Introduction
The majority (90%) of cases of esophageal cancer in
China are esophageal squamous cell carcinomas (ESCC). The high
incidence of ESCC in China is part of the aptly named ‘Asian
Esophageal Cancer Belt’, which is characterized by a low 5-year
survival rate and high incidence and mortality rates, although,
contributing risk factors and mechanisms of ESCC remain unknown
(1,2).
Tumor necrosis factor-α (TNF-α) is a key cytokine
involved in the onset of chronic inflammation, which plays a major
role in the tumor microenvironment. There is a vicious cycle
between tumor formation and inflammation, in which inflammation
promotes tumor formation, and tumor formation promotes inflammation
(3). TNFα is a major mediator of
tumor-related inflammation in the tumor microenvironment and
abundant evidence has demonstrated that TNFα promotes tumor growth,
metastatic and invasive activities, and stimulation of
neoangiogenesis in the tumor microenvironment in breast, ovarian,
colorectal, prostate, bladder and renal cancers, as well as
melanoma and esophageal carcinoma (3–8).
However, the mechanisms through which TNFα promotes tumor
progression remain unknown.
TNF-α-induced protein 2 (TNFAIP2) is located on
chromosome 14q32 and encodes a protein consisting of 654 amino
acids. TNFAIP2 is a member of the Sec6 family and was originally
identified as a TNF-α-induced protein in human endothelial cells
(9). Rusiniak et al found
that TNFAIP2 is a potential target for transcriptional repression
by the acute promyelocytic leukemia (PML)-retinoic acid receptor α
fusion protein (10). Moreover,
TNFAIP2, as an independent prognostic indicator in nasopharyngeal
carcinoma, is highly expressed in nasopharyngeal carcinoma, and
promotes migration and invasion likely by modulating actin
organization (11,12).
Based on these pivotal findings, we hypothesized
that TNFAIP2 plays a role in the tumorigenesis and metastasis of
ESCC and thus is a potential therapeutic target for ESCC. However,
there are relatively few studies of the molecular functions of
TNFAIP2, and the possible associations with tumorigenesis and
metastasis in ESCC remain unclear.
Materials and methods
Patients and clinical specimens
A total of 79 ESCC specimens (55 paraffin-embedded
and 24 fresh) with adjacent free-cancerous esophageal tissues that
were frozen and stored in liquid nitrogen were histopathologically
diagnosed at the Department of Pathology of Chongqing Medical
University (Chongqing, China), and the Department of Cardiothoracic
Surgery of The First Affiliated Hospital of Chongqing Medical
University (Chongqing, China), respectively, from 2013 to 2015.
Clinical and tumor (T)-lymph node (N)-metastasis (M) staging was
performed in accordance with the guidelines of the 6th edition of
the International Union Against Cancer (UICC). None of the patients
received radiation or chemotherapy before surgery. Clinical patient
information is summarized in Table
I. The study protocol was approved by the Institutional
Research Ethics Committee of The First Affiliated Hospital of
Chongqing Medical University and informed consent was obtained from
all patients.
 | Table I.Relationships between TNFAIP2
expression and clinicopathological features of the ESCC
patients. |
Table I.
Relationships between TNFAIP2
expression and clinicopathological features of the ESCC
patients.
| Characteristics | Total (n) | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.66 |
|
≥63b | 32 | 19 | 13 |
|
|
<63 | 23 | 15 | 8 |
|
| Gender |
|
|
| 0.288 |
|
Male | 43 | 25 | 18 |
|
|
Female | 12 | 9 | 3 |
|
|
Differentiation |
|
|
| 0.408 |
| G1 | 16 | 8 | 8 |
|
| G2 | 24 | 17 | 7 |
|
| G3 | 15 | 9 | 6 |
|
| T stage |
|
|
| 0.041a |
| T1 | 8 | 2 | 6 |
|
| T2 | 9 | 4 | 5 |
|
| T3 | 28 | 20 | 8 |
|
| T4 | 10 | 8 | 2 |
|
| N stage |
|
|
|
0.019a |
| N0 | 28 | 13 | 15 |
|
| N1 | 14 | 9 | 5 |
|
| N2 | 13 | 12 | 1 |
|
| UICC stage |
|
|
| 0.023a |
| I | 11 | 3 | 8 |
|
| II | 23 | 15 | 8 |
|
|
III | 21 | 16 | 5 |
|
| β-catenin |
|
|
| 0.006a |
|
High | 36 | 27 | 9 |
|
|
Low | 19 | 7 | 12 |
|
| E-cadherin |
|
|
| <0.001 |
|
High | 18 | 5 | 13 |
|
|
Low | 37 | 29 | 8 |
|
Immunohistochemical analysis
Immunohistochemical analysis of 55 human
paraffin-embedded ESCC samples was performed to study the
expression profiles of TNFAIP2, β-catenin and E-cadherin using
anti-human TNFAIP2 antibody (dilution, 1:100; cat. no. sc-30138;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), β-catenin
antibody (dilution, 1:100; cat. no. 8480) and E-cadherin antibody
(dilution, 1:400; cat. no. 3195) (both from Cell Signaling
Technology, Inc., Danvers, MA, USA). The procedure was similar to
previously described methods (11,13–15).
The histopathological features and patient data of the samples were
evaluated via the H-scores method by two board-certified
pathologists who were blinded to patient data. The intensity of
specific staining was defined as negative (0), weakly positive
(1+), somewhat positive (2+), strongly positive (3+) and very
strongly positive (4+). H-score = Σ Pi (i
+ 1), where Pi is the percentage of stained cells
in each intensity category; i = 1-4. Total scores ≥300 were
characterized as a high-level expression (16,17).
Western blot analysis
Protein extraction and western blot analysis were
performed as previously described (15,18).
Briefly, the cells were lysed with lysis buffer containing protease
inhibitors and the lysates (40 µg/lane) were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Then, proteins were transferred to a polyvinylidene fluoride
membrane, which was incubated with appropriate secondary antibodies
[i.e., anti-TNFAIP2 (dilution, 1:1,000; cat. no. sc-30138; Santa
Cruz Biotechnology, Inc.), β-catenin (1:1,000; cat. no. 8480),
E-cadherin (dilution, 1:1,000; cat. no. 3195; both from Cell
Signaling Technology, Inc.), c-myc (dilution, 1:1,000; cat. no.
ab32072; Abcam, Cambridge, UK), cyclin D1 (dilution, 1:1,000; cat.
no. 2978), MMP-7 (dilution, 1:1,000; cat. no. 3801), p-GSK-3β
(dilution, 1:1,000; cat. no. 9322), GSK-3β (1:1,000; cat. no.
12456), Snail (dilution, 1:1,000; cat. no. 3879) (all from Cell
Signaling Technology, Inc.), and β-actin, respectively] that were
detected and visualized using an enhanced chemiluminescence
detection system (BeyoECL Plus; Beyotime Institute of
Biotechnology, Shanghai, China).
Cell culture
Eca109 and Kyse150 cells, both ESCC cell lines, were
maintained in Roswell Park Memorial Institute (RPMI)-1640 medium
(Corning, Inc., Corning, NY, USA) supplemented with 10% fetal
bovine serum (FBS), 100 U/ml of penicillin, and 100 µg/ml of
streptomycin, and incubated in a humidified atmosphere of 5%
CO2 at 37̊C.
Treatment of cells with recombinant
human (rh)-TNFα
rh-TNFα (cat. no. 10602; Sino Biological, Inc.,
Beijing, China) was diluted with complete medium to a concentration
of 25 ng/ml. Cells were treated with rh-TNFα for 48 h and TNFAIP2
mRNA and protein expression levels were detected by quantitative
real-time PCR (qRT-PCR) and western blot analysis, respectively.
All experiments were repeated three times. The results of qRT-PCR
were normalized with β-actin using the 2−∆∆Ct method
(19).
Production of lentiviral constructs
and infection
Lentivirus RNA interference of TNFAIP2 (LV-RNAi
TNFAIP2 shRNA, 5′-TCTTCACCAAAGGGAAGAA-3′); and a negative control
lentiviral vector (LV-CON shRNA, 5′-TTCTCCGAACGTGTCACGT-3′) were
designed and synthesized by GeneChem Co., Ltd. (Shanghai, China).
The lentivirus titers were 5×108 infectious U/ml.
To determine the multiplicity of infection (MOI) of
the Eca109 and Kyse150 cell lines, a pre-experiment was performed.
Briefly, Eca109 and Kyse150 cells (2×103 cells/well)
were cultured in 96-well plates for 12 h, and then infected with
0.2 µl (MOI=10), 0.4 µl (MOI=20) or 0.6 µl (MOI=30) of lentivirus
diluted with serum-free medium to 1×108 infectious U/ml,
respectively. After 12 h, the medium was replaced with fresh
complete medium. After three days, 95% transduction efficiencies of
the Eca109 and Kyse150 cells were infected at an MOI of 30 and 20,
respectively.
According to the results of the pre-experiment, the
Kyse150 and Eca109 cells (2×104 cells/well) were
cultured in 6-well plates and transfected with 4 and 6 µl of the
diluted lentivirus, respectively. To obtain stable transfection,
puromycin (1 µg/ml) was added to the cultures at 72 h after
transfection. The RNAi effectiveness was validated by qRT-PCR and
western blot analysis.
Total RNA extraction, reverse
transcription and qRT-PCR
Total RNA was isolated from esophagus carcinoma
tissues and Ec109 and Kyse150 cells, using RNAiso Plus Total RNA
extraction reagent (cat. no. 9108; Takara Bio, Inc., Otsu, Japan).
The PrimeScript™ RT reagent kit with gDNA Eraser (Perfect
Real-Time) (cat. no. RR047A; Takara Bio, Inc.) was used for reverse
transcription of total RNA (1 µg). qRT-PCR was performed with an
Applied Biosystems 7500 Real-Time PCR System (Life Technologies,
Carlsbad, CA, USA) using SYBR® Premix Ex Taq™ II
kit (Tli RnaseH Plus) (cat. no. RR820A, Takara Bio, Inc.) according
to the manufacturers instructions. β-actin was used as an
endogenous control to normalize the relative expression level of
TNFAIP2. The following primer pairs were used to amplify the mRNA
of TNFAIP2 and β-actin: (F) 5′-CCTGCTCTCCCTACGC-3′ and (R)
5′-CGTCCAAGATGCTCCG-3′ (11); and
(F) 5′-CTCCTCCTGAGCGCAAGTACTC-3′ and (R) 5′-TCCTGCTTGCTGATCCACATC-3
(13), respectively.
Cell migration and invasion
assays
Migration and invasion assays were performed as
described by Huang et al and Whitington et al
(15,20). Briefly, lentivirus-transfected
Eca109 and Kyse150 cells were plated at concentrations of
5×104 and 1×105 cells/8-µm Transwell insert
with or without Matrigel coating (both from Corning, Inc.) and
incubated for 48 h, respectively. Cells that had penetrated the
membranes were quantified by counting the number of cells in five
random microscopic fields. All experiments were performed in
triplicate.
Cell colony formation and
proliferation assays
LV-RNAi TNFAIP2 and LV-CON group cells were
trypsinized and resuspended at 2×104 cells/ml. Then, 25
µl (for colony formation; total volume, 2 ml) and 100 µl (for
proliferation) of the cell suspensions were added to each well of
6- and 96-well plates, respectively. Cells plated in the 6-well
plates were incubated for 10–14 days and the formed colonies were
fixed with 4% paraformaldehyde and stained with crystal violet for
20 min, respectively. After 24, 48 and 72 h, 10 µl of Cell Counting
Kit-8 (CCK-8; reagent) (Dojindo Molecular Technologies, Inc.,
Tokyo, Japan) was added to each well of the 96-well plates. Cell
proliferation was determined using a microplate reader (Infinite
200 PROs; Tecan Trading AG, Männedorf, Switzerland) at designed
time points by reading the absorbance at 450 nm after CCK-8
treatment for 2 h. Values of proliferation were obtained from five
replicate wells for each treatment and time point. Each experiment
was performed in triplicate.
Cell cycle and apoptosis analysis
Trypsinized cells were resuspended, washed,
centrifuged with phosphate-buffered saline (PBS) at 4̊C three
times, and then fixed with ice-cold 70% ethanol overnight at 4̊C
for the cell cycle assay or resuspended in 1,000 µl of PBS for the
cell apoptosis assay. The cell cycle and apoptosis assays were
conducted at Chongqing Medical University Life Sciences Institute.
Each assay was independently repeated three times.
Statistical analysis
SPSS 22 statistical software package (SPSS, Inc.,
Chicago, IL, USA) was used to perform all statistical analyses. The
statistical significance of the qRT-PCR results for TNFAIP2 gene
expression and for the observations on the basis of cell
proliferation, colony formation, migration, invasion, cycle and
apoptosis were assessed using Student's t-tests (two-tailed). The
Pearson χ2 test was applied to evaluate the
relationships between TNFAIP2 expression and expression of
β-catenin and E-cadherin and various clinicopathological
characteristics. The Kaplan-Meier method and the log-rank test were
used to plot and compare the survival curves, respectively. Data
shown are presented as the mean ± SD or SEM from triplicate
experiments as indicated. Significant differences were P-value
<0.05.
Results
TNFAIP2 mRNA and protein
overexpression in ESCC clinical tissue specimens
TNFAIP2 was identified as an angiogenic factor and
can be induced in umbilical vein endothelial cells by TNF-α
(9). Furthermore, TNFAIP2 regulates
invasion and metastasis in nasopharyngeal carcinoma (11), and its genetic polymorphism was
found to influence ESCC, squamous cell carcinoma of the head and
neck and gastric cancer (21–23).
However, the mechanisms underlying TNFAIP2 regulation in ESCC
remain unknown. Hence, immunohistochemical staining with
anti-TNFAIP2 antibody was used to examine TNFAIP2 protein
expression in 55 paraffin-embedded ESCC specimens which showed high
levels in 34 (62%) samples and low levels in 21 (38%) samples,
respectively. In contrast, TNFAIP2 was undetectable in the adjacent
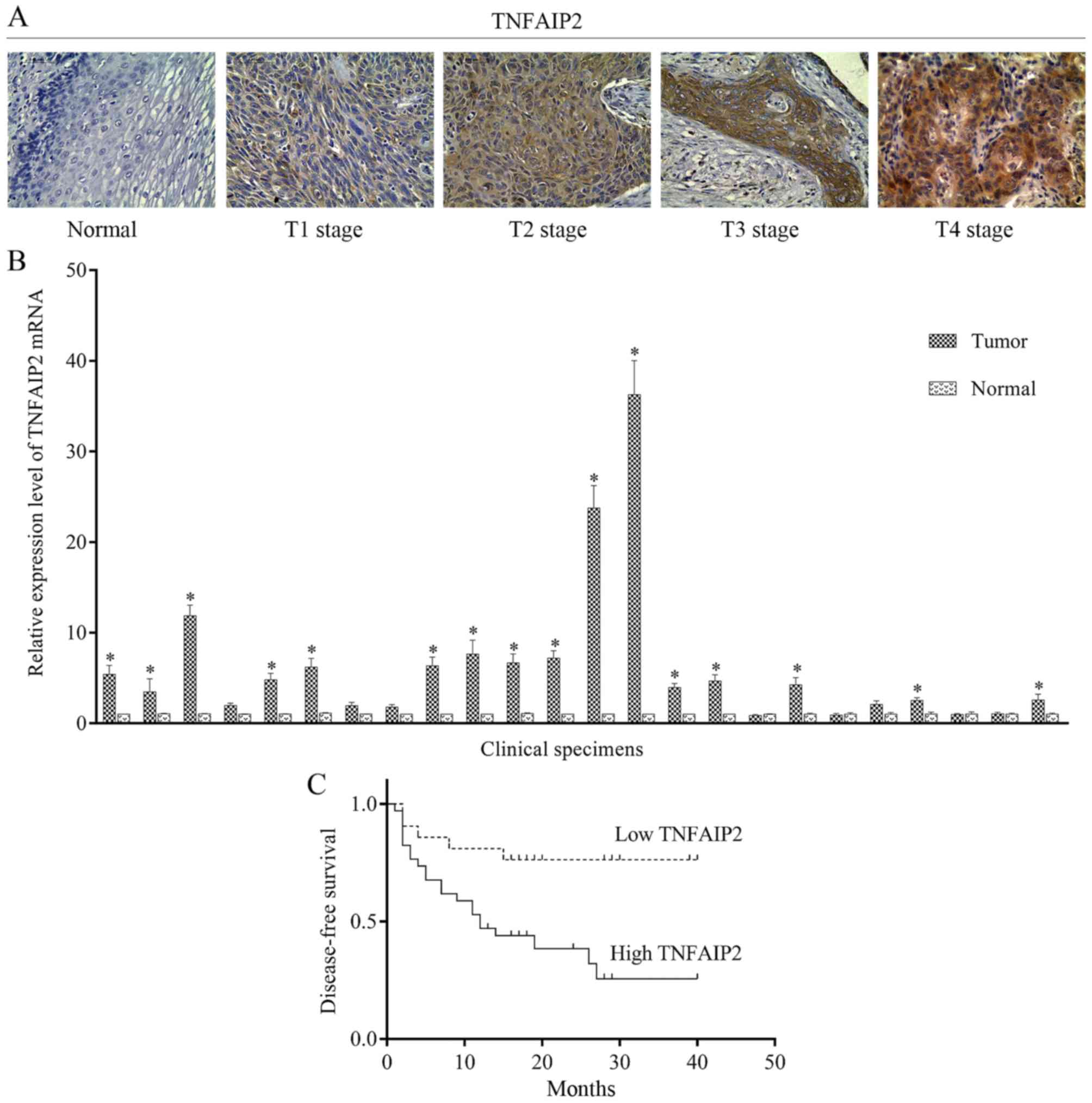
normal epithelium (Fig. 1A).
Moreover, TNFAIP2 mRNA expression was quantified by qRT-PCR, which
showed that mRNA levels in 16 (67%) of 24 samples were higher than
levels noted in the adjacent normal tissues (Fig. 1B).
Correlations between patient
characteristics, clinical features and TNFAIP2 expression
The patient characteristics and clinical features
shown in Table I were evaluated to
identify correlations between TNFAIP2 and clinicopathological
characteristics of ESCC patients, which showed that there were no
significant correlations between TNFAIP2 expression and age, gender
and cellular differentiation. However, there were significant
associations between TNFAIP2 expression and T, N and UICC stage. As
shown in Fig. 1A, TNFAIP2
expression gradually increased along with the T stage.
Association between TNFAIP2 and
disease-free survival (DFS)
Kaplan-Meier survival analysis and the log-rank test
were used to investigate potential impacts of TNFAIP2 expression on
the DFS of patients. The median follow-up period was 20 months and
at the time of the present study 28 patients survived and 27 had
died. Kaplan-Meier survival curves showed that DFS was obviously
higher with low TNFAIP2 expression than high expression (Fig. 1C). Moreover, univariate Cox
regression analysis revealed a correlation between DFS and TNFAIP2
expression (p=0.023; regression coefficient=1.047; hazard
ratio=2.849; 95% confidence interval (CI)=1.152–7.044). These
results indicate that elevated TNFAIP2 expression may be a risk
factor for ESCC-associated mortality.
TNFAIP2 mRNA and protein
overexpression in TNFα-treated cells
The results of the present study showed that
aberrant TNFAIP2 expression in ESCC clinical specimens was
correlated with intratumoral T, N and UICC stage. qRT-PCR and
western blotting were used to detect THFAIP2 expression levels in
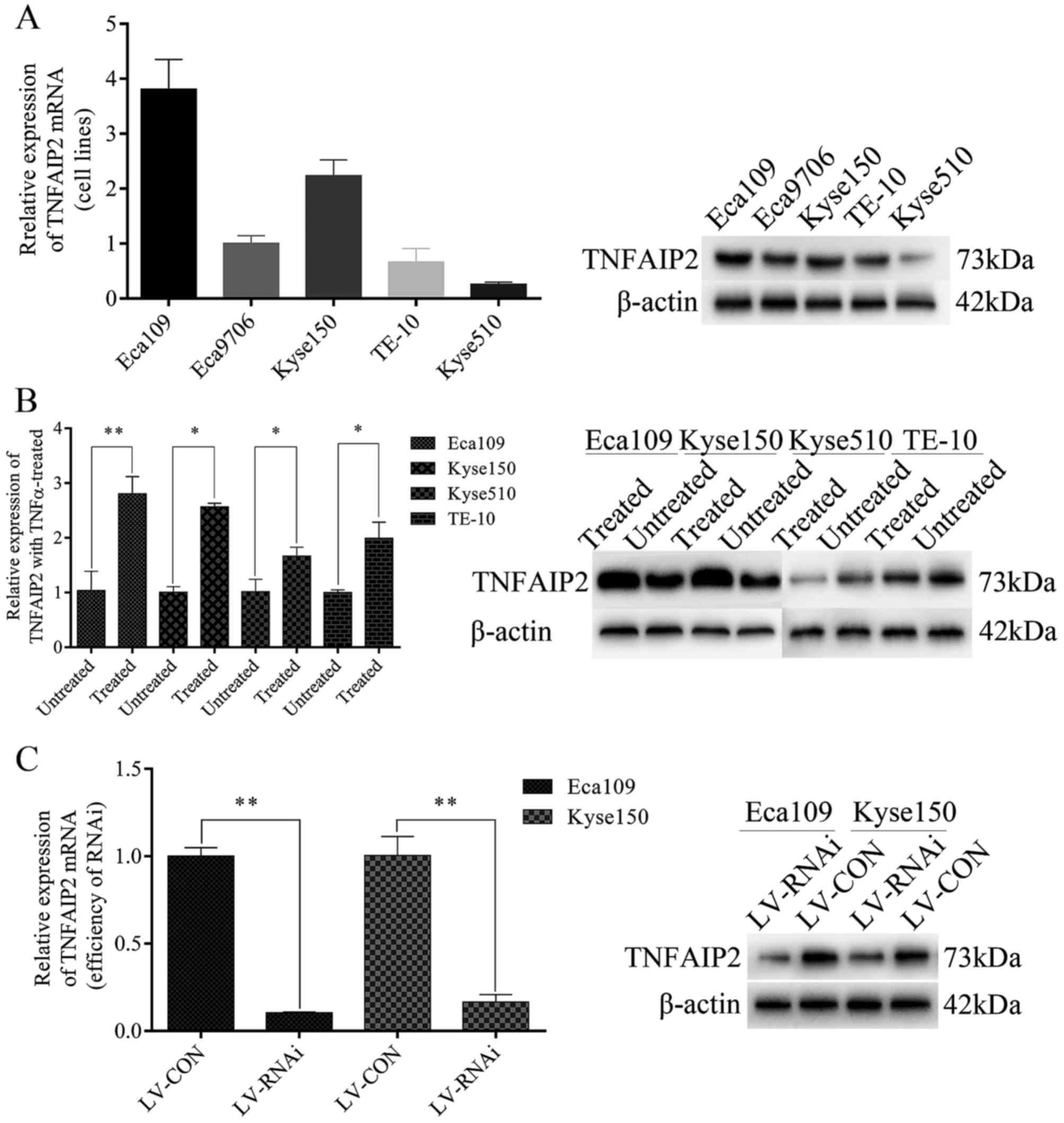
Eca109, Eca9706, TE-10, Kyse150 and Kyse510 cells. As shown in
Fig. 2A, Eca109 and Kyse150 were
used to study their biological functions influenced by TNFAIP2
in vitro. In this experiment, Eca109, Kyse150, Kyse510 and
TE-10 cells were treated with 25 ng/ml of rh-TNFα for 48 h. As
shown by the results in Fig. 2B,
there were significant differences in TNFAIP2 mRNA and protein
expression levels between treated and untreated cells, indicating
that TNFAIP2 can be induced and regulated by TNFα.
LV-RNAi-mediated TNFAIP2 knockdown
decreases tumorigenic and metastatic properties in vitro
Cell colony formation, proliferation, cell cycle,
apoptosis, migration and invasive ability play significant roles in
tumor tumorigenic and metastatic properties. Therefore, lentiviral
RNA interference (LV-RNAi) was used to knock down TNFAIP2 in the
ESCC cell lines Eca109 and Kyse150 using techniques to study
biological functions of cell lines in vitro with an empty
lentiviral vector as a negative control (LV-CON). The efficiencies
of knockdown and transfection were >75 and 90% at the mRNA and
protein levels (Fig. 2C),
respectively.
Colony formation was significantly inhibited in the
LV-RNAi group, as compared with the LV-CON group (Fig. 3A1 and A2). LV-RNAi TNFAIP2 resulted
in obvious decreased proliferation of the Eca109 and Kyse150 cells
compared with the LV-CON group (Fig.
3A3 and A4).
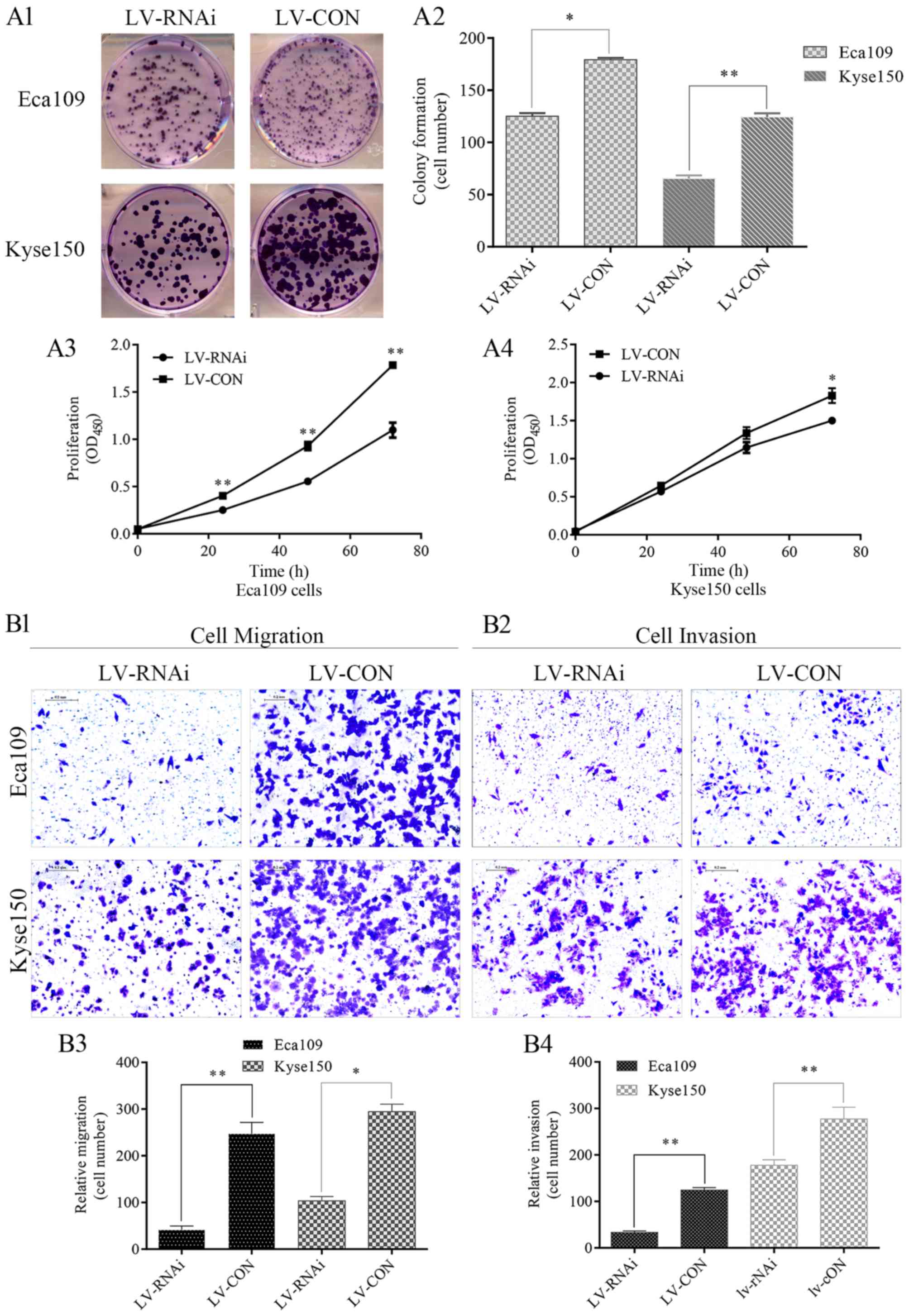 | Figure 3.Lentivirus-mediated RNA interference
(LV-RNAi) of TNFAIP2 decreased cell colony formation,
proliferation, migration and invasion, and arrested the cell cycle
in the G0/G1 phase. (A1-A4) Colony formation (cell number) and cell
proliferation (OD450) were inhibited in the LV-RNAi
group as compared with the LV-CON group. The data from three
independent experiments are presented as the mean ± standard
deviation; *p<0.05, **p<0.001. (B1-B4) Migration and invasion
were partially inhibited in the LV-RNAi group. Cells were counted
in five random fields with magnifications, of ×10. The experiments
were performed in triplicate, and the results are presented as the
mean ± standard deviation; *p<0.05, **p<0.001. (C1-C4) Cell
cycle was mainly arrested in the G0/G1 phase in the LV-RNAi group
and the decreased proportion of cells in S phase was attributed to
the cells arrested in G0/G1 phase. Three independent experiments
were performed, and the results are presented as the mean ±
standard deviation; *p<0.05. (D) TNFAIP2 regulate the expression
of genes upstream and downstream of Wnt/β-catenin. LV-RNAi-mediated
TNFAIP2 knockdown increased the expression level of p-GSK-3β and
downregulated the expression of β-catenin, which is a key factor in
the Wnt/β-catenin signaling pathway. Expression profiles of cyclin
D1, c-myc, MMP-7, Snail and E-cadherin between the LV-RNAi and
LV-CON group were compared. β-actin was used as a loading
control. |
A Transwell assay was employed to examine the role
of TNFAIP2 in cellular migration and invasion. As shown by the
Transwell assay results presented in Fig. 3B1-B4, cell migration and invasive
ability were significantly declined in the LV-RNAi group as
compared with the LV-CON group. To investigate potential mechanisms
underlying LV-RNAi-induced inhibition of colony formation and
proliferation, cell cycle distribution and apoptosis of cells in
the LV-RNAi and LV-CON groups were detected by flow cytometry,
which showed that in the LV-RNAi group, the Eca109 and Kyse150
cells were mainly arrested in the G0/G1 phase and the percentages
of cells in the G2 and S phases were decreased (Fig. 3C1-C4), but, there were no
significant differences in apoptosis between the LV-RNAi and LV-CON
groups (p=0.292). Notably, these findings demonstrated that an
abundance of TNFAIP2 plays an oncogenic role in ESCC.
Expression of β-catenin is correlated
with TNFAIP2 expression
Several pathways were investigated to elucidate the
molecular mechanisms underlying LV-RNAi-mediated inhibition of
tumorigenic and metastatic properties. The results showed
discrepancies between the interrelated members of the Wnt/β-catenin
signaling pathway expressed in the LV-RNAi TNFAIP2 group and LV-CON
group cells. On the assumption that β-catenin expression could be
upregulated by TNFAIP2, ESCC cell lines stably transfected with
lentiviral particles carrying TNFAIP2 RNAi were used to identify
the underlying mechanisms by western blot and qRT-PCR analyses of
the cell lysates. Obviously, when TNFAIP2 expression was
downregulated, the protein expression levels of β-catenin were
decreased synchronously (Fig. 3D).
Moreover, expression levels of p-GSK-3β (upstream of β-catenin),
c-Myc, cyclin D1, matrix metalloproteinase-7 (MMP-7), Snail
(downstream) and E-cadherin were evaluated by western blot
analysis, which indicated that expression of c-Myc, cyclin D1,
MMP-7 and Snail decreased, while expression levels of E-cadherin
and p-GSK-3β were increased in the LV-RNAi group, as compared with
the LV-CON group (Fig. 3D).
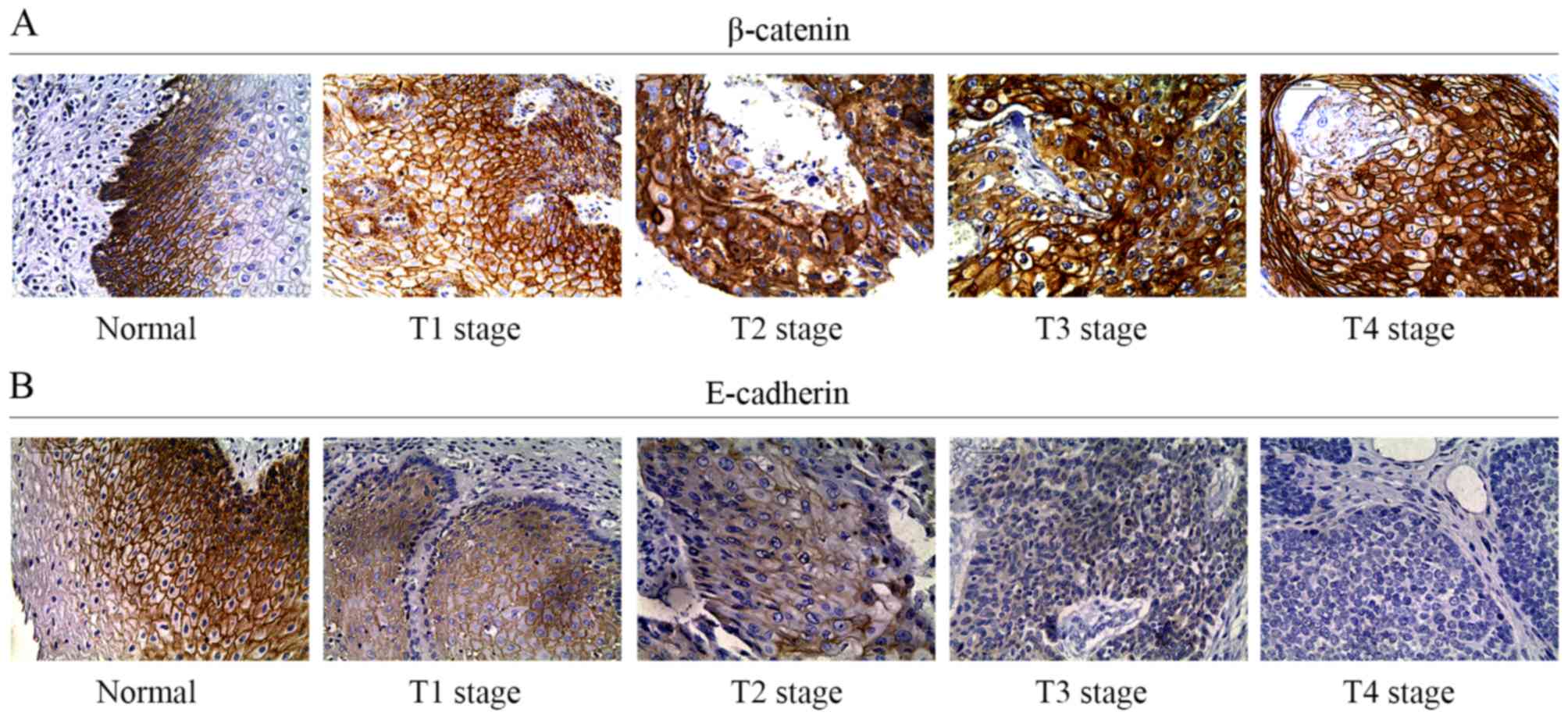
To elucidate potential associations between TNFAIP2
and β-catenin, immunohistochemical staining analysis with β-catenin
antibody was conducted using 55 paraffin-embedded specimens. As
shown in Fig. 4A, β-catenin
expression (in cytoplasm or nucleus) was relatively high in 36
(65%) samples and relatively low in 19 (35%). β-catenin expression
was found to be upregulated in the cytoplasm of cells from tumors
with an advanced T stage. The results of the Pearson Chi-squared
test confirmed the correlations between TNFAIP2 and β-catenin
expression profiles (Table I).
Based on the western blot results of Snail and
E-cadherin, we hypothesized that TNFAIP2 regulated E-cadherin
expression via β-catenin. Hence, immunohistochemical staining
analysis with E-cadherin antibody was conducted with 55
paraffin-embedded specimens to identify correlations between the
expression profiles of TNFAIP2 and E-cadherin. The results showed
that low and high E-cadherin expression levels were detected in 37
(67%) and 18 (33%) samples, respectively, as compared with adjacent
normal epithelium (Fig. 4B).
Pearson Chi-squared test results revealed a correlation between
TNFAIP2 and E-cadherin expression levels, as shown in Table I. Moreover, the Pearson Chi-squared
test results showed that β-catenin and E-cadherin expression levels
were correlated with T stage (p=0.001 and p<0.001, respectively)
and immunohistochemical staining analysis (Fig. 4).
Based on these results, we extrapolated that TNFAIP2
operates through the Wnt/β-catenin signaling pathway.
Discussion
The aim of the present study was to determine
whether TNFAIP2 overexpression promotes cellular proliferation,
migration and invasion via activation of the Wnt/β-catenin
signaling pathway in ESCC. TNFAIP2, a major response gene to the
B94 protein, was found to be upregulated by TNFα in human
endothelial cells (9). The
biological function of TNFAIP2 in ESCC remains vague as there is a
limited number of studies detailing the mechanisms underlying the
influence of TNFAIP2 expression in biological functions of tumors.
Nonetheless, some of these studies state that EBV-encoded LMP1
potently induces TNFAIP2 expression via necrosis factor-κB, which
presents a possible independent prognostic indicator of the
migration and invasion of nasopharyngeal carcinoma (11,12).
High levels of TNFAIP2 mRNA in unfractionated bone marrow and
APL-treated blasts with all-trans-retinoic acid was found to
result in the upregulation of TNFAIP2 (10), and the malignant Reed-Sternberg
cells in classical Hodgkin's lymphoma, LP cells in nodular
lymphocyte-predominant Hodgkin lymphoma, and B-cells in primary
mediastinal B-cell lymphoma aberrantly express TNFAIP2 (24). Accordingly, it is essential to
examine TNFAIP2 expression and to investigate its biological
functions in ESCC.
TNFAIP2 may play a significant role as a potential
prognostic factor and therapeutic target in ESCC, as TNFAIP2 is
overexpressed in ESCC and is pivotally associated with T, N and
UICC stages in ESCC patients. Moreover, lower TNFAIP2 expression is
more closely related with T, N and UICC stages, as compared with
higher TNFAIP2 expression, particularly in advanced T and UICC
stages. Moreover, Kaplan-Meier analysis indicated that overall
survival in patients with TNFAIP2 overexpression was correlated
with low TNFAIP2 expression. Accordingly, TNFAIP2 may be a
potential prognostic factor in ESCC and associated with tumor
progression.
TNFα, as a major cytokine in the tumor
microenvironment, is capable of stimulating tumor cell growth and
dissemination (3). Based on the
findings of the aforementioned and present studies (9–11,24),
TNFAIP2 was overexpressed in TNFα-treated ESCC cell lines. Hence,
we speculated that LV-RNAi-mediated TNFAIP2 knockdown could stifle
cell growth, colony formation, proliferation, migration, invasion
and the cell cycle of the tumorigenic ESCC cell lines Eca109 and
Kyse150, which demonstrated that TNFAIP2 plays an important role in
the tumor-facilitating effects of TNFα in ESCC. The study results
also showed that the tumor-prohibitive function (cell growth,
colony formation, proliferation and cell cycle) of LV-RNAi-mediated
TNFAIP2 knockdown was mainly correlated with its role in arresting
the cell cycle at the G0/G1 stage. LV-RNAi-mediated TNFAIP2
knockdown could downregulate cyclin D1 and c-Myc, which are closely
associated with tumorigenesis (25–27).
Meanwhile, LV-RNAi-mediated TNFAIP2 knockdown decreased MMP-7 and
Snail expression, but increased expression of E-cadherin. To the
best of our knowledge, ectopic expression of MMP-7, β-catenin and
E-cadherin plays an indispensable role in facilitating invasiveness
and decreasing cell-to-cell adhesion in ESCC (28).
Taking previous studies (29–31)
and our data into consideration, we believe that expression levels
of cyclin D1, c-Myc, MMP-7 and Snail (all of them are downstream
targets of β-catenin) were mainly decreased via LV-RNAi-mediated
TNFAIP2 knockdown in the ESCC cell lines. We further demonstrated
that β-catenin mRNA and protein expression levels were
downregulated through LV-RNAi-mediated TNFAIP2 knockdown.
Meanwhile, p-GSK-3β, which is located upstream of β-catenin, was
upregulated by LV-RNAi-mediated TNFAIP2 knockdown. We hypothesized
that β-catenin and E-cadherin expression levels were closely
correlated with TNFAIP2 overexpression. To confirm this hypothesis,
immunohistochemical staining analysis was performed to validate the
suspected correlations between TNFAIP2 and β-catenin and
E-cadherin. The results of the Pearson Chi-squared test
immunohistochemical analysis were coincident with our
hypothesis.
Although, significant findings were revealed in the
present study, there were some limitations that should be
addressed. For example, since there was a limited number of
adequate clinical samples and limited follow-up, we only
investigated whether TNFAIP2 expression is an independent risk
factor for ESCC using univariate Cox regression analysis, which
showed that there were no significant differences with TNFAIP2,
β-catenin, E-cadherin, T, UICC and N stage, as covariates.
Notwithstanding these limitations, further studies are warranted to
investigate the function of TNFAIP2 in ESCC.
In summary, we identified a potential role of
TNFAIP2 overexpression in ESCC as an oncogene. Therefore, TNFAIP2
overexpression facilitates proliferation and metastasis via
activation of the Wnt/β-catenin pathway in ESCC. Meanwhile, TNFAIP2
may be a potential and momentous prognostic indicator in ESCC
patients.
Acknowledgements
We thank Professor Tingxiu Xiang (Chongqing Key
Laboratory of Molecular Oncology and Epigenetics) and Ke Yang
(Department of Pathological Center, Chongqing Medical University)
for providing technical support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crusz SM and Balkwill FR: Inflammation and
cancer: Advances and new agents. Nat Rev Clin Oncol. 12:584–596.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenzato G: Tumour necrosis factor: A
cytokine with multiple biological activities. Br J Cancer.
61:354–361. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szlosarek P, Charles KA and Balkwill FR:
Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer.
42:745–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F: F. B: Tumour necrosis factor
and cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J, Han J, Zhao Y, Zhu Q and Hu J:
Curcumin induces apoptosis in tumor necrosis factor-alpha-treated
HaCaT cells. Int Immunopharmacol. 13:170–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarma V, Wolf FW, Marks RM, Shows TB and
Dixit VM: Cloning of a novel tumor necrosis factor-alpha-inducible
primary response gene that is differentially expressed in
development and capillary tube-like formation in vitro. J Immunol.
148:3302–3312. 1992.PubMed/NCBI
|
|
10
|
Rusiniak ME, Yu M, Ross DT, Tolhurst EC
and Slack JL: Identification of B94 (TNFAIP2) as a potential
retinoic acid target gene in acute promyelocytic leukemia. Cancer
Res. 60:1824–1829. 2000.PubMed/NCBI
|
|
11
|
Chen LC, Chen CC, Liang Y, Tsang NM, Chang
YS and Hsueh C: A novel role for TNFAIP2: Its correlation with
invasion and metastasis in nasopharyngeal carcinoma. Mod Pathol.
24:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CC, Liu HP, Chao M, Liang Y, Tsang
NM, Huang HY, Wu CC and Chang YS: NF-κB-mediated transcriptional
upregulation of TNFAIP2 by the Epstein-Barr virus oncoprotein,
LMP1, promotes cell motility in nasopharyngeal carcinoma. Oncogene.
33:3648–3659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hadisaputri YE, Miyazaki T, Suzuki S,
Yokobori T, Kobayashi T, Tanaka N, Inose T, Sohda M and Kuwano H:
TNFAIP8 overexpression: Clinical relevance to esophageal
squamous cell carcinoma. Ann Surg Oncol. 19:(Suppl 3). S589–S596.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida A, Tsuta K, Ohno M, Yoshida M,
Narita Y, Kawai A, Asamura H and Kushima R: STAT6
immunohistochemistry is helpful in the diagnosis of solitary
fibrous tumors. Am J Surg Pathol. 38:552–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo J, Zhang C, Wang C, Li L, Li C, Li Q,
Zhang M and Wu Q: Miz-1 promotes the proliferation of esophageal
cancer cells via suppression of p21 and release of p21-arrested
cyclin D1. Oncol Rep. 35:3532–3540. 2016.PubMed/NCBI
|
|
16
|
Budwit-Novotny DA, McCarty KS, Cox EB,
Soper JT, Mutch DG, Creasman WT, Flowers JL and McCarty KS Jr:
Immunohistochemical analyses of estrogen receptor in endometrial
adenocarcinoma using a monoclonal antibody. Cancer Res.
46:5419–5425. 1986.PubMed/NCBI
|
|
17
|
Smith J, Robida MD, Acosta K, Vennapusa B,
Mistry A, Martin G, Yates A and Hnatyszyn HJ: Quantitative and
qualitative characterization of Two PD-L1 clones: SP263 and E1L3N.
Diagn Pathol. 11:442016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kreso A, van Galen P, Pedley NM,
Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W,
Sydorenko N, et al: Self-renewal as a therapeutic target in human
colorectal cancer. Nat Med. 20:29–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Q, Whitington T, Gao P, Lindberg JF,
Yang Y, Sun J, Väisänen MR, Szulkin R, Annala M, Yan J, et al: A
prostate cancer susceptibility allele at 6q22 increases RFX6
expression by modulating HOXB13 chromatin binding. Nat Genet.
46:126–135. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Ma H, Yu H, Liu Z, Wang LE, Tan D,
Muddasani R, Lu V, Ajani JA, Wang Y, et al: The miR-184
binding-site rs8126 T>C polymorphism in TNFAIP2 is
associated with risk of gastric cancer. PLoS One. 8:e649732013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Yu H, Zhang Y, Zhang X, Zheng G,
Gao Y, Wang C and Zhou L: A functional TNFAIP2 3-UTR rs8126
genetic polymorphism contributes to risk of esophageal squamous
cell carcinoma. PLoS One. 9:e1093182014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Wei S, Ma H, Zhao M, Myers JN,
Weber RS, Sturgis EM and Wei Q: A functional variant at the miR-184
binding site in TNFAIP2 and risk of squamous cell carcinoma
of the head and neck. Carcinogenesis. 32:1668–1674. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondratiev S, Duraisamy S, Unitt CL, Green
MR, Pinkus GS, Shipp MA, Kutok JL, Drapkin RI and Rodig SJ:
Aberrant expression of the dendritic cell marker TNFAIP2 by the
malignant cells of Hodgkin lymphoma and primary mediastinal large
B-cell lymphoma distinguishes these tumor types from
morphologically and phenotypically similar lymphomas. Am J Surg
Pathol. 35:1531–1539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhou X, Zhu H, Liu S, Zhou C,
Zhang G, Xue L, Lu N, Quan L, Bai J, et al: Overexpression of EB1
in human esophageal squamous cell carcinoma (ESCC) may promote
cellular growth by activating beta-catenin/TCF pathway. Oncogene.
24:6637–6645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao Y, Song C, Hui L, Li CY, Wang J, Tian
Y, Han X, Chen Y, Tian DL, Qiu X, et al: Overexpression of
RNF146 in non-small cell lung cancer enhances proliferation
and invasion of tumors through the Wnt/β-catenin signaling pathway.
PLoS One. 9:e853772014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin YR, Tang H, Xie F, Liu H, Zhu Y, Ai J,
Chen L, Li Y, Kwong DL, Fu L, et al: Characterization of
tumor-suppressive function of SOX6 in human esophageal
squamous cell carcinoma. Clin Cancer Res. 17:46–55. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada Y and Sato F: Molecular factors
related to metastasis of esophageal squamous cell carcinoma.
Esophagus. 4:7–18. 2007. View Article : Google Scholar
|
|
29
|
Qiu HB, Zhang LY, Ren C, Zeng ZL, Wu WJ,
Luo HY, Zhou ZW and Xu RH: Targeting CDH17 suppresses tumor
progression in gastric cancer by downregulating Wnt/β-catenin
signaling. PLoS One. 8:e569592013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moon RT: Wnt/beta-catenin pathway. Sci
STKE. 2005:cm1. 2005.PubMed/NCBI
|