Introduction
Cervical cancer is the second most common cancer in
women following breast cancer in developing countries (1). In China, the incidence of cervical
cancer is 28.2/1,000 among the 30–44 female age group according to
recent statistical data (2). It has
now been established that persistent infection of high-risk human
papillomavirus (HPV) is the major risk factor of cervical cancer,
and the expression of HPV oncogenes E6 and E7 play an important
role during neoplastic growth (3).
However, a majority of sexually active women get transient
infections more than once in their lifetimes, which causes the low
specificity of HPV testing (4). The
delayed use of the HPV vaccine in developing countries incurred a
large amount of cervical cancer patients. Consequently, there is an
urgent need for specific diagnostic methods and therapeutic
approaches against cervical cancer.
The B7 protein superfamily provides positive and
negative signals that modulate immunological functions, including
B7-1 (CD80), B7-2 (CD86), PD-L1 and PD-L2, which bind to
coinhibitory molecules CTLA-4 and PD-1, downregulating T-cell
function (5). B7-H4 was discovered
as a B7 family member in 2003. It is also known as B7x, B7S1, VTCN1
and DD-O110 (6), and was reported
to serve as a negative modulator in antitumor responses by
inhibiting the functions of CD4+ and CD8+
cells (7–9). However, there have also been studies
reporting the positive regulation of the antitumor immunity of
B7-H4, and it is presumed that there are at least two independent
receptors which are differentially expressed under certain
conditions causing the inverse effects of B7-H4 (10,11).
B7-H4 is widely expressed in many types of cancer,
including lung and breast cancer, renal cell and colorectal
carcinoma, and ovarian cancer (12–16).
This molecule has demonstrated great diagnostic potential in
ovarian cancer particularly (17,18).
To evaluate the prognostic role of B7-H4 in cervical cancer, we
sampled the tumor serum and tissue samples obtained from cervical
intraepithelial neoplasia (CIN) and cervical cancer patients and
examined their B7-H4 expression levels. Through gene silencing or
overexpression of B7-H4 in cervical cancer cell lines, we described
the effects of B7-H4 on proliferation, cell cycle arrest,
apoptosis, migration and invasion of cancer cells.
Materials and methods
Serum and tissue samples of
patients
Thirty-four normal matched controls (healthy or
uterus benign tumor cases), 20 CIN and 100 cervical cancer patients
(providing serum or tissue samples) admitted to Qilu Hospital of
Shandong University were enrolled in the present study, between
2015 and 2016. The CIN and cervical cancer patients were staged
according to the 2009 FIGO staging guidelines (19). For all these patients, the records
containing the age, HPV infection history, TCT, colposcopy and
pathology were examined. The present study, was approved by the
Ethics Committee of Qilu Hospital.
Cell lines and culture conditions
The human cervical carcinoma (HeLa, SiHa and CaSki)
and E6/E7 immortalized human cervical epithelial (H8) cell lines
were obtained from the Cancer Center Laboratory of Shandong
University (Jinan, Shandong, China). The HeLa and H8 cells were
maintained in Dulbecco's modified Eagles medium (DMEM), SiHa cells
were maintained in minimum essential medium (MEM), CaSki cells were
maintained in Roswell Park Memorial Institute (RPMI)-1640 medium,
and all of the media (Hyclone Laboratories, Logan, UT, USA) were
supplemented with 10% fetal bovine serum (FBS; Gibco, Sydney,
Australia). The four cell lines were incubated in a humidified
atmosphere at 37̊C with 5% CO2. In addition, we
collected cervical fall off epithelia tissue from healthy women
volunteers as normal uterine cervix (NUC) cells.
Immunofluorescence staining
The SiHa and HeLa cells were washed there times with
phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde
for 15 min. After permeation with 0.2% Triton X-100 in PBS for 10
min, the cells were immunostained with rabbit anti-B7-H4 monoclonal
antibody (1:100 dilution in PBS; Abcam, Cambridge, MA, USA) at 4̊C
overnight. The cells were washed three times with PBS for 10 min
each, and incubated for 1 h with a secondary goat anti-rabbit IgG
antibody (1:200 dilution in PBS; Zhongshan Jinqiao Biotechnology
Co., Ltd., Beijing, China) at room temperature. Cells were washed
in 4,6-diamidine-2-phenylindole dihydrochloride (DAPI) (Yusen
Biotech Inc., Shanghai, China) for 3 min away from the light.
Microscopic observations were performed using the Olympus IX51
inverted microscope.
Western blotting
The transfected cells were washed three times with
PBS and lysed on ice using radio immunoprecipitation assay buffer
(RIPA; Beyotime Institute of Biotechnology, Haimen, China) with 1%
phenylmethylsulfonyl fluoride (PMSF) and 1% NaF for 30 min. The
normal and tumor tissues were cut into pieces using tissue
scissors, and homogenized by a tissue grinder (Tiangen, Beijing,
China). The reagents were added into the tissue homogenates as
aforementioned. Cell and tissue lysis were centrifuged at 12,000
rpm for 10 min at 4̊C and the protein extracts (30–50 µg) were
loaded in each lane and separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred to
polyvinylidene fluoride membranes (Millipore, Darmstadt, Germany).
The membranes were blocked with 5% skim milk and probed with
anti-E2F (ProteinTech Group, Inc., Chicago, IL, USA), anti-B7-H4,
anti-phosphorylated Rb-(pRb), anti-P16, anti-P21, anti-Bcl-2,
anti-cleaved PARP, anti-cleaved caspase-3 (Abcam) and anti-GAPDH
[Cell Signaling Technology (CST), Danvers, MA, USA] antibodies. The
membranes were then incubated with anti-rabbit and anti-mouse IgG
(Millipore), and detected by enhanced chemiluminescence (ECL) using
ImageQuant LAS 4000 (GE Healthcare Life Sciences, Logan, UT, USA).
The results were analyzed by ImageJ software (NIH, Bethesda, MD,
USA).
Reverse transcription and real-time
PCR
The cellular RNA from the tumor cells and tissue
samples was extracted using TRIzol reagent (Life Technologies,
Carlsbad, CA, USA) and the cDNA was synthesized from 3–5 µg of RNA
using M-MLV reverse transcriptase (Invitrogen, Shanghai, China)
according to the manufacturer's instructions. In addition,
primer-probe sets for qPCR for each gene were designed using the
PrimerBank and are listed in Table
I. The data were collected using the StepOnePlus™ software
(Applied Biosystems, Shanghai, China) and quantified using the
2−ΔΔCt method.
 | Table I.Primers used in RT-PCR analysis and
the small interfering RNA sequences. |
Table I.
Primers used in RT-PCR analysis and
the small interfering RNA sequences.
| Gene |
| Primer
sequence | Species |
|---|
| B7-H4 | Forward |
5′-CCCAATCCGAAGTGTCAACT-3′ | Human |
|
| Reverse |
5′-TATCCTGGTGCCCGATAGAG-3′ |
|
| Si-B7-H4 | Forward |
5′-GUCACCUACAGCUGCUAAATT-3′ | Human |
|
| Reverse |
5′-UUUAGCAGCUGUAGGUGACTT-3′ |
|
| Si-control | Forward |
5′-UUCUCCGAACGUGUCACGUTT-3′ | Human |
|
| Reverse |
5′-ACGUGACACGUUCGGAGAATT-3′ |
|
| E7 | Forward |
5′-AGTGTGACTCTACGCTTCGG-3′ | Human |
|
| Reverse |
5′-TGTGCCCATTAACAGGTCTT-3′ |
|
| Rb | Forward |
5′-ATGCCCCAGAACCCTTGTATC-3′ | Human |
|
| Reverse |
5′-GCCCATAGCCTTCCTTCTGAT-3′ |
|
| MMP-2 | Forward |
5′-TACAGGATCATTGGCTACACACC-3′ | Human |
|
| Reverse |
5′-GGTCACATCGCTCCAGACT-3′ |
|
| MMP-9 | Forward |
5′-TGTACCGCTATGGTTACACCTCG-3′ | Human |
|
| Reverse |
5′-GGCAGGGACAGTTGCTTCT-3′ |
|
| VEGF | Forward |
5′-TCTCTACCCCAGGTCAGACG-3′ | Human |
|
| Reverse |
5′-AGCAATGTCCTGAAGCTCCC-3′ |
|
| GAPDH | Forward |
5′-TGCACCACCTGCTTAGC-3′ | Human |
|
| Reverse |
5′-GGCATGGACTGTGGTCATGAG-3′ |
|
ELISA assay
The serum samples for the detection of B7-H4 were
kept frozen at −80̊C until use. The ELISA kit for the detection of
B7-H4 was obtained from LifeSpan Biosciences, Inc. (Seattle, WA,
USA) and used according to the manufacturer's recommendations.
Immunohistochemical staining and
evaluation
The tissues were cut into 4-µm sections. Standard SP
immunohistochemistry (Maxim, Fuzhou, China) was performed with the
B7-H4 antibody (1:500 dilution in PBS; Abcam) and a Polink-2 Plus
Polymer HRP Detection System (ZSGB-BIO, Beijing, China). The
detection of B7-H4 in cancer cells was assessed by two independent
investigators. Quantifications were recorded as follows: <10%
positive cells, 0; 10–25%, 1; 26–50%, 2; 51–75%, 3; >75%
positive cells, 4. Staining intensity was scored as: absent, 0;
weak, 1; moderate, 2; and strong, 3. The final score was the
multiplication of the quantification and staining intensity. A
final score of 0–1 was classified as negative, and ≥2 was
considered positive.
Silencing of the B7-H4 gene in SiHa
cells
The small interfering RNA sequences targeting human
B7-H4 (Si-B7-H4) and its control sequence (Si-control) were
designed by GenePharma Co., Ltd. (Shanghai, China). The SiHa cells
were seeded in a 6-well plate with 4×104 cells/ml/well.
Twenty-four hours later, cells were transfected with 50 nM of the
Si-B7-H4 or control sequences using Lipofectamine 2000 (Invitrogen
Life Technologies). The transfected cells were harvested 48 h
post-transfection for the follow-up experiments. The Si-B7-H4 and
Si-control sequences are listed in Table I.
Overexpression of the B7-H4 gene in
HeLa cells
The B7-H4 plasmid was designed by GenePharma Co.,
Ltd., and the lentiviral vector pLenti-C-Myc-DDk-IRES-Puro (7.6 kb)
under the control of a cytomegalovirus promoter was obtained from
Vector Gene Technology Co., Ltd. (Beijing, China). After the
transfection and virus package, puromycin dihydrochloride (2 µg/ml;
Amresco, Solon, OH, USA) was used to generate HeLa cell lines that
stably express B7-H4 (pCMV-B7-H4) and its negative control
(pCMV-myc).
Cell proliferation assay
Cell viability was assessed using Cell Counting
Kit-8 (CCK-8) (Tongren, Shanghai, China). According to the product
manual, 2×103 cells were seeded in each well of a
96-well plate, and incubated for 0, 24, 48, 72 and 96 h, and 10 µl
of CCK-8 reagent was added to each well at 4 h before assessing the
optical density (OD) at 450 nm using a microplate reader (Infinite
2000; Tecan, Männedorf, Switzerland).
Flow cytometry
Cells were washed twice using precooling PBS, and
then digested in 75% alcohol at 4̊C overnight. Propidium iodide
(PI) was added into the cell suspensions and the cell cycle
distribution was detected by FACSCalibur flow cytometer (both from
BD Biosciences, Franklin Lakes, NJ, USA). As for cell apoptosis, we
used two apoptosis kits, one was the Annexin V-PE/PI, the other was
the Annexin V-FITC/7-AAD. The results were analyzed following the
manufacturer's protocol using the flow cytometer as
aforementioned.
Transwell migration and invasion
assays
After the transfection of Si-B7-H4 or pCMV-B7-H4 as
well as their negative controls, 6×104 SiHa or
4×104 HeLa cells were digested and suspended in 100 µl
serum-free MEM and DMEM, respectively, then the cells were seeded
into the upper Transwell chamber (8.0-µm pore size; Costar,
Cambridge, MA, USA), in the absence or presence of 100 µl of
Matrigel (1:8 dilution in serum-free medium; Corning, Corning, NY,
USA). Medium with 20% FBS was added to the lower chamber as a
chemoattractant. After 24 h, the cells passing through the filter
were stained with 0.1% crystal violet, and the images were captured
by the Olympus IX51 inverted microscope. The number of migrated or
invaded cells was counted in five random fields (magnification,
×200) of each chamber under the microscope.
Statistical analysis
GraphPad Prism version 5.01 (GraphPad Software Inc.,
San Diego, CA, USA) was used for statistical analysis. In the
present study, data are expressed as the means with standard
deviations (SDs), and statistical comparisons were performed using
a Student's t-test or a Chi-squared test. P<0.05 was considered
to indicate a statistically significant result.
Results
Expression of B7-H4 in human cervical
cancer tissues and cell lines
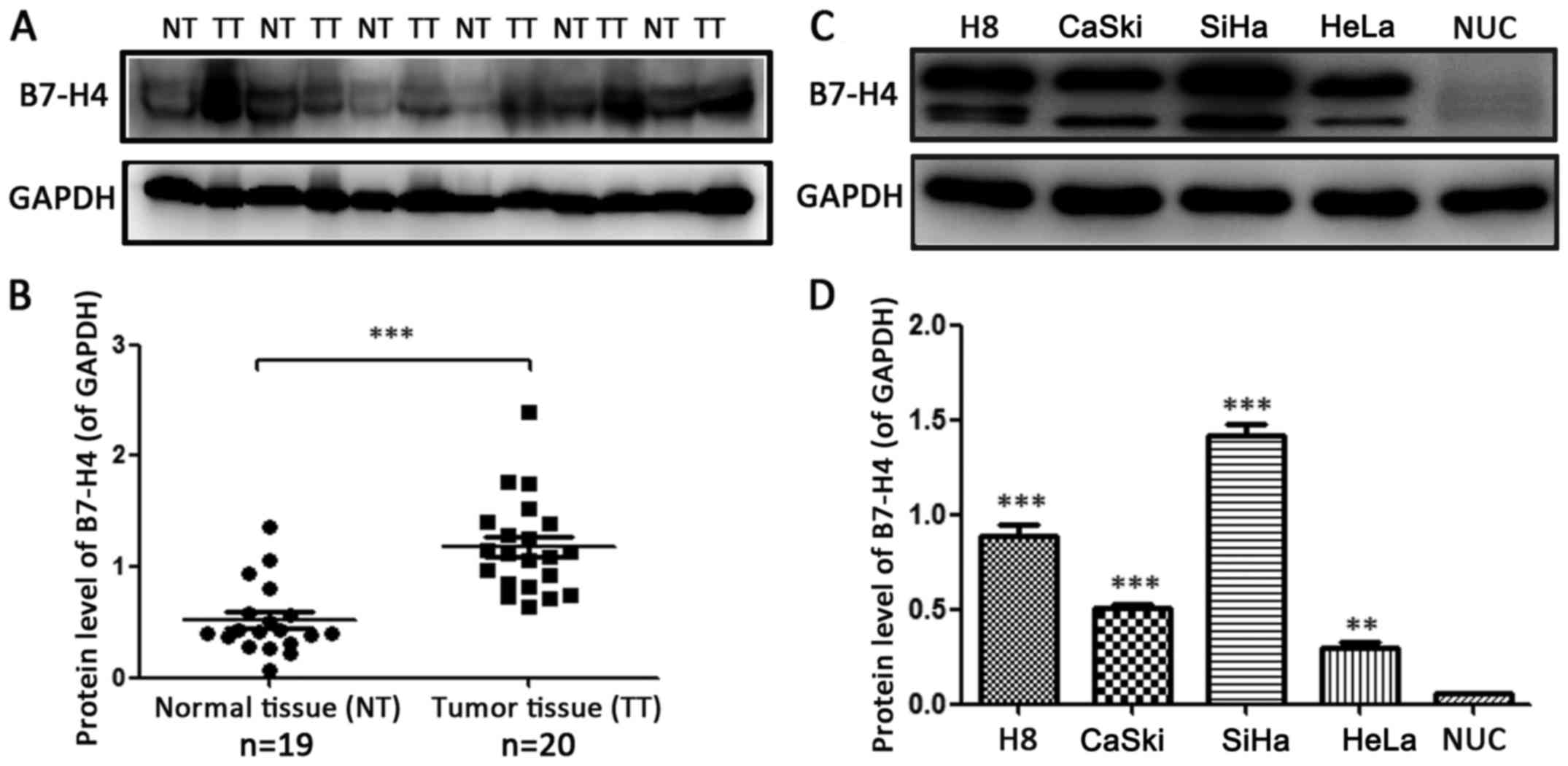
To examine whether B7-H4 is expressed in cervical
cancer, the relative protein expression levels of B7-H4 in 19
normal cervical and 20 cervical cancer tissues were assessed by
western blotting (Fig. 1A). The
protein level of B7-H4 in the cervical cancer samples was
significantly higher than that in the normal tissues (P<0.001).
We also detected the B7-H4 protein expression in H8 and three
cervical cancer cell lines including CaSki, HeLa and SiHa (Fig. 1C). The protein expression level of
B7-H4 was the lowest in the NUC cells and the highest in the SiHa
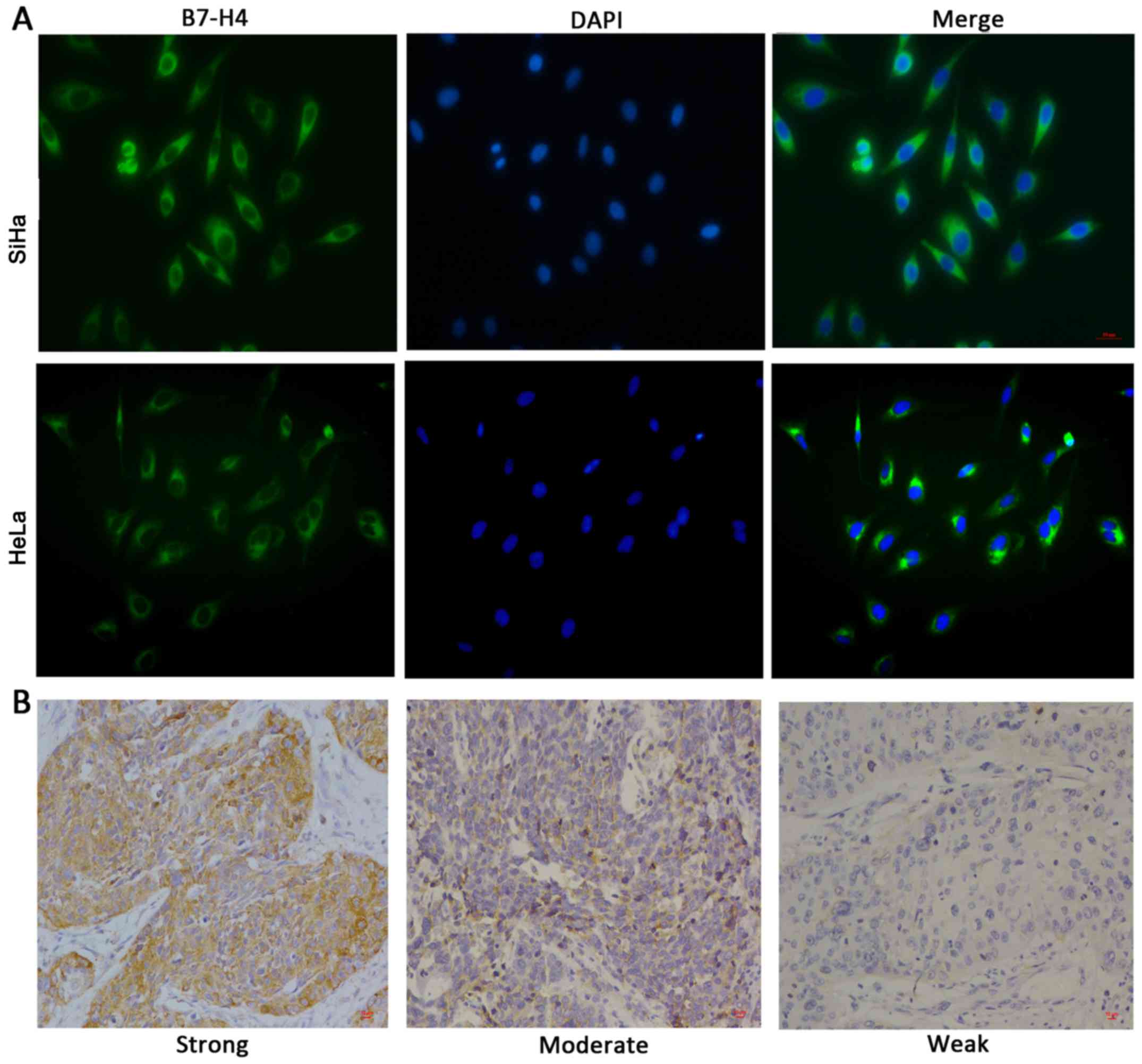
cell line. To investigate the cellular location of B7-H4,
immunofluorescence staining (Fig.
2A) and immunohistochemical staining (Fig. 2B) were performed and the results
suggested that B7-H4 was mainly distributed in the cytoplasm of the
cervical cancer cells. Collectively, these data suggest that B7-H4
was highly expressed in the cytoplasm of cervical cancer cells.
However, it was not equally expressed in the different cervical
cancer cell lines. Thus, knockdown of B7-H4 in the SiHa cell line
and overexpression of it in the HeLa cell line was employed to
study the functions of B7-H4.
Expression of sB7-H4 in CIN and
cervical cancer patients
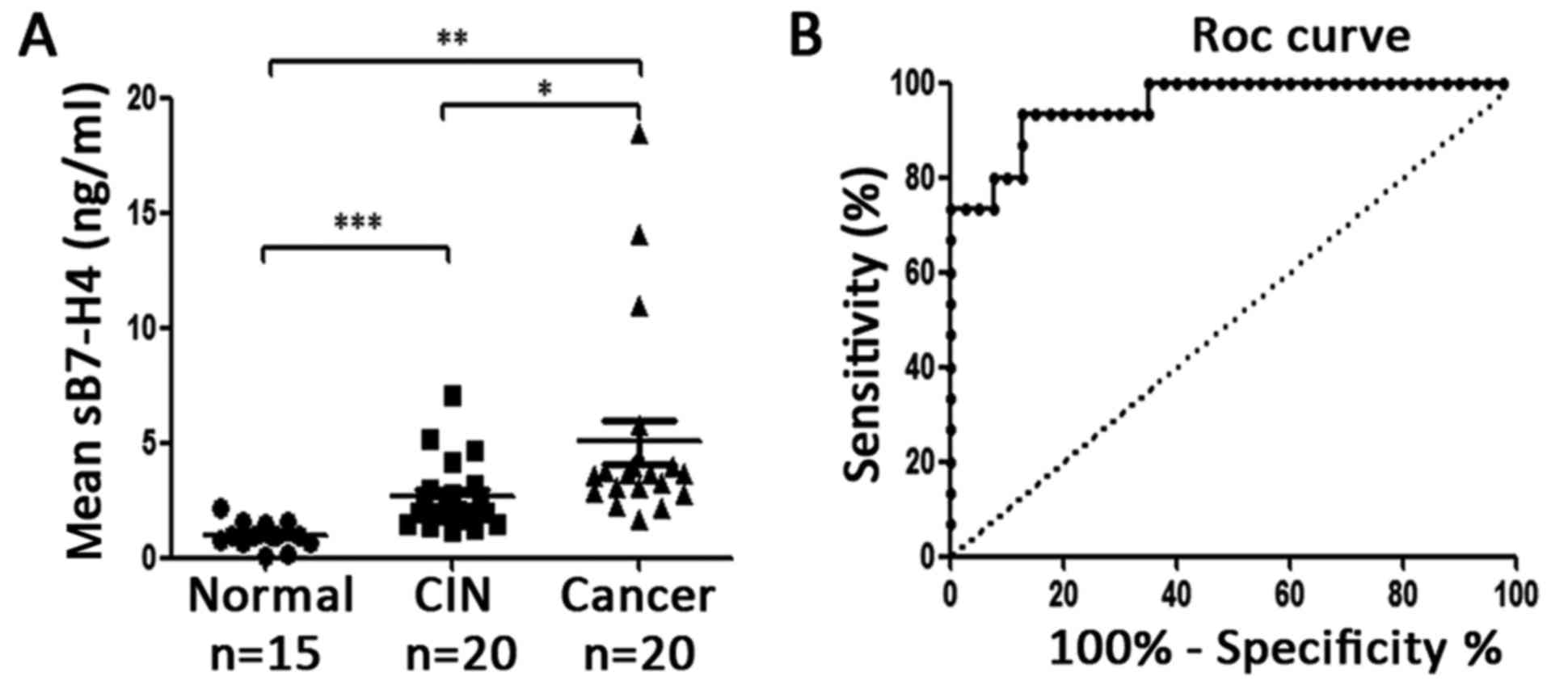
To investigate the expression of B7-H4 in blood,
ELISA assay was performed to detect the sB7-H4 in 15 healthy
volunteers, 20 CIN and 20 cervical cancer patients. The results
(Fig. 3A; Table II) revealed that the level of
sB7-H4 in CIN patients was higher than that of the healthy
volunteers (Fig. 3A, 2.654±1.533
vs. 1.000±0.557; P=0.0004), and in cervical cancer patients, sB7-H4
was higher than that of the CIN patients (Fig. 3A, 5.042±4.336 vs. 2.654±1.533;
P=0.0257) and healthy volunteers (Fig.
3A, 5.042±4.336 vs. 1.000±0.557; P=0.0011). There was no
difference in CIN I and CIN II–III (P=0.469) or in stage I and II
cervical cancer patients (P=0.205), but the level of sB7-H4 in
adenocarcinoma was higher than that in squamous carcinoma patients
(Table II, 7.101±7.568 vs.
4.527±3.307; P=0.044). The receiver operating characteristic curve
(ROC) is presented in Fig. 3B, and
the area under the curve (AUC) was 0.955, and by using the
concentration of sB7-H4 ≥1.638 as a critical value to predict CIN
and cervical cancer, its sensitivity and specificity were 93.33 and
87.50%, respectively. Collectively, these data demonstrated that
sB7-H4 has the potential to become an early diagnostic indicator of
cervical cancer.
 | Table II.Expression of sB7-H4 in CIN and
cervical cancer patients and the relationship with their grades and
histologies. |
Table II.
Expression of sB7-H4 in CIN and
cervical cancer patients and the relationship with their grades and
histologies.
|
| Expression of
sB7-H4 |
|
|
|---|
|
|
|
|
|
|---|
| Subjects | No. | Mean (ng/ml) | SD | P-value |
|---|
| Normal-control | 15 | 1.000 | 0.557 |
|
| CIN | 20 | 2.654 | 1.533 |
|
| CIN I
(LSIL) | 5 | 2.141 | 0.783 | 0.469 |
| CIN
II/III (HSIL) | 15 | 2.782 | 1.663 |
|
| Cervical
cancer | 20 | 5.042 | 4.336 |
|
| Stage
I | 15 | 5.571 | 0.690 | 0.205 |
| Stage
II | 5 | 3.452 | 4.916 |
|
|
Squamous carcinoma | 15 | 4.527 | 3.307 | 0.044a |
|
Adenocarcinoma | 5 | 7.101 | 7.56 |
|
Relationship between B7-H4 expression
in cervical cancer tissues and clinicopathological factors
Next, we performed immunohistochemical staining and
the results are listed in Table
III. We found that the expression level of B7-H4 in cervical
cancer tissues was not correlated with age (P=0.241), histology
(P=0.536), tumor differentiation (P=0.419), clinical stage
(P=0.540), tumor size (P=0.183), lymph vascular space involvement
(LVSI; P=1.000), lymph node metastasis (LNM; P=0.620) and deep
stromal invasion (DSI; P=0.993).
 | Table III.Relationship between B7-H4 expression
and clinicopathological factors. |
Table III.
Relationship between B7-H4 expression
and clinicopathological factors.
|
|
| Expression of
B7-H4 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | No. | Negative | Positive | P-value |
|---|
| Age (years) |
|
|
| 0.241 |
|
≤45 | 35 | 26 | 9 |
|
|
>45 | 25 | 15 | 10 |
|
| Histology |
|
|
| 0.536 |
|
SCC | 53 | 35 | 18 |
|
|
Adenocarcinoma | 7 | 6 | 1 |
|
|
Differentiation |
|
|
| 0.419 |
|
Low | 27 | 17 | 10 |
|
|
Moderate/high | 33 | 24 | 9 |
|
| Clinical stage |
|
|
| 0.540 |
| I | 46 | 30 | 16 |
|
| II | 14 | 11 | 3 |
|
| Tumor size
(cm) |
|
|
| 0.183 |
|
<4 | 42 | 26 | 16 |
|
| ≥4 | 18 | 15 | 3 |
|
| LVSI |
|
|
| 1.000 |
|
Negative | 52 | 36 | 16 |
|
|
Positive | 8 | 5 | 3 |
|
| LNM |
|
|
| 0.620 |
|
Negative | 50 | 33 | 17 |
|
|
Positive | 10 | 8 | 2 |
|
| DSI |
|
|
| 0.993 |
|
≥1/2 | 22 | 15 | 7 |
|
|
<1/2 | 38 | 26 | 12 |
|
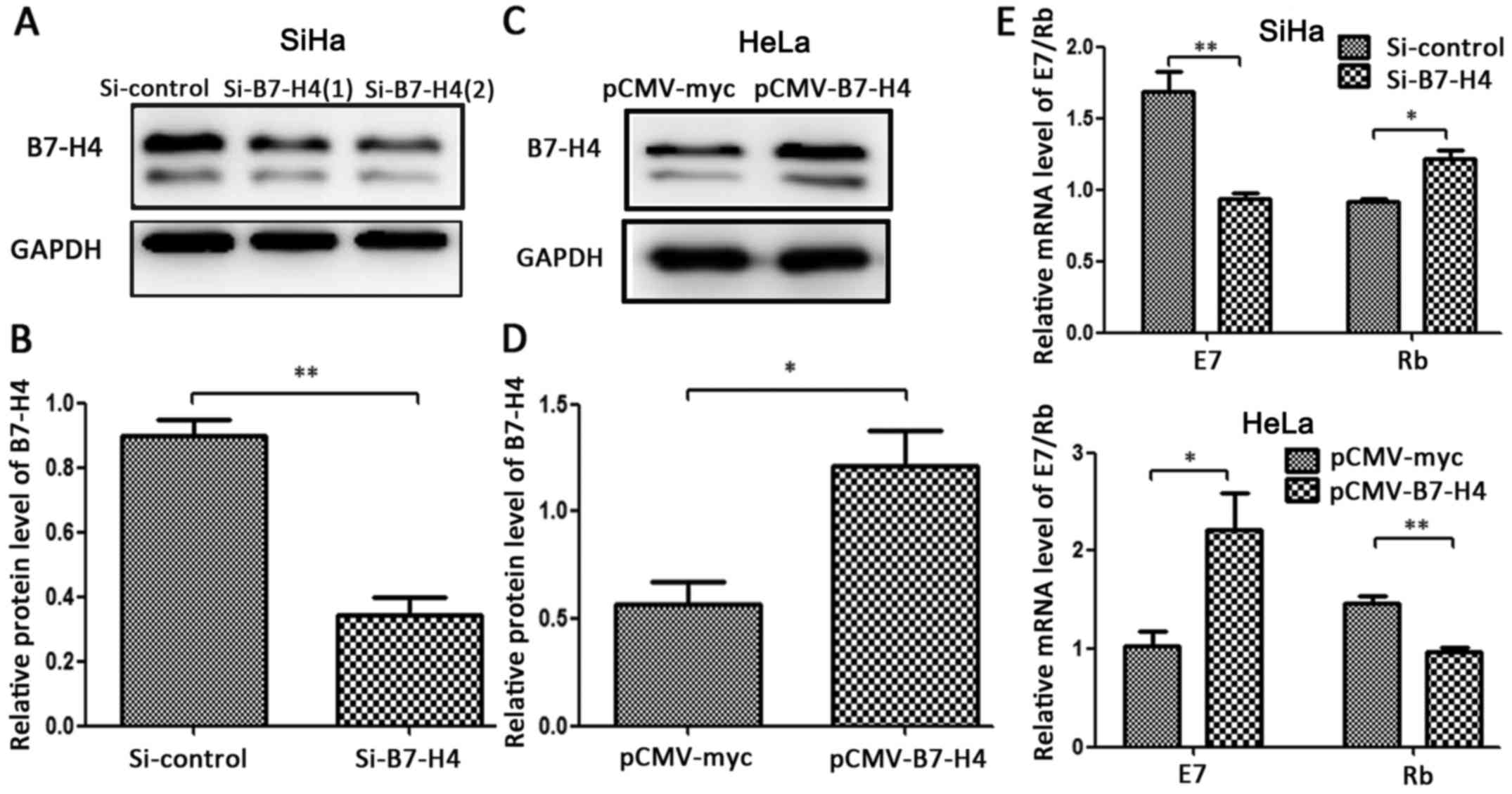
Effects of B7-H4 silencing and
overexpression on E7/Rb mRNA
The HPV oncoprotein E7 forms a complex with tumor
suppressor Rb and inhibits the activities of the proteins in cell
cycle regulatory systems, which is essential for immortalization
and transformation of human cervical squamous epithelial cells
(20,21). To investigate whether B7-H4 could
affect E7/Rb at the transcriptional level, we examined the effect
of silencing and overexpression of B7-H4 on the mRNA level of
E7/Rb. Compared with the control groups, the B7-H4 protein levels
were significantly decreased in the SiHa cell line (Fig. 4A and B) and markedly increased in
the HeLa cell line (Fig. 4C and D)
by 2-fold. In addition, the silencing of B7-H4 in the SiHa cell
line resulted in the downregulation of E7 mRNA and the upregulation
of Rb mRNA, and the overexpression of B7-H4 in the HeLa cell line
led to the opposite effect (Fig. 4E and
F). These findings revealed that B7-H4 may take part in the
formation of cervical cancer by influencing the E7/Rb pathway.
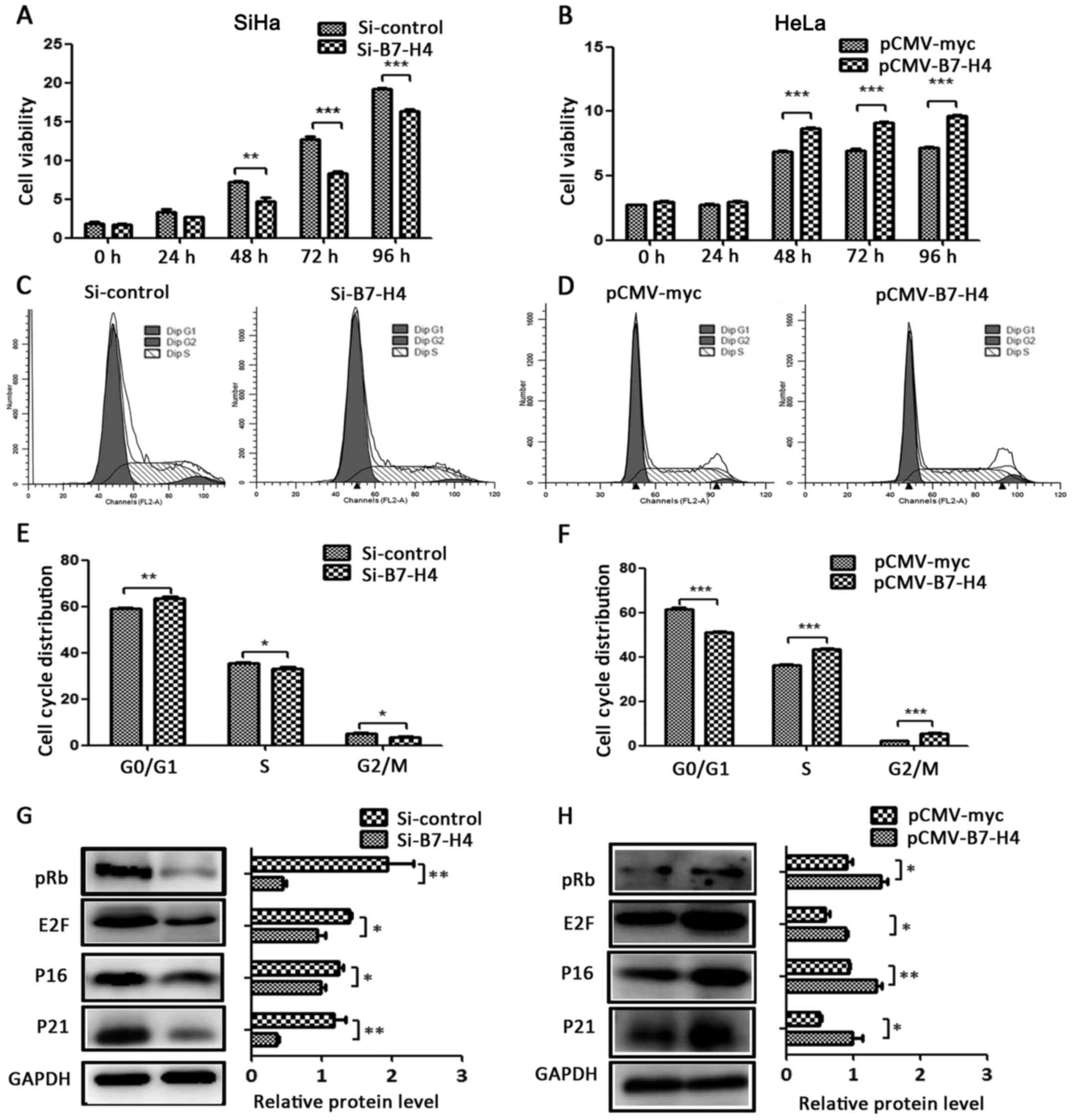
B7-H4 increases cell viability and
accelerates the cell cycle
To examine whether B7-H4 could affect cell viability
and the cell cycle, we performed CCK-8 and flow cytometric assays
(15). The cell viability of the
SiHa Si-B7-H4 group was decreased at 48, 72 and 96 h (Fig. 5A), and the cell viability of the
HeLa pCMV-B7-H4 group was increased at 48, 72 and 96 h (Fig. 5B), in comparison with their control
groups. We also detected the cell cycle distribution using flow
cytometry. Compared with the control groups, the SiHa cells treated
with Si-B7-H4 were arrested in the G0/G1 phase (Fig. 5C) and the HeLa cells with
overexpression of B7-H4 demonstrated accelerated S to G2/M phase
transition (Fig. 5D). We also found
that the cell cycle regulatory proteins, including pRB, E2F, P16
and P21, were influenced by B7-H4 expression. Specifically, the
protein expression levels of pRB, E2F, P16 and P21 were altered
with the expression change of B7-H4. These proteins were
downregulated in the SiHa cells with silenced B7-H4 (Fig. 5G) and upregulated in the pCMV-B7-H4
group of the HeLa cell line (Fig.
5H) in comparison with their control groups.
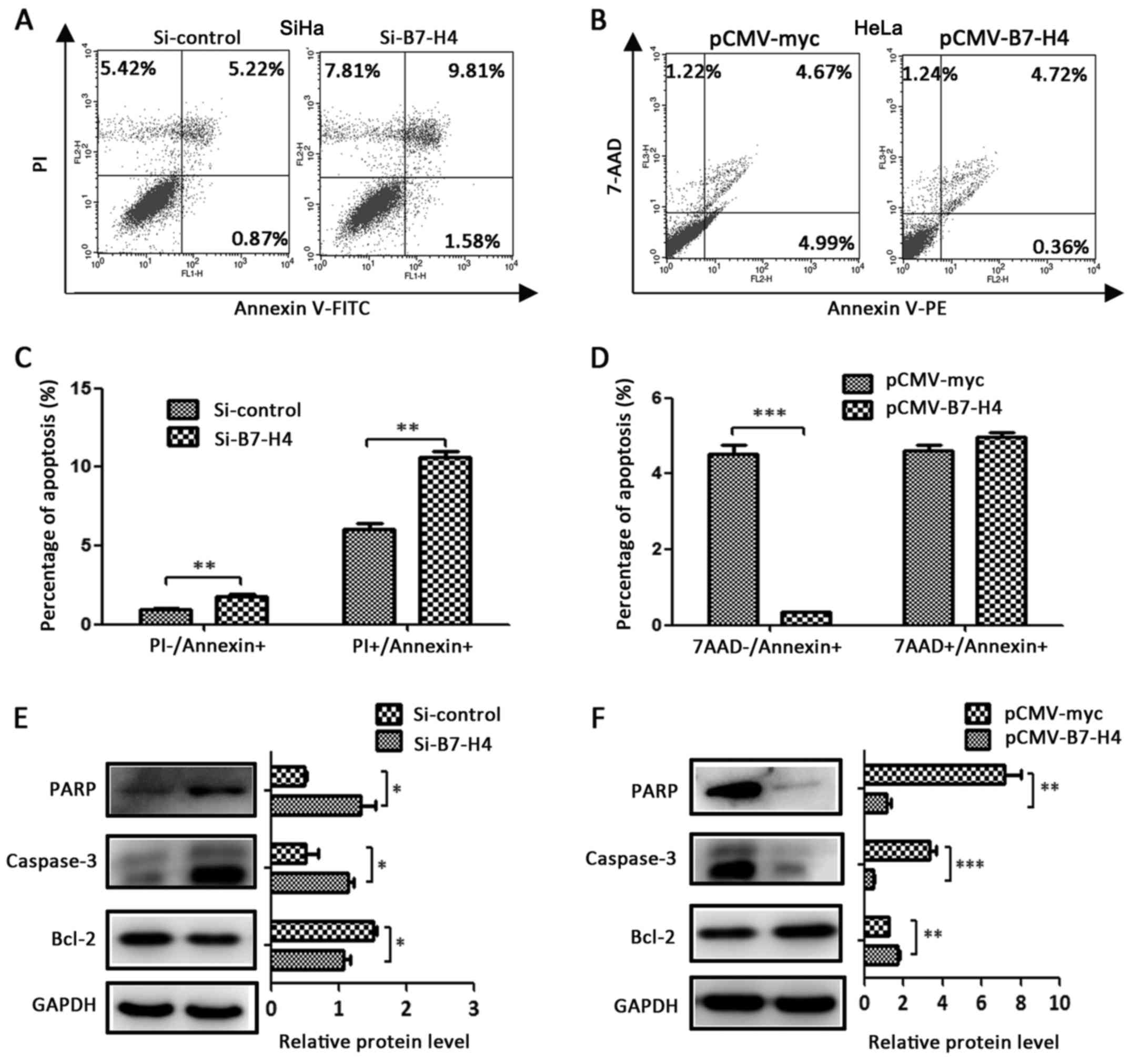
B7-H4 inhibits cell apoptosis
To determine whether B7-H4 expression affects
cervical cancer cell death, the knockdown of B7-H4 in the SiHa cell
line led to an increase in early apoptosis
(PI−/Annexin+) as well as late apoptosis
(PI+/Annexin+) (Fig. 6A and C), and the overexpression of
B7-H4 in the HeLa cell line led to a decrease of early apoptosis
(7AAD−/Annexin+) (Fig. 6B and D) when compared with the
control groups. The western blotting results revealed that the
apoptosis-related proteins, including cleaved PARP and cleaved
caspase-3, were upregulated in the Si-B7-H4 group of the SiHa cell
line (Fig. 6E) and downregulated in
the pCMV-B7-H4 group of the HeLa cell line (Fig. 6F) in comparison with the control
groups, and the anti-apoptosis protein Bcl-2 was in inverse
proportion (Fig. 6E and F).
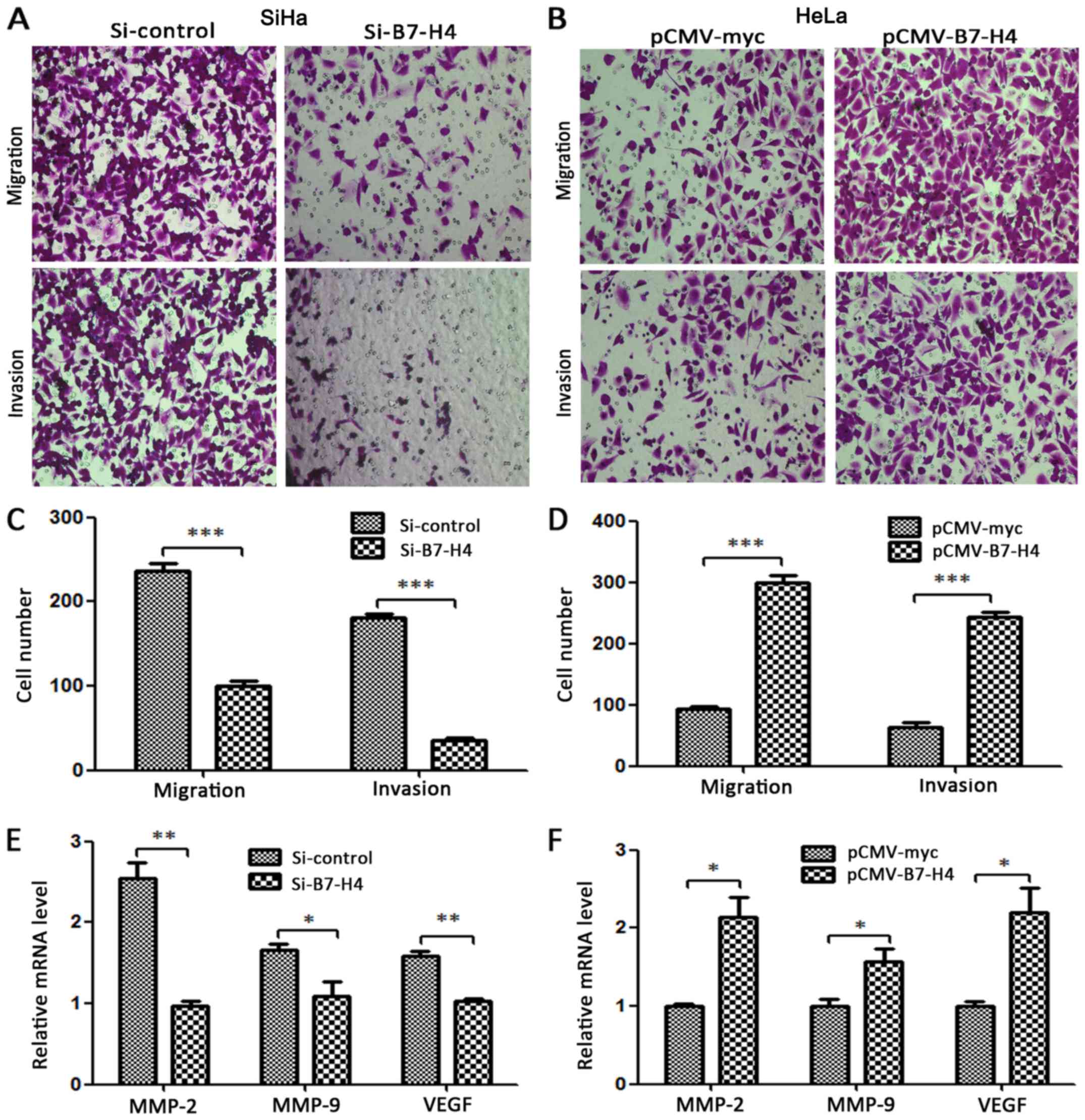
B7-H4 promotes migration and
invasion
Compared with the control groups, the migrated and
invasive number of cells decreased significantly in the SiHa
Si-B7-H4 group and increased markedly in the HeLa pCMV-B7-H4 group
(Fig. 7A and B). Matrix
metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth
factor (VEGF) have been reported to be associated with the
migration and invasion of various cancer cell lines (22–24).
To study whether B7-H4 affected MMP-2, MMP-9 and VEGF at the
transcriptional level, we examined the effect of the silencing and
overexpression of B7-H4 at the mRNA level of MMP-2, MMP-9 and VEGF,
and found that these three molecules were regulated with the
expression change of B7-H4 as determined by RT-PCR. Specifically,
the silencing of B7-H4 in the SiHa cell line decreased the mRNA
expression of these molecules (Fig.
7E) while the overexpression of B7-H4 in the HeLa cell line
increased their mRNA expression (Fig.
7F).
Discussion
B7-H4 has been reported be involved in the
occurrence and development of tumors through the regulation of the
immune system (7,8,25,26)
and plays additional roles in the carcinogenic process directly
(15,16,27).
In the present study, we provided evidence that B7-H4 expression is
present in CIN and cervical cancer patients and we elucidated the
potential underlying mechanism of their carcinogenic effect.
B7-H4 was mainly distributed in the cytoplasm of the
cervical cancer cells according to our experimental results, and
Zhang et al reported that B7-H4 was also a
cytoplasmic-nuclear shuttling protein and could perform different
functions in its different subcellar locations (27).
Through the detection of serum B7-H4 in healthy
women, CIN and cervical cancer patients, we found that the AUC of
sB7-H4 was 0.955, and the sensitivity and specificity reached 93.33
and 87.50% (Fig. 3), respectively.
Compared with the most commonly used HPV testing (28,29),
the sensitivity was approximately the same, but the specificity was
greatly improved. Thus, we tentatively conclude that serum B7-H4
could be used for the early detection and diagnosis of CIN and
cervical cancer in the future; however, more direct human serum
samples are essential to ascertain this conclusion. The results of
immunohistochemistry (Table III)
revealed that the expression of B7-H4 was not associated with
clinicopathological characteristics such as age, histology,
diffentation, clinical stage, tumor size, LVSI, LNM and DSI, which
is consistent with previous studies (12,30).
It is known that the E7/Rb pathway plays a crucial
role in the carcinogenic process caused by the persistent infection
of high risk HPV (3). E7 is an
HPV-derived oncogenic protein, and it combines with the
tumor-suppressor molecule Rb, leading to the release of
transcripton activator E2F (3,31–33).
Our data suggest that B7-H4 participates and influences this
process: the silencing of B7-H4 in the SiHa cell line resulted in
the increase of E7 mRNA and the decrease of Rb mRNA, along with an
increase in the protein level of E2F, and the overexpression of
B7-H4 in the HeLa cell line had the opposite effect (Figs. 4E and F, and 5G and H). The follow-up proliferation
assay results confirmed this hypothesis. However, the cell cycle
regulation proteins phosphorylated Rb, P16 and P21 were also
altered with the regulation of B7-H4. The overexpression of
B7-H4 protected cancer cells from apoptosis, and the
silencing of B7-H4 enhanced intracellular caspase activity, leading
to the acceleration of cervical cancer cell apoptosis. This can
account for the alteration of apoptosis-related proteins including
cleaved PARP and cleaved caspase-3 as well as the anti-apoptosis
protein Bcl-2. This finding was consistent with a study by Salceda
et al (13). B7-H4 promoted
the mRNA expression of MMP-2, MMP-9 and VEGF. According to previous
studies, these molecules are related to the migration and invasion
of tumor cells (22–24), suggesting that B7-H4 may be a
potential therapeutic target for cervical cancer. We did not
conduct a tumorigenicity assay in vitro due to the
limitations of the experimental conditions, and the effect of B7-H4
on tumor formation in the LoVo colorectal cell line and in HEK293
cells has previously been confirmed by different groups (15,27).
In conclusion, our findings suggest that the
inhibitory molecule B7-H4 is involved in cervical cancer
progression. Serum B7-H4 could be used as a valuable prognostic
indicator for CIN and cervical cancer patients, and targeting B7-H4
may be a promising treatment of cervical cancer.
Acknowledgements
The present study was conducted at Qilu Hospital,
Shandong University, and was supported by the Science and
Technology Developing Planning of Shandong Province (2014GH218029),
the National Natural Science Foundation of China (NSFC; 81572559),
and the National Science and Technology Project of China
(2015BAI13B05).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zur Hausen H: Papillomaviruses causing
cancer: Evasion from host-cell control in early events in
carcinogenesis. J Natl Cancer Inst. 92:690–698. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Y, Li L, Hu Z, Li S, Wang S, Liu J, Wu
C, He L, Zhou J, Li Z, et al: A genome-wide association study
identifies two new cervical cancer susceptibility loci at 4q12 and
17q12. Nat Genet. 45:918–922. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: A widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahbar R, Lin A, Ghazarian M, Yau HL,
Paramathas S, Lang PA, Schildknecht A, Elford AR, Garcia-Batres C,
Martin B, et al: B7-H4 expression by nonhematopoietic cells in the
tumor microenvironment promotes antitumor immunity. Cancer Immunol
Res. 3:184–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahbar R and Ohashi PS: B7-H4 is a
positive regulator of antitumor immunity. OncoImmunology.
5:e10505752015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng HX, Wu WQ, Yang DM, Jing R, Li J,
Zhou FL, Jin YF, Wang SY and Chu YM: Role of B7-H4 siRNA in
proliferation, migration, and invasion of LOVO colorectal carcinoma
cell line. BioMed Res Int. 2015:3269812015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Jiang J, Gao R, Wei S, Nan F, Li
S and Kong B: B7-H4 expression promotes tumorigenesis in ovarian
cancer. Int J Gynecol Cancer. 19:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simon I, Liu Y, Krall KL, Urban N, Wolfert
RL, Kim NW and McIntosh MW: Evaluation of the novel serum markers
B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of
ovarian cancer. Gynecol Oncol. 106:112–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Xu C, Wang Y, Yu L and Zhang X:
Prognostic values of B7-H4 in non-small cell lung cancer.
Biomarkers. 1–16. 2016. View Article : Google Scholar
|
|
19
|
Pecorelli S, Zigliani L and Odicino F:
Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol
Obstet. 105:107–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMurray HR, Nguyen D, Westbrook TF and
McAnce DJ: Biology of human papillomaviruses. Int J Exp Pathol.
82:15–33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaur P, McDougall JK and Cone R:
Immortalization of primary human epithelial cells by cloned
cervical carcinoma DNA containing human papillomavirus type 16
E6/E7 open reading frames. J Gen Virol. 70:1261–1266. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Lai L, Liu S, Zhou C, Wu C, Huang
M and Lin Q: Targeting HIF-1α and VEGF by lentivirus-mediated RNA
interference reduces liver tumor cells migration and invasion under
hypoxic conditions. Neoplasma. 63:934–940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu X, Duan L, Xie H, Lu X, Lu D, Lu D,
Jiang N and Chen Y: Evaluation of MMP-9 and MMP-2 and their
suppressor TIMP-1 and TIMP-2 in adenocarcinoma of esophagogastric
junction. Onco Targets Ther. 9:4343–4349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie B, Zhang Z, Wang H, Chen Z, Wang Y,
Liang H, Yang G, Yang X and Zhang H: Genetic polymorphisms in MMP
2, 3, 7, and 9 genes and the susceptibility and clinical outcome of
cervical cancer in a Chinese Han population. Tumour Biol.
37:4883–4888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fauci JM, Straughn JM Jr, Ferrone S and
Buchsbaum DJ: A review of B7-H3 and B7-H4 immune molecules and
their role in ovarian cancer. Gynecol Oncol. 127:420–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang
W, Zhang Y and Geng W: B7-H4 overexpression impairs the immune
response of T cells in human cervical carcinomas. Hum Immunol.
75:1203–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Wu H, Lu D, Li G, Sun C, Song H,
Li J, Zhai T, Huang L, Hou C, et al: The costimulatory molecule
B7-H4 promote tumor progression and cell proliferation through
translocating into nucleus. Oncogene. 32:5347–5358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu H, Lin A, Shao X, Shi W, Zhang Y and
Yan W: Diagnostic accuracy of high-risk HPV genotyping in women
with high-grade cervical lesions: Evidence for improving the
cervical cancer screening strategy in China. Oncotarget.
7:83775–83783. 2016.PubMed/NCBI
|
|
29
|
Park IU, Wojtal N, Silverberg MJ, Bauer
HM, Hurley LB and Manos MM: Cytology and human papillomavirus
co-test results preceding incident high-grade cervical
intraepithelial neoplasia. PLoS One. 10:e01189382015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Cao NN, Wang S, Man HW, Li PF and
Shan BE: Roles of coinhibitory molecules B7-H3 and B7-H4 in
esophageal squamous cell carcinoma. Tumour Biol. 37:2961–2971.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balsitis S, Dick F, Dyson N and Lambert
PF: Critical roles for non-pRb targets of human papillomavirus type
16 E7 in cervical carcinogenesis. Cancer Res. 66:9393–9400. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Watt PJ, Ngarande E and Leaner VD:
Overexpression of Kpnβ1 and Kpnα2 importin proteins in cancer
derives from deregulated E2F activity. PLoS One. 6:e277232011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|