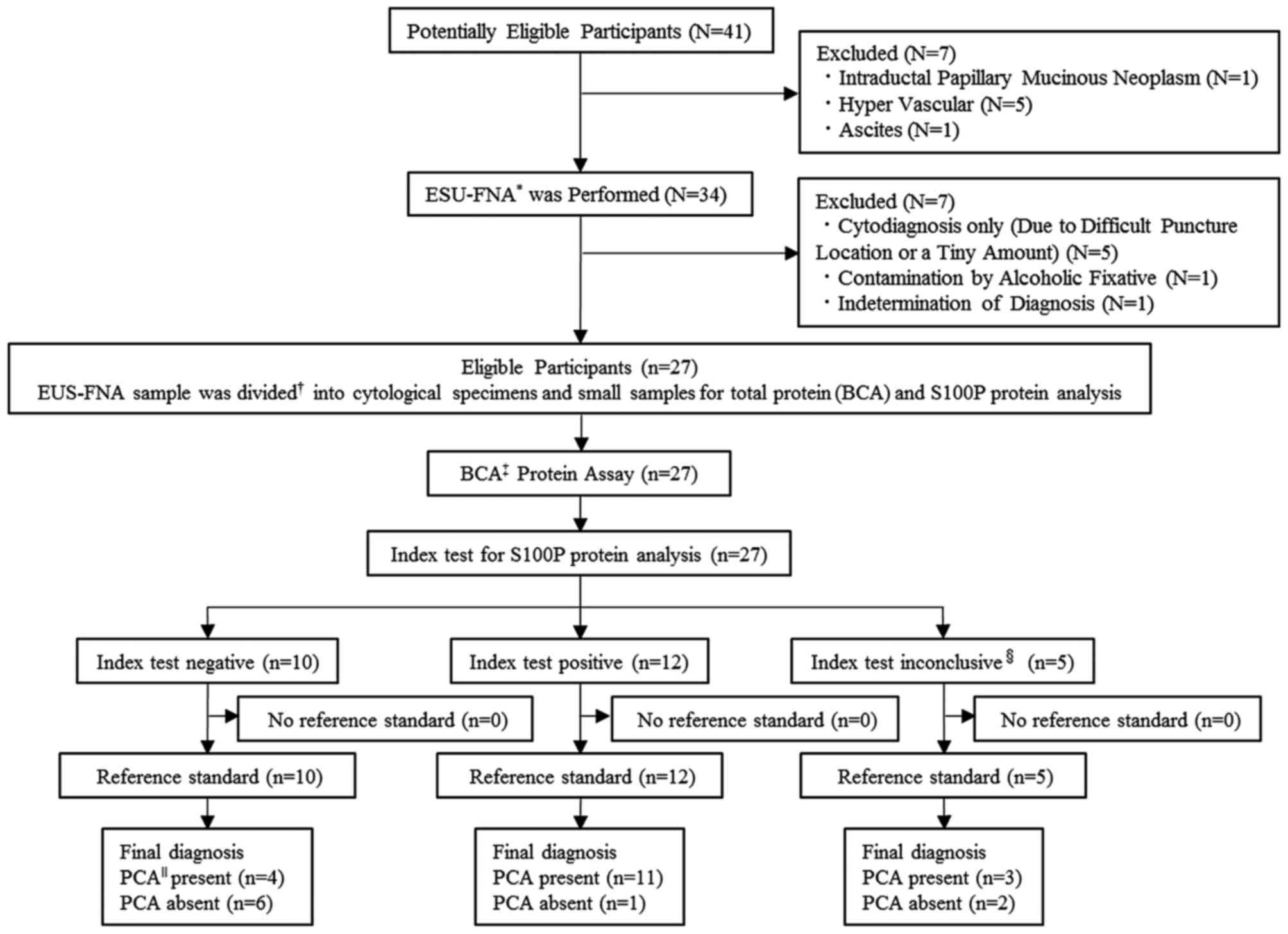
A total of 41 adult patients with a pancreatic mass
were enrolled in the first entry, and 7 patients were excluded due
to intraductal papillary mucinous neoplasm (n=1), the presence of
intervening blood vessels (n=5) and ascites (n=1). A total of 34
patients underwent diagnostic EUS-FNA. No procedure-related adverse
events were observed during the present study. Of the 34 patients
that underwent EUS-FNA, 7 patients were excluded due to
cytodiagnosis only owing to difficult puncture location or a tiny
amount of tissue (n=5), contamination by alcoholic fixative (n=1)
and indetermination of final diagnosis (n=1).
Finally, 27 patients were enrolled in the present
study, according to the inclusion and exclusion criteria, and
cytology and S100P protein analysis were performed on all patients
(Fig. 2). The baseline demographic
and clinical characteristics of the patients are shown in Table I. There was no patient lost in
follow-up in the present study.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | Patients
(n=27) |
|---|
| Age, mean (range),
years | 70.0 (37–86) |
| Gender
(male/female) | 16/11 |
| Final
diagnosisa |
|
|
Pancreatic adenocarcinoma |
|
|
Clinical
stageb |
|
|
IIb | 6 |
|
III | 1 |
|
IV | 11 |
| Chronic
pancreatitis | 3 |
|
Autoimmune pancreatitis | 5 (type
1)c |
| Normal
pancreatic tissue | 1 |
| Size of pancreatic
lesion, mean (range), mm | 29.5
(13.0–42.0) |
| No. of needle
passes, mean (SD)d | 2.6 (0.85) |
| Needle size
(gauge) |
|
| 19 | 1 |
| 22 | 19 |
| 25 | 4 |
| 22 and
25 | 3 |
| CEAe in PCA/BPL, mean (SD), ng/ml | 10.8 (11.7)/4.0
(1.6) |
| CA19-9e in PCA/BPL, mean (SD), µ/ml | 1465.8
(2535.1)/232.7 (407.3) |
The final diagnosis was PCA in 18 patients, chronic
pancreatitis in 3, autoimmune pancreatitis (AIP) in 5, and a normal
pancreas in 1. Six patients among the 18 with PCA underwent
surgical resection. For the patients with PCA, the clinical stage
at the time of enrollment according to the American Joint Committee
on Cancer TNM staging was stage IIb in 6 patients, stage III in 1
patient, and stage IV in 11 patients; there were no patients with
stage Ia, Ib or IIa in the present study. The mean serum level of
CA19-9 and CEA in patients with PCA was 1,465.8 U/ml and 10.8
ng/ml, respectively, and in patients with BPL it was 232.7 U/ml and
4.0 ng/ml, respectively.
The S100P protein could not be quantified in the
EUS-FNA samples of 5 (3 with PCA, 1 with AIP and 1 with chronic
pancreatitis) of the 27 patients due to low total protein
concentrations of <10 mg/ml (index test inconclusive, Fig. 2). In fact, for these samples, serial
dilutions in the range of the assay produced non-linear values for
the S100P protein. Thus, EUS-FNA conducted in our routine manner
(32) yielded samples that were
adequate for S100P protein quantification in 22 of the 27 patients
(81.5%). In these 22 patients, the S100P protein concentration was
assessed as the index test (Fig.
2).
When the 22 patients with a conclusive index test
were divided into 2 groups, PCA (n=15) and BPL (n=7) (the latter
consisting of 2 patients with chronic pancreatitis, 4 with AIP, and
1 with a normal pancreas), the mean total protein concentration was
75.7 mg/ml (range, 10.2–155.0 mg/ml) and 43.5 mg/ml (range,
17.9–70.1 mg/ml), for PCA and BPL, respectively. The mean volume of
EUS-FNA sample in the PCA and BPL groups was 61.4 µl (range,
0.5–320.0 µl) and 7.0 µl (2.0–12.0 µl), respectively. The smallest
EUS-FNA sample volume was 0.5 µl from a patient in the PCA group,
however the S100P protein was still assessable at 318.9 ng/ml.
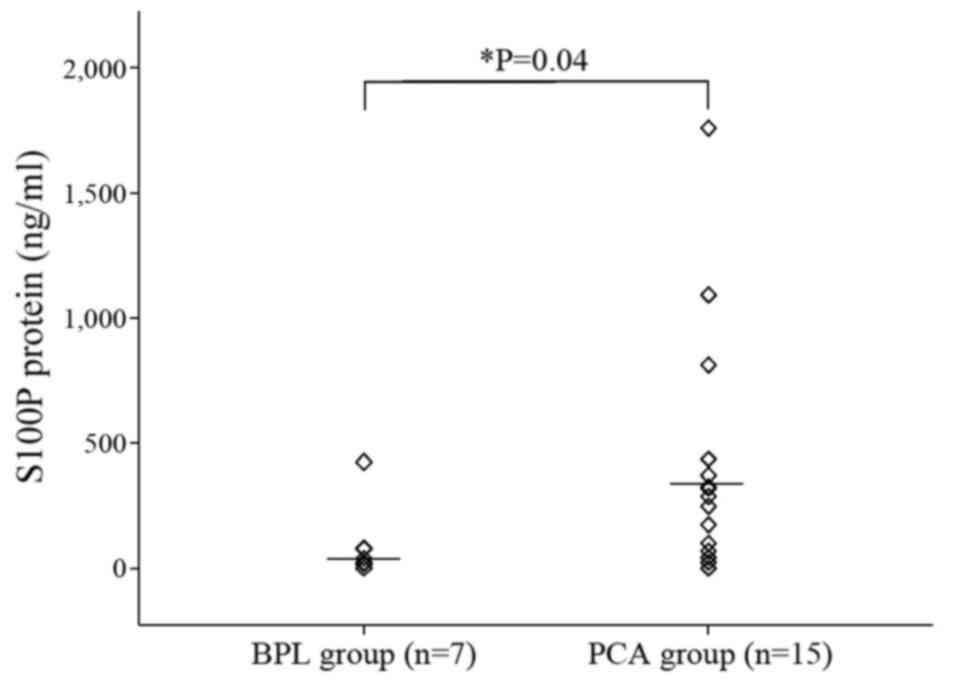
S100P protein concentrations in FNA samples from the
PCA group were significantly higher than in the BPL group
(404.5±480.5 vs. 94.0±149.4 ng/ml, respectively; P=0.04, Fig. 3). In addition, the FNA samples from
the PCA group had significantly higher S100P protein concentrations
than AsPC-1 cultured cells and AsPC-1 xenograft tumors (404.5 vs.
4.09 vs. 10.85 ng/ml, respectively; P<0.05, Figs. 1 and 3).
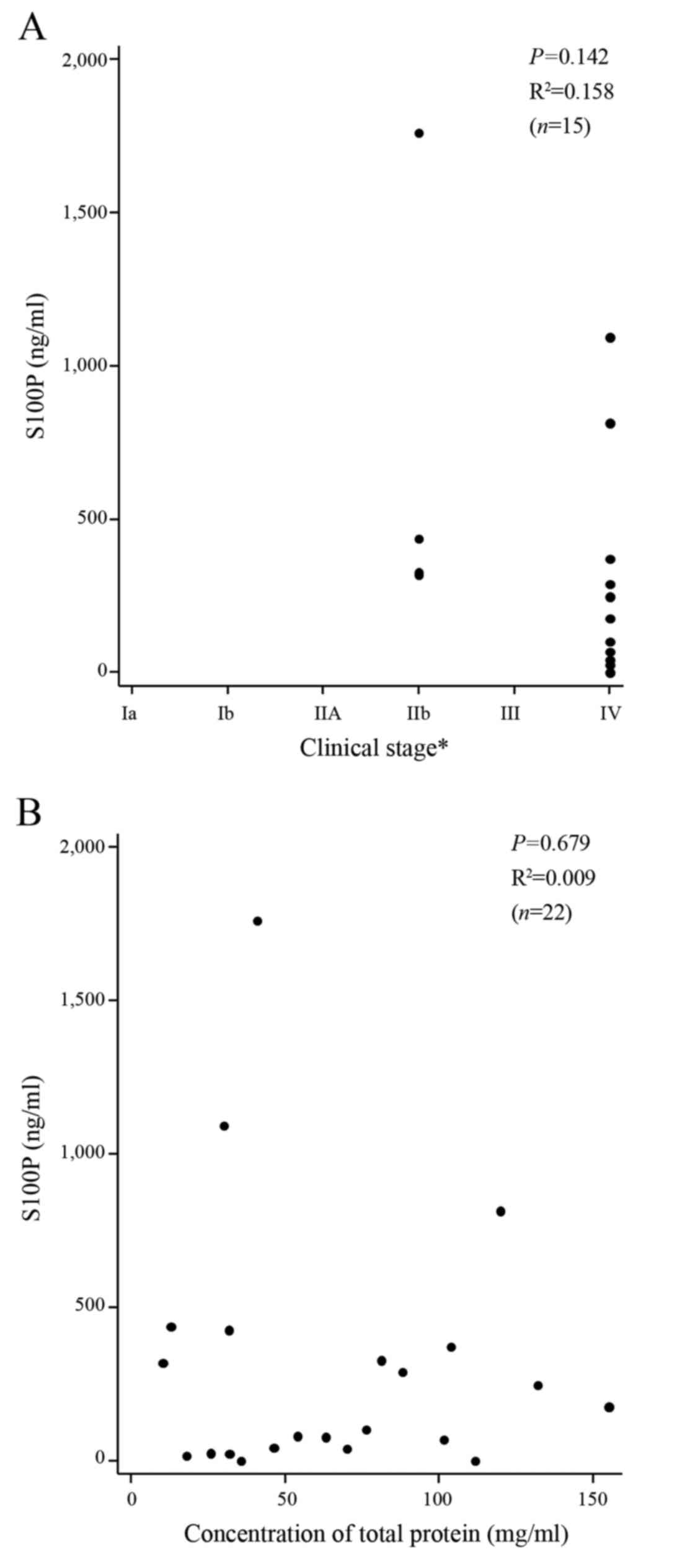
Excluding the 5 patients with the inconclusive index
tests, linear regression analysis revealed that there was no
correlation between the progression of PCA as assessed by the
clinical stage and the concentration of the S100P protein (n=15,
P=0.142, R2=0.158; Fig.
4A). There was also no correlation between the concentrations
of the S100P protein and the total protein (n=22, P=0.679,
R2=0.009, Fig. 4B),
demonstrating the specificity and lack of cross-reactivity with
other relevant proteins in the S100P protein assay.
Diagnostic accuracy for the
quantitative analysis of the S100P protein, EUS-FNA cytology and
both tests combined
A ROC curve analysis was performed using the results
of the 22 EUS-FNA samples with conclusive index tests to determine
the cut-off value for the S100P protein concentration for the
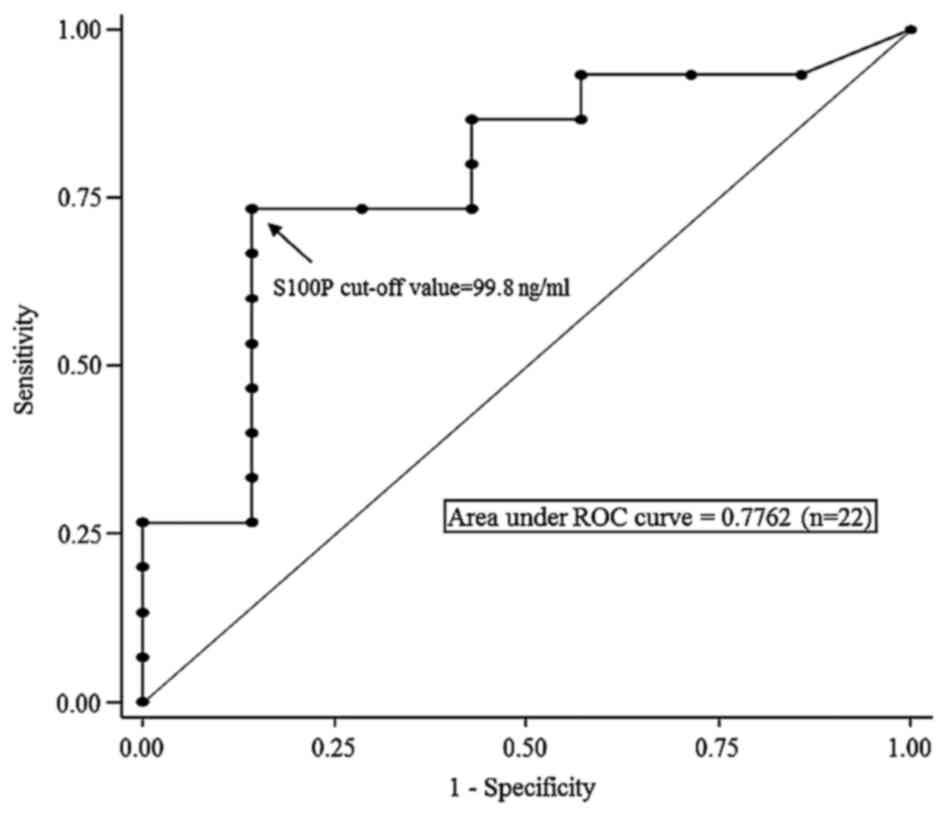
detection of PCA. The ROC curve analysis determined an S100P
protein concentration of 99.8 mg/ml to be the cut-off value for the
detection of PCA (Fig. 5). Using
this cut-off value, among the 12 patients with a positive index
test, 11 patients were finally diagnosed with PCA, and 1 patient
with BPL (S100P protein concentration, 425.8 ng/ml). In contrast,
among the 10 patients with negative index tests, 4 patients were
finally diagnosed with PCA and 6 with BPL (Fig. 2). The sensitivity, specificity,
positive predictive value (PPV), NPV, accuracy and AUC of S100P
protein concentrations to detect PCA were 73.3, 85.7, 91.7, 60.0,
77.3 and 0.78, respectively (Table
II and Fig. 5).
 | Table II.Diagnostic performances of the
quantitative analysis of S100P protein, endoscopic
ultrasound-guided fine needle aspiration (EUS-FNA) cytology, and
the combination of EUS-FNA cytology and S100P protein
quantification for detecting PCA. |
Table II.
Diagnostic performances of the
quantitative analysis of S100P protein, endoscopic
ultrasound-guided fine needle aspiration (EUS-FNA) cytology, and
the combination of EUS-FNA cytology and S100P protein
quantification for detecting PCA.
|
| S100P protein
ELISAa (%) | EUS-FNA
cytologyb (%) |
Combinationb,c (%) |
|---|
| Sensitivity (95%
CI) | 73.3
(44.9–92.2) | 77.8
(52.4–93.6) | 94.4
(75.7–99.1) |
| Specificity (95%
CI) | 85.7
(42.1–99.6) | 100 (66.4-100) | 88.9
(51.8–99.7) |
| Positive predictive
value (95% CI) | 91.7
(61.5–99.8) | 100 (76.8-100) | 94.4
(72.7–99.9) |
| Negative predictive
value (95% CI) | 60.0
(26.2–87.8) | 69.2
(38.6–90.9) | 88.9
(51.8–99.7) |
| Accuracy (95%
CI) | 77.3
(54.6–99.8) | 85.2
(66.3–95.8) | 92.6
(75.7–99.1) |
| Area under the
curve (95% CI) | 0.78
(0.55–1.00) | 0.89
(0.79–0.99) | 0.92
(0.79–1.00) |
In addition, the sensitivity, specificity, PPV, NPV,
accuracy and AUC of EUS-FNA cytology alone to detect PCA in all 27
patients, including the 5 patients with the inconclusive index
tests, were 77.8, 100, 100, 69.2, 85.2 and 0.89%, respectively
(Table II).
The sensitivity, specificity, PPV, NPV, accuracy and
AUC of CEA/CA19-9 to detect PCA, according to our institutional
cut-off value (5.8 ng/ml of CEA and 37.0 U/ml of CA19-9), in all 27
patients were 58.8/94.4, 77.7/55.6, 83.3/80.9, 50.0/83.3, 65.4/81.4
and 0.75/0.81%, respectively (data not shown).
When EUS-FNA cytology was combined with the
quantitative analysis of S100P protein, 1 patient with BPL and 2
patients with PCA revealed negative cytology results but positive
index tests for S100P protein. Therefore, the sensitivity,
specificity, PPV, NPV, accuracy and AUC of S100P protein
concentrations combined with EUS-FNA cytology to detect PCA in the
27 patients were 94.4, 88.9, 94.4, 88.9, 92.6 and 0.92%,
respectively (Table II).
Discussion
To date, evaluation of S100P using tissue samples
has been conducted retrospectively using qualitative real-time
reverse transcription PCR (RT-PCR) or IHC (10,19,21–27,29).
In addition, limitations of real-time RT-PCR using pancreatic
tissue have been reported, such as the frequent occurrence of
fragmentation or degradation of S100P mRNA due to rich RNase in the
pancreas (4,30). In fact, nearly 50% of FNAs cannot be
reliably examined using mRNA analysis (4). Moreover, in order to assess the
expression of S100P expression while preventing the fragmentation
of mRNA in real-time RT-PCR, the separation of pure PCA cells from
strong alkaline pancreatic juice, and the isolation of total
cellular RNA from PCA tissue, are required (4,22,23,26,30,38).
Thus, evaluation of S100P in pancreatic tissue samples with
real-time RT-PCR includes cumbersome preparatory procedures. IHC
does not have the limitations of real-time RT-PCR and can be
achieved even in EUS-FNA samples. However, IHC can only be
performed when adequate amounts of cells are collected, fixed and
embedded in paraffin (4,29). Staining edge artifacts and
non-specific background staining from contamination can also be a
problem with IHC (29). In
addition, when EUS-FNA of the pancreatic mass is performed via a
transgastric puncture, a diagnosis of PCA should be made carefully,
since the EUS-FNA sample could contain gastric tissue, and the
normal gastric epithelium is strongly stained by S100P (27,29,39).
EUS-FNA samples of PCA and in vivo AsPC-1
xenograft tumors showed higher concentrations of S100P protein than
in vitro AsPC-1 cultured cells, even though AsPC-1 cultured
cells had 100% pure cellularity. Along with our novel finding of
S100P protein production in PANC-1 xenograft tumors, these results
suggest that in vivo samples may be more activated to
produce S100P protein, and fewer PCA cells may be required to
quantify the S100P protein, compared to in vitro samples.
Activation of the S100P production may be associated with various
environmental factors. In fact, S100P plays a significant role in
cancer activation, proliferation and immortalization (40–43).
In the different cell lines, the high levels of
S100P protein in MCF-7 and the low levels in MIA PaCa-2 and PANC-1
cells are in agreement with studies using PCR methods (20,22,41,44) or
immunoblotting (42). These
findings indicate that the degree of S100P protein production is
associated not only with S100P activation, but also the type of
cancer cell. S100P activates mitogen-activated protein kinase and
nuclear factor-κB pathways through the receptor of advanced
glycation end products, and its expression increases tumor growth
and metastasis (40–42). Therefore, our results indicate that
the activation of PCA and its potential aggressiveness may be
evaluated with the quantitative analysis of the S100P protein
before surgery or chemotherapy. However, the present study showed
no correlation between the clinical stage of PCA and the
concentrations of the S100P protein in EUS-FNA-samples, although
other studies have proposed that the expression levels of S100P
increase during pancreatic cancer progression from the early stage
to invasive adenocarcinoma (21,41).
Other investigations of cholangiocarcinoma also showed that
patients with high expression of S100P had a more advanced stage
and a higher mortality and metastasis rate than patients with low
expression of S100P (45). The
discrepancy between the present study and other research may be
partly explained by the variety of sample volumes and the
concentration of total protein in the EUS-FNA samples. In addition,
the present study did not contain early-stage cases of PCA.
Therefore, larger sample sizes with early-stage cases may be needed
to confirm the prognostic prediction of the S100P protein in
EUS-FNA samples.
EUS-FNA samples with low protein concentration
assessed by the BCA protein assay showed inconclusive results for
the S100P protein. In contrast, the S100P protein was detectable in
EUS-FNA samples with a small volume of 0.5 µl. These results show
that the limiting factor for the S100P analysis is not the volume
of the sample but the total protein concentration in the EUS-FNA
sample. Moreover, the results indicate that EUS-FNA samples with
low protein concentrations may only have a few PCA cells, since in
some cases a major portion of the EUS-FNA sample can be comprised
of other contaminants. However, the pathological assessment of poor
cellularity or contamination was virtually impossible in the
present study, since EUS-FNA samples were all solubilized for S100P
protein analyses due to the ultra-low volume produced by one needle
pass.
An average of 2.6 EUS-FNA passes resulted in a high
sensitivity of 94.4% using EUS-FNA cytology combined with S100P
protein quantification. Suzuki et al (14) reported an association between the
sensitivity and the pass number with a 25-gauge needle for EUS-FNA
for solid pancreatic lesions. The sensitivity was increased by
adding passes; 53% by 1 pass, 73% by 2 passes, 87% by 3 passes, and
93% by 4 passes. It should be noted, however, that our pilot study
cannot be compared with Suzuki's study, since the study designs and
sample sizes varied. However, our average of 2.6 passes with a high
diagnostic value indicates that the combination of EUS-FNA cytology
plus S100P protein assessment produces a high sensitivity while
decreasing the number of passes as well as a variety of risks, such
as bleeding, pancreatitis, peritoneal dissemination, procedure
time, costs and patient discomfort. In addition, our results show a
high NPV (88.6%) for the combination analysis, which indicates that
this method holds the potential for improving the limitations of
EUS-FNA with low NPV (17).
