Introduction
As a malignant bone tumor, osteosarcoma (OS) is most
prevalent in children and young adults. It accounts for ~2.4 and
20% of all pediatric cancers and primary bone malignancies,
respectively (1–3). The current treatment of OS is mainly
based on surgery and chemotherapy, and the treatment outcomes are
limited (4). Chemotherapy drugs,
such as methotrexate, doxorubicin, cisplatin, oxaliplatin (OXA),
5-fluorouracil (5-FU), bleomycin, Taxol and etoposide are not
effective and even possess numerous side-effects and toxicities
(5,6). Therefore, understanding the molecular
mechanisms of OS cells is highly beneficial, and more efficient and
novel pharmaceutical products for OS are pressingly needed.
Recently, traditional Chinese medicine (TCM) has successfully
gained people's attention. Moreover, a large number of
plant-derived bioactive compounds used in TCM are now used to treat
different types of malignant cancer.
As a natural product extracted from Chinese herbs,
tetrandrine (TET)
[(1b)-6,6′,7,12-tetramethoxy-2,2′-dimethyl-berbaman] is a
bisbenzylisoquinoline alkaloid isolated from the Chinese herb
Stephania tetrandra S. Moore (7). TET has been used for years as a
clinical drug in China to treat patients with silicosis, autoimmune
disorders, inflammatory pulmonary and cardiovascular diseases, and
hypertension (8). Recently,
evidence has indicated that TET possesses an antitumor effect
through its antiproliferative and apoptosis-inducing abilities in
various malignant cancers, such as gastric (9), bladder (10), prostate (11) and colon cancer (12), gallbladder carcinoma (13) and OS (14). Tao et al reported that TET
induces the apoptosis of human OS cells through the activation of
the mitochondrial pathway (15).
However, there are still few studies exploring the possible
mechanisms underlying the TET-induced antiproliferation and
apoptosis in OS cells.
Identified as a tumor-suppressor gene, phosphatase
and tensin homolog (PTEN) functions as a lipid phosphatase. PTEN
dephosphorylates phosphatidylinositol (3,4,5)-triphosphate
(PIP3), and counteracts the activity of
phosphatidylinositol-3-kinase (PI3K). PI3K phosphorylates
phosphatidylinositol (4,5)-bisphosphate (PIP2) to
generate PIP3. In addition, PIP3 is the
membrane anchor and ligand of the pleckstrin homology (PH) domain
of AKTs (16). Moreover, PTEN
negatively regulates not only PI3K signaling, but also
mitogen-activated protein kinases (MAPKs), which are downstream of
RAS (17). Deletions or the
inactivation of mutations of PTEN are observed in a large number of
various cancers at high frequency. MAPK consist of a family of
protein kinases such as ERK, JNK and p38, which phosphorylate
specific serines and threonines of target protein substrates and
regulate cellular activities including gene expression, mitosis,
motility, metabolism and programmed death (18). Among the MAPKs family, it has been
recently found that p38 MAPK plays a significant role in the
induction of apoptosis in various cell systems (19).
It has been recently reported that p38 MAPK and PTEN
can affect each other mutually in numerous types of cancer. For
example, Li et al reported that the phosphorylated form of
PTEN enhances p38 signaling (20),
while Wu et al reported that p38 MAPK enhances PTEN
signaling (21). However, to date,
there is no study addressing the relationship between p38 MAPK and
PTEN in OS. We hypothesized that p38 MAPK may interact with PTEN in
the TET-induced antiproliferation process in OS. In addition, our
data ascertained that TET can inhibit the proliferation of OS
cells, and this process may be mediated by the upregulation of PTEN
through at least in part the activation of p38 MAPK.
Materials and methods
Agent preparations and cell
culture
TET (purity=99.2%) was obtained from Hao-Xuan
Bio-Technology Co., Ltd. (Xi'an, China), and was dissolved in
dimethyl sulfoxide (DMSO) for in vitro experiments as well
as prepared with 0.4% carboxymethylcellulose sodium (CMC-Na) for
in vivo experiments. Antibodies were obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). The p38 inhibitor
SB203580 (#S1076) was purchased from Selleckchem (Houston, TX,
USA). The human OS cell line 143B was purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum (FBS), 100 U/ml of penicillin and 100 µg/ml of
streptomycin at 37̊C in 5% CO2.
Cell Counting Kit-8 (CCK-8) assay
Sub-confluent 143B cells were seeded into 96-well
plates (150 µl, 1,500 cells/well) and cultured for 24 h (37̊C, 5%
CO2). Then, cells were treated with pre-designated
concentrations (1, 2, 3, 4 and 5 µM) of TET, recombinant adenovirus
or DMSO. After 24, 48 and 72 h, CCK-8 (10 µl/well; 7Sea Biotech,
Shanghai, China) was added to the cells, and the 96-well plate was
continuously incubated for 2 h. Subsequently, the OD value for each
well was read at a wavelength of 450 nm to determine the cell
viability on a microplate reader. Each condition was carried out in
triplicate. The cell viability was calculated as follows:
Cellviability(%)=OD(experiment)–OD(blank)OD(control)–OD(blank)×100
Construction of recombinant
adenovirus
Recombinant adenoviruses expressing PTEN (AdPTEN)
and small interfering RNA fragments targeting PTEN (AdsiPTEN) were
constructed with the AdEasy system (22). AdPTEN and AdsiPTEN were tagged with
red fluorescence protein (RFP). The recombinant adenovirus
expression RFP alone was used as a vector control. There were 4
fragments targeting PTEN in AdsiPTEN, and each of the fragments top
line sequence is as follows: AGCTAAAGGTGAAGATATA; AGTAAGGACCA
GAGACAAA; CAGATAATGACAAGGAATA; AGAAAGA CTTGAAGGCGTA.
