Introduction
Ampelopsis sinica (Miq.) W.T. Wang, belongs
to the genus of Ampelopsis (Vitaceae family), the root of
which has been used as folk traditional Chinese medicine for
heat-clearing and detoxifying, antibacterial and antivirus, and
trauma disease. In 1987, Yang et al documented that A.
sinica root could significantly decrease elevated
glutamic-pyruvic transaminase (GPT) level caused by carbon
tetrachloride and galactosamine (1). According to Japanese patent
publication, A. sinica root contains an effective
anti-hepatic fibrosis element. Studies worldwide indicate that the
extracts of A. sinica root has significant effects on
hepatoprotection, descending transaminase, anti-hepatitis B virus
and suppressing free radical damage (2–6). Thus,
we speculated that A. sinica root has anti-hepatoma
activity.
Our
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
pilot experiments showed that the ethyl acetate part of A.
sinica root had the best antitumor activity compared to
petroleum ether, n-butyl alcohol and water-soluble part. This
experimental study was performed to investigate the suppression
effects of the ethyl acetate extract of A. sinica root on
growth of HepG2 cells and to clarify the possible mechanism of its
anti-hepatoma activity.
Materials and methods
Plant material preparation and
extraction
A. sinica root (ASR) was collected in August
2013 from Macheng in Hubei. It was authenticated by Professor Keli
Chen and the voucher specimens were deposited in the herbarium of
Hubei University of Chinese Medicine (Wuhan, China). The ethanol
extract was prepared as previously described (2). The total ethanol extract was extracted
with ethyl acetate to obtain the A. sinica root ethyl
acetate extract (ASRE) which was lyophilized and dissolved in
dimethyl sulfoxide (DMSO) for use in the follow-up experiments. The
purity of ASRE was ~1.5%.
Reagents and cell cultures
Acetonitrile (chromatographically pure) and formic
acid (mass spectrum magnitude) were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Gallic acid (batch lot.
110831-201204) and catechin (batch lot. 878-200102) were purchased
from the National Institute for the Control of Pharmaceutical and
Biological Products (Beijing, China).
Dulbecco's modified Eagle's medium (DMEM), trypsin
and penicillin/streptomycin were obtained from Gibco (Grand Island,
NY, USA). Fetal bovine serum (FBS) was purchased from HyClone
(Logan, UT, USA). TRIzol, MTT and DMSO were from Sigma-Aldrich (St.
Louis, MO, USA). All chemicals and reagents were of analytical
reagent grade. HepG2, hepatocellular carcinoma (HCC); A549, lung
carcinoma; PC3, prostate carcinoma; MCF7, breast carcinoma; MGC803,
gastric carcinoma; and HL-7702, normal human liver cells were
obtained from China Center for Type Culture Collection of Wuhan
University (Wuhan, China). All the cells were routinely cultured in
DMEM supplemented with 10% FBS and 1% streptomycin/penicillin at
37°C in a humidified incubator with 5% CO2 under
saturating humidity. The cells were subcultured with 0.25% trypsin
when they were 80% confluent. Then, the cells in exponential growth
phase were collected for the following experiments.
UPLC-Q-TOF MSE
analysis
ACQUITY UPLC/Xevo G2-XS QTof system coupled with an
ACQUITY UPLC HSS T3 (50 mm × 2.1 mm, 1.7 µm) was used for the
component analysis of ASRE. The mobile phase was composed of
solvent A (0.1% formic acid in water) and solvent B (acetonitrile).
The optimized elution conditions were as follows: 0.00–1.50 min,
5–23% B; 1.50–4.00 min, 23% B; 4.00–7.00 min, 23–95% B. The column
temperature was maintained at 45°C. The flow rate was set at 500
µl/min. The injection volume was 2 µl.
Leucine enkephaline was used for the lock mass. Each
sample was analyzed in ESI− mode. The scan range was
from 50 to 2,000 m/z. Desolvation gas flow and cone gas flow were
set at 600 and 50 l•h−1, respectively. The source
temperature and the desolvation temperature were set at 100 and
450°C, respectively. The capillary and cone voltage were set at 0.5
kV and 40 V, respectively. An alternation low-energy scan (off) and
high-energy scan (collision energy ramped from 15 to 30 eV) were
used to acquire the precursor (MS) and their fragments
(MSE) in ESI− mode. The instrument was
controlled with MassLynx 4.1 software (Waters Corporation, Milford,
MA, USA).
Cell viability assay
The in vitro antitumor activity of ASRE was
assessed by MTT assay as described in detail elsewhere (7) on five different cancer cell lines.
Briefly, the logarithmic growth phase cells were dispensed into a
96-well microplate at a density of 2×104 cells/ml and
cultured at 37°C in 5% CO2. Then the cells were exposed
to ASRE at various concentrations ranging from 12.5 to 100 µg/ml
for 72 h and the wells without ASRE treatment were considered as
control. After the exposure period, the cells were cultured for an
additional 4 h with 0.5 mg/ml MTT to product formazan. Then, 100 µl
DMSO was added to each well to dissolve the formed formazan
crystals. An automated microplate reader was used to determine the
optical density at a wavelength of 490 nm. Relative cell growth
inhibition rate (%) = (1 -
ODtreated/ODcontrol) × 100% (8). Cytotoxicity was evaluated by
IC50 (50% inhibition of viability) which was obtained
from a regression analysis of dose-response curves. Assays were
done with 3 parallel wells on three independent trials.
Inverted microscope observation
HepG2 cells were seeded into 24-well plates and
cultured overnight, followed by exposed to different doses of ASRE
(0, 50, 75 and 100 µg/ml). The morphological changes and growth
status were observed and photographed using inverted light
microscopy (IX73; Olympus, Tokyo, Japan).
Hoechst 33258 staining test
Hoechst 33258 staining was used to observe the
morphology of apoptotic cells, as previously described (9). The 1×105 cells/ml
exponentially growing HepG2 cells were seeded in 6-well plates and
incubated for 24 h. After the cells treated with various
concentrations of ASRE (0, 50, 75 and 100 µg/ml) for 24 h, the
cells were fixed with 70% ethanol for 20 min at room temperature.
Then, after being washed with phosphate-buffered saline (PBS), the
cells in each well were stained with 0.5 ml Hoechst 33258 solution
at room temperature for 5 min. The cells were photographed under a
inverted fluorescence microscopy (IX73; Olympus) at ×400
magnification to observe morphological changes after being washed
with PBS.
Scanning electron microscopy (SEM)
observation
In order to accurately observe the details of
morphologic changes of HepG2 cells treated with different
concentrations of ASRE, the SEM observation was determined as
previously described (10) with
slight modifications. Briefly, HepG2 cells were cultured on sterile
glass slides to cell slides. The slides of cells with or without
ASRE were fixed with 2.5% glutaraldehyde overnight, and then fixed
with 1% osmic acid for 4 h after several washes with PBS. The
slides dehydrated in an ethanol series and dried with the
lyophilizer. After gold sputtering, the slides were detected by a
SEM (JSM-6501; JEOL Ltd., Tokyo, Japan).
Flow cytometry analysis
HepG2 cells were seeded into 6-well plates and
cultured overnight. Then the cells were treated with ASRE at
different concentrations (0, 50, 75 and 100 µg/ml) for 24 h or
treated with ASRE at 75 µg/ml for different times (0, 12, 18 and 24
h). After drug incubation time, all cells were harvested with
trypsin and washed twice with PBS, and resuspended in 400 µl
Annexin V binding buffer. Then the cells were stained with 5 µl
Annexin V-FITC for 15 min and 10 µl propidium iodide (PI) for 5 min
at 4°C. This assay was performed exactly as indicated in the
manufacturer's instructions of the Annexin V-FITC Cell Apoptosis
Detection kit (BestBio, Shanghai, China). A FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA) was used to detect
fluorescence and the percentage of apoptotic cells were calculated
by the internal software system of the FACSCalibur. Approximately
104 cells were analyzed for each trail.
RT-PCR analysis
TRIzol was used to extract total cellular RNA from
HepG2 cultured as above in the presence of ASRE for indicated time
(0, 1.5, 3, 6, 9 and 12 h). Cells were grown without ASRE as
control. RNA quantity and purity were assessed by UV
spectrophotometer (TU-1901; Beijing Purkinje General Instrument
Co., Ltd., Beijing, China) based on the absorbance measurement at
260 and 280 nm. Total RNA (1.5 µg) was quantitated and then was
reverse transcribed with a Script RT kit (Tiangen Biotech Co.,
Ltd., Beijing, China) according to the manufacturer's instructions
to synthesise complementary DNA (cDNA). The levels of
cyclooxygenase-2 (COX-2), 5-lipoxygenase (5-LOX), FLAP, bax, bcl-2,
caspase-3 and survivin mRNA expression were measured by RT-PCR
using Tiangen SuperReal PreMix Plus (Tiangen Biotech Co., Ltd.)
with specific primers (Table I).
The actin gene was used as reference gene. The 20 µl reaction
system contained 1 µl resulting cDNA template, 1 µl of specific
sense primer, 1 µl of specific antisense primer and 10 µl 2X
SYBR-Green SuperReal PreMix Plus, 7 µl RNase-free ddH2O.
PCR amplification was initiated by 15 min of denaturation at 95°C,
and then followed by 40 cycles of 95°C for 10 sec, 59–63°C
(annealing temperature) for 20 sec and 72°C for 30 sec, and a final
incubation at 72°C for 5 min. All RT-PCR reactions for each sample
were performed in duplicate. The obtained cycle threshold number of
each gene (Ct value) was normalized into fold of relative changes
according to the equation of 2−ΔΔCT method (11). The amplification products were
analyzed on 1% agarose gel electrophoresis in 1X TAE buffer by
ethidium bromide staining.
 | Table I.Primer list. |
Table I.
Primer list.
| Genes | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| 5-LOX |
GCCTCCCTGTGCTTTCC |
ACCTGGTCGCCCTCGTA |
| FLAP |
GCTGCGTTTGCTGGACTGATGTA |
TAGAGGGGAGATGGTGGTGGAGAT |
| COX-2 |
TATGAGTGTGGGATTTGACCAG |
TCAGCATTGTAAGTTGGTGGAC |
| bcl-2 |
GTGGAGGAGCTCTTCAGGGA |
AGGCACCCAGGGTGATGCAA |
| bax |
GGCCCACCAGCTCTGAGCAGA |
GCCACGTGGGCGTCCCAAAGT |
| Caspase-3 |
ATGGAGAACACTGAAAACTCAGT |
TTAGTGATAAAAATAGAGTTCTTTTGT |
| Survivin |
ATGGGTGCCCCGACGTTGCCCCCT |
TCAATCCATGGCAGCCAGCTGCTCG |
| β-actin |
TGACGTGGACATCCGCAAAG |
CTGGAAGGTGGACAGCGAGG |
Immunofluorescent assay
HepG2 cells were cultured on sterile glass slides in
6-well plates. After 60% confluency, the cells were exposed to
varying concentrations of ASRE (0, 50, 75 and 100 µg/ml) for 24 h.
Cells were removed from the culture medium and washed 3 times with
cold PBS. Then the cells in each well were fixed with 70% ice-cold
ethanol in PBS at room temperature for 20 min. Triton X-100 (0.1%)
in PBS was used to enhance the permeability of HepG2 cell membrane.
The cells slides were blocked with 10% normal goat serum (Boster
Biotechnology Co., Ltd., Wuhan, China) in the wet box for 1 h at
room temperature after PBS washed 3 times for 5 min each. One hour
later, the cells were washed gently with PBS and then incubated
with the primary antibody, p53 antibody (FL-393; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) which is a rabbit
polyclonal IgG, in the wet box at 4°C overnight. After the primary
antibody incubation steps, HepG2 cell slides were gently washed in
PBS at least 3 times for 5 min each and then incubated with the
FITC fluorescent-labeled goat anti-rabbit secondary antibody
(Boster Biotechnology Co., Ltd.) in the wet box for 30 min at room
temperature away from light. At the end of the immunofluorescent
assay, the cells climbing should be always extensively washed with
PBS, at least 3 times for 5 min each, and then should be dropped in
antifade solution to avoid the attenuation of the FITC fluorescent.
The samples were observed by an inverted fluorescence microscope
(IX73; Olympus) at ×200 magnification.
In vivo antitumor activity
Kunming mice (male, 20 ± 2 g) were procured from the
Center of Experimental Animals in Wuhan University (Wuhan, China).
The animal experimental protocol was approved by the Institutional
Animal Care and Use Committee and the local experimental Ethics
Committee (Laboratory Animal Certificate no. SYXK 2012–0068). The
in vivo antitumor activity of ASR ethanol extract was
further tested in hepatoma H22 tumor cell-bearing mouse model.
Briefly, mice were injected with hepatoma H22 cells in the armpit
respectively except the normal group mice. The recipient mice were
randomly separated into five groups (each group 12 mice): a normal
group, a negative control group, a positive control group and ASR
ethanol extracts low- and high-dose groups. 5-Fu was given to the
positive control group mice by intraperitoneal injection every
other day. The negative control and normal groups were treated
every day with normal saline only. The ASR ethanol extract low- and
high-dose groups were administered with 1 and 5 g/kg ethanol
extract by gavage every day separately. After 10 days of treatment,
all mice were sacrificed, and the tumors, spleens and thymus were
excised for weighing. Spleen and thymus index is the ratio of
spleen and thymus weight (mg) to the body weight of the mouse
(g).
Statistical analysis
The experiments were performed at least 3 times. All
values are presented as the means ± standard error of the mean
(SEM). Variance of P-values obtained was calculated by means of a
single-factor analysis of variance (ANOVA) test. Differences were
considered to indicate a statistically significant result, at
P-value <0.05.
Results
UPLC-Q-TOF MSE assay
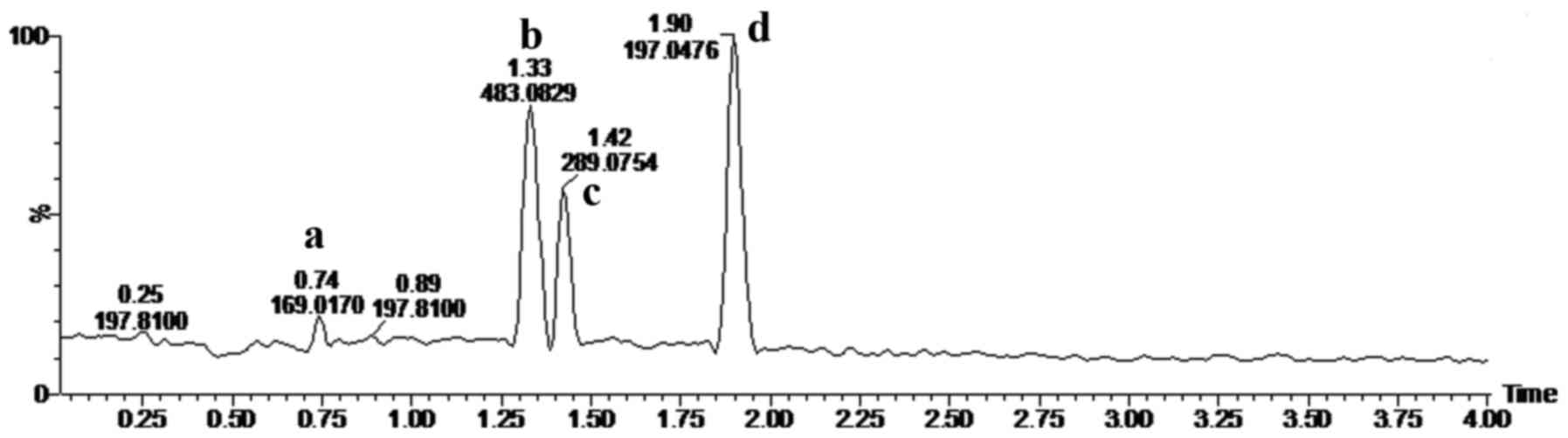
Fig. 1 shows the
UPLC-Q-TOF MS base peak ion (BPI) chromatograms of ASRE in negative
ESI-MSE mode. Four main peaks appeared in the
chromatograms and their retention times (RT) were: 0.74, 1.32, 1.42
and 1.898 min, respectively. The quasi-molecular ions at m/z
169.0170, 483.0829, 289.0754 and 197.0476 [M-H]− in the
negative ESI mode suggested the molecular formula
C7H6O5,
C20H20O14,
C15H14O6 and
C9H10O5. According to the
molecular weight, related literature (12–15),
and standard compound peaks, we initially speculated that m/z
169.0170, 289.0754 and 197.0476 [M-H]− were gallic acid,
catechin and gallic acid ethyl ester, respectively. Based on the
main fragmentations of mass spectrums in negative
ESI-MSE mode, we deduced that compound
C20H20O14 was most likely
1,2-Bis-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranose.
Cell viability assay
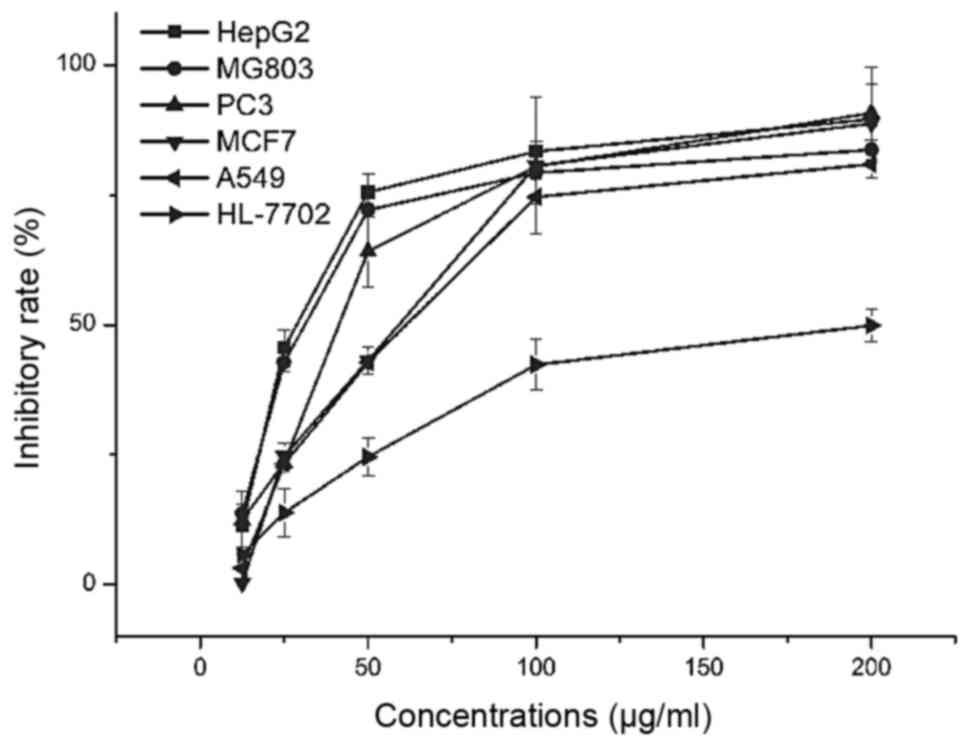
To evaluate the effect of ASRE on cell growth, the
proliferation of five human tumor cell lines HepG2, PC3, MCF7,
MG803, A549 and one normal cell HL-7702 were determined by MTT
method. The IC50 values which quantitatively expressed
the potency of ASRE in inhibiting the cancer cells are shown in
Table II, the ASRE exhibited
highest antitumor activity against the HepG2 cell
(IC50=32.9 µg/ml) compared to the other four tumor
cells. In addition, for normal cell HL-7702 (normal human liver),
ASRE had the lowest cytotoxicity compare to tumor cells. As shown
in Fig. 2, ASRE reduced tumor cell
viability in a dose-dependent manner and the viability of HepG2
cells was significantly affected when the cells were treated with
ASRE at the concentration of 25 µg/ml or above.
 | Table II.IC50 values for ASRE
extracts on the proliferation of different cell lines (µg/ml). |
Table II.
IC50 values for ASRE
extracts on the proliferation of different cell lines (µg/ml).
|
| HepG2 | MG803 | PC3 | MCF7 | A549 | HL-7702 |
|---|
| ASRE extract | 32.9±0.22 | 35.6±2.47 | 48.7±2.37 | 55.8±8.9 | 61.9±5.4 | >200 |
The ASRE at doses up to 200 µg/ml caused no more
than 50% growth inhibition of the normal HL-7702 cells. The MTT
results identified that the ASRE has antitumor activity on human
tumor cells, especially HepG2 cell, and lower cytotoxicity on
normal cells than against the cancer cells.
Morphological changes of HepG2 cells
induced by ASRE under inverted microscope
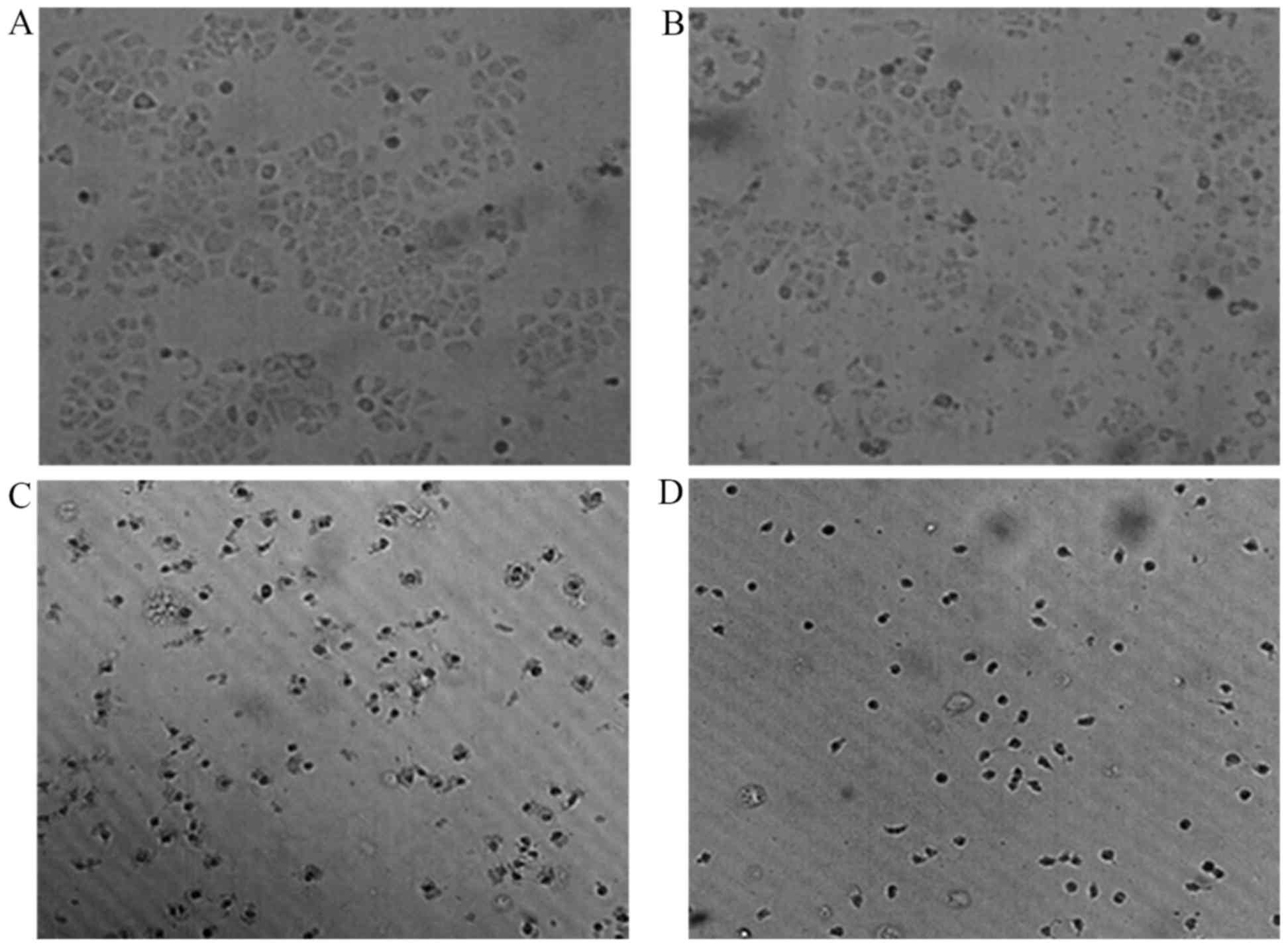
As shown in Fig. 3,
marked morphological changes were observed as compared with
untreated cells, while HepG2 cells were incubated with different
doses of ASRE (25, 50 and 100 µg/ml) for 24 h. Untreated HepG2
cells attached closely on the culture in a homogeneous size with
few floating cells and some of them contacted each other to form
colonies (Fig. 3A). However, ASRE
treated cells made fewer cellular contacts and became round in
shape with shrunken nuclei. It was observed that the adherent cells
were reduced and the floating cells increased. Furthermore, these
cell morphological changes were dose-dependent (Fig. 3B-D).
Hoechst 33258 staining
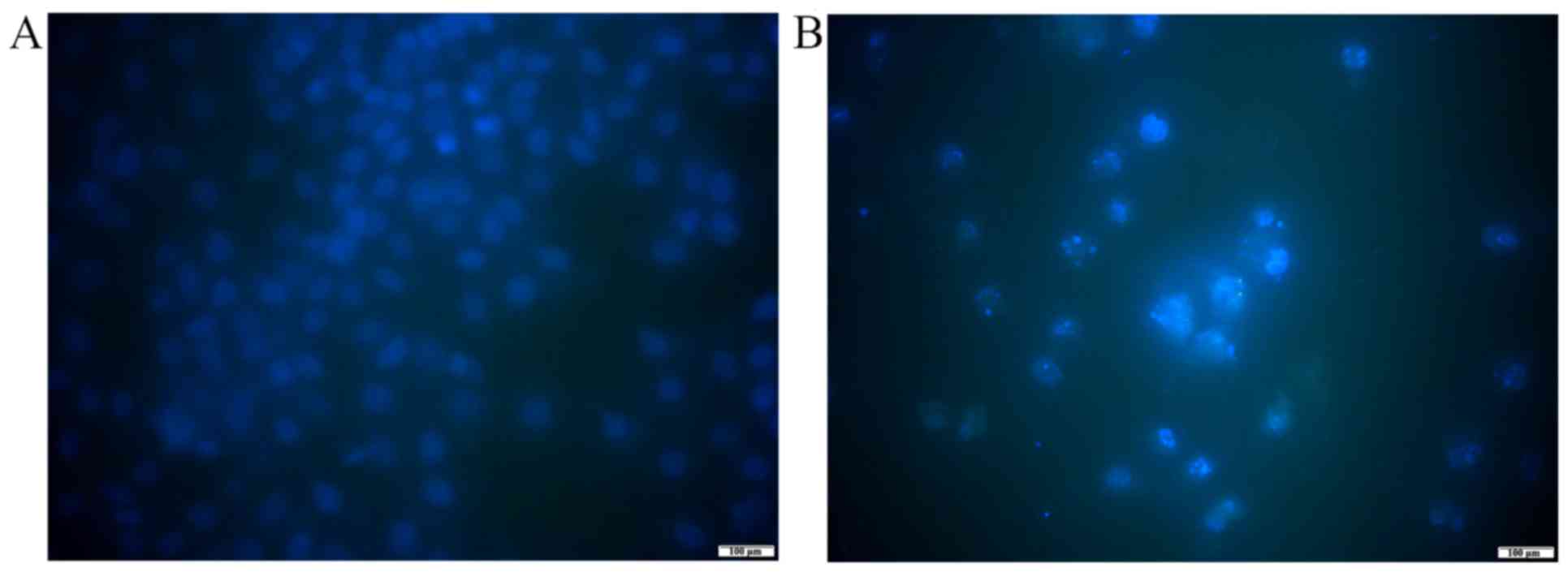
To analysis the effect of ASRE on the morphology of
apoptotic cells, Hoechst 33258 staining test was conducted. After
exposing to 100 µg/ml ASRE for 48 h, distinct morphological changes
in chromatin morphology such as crenation, condensation and
fragmentation were observed in HepG2 cells (Fig. 4).
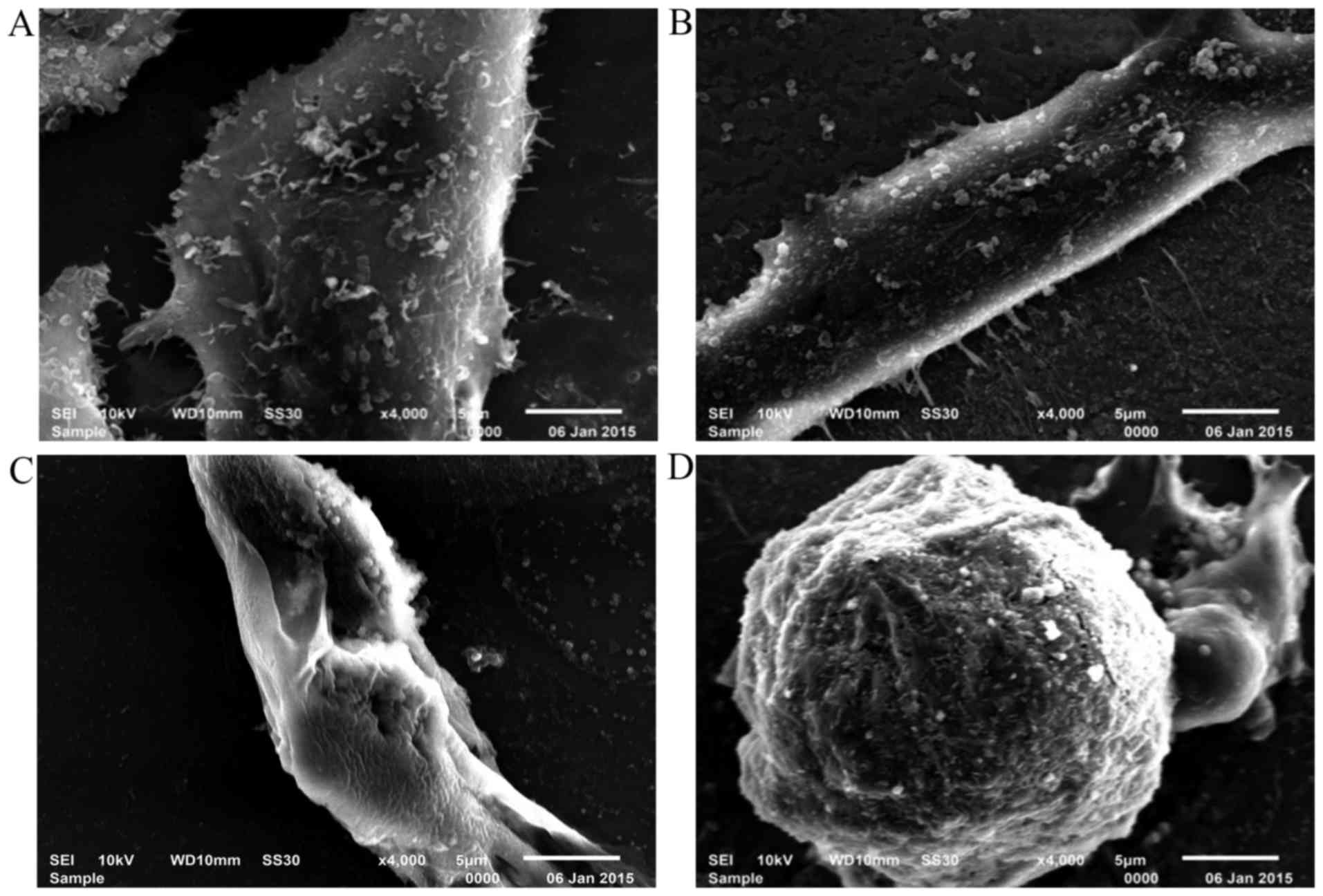
SEM observation
The ultrastructural changes of HepG2 cells after
respectively exposed to various concentrations (0, 50, 75 and 100
µg/ml) were observed under SEM. As shown in Fig. 5A-C, with the increase of the ASRE
concentration, the treated cells became shrunken and displayed
spheres, adherence was not good gradually, showed less or no
microvilli, and formation of apoptotic bodies was observed
(Fig. 5D).
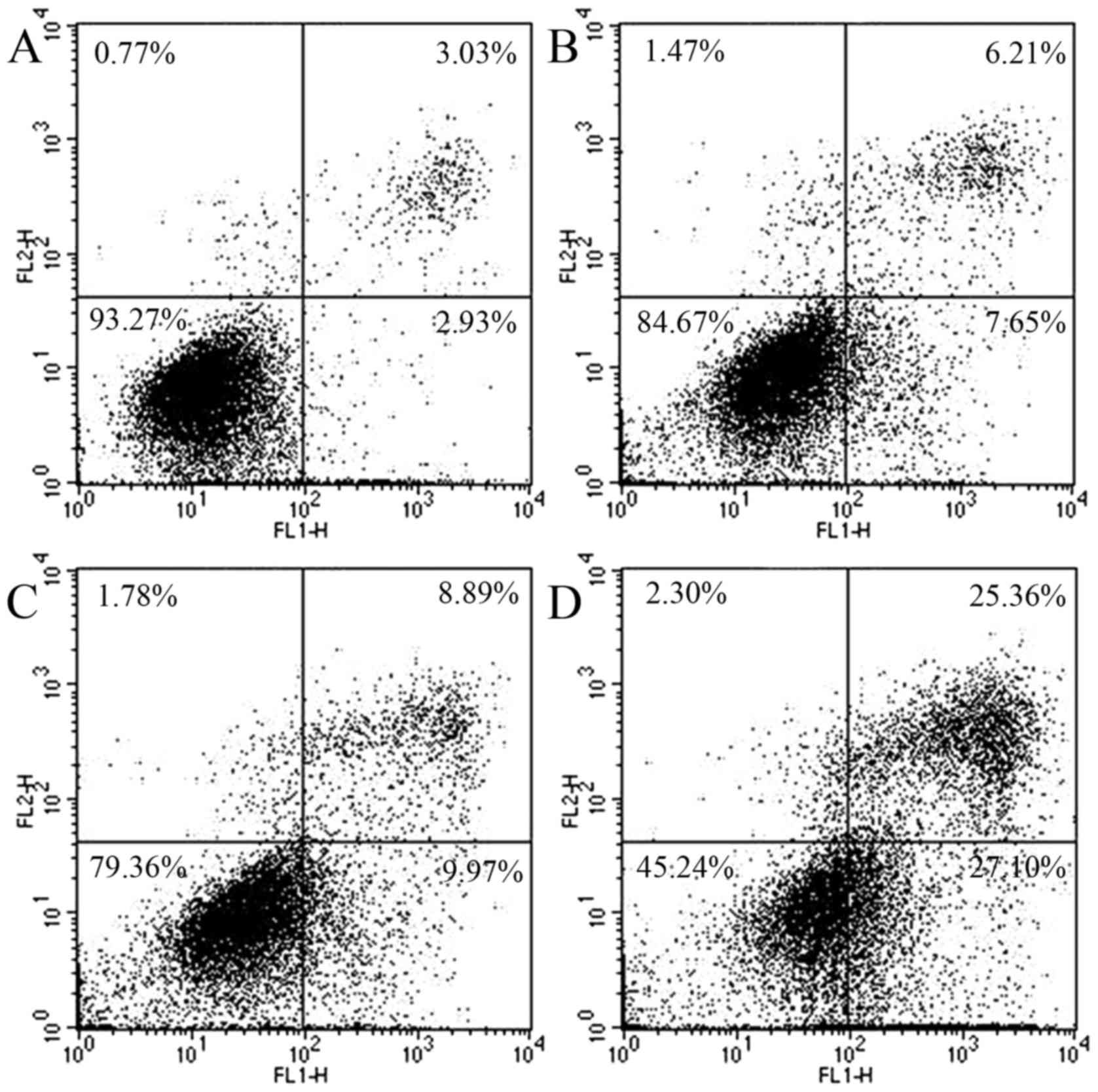
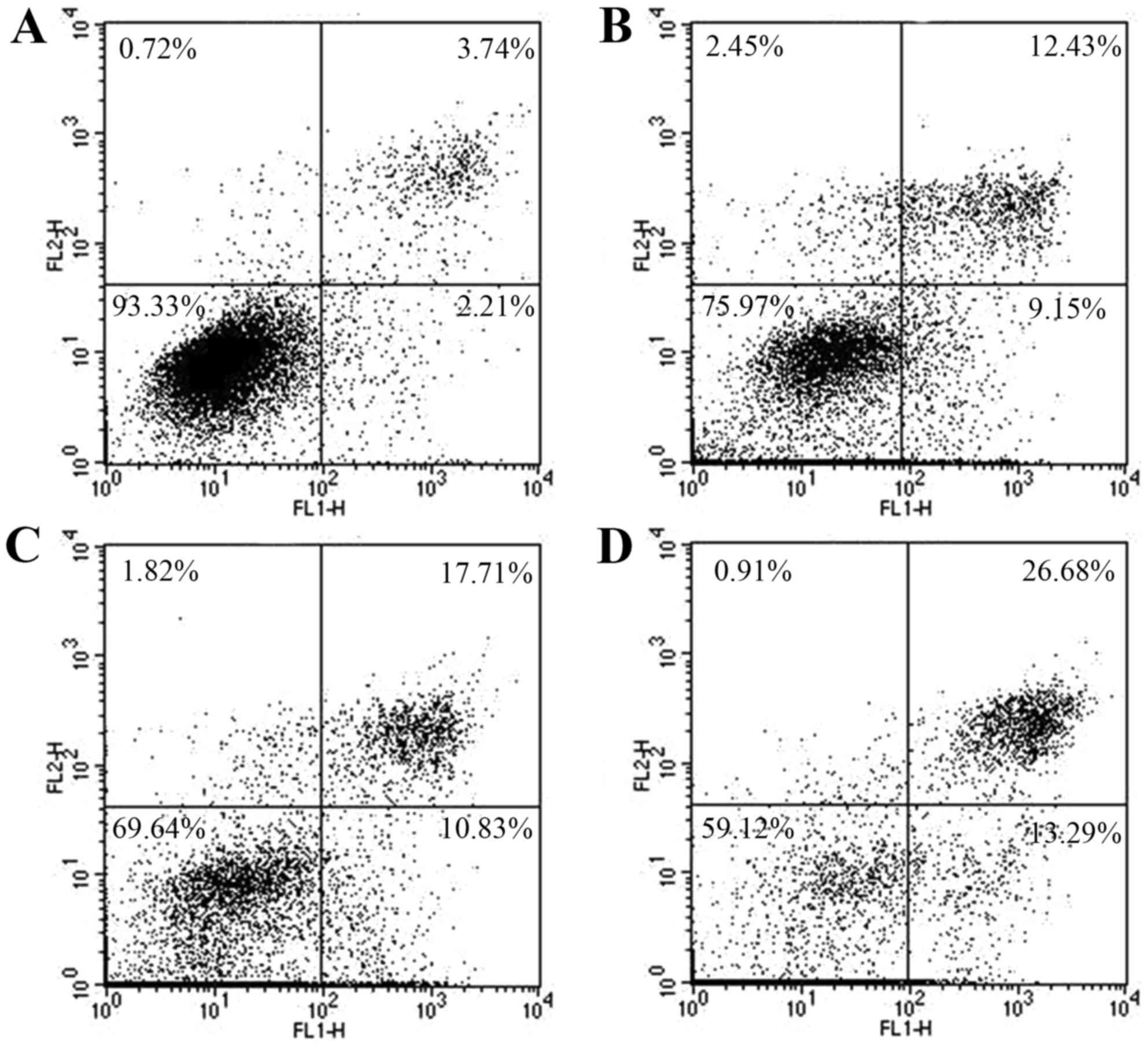
ASRE induces apoptosis in HepG2 cells
in vitro
To confirm whether ASRE induced cell death via
apoptosis, the cells stained with Annexin V-FITC and PI were
analyzed by flow cytometry. In the early stages of apoptosis,
phosphatidylserine (PS) was everted from the internal layer to the
outer face of the plasma membrane. Annexin V was functioned as a
sensitive probe of apoptosis in early phase for its high affinity
to PS on the cell membrane surface. The Annexin V-FITC and PI dual
staining method has been widely used to differentiate viable cells
(Annexin V−/PI−), early apoptotic cells
(Annexin V+/PI−), late apoptotic cells
(Annexin V+/PI+) and necrotic cells (Annexin
V−/PI+). In this study, we accept the Annexin
V-FITC positively stained (early and late stage of apoptosis) cells
as apoptotic population. As shown in Fig. 6, the apoptosis population (%) of
HepG2 cells increased from 5.96 to 52.46% when ASRE concentration
increased from 0 to 100 µg/ml. Similarly, the apoptosis population
(%) of HepG2 cells increased from 5.95 to 39.97% when the acting
time of ASRE increased from 0 to 24 h (Fig. 7). The results support time- and
dose-dependent late apoptotic induction properties of ASRE on HepG2
cells.
Effect of ASRE on inflammation factors
and apoptosis-related gene expression
To further explore the potential mechanism of ASRE
on inhibiting cell growth and inducing apoptosis, mRNA expression
of several inflammation-related genes and apoptosis-related genes
were investigated by RT-PCR assay. As shown in Fig. 8, the level of COX-2, 5-LOX and FLAP
mRNA was gradually decreased with the incubation time increasing
from 0 up to 12 h, which implied ASRE may prevent inflammatory
response related factors production at gene level. The treatment of
ASRE on HepG2 cells for 12 h inhibited COX-2, 5-LOX and FLAP mRNA
production up to 73.47, 63.51 and 60.82%, respectively.
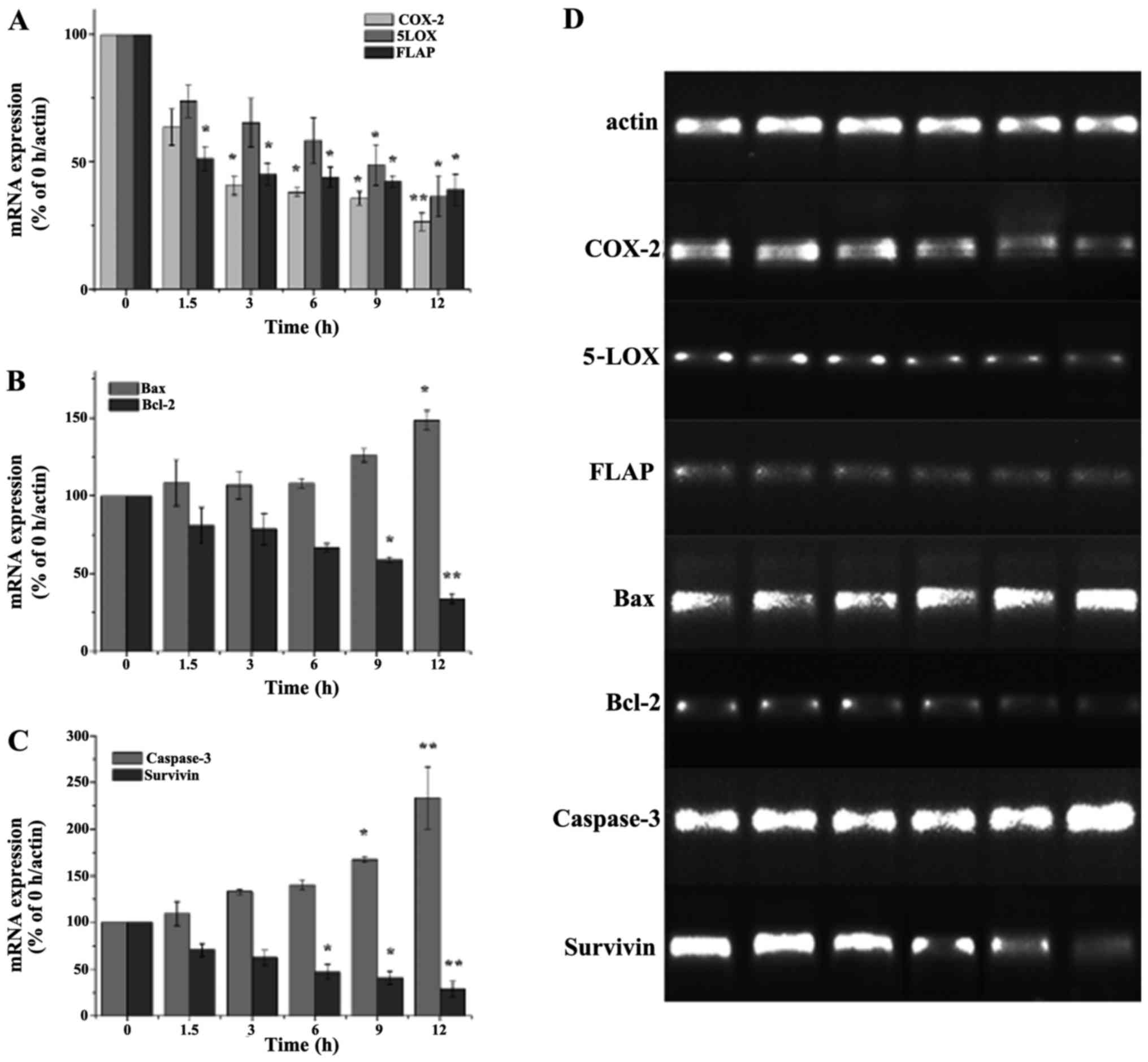 | Figure 8.Effects of ASRE on COX-2, 5-LOX,
FLAP, bax, bcl-2, caspase and survivin mRNA expression. (A-D) HepG2
cells were treated with ASRE for 0, 1.5, 3, 6, 9 and 12 h. Values
are means ± SD (n=5). *P<0.05 vs. the control group; **P<0.01
vs. the control group. ASRE, ethyl acetate extract from
Ampelopsis sinica root; COX-2, cyclooxygenase-2; 5-LOX,
5-lipoxygenase. |
In another aspect, bax and bcl-2 are a pair of most
valuable genes for research in bcl-2 family, and the ratio of
bax/bcl-2 determines whether a cell undergoes apoptosis. Caspase
activation is known as a major step in apoptosis. Caspase-3 plays
key role in the process of transmitting apoptosis signs, and
survivin could be one of the most powerful anti-apoptosis factors
discovered. Our data suggested that the mRNA expression of bax and
caspase-3 were upregulated by ~1.48- and 2.33-fold, respectively,
while bcl-2 and survivin were downregulated by ~66.14 and 71.5%,
after treatment of ASRE for 12 h. In addition, the mRNA expression
levels of bax, bcl-2, caspase-3 and survivin change regularly with
the action time of ASRE extending to 12 h.
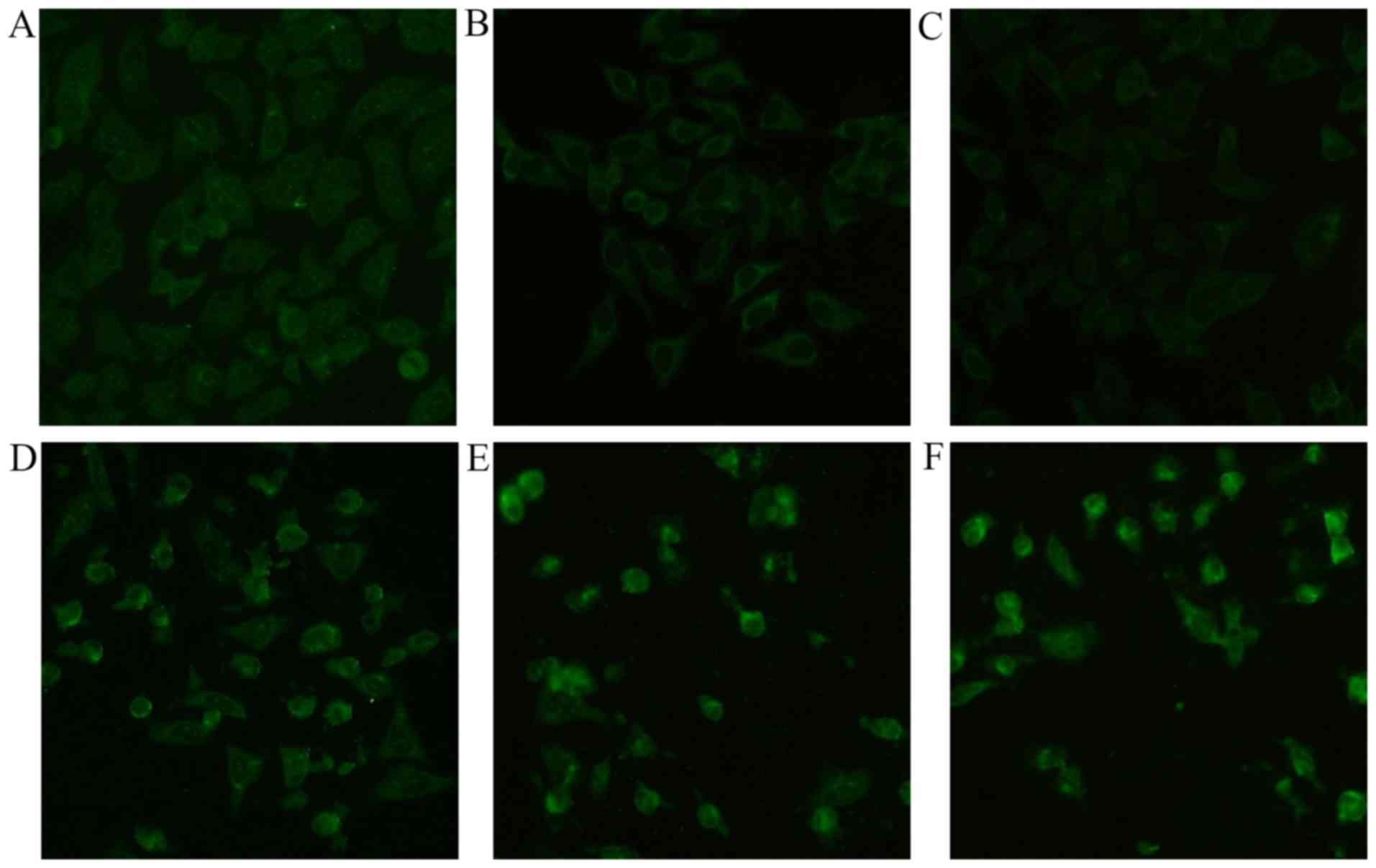
Immunofluorescence assay
To further clarify the involvement of apoptosis
pathway induced by ASRE in HepG2 cells, expression of
apoptosis-related protein p53 was measured by immunofluorence
stain. Fig. 9 clearly illustrates
that the fluorescence intensity increased with the increasing of
ASRE concentration, which revealed ASRE treatment resulted in
upregulation of the tumor suppressor protein p53 expression.
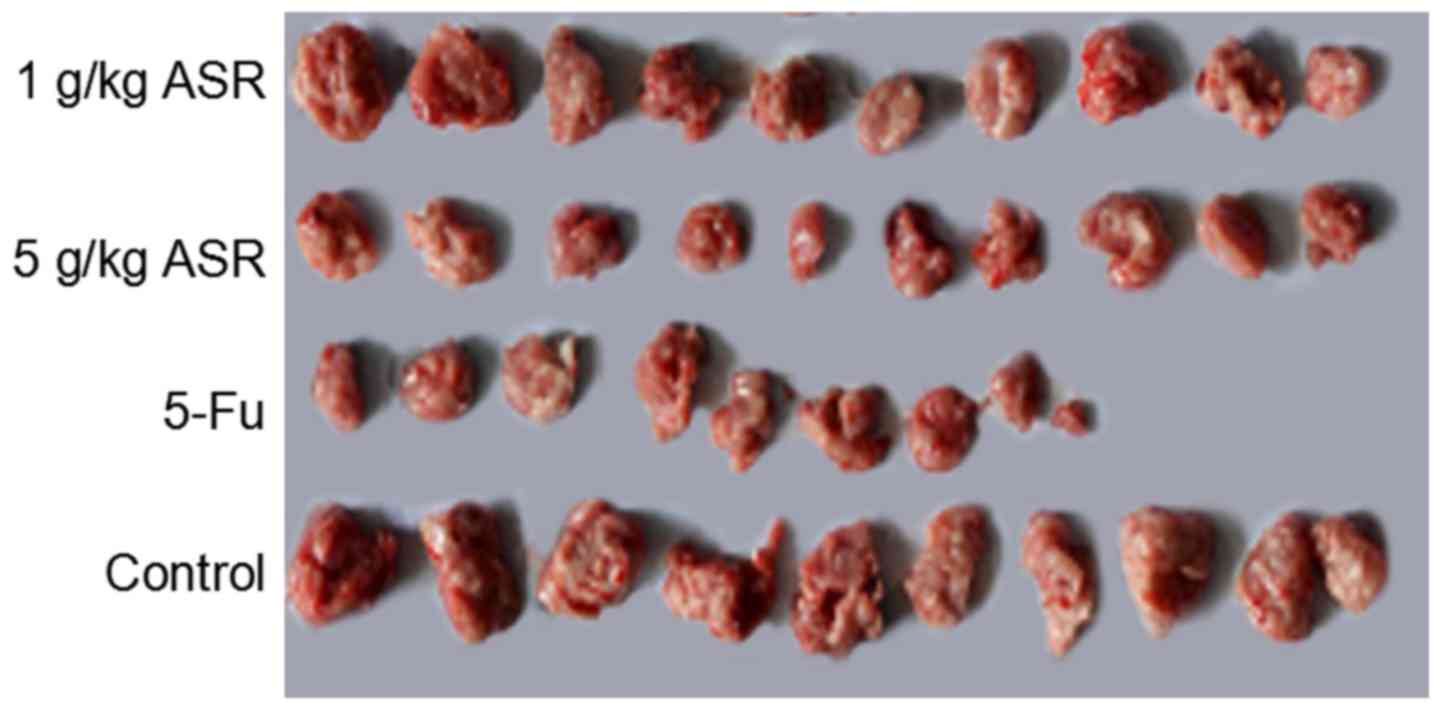
Antitumor activity in vivo
Due to the good antitumor activities in vitro
of ASRE, we conducted animal research to evaluate the antitumor
effect of ASR ethanol extract in vivo. As daily observation,
no mouse died when treated with ASR ethanol extracts below the
dosages of 5 g/kg/day. The effects of ASR ethanol extract on mice
transplanted with hepatoma H22 are presented in Table III. The inhibitory rates were
27.00 and 59.14% at the dosages of 1, 5 g/kg/day, respectively. The
results revealed that the higher dose ASR ethanol extracts (5
g/kg/day) significantly decreased the tumor weights of H22
tumor-bearing mice compared to the control group (P<0.01).
Furthermore, ASR ethanol extracts did not decrease the spleen and
thymus index of the tumor-bearing mice (Table III; Fig. 10).
 | Table III.Antitumor effects of ASR ethanol
extracts against tumor growth on hepatoma H22 tumor-bearing
mice. |
Table III.
Antitumor effects of ASR ethanol
extracts against tumor growth on hepatoma H22 tumor-bearing
mice.
| Treatment | Tumor weight
(g) | Tumor growth
inhibition (%) | Spleen index (mg/10
g) | Thymus index (mg/10
g) |
|---|
| Control | 1.33±0.39 |
| 5.96±1.08 | 2.01±0.65 |
| ASR |
|
|
|
|
| 1
g/kg | 1.03±0.30 | 27.00 | 5.44±1.02 | 1.97±0.60 |
| 5
g/kg |
0.54±0.27b | 59.14 | 5.70±0.60 | 1.97±0.60 |
| 5-Fu |
0.53±0.27b | 60.24 |
4.68±0.94b |
1.78±0.60a |
Discussion
The majority of HCC cases arise in the setting of
underlying liver disease, such as chronic hepatitis B or C which
lead to inflammation-induced lesions in the liver, hepatic fat
accumulation, progressive fibrosis and irreversible cirrhosis
(16–18). Studies worldwide have indicated that
the extract of ASR has significant effect on hepatoprotection,
descending transaminase, anti-hepatitis B virus, and suppressing
free radical damage (2–6). Thus, we hypothesized that ASR had
anti-hepatoma activity. The findings in this study showed that ASRE
had dose-dependent antitumor activity against various tumor cells,
especially on HepG2 cells (IC50=32.9 µg/ml), and was
less harmful to HL-7702 cells (IC50 >150 µg/ml) than
the tested tumor cells, which indicated that ASRE inhibited
proliferation of cancer cells selectively.
To date, several phytocompounds have been isolated
from ASR, and both the pure compounds and crude extracts have
displayed antioxidative effects and anti-inflammatory activities
(2). The ethyl acetate portion of
ASR showed stronger antitumor activity in pre-test than other
portions probably due to the principal polyhydroxylphenolic
compounds, such as gallic acid and catechin. Previous studies have
indicated that gallic acid (an important phytochemical in
pomegranates) and catechin (the primary phytochemical in green tea)
have cancer-preventing activities in a variety of cancer cell types
(19–22), and their cancer-preventing
activities have a certain relationship with their antioxidant and
anti-inflammatory activities (23–26).
According to our experiment and literature, ASRE was found to
exhibit higher anti-hepatoma activity than both gallic acid
(IC50=106.85 µg/ml) (27) and catechin which inhibited the
growth of HepG2 cell lines at a concentration of 100–200 µg/ml
(28). Similar results were seen in
co-treatment of catechin and caffeine which resulted in a greater
degree of cell inhibition than catechin or caffeine alone (29). Thus, further research on testing the
effects of catechin and gallic acid in combination are needed.
The close association between cancer and chronic
inflammation has been recognized for centuries. COX-2, 5-LOX and
FLAP have been confirmed to be associated with occurrence,
development and metastasis of several kinds of human tumors
(30–32). Research has shown that excessive
expression of COX-2 which plays its carcinogenesis role by
inhibiting apoptosis, promoting cell division and proliferation,
accelerating angiogenesis and blocking cell cycle (33–35) is
emerged in malignant tumors, such as human breast cancer,
esophageal squamous cell carcinomas, prostate cancer and colon
cancer (36–39). Several groups have shown that 5-LOX
pathway plays an important role in tumor growth (40,41).
As a 5-LOX activating protein, FLAP plays an important role in
5-LOX activity (42). Based on the
RT-PCR results, the level of COX-2, 5-LOX and FLAP mRNA was
gradually decreased with the incubation time after ASRE treatment,
it is likely that the inhibitory effect of ASRE on HepG2 cell is
associated with its anti-inflammatory properties.
The process of apoptosis is complex, and it is
well-known that there are two main apoptotic signaling pathways,
the mitochondria-mediated pathway and the death receptor-mediated
pathway. In mitochondrial-mediated apoptosis pathway, the bcl-2
family members such as pro-apoptotic member bax and anti-apoptotic
member bcl-2 have been considered to be vital regulators and the
ratio of bax/bcl-2 largely determines whether cells will experience
apoptosis (43–45). The mitochondria and the death
receptor-mediated pathways converge to caspase-3, the activation of
which means cells would undergo apoptosis unavoidably. p53
functions as a transcription factor regulating downstream genes
important in cell cycle arrest, DNA repair, and apoptosis, loss of
p53 in many cancers leads to apoptosis inhibition (46). In our study, ASRE treatment resulted
in an upregulation of bax and downregulation of bcl-2, increased
the ratio of bax/bcl-2, activated caspase-3 and promoted p53
protein expression. Combined with the findings in our experimental
study, it could be assumed that ASRE lead to apoptosis of cancer
cells through inflammatory cytokines mediated pathway and
mitochondria-mediated pathway. Increasing expression of p53 protein
after ASRE treatment indicated the antitumor mechanisms of ASRE are
also connected to the upregulation of p53 protein.
In conclusion, the present study demonstrated that
ASRE has a powerful anticancer activity in vitro and in
vivo without obvious toxicity. The mechanism of apoptosis
induced by ASRE may be associated with downregulation of
inflammatory cytokines including COX-2, 5-LOX and FLAP, the
increase of the ratio of bax/bcl-2, activation of caspase-3 and
inhibition of survivin, and the increasing expression of p53
protein, may provide experimental evidence for the possibility of
ASRE being a potential therapeutic agent in the treatment and/or
prevention of HCC.
Acknowledgements
This study was supported by Natural Science
Foundation of Hubei Province of China (96J099), New Products of TCM
Senile Diseases Co-Innovation Center of Hubei.
Glossary
Abbreviations
Abbreviations:
|
ASR
|
Ampelopsis sinica (Miq.) W.T.
Wang root
|
|
ASRE
|
ethyl acetate extract from
Ampelopsis sinica root
|
|
PI
|
propidium iodide
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
FBS
|
fetal bovine serum
|
|
IC50
|
concentration of 50% inhibition
|
|
SEM
|
scanning electron microscopy
|
|
COX
|
cyclooxygenase
|
|
LOX
|
lipoxygenase
|
|
PS
|
phosphatidylserine
|
|
HCC
|
hepatocellular carcinoma
|
References
|
1
|
Yang LL, Yen KY, Kiso Y and Hikino H:
Antihepatotoxic actions of Formosan plant drugs. J Ethnopharmacol.
19:103–110. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen K, Plumb GW, Bennett RN and Bao Y:
Antioxidant activities of extracts from five anti-viral medicinal
plants. J Ethnopharmacol. 96:201–205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen K and Yang Z: The acting part anti
HSV-1 in infected cells of extract from Ampelopsis sinica
roots. J Chin Druggist. 2:225–226. 1999.
|
|
4
|
Chen K, Li H, Chen Y and Zhang C:
Inhibition of extracts of Ampelopsis sinica roots on DHBVsAg
in sera of ducklings. Zhong Yao Cai. 23:41–42. 2000.(In Chinese).
PubMed/NCBI
|
|
5
|
Pang R, Tao JY, Zhang SL, Chen KL, Zhao L,
Yue X, Wang YF, Ye P, Zhu Y and Wu JG: Ethanol extract from
Ampelopsis sinica root exerts anti-hepatitis B virus
activity via inhibition of p53 pathway in vitro. Evid Based
Complement Alternat Med. 2011:9392052011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen KL, Zhang XM, Li HM and Zhang CZ:
Anti-hepatic injury induced by D-GalN of three kinds of Radix
Ampelopsis Sincae. Zhong Yao Cai. 7:353–354. 1999.(In Chinese).
|
|
7
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu F, Wang JG, Wang SY, Li Y, Wu YP and
Xi SM: Antitumor effect and mechanism of Gecko on human
esophageal carcinoma cell lines in vitro and xenografted sarcoma
180 in Kunming mice. World J Gastroenterol. 14:3990–3996. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi FH, Li AY, Lv H, Zhao L, Li JJ, Gao B
and Tang W: Apoptosis-inducing effect of cinobufacini, Bufo bufo
gargarizans Cantor skin extract, on human hepatoma cell line
BEL-7402. Drug Discov Ther. 2:339–343. 2008.PubMed/NCBI
|
|
10
|
Pesce M and De Felici M: Apoptosis in
mouse primordial germ cells: a study by transmission and scanning
electron microscope. Anat Embryol (Berl). 189:435–440. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong ML and Medrano JF: Real-time PCR for
mRNA quantitation. Biotechniques. 39:75–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng QR, Chen KL and Ye CJ: The
comparative study on the chemical components of four kinds of
Ampelopsis sinica roots. Asia-Pacific Traditi Med. 3:55–56.
