Introduction
State-of-the-art advanced gastric cancer (GC)
treatment mainly involves chemotherapy and targeted therapy. In
most cases, however, intrinsic or acquired chemoresistance occurs
and results in a 5-year survival rate of less than 10% in patients
diagnosed with advanced disease (1). Therefore, chemoresistance is the main
cause of the poor prognosis in GC. As a third-generation platinum
derivative, oxaliplatin has been successfully applied in treating
gastrointestinal tumors. Although, responsiveness to oxaliplatin is
initially high, patients ultimately develop oxaliplatin resistance
(2). Nevertheless, the exact
mechanisms involved in oxaliplatin resistance in GC have not been
elucidated. Thus, it is essential to identify novel therapeutic
strategies for decreasing the resistance of GC to oxaliplatin-based
chemotherapy.
Metastasis-associated in colon cancer 1
(MACC1), as the name suggests, was originally identified as
a colon cancer oncogene promoting metastasis (3). Our previous studies demonstrated that
MACC1 is involved in the process of gastric malignancy, enhances GC
cell proliferation and invasiveness, and is associated with
abnormal glucose metabolism (4,5). MACC1
has been confirmed to be a metabolic oncogene, however, little has
been reported concerning MACC1 and oxaliplatin resistance in GC.
Emerging evidence suggests that altered metabolism in cancer cells
is fundamentally involved in the development of drug resistance
(6–8). The targeting of key metabolic enzymes
which sustain these cancerous metabolic adaptations bears great
promise for improving treatment efficacy in patients with
metastatic diseases (7,9). Hence, we hypothesized that metabolism
bridges MACC1 and oxaliplatin resistance. Various studies have
found that the key enzymes of lipid metabolism are involved in drug
resistance (10–12). Furthermore, through the
bioinformatic analysis from cBio Cancer Genomics Portal (http://www.cbioportal.org), we explored the
relationships between MACC1 and key enzymes in lipid metabolism and
found that the expression of MACC1 is positively associated with
fatty acid synthase (FASN) in GC.
The multi-enzyme protein FASN is a key enzyme in
lipid metabolism, whose expression has great potential in helping
to understand disorders of lipid metabolism, which is of great
importance in GC progression (13,14).
In previous studies, we found that FASN was highly expressed in GC
tissues and that the overexpression of FASN was associated with the
poor prognosis of GC patients (15–17).
More importantly, FASN is reported to be a druggable gene and has
contributed to platinum-resistance in several types of tumors
(18–20). However, the probable underlying
mechanisms remain unknown. Therefore, we hypothesized that FASN
inhibition contributes to oxaliplatin resistance in GC and we
further explored the probable mechanisms involved.
Hence, in the present study, we tried to determine
the correlation of MACC1, FASN and oxaliplatin resistance in GC.
For this purpose, IHC, in vivo and in vitro studies
were conducted to clarify the detailed relationship between MACC1
and FASN. Moreover, drug-sensitivity experiments were also
performed to ascertain that MACC1 decreased the chemosensitivity of
GC cells to oxaliplatin through the regulation of FASN
expression.
Materials and methods
Patients and tissue specimens
The present study was approved by the Ethics Review
Board of Nanfang Hospital, Southern Medical University (Guangzhou,
China). The present study was conducted on tissue specimens from
167 patients who had been histologically diagnosed with GC at
Nanfang Hospital from 2005 to 2012 and tumor stage was defined
according to the AJCC Cancer Staging Manual (7th edition, 2010).
Among them, 131 stage I–III patients received radical resection
(19, 49 and 63 for stages I, II and III, respectively), and 36
stage IV patients (with metastasis in distant organs) underwent
palliative surgery and/or chemotherapy. Postoperative follow-up
ranging from 0.5 to 80.0 months was obtained for all patients.
Twelve paired tumors and corresponding normal gastric mucosa were
rapidly removed at surgery and stored at −80̊C in a freezer for RNA
or protein extraction.
Cell culture and transfection
assays
Human gastric adenocarcinoma poorly differentiated
BGC-823 and well-differentiated MKN-28 cell lines were obtained
from Foleibao Biotechnology Development Co. (Shanghai, China).
Stably-transfected MACC1-overexpressing and silenced cell lines
were established and cultured as described in our previous
published study (16). Transient
transfection of FASN-specific small interfering RNAs (siRNAs)
(RiboBio Co., Guangzhou, China) was performed using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA) on BGC-823 and MKN-28 cell
lines. A non-specific scrambled siRNA (Ctrl) was used as a negative
control. The silencing sequences of FASN-siRNAs are listed in
Table I.
 | Table I.FASN-siRNA silencing sequences. |
Table I.
FASN-siRNA silencing sequences.
| siRNA1 | F |
GGACCUGUCUAGGUUUGAUdTdT |
|
| R |
dTdTCCUGGACAGAUCCAAACUA |
| siRNA2 | F |
UGGAGCGUAUCUGUGAGAAdTdT |
|
| R |
dTdTACCUCGCAUAGACACUCUU |
| siRNA3 | F |
CCUGCGUGGCCUUUGAAAUdTdT |
|
| R |
dTdTGGACGCACCGGAAACUUUA |
MTT and EdU assays
The effects of siFASN and FASN inhibitor C75 on cell
proliferation were determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and EdU incorporation assays. Briefly, GC cells were seeded in a
96-well plate 1 day before treatment with 10 µg/ml C75 for 24 h or
50 nM siFASN for 48 h. After incubation, MTT solution [5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well in an
amount equal to 10% of the culture medium volume. Cells were then
incubated for 4 h at 37̊C, and the absorbance was evaluated at 570
nm using a microplate spectrophotometer (Thermo Scientific,
Franklin, MA, USA). Each assay was performed in triplicate. All
experiments were repeated three times. In addition, EdU
incorporation assay was conducted using the EdU assay kit (RiboBio
Co.) according to the manufacturer's instructions. Cells were
incubated with 50 nM of EdU for an additional 2 h at 37̊C after
treatment. Cells were fixed with 4% formaldehyde for 15 min at room
temperature and treated with 0.5% Triton X-100 for 20 min at room
temperature to permeate cell membranes. After being washed with PBS
three times, the cells were incubated with 1X Apollo reaction
cocktail (100 µl/well) for 30 min. DNA was stained with 10 µg/ml of
Hoechst 33342 stain (100 µl/well) for 20 min and images were
visualized with fluorescence microscopy. Five fields of view were
randomly selected for each sample. EdU-positive cells were stained
with red dye, and the relative proliferation-positive ratios were
calculated from the average cell count of the five visual
fields.
