Introduction
Lung cancer is the most common cancer worldwide, and
more than 1.8 million new cases and almost 1.6 million deaths were
estimated in 2012, with most of these cases (80–85%) categorized as
non-small cell lung cancer (NSCLC) (1). In China, lung cancer is the most
frequently diagnosed cancer and the leading cause of cancer-related
death (2). NSCLC treatments include
surgery, chemotherapy, and radiation therapy, which are determined
by the type and stage of cancer. Surgery is the only curative
treatment of NSCLC; however, less than 20% of NSCLC patients who
were not diagnosed at advanced stages of the disease are
potentially curable with surgical resection (3). Numerous clinical studies have
confirmed the effectiveness of chemotherapy combined with external
beam irradiation (EBRT) in the treatment of patients with NSCLC
presenting with locally advanced malignancy (4–7). To
our disappointment, they have not greatly affected outcomes, and
the gain often comes with substantial severe toxicity
(myelosuppression, nausea, vomiting and radiation pneumonitis),
especially affecting important organs and tissues (heart,
esophagus, and large blood vessels) (8,9). A
great number of patients with advanced NSCLC also cannot tolerate
the currently available treatment modalities mainly owing to their
poor general condition, especially with other complication.
Therefore, novel therapeutic approaches are urgently needed to
effectively prolong survival time and obviously improve the quality
of life in advanced patients.
In recent years, 125I implantation has been widely
used to treat advanced and inoperable prostate cancer, lung cancer,
pancreatic cancer, colorectal cancer and head and neck cancer
because of accurate positioning, little trauma, high doses in the
target volume, few normal tissues exposed and minimal complications
(10–14). The advantages of 125I have led to
its widespread application in China (11,15).
Although many clinical trials have reported that
125I seed radiation is a feasible adjuvant treatment to control
local symptoms and prolong survival for advanced or unresectable
NSCLC (16,17), its biological effects and underlying
molecular mechanisms are far from fully understood.
Qu and colleagues (18) demonstrated that a continuous
low-dose rate of irradiation (CLDR) induced stronger growth
inhibition in A549 cells than single EBRT due to the aggravation of
G2/M arrest, and increased apoptosis. Takabayashi and colleagues
(19) demonstrated that 125I seed
irradiation induced apoptosis and suppressing proliferation in
histologically varied gastric cancers to exert antitumor effects.
The tumor microenvironment is often hypoxic, and tumor hypoxia is
associated with formation of new capillaries and resistance to
radiation therapy. HIF (e.g. HIF-1α, HIF-2α, HIF-3α) are activated
in response to hypoxia, which upregulate numerous genes affecting
angiogenesis. VEGF as the best known and the most efficient
angiogenic factor is constitutively activated. It is thus inferred
that the HIF upregulation of VEGF is most likely responsible for
angiogenesis in tumors (20,21).
It is well known that angiogenesis is absolutely required for tumor
growth. Therefore, inhibition of angiogenesis can prevent tumor
growth (22). This study was
designed to investigate 125I-induced cell apoptosis, suppressing
proliferation and angiogenesis and the possible role of HIF-1α and
VEGF in the process of 125I brachytherapy in lung carcinoma.
Herein, we examined its biological effects on adenocarcinoma cells,
the most common pathological type of NSCLC, using xenograft
models.
Materials and methods
Cell line and cell culture
The human lung adenocarcinoma cell line A549 was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The cells were cultured in RPMI-1640 (Gibco,
Carlsbad, CA, USA) medium supplemented with 10% heat-inactivated
fetal bovine serum and 1% pen-strep (100 U/ml penicillin and 100
mg/ml streptomycin) (Gibco) in a 37°C humidified incubator
containing 5% CO2. All experiments were performed using
cells grown to 60–80% confluence. For in vivo injections,
suspensions with >95% viability were used, as determined by
trypan blue exclusion.
Animal model
Female BALB/c nude mice, 4–6 weeks old and weighing
17–20 g, were purchased from Institute of Chinese Academy of
Medical Sciences and allowed to acclimatize for 1 week under
specific pathogen-free conditions (23±2°C and 55±5% humidity) in
the Animal Care Facility before any intervention was initiated. The
mice were housed and maintained under specific pathogen-free
conditions in facilities approved by the Animal Care and Use
Committee of Qingdao University School of Medicine. A549 cells
(5×106) in 0.2 ml PBS were injected subcutaneously into
the right dorsal flank of each mouse. Mice were monitored daily for
tumor development and total body weight, tumor incidence and mass,
were recorded every four days till the end of study. The tumor
volume (V) was calculated by the following formula: V
(mm3)=LxW2x1/2 (L, length of tumor; W, width
of tumor).
125I seeds
The 125I seeds were provided by Invasive Technology
Department of The Affiliated Hospital of Qingdao University, with a
diameter of 0.8 mm, and a length of 4.5 mm. The average energy was
27.4 to 35.5 keV, with a half-life of 59.6 days. After decaying
into the organs, the 125I seeds released continuous low-dose γ-ray
and soft X-ray (5% of 35 keV and 95% of 28 keV, respectively). The
internal irradiation was relatively long-acting, 93–97% of the
brachytherapy dose was delivered within 8–10 months.
125I brachytherapy seed implant
As tumors reached a size of approximately 300
mm3 in about 24 days, mice were randomly divided into 4
groups (n=10/group). Four groups were grouped as follows:
non-implanted control group; sham seed implant group; 125I seed
(0.6 mCi) implant group; and 125I seed (0.8 mCi) implant group.
Before in vivo implantation, each mouse was anesthetized via
inhalation of diethyl ether (provided by Pharmacy Department of the
Affiliated Hospital of Qingdao University). The visible mass in
mice was punctured by the 18-gauge needles of the Mick-applicator
through which seeds were implanted. After 30 days of treatment, all
mice were humanely sacrificed, and tumors were harvested and
weighed, then fixed in 4% paraformaldehyde (PFA) or flash frozen in
liquid nitrogen.
Immunohistochemistry for Ki67 and
CD34
Immunohistochemical procedures of Ki67 and CD34 were
done as described previously by Lee and colleagues (23). In brief, samples of the tumors were
fixed in 4% PFA for 24 h, embedded with paraffin, then sectioned (4
µm) longitudinally and stained with hematoxylin and eosin
(H&E). Following staining cell proliferation and microvessel
density was assessed by quantitative morphometric analysis of Ki67
and CD34 expression, respectively. For detecting CD34 and Ki67
immunoreactivity, tissue sections were deparaffinized with
sequential washing of xylene, rehydrated with descending grades of
ethanol, followed by antigen retrieval and endogenous peroxidase
treatment. After blocking endogenous peroxides and nonspecific
proteins, sections were incubated at 4°C overnight with rabbit
anti-mouse monoclonal anti-Ki67 antibody (Abcam, Cambridge, MA,
USA) at a 1:100 dilution and rabbit anti-mouse monoclonal anti-CD34
antibody (Cell Signaling Technology, Boston, MA, USA) at a 1:85
dilution, respectively. Subsequently, slides were washed three
times in Tris-buffered saline (TBS), and incubated for 20 min at
room temperature with peroxidase-conjugated goat anti-rabbit IgG
secondary antibodies (Abcam). Negative controls were incubated with
PBS instead of the primary antibody. Visualization was achieved
with a diaminobenzidine (DAB) substrate system in which nuclei with
DNA fragmentation or endothelial cells were stained brown.