The significant difference between the PCA and BPL
groups for the S100P protein concentrations indicates that our
quantitative S100P analysis may be a promising new method for the
diagnosis of PCA from EUS-FNA samples. This analysis combined with
EUS-FNA cytology showed high performance, while the cut-off value
could be used to evaluate S100P protein concentrations in ultra-low
volume FNA samples as an auxiliary diagnosis. Our results
correspond with past studies using RT-PCR or IHC that demonstrated
that S100P is not highly expressed in chronic pancreatitis, normal
pancreatic tissue, fibrous stroma or other necrotic tissue, unlike
PCA cells (18–20,22,27,46,47).
When the cut-off value for the S100P protein was set at 99.8 ng/ml,
1 out of 5 patients with AIP showed a high concentration of S100P
protein (425.8 ng/ml). In this patient, our novel quantitative
analysis possibly detected S100P protein originating from
inflammatory cells such as leukocytes, since mRNA levels of the
S100P protein are moderately high in leukocytes (39), and pathologically, type I AIP has
many inflammatory cells, as its histopathological name,
lymphoplasmacytic sclerosing pancreatitis, suggests (48).
The following represent potential limitations of the
present study. The present study was a single-center based trial
with a small sample size. Even though the cytology sample volumes
had, on average, 1.6 more passes than the S100P protein analysis
samples (1 pass), the accuracy of the cytological diagnosis may be
affected to some degree. In the present study, results for EUS-FNA
cytology alone showed a lower sensitivity (77.8%) compared with
previous studies (1,16,17).
The splitting method used in the present study may decrease the
volume of sample for cytology. However, there is a potential
difference in the puncture location and sample component for the
cytology samples compared to the protein analysis samples.
In conclusion, we successfully established a novel,
simple quantitative test for detecting small amounts of PCA cells
in tiny samples of EUS-FNA by means of a high sensitivity sandwich
ELISA for the S100P protein. Secondly, the S100P protein
concentrations differed between cell lines, xenograft tumors and
human EUS-FNA samples. Thirdly, quantification of the S100P protein
can provide reliable discrimination between PCA and BPL, as a
simple and objective method for an auxiliary diagnosis using the
cut-off value we established. Finally, the combination of EUS-FNA
cytology plus S100P protein quantification showed high accuracy for
diagnosing PCA from pancreatic masses by EUS-FNA specimens, in the
setting of a prospective continuous series.
Acknowledgements
We would like to thank Professor Masato Matsushima
and Dr Masako Nishikawa for their assistance with the statistical
analyses included in the present study. Special thanks are
expressed to Yumi Takagi for technical assistance and performing
the ELISA assays. In addition, we would like to thank the
Department of Surgery and the Division of Gastroenterology and
Hepatology for patient recruitment. The manuscript has been revised
by a specialist native English editor, Fiona Wylie with a PhD in
Biomedical Sciences.
References
|
1
|
Puli SR, Bechtold ML, Buxbaum JL and
Eloubeidi MA: How good is endoscopic ultrasound-guided fine-needle
aspiration in diagnosing the correct etiology for a solid
pancreatic mass?: A meta-analysis and systematic review. Pancreas.
42:20–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savides TJ, Donohue M, Hunt G, Al-Haddad
M, Aslanian H, Ben-Menachem T, Chen VK, Coyle W, Deutsch J, DeWitt
J, et al: EUS-guided FNA diagnostic yield of malignancy in solid
pancreatic masses: A benchmark for quality performance measurement.
Gastrointest Endosc. 66:277–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dina R, Tran-Dang MA, Mauri F, Gudi M,
Cohen P, Ahmad R, Batav L, Vlavianos P and Spalding D:
Pancreatobiliary cytology in the multidisciplinary setting.
Cytopathology. 24:150–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bournet B, Gayral M, Torrisani J, Selves
J, Cordelier P and Buscail L: Role of endoscopic ultrasound in the
molecular diagnosis of pancreatic cancer. World J Gastroenterol.
20:10758–10768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fritscher-Ravens A, Brand L, Knöfel WT,
Bobrowski C, Topalidis T, Thonke F, de Werth A and Soehendra N:
Comparison of endoscopic ultrasound-guided fine needle aspiration
for focal pancreatic lesions in patients with normal parenchyma and
chronic pancreatitis. Am J Gastroenterol. 97:2768–2775. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varadarajulu S, Tamhane A and Eloubeidi
MA: Yield of EUS-guided FNA of pancreatic masses in the presence or
the absence of chronic pancreatitis. Gastrointest Endosc.