Flow cytometric analysis for apoptosis
and cell cycle
Sub-confluent 143B cells were seeded into 6-well
plates, and treated with pre-designated concentrations (1, 2 and 4
µM) of TET, recombinant adenovirus or DMSO for 24 h. For the cell
cycle analysis, cells were harvested and washed with
phosphate-buffered saline (PBS) (4̊C), collected with cold 70%
ethanol (4̊C) and finally stained with 1 ml of propidium iodide
(PI) (20 mg/ml) containing RNase (1 mg/ml) in PBS for 30 min. Then
the cells were detected with fluorescence activated cell sorting
(FACS). For the apoptosis assay, the cells were washed with PBS
(4̊C), incubated with PI and Annexin V-FITC following the
instructions of the kits (KeyGen Biotech, Nanjing, China).
Subsequently, the cells were analyzed by FACS.
Xenograft tumor model of human OS
The use and care of animals was approved by the
Institutional Animal Care and Use Committee (IACUC) of Chongqing
Medical University. Athymic nude mice (female, 4–6 weeks old, 4
mice/group) were purchased from the Animal Center of Chongqing
Medical University (Chongqing, China). Sub-confluent cells (143B)
were washed and re-suspended in PBS (4̊C) to a density of
4×107 cells/ml and then were subcutaneously injected
into the right flanks of athymic nude mice using a 27-G needle (50
µl, ~2×106 cells). One week after the injections, the
mice were treated with pre-designated doses (40 and 80 mg/kg) of
TET or solvent by intragastric administration daily for 4 weeks.
Then, the mice were sacrificed and samples were harvested for
histological evaluation.
Protein harvest and western blot
analysis (WB) assay
143B cells were seeded into a 6-well plate and
treated with pre-designated concentrations (1, 2 and 4 µM) of TET,
SB203580 or DMSO. At pre-designated time points, cells were washed
with PBS (4̊C) and lysed with ice-cold lysis buffer (300 µl). The
lysates were boiled for 10 min. After electrophoresis, proteins
were transferred to polyvinylidene fluoride (PVDF) membranes, then
blotted with corresponding primary antibodies and incubated with
HRP-labeled secondary antibodies successively. The target proteins
were developed with the SuperSignal West Pico Substrate (Pierce,
Rockford, IL, USA). Each condition was carried out in
triplicate.
Histological evaluation and
immunohistochemical (IHC) staining
Retrieved tumor masses were fixed with
paraformaldehyde (4%) and embedded with paraffin, respectively.
Serial sections were stained with hematoxylin and eosin (H&E).
For IHC staining, the processed slides were deparaffinized and
rehydrated in a graduated manner. The deparaffinized sections were
incubated with proliferating cell and nuclear antigen (PCNA)
antibody (1:100 dilution) or PTEN antibody (1:100 dilution) or
isotype IgG as a control. Finally, the sections were incubated with
streptavidin-labeled secondary antibodies and then visualized with
diaminobenzidine (DAB) staining.
Statistical analysis
The results of all experiments are presented as the
mean ± standard deviation (SD) of at least 3 independent tests.
Statistical analysis of the results was conducted using a t-test by
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA), and
a P-value <0.05 was considered to indicate a statistically
significant result.
Results
TET exhibits an antiproliferative
effect and arrests the cell cycle at the G1 phase in 143B
cells
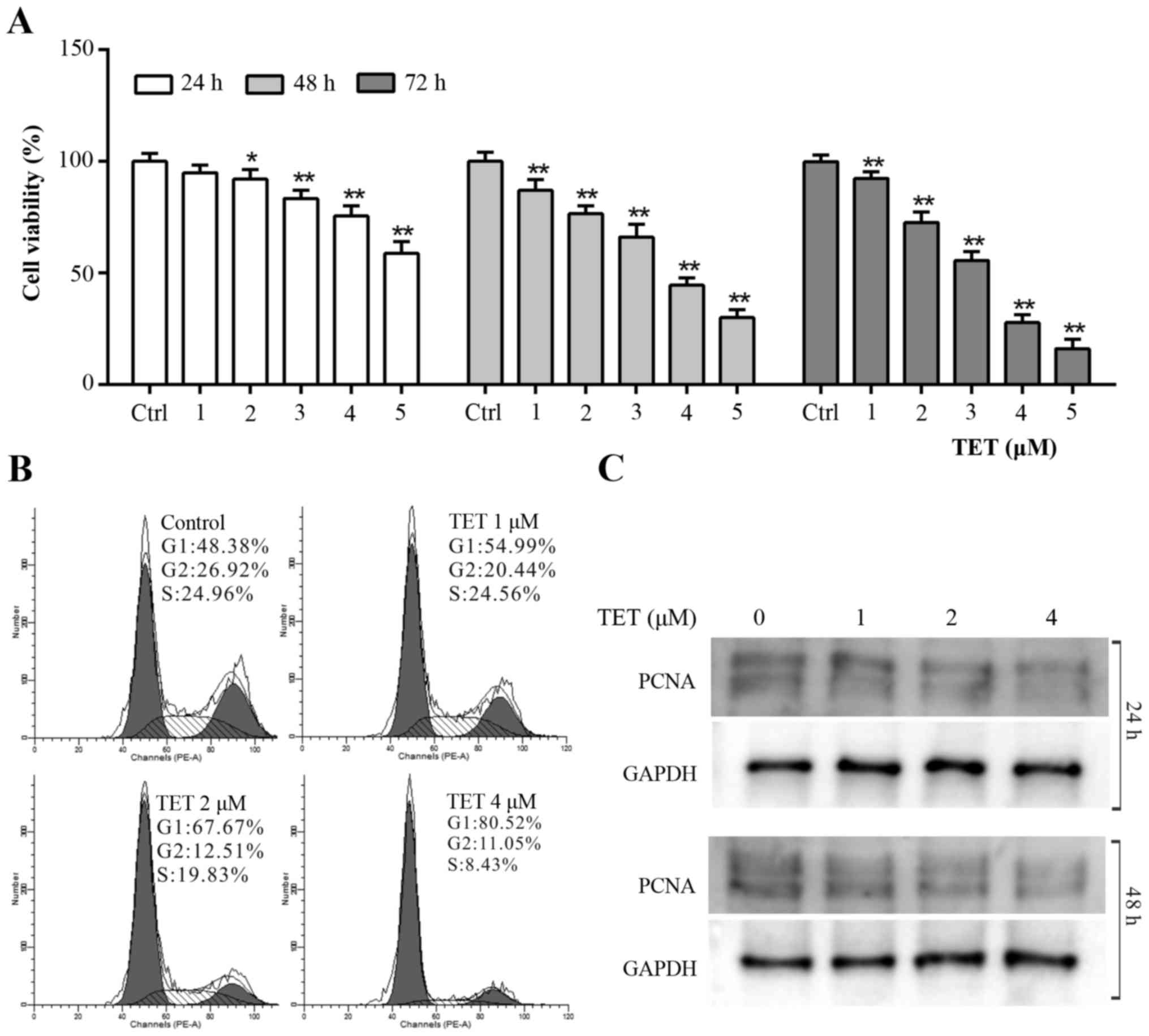
To validate whether TET could be used as a
chemotherapeutic agent for OS patients, CCK-8 assay was applied to
explore the antiproliferative effect of TET on 143B cells. The
results revealed that TET inhibited the proliferation of 143B cells
effectively in a concentration- and time-dependent manner (Fig. 1A). According to the CCK-8 assay
result, we chose 1, 2 and 4 µM of TET to perform the following
experiments. Cell cycle analysis results revealed that TET induced
cell cycle arrest at the G1 phase in 143B cells, and that the cell
cycle arrest effect was positively related with the concentration
of TET (Fig. 1B). The WB assay
showed that TET significantly inhibited the expression of
proliferating cell nuclear antigen (PCNA) (Fig. 1C). These data suggest that TET may
be used as a chemotherapeutic agent or adjuvant for human OS
treatment.