Quantitative real-time PCR
GC tissue specimens for quantitative real-time PCR
(qRT-PCR) analysis were obtained from 20 patients, who underwent
surgery from November-December 2011. None of these patients had
received chemotherapy prior to surgery. Primer sequences involved
in the present study are summarized in Table II. A TRIzol kit (Life Science,
Carlsbad, CA, USA) for total RNA extraction and a reverse
transcriptase kit (Roche, Penzberg, Germany) for cDNA synthesis
were used according to the manufacturers protocols. qRT-PCR was
performed using SYBR-Green I Master kit (Roche) on a LightCycler
480 system.
 | Table II.Primer sequences for quantitative
real-time PCR. |
Table II.
Primer sequences for quantitative
real-time PCR.
| Gene name |
| Primer sequences (5
to 3) | Primer
length(bps) |
|---|
| MACC1 | F |
GGCTGTGATGCTACGAGATA | 20 |
|
| R |
ACACCAGGACAATGCCTACT | 20 |
| FASN | F |
CGACAGCACCAGCTTCGCCA | 20 |
|
| R |
CACGCTGGCCTGCAGCTTCT | 20 |
| ACC | F |
TCAAACTGCAGGTATCCCAAC | 20 |
|
| R |
ATTTTCCTGCCAGTCCACAC | 20 |
| ACLY | F |
GAAGGGAGTGACCATCATCG | 20 |
|
| R |
TTAAAGCACCCAGGCTTGAT | 20 |
| GAPDH | F |
ACTTCAACAGCGACACCCACTC | 22 |
|
| R |
TACCAGGAAATGAGCTTGACAAAG | 24 |
Western blotting
Cell lysates were first loaded onto SDS-PAGE for
electrophoresis, then, transferred to polyvinylidene difluoride
membranes. The membranes were blocked with 5% non-fat milk for 1 h,
and probed with anti-FASN (Cell Signaling Technology, Danvers, MA,
USA) and anti-GAPDH (Novus, St. Charles, MO, USA) antibodies at 4̊C
overnight. Membranes were washed in Tris-Tween buffer saline, and
incubated with horseradish peroxide-conjugated secondary antibodies
(Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) at
room temperature for 1 h. The enhanced chemiluminescence method
with a western blotting detection system (Kodak Digital Science,
Rochester, NY, USA) was used. Image software Quantity One v4.6.2
was used for the intuitionistic and quantitative analysis.
Immunohistochemistry
Immunohistochemical staining was carried out
according to the Dako EnVision System (Dako, Glostrup, Denmark).
The sections were deparaffinized, rehydrated in serially graded
ethanol, and heated 5 min in citric buffer (pH 6.0) once for
antigen retrieval. They were then washed with distilled water,
blocked with 3% hydrogen peroxide and incubated overnight with the
primary antibodies including MACC1 (Abcam, Cambridge, UK), FASN
(Cell Signaling Technology, Inc., Danvers, MA, USA) at 4̊C. After
being washed with a 0.01 M concentration of PBS, a secondary
antibody solution (biotinylated antibody solution; Dako) was added
for 1 h at room temperature. Subsequently, the sections were
washed, stained with 3,3′-diaminobenzidine (DAB) chromogen (Dako)
and counterstained with hematoxylin (17). Target protein expression levels in
tumor tissues were scored using a semi-quantitative method as
previously described with modifications.
Animals and establishment of
subcutaneous tumor models
Four-week-old male athymic BALB/c nude mice were
obtained from Sun Yat-Sen University (Guangzhou, China). All animal
experiments followed the ethical guidelines formulated by the
Institute of Experimental Animal of Southern Medical University.
Mice were housed in specific pathogen-free conditions with standard
laboratory food for mice and water. BGC823 GC cells
(1×107) in 0.3 ml of PBS were subcutaneously injected
into the left and right posterior flank regions of each mouse, and
tumor growth was assessed every three days using calipers. Mice
were randomly chosen and assigned to two groups (five mice/group)
according to different expression levels of MACC1 (oxMACC1 and the
control group, shMACC1 and the control group). After 28 days, mice
were sacrificed and the tumor volumes were determined in accordance
with the formula: V = a×b×(a + b)/2, where ‘a’ is length and ‘b’ is
width of the tumor, respectively.
Statistical analysis
Statistical calculations were performed using SPSS
20.0 software (version 20.0; SPSS, Inc., Chicago, IL, USA).
Survival analysis was performed from the date of surgery to the
time of diagnosis of recurrence or death using the Kaplan-Meier
method. The relationship between MACC1 and FASN was analyzed by
statistical linear regression. The significant differences were
calculated using Student's t-test for continuous variables. Results
of the MTT assay were analyzed by repeated measures. The
quantitative data are expressed as the mean of three independent
experiments. P-values <0.05 were defined as statistically
significant.
Results
MACC1 and FASN are positively
correlated and responsible for the poor prognoses in GC
patients
We demonstrated that MACC1 was overexpressed in GC
and was related to glycometabolism in previous studies (4,5). In
the present study, we focused on the relevance between MACC1 and
tumor lipid metabolism. We commenced a systematic research on the
relationship between MACC1 and three key enzymes in lipid
metabolism, namely Acetyl-CoA carboxylase (ACC), ATP-citrate lysase
(ACLY) and FASN. First, bioinformatics website http://www.cbioportal.org was employed to explore
whether there was a correlation between MACC1 and these three key
enzymes. A marked correlation was found between MACC1, ACLY and
FASN in GC, with the correlation coefficients listed in Table III.
 | Table III.Correlation between MACC1 and three
key enzymes of lipogenesis. |
Table III.
Correlation between MACC1 and three
key enzymes of lipogenesis.
| Gene symbol | Pearson score | Spearman score |
|---|
| ACC | – | – |
| ACLY | 0.21 | 0.32 |
| FASN | 0.09 | 0.21 |
To confirm the reliability of the data, we collected
GC tissues from 9 clinical cases and the total RNA was extracted.
The gene expression of MACC1 and these three key enzymes of
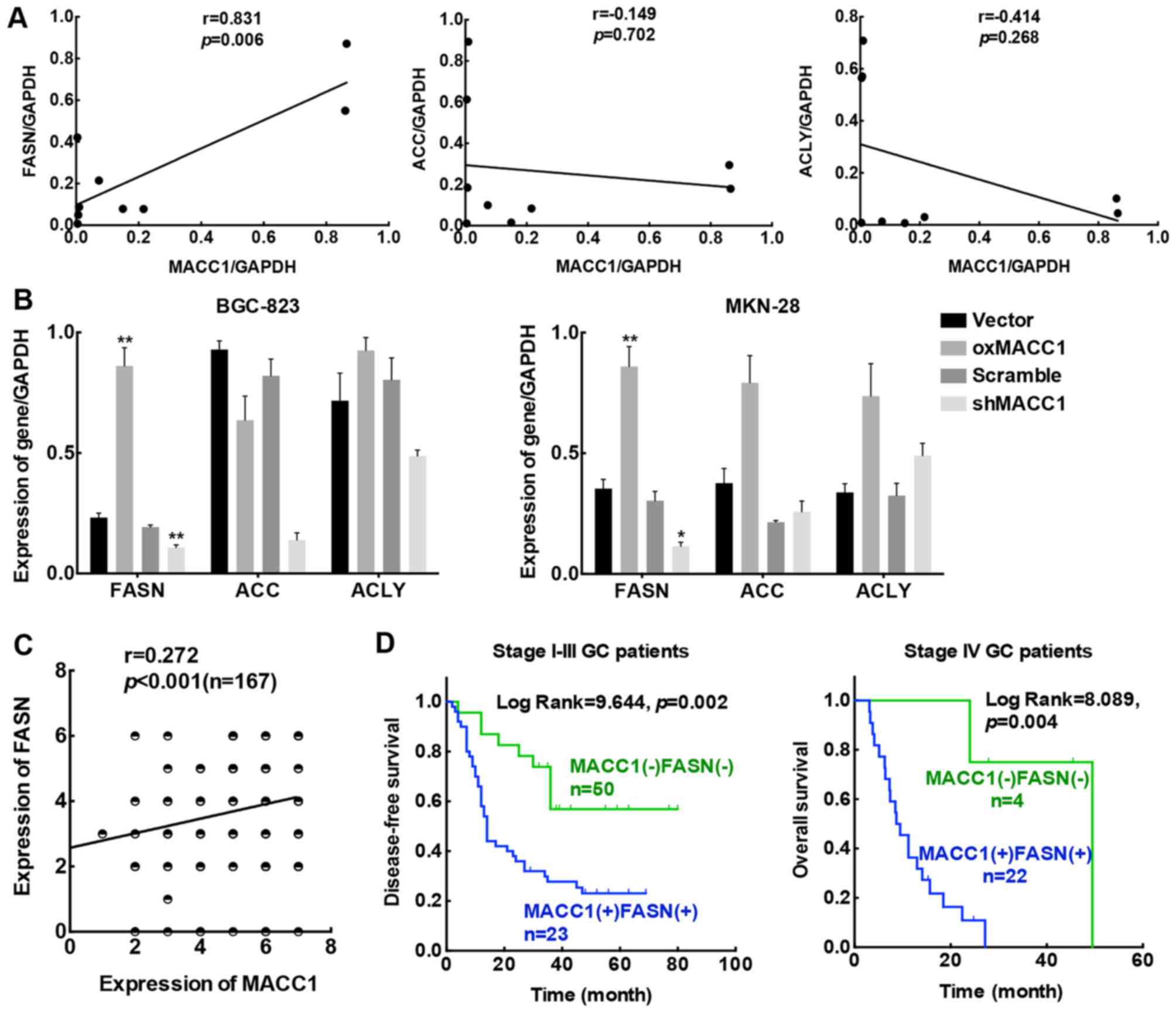
lipogenesis were synchronously analyzed. As indicated in Fig. 1A, the expression of MACC1 and FASN
was significantly correlated (r=0.831, p=0.003), while no
significant correlation was found between MACC1 and ACC (r=−0.149,
p=0.351) or ACLY (r=−0.414, p=0.134). Based on these results, we
suggested that MACC1 may be correlated with FASN, one of the key
enzymes of lipogenesis. Next, we conducted qRT-PCR to assess the
mRNA levels of these three enzymes in the MACC1 stably-transfected
GC cell lines. According to our results, overexpression of MACC1
(oxMACC1) stimulated FASN gene expression efficiently compared to
its negative control (vector), while knockdown of MACC1 (shMACC1)
reversed this tendency when compared to its control cell line
(scramble) (Fig. 1B). Based on the
results obtained, we considered that among these three key enzymes
involved in the process of de novo fatty acid synthesis,
FASN was the main enzyme that had a positive correlation with MACC1
in GC.
 | Figure 1.MACC1 and FASN are positively
correlated and responsible for poor prognoses in GC. (A) The
correlation between MACC1 and three key enzymes of lipogenesis,
ACC, ACLY and FASN in 9 clinical specimens. (B) Gene expression
levels of ACC, ACLY and FASN in MACC1 stably-transfected GC cells.
Data are the results obtained from triple independent experiments
and are expressed as the mean ± SD. (C) Linear regression analysis
of MACC1 and FASN protein expression in 167 GC patient tissues. (D)
Kaplan-Meier survival plots of DFS for stage I–III and OS for stage
IV patients according to MACC1 and FASN expression; *p<0.05,
**p<0.01. MACC1, metastasis-associated in colon cancer 1; FASN,
fatty acid synthase; GC, gastric cancer; ACC, acetyl-CoA
carboxylase; ACLY, ATP-citrate lysase; DFS, disease-free survival;
OS, overall survival. |
Statistical analysis was then conducted based on the
immunohistochemical staining of the protein levels of MACC1 and
FASN in the 167 cases of GC patients. Linear-regression analysis
indicated a marked correlation between MACC1 and FASN protein
expression (p<0.001; Fig. 1C).