62:728–736; quiz 751, 753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krishna NB, Mehra M, Reddy AV and Agarwal
B: EUS/EUS-FNA for suspected pancreatic cancer: Influence of
chronic pancreatitis and clinical presentation with or without
obstructive jaundice on performance characteristics. Gastrointest
Endosc. 70:70–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribeiro A, Peng J, Casas C and Fan YS:
Endoscopic ultrasound guided fine needle aspiration with
fluorescence in situ hybridization analysis in 104 patients with
pancreatic mass. J Gastroenterol Hepatol. 29:1654–1658. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin F and Staerkel G: Cytologic criteria
for well differentiated adenocarcinoma of the pancreas in
fine-needle aspiration biopsy specimens. Cancer. 99:44–50. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dim DC, Jiang F, Qiu Q, Li T, Darwin P,
Rodgers WH and Peng HQ: The usefulness of S100P, mesothelin,
fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing
pancreatic adenocarcinoma in cytological specimens obtained by
endoscopic ultrasound guided fine-needle aspiration. Diagn
Cytopathol. 42:193–199. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siddiqui AA, Brown LJ, Hong SK,
Draganova-Tacheva RA, Korenblit J, Loren DE, Kowalski TE and
Solomides C: Relationship of pancreatic mass size and diagnostic
yield of endoscopic ultrasound-guided fine needle aspiration. Dig
Dis Sci. 56:3370–3375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iglesias-Garcia J, Dominguez-Munoz JE,
Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A and
Forteza-Vila J: Influence of on-site cytopathology evaluation on
the diagnostic accuracy of endoscopic ultrasound-guided fine needle
aspiration (EUS-FNA) of solid pancreatic masses. Am J
Gastroenterol. 106:1705–1710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camellini L, Carlinfante G, Azzolini F,
Iori V, Cavina M, Sereni G, Decembrino F, Gallo C, Tamagnini I,
Valli R, et al: A randomized clinical trial comparing 22G and 25G
needles in endoscopic ultrasound-guided fine-needle aspiration of
solid lesions. Endoscopy. 43:709–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki R, Irisawa A, Bhutani MS, Hikichi
T, Takagi T, Sato A, Sato M, Ikeda T, Watanabe K, Nakamura J, et
al: Prospective evaluation of the optimal number of 25-gauge needle
passes for endoscopic ultrasound-guided fine-needle aspiration
biopsy of solid pancreatic lesions in the absence of an onsite
cytopathologist. Dig Endosc. 24:452–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasuda I, Iwashita T and Doi S: Tips for
endoscopic ultrasound-guided fine needle aspiration of various
pancreatic lesions. J Hepatobiliary Pancreat Sci. 21:E29–E33. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hewitt MJ, McPhail MJ, Possamai L, Dhar A,
Vlavianos P and Monahan KJ: EUS-guided FNA for diagnosis of solid
pancreatic neoplasms: A meta-analysis. Gastrointest Endosc.
75:319–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartwig W, Schneider L, Diener MK,
Bergmann F, Büchler MW and Werner J: Preoperative tissue diagnosis
for tumours of the pancreas. Br J Surg. 96:5–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Logsdon CD, Simeone DM, Binkley C,
Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R and Hanash
S: Molecular profiling of pancreatic adenocarcinoma and chronic
pancreatitis identifies multiple genes differentially regulated in
pancreatic cancer. Cancer Res. 63:2649–2657. 2003.PubMed/NCBI
|
|
19
|
Crnogorac-Jurcevic T, Missiaglia E,
Blaveri E, Gangeswaran R, Jones M, Terris B, Costello E,
Neoptolemos JP and Lemoine NR: Molecular alterations in pancreatic
carcinoma: Expression profiling shows that dysregulated expression
of S100 genes is highly prevalent. J Pathol. 201:63–74. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato N, Fukushima N, Matsubayashi H and
Goggins M: Identification of maspin and S100P as novel
hypomethylation targets in pancreatic cancer using global gene
expression profiling. Oncogene. 23:1531–1538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dowen SE, Crnogorac-Jurcevic T,
Gangeswaran R, Hansen M, Eloranta JJ, Bhakta V, Brentnall TA,
Lüttges J, Klöppel G and Lemoine NR: Expression of S100P and its
novel binding partner S100PBPR in early pancreatic cancer. Am J
Pathol. 166:81–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohuchida K, Mizumoto K, Egami T, Yamaguchi
H, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M and Tanaka
M: S100P is an early developmental marker of pancreatic
carcinogenesis. Clin Cancer Res. 12:5411–5416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Zheng B, Robbins DH, Lewin DN,
Mikhitarian K, Graham A, Rumpp L, Glenn T, Gillanders WE, Cole DJ,
et al: Accurate discrimination of pancreatic ductal adenocarcinoma
and chronic pancreatitis using multimarker expression data and
samples obtained by minimally invasive fine needle aspiration. Int
J Cancer. 120:1511–1517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin F, Shi J, Liu H, Hull ME, Dupree W,
Prichard JW, Brown RE, Zhang J, Wang HL and Schuerch C: Diagnostic
utility of S100P and von Hippel-Lindau gene product (pVHL) in
pancreatic adenocarcinoma-with implication of their roles in early
tumorigenesis. Am J Surg Pathol. 32:78–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kosarac O, Takei H, Zhai QJ, Schwartz MR
and Mody DR: S100P and XIAP expression in pancreatic ductal
adenocarcinoma: Potential novel biomarkers as a diagnostic adjunct
to fine needle aspiration cytology. Acta Cytol. 55:142–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bournet B, Pointreau A, Souque A, Oumouhou
N, Muscari F, Lepage B, Senesse P, Barthet M, Lesavre N, Hammel P,
et al: Gene expression signature of advanced pancreatic ductal
adenocarcinoma using low density array on endoscopic
ultrasound-guided fine needle aspiration samples. Pancreatology.
12:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Shi J, Anandan V, Wang HL, Diehl D,
Blansfield J, Gerhard G and Lin F: Reevaluation and identification
of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3)
for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med.
136:601–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu H, Zhang Q, Huang C, Shen Y, Chen X,
Shi X and Tang W: Diagnostic value of S100P for pancreatic cancer:
A meta-analysis. Tumour Biol. 35:9479–9485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng H, Shi J, Wilkerson M, Meschter S,
Dupree W and Lin F: Usefulness of S100P in diagnosis of
adenocarcinoma of pancreas on fine-needle aspiration biopsy
specimens. Am J Clin Pathol. 129:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Augereau C, Lemaigre FP and Jacquemin P:
Extraction of high-quality RNA from pancreatic tissues for gene
expression studies. Anal Biochem. 500:60–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
The National Cancer Institute (NCI):
Cancer Clinical Trials: The Basic Workbook [NCI Web site].
September;2002 https://accrualnet.cancer.gov/sites/accrualnet.cancer.gov/files/BasicsWorkbook_m.pdfAccessed.
October 15–2016.
|
|
32
|
Imazu H, Uchiyama Y, Kakutani H, Ikeda K,
Sumiyama K, Kaise M, Omar S, Ang TL and Tajiri H: A prospective
comparison of EUS-guided FNA using 25-gauge and 22-gauge needles.
Gastroenterol Res Pract. 2009:5463902009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Des Jarlais DC, Lyles C and Crepaz N:
TREND Group: Improving the reporting quality of nonrandomized
evaluations of behavioral and public health interventions: The
TREND statement. Am J Public Health. 94:361–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis
CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC,
et al: STARD Group: STARD 2015: An Updated List of Essential Items
for Reporting Diagnostic Accuracy Studies. Clin Chem. 61:1446–1452.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pitman MB, Centeno BA, Ali SZ, Genevay M,
Stelow E, Mino-Kenudson M, Fernandez-del Castillo C, Max Schmidt C,
Brugge W and Layfield L: Papanicolaou Society of Cytopathology:
Standardized terminology and nomenclature for pancreatobiliary
cytology: The Papanicolaou Society of Cytopathology guidelines.
Diagn Cytopathol. 42:338–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pitman MB and Layfield LJ: Guidelines for
pancreaticobiliary cytology from the Papanicolaou Society of
Cytopathology: A review. Cancer Cytopathol. 122:399–411. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Layfield LJ and Pitman MB: The
Papanicolaou Society of Cytopathology guidelines for
pancreaticobiliary tract cytology: A new installment in the
‘Bethesda’ style of guidelines from the Papanicolaou Society of
Cytopathology. Diagn Cytopathol. 42:283–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lekanne Deprez RH, Fijnvandraat AC,
Ruijter JM and Moorman AF: Sensitivity and accuracy of quantitative
real-time polymerase chain reaction using SYBR green I depends on
cDNA synthesis conditions. Anal Biochem. 307:63–69. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parkkila S, Pan PW, Ward A, Gibadulinova
A, Oveckova I, Pastorekova S, Pastorek J, Martinez AR, Helin HO and
Isola J: The calcium-binding protein S100P in normal and malignant
human tissues. BMC Clin Pathol. 8:26890-8–2. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arumugam T, Simeone DM, Schmidt AM and
Logsdon CD: S100P stimulates cell proliferation and survival via
receptor for activated glycation end products (RAGE). J Biol Chem.
279:5059–5065. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arumugam T, Simeone DM, Van Golen K and
Logsdon CD: S100P promotes pancreatic cancer growth, survival, and
invasion. Clin Cancer Res. 11:5356–5364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arumugam T, Ramachandran V and Logsdon CD:
Effect of cromolyn on S100P interactions with RAGE and pancreatic
cancer growth and invasion in mouse models. J Natl Cancer Inst.
98:1806–1818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arumugam T and Logsdon CD: S100P: A novel
therapeutic target for cancer. Amino Acids. 41:893–899. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou C, Zhong Q, Rhodes LV, Townley I,
Bratton MR, Zhang Q, Martin EC, Elliott S, Collins-Burow BM, Burow
ME, et al: Proteomic analysis of acquired tamoxifen resistance in
MCF-7 cells reveals expression signatures associated with enhanced
migration. Breast Cancer Res. 14:R452012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu Z, Boonmars T, Nagano I, Boonjaraspinyo
S, Srinontong P, Ratasuwan P, Narong K, Nielsen PS and Maekawa Y:
Significance of S100P as a biomarker in diagnosis, prognosis and
therapy of opisthorchiasis-associated cholangiocarcinoma. Int J
Cancer. 138:396–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Missiaglia E, Blaveri E, Terris B, Wang
YH, Costello E, Neoptolemos JP, Crnogorac-Jurcevic T and Lemoine
NR: Analysis of gene expression in cancer cell lines identifies
candidate markers for pancreatic tumorigenesis and metastasis. Int
J Cancer. 112:100–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fukushima N, Sato N, Prasad N, Leach SD,
Hruban RH and Goggins M: Characterization of gene expression in
mucinous cystic neoplasms of the pancreas using oligonucleotide
microarrays. Oncogene. 23:9042–9051. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shimosegawa T, Chari ST, Frulloni L,
Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM,
Löhr M, et al: International Association of Pancreatology:
International consensus diagnostic criteria for autoimmune
pancreatitis: Guidelines of the International Association of
Pancreatology. Pancreas. 40:352–358. 2011. View Article : Google Scholar : PubMed/NCBI
|