TET induces apoptosis in 143B
cells
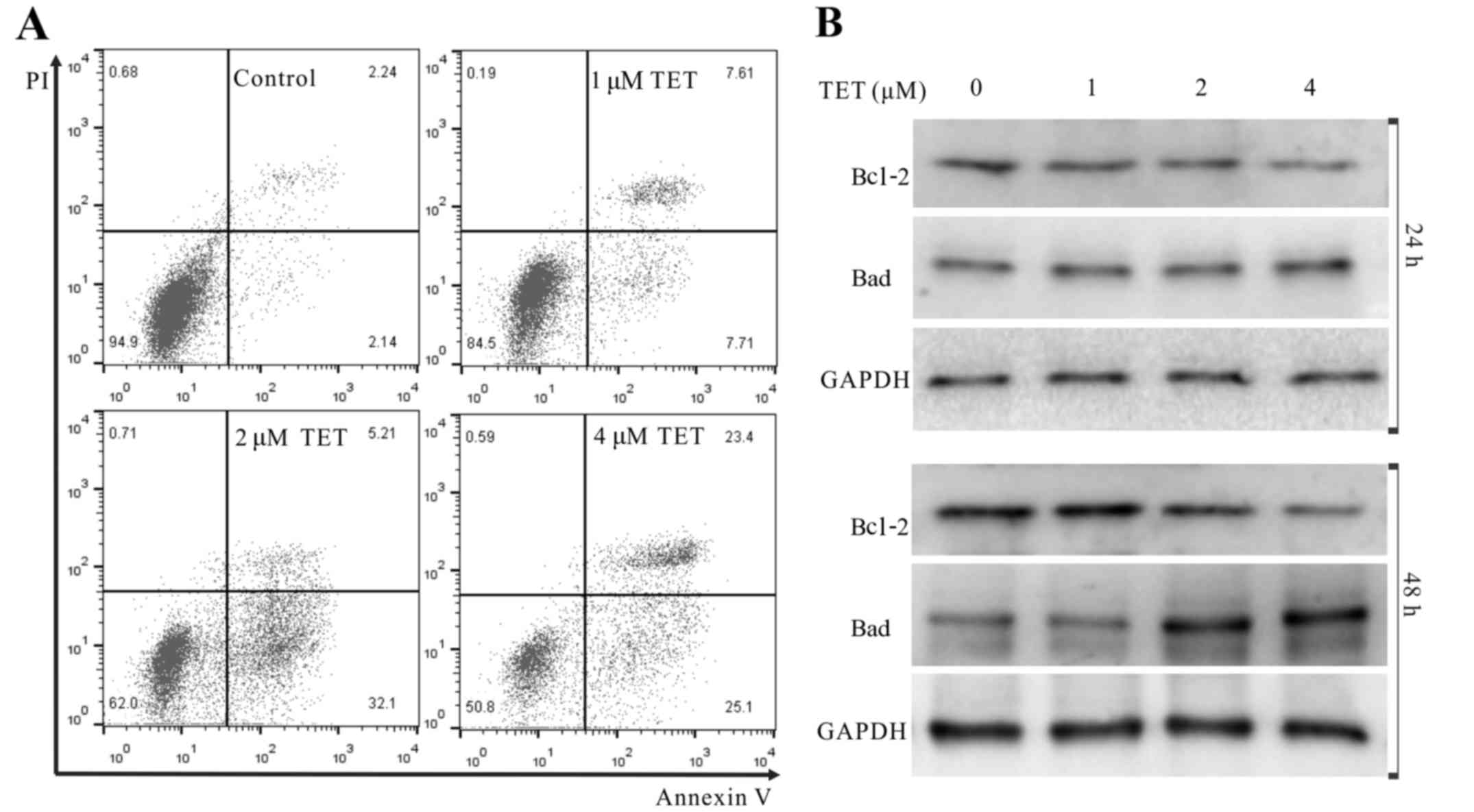
We next conducted further analyses to demonstrate
whether TET induced apoptosis of 143B cells. The results showed
that TET increased the apoptotic cell rate
concentration-dependently (Fig.
2A). WB revealed that TET substantially upregulated the protein
level of Bad, but downregulated the protein level of Bcl-2 in the
143B cells (Fig. 2B). These results
strongly indicate that TET is a potent apoptosis inducer in 143B
cells.
TET inhibits human OS growth in a
xenograft tumor model
To investigate the in vivo anticancer
activity of TET, we applied a well-established xenograft OS model.