TUNEL staining
For the detection of apoptosis, tumor specimens were
subjected to a TUNEL assay using the In Situ Cell Death
Detection kit (Roche, Basel, Switzerland), in accordance with the
manufacturer's instructions. Briefly, 4% PFA fixed tissues were
permeabilized with Proteinase K working solution (provided in the
kit) at 37°C for 30 min. The slides were washed twice in PBS and
incubated with 50 µl of TUNEL reaction mixture (provided in the
kit) in a dark and humid atmosphere for 60 min at 37°C, followed by
incubation with Converter-POD solution (provided in the kit) for 30
min at 37°C. Slides were developed using DAB, mounted under glass
coverslip, and then analyzed under a light microscope.
Microscopic quantitative analyses of
Ki67, CD34, and TUNEL
To quantify MVD, 5 random fields at ×400
magnification per slide were examined for each tumor (one slide per
mouse, 5 slides of each treatment group) and microvessel (MV)
counts per field were done by two investigators in a blinded
fashion. A single MV was defined as a cluster clearly separate from
adjacent MVs or single cell stained positive for CD34 with the
presence of a lumen. To quantify Ki67 expression, the number of
Ki67-positive cells and the total number of tumor cells were
counted in 5 random fields at ×400 magnification. Proliferative
index was calculated by the percentage of positive cells to total
tumor cells. For quantification of TUNEL positive cells, the number
of TUNEL-positive and total tumor cells was counted in 5 random
fields at ×400 magnification. The apoptosis index was calculated as
the ratio of TUNEL-positive to total tumor cells. DAB-stained
sections were examined via a BX51T-PHD-J11 microscope (Olympus Co.,
Tokyo, Japan). Images were captured and analyzed using Image-Pro
Plus software (Media Cybernetics, Rockville, MD, USA).
Total RNA extraction and real-time
PCR
Total RNA was extracted from flash-frozen tumor
tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. Then 5 µg of total
RNA was reverse-transcribed in a 50 µl reaction by TIANScript RT
kit (Tiangen Biotech, Beijing, China). Real-time PCR was performed
with Super Real PreMix Plus (Tiangen Biotech) in the ABI 7500
Real-time PCR System (Applied Biosystems, Foster City, CA, USA).
The thermocycling conditions were as follows: pre-denaturation at
95°C for 30 sec, followed by 40 cycles at 95°C for 10 sec, 58°C for
30 sec, and 70°C for 30 sec. We determined the expression of VEGF
and HIF-1α mRNAs, using the following PCR primers: for VEGF,
forward 5′-GGAGCGTTCACTGTGAGC-3′, reverse 5′-GCGAGTCTGTGTTTTTGC-3′,
and amplified fragment length of 96 bp; for HIF-1α, forward
5′-CTGGAAACGAGTGAAAGG-3′, reverse 5′-ATGCTAAATCGGAGGGTA-3′, and
amplified fragment length of 86 bp; for β-actin, forward
5′-GGCACCACACCTTCTAC-3′, reverse 5′-CTGGGTCATCTTTTCAC-3′, and
amplified fragment length of 107 bp. The housekeeping gene β-actin
served as an internal control. Relative mRNA expression levels were
calculated by the 2−∆∆Ct method in the ABI 7500 Sequence
Detection system (Applied Biosystems), according to the
manufacturer's protocol.
Protein extraction and western
blotting assay
Total protein was extracted from tumor tissues with
lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). The lysates were ultra-sonicated and centrifuged at 12,000
× g at 4°C for 10 min. Subsequently, the supernatant was
transferred into new tubes. Protein concentrations were determined
by BCA assay (Beyotime Institute of Biotechnology). Protein lysates
(20 µg) were then separated by 10% SDS-PAGE, and transferred to
PVDF membranes. After blocking with TBS/5% skim milk, the membranes
were incubated with rabbit anti-mouse monoclonal anti-VEGF antibody
(1:1000; Abcam) and rabbit anti-mouse monoclonal anti-HIF-1α
antibody (1:1000; Abcam) for 2 h at room temperature, respectively.
They were then washed with TBST, and incubated with HRP-conjugated
goat anti-rabbit IgG secondary antibodies (1:10,000; CoWin Biotech,
Beijing, China) for 1.5 h at room temperature. As a loading
control, the blots were also probed with rabbit anti-mouse
monoclonal anti-β-actin antibody (1:1,000; Abcam). Proteins were
then visualized using ECl Plus Detection Reagents (Beyotime
Institute of Biotechnology) with UVP GDS-8000 System (Thermo
Scientific Inc., Waltham, MA, USA). Bands were quantified using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The data were obtained from at least three
independent experiments and each experiment used three parallel
samples. All results are presented as mean ± standard error.
Differences between means in more than two groups were analyzed by
ANOVA followed by Student-Newman-Keuls test. Differences between
means in two groups were compared using the unpaired-sample t-test.
All statistical analyses were done using SPSS software, version
22.0 for Windows (SAS Institute, Cary, NC, USA). Differences were
considered significant at P-values <0.05.
Results
Effect of 125I seed irradiation on
tumor growth of lung cancer
Tumor xenografts consisting of transplanted human
lung adenocarcinoma A549 cells were used to evaluate the antitumor
effects of the 125I seeds. When tumors reached approximately 300
mm3 at day 24, twenty seeds with the radio dosage of 0.6
or 0.8 mCi were implanted into mice of respective groups. In
addition, to account for possibility of local effects, we also
implanted sham seed with the radio dosage of 0 mCi on a separate
group of animals. All seeds were well located without loss and
displacement and removed. To confirm its enhanced tumor
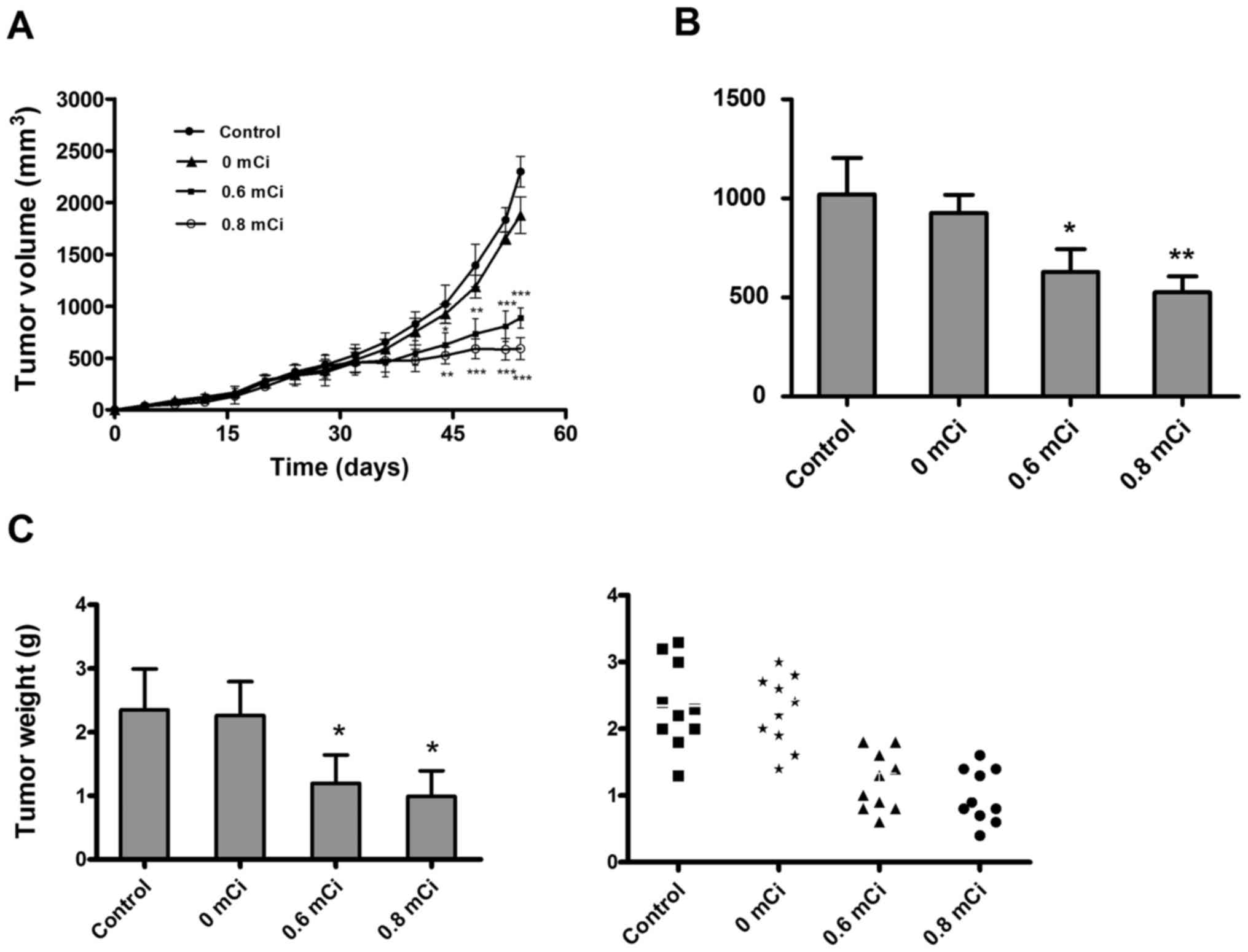
suppression, we tested tumor growth. As shown in Fig. 1A, there were no significant changes
in tumor volume during the first 3 weeks after seed implantation,
but after that, tumors of 0.6 or 0.8 mCi group were much smaller
than 0 mCi or the control group and significant differences in
tumor volumes were observed between the 0.6 or 0.8 mCi group and
the control group (all P<0.05). Tumor volumes on day 44 (day 20
after treatment) in 0.6 mCi (627.517±116.251) or 0.8 mCi
(525.763±81.141) groups were significantly suppressed when compared
with control group (1021.040±183.301; P=0.012, P=0.006), with an
average decrease in tumor size of about 38% or 49% (Fig. 1B). Tumor volumes in 0 mCi or control
groups increased exponentially with no regression, and no
significant differences in tumor volumes were observed on day 54
between 0 mCi and control groups (P=0.124). 0.6 or 0.8 mCi group
also decreased tumor weight by 49 and 62% compared with control
group (all P<0.05; Fig. 1C).
This indicated that 125I seed irradiation significantly inhibited
growth of the tumors during the 4- to 5-week treatment. Besides,
none of the mice died during the treatment, and no significant
complications associated with seed implantation were observed in
vital organs. These results underscore the safety of 125I seed
treatment.
Effect of 125I seed irradiation on
morphology of lung cancer
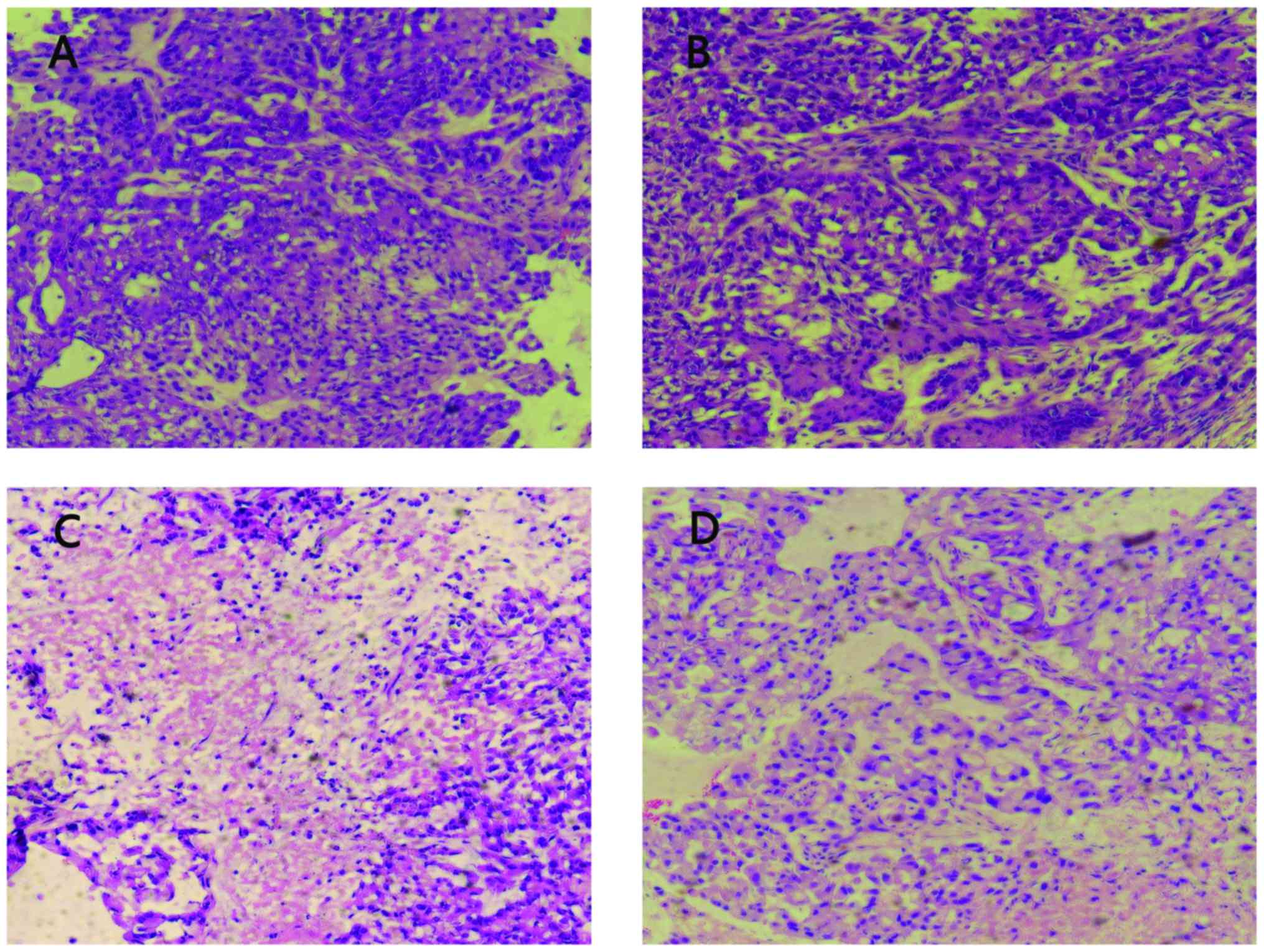
To investigate the effect of the 125I seed
irradiation on the histology of the A549 xenografts, tumor sections
obtained from mice in four groups were stained using H&E. As
shown in Fig. 2, the histological
appearance of the tumors in 0 mCi and control groups was quite
different from that in 0.6 and 0.8 mCi groups. In 0 mCi and control
groups, the cancer cells were densely arranged with large darkly
stained nuclei and obvious karyokinesis (Fig. 2A and B). Whereas, the cancer cells
around the 125I seed in 0.6 and 0.8 mCi groups revealed broad
necrosis after treatment (Fig. 2C and
D). The cancer cells adjacent to the necrotic region were
loosely arranged with condensed nuclei and reduced eosinophilic
cytoplasm. These results indicated that 0.6 or 0.8 mCi seed
implantation caused growth inhibition of cancer cells in the A549
xenografts.
Effect of 125I seed irradiation on
proliferation, MVD, and apoptosis
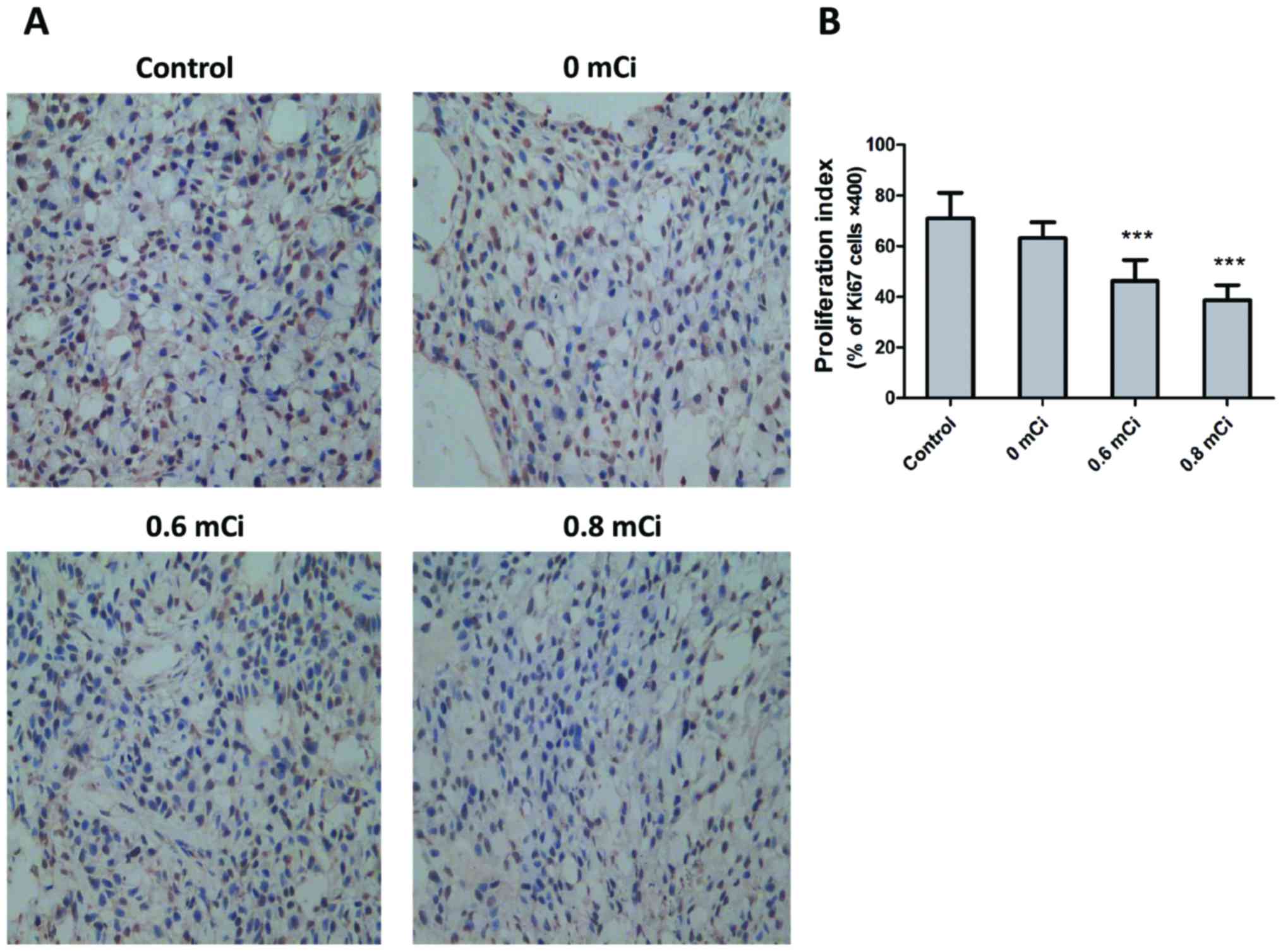
To determine possible mechanisms underlying 125I
seed-mediated suppression of tumor growth, we hypothesized that
125I seed mediates its antitumor effects via inhibition of
proliferation. Therefore, we first examined its anti-proliferative
effects by performing Ki67 immunohistochemistry on tumors obtained
at necropsy from the four groups. The results in Fig. 3A and B showed that the proliferation
indices were significantly decreased in 0.6 mCi (46.200±8.349) and
0.8 mCi (38.600±6.025) group compared with control group
(71.000±10.000; P<0.001, both). However, there was no
significant difference in the proliferation indices between 0 mCi
group (63.200±6.221) and control group (P=0.059). The tumor tissues
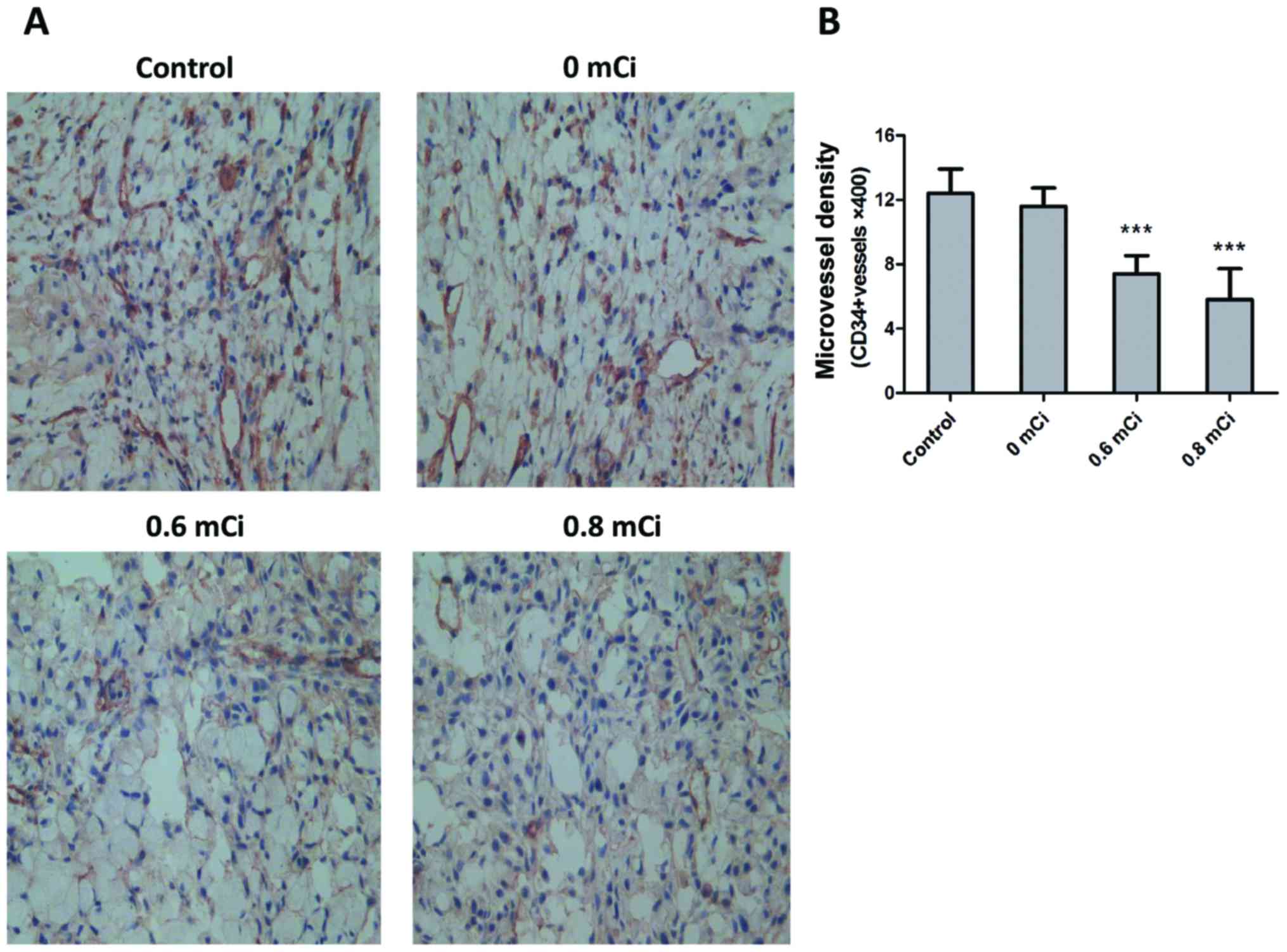
were stained with CD34, an endothelial marker, to measure MVD and
thus ascertain whether tumor angiogenesis might be affected
following 125I seed therapy. MVD, calculated as a measure of
angiogenesis, was significantly lower in 0.6 mCi (7.400±1.140) and
0.8 mCi (5.800±1.924) groups compared with control group
(12.400±1.517; P<0.001, both; Fig.
4A and B). Treatment with 0.6 or 0.8 mCi seed implantation
resulted in a 40% or 53% decrease in MVD compared with controls,
respectively. Treatment with 0 mCi (11.600±1.140) seed implantation
did not result in a significant decrease in MVD compared with
controls (P=0.366). To further elucidate the mechanism by which
125I seed irradiation elicits its antitumor effects, we examined
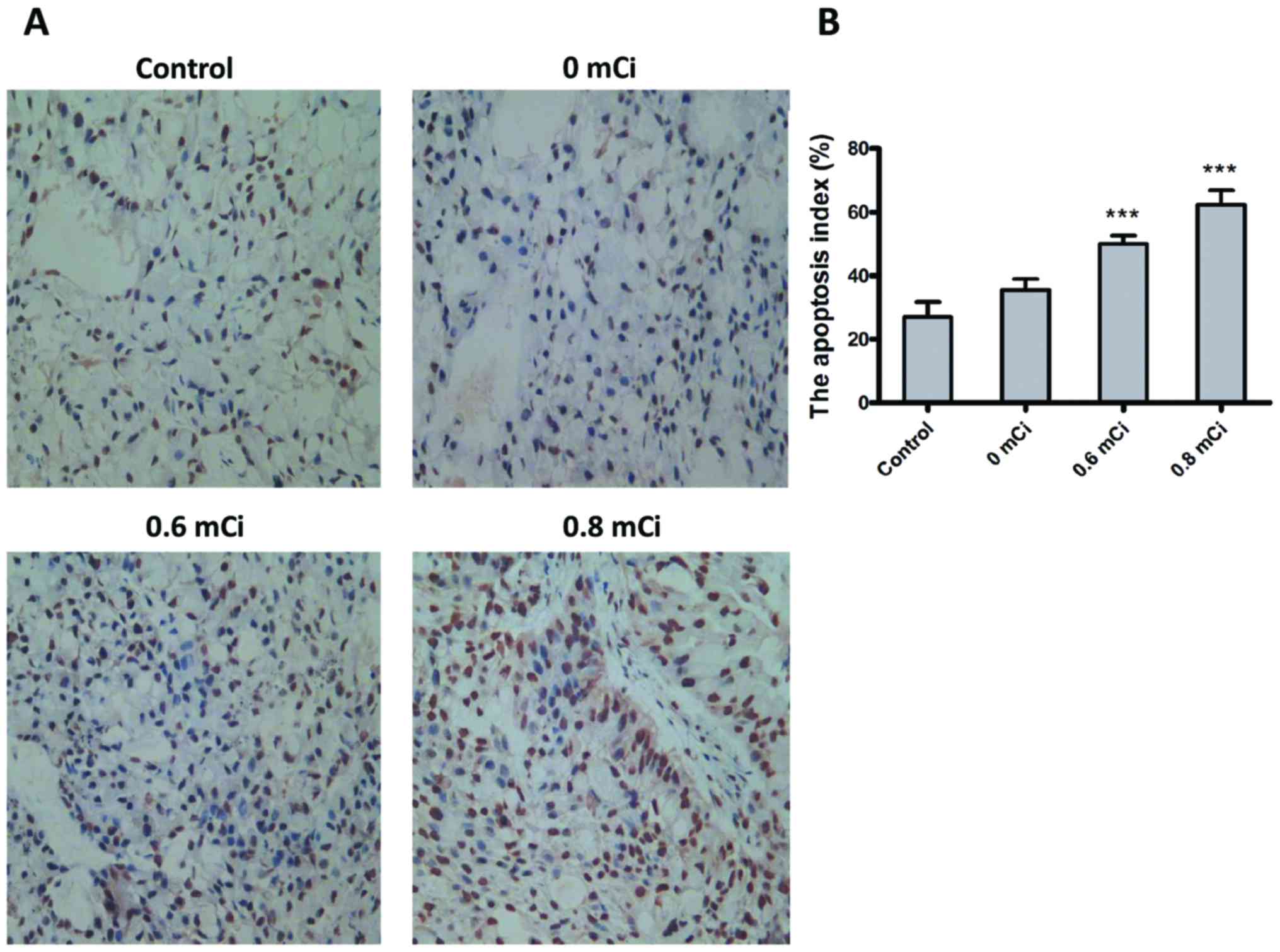
tumor cell apoptosis using the TUNEL assay. As shown in Fig. 5, the apoptotic indices in control
and 0 mCi groups were 27.00±4.69% and 35.50±3.42%, respectively.
Further, the apoptotic indices in 0.6 and 0.8 mCi groups were
50.00±2.58% and 62.33±4.51%. There was statistically significant
difference in apoptotic indices between 0.6 and 0.8 mCi groups and
control group (P<0.001, both). These findings suggest that
treatment with 125I seed irradiation causes apoptosis in tumor
cells.
125I seed irradiation downregulates
the expression of VEGF and HIF-1α in tumors
It has been demonstrated that a high level of HIF-1α
in the tumor microenvironment leads to enhanced angiogenesis and
proliferation. Given that its gene target VEGF is also a critical
regulator for neovascularization (24,25),
we questioned whether 125I seeds mediate its effects through
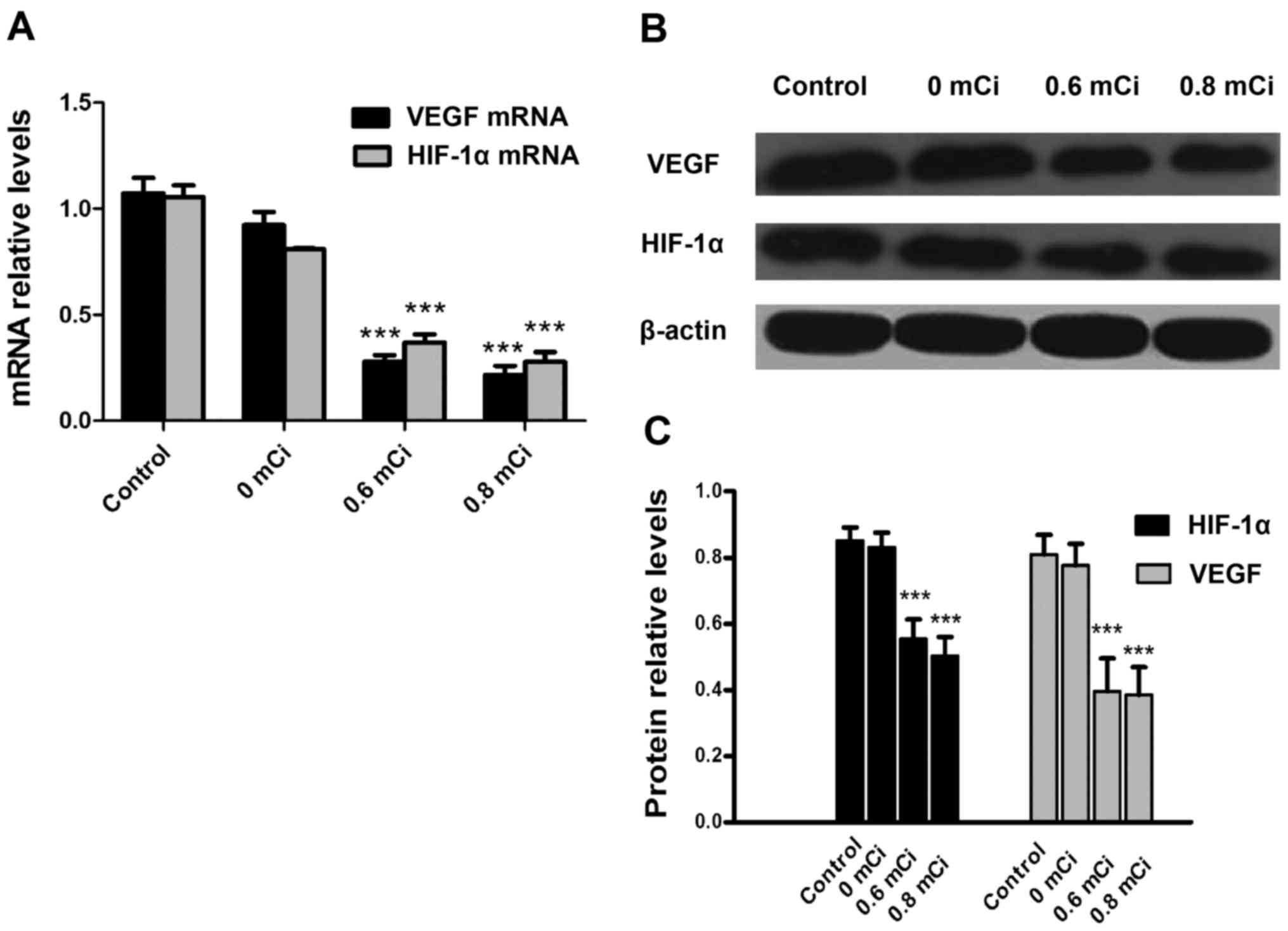
modulation of HIF-1α and VEGF. Therefore, we determined the HIF-1α
and VEGF mRNA expression by RT-PCR in A549 xenograft after
treatment in all four cohorts of animals. Expression of HIF-1α and
VEGF mRNA in the 0.6 mCi (0.370±0.086, 0.279±0.066) and 0.8 mCi
(0.278±0.065, 0.215±0.062) groups were significantly lower than in
the control group (1.055±0.078, 1.073±0.103, all P<0.001;
Fig. 6A). Moreover, HIF-1α and VEGF
mRNA expression did not differ between 0 mCi group (0.810±0.006,
0.923±0.089) and control group (P=0.150, 0.236). These data suggest
that 125I seed irradiation significantly affects the expression of
HIF-1α and VEGF mRNA. Expression of HIF-1α and VEGF were also
examined by western blotting (Fig.
6B). As shown in Fig. 6C,
HIF-1α and VEGF protein expression decreased in the 0.6 mCi
(0.554±0.0585, 0.396±0.010) and 0.8 mCi (0.502±0.0582,
0.385±0.0842) groups compared with the control group (0.852±0.0395,
0.810±0.0593; all P<0.001). However, there were no significantly
statistical differences in HIF-1α and VEGF protein expression
between 0 mCi group (0.810±0.006, 0.923±0.089) and control group
(P=0.157, P=0.191). These data suggest that 125I irradiation
significantly affects HIF-1α and VEGF protein expression.
Discussion
NSCLC is the most prevalent type of lung cancer.
Despite therapeutics advances in surgery, chemotherapy, and
radiotherapy, the five-year overall survival rate is poor.
Radiotherapeutics approach has recently started to play an
important role in the treatment of advanced lung cancer. However,
the adverse effects of conventional EBRT on surrounding organs pose
a major problem. The advantages of 125I seed radiation are the low
dose rates and conformal irradiation which increases the dose
applied within the target area, thus decreasing the incidental
radiation injury to normal tissues and the attendant complications
(26,27). Several recent studies suggest that
apoptosis and proliferative inhibition may have important roles in
the therapeutic effects of 125I seeds (18,28),
but the mechanism in the treatment of lung cancer, especially in
animal models, is not known completely. In addition, the HIF-1α and
VEGF may be involved in the lung cancer tumorigenesis (29). Irradiation therapy may downregulate
the expression of HIF-1α and VEGF in tumors, thereby affecting
angiogenesis and then affecting tumor growth (30,31).
However, comprehensive knowledge on this topic, particularly at the
molecular level, is still lacking. In this study, we assessed the
radiobiological effects of 125I seeds on human lung adenocarcinoma
cells in vivo.
A549 lung cancer cells were cultured ex vivo
and implanted subcutaneously into the nude mice to create the
animal model. We examined the effects of irradiation on tumor
growth and found that radioactive 125I seed significantly inhibited
tumor growth in nude mice. By observing H&E-stained slides,
large necrotic regions were observed around the 125I seed in
tumors. Immunohistochemistry staining indicated that the expression
of Ki67 and microvessel density (CD34) was inhibited by 125I seed
irradiation in tumor tissues. The Ki67, which is a proliferative
cell marker expressed in all phases of the cell cycle except the G0
stage, is considered to be a reliable index of the proliferation
rate (32). Angiogenesis which is
required for the delivery of oxygen and nutrients in the tumor
tissues, possibly resulting in the apoptosis in vivo, was
inhibited by 125I seeds. To test the effect of 125I seed radiation
on apoptosis, we assayed apoptosis using TUNEL staining. Our data
showed that 125I seed significantly induced apoptosis in tumor
tissues, indicating that 125I seed may indeed inhibit tumor growth
through the inhibition of angiogenesis. These data suggest that
125I seed radiation is an effective radiotherapeutics approach for
lung cancer through inhibiting cell proliferation, angiogenesis and
inducing apoptosis in vivo.
Hypoxia is known to be a hallmark of solid tumors
and increased angiogenesis (33);
under hypoxia condition, HIF-1 regulates expression of numerous
angiogenic genes and stimulate angiogenesis as a long-term solution
to the hypoxic conditions (34,35).
HIF-1 is a heterodimeric transcription factor consisted of HIF-1α
and HIF-1β subunits (36). HIF-1α
is an important mediator induced by hypoxia, growth factors, and
oncogenes (37). As a transcription
factor involved in the process of gene related hypoxic adaptation
of neoplasm, HIF-1α is often upregulated in human cancers. HIF-1α
activates the transcription of many genes such as VEGF,
endothelin-1, and inducible nitric oxide synthase, which are
involved in vasodilation, neovascularization, and tumor metastasis
(38). Many investigations have
revealed that the relationships between HIF-1α, VEGF and
angiogenesis resulted from hypoxic condition (39,40).
To test whether 125I seed radiation affects HIF-1α levels, we
analyzed HIF-1α expression by real-time PCR and western blotting
and showed that 125I seed specifically inhibited HIF-1α expression
in cancer cells.
VEGF is known to be an important angiogenic factor
for endothelial cells. Many studies recently showed that VEGF
levels correspond with advanced lung cancer. The increased VEGF
levels were found to be associated with poor prognosis in patients
with NSCLC (41,42). In addition, the activation of VEGF
expression can be induced by HIF-1α, as indicated above. Our data
showed that 125I seed radiation inhibited VEGF expression at the
transcriptional level and protein level. HIF-1 expression is known
to play an important role in VEGF transcriptional activation in
response to hypoxia (34). Thus,
125I seed radiation may inhibit VEGF transcriptional level through
the decrease of HIF-1 expression in cancer cells. This result is
consistent with the data showing the inhibition of angiogenesis by
125I seed because HIF-1α and VEGF are important for tumor
angiogenesis. Our study demonstrated that HIF-1α and VEGF play an
important role in the therapeutic effects of continuous low-energy
125I irradiation and are involved in the mechanism of the 125I seed
implantation therapy process.
Collectively, it was shown that 125I seed radiation
can elicit significant anticancer effects by inhibiting
proliferation and inducing apoptosis in vivo. In the tumor
microenvironment, reducing the levels of HIF-1α and VEGF induced by
125I seed might inhibit angiogenesis. These findings suggest that
the 125I seed radiation may be a promising therapeutics approach
against lung cancer.
Glossary
Abbreviations
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
EBRT
|
external beam irradiation
|
|
TUNEL
|
transferase-mediated fluorescein
deoxy-UTP nick-end labeling
|
References
|
1
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toyokawa G, Takenoyama M and Ichinose Y:
Multimodality treatment with surgery for locally advanced
non-small-cell lung cancer with n2 disease: A review article. Clin
Lung Cancer. 16:6–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cardenal F, Nadal E, Jové M and
Faivre-Finn C: Concurrent systemic therapy with radiotherapy for
the treatment of poor-risk patients with unresectable stage III
non-small-cell lung cancer: A review of the literature. Ann Oncol.
26:278–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfister DG, Johnson DH, Azzoli CG, Sause
W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT,
et al: American Society of Clinical Oncology: American Society of
Clinical Oncology treatment of unresectable non-small-cell lung
cancer guideline: Update 2003. J Clin Oncol. 22:330–353. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dillman RO, Hemdon J, Seagren SL, Eaton WL
Jr and Green MR: Improved survival in stage III non-small-cell lung
cancer seven-year follow-up of cancer and leukemia group B (CALGB)
8433 trial. J Natl Cancer Inst. 88:1210–1215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wagner TD and Yang GY: The role of
chemotherapy and radiation in the treatment of locally advanced
non-small cell lung cancer (NSCLC). Curr Drug Targets. 11:67–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liew MS, Sia J, Starmans MH, Tafreshi A,
Harris S, Feigen M, White S, Zimet A, Lambin P, Boutros PC, et al:
Comparison of toxicity and outcomes of concurrent radiotherapy with
carboplatin/paclitaxel or cisplatin/etoposide in stage III
non-small cell lung cancer. Cancer Med. 2:916–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cannon DM, Mehta MP, Adkison JB, Khuntia
D, Traynor AM, Tomé WA, Chappell RJ, Tolakanahalli R, Mohindra P,
Bentzen SM, et al: Dose-limiting toxicity after hypofractionated
dose-escalated radiotherapy in non-small-cell lung cancer. J Clin
Oncol. 31:4343–4348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park DS, Gong IH, Choi DK, Hwang JH, Shin
HS and Oh JJ: Radical prostatectomy versus high dose permanent
prostate brachytherapy using iodine-125 seeds for patients with
high risk prostate cancer: A matched cohort analysis. World J Urol.
31:1511–1517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ZM, Lu J, Liu T, Chen KM, Huang G and
Liu FJ: CT-guided interstitial brachytherapy of inoperable
non-small cell lung cancer. Lung Cancer. 74:253–257. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joyce F, Burcharth F, Holm HH and Strøyer
I: Ultrasonically guided percutaneous implantation of iodine-125
seeds in pancreatic caicinoma. Int J Radiat Oncol Biol Phys.
19:1049–1052. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JJ, Yuan HS, Li JN, Jiang WJ, Jiang
YL and Tian SQ: Interstitial permanent implantation of 125I seeds
as salvage therapy for re-recurrent rectal carcinoma. Int J
Colorectal Dis. 24:391–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Y-L, Meng N, Wang J-J, Ran WQ, Yuan
HS, Qu A and Yang RJ: Percutaneous computed
tomography/ultrasonography-guided permanent iodine-125 implantation
as salvage therapy for recurrent squamous cell cancers of head and
neck. Cancer Biol Ther. 9:959–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang FJ, Li CX, Zhang L, Wu PH, Jiao DC
and Duan GF: Short- to mid-term evaluation of CT-guided 125I
brachytherapy on intra-hepatic recurrent tumors and/or
extra-hepatic metastases after liver transplantation for
hepatocellular carcinoma. Cancer Biol Ther. 8:585–590. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heelan RT, Hilaris BS, Anderson LL, Nori
D, Martini N, Watson RC, Caravelli JF and Linares LA: Lung tumors:
Percutaneous implantation of I-125 sources with CT treatment
planning. Radiology. 164:735–740. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Guan J, Yang L, Zheng X, Yu Y and
Jiang J: Iodine-125 brachytherapy improved overall survival of
patients with inoperable stage III/IV non-small cell lung cancer
versus the conventional radiotherapy. Med Oncol. 32:3952015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu A, Wang H, Li J, Wang J, Liu J, Hou Y,
Huang L and Zhao Y: Biological effects of (125)i seeds radiation on
A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer
Invest. 32:209–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takabayashi K, Kashiwagi K, Kawata T, Sato
T, Matsuoka K, Hisamatsu T, Takaishi H, Hibi T, Ogata H, Yahagi N,
et al: Continuous low-dose irradiation by I-125 seeds induces
apoptosis of gastric cancer cells regardless of histological
origin. Cancer Biol Ther. 15:81–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carmeliet P: Manipulating angiogenesis in
medicine. J Intern Med. 255:538–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acker T and Plate KH: A role for hypoxia
and hypoxia-inducible transcription factors in tumor physiology. J
Mol Med (Berl). 80:562–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G,
Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, et al:
Surgical stress promotes tumor growth in ovarian carcinoma. Clin
Cancer Res. 15:2695–2702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong S-S, Lee H and Kim K-W: HIF-1α: A
valid therapeutic target for tumor therapy. Cancer Res Treat.
36:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thangarajah H, Yao D, Chang EI, Shi Y,
Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, et al:
The molecular basis for impaired hypoxia-induced VEGF expression in
diabetic tissues. Proc Natl Acad Sci USA. 106:13505–13510. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peretz T, Nori D, Hilaris B, Manolatos S,
Linares L, Harrison L, Anderson LL, Fuks Z and Brennan MF:
Treatment of primary unresectable carcinoma of the pancreas with
I-125 implantation. Int J Radiat Oncol Biol Phys. 17:931–935. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazeron JJ, Noël G, Simon JM, Racadot S
and Jauffret E: Brachytherapy in head and neck cancers. Cancer
Radiother. 7:62–72. 2003.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu L, Chen H and Cheng W: Influence of
~(125)I continuous low dose-rate irradiation to apoptosis and
DNA-PK expression of human lung carcinoma cell lines. Chinese J
Clin Med. 6:2007.
|
|
29
|
Lin X, Li HR, Lin XF, Yu ME, Tu XW, Hua
ZD, Lin M, Xu NL, Han LL and Chen YS: Silencing of Livin inhibits
tumorigenesis and metastasis via VEGF and MMPs pathway in lung
cancer. Int J Oncol. 47:657–667. 2015.PubMed/NCBI
|
|
30
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aita M, Fasola G, Defferrari C, Brianti A,
Bello MG, Follador A, Sinaccio G, Pronzato P and Grossi F:
Targeting the VEGF pathway: Antiangiogenic strategies in the
treatment of non-small cell lung cancer. Crit Rev Oncol Hematol.
68:183–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
33
|
Semenza GL: Involvement of
hypoxia-inducible factor 1 in human cancer. Intern Med. 41:79–83.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Semenza GL: Hypoxia-inducible factor 1:
Master regulator of O2 homeostasis. Curr Opin Genet Dev.
8:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukuda R, Hirota K, Fan F, Jung YD, Ellis
LM and Semenza GL: Insulin-like growth factor 1 induces
hypoxia-inducible factor 1-mediated vascular endothelial growth
factor expression, which is dependent on MAP kinase and
phosphatidylinositol 3-kinase signaling in colon cancer cells. J
Biol Chem. 277:38205–38211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kerbel RS: New targets, drugs, and
approaches for the treatment of cancer: An overview. Cancer
Metastasis Rev. 17:145–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mouriaux F, Sanschagrin F, Diorio C,
Landreville S, Comoz F, Petit E, Bernaudin M, Rousseau AP, Bergeron
D and Morcos M: Increased HIF-1α expression correlates with cell
proliferation and vascular markers CD31 and VEGF-A in uveal
melanoma. Invest Ophthalmol Vis Sci. 55:1277–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Harada H: How can we overcome tumor
hypoxia in radiation therapy? J Radiat Res (Tokyo). 52:545–556.
2011. View Article : Google Scholar
|
|
41
|
Iwasaki A, Kuwahara M, Yoshinaga Y and
Shirakusa T: Basic fibroblast growth factor (bFGF) and vascular
endothelial growth factor (VEGF) levels, as prognostic indicators
in NSCLC. Eur J Cardiothorac Surg. 25:443–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kishiro I, Kato S, Fuse D, Yoshida T,
Machida S and Kaneko N: Clinical significance of vascular
endothelial growth factor in patients with primary lung cancer.
Respirology. 7:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|