2007.
|
|
13
|
Xu ZH, Liu X and Xu G: Research on
chemical constituents of Ampelopsis sinica root. J Tradit
Chinese Med. 8:484–485. 1995.(In Chinese).
|
|
14
|
Yang L, Chen KL and Ye CJ: The quality
standard of Ampelopsis sinica root ointment. J Central South
Univ National (Natural Science Edition). 26:26–29. 2007.
|
|
15
|
Chen KL: Research on chemical constituents
of Ampelopsis sinica root. J Tradit Chinese Med. 5:294–295.
1996.(In Chinese).
|
|
16
|
Chen VL, Le AK, Kim NG, Kim LH, Nguyen NH,
Nguyen PP, Zhao C and Nguyen MH: Effects of cirrhosis on short-term
and long-term survival of patients with hepatitis B-related
hepatocellular carcinoma. Clin Gastroenterol Hepatol. 14:887–895.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vescovo T, Refolo G, Vitagliano G, Fimia
GM and Piacentini M: Molecular mechanisms of hepatitis C
virus-induced hepatocellular carcinoma. Clin Microbiol Infect.
22:853–861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arzumanyan A, Reis HM and Feitelson MA:
Pathogenic mechanisms in HBV- and HCV-associated hepatocellular
carcinoma. Nat Rev Cancer. 13:123–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Al-Hendy M, Richard-Davis G,
Montgomery-Rice V, Sharan C, Rajaratnam V, Khurana A and Al-Hendy
A: Green tea extract inhibits proliferation of uterine leiomyoma
cells in vitro and in nude mice. Am J Obstet Gynecol. 202:2892010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mak JC: Potential role of green tea
catechins in various disease therapies: progress and promise. Clin
Exp Pharmacol Physiol. 39:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawada M, Ohno Y, Ri Y, Ikoma T, Yuugetu
H, Asai T, Watanabe M, Yasuda N, Akao S, Takemura G, et al:
Anti-tumor effect of gallic acid on LL-2 lung cancer cells
transplanted in mice. Anticancer Drugs. 12:847–852. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You BR and Park WH: Gallic acid-induced
lung cancer cell death is related to glutathione depletion as well
as reactive oxygen species increase. Toxicol In Vitro.
24:1356–1362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lambert JD and Elias RJ: The antioxidant
and pro-oxidant activities of green tea polyphenols: a role in
cancer prevention. Arch Biochem Biophys. 501:65–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saito Y, Shimada M, Utsunomiya T, Imura S,
Morine Y, Ikemoto T, Mori V, Hanaoka J and Kanamoto M: Green tea
catechins improve liver dysfunction following massive hepatectomy
through anti-oxidative and anti-inflammatory activities in rats.
Gastroenterology. 140:S9282011.
|
|
25
|
Inoue M, Sakaguchi N, Isuzugawa K, Tani H
and Ogihara Y: Role of reactive oxygen species in gallic
acid-induced apoptosis. Biol Pharm Bull. 23:1153–1157. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Locatelli C, Filippin-Monteiro FB, Centa A
and Creczinsky-Pasa TB: Antioxidant, antitumoral and
anti-inflammatory activities of gallic acid. Handbook on Gallic
Acid: Natural Occurrences, Antioxidant Properties and Health
Implications. Thompson MA and Collins PB: 4th. Nova Science
Publishers; Hauppauge, NY: pp. 1–23. 2013
|
|
27
|
Lim FPK, Bongosia LFG, Yao NBN and
Santiago LA: Cytotoxic activity of the phenolic extract of virgin
coconut oil on human hepatocarcinoma cells (HepG2). Int Food Res J.
21:729–733. 2014.
|
|
28
|
Jain P, Kumar N, Josyula VR, Jagani HV,
Udupa N, Mallikarjuna-Rao C and Vasanth-Raj P: A study on the role
of (+)-catechin in suppression of HepG2 proliferation via caspase
dependent pathway and enhancement of its in vitro and in vivo
cytotoxic potential through liposomal formulation. Eur J Pharm Sci.
50:353–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haddad L and Rowland-Goldsmith M:
Assessment of the effects of caffeine, gallic acid, and
epigallocatechin-3-gallate on cell inhibition, PIM-3 and E.
cadherin protein levels in two lines of pancreatic cancer cells.
Presented at the Fall 2014 Undergraduate Student Research Day at
Chapman University. Student Research Day Abstracts and Posters.
76:2014.http://digitalcommons.chapman.edu/cusrd_abstracts/76
|
|
30
|
Rao CV, Janakiram NB and Mohammed A:
Lipoxygenase and cyclooxygenase pathways and colorectal cancer
prevention. Curr Colorectal Cancer Rep. 8:316–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneider C and Pozzi A: Cyclooxygenases
and lipoxygenases in cancer. Cancer Metastasis Rev. 30:277–294.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fürstenberger G, Krieg P, Müller-Decker K
and Habenicht AJ: What are cyclooxygenases and lipoxygenases doing
in the driver's seat of carcinogenesis? Int J Cancer.
119:2247–2254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Y, Tang XM, Half E, Kuo MT and
Sinicrope FA: Cyclooxygenase-2 overexpression reduces apoptotic
susceptibility by inhibiting the cytochrome c-dependent
apoptotic pathway in human colon cancer cells. Cancer Res.
62:6323–6328. 2002.PubMed/NCBI
|
|
34
|
Yao L, Liu F, Hong L, Sun L, Liang S, Wu K
and Fan D: The function and mechanism of COX-2 in angiogenesis of
gastric cancer cells. J Exp Clin Cancer Res. 30:132011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trifan OC, Smith RM, Thompson BD and Hla
T: Overexpression of cyclooxygenase-2 induces cell cycle arrest.
Evidence for a prostaglandin-independent mechanism. J Biol Chem.
274:34141–34147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang WG, Douglas-Jones A and Mansel RE:
Levels of expression of lipoxygenases and cyclooxygenase-2 in human
breast cancer. Prostaglandins Leukot Essent Fatty Acids.
69:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takatori H, Natsugoe S, Okumura H,
Matsumoto M, Uchikado Y, Setoyama T, Sasaki K, Tamotsu K, Owaki T,
Ishigami S, et al: Cyclooxygenase-2 expression is related to
prognosis in patients with esophageal squamous cell carcinoma. Eur
J Surg Oncol. 34:397–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshimura R, Sano H, Masuda C, Kawamura M,
Tsubouchi Y, Chargui J, Yoshimura N, Hla T and Wada S: Expression
of cyclooxygenase-2 in prostate carcinoma. Cancer. 89:589–596.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ogino S, Kirkner GJ, Nosho K, Irahara N,
Kure S, Shima K, Hazra A, Chan AT, Dehari R, Giovannucci EL, et al:
Cyclooxygenase-2 expression is an independent predictor of poor
prognosis in colon cancer. Clin Cancer Res. 14:8221–8227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Romano M and Claria J: Cyclooxygenase-2
and 5-lipoxygenase converging functions on cell proliferation and
tumor angiogenesis: implications for cancer therapy. FASEB J.
17:1986–1995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghosh J and Myers CE: Arachidonic acid
stimulates prostate cancer cell growth: critical role of
5-lipoxygenase. Biochem Biophys Res Commun. 235:418–423. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Datta K, Biswal SS and Kehrer JP: The
5-lipoxygenase-activating protein (FLAP) inhibitor, MK886, induces
apoptosis independently of FLAP. Biochem J. 340:371–375. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ghosh J and Myers CE: Inhibition of
arachidonate 5-lipoxygenase triggers massive apoptosis in human
prostate cancer cells. Proc Natl Acad Sci USA. 95:13182–13187.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu W, Lee SK, Jung MJ, Heo SI, Hur JH and
Wang MH: Induction of cell cycle arrest and apoptosis by the ethyl
acetate fraction of Kalopanax pictus leaves in human colon
cancer cells. Bioresour Technol. 101:9366–9372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun B, Geng S, Huang X, Zhu J, Liu S,
Zhang Y, Ye J, Li Y and Wang J: Coleusin factor exerts cytotoxic
activity by inducing G0/G1 cell cycle arrest and apoptosis in human
gastric cancer BGC-823 cells. Cancer Lett. 301:95–105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qi F, Li A, Inagaki Y, Xu H, Wang D, Cui
X, Zhang L, Kokudo N, Du G and Tang W: Induction of apoptosis by
cinobufacini preparation through mitochondria- and Fas-mediated
caspase-dependent pathways in human hepatocellular carcinoma cells.
Food Chem Toxicol. 50:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|