Compared with the solvent control group, TET suppressed the tumor
growth dose-dependently (Fig. 3A and
B). Upon H&E staining, more necrotic cells were found in
the TET-treated tumors than this number in the control group, and
more necrotic cells were found with the higher dose of TET
(Fig. 3C). To investigate any
potential cytotoxic effects of TET, we measured the weight of the
kidney, spleen, liver and the mouse body weight for each group, and
no statistical difference was found among the groups. In addition,
liver and kidney sections were dealt with H&E staining, and no
obvious morphological change was discovered, indicating that the
present dose of TET is nontoxic (Fig.
3B and C). IHC staining results showed that PCNA was
dose-dependently downregulated by TET in OS (Fig. 3D). These results showed that TET can
inhibit the proliferation of 143B cells in vivo and is
relatively safe. These results further demonstrated that TET may be
a potential anticancer agent for OS.
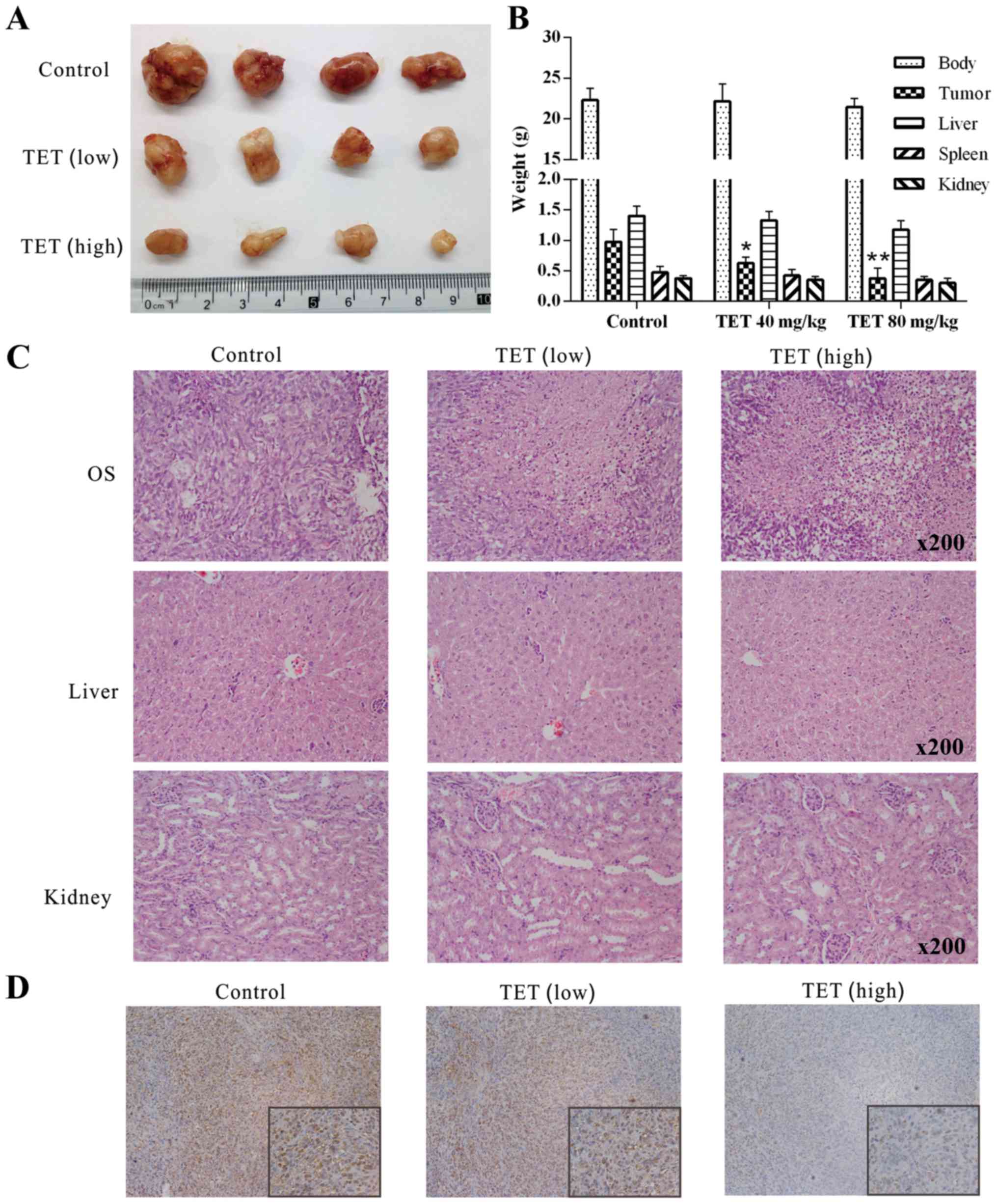 | Figure 3.Effects of TET on the tumor growth of
OS. (A) Tumor masses collected from nude mice revealed the
anticancer effect of TET on OS. (B) Quantitative results of the
weight of the kidney, spleen and liver, mouse body weight and tumor
weight of each group showed that TET had an antiproliferative
effect on OS without having toxic side-effects (*P<0.05,
compared with the control; **P<0.01, compared with the control).
