Introduction
Cancer is one of the major life threatening diseases
worldwide (1). Colorectal cancer is
the third leading cause of cancer-associated mortality in both men
and women in the world (2). Regular
screening increases the likelihood that colorectal cancer is
detected at an early stage, more likely to be cured and with faster
recovery (3). Although significant
progress has been made over the past few decades, effective
treatment of this disease is still lacking. It is believed that
targeted therapy that focuses on specific molecules or signalling
pathways hold the key for more effective treatment of this disease
than the currently used chemotherapy.
Lectins, a group of highly diverse proteins of
non-immune origin that are ubiquitous in nature, recognize specific
carbohydrate structures. Tumour cells in general display different
carbohydrate profiles on the cell surface compared to
non-transformed cells which can be detected using lectins. Lectins
can reasonably differentiate malignant tumours from normal cells,
and hence are used for cancer diagnosis and some are also being
studied for their possible use as therapeutic agents (4). Lectins from edible mushrooms, such as
Agaricus bisporus, Agrocybe aegerita I & II,
Kurkowa lectin Pleurotuseous, Tricholomamongolicum
and Pholiotaadiposa, have been shown to exert
antiproliferative effects in cancer in vitro and antitumor
effects in vivo (5–11). The two main properties of lectins
that is, selectivity and cytotoxicity, have been exploited for
devising therapeutic strategies against cancer.
Sclerotium rolfsii, lectin (SRL) purified
from the fungus Sclerotium rolfsii (12) has been shown to have exquisite
binding specificity toward Thomsen-Friedenreich antigen (TF-Ag;
Galβ1-3GalNAcα-O-Ser/Thr) and its derivatives (13). The TF antigen is highly expressed by
>90% of tumours and rarely occurs in normal tissues (14). The crystal structure of SRL
determined at 1.1 Å resolution, revealed two carbohydrate binding
sites (15). Our previous studies
have shown that SRL binds specifically to human cancerous colon but
not to normal colon and exhibits strong antiproliferative activity
in human colon cancer (HT29 and DLD1) (16), breast cancer (MCF-7 and ZR-75) and
ovarian cancer (PA-1) cells by inducing cellular apoptosis
(17,18). Recently, we demonstrated that the
presence of SRL affects multiple cell signalling pathways with
early effects on MAPK- and c-JUN-associated cell proliferation
pathways followed by miRNA-associated cell activity and apoptosis
pathways. Lectin affinity purification has extracted a total of 25
proteins in HT29 cells, including keratins, heat shock proteins
HSP-60 and HSP-90, ATP synthase subunit α, mitochondrial retinal
dehydrogenase 1, actin cytoplasmin 2, tubulins, pyruvate kinase-M,
Annexin A2, peroxiredoxin-1, prohibitin, α-enolase and ADP/ATP
translocase 2 that are known to be involved in cell survival,
apoptosis and tumorigenesis (19,20).
These effects of SRL together with its exquisite binding
specificity towards TF-Ag-associated glycans prompted us to
investigate the possibility of developing SRL as a therapeutic
agent.
Materials and methods
Materials
Protease Inhibitor Cocktail Set III was obtained
from Calbiochem (Darmstadt, Germany); CD-31 (PECAM-1) was obtained
from Invitrogen (Carlsbad, CA, USA) and Dulbeccos modified Eagles
medium (DMEM) and fetal calf serum (FCS) were obtained from
Gibco-Invitrogen (Paisley, UK). Bovine serum albumin (BSA),
streptavidin-horseradish peroxidase, 3,3,5,5-tetramethylbenzidine
(TMB) and poly-L-lysine solution were obtained from Sigma-Aldrich
(St. Louis, MO, USA). Taq DNA polymerase was obtained from
Invitrogen. Oligonucleotide primers and Tris buffer were obtained
from Sigma-Aldrich and dNTPs from Fermentas (Waltham, MA, USA).
3-3-Diaminobenzidine chromogen/H2O2 substrate
in buffer solution (pH 7.5) (DAB kit) and rabbit anti-SRL
polyclonal antisera were obtained from Bangalore Genei India Pvt.
Ltd. (Bangalore India). Caspase-3 (3G2) mouse mAb was procured from
Cell Signaling Technology, Inc. (Danvers, MA, USA).
Species-specific HRP-labelled secondary antibodies were procured
from Bio-Rad Laboratories (Hercules, CA, USA). Anticoagulated tubes
were obtained from Greiener Bio-One (1 ml K3E K3EDTA;
Frickenhausen, Germany) and a flat bottom 96-well micro-test plate
were obtained from Tarsons (Kolkata, India). All other reagents
were of analytical grade and Milli-Q water was used for all the
preparations.
Purification of Sclerotium rolfsii
lectin (SRL)
Purification of SRL from the sclerotial bodies was
carried out as previously described (12). Briefly, SRL was extracted from
powdered sclerotial bodies with 50 mM acetate buffer containing 100
mM NaCl (pH 4.3) and subjected to 30% methanol precipitation
followed by ion exchange chromatography on CM cellulose matrix and
finally purified on a Superdex G-75 gel filtration column. The
purified lectin was used for biodistribution and efficacy studies.
The lectin activity was routinely determined by the 2-fold serial
dilution method using trypsinised human erythrocytes.
Purification of polyclonal SRL
antibodies from Protein A agarose column
Custom designed anti-SRL polyclonal antibodies were
purified from immunized rabbit serum (from Bangalore Genei India)
by subjecting to 50% ammonium sulphate precipitation, followed by
affinity chromatography on a Protein A agarose column equilibrated
with 100 mM phosphate buffer (pH 7.2) containing 150 mM NaCl
phosphate-buffered saline (PBS). Bound SRL antibodies were eluted
with 100 mM glycine-HCl (pH 2.5), dialysed against PBS and stored
at −20°C.
Cell culture
Human colon cancer HT29 cells were obtained from the
European Cell Culture Collection via the Public Health Laboratory
Service (Porton Down, UK). HT29 cells were cultured in DMEM
supplemented with 10% FBS, 100 units/ml penicillin and 100 µg/ml
streptomycin (complete DMEM) at 37°C in 5% CO2.
Mice
All animal experiments were carried out at the
Advanced Centre for Treatment, Research and Education in Cancer
with approval from the Ethics Committee (IRB approval no. 07/2012)
of Tata Memorial Centre-ACTREC-IAEC (Institutional Animal Ethics
Committee). Inbred female BALB/c nude mice (6–8 weeks old, weighing
18–22 g) for efficacy studies and male Swiss albino mice (6–8 weeks
old, weighing 20–25 g) for pharmacokinetics and biodistribution
studies were used. The animals were housed in groups of 4–5 per
polycarbonate cage under individually ventilated caging systems
(IVCs) under controlled conditions of 55±5% humidity, 22±2°C
temperature, and light cycle (12:12 h). All mice were provided with
easy access to in-house pelleted feed and with ad libitum
UV-treated water and were monitored daily for general health
status.
Efficacy studies of SRL on tumour
growth in BALB/c nude mice
HT29 cells were suspended at 1×107
cells/ml in DMEM and a cell suspension (0.1 ml) was injected
subcutaneously into two donor mice. After 4 weeks, the tumours from
these donor mice were excised, chopped into 2- to 3-mm fragments
and a single piece of tumour was transplanted subcutaneously into
each of 12 BALB/c nude mice. Tumours were allowed to grow to reach
the appropriate size of 5–6 mm, i.e. 80–120 mm3 in
volume and animals were divided randomly into 2 groups of 5 mice in
each group, one for SRL treatment (T) and other used as a control
(TBS, C). Mice were intraperitoneally administered SRL (30 mg/kg
body weight/mouse) in TBS (pH 7.5) on every other days for 17 days
for a total of 9 doses. The mice in the control group were injected
intraperitoneally with TBS at the same units (5–10 UI) and
maintained for 2 weeks after the last dose. The mice were weighed
and tumour volume was also determined by measuring the length (L)
and width (W) of the tumour after each injection. The tumour volume
at day n (TVn) was calculated as TV (mm3) =
(L×W2)/2. The relative tumour volume at day n (RTVn) vs.
day 0 was expressed according to the formula: RTVn = TVn/TV0
(21,22). Regression of tumour T/C (%) in the
treated vs. control mice was calculated using: T/C (%) = (mean RTV
of treated/control group) × 100. The changes in the tumour size of
the treated mice at different time-points compared to the control
were used for monitoring the efficacy of the lectin. Mice were
sacrificed 2 weeks after the last dose, and whole blood was
collected in tubes coated with anticoagulant and used for analysis
of total blood cell count, hemoglobin content, WBC and platelet
count. Tumours were excised and organs such as liver, kidney and
lung were collected directly in PBS to remove the blood traces,
wrapped in aluminium foil and stored at −80°C until further use for
immunohistological analysis. Excised tumours were also snap frozen
and stored at −80°C for STR analysis.
PCR analysis of short tandem repeat
(STR)
In order to ascertain the human origin of the
tumours using short tandem repeat (STR) method (23), a small piece of the tumour tissues
was collected from BALB/c mice with HT-29 xenograft, excised after
2 weeks from the last dose of injection. These tissues were snap
frozen and stored at −80°C for extraction of DNA. DNA was extracted
from the samples using phenol chloroform extraction followed by
ethanol precipitation. The DNA precipitates were suspended in
Tris-EDTA (TE) buffer, pH 8.4. The extracted DNA was subjected to
PCR amplification using human-specific microsatellite primers
(forward, 5′-TGCCATTTGTGGGTACATTC-3′ and reverse,
5′-TTGTGTTTCTTTTTCTGTTCCTACA-3′). In brief, 100 ng of target DNA
was amplified in a 15-µl reaction volume containing 5 IU of
Taq DNA polymerase, 0.4 pM each oligonucleotide primer, 10
mM Tris buffer, 3.5 mM MgCl2, 0.4 mM each of dNTPs. PCR
was performed with a 5-min denaturation at 94°C followed by 34
cycles of a 20-sec denaturation at 94°C, a 20-sec annealing at
58°C, and a 30-sec elongation at 72°C in a thermal cycler
(Eppendorf, Hauppauge, NY, USA). Amplicon was separated on 2%
agarose gel and visualized by UV light.
Pharmacokinetics and biodistribution
studies
Swiss albino mice were randomized into two groups,
the control group (TBS) and the SRL-treated group which included 4
time-points (1, 6, 24 and 48 h) with 3 animals for each time-point.
Lectin was prepared in TBS (pH 7.5), hence TBS served as the
vehicle control. Animals were injected intraperitoneally with TBS
and SRL (24 mg/kg body weight/mouse) and maintained for different
time-points and blood was collected from the treated and control
mice by retro-orbital bleeding. After incubation of the blood at
room temperature (RT) for 15 min, serum was collected by
centrifuging the sample at 2,500 rpm for 15 min at 4°C and stored
at −80°C until further use. The pharmacokinetic profile of the
serum samples was evaluated by ELISA. Serum samples collected at 24
h were also analysed for biochemical parameters of toxicity such as
serum liver enzymes (aspartate transaminase and alanine
transaminase), lactate dehydrogenase (LDH), blood urea nitrogen
(BUN) and creatinine levels [carried out at Unique Bio Diagnostics
Enterprises (Mumbai, India)]. Animals were then sacrificed by
cervical dislocation, and the organs (liver, kidney, heart,
stomach, lungs, spleen, intestine and brain) were collected
directly in PBS to remove the blood traces, wrapped in aluminium
foil and stored at −80°C until further use for biodistribution
studies by ELISA or histology.
Tissue homogenization
Tissue homogenate was prepared by taking a part of
the tissue from each organ, thawed on ice, weighed in a 2-ml
Eppendorf tube and extracted in extraction buffer, PBS pH 7.4 (four
volume/g of organ weight). Protease Inhibitor Cocktail Set III was
added to the extraction buffer (1/20th volume of the tissue weight)
and subjected to homogenization using an electric homogenizer
(24,25). Prior to homogenization, the probe
was dipped in ice and the tissue was homogenized at 4°C with 8–10
oscillate up- and down-strokes for soft tissues derived from organs
such as the liver, stomach, small intestine, lung, spleen, kidney
and brain; whereas, hard tissues from the heart required more
strokes and agitation. After homogenization, each sample was
centrifuged for 20 min at 16,000 rpm at 4°C and the supernatant was
collected and stored at −80°C until further use for analysis.
Bioanalytical validation
A calibration curve was constructed using different
concentrations of SRL (0.002–0.312 µg/ml and 0.002–0.078 µg/ml)
including a lower limit of quantification (LLOQ) for evaluating SRL
in organ and serum extracts, respectively. Analytical recovery by
ELISA was evaluated by adding serum and organ extracts at a 1:100
dilution and using the respective calibration curves and measuring
its recovery using the same procedure and conditions as described
in the ELISA experiments. The absorbance was measured at 450 nm and
values were plotted against the concentrations. The LLOQ was set as
the lowest measurable concentration with acceptable accuracy and
precision not >20% of the expected values (26,27).
Precision and accuracy were determined by analyzing quality control
samples (QCs). Recovery was computed by comparing responses in
triplicate of extracted QCs (28,29).
ELISA
Indirect ELISA was performed using 96-well plates.
The plates were pre-coated with a known concentration of SRL
(0.002–0.3 µg/100 µl/well), serum samples or tissue homogenate
(1:100 dilution) in coating buffer (15 mM sodium carbonate and 17
mM bicarbonate buffer, pH 9.6, containing 3 mM sodium azide) and
incubated overnight at 4°C. Wells were washed thrice with 50 mM
PBS, at pH 7.4 containing 0.1% (v/v) Tween-20 (PBST) to remove the
unbound proteins. Non-specific binding was blocked by incubating
with 100 µl of 1% BSA in PBS for 2 h at RT at 37°C, followed by
washing twice with PBST to remove excess blocking agent. Wells were
incubated with rabbit anti-SRL polyclonal antibodies (100 µl/well,
1:10,000 dilutions in PBS) overnight at 4°C. After washing,
HRP-conjugated anti-rabbit IgG secondary antibodies (Sigma-Aldrich)
at 1:10,000 dilutions were added and incubated for 2 h at RT at
37°C. The colour was developed by the addition of
3,3,5,5-tetramethylbenzidine (TMB; Sigma-Aldrich). The reaction was
stopped using 4 M H2SO4 (100 µl/well) and the
absorbance was recorded at 450 nm on a microtiter plate reader
(Tecan, Crailsheim, Germany).
Histological analysis
Histochemical examinations were performed using the
tissue samples of the tumors and organs including liver, spleen,
kidney, heart, lung, stomach, small intestine and brain. Excised
tumours were collected and fixed in 10% formalin and embedded in
paraffin blocks, and 5-µm sections were used for hematoxylin and
eosin (H&E) staining and for immunohistochemistry (IHC). The
histological sections were observed under an optical microscope and
photographed.
Immunohistochemical staining
Sections (5-µm thick) of the SRL-treated tumours
with the controls were prepared by using the tumour tissues
collected from the snap-frozen samples stored at −80°C and stained
with a specific mouse anti-CD31 (PECAM-1) antibody (clone MEM-05)
according to the manufacturers instructions. The primary antibody
(1:500) was diluted in PBS and incubated with samples at RT for 1 h
followed by washing with TBST and then with HRP-conjugated goat
anti-mouse IgG (H+L) secondary antibodies (1:3,000) diluted in PBS
and incubated at RT for 1 h. Finally the slides were developed
using 3,3-diaminobenzidine (DAB) substrate and counterstained with
hematoxylin. Images (magnification, ×10) were captured to monitor
CD31 expression.
Statistical analysis
The antitumor activity (as measured by differences
in tumor mass) was expressed as mean ± standard deviation (SD).
In vivo data and ELISA calibration curve with unknowns was
analyzed using GraphPad Prism 6. The statistical significance of
differences in tumor volume was determined by one-way analysis of
variance (ANOVA), followed by Sidak test for three or more groups;
a value of P<0.05 was considered as significant.
Results
SRL inhibits tumour growth in BALB/c
nude mice bearing HT-29 xenografts
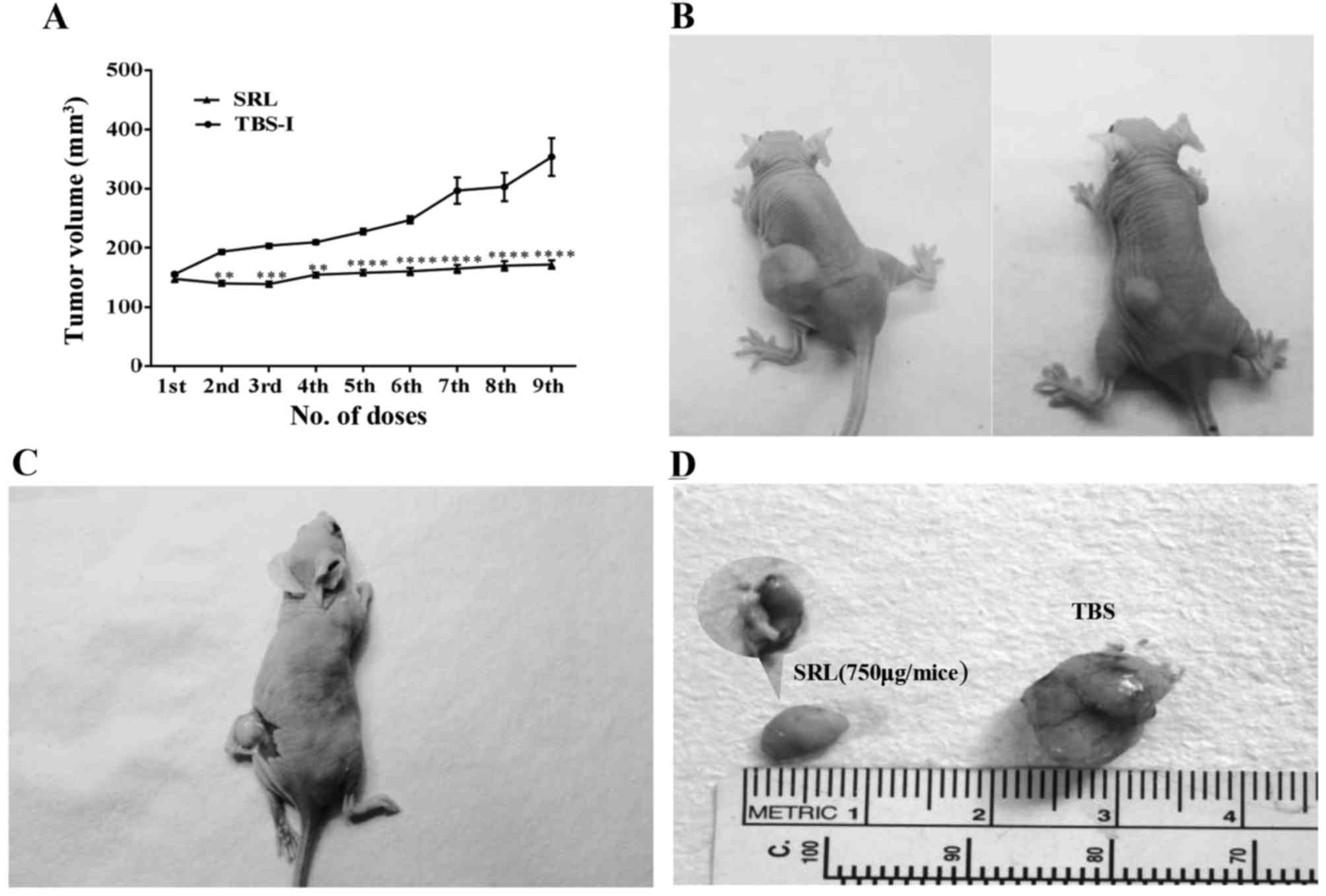
Mice injected intraperitoneally with SRL (30 mg/kg
body weight/mouse) showed significant inhibition (35.8%,
P<0.0001) of tumour growth when compared with that observed in
the control group (Fig. 1). The
average initial tumour volume in the TBS and SRL-treated groups was
155±4.7 and 147±10.9 mm3, respectively before tumour
cell treatment and the average tumour volume after completion of 9
doses was 353±71.2 and 171±16 mm3, respectively
(P<0.001). No noticeable change in the weight and tumour volume
of either control or treated group was observed after maintaining
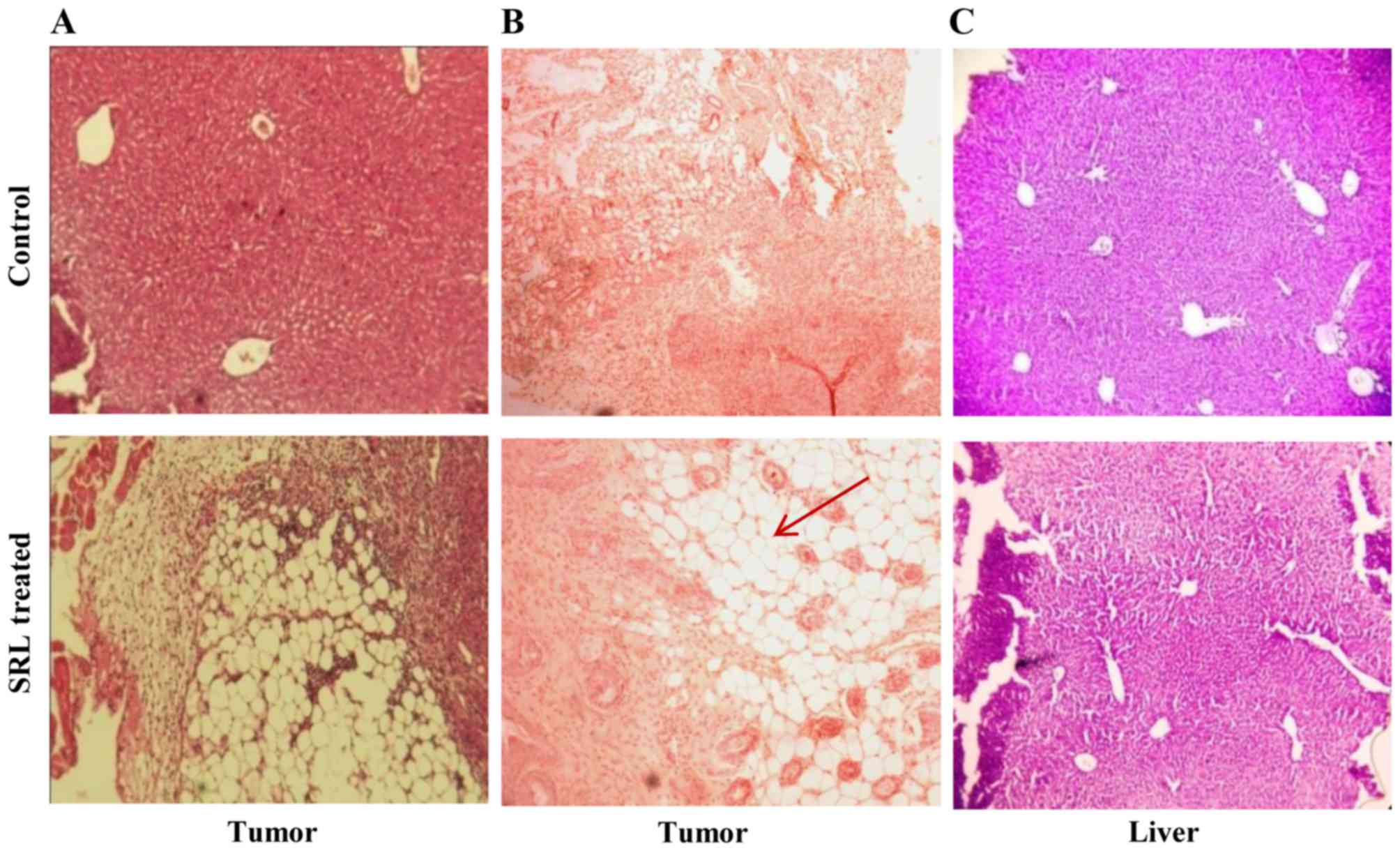
treatment for 14 days. The histology of the tumour sections of the
SRL-treated and untreated animals showed marked differences. The
SRL-treated mouse tumours appeared more like normal ones while the
TBS-treated control mouse tumours showed a characteristic tumour
appearance. The histology of liver sections showed that SRL-treated
mice appeared normal compared to the control-treated mice bearing
tumours, indicating the tumour-regressing effect of SRL (Fig. 2). Moreover, all the treated animals
were healthy without any significant changes in body weight
compared to the controls. Complete blood count (CBC) revealed no
detectable changes in the SRL-treated and TBS-treated groups
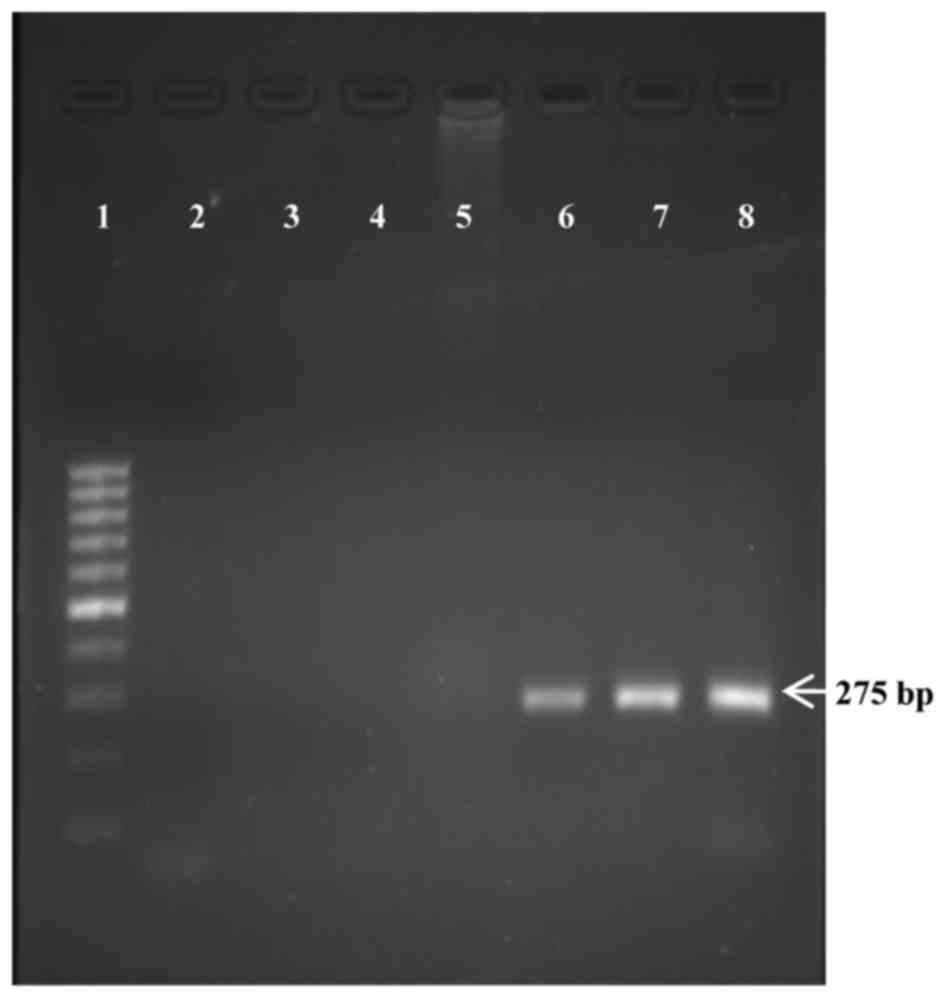
(Table I). PCR analysis of the
tumours obtained from the experimental mice showed similar product
size of 275 bp as that of the implanted HT29 cells, indicating that
the tumours which developed in nude mice were from the implanted
human cancer cells and not from the mice (Fig. 3).
 | Table I.Toxicity studies of SRL in BALB/c
nude mice. |
Table I.
Toxicity studies of SRL in BALB/c
nude mice.
| Parameters | SRL (750
µg/mice) | Control (TBS) |
|---|
| Initial body weight
(g) | 20.31 | 20.62 |
| Final body weight
(g) | 19.97 | 20.94 |
| Mortality | None | None |
| RBC
(106/µl) | 9.69 | 10.33 |
| WBC
(103/µl) | 1.81 | 1.38 |
| Platelet count
(103/µl) | 1009 | 990 |
| Hemoglobin
(g/dl) | 14.4 | 15.73 |
Anti-angiogenic effect of SRL
SRL-treated animals also showed clear reduction in
the number of blood vessels in the excised tumours compared with
that from the control-treated animals (Fig. 1), indicating an effect of SRL
treatment on suppression of angiogenesis. The anti-angiogenesis
effect of SRL was also supported by the immunohistological analysis
in which the expression of PECAM (CD31), an angiogenesis marker,
was observed to be much lower in the SRL-treated animals than that
in the control-treated animals (Fig.
2B).
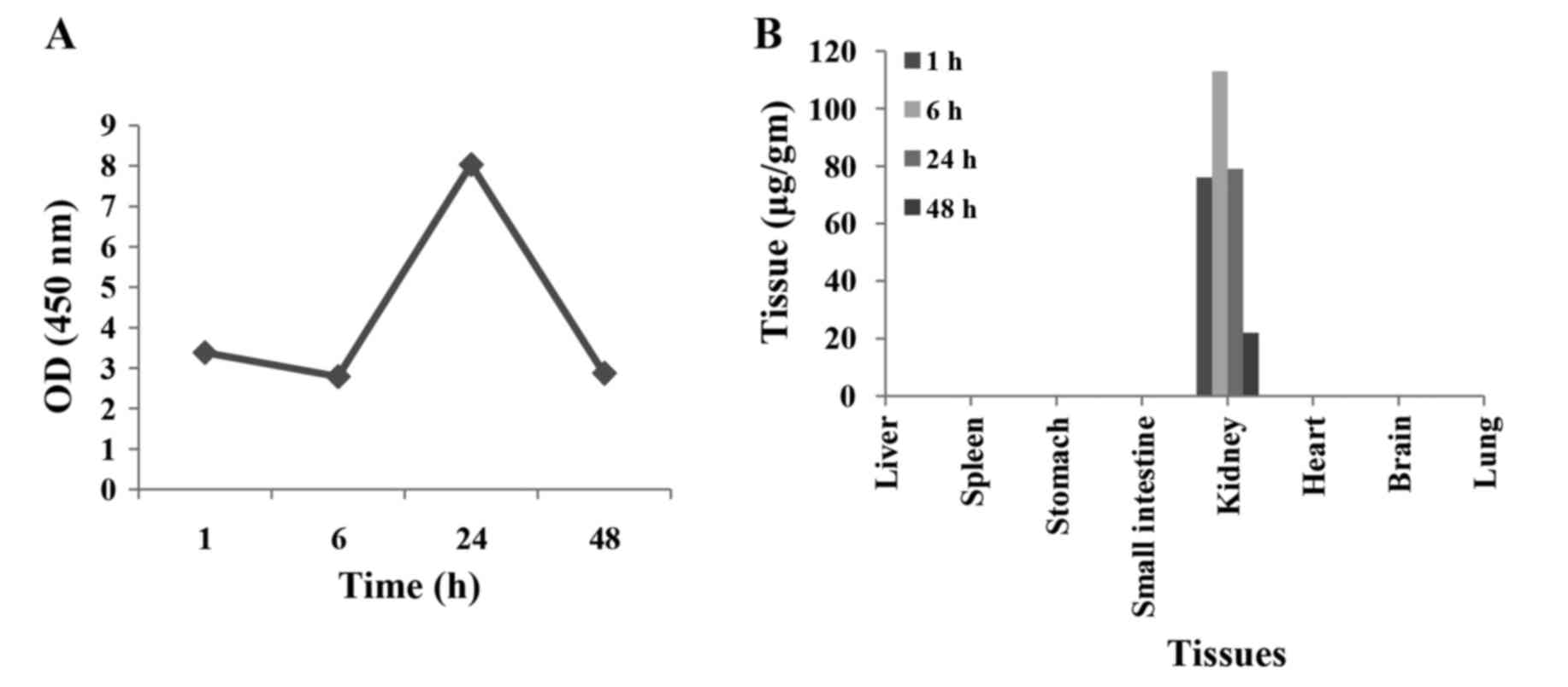
Pharmacokinetic profile
Pharmacokinetic profile of SRL in Swiss albino mice
was obtained by evaluation of serum samples from the SRL-treated
mice by ELISA. Mice injected with SRL (24 mg/kg body weight/mouse)
intraperitoneally showed maximal SRL concentrations in serum at 24
h (8.02 µg/ml) (Fig. 4A). These
results indicate that SRL was not accumulated in the body and was
eliminated. Biochemical parameters in serum samples collected at 24
h and analysed for hepatic function namely SGPT, SGOT, bilirubin
and cholesterol exhibited no significant alterations between the
SRL-treated mice and control-treated mice. Biochemical parameters
related to kidney functions including urea, uric acid creatinine
and total protein also showed no significant differences between
the SRL-treated and control group (Table II). These results indicate the
non-toxic nature of SRL treatment in the mice.
 | Table II.Toxicity studies of SRL in Swiss
albino mice. |
Table II.
Toxicity studies of SRL in Swiss
albino mice.
| Parameters | Std. values | TBS | SRL |
|---|
| OT IU/l | 54–298 | 98.6 | 64.5 |
| PT IU/l | 17–77 | 43 | 25.5 |
| CREAT mg/dl | 0.3–1 | 0.43 | 0.4 |
| BUN mg/dl | 8–33 | 40.7 | 33.6 |
| LDH g/dl | 159–1045 | 1132.3 | 557.5 |
In vivo biodistribution
The presence of SRL in various organs including the
liver, kidney, lung, spleen, stomach, small intestine, heart and
brain, respectively was analysed. The lectin was detected in trace
amounts in the kidney but not in any other organ (Fig. 4B). SRL at 76, 113, 79 and 22 µg/g
was detected in the kidney at 1, 6, 24 and 48 h, respectively.
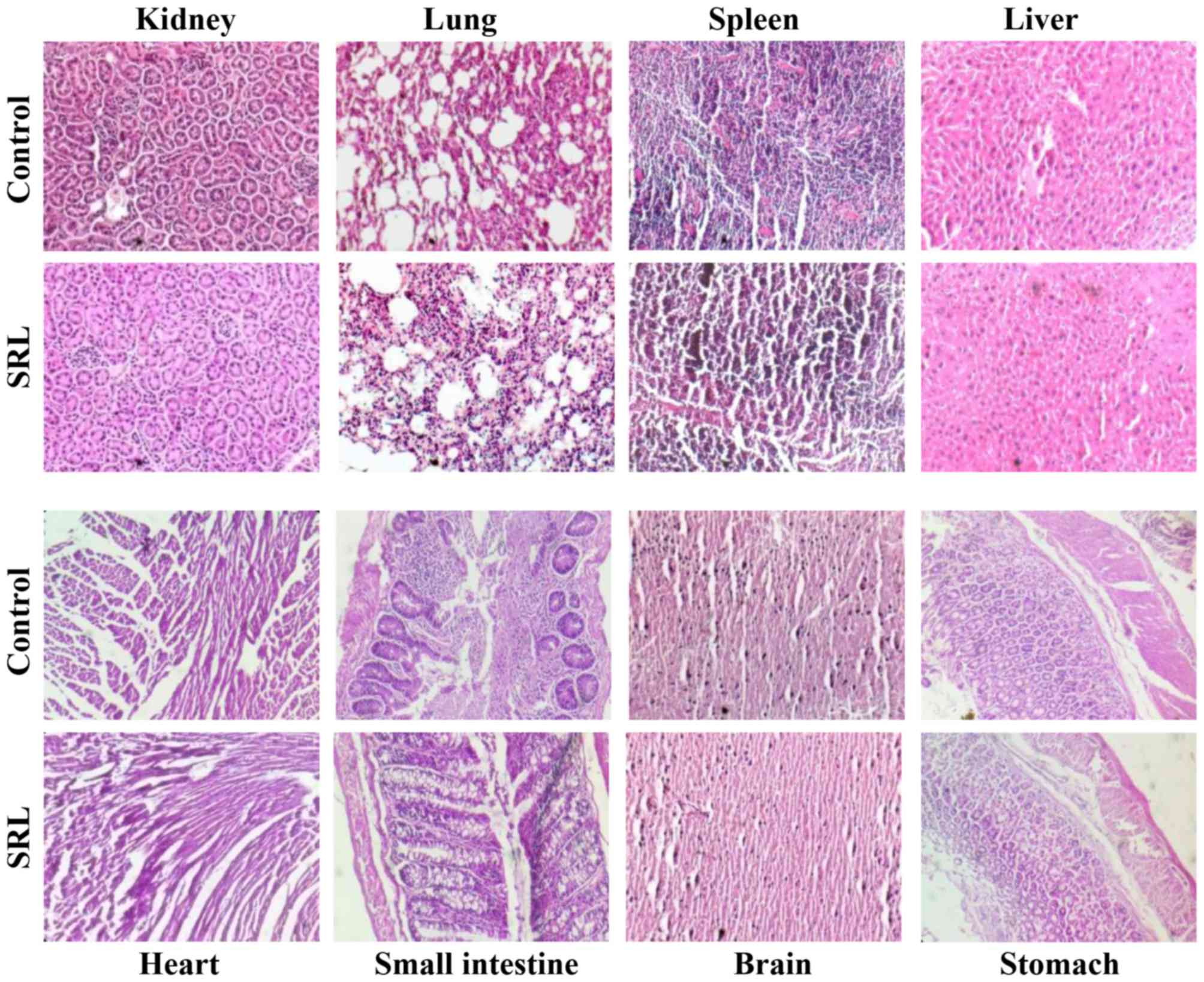
H&E staining of tissue sections of the different organs showed
no morphological changes at 24 h between the SRL-treated or
control-treated animals (Fig. 5).
These results suggest that SRL is effectively cleared in the body
and is non-toxic to mice.
Discussion
The present study reports that when administered
intraperitoneally, SRL reduces tumour growth and inhibits
angiogenesis in human colon cancer HT-29 cell xenografts in mice
and is non-toxic. SRL was demonstrated to have good renal clearance
and was eliminated without causing any sign of toxicity or
significant changes in biochemical parameters of the mice.
Previous studies have shown that SRL induces cell
apoptosis and inhibits cell growth in human colon cancer cells
in vitro and suppresses tumour growth in NOD-SCID mice
bearing HT-29 xenografts in vivo when injected
intratumourally (16). SRL was
shown to affect multiple signalling pathways in HT-29 cells by
interacting with cell membrane proteins involved in cell survival,
apoptosis and tumorigenesis (19,20).
The present study revealed that intraperitoneal introduction of SRL
suppressed tumour growth in the xenograft mouse model. H&E
staining of the liver sections of the SRL-treated and
control-treated mice showed no difference in morphology of the
liver but the expression of CD-31 was substantially lower in the
SRL-treated mice. This suggests that SRL treatment also inhibits
angiogenesis. After SRL intraperitoneal injection, the level of SRL
in the blood was seen to be peaked at 24 h. It is known that almost
all drugs, chemicals and xenobiotics are eliminated through renal
excretion; hence it was necessary to estimate the effects of SRL on
kidney function (30,31). There were no changes in biochemical
parameters in the SRL-treated mice compared to the control-treated
mice group indicating no alterations in the animal hepatic and
renal functions. Biodistribution analysis showed that SRL did not
accumulated in any organ apart from its temporary appearance in the
kidney. Pharmacokinetic and biodistribution patterns indicate that
SRL has a very fast rate of absorption and is rapidly distributed
to the entire body through the circulation. SRL appears to be
quickly eliminated from the body through the kidney, indicating a
beneficial property of SRL as a therapeutic agent.
SRL binds to the oncofetal TF antigen and TF-related
carbohydrate structures (12,13).
In colorectal cancer, increased expression of oncofetal TF and
sialylated Tn is common (32–37)
and can be detected using lectins including peanut lectin,
Arachis hypogea (PNA) (38)
and Sambucus nigra lectin (SNA). Many lectins have shown
anticancer effects when tested in vitro and in some cases
also in vivo but not many have reached clinical trials
partly due to their cytotoxicity (39). Tn antigen (GalNAc-Ser/Thr)-binding
Helix pomatia lectin (HPA) identifies a subset of metastatic
colorectal cancer. HPA binding also reveals overexpression of
sialyl-Lewis X carbohydrate antigen which is correlated with poor
prognosis of colorectal cancer (40,41).
Viscum album var. coloratum agglutinin (VCA), binding lectin
from Korean mistletoe has been used in human clinical trials at low
doses for the treatment of different cancers without serious
side-effects and the action seems to be beneficial (39). A mannose/sialic acid-binding plant
lectin from Polygonatum cyrtonema lectin (PCL) has shown
antitumour effects by inducing apoptosis and autophagy in cancer
cells (42,43). Two cytotoxic mistletoe isolectins
designated as KML-IIU and KML-IIL were found to exhibit
cytotoxicity in various human and mouse cancer cell lines (44). Mistletoe lectin has been extensively
studied for its clinical use and has reached clinical trials
(45,46). Reports are available on the
antitumor effects of Concanavalin A lectin, one of the most
extensively studied lectin. Clinical use of Con A is questionable
due to its strong cytotoxic effects including the induction of
hepatitis (47,48).
The strong anti-proliferative, antitumour and
anti-angiogenesis effects of SRL shown in vitro and in
vivo in the present and previous studies with no detectable
cytotoxic effects confirm the high potential of SRL as a
therapeutic agent for cancer treatment.
Acknowledgements
The present study was supported by funding from the
British Council under UKIERI, UGC under UPE and CPEPA and DBT-BIRAC
programme.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
SRL
|
Sclerotium rolfsii lectin
|
|
HT-29
|
human colorectal adenocarcinoma grade
II cell line
|
|
TF
|
Thomsen-Friedenreich antigen
|
|
AAL
|
Agrocybe aegerita lectin
|
|
ABL
|
Agaricus bisporus lectin
|
|
PNA
|
peanut agglutinin
|
|
SNA
|
Sambucus nigra lectin
|
|
HPA
|
Helix pomatia lectin
|
|
VCA
|
Viscum album var. coloratum
agglutinin
|
|
PCL
|
Polygonatum cyrtonema
lectin
|
|
Con A
|
Concanavalin A lectin
|
|
BSA
|
bovine serum albumin
|
|
DMEM
|
Dulbeccos modified Eagles medium
|
|
FCS
|
fetal calf serum
|
|
GalNAc
|
N-acetyl-D-galactosamine
|
|
GlcNAc
|
N-acetyl-D-glucosamine
|
|
HRP
|
horseradish peroxidase
|
|
PBS
|
phosphate-buffered saline
|
|
SDS
|
sodium dodecyl sulphate
|
|
TBS
|
Tris-buffered saline
|
|
RT
|
room temperature
|
|
PBST
|
phosphate-buffered saline Tween-20
|
References
|
1
|
World Health Organization: Global Health
Observatory data repository 2011. Number of deaths (world) by
cause; http://apps.who.int/gho/data/node.main.CODWORLD?lang=enAccessed.
October 31–2013
|
|
2
|
American Cancer Society: Cancer Facts
& Figures 2015. US Mortality Data, National Center for Health
Statistic Center for Disease, Control & Prevention.
2015.https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin MD, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bies C, Lehr CM and Woodley JF:
Lectin-mediated drug targeting: History and applications. Adv Drug
Deliv Rev. 56:425–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu LG, Fernig DG, White MR, Spiller DG,
Appleton P, Evans RC, Grierson I, Smith JA, Davies H, Gerasimenko
OV, et al: Edible mushroom (Agaricus bisporus) lectin, which
reversibly inhibits epithelial cell proliferation, blocks nuclear
localization sequence-dependent nuclear protein import. J Biol
Chem. 274:4890–4899. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu L, Fernig DG, Smith JA, Milton JD and
Rhodes JM: Reversible inhibition of proliferation of epithelial
cell lines by Agaricus bisporus (edible mushroom) lectin.
Cancer Res. 53:4627–4632. 1993.PubMed/NCBI
|
|
7
|
Zhao C, Sun H, Tong X and Qi Y: An
antitumour lectin from the edible mushroom Agrocybe
aegerita. Biochem J. 374:321–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang S, Chen Y, Wang M, Yin Y, Pan Y, Gu
B, Yu G, Li Y, Wong BH, Liang Y, et al: A novel lectin from
Agrocybe aegerita shows high binding selectivity for
terminal N-acetylglucosamine. Biochem J. 443:369–378. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HX, Liu WK, Ng TB, Ooi VE and Chang
ST: The immunomodulatory and antitumor activities of lectins from
the mushroom Tricholoma mongolicum. Immunopharmacology.
31:205–211. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YR, Liu QH, Wang HX and Ng TB: A novel
lectin with potent antitumor, mitogenic and HIV-1 reverse
transcriptase inhibitory activities from the edible mushroom
Pleurotus citrinopileatus. Biochim Biophys Acta. 1780:51–57.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang GQ, Sun J, Wang HX and Ng TB: A
novel lectin with antiproliferative activity from the medicinal
mushroom Pholiota adiposa. Acta Biochim Pol. 56:415–421.
2009.PubMed/NCBI
|
|
12
|
Swamy BM, Hegde GV, Naik RS and Inamdar
SR: T-antigen binding lectin from the phytopathogenic fungus
Sclerotium rolfsii. Lect. Biol Biochem Clin Biochem.
15:45–55. 2001.
|
|
13
|
Chachadi VB, Inamdar SR, Yu LG, Rhodes JM
and Swamy BM: Exquisite binding specificity of Sclerotium
rolfsii lectin toward TF-related O-linked mucin-type glycans.
Glycoconj J. 28:49–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu LG: The oncofetal Thomsen-Friedenreich
carbohydrate antigen in cancer progression. Glycoconj J.
24:411–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leonidas DD, Swamy BM, Hatzopoulos GN,
Gonchigar SJ, Chachadi VB, Inamdar SR, Zographos SE and Oikonomakos
NG: Structural basis for the carbohydrate recognition of the
Sclerotium rolfsii lectin. J Mol Biol. 368:1145–1161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inamdar SR, Savanur MA, Eligar SM,
Chachadi VB, Nagre NN, Chen C, Barclays M, Ingle A, Mahajan P,
Borges A, et al: The TF-antigen binding lectin from Sclerotium
rolfsii inhibits growth of human colon cancer cells by inducing
apoptosis in vitro and suppresses tumor growth in vivo.
Glycobiology. 22:1227–1235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savanur MA, Eligar SM, Pujari R, Chen C,
Mahajan P, Borges A, Shastry P, Ingle A, Kalraiya RD, Swamy BM, et
al: Sclerotium rolfsii lectin induces stronger inhibition of
proliferation in human breast cancer cells than normal human
mammary epithelial cells by induction of cell apoptosis. PLoS One.
9:e1101072014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eligar SM, Pujari R, Swamy BM, Shastry P
and Inamdar SR: Sclerotium rolfsii lectin inhibits
proliferation and induces apoptosis in human ovarian cancer cell
line PA-1. Cell Prolif. 45:397–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barkeer S, Guha N, Hothpet V, Adavigowda
Saligrama D, Hegde P, Padmanaban A, Yu LG, Swamy BM and Inamdar SR:
Molecular mechanism of anticancer effect of Sclerotium
rolfsii lectin in HT29 cells involves differential expression
of genes associated with multiple signaling pathways: A microarray
analysis. Glycobiology. 25:1375–1391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barkeer S, Gudihal R, Eligar SM, Hegde P,
Lu GY, Bale MS and Inamdar SR: Identification and characterization
of Sclerotium rolfsii lectin (SRL) binding proteins from
human colon epithelial cancer HT29 cells. Translational Biomedicine
ISSN 2172-0479. 2015.
|
|
21
|
Sharma SK, Pedley RB, Bhatia J, Boxer GM,
El-Emir E, Qureshi U, Tolner B, Lowe H, Michael NP, Minton N, et
al: Sustained tumor regression of human colorectal cancer
xenografts using a multifunctional mannosylated fusion protein in
antibody-directed enzyme prodrug therapy. Clin Cancer Res.
11:814–825. 2005.PubMed/NCBI
|
|
22
|
Vallespí MG, Pimentel G, Cabrales-Rico A,
Garza J, Oliva B, Mendoza O, Gomez Y, Basaco T, Sánchez I, Calderón
C, et al: Antitumor efficacy, pharmacokinetic and biodistribution
studies of the anticancer peptide CIGB-552 in mouse models. J Pept
Sci. 20:850–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ingle AD and Hosetti BB: Use of
immuno-compromised mouse model for establishment and study of human
animal tumours. Indian J Vet Pathol. 34:156–161. 2010.
|
|
24
|
Simpson RJ: Homogenization of mammalian
tissue. Cold Spring Harb Protoc pdb prot 5455. 2010.https://doi.org/10.1101/pdb.prot5455https://doi.org/10.1101/pdb.prot5455.
View Article : Google Scholar
|
|
25
|
Burden DW: Guide to the Homogenization of
Biological Samples. Random Primers. 7:2008.1–14, 2008. http://www.opsdiagnostics.com/notes/ranpri/Homogenization%20Guide%20ver.pdf
|
|
26
|
Hartmann C, Smeyers-Verbeke J, Massart DL
and McDowall RD: Validation of bioanalytical chromatographic
methods. J Pharm Biomed Anal. 17:193–218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn JE, Karlsson MO, Dunne A and Ludden
TM: Likelihood based approaches to handling data below the
quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn.
35:401–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jusko WJ: Use of pharmacokinetic data
below lower limit of quantitation values. Pharm Res. 29:2628–2631.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beal SL: Ways to fit a PK model with some
data below the quantification limit. J Pharmacokinet Pharmacodyn.
28:481–504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sallam H, El-Serafi I, Meijer L and Hassan
M: Pharmacokinetics and biodistribution of the cyclin-dependent
kinase inhibitor -CR8- in mice. BMC Pharmacol Toxicol. 14:502013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khlebtsov N and Dykman L: Biodistribution
and toxicity of engineered gold nanoparticles: A review of in vitro
and in vivo studies. Chem Soc Rev. 40:1647–1671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghazarian H, Idoni B and Oppenheimer SB: A
glycobiology review: Carbohydrates, lectins and implications in
cancer therapeutics. Acta Histochem. 113:236–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Campbell BJ, Finnie IA, Hounsell EF and
Rhodes JM: Direct demonstration of increased expression of
Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and
ulcerative colitis mucin and its concealment in normal mucin. J
Clin Invest. 95:571–576. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh R, Campbell BJ, Yu LG, Fernig DG,
Milton JD, Goodlad RA, FitzGerald AJ and Rhodes JM: Cell
surface-expressed Thomsen-Friedenreich antigen in colon cancer is
predominantly carried on high molecular weight splice variants of
CD44. Glycobiology. 11:587–592. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hanisch FG and Baldus SE: The
Thomsen-Friedenreich (TF) antigen: A critical review on the
structural, biosynthetic and histochemical aspects of a
pancarcinoma-associated antigen. Histol Histopathol. 12:263–281.
1997.PubMed/NCBI
|
|
36
|
Almogren A, Abdullah J, Ghapure K,
Ferguson K, Glinsky VV and Rittenhouse-Olson K:
Anti-Thomsen-Friedenreich-Ag (anti-TF-Ag) potential for cancer
therapy. Front Biosci (Schol Ed). 4:840–863. 2012.PubMed/NCBI
|
|
37
|
Belov L, Zhou J and Christopherson RI:
Cell surface markers in colorectal cancer prognosis. Int J Mol Sci.
12:78–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh R, Subramanian S, Rhodes JM and
Campbell BJ: Peanut lectin stimulates proliferation of colon cancer
cells by interaction with glycosylated CD44v6 isoforms and
consequential activation of c-Met and MAPK: Functional implications
for disease-associated glycosylation changes. Glycobiology.
16:594–601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yau T, Dan X, Ng CC and Ng TB: Lectins
with potential for anti-cancer therapy. Molecules. 20:3791–3810.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mitchell BS and Schumacher U: The use of
the lectin Helix pomatia agglutinin (HPA) as a prognostic
indicator and as a tool in cancer research. Histol Histopathol.
14:217–226. 1999.PubMed/NCBI
|
|
41
|
Peiris D, Ossondo M, Fry S, Loizidou M,
Smith-Ravin J and Dwek MV: Identification of O-linked
glycoproteins binding to the lectin Helix pomatia agglutinin
as markers of metastatic colorectal cancer. PLoS One.
10:e01383452015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu B, Wu JM, Li J, Liu JJ, Li WW, Li CY,
Xu HL and Bao JK: Polygonatum cyrtonema lectin induces
murine fibrosarcoma L929 cell apoptosis and autophagy via blocking
Ras-Raf and PI3K-Akt signaling pathways. Biochimie. 92:1934–1938.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang SY, Yu QJ, Bao JK and Liu B:
Polygonatum cyrtonema lectin, a potential antineoplastic
drug targeting programmed cell death pathways. Biochem Biophys Res
Commun. 406:497–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kang TB, Song SK, Yoon TJ, Yoo YC, Lee KH,
Her E and Kim JB: Isolation and characterization of two Korean
mistletoe lectins. J Biochem Mol Biol. 40:959–965. 2007.PubMed/NCBI
|
|
45
|
Yoon TJ, Yoo YC, Kang TB, Song SK, Lee KB,
Her E, Song KS and Kim JB: Antitumor activity of the Korean
mistletoe lectin is attributed to activation of macrophages and NK
cells. Arch Pharm Res. 26:861–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rostock M, Huber R, Greiner T, Fritz P,
Scheer R, Schueler J and Fiebig HH: Anticancer activity of a
lectin-rich mistletoe extract injected intratumorally into human
pancreatic cancer xenografts. Anticancer Res. 25:1969–1975.
2005.PubMed/NCBI
|
|
47
|
Miyagi T, Takehara T, Tatsumi T, Suzuki T,
Jinushi M, Kanazawa Y, Hiramatsu N, Kanto T, Tsuji S, Hori M, et
al: Concanavalin a injection activates intrahepatic innate immune
cells to provoke an antitumor effect in murine liver. Hepatology.
40:1190–1196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lei HY and Chang CP: Lectin of
Concanavalin A as an anti-hepatoma therapeutic agent. J Biomed Sci.
16:102009. View Article : Google Scholar : PubMed/NCBI
|