In addition, Kaplan-Meier survival analysis revealed a shorter
disease-free survival (DFS) for stage I–III and overall survival
(OS) for stage IV patients with high expression of both MACC1 and
FASN than those with low expression of MACC1 and FASN (Fig. 1D). Thus, we ascertained that the
expression of MACC1 was positively correlated with FASN in GC
tissues. Moreover, co-expression of MACC1 and FASN predicted a poor
prognosis for GC patients. These findings highly suggest that MACC1
contributes to GC progression partly through abnormal tumor lipid
metabolism.
MACC1 positively regulates FASN
expression in vitro and in vivo
According to the clinical data, we concluded that
the expression of MACC1 and FASN were closely correlated at both
mRNA and protein levels. Therefore, we next investigated their
relationship both in vitro and in vivo. To begin
with, we extracted the total protein of two MACC1
stably-transfected GC cell lines (vector, oxMACC1, scramble and
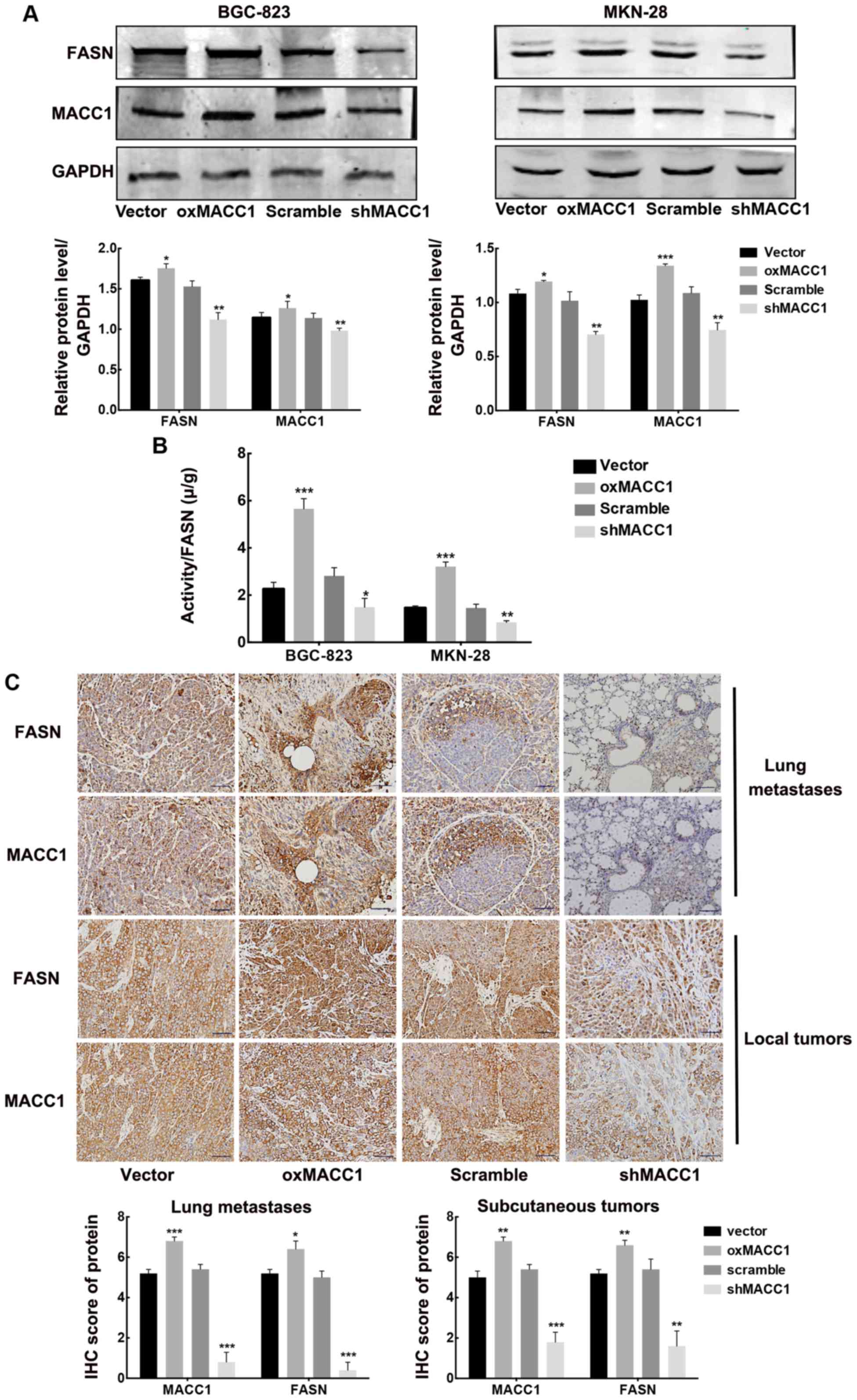
shMACC1 for BGC-823 and MKN-28 cell lines). Western blotting
results revealed that the protein levels of FASN were significantly
increased in the oxMACC1 group and concurrently downregulated after
silencing MACC1 (Fig. 2A).
Considering that after stable-transfection of MACC1,
FASN protein expression was altered, we next detected whether the
enzyme activity of FASN was concomitantly changed. We found that
MACC1 overexpression markedly enhanced the activity of FASN, while
silencing of MACC1 significantly downregulated the enzyme activity,
respectively (Fig. 2B). These
results demonstrated that interference with MACC1 could not only
alter the expression of FASN in both gene and protein levels, but
also modulate its enzyme activity.
Finally, we established xenograft gastric tumor
models by subcutaneously injecting MACC1 stably-transfected GC
BGC-823 cells into nude mice. After four weeks when xenograft
tumors formed, the local tumor and lung metastatic lesions were
both utilized for IHC staining. We found that in both xenograft and
metastatic tissues, tumors from the groups injected with the
oxMACC1 cells exhibited high expression of FASN, while the tumors
derived from the shMACC1 cells exhibited significantly low
expression of FASN, compared to the control groups, respectively
(Fig. 2C). We demonstrated that
MACC1 upregulated FASN expression in vitro and in
vivo at both the gene and protein levels.
FASN inhibition attenuates proliferation in GC cell
lines without MACC1 variation. The foregoing experiments indicated
that MACC1-overexpressing BGC-823 and MKN-28 cells had upregulated
FASN expression at both the mRNA and protein levels. Therefore, we
introduced a synthesized FASN inhibitor (C75) and siRNA separately
to suppress the expression of FASN in the MACC1 stably-transfected
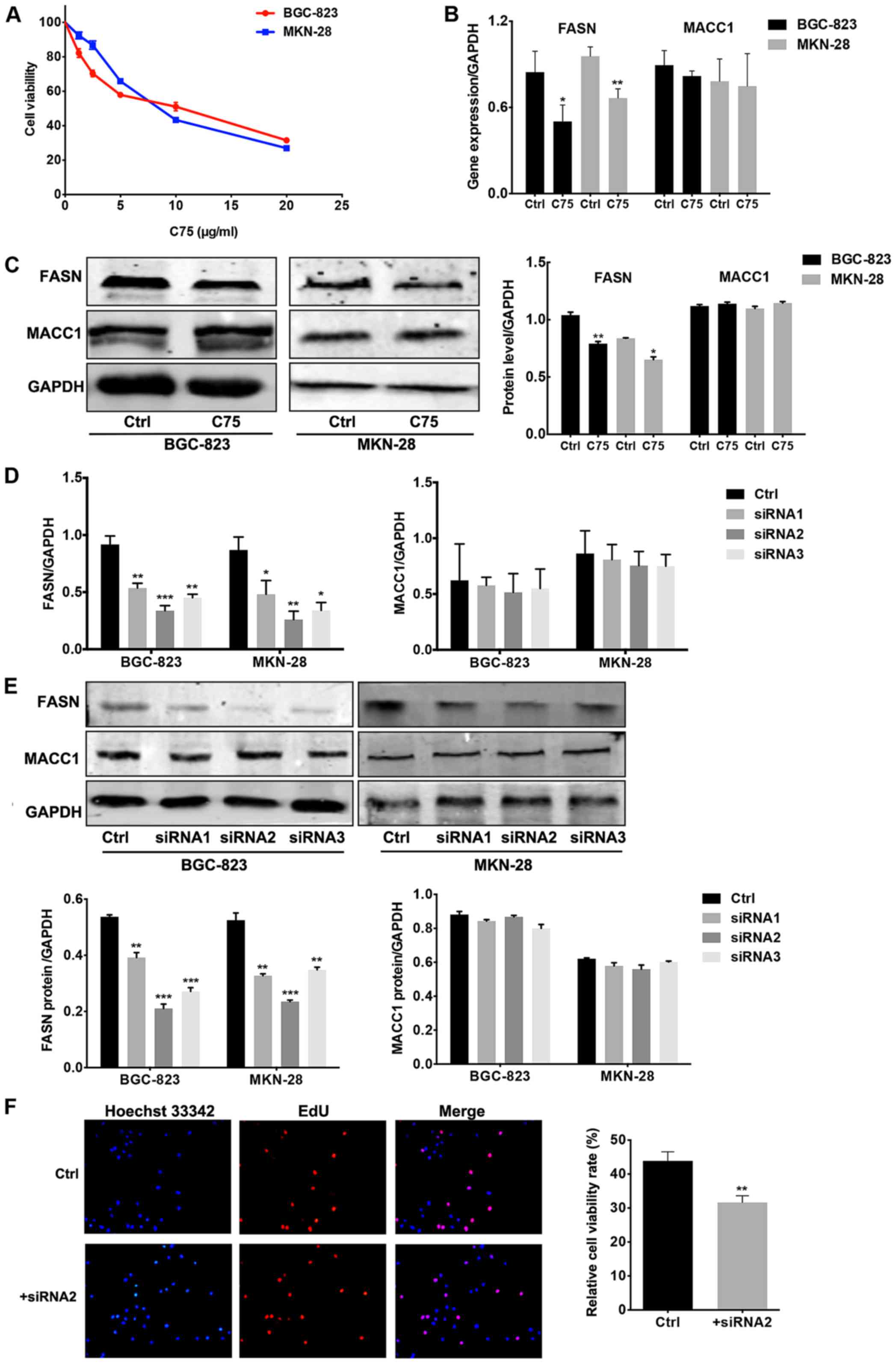
cell lines. First of all, the optimal concentration of C75 in the
GC cell lines was determined using an MTT assay. The results
indicated a marked decrease in cell viability as C75 concentration
increased (Fig. 3A). Next, C75 or
the control blank solvent were added into the cell culture medium
of wild-type GC cells for a 24-h incubation, after which cells were
collected for qRT-PCR and western blotting. In both wild-type
BGC-823 and MKN-28 cells, C75 treatment generated a significant
decrease in FASN mRNA levels compared to the control group
(p<0.05 for BGC-823; p<0.01 for MKN-28). The inhibitory rate
for FASN was 58.8 and 69.8%, respectively, but no difference in
MACC1 mRNA expression was observed in both groups (p>0.05;
Fig. 3B). Similarly, FASN
expression was significantly suppressed by C75 at the protein level
in GC cells, while expression of MACC1 was not significantly
decreased by the FASN inhibitor (Fig.
3C). These results suggested that MACC1, working as the
upstream molecule, regulated the expression of FASN.
The aforementioned results indicated that exogenous
blockade of FASN had no influence on MACC1 expression. For a more
thorough and comprehensive elucidation, siRNA was introduced.
FASN-siRNA was transfected into GC cells using Lipofectamine 2000
to suppress FASN expression endogenously. BGC-823 and MKN-28 cells
were both Transit-transfected with FASN-siRNA1, siRNA2, siRNA3 and
control siRNA at the optimal concentration following the protocol
instructions. After a 48-h transfection, cells were collected for
qRT-PCR and western blotting to analyze the perturbation efficiency
of FASN and the expression status of both FASN and MACC1. The
expression of FASN was significantly downregulated by siRNAs in the
poorly differentiated BGC-823 cells and the well-differentiated
MKN-28 cells at both the mRNA and protein levels (Fig. 3D and E). We compared the effect of
the three FASN-siRNAs with different sequences on FASN blockade at
both the gene and protein levels in BGC-823 and MKN-28 cells and
selected the most effective sequence FASN-siRNA2 for follow-up
function experiments.
In addition, we further explored the detailed
function of FASN in GC progression. Endogenous FASN in poorly
differentiated BGC-823 cells was blocked through FASN-siRNA
transfection. An EdU proliferation assay was conducted to explore
cell proliferation activity. As expected, the cellular fluorescent
images clearly revealed decreased cell proliferation with FASN
inhibition. The quantitative analysis indicated a significant
difference (p<0.01; Fig. 3F).
These results provide evidence that FASN inhibition attenuates
proliferation in GC cell lines without MACC1 variation.
Overexpression of MACC1 attenuates
chemosensitivity, while silencing of FASN restores drug sensitivity
to oxaliplatin in GC
As numerous studies have confirmed that MACC1 and
FASN are both druggable targets, which contribute to
platinum-resistance in several tumors (10,24,25),
the present experiment explored whether MACC1 and FASN affect the
chemosensitivity of GC cells to oxaliplatin. We first studied the
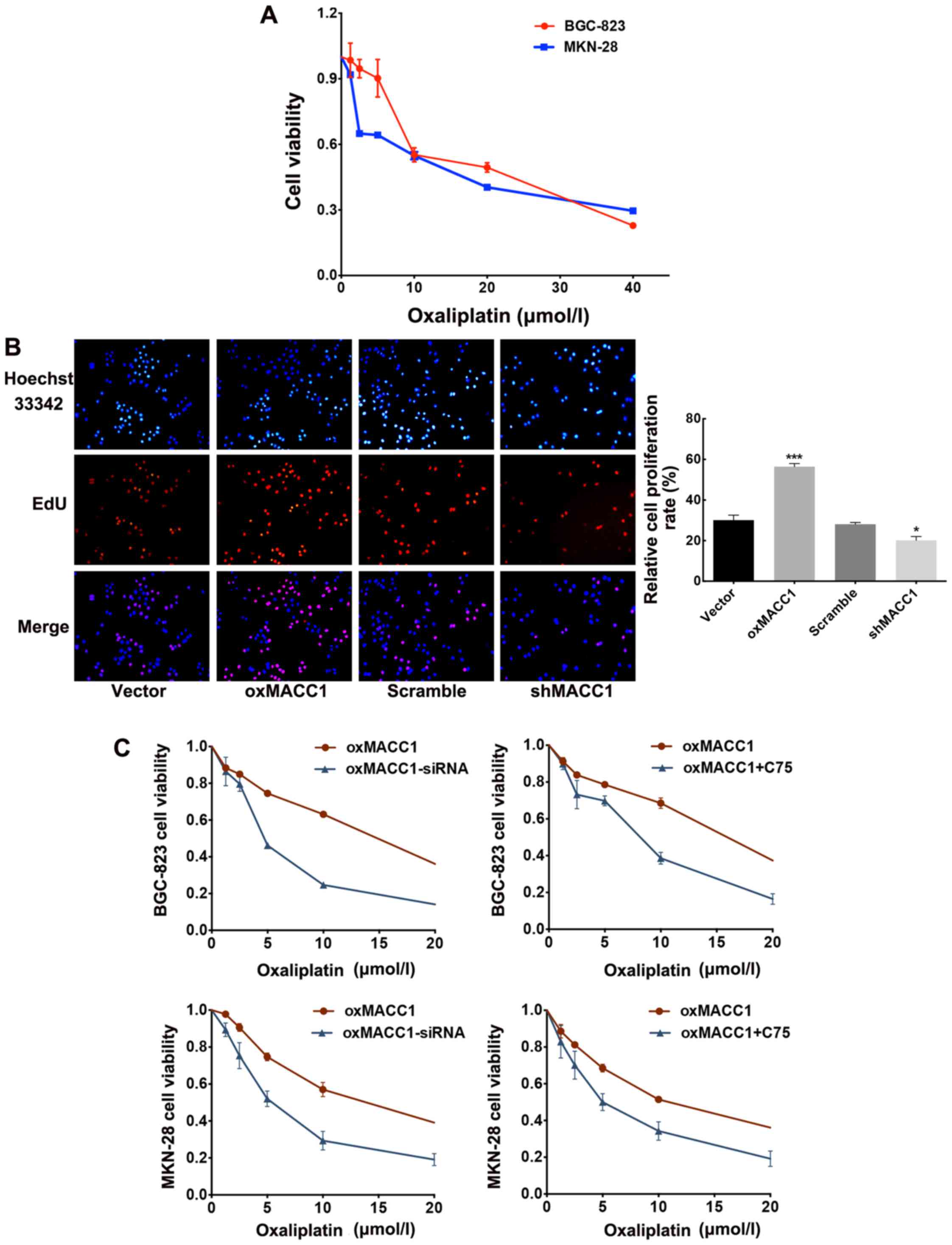
inhibitory effect of oxaliplatin on wild-type GC cells. A gradient
of six concentrations (0, 1.25, 2.5, 5, 10 and 20 µmol/l) of
oxaliplatin was employed separately to stimulate BGC-823 and MKN-28
cells for 24 h and the resulting cell viability was assessed with
an MTT assay (Fig. 4A). We noted
that in both cell lines, cell viability was decreased as drug
dosage increased. A high concentration of oxaliplatin had a more
obvious inhibitory effect on cell proliferation and cytotoxicity
increased as well. Therefore, we calculated the ID50
value of oxaliplatin for both cell lines and decided that the
optimal concentration for subsequent experiments was 10 µmol/l.
Ensuingly, we employed EdU cell proliferation assay
on MACC1 stably-transfected cells after oxaliplatin incubation (10
µmol/l) for 24 h. Cellular fluorescence intuitively revealed higher
proliferation activity in the MACC1-overexpressing cells than that
noted in the control vector group (p<0.001; Fig. 4B); conversely, shMACC1 and its
control scramble cells demonstrated a reverse tendency, which
exhibited decreased proliferation activity in the shMACC1 cells
compared with the scramble group (p<0.005).
The aforementioned results suggested that MACC1
suppressed the drug-sensitivity of GC cells to oxaliplatin. We
further explored the probable underlying mechanism in the following
experiment. With this in mind, we assumed that MACC1 regulated the
drug sensitivity of GC cells to oxaliplatin through the modulation
of the key enzyme of lipogenesis FASN. To confirm our conjecture,
we treated MACC1-overexpressing cells with either C75 or FASN-siRNA
for 24 h to suppress the expression of FASN. Then, an MTT assay was
performed to assess the inhibition efficacy of oxaliplatin on cell
proliferation. We found that after inhibition of FASN by exogenous
artificially synthesized compound C75, oxaliplatin slowed cell
proliferation more efficiently than the untreated control group.
Furthermore, we introduced FASN-siRNA in the MACC1-overexpressing
GC cells to block endogenous FASN expression at the genetic level.
The same result was obtained which indicated an enhanced
sensitivity to oxaliplatin and a decreased proliferation ability of
MACC1-overexpressing cells after FASN blockade (Fig. 4C). In general, the aforementioned
experiments ascertained our previous hypothesis that upregulation
of FASN by MACC1 attenuated drug sensitivity of GC cells to
oxaliplatin.
Discussion
Tumor cells are commonly characterized by high
glucose uptake and consumption ascribed to the low efficiency of
aerobic glycolysis (21), and
lipometabolism is an additional pathway which is crucial in
sustainable energy supplementation for its rapid proliferation.
Within the organism, a set of precise lipometabolic networks
regulate the process of lipid biosynthesis and lipolysis (22), which ensure the normal structure and
function of cells. Multiple studies have shown that lipometabolic
disorders are associated with the occurrence and development of
many diseases including tumors (23). Our preliminary study revealed that
MACC1 is a critical protein related to GC cell metabolism, which
mediates GC glucose metabolism, neovascularization and lymph
angiogenesis (4,24). However, to date there has been no
investigation on the role of MACC1 in GC cell lipometabolism. Using
bioinformatic analysis from cBio Cancer Genomics Portal (http://www.cbioportal.org), we found that the
expression of MACC1 is closely associated with key enzymes of
lipometabolism (ACLY and FASN) in GC, highly suggesting that MACC1
is associated with lipid metabolism. Notably, confirmation of this
theory in GC cells and tissues using qRT-PCR demonstrated that only
FASN expression is correlated to MACC1. Therefore, we focused on
FASN and MACC1 in the following experiment.
FASN is a key enzyme in lipid metabolism, whose
expression has great potential in helping to understand
lipidmetabolism disorders (25,26).
In the present study, we generally clarified the relationship
between FASN and MACC1 in vivo and in vitro. We first
confirmed that MACC1 was positively associated with FASN both at
the mRNA and protein levels. More importantly, we found that MACC1
could regulate the expression and enzyme activity of FASN, while
FASN inhibition could not alter the expression of MACC1. This
successfully confirmed that MACC1 was the upstream regulator of
FASN.
As aforementioned, chemoresistance is the main cause
for the poor prognosis in GC (27).
In the present study, we found that MACC1 overexpression was
correlated with a poorer prognosis in GC patients who received
oxaliplatin treatment, highly indicating that MACC1 works as a
druggable gene and contributes to oxaliplatin resistance in GC. In
fact, this is not the first time MACC1 has been found to play a
role in drug resistance. It was reported that MACC1 overexpression
induced cisplatinum resistance in ovarian cancer (28,29).
Therefore, it is possible and reasonable that MACC1 contributes to
oxaliplatin resistance in GC. We further explored the detailed
mechanism and found that FASN participated in MACC1-induced
oxaliplatin resistance.
FASN is also considered to be a druggable gene, and
FASN overexpression is correlated with tumor drug-resistance, which
can partially explain the relationship between FASN and poor
prognosis. In the present study, an siRNA targeted to FASN or
semi-synthetic inhibitor C75, were employed to clarify the role of
FASN in MACC1-induced oxaliplatin resistance. We found that both
siRNA and C75 significantly reversed the downregulation of
chemosensitivity to oxaliplatin induced by MACC1 overexpression,
greatly indicating that FASN was the key link in MACC1-induced
oxaliplatin resistance and inhibition of FASN may be a therapeutic
strategy for oxaliplatin resistance.
A previous study utilized gene expression profile
microarray analysis and found that pro-apoptotic genes and proteins
were increased in FASN-knockout breast cancer cells (30). It has been considered that knockout
of FASN plays a dominant role in the upregulation of ceramide
levels and consequently upregulates the expression of these
pro-apoptotic genes. However, the concrete underlying mechanism
remains unclear. Liu et al found that FASN overexpression in
breast cancer could suppress doxorubicin-induced cell apoptosis and
caspase-8 activation (10). The
present study contends that apoptosis or DNA-damage are among the
main mechanisms of FASN-mediated drug-resistance. However, whether
MACC1-facilitated oxaliplatin resistance of GC is associated with
those factors requires further investigation. Undeniably, MACC1 had
an impact on GC chemosensitivity to oxaliplatin through interaction
with FASN.
In conclusion, our study explored lipometabolism,
confirmed the relationship between MACC1 and the key enzyme of
lipogenesis, FASN, and further elucidated the role of MACC1 in
cancer metabolism. In combination with clinical data, we revealed
for the first time the role of MACC1 in the chemosensitivity of
oxaliplatin, confirmed that silencing of MACC1 improved the
chemosensitivity of GC cells to oxaliplatin by downregulation of
FASN, and highly suggest that targeting FASN may be a new
therapeutic strategy for the reversal of
oxaliplatin-resistance.
Acknowledgements
The present study was funded by grants from the
National Natural Science Foundation of China (81502116 to L.S.),
grants from the Scientific and Technological Projects of Nanshan
District, Shenzhen (2015011 to J.-M.D.), the Team Program of
Natural Science Foundation of Guangdong Province, China (no.
S2011030003134 to W.-J.L.), the Team Program of Natural Science
Foundation of Guangdong Province, China (2015A030310085 to L.S.),
and the Key Clinical Specialty Discipline Construction Program of
China (to the Department of Oncology, Nanfang Hospital).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peinert S, Grothe W, Stein A, Müller LP,
Ruessel J, Voigt W, Schmoll HJ and Arnold D: Safety and efficacy of
weekly 5-fluorouracil/folinic acid/oxaliplatin/irinotecan in the
first-line treatment of gastrointestinal cancer. Ther Adv Med
Oncol. 2:161–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein U, Walther W, Arlt F, Schwabe H,
Smith J, Fichtner I, Birchmeier W and Schlag PM: MACC1, a newly
identified key regulator of HGF-MET signaling, predicts colon
cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin L, Huang H and Liao W, Ma H, Liu J,
Wang L, Huang N, Liao Y and Liao W: MACC1 supports human gastric
cancer growth under metabolic stress by enhancing the Warburg
effect. Oncogene. 34:2700–2710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Wu Y, Lin L, Liu P, Huang H, Liao
W, Zheng D, Zuo Q, Sun L, Huang N, et al: Metastasis-associated in
colon cancer-1 upregulation predicts a poor prognosis of gastric
cancer, and promotes tumor cell proliferation and invasion. Int J
Cancer. 133:1419–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Djiogue S, Kamdje Nwabo AH, Vecchio L,
Kipanyula MJ, Farahna M, Aldebasi Y and Seke Etet PF: Insulin
resistance and cancer: The role of insulin and insulin-like growth
factors. Endocr Relat Cancer. 20:R1–R17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiller K and Metallo CM: Profiling
metabolic networks to study cancer metabolism. Curr Opin
Biotechnol. 24:60–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamada M, Nagano O, Tateyama S, Ohmura M,
Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, et
al: Modulation of glucose metabolism by CD44 contributes to
antioxidant status and drug resistance in cancer cells. Cancer Res.
72:1438–1448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y and Yang JM: Altered energy
metabolism in cancer: A unique opportunity for therapeutic
intervention. Cancer Biol Ther. 14:81–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Wu X, Dong Z, Luo Z, Zhao Z, Xu Y
and Zhang JT: Fatty acid synthase causes drug resistance by
inhibiting TNF-α and ceramide production. J Lipid Res. 54:776–785.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider
M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L, et al: Role of
fatty acid synthase in gemcitabine and radiation resistance of
pancreatic cancers. Int J Biochem Mol Biol. 2:89–98.
2011.PubMed/NCBI
|
|
12
|
Wangpaichitr M, Sullivan EJ,
Theodoropoulos G, Wu C, You M, Feun LG, Lampidis TJ, Kuo MT and
Savaraj N: The relationship of thioredoxin-1 and cisplatin
resistance: Its impact on ROS and oxidative metabolism in lung
cancer cells. Mol Cancer Ther. 11:604–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuhajda FP: Fatty-acid synthase and human
cancer: New perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang HG, Hao J, Zhang WJ, Lu WJ, Dong P,
Liu YB and Chen L: Expression of fatty acid synthase negatively
correlates with PTEN and predicts peritoneal dissemination of human
gastric cancer. Asian Pac J Cancer Prev. 16:6851–6855. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashimoto T, Kusakabe T, Sugino T, Fukuda
T, Watanabe K, Sato Y, Nashimoto A, Honma K, Kimura H, Fujii H, et
al: Expression of heart-type fatty acid-binding protein in human
gastric carcinoma and its association with tumor aggressiveness,
metastasis and poor prognosis. Pathobiology. 71:267–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan J, Sun L, Huang H, Wu Z, Wang L and
Liao W: Overexpression of fatty acid synthase predicts a poor
prognosis for human gastric cancer. Mol Med Rep. 13:3027–3035.
2016.PubMed/NCBI
|
|
18
|
Shiragami R, Murata S, Kosugi C, Tezuka T,
Yamazaki M, Hirano A, Yoshimura Y, Suzuki M, Shuto K and Koda K:
Enhanced antitumor activity of cerulenin combined with oxaliplatin
in human colon cancer cells. Int J Oncol. 43:431–438.
2013.PubMed/NCBI
|
|
19
|
Liu H, Liu Y and Zhang JT: A new mechanism
of drug resistance in breast cancer cells: Fatty acid synthase
overexpression-mediated palmitate overproduction. Mol Cancer Ther.
7:263–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauerschlag DO, Maass N, Leonhardt P,
Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba
R, Meinhold-Heerlein I, et al: Fatty acid synthase overexpression:
Target for therapy and reversal of chemoresistance in ovarian
cancer. J Transl Med. 13:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menendez JA: Fine-tuning the
lipogenic/lipolytic balance to optimize the metabolic requirements
of cancer cell growth: Molecular mechanisms and therapeutic
perspectives. Biochim Biophys Acta. 1801:381–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furuta E, Okuda H, Kobayashi A and Watabe
K: Metabolic genes in cancer: Their roles in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.PubMed/NCBI
|
|
24
|
Sun L, Duan J, Jiang Y, Wang L, Huang N,
Lin L, Liao Y and Liao W: Metastasis-associated in colon cancer-1
upregulates vascular endothelial growth factor-C/D to promote
lymphangiogenesis in human gastric cancer. Cancer Lett.
357:242–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hopperton KE, Duncan RE, Bazinet RP and
Archer MC: Fatty acid synthase plays a role in cancer metabolism
beyond providing fatty acids for phospholipid synthesis or
sustaining elevations in glycolytic activity. Exp Cell Res.
320:302–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang L, Wu P, Senthilkumar R, Tian X, Liu
H, Shen X, Tao Z and Huang P: Loss of fatty acid synthase
suppresses the malignant phenotype of colorectal cancer cells by
down-regulating energy metabolism and mTOR signaling pathway. J
Cancer Res Clin Oncol. 142:59–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang D and Fan D: New insights into the
mechanisms of gastric cancer multidrug resistance and future
perspectives. Future Oncol. 6:527–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen ZM, Shi HR, Li X, Deng YX and Zhang
RT: Downregulation of MACC1 expression enhances cisplatin
sensitivity in SKOV-3/DDP cells. Genet Mol Res. 14:17134–17144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang R, Shi H, Ren F, Li X, Zhang M, Feng
W and Jia Y: Knockdown of MACC1 expression increases cisplatin
sensitivity in cisplatin-resistant epithelial ovarian cancer cells.
Oncol Rep. 35:2466–2472. 2016.PubMed/NCBI
|
|
30
|
Hilvo M, Denkert C, Lehtinen L, Müller B,
Brockmöller S, Seppänen-Laakso T, Budczies J, Bucher E, Yetukuri L,
Castillo S, et al: Novel theranostic opportunities offered by
characterization of altered membrane lipid metabolism in breast
cancer progression. Cancer Res. 71:3236–3245. 2011. View Article : Google Scholar : PubMed/NCBI
|