(C) H&E staining results show the effect of TET on OS, liver
and kidney samples; representative results are shown. (D) IHC
staining show that TET affects the expression level of PCNA in OS;
representative results are shown. TET, tetrandrine; OS,
osteosarcoma; H&E, hematoxylin and eosin; IHC,
immunohistochemical; PCNA, proliferating cell nuclear antigen. |
PTEN is involved in the
antiproliferative effect of TET in 143B cells
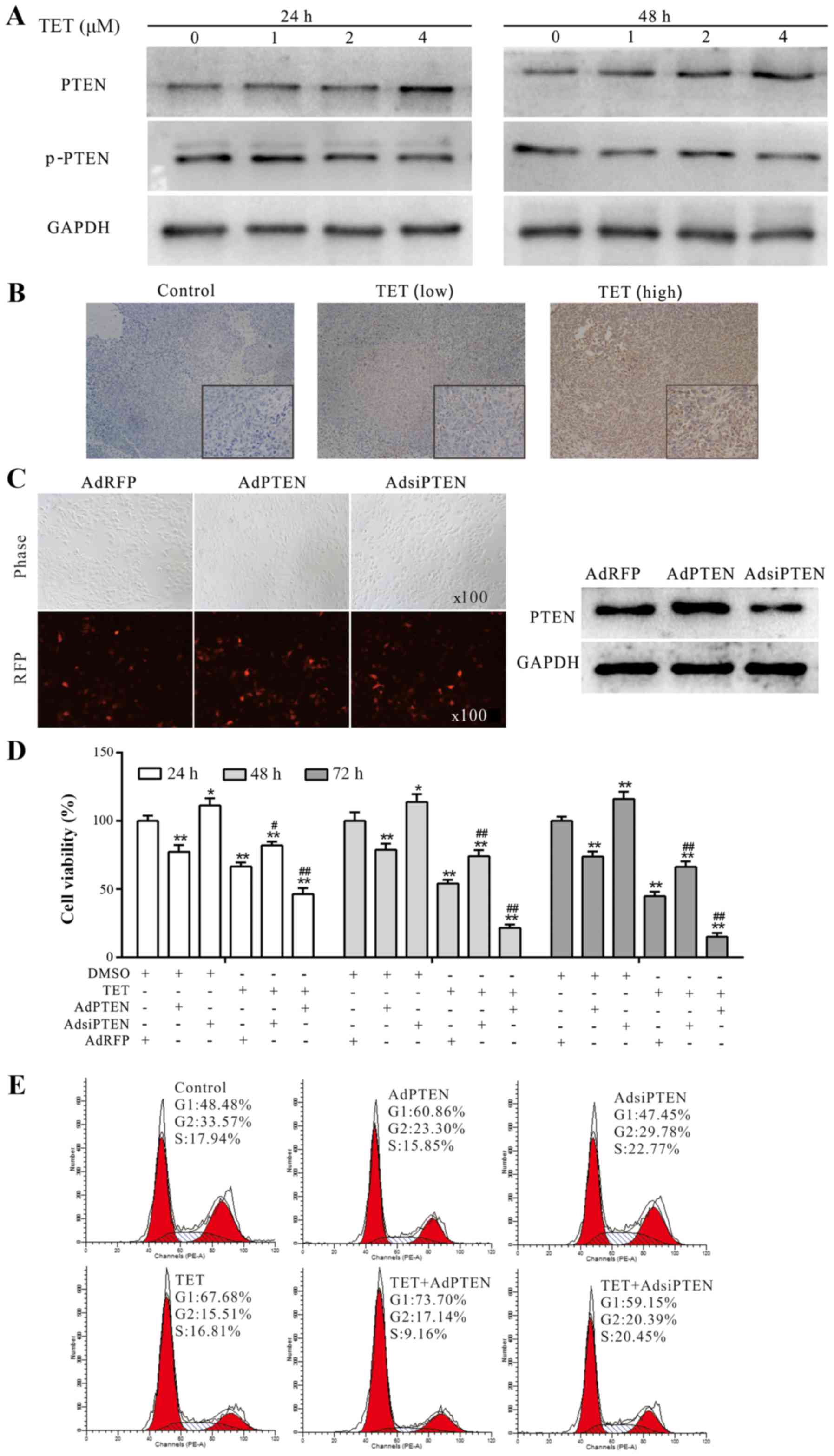
We investigated whether PTEN is involved in the
antiproliferative effect of TET in 143B cells as in other cancer
cells (23). The WB assay results
showed that TET concentration- and time-dependently induced the
expression of PTEN (Fig. 4A). The
IHC staining results revealed that PTEN was upregulated
dose-dependently in vivo after treatment with TET (Fig. 4B). Before being used in the present
study, the recombinant adenoviruses were ascertained for proper
functioning in 143B cells by fluorescence images and WB assay
(Fig. 4C). The CCK-8 assay
demonstrated that exogenous expression of PTEN substantially
enhanced the antiproliferative effect of TET, while knockdown of
PTEN decreased this effect of TET in the 143B cells (Fig. 4D). Moreover, flow cytometric
analysis revealed that the TET-induced cell cycle arrest in 143B
cells could be strengthened by the exogenous expression of PTEN,
and weakened by the knockdown of PTEN (Fig. 4E). These results consequently
indicated that PTEN is involved in the antiproliferative effect of
TET in 143B cells.
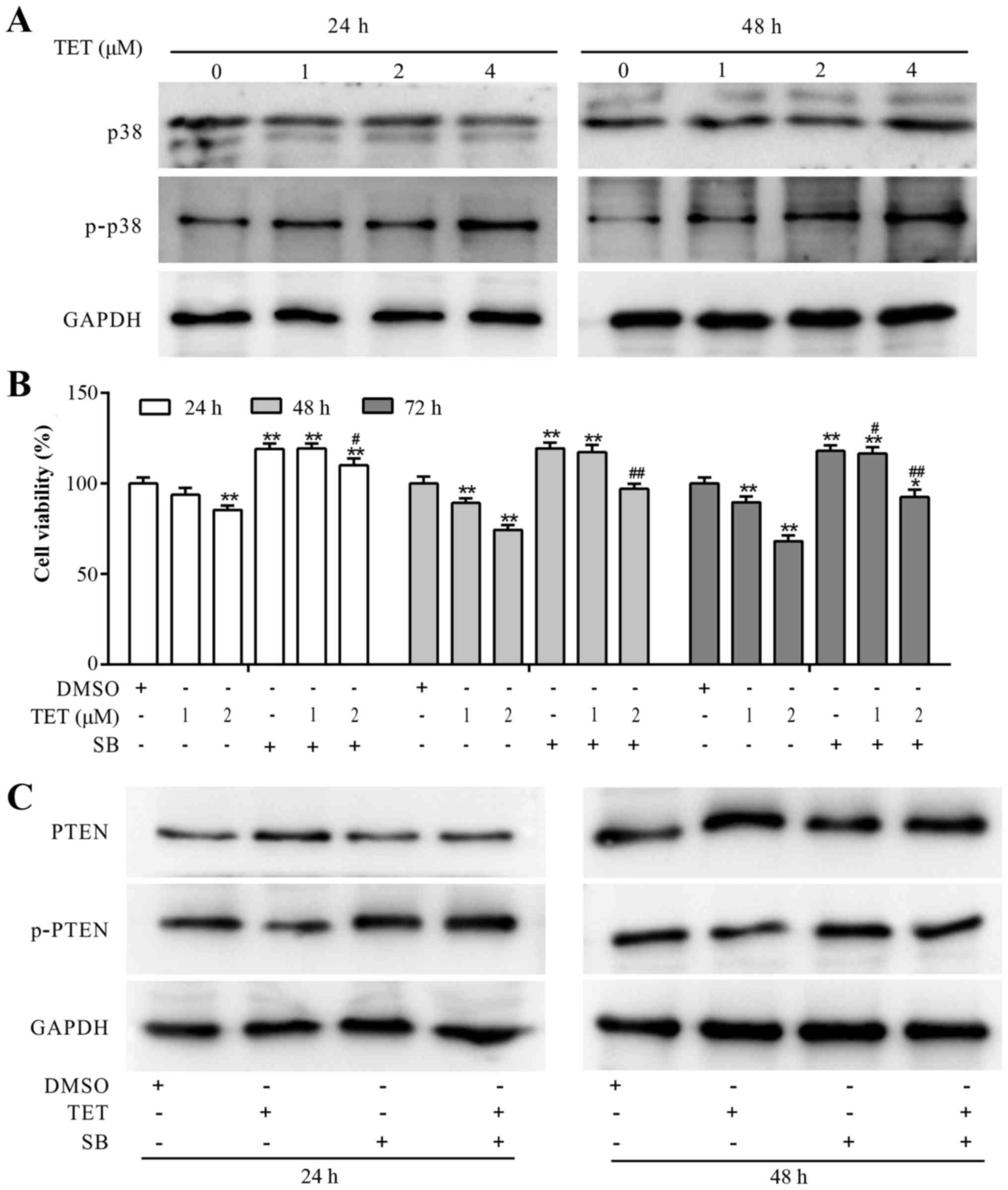
TET upregulates PTEN by activating p38
MAPK in 143B cells
Although, PTEN partly mediates the antiproliferative
effect of TET in 143B cells, the mechanism involved in the
upregulation of PTEN by TET remains unknown. With further analysis,
we found that TET concentration-dependently increased the
phosphorylation of p38 MAPK (Fig.
5A). CCK-8 assay results demonstrated that the
antiproliferative effect of TET was partly reversed by SB203580
(SB) in the 143B cells (Fig. 5B).
When TET was combined with SB to treat 143B cells, the expression
level of PTEN was decreased, and the expression level of p-PTEN was
markedly increased (Fig. 5C). These
results suggested that the upregulation of PTEN may have resulted
from the activation of p38 MAPK, which may block the
phosphorylation of PTEN in the 143B cells.
Discussion
In the present study, we demonstrated that TET may
be a potential and effective antiproliferation drug for OS.
Mechanistically, we discovered that the anticancer ability of TET
may be partly mediated by upregulating PTEN signaling. Moreover,
the upregulation of PTEN signaling is mediated by the activation of
p38 MAPK.
As one of the most frequent primary malignant bone
tumors that occurs in childhood, OS is conventionally treated with
chemotherapy agents. However, the chemotherapy drugs used currently
do not only exhibit low efficiency, but also produce numerous
side-effects (6,24). Thus, it is of high significance to
take notice of new drug research and developments, so as to improve
the quality of life of patients. TET is a bisbenzylisoquinoline
alkaloid which is extracted from the dried root of Chinese herb
medicinal plant Hang Fang Ji. In addition, recent studies have
shown no side-effects of TET in the treatment of silicosis and lung
cancer (25), which makes it an
ideal anticancer drug. Moreover, with definite knowledge on the
synthesis, distribution, extraction, structural elucidation and
pharmacological properties of TET, this very herb has been
confirmed as a potential agent capable of inhibiting proliferation
and inducing apoptosis in many malignant cancer cells (12,25).
In the present study, we found that TET has antiproliferative and
apoptosis-inducing effects in 143B cells. Moreover, TET has been
reported to have the ability to arrest the cell cycle at different
phases in various cancer cells (9,13,26).
In our research, TET arrested the cell cycle at the G1 phase in
143B cells. Moreover, TET inhibited human OS growth in a xenograft
tumor model. Thus, it is highly suggested that TET could be used as
a potential anticancer drug for human OS.
The effect of the pharmacological property of TET on
malignant cancer cells has been confirmed to be achieved through
different pathways such as the calcium channel (27), reactive oxygen species (28), caspase-dependent pathway (29), reversal of multidrug resistance
(30), decrease of phosphorylated
Akt (31) and activation of Jnk1/2
(32). For many malignant cancers,
such as prostate cancer, the mutation of PTEN plays an essential
role (33) and overexpression of
PTEN results in a higher chemosensitivity (34) which indicates that PTEN is a
tumor-suppressor gene. Thus, PTEN may be a potential target for OS
treatment. However, this concept warrants further study. Therefore,
we investigated whether PTEN is involved in the antiproliferative
effect of TET in 143B cells. As a lipid phosphatase catalyzing
PIP3 dephosphorylation resulting in the production of
PIP2, PTEN inhibits the activation of the PI3K/Akt/mTOR
pathway, which is implicated in many cellular functions including
cell proliferation, survival and inhibition of apoptosis (35). Moreover, the phosphorylation of PTEN
inactivates PTEN from its anticancer ability, which means the ratio
of p-PTEN/PTEN is positively related with the inactivation level of
PTEN (36). In the present study,
TET increased the protein level of PTEN and decreased the level of
p-PTEN. Exogenous expression of PTEN substantially enhanced the
antiproliferation and cell cycle arrest produced by TET, while
knockdown of PTEN partly reduced this effect of TET in 143B cells.
Hence, we speculated that TET may increase the protein level of
PTEN as well as decrease its phosphorylation. Although, the precise
mechanism underlying this process remains unclear, we have reason
to speculate that the antiproliferative and apoptosis-inducing
effect of TET is related with the PTEN pathway.
As a subgroup of the MAPKs, p38 was first described
as a transducer of the response to environmental stress conditions
and as a critical mediator of inflammatory cytokines. Subsequently,
p38 was demonstrated to have the ability to regulate different
processes including cell cycle, differentiation, inflammation,
senescence, autophagy and apoptosis (37). p38 MAPKs are phosphorylated and
activated by MAPK kinases. Once activated, p38 MAPKs phosphorylate
the serine/threonine residues of their substrates, which include
several transcription factors as well as protein kinases (38). It has been reported that the
activation and phosphorylation of p38 MAPK mediates the anticancer
activity in numerous malignant cancers such as pancreatic (39) and gastric cancer (40), and head and neck carcinoma (41).
We hypothesized that in OS, p38 MAPK may be
associated with PTEN in a direct or indirect way. In the present
study, TET activated and phosphorylated p38 MAPK in 143B cells,
while upregulating PTEN and decreasing the level of p-PTEN. In
addition the p38 MAPK inhibitor was partly capable of reversing the
TET-induced antiproliferation in 143B cells, the upregulation of
PTEN and the downregulation of p-PTEN. Consequently, it is strongly
suggested that the upregulation of PTEN by TET may be the result of
the decrease in p-PTEN, which may be mediated by the TET-induced
activation and phosphorylation of p38 MAPK.
In summary, we demonstrated that TET can be used as
an effective chemotherapeutic agent for human OS. The anticancer
activity of TET in 143B cells may be mediated by an increase in
PTEN, which may result from the TET-induced activation of p38 MAPK.
Future studies should be carried out to investigate the possible
molecular mechanism of TET on the activation of p38 MAPK, and
elucidate the possible interaction between p38 MAPK and PTEN
phosphorylation in 143B cells. Furthermore, as the present study
did not involve the migration and metastasis of OS cells, a number
of subsequent experiments and pre-clinical assessments should be
carried out for further evaluation of this drug.
Acknowledgements
The present study was supported in part by grants
from the National Natural Science Foundation of China (grants no.
81672230), and the Natural Science Foundation of Chongqing (grant
no. cstc2013jjB10021). Sincerely, we thank Dr T.C. He (University
of Chicago Medical Center, USA) for his generous gift of all
recombinant adenoviruses.
References
|
1
|
Wan G, Tao JG, Wang GD, Liu SP, Zhao HX
and Liang QD: In vitro antitumor activity of the ethyl
acetate extract of Potentilla chinensis in osteosarcoma
cancer cells. Mol Med Rep. 14:3634–3640. 2016.PubMed/NCBI
|
|
2
|
Meng ZJ, Wu N, Liu Y, Shu KJ, Zou X, Zhang
RX, Pi CJ, He BC, Ke ZY, Chen L, et al: Evodiamine inhibits the
proliferation of human osteosarcoma cells by blocking PI3K/Akt
signaling. Oncol Rep. 34:1388–1396. 2015.PubMed/NCBI
|
|
3
|
Scholten DJ II, Timmer CM, Peacock JD,
Pelle DW, Williams BO and Steensma MR: Down regulation of Wnt
signaling mitigates hypoxia-induced chemoresistance in human
osteosarcoma cells. PLoS One. 9:e1114312014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pruksakorn D, Teeyakasem P, Klangjorhor J,
Chaiyawat P, Settakorn J, Diskul-Na-Ayudthaya P, Chokchaichamnankit
D, Pothacharoen P and Srisomsap C: Overexpression of KH-type
splicing regulatory protein regulates proliferation, migration, and
implantation ability of osteosarcoma. Int J Oncol. 49:903–912.
2016.PubMed/NCBI
|
|
5
|
Ma K, Huang MY, Guo YX and Hu GQ:
Matrine-induced autophagy counteracts cell apoptosis via the ERK
signaling pathway in osteosarcoma cells. Oncol Lett. 12:1854–1860.
2016.PubMed/NCBI
|
|
6
|
Sarman H, Bayram R and Benek SB:
Anticancer drugs with chemotherapeutic interactions with
thymoquinone in osteosarcoma cells. Eur Rev Med Pharmacol Sci.
20:1263–1270. 2016.PubMed/NCBI
|
|
7
|
Wei X, Qu TL, Yang YF, Xu JF, Li XW, Zhao
ZB and Guo YW: Design and synthesis of new tetrandrine derivatives
and their antitumor activities. J Asian Nat Prod Res. 18:966–975.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016.PubMed/NCBI
|
|
9
|
Lei RR, Hu HF, Bai F, Liu Y, Wu CZ, Huang
XX, Xie LP and Hu YJ: Anti-proliferative and apoptotic effects of
S1, a tetrandrine derivative, in human gastric cancer BGC-823
cells. Chin J Nat Med. 14:527–533. 2016.PubMed/NCBI
|
|
10
|
Zhang Y, Liu W, He W, Zhang Y, Deng X, Ma
Y, Zeng J and Kou B: Tetrandrine reverses epithelial-mesenchymal
transition in bladder cancer by downregulating Gli-1. Int J Oncol.
48:2035–2042. 2016.PubMed/NCBI
|
|
11
|
Li D, Lu Y, Sun P, Feng LX, Liu M, Hu LH,
Wu WY, Jiang BH, Yang M, Qu XB, et al: Inhibition on proteasome β1
subunit might contribute to the anti-cancer effects of
fangchinoline in human prostate cancer cells. PLoS One.
10:e01416812015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH, et al: The role of IGFBP-5 in
mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015.PubMed/NCBI
|
|
13
|
Zhu R, Liu T, Tan Z, Wu X, Li M, Jiang L,
Bao R, Shu Y, Lu A and Liu Y: Tetrandrine induces apoptosis in
gallbladder carcinoma in vitro. Int J Clin Pharmacol Ther.
52:900–905. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian Y, Yin H and Xu H: Enhanced
pro-apoptotic effect of tetrandrine loaded nanoparticles against
osteosarcoma cells. Curr Drug Deliv. 13:946–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao LJ, Zhou XD, Shen CC, Liang CZ, Liu B,
Tao Y and Tao HM: Tetrandrine induces apoptosis and triggers a
caspase cascade in U2-OS and MG-63 cells through the intrinsic and
extrinsic pathways. Mol Med Rep. 9:345–349. 2014.PubMed/NCBI
|
|
16
|
Shojaee S, Chan LN, Buchner M, Cazzaniga
V, Cosgun KN, Geng H, Qiu YH, von Minden MD, Ernst T, Hochhaus A,
et al: PTEN opposes negative selection and enables oncogenic
transformation of pre-B cells. Nat Med. 22:379–387. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YL and Yan Y: Timing of the loss of
Pten protein determines disease severity in a mouse model of
myeloid malignancy. 127:1912–1922. 2016.
|
|
18
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banerjee B, Nandi P, Chakraborty S, Raha
S, Sen PC and Jana K: Resveratrol ameliorates
benzo(a)pyrene-induced testicular dysfunction and apoptosis:
Involvement of p38 MAPK/ATF2/iNOS signaling. J Nutr Biochem.
34:17–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Zhang J, Tang M, Xin J, Xu Y, Volk
A, Hao C, Hu C, Sun J, Wei W, et al: Hematopoietic stem cell
activity is regulated by Pten phosphorylation through a
niche-dependent mechanism. Stem Cells. 34:2130–2144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He
BC and Wu K: Oridonin upregulates PTEN through activating p38 MAPK
and inhibits proliferation in human colon cancer cells. Oncol Rep.
35:3341–3348. 2016.PubMed/NCBI
|
|
22
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rhei E, Kang L, Bogomolniy F, Federici MG,
Borgen PI and Boyd J: Mutation analysis of the putative tumor
suppressor gene PTEN/MMAC1 in primary breast carcinomas.
Cancer Res. 57:3657–3659. 1997.PubMed/NCBI
|
|
24
|
Wang L, Wang W, Rui Z and Zhou D: The
effective combination therapy against human osteosarcoma:
Doxorubicin plus curcumin co-encapsulated lipid-coated polymeric
nanoparticulate drug delivery system. Drug Deliv. 23:3200–3208.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhagya N and Chandrashekar KR: Tetrandrine
- A molecule of wide bioactivity. Phytochemistry. 125:5–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao W, Jiang Y, Men Q, Yuan L, Huang Z,
Liu T, Li W and Liu X: Tetrandrine induces G1/S cell cycle arrest
through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis
in vivo. Int J Oncol. 46:360–368. 2015.PubMed/NCBI
|
|
27
|
Chiou WF, Lee WS and Yeh PH: Tetrandrine
selectively protects against amyloid-beta protein - but not against
MPTP-induced cytotoxicity in SK-N-SH neuroblastoma cells. Planta
Med. 72:1300–1304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen YC, Chen CF, Wang SY and Sung YJ:
Impediment to calcium influx and reactive oxygen production
accounts for the inhibition of neutrophil Mac-1 Up-regulation and
adhesion by tetrandrine. Mol Pharmacol. 55:186–193. 1999.PubMed/NCBI
|
|
29
|
Oh SH and Lee BH: Induction of apoptosis
in human hepatoblastoma cells by tetrandrine via caspase-dependent
Bid cleavage and cytochrome c release. Biochem Pharmacol.
66:725–731. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu M, Sheng LH, Zhu XH, Zeng SB and Zhang
GJ: Reversal effect of Stephania tetrandra-containing Chinese herb
formula SENL on multidrug resistance in lung cancer cell line
SW1573/2R120. Am J Chin Med. 38:401–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing ZB, Yao L, Zhang GQ, Zhang XY, Zhang
YX and Pang D: Fangchinoline inhibits breast adenocarcinoma
proliferation by inducing apoptosis. Chem Pharm Bull. 59:1476–1480.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaudhary P and Vishwanatha JK: c-Jun
NH2-terminal kinase-induced proteasomal degradation of
c-FLIPL/S and Bcl2 sensitize prostate cancer
cells to Fas- and mitochondria-mediated apoptosis by tetrandrine.
Biochem Pharmacol. 91:457–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fallahabadi ZR, Daloii Noori MR, Mahdian
R, Behjati F, Shokrgozar MA, Abolhasani M, Asgari M and Shahrokh H:
Frequency of PTEN alterations, TMPRSS2-ERG fusion and their
association in prostate cancer. Gene. 575:755–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang N, Zhou X, Zhao M, Zhao D, Zhu Z, Li
S and Yang H: Down-regulation of microRNA-26b modulates non-small
cell lung cancer cells chemoresistance and migration through the
association of PTEN. Acta Biochim Biophys Sin. 47:530–538. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mi S, Xiang G, Yuwen D, Gao J, Guo W, Wu
X, Wu X, Sun Y, Su Y, Shen Y, et al: Inhibition of autophagy by
andrographolide resensitizes cisplatin-resistant non-small cell
lung carcinoma cells via activation of the Akt/mTOR pathway.
Toxicol Appl Pharmacol. 310:78–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiong J, Li Z, Zhang Y, Li D, Zhang G, Luo
X, Jie Z, Liu Y, Cao Y, Le Z, et al: PRL-3 promotes the peritoneal
metastasis of gastric cancer through the PI3K/Akt signaling pathway
by regulating PTEN. Oncol Rep. 36:1819–1828. 2016.PubMed/NCBI
|
|
37
|
Šrámek J, Němcová-Fürstová V and Kovář J:
Kinase signaling in apoptosis induced by saturated fatty acids in
pancreatic β-cells. Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
38
|
Segalés J, Perdiguero E and Muñoz-Cánoves
P: Regulation of muscle stem cell functions: A focus on the p38
MAPK signaling pathway. Front Cell Dev Biol. 4:912016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Bai YY, Yang Y, Hu F, Wang Y, Yu
Z, Cheng Z and Zhou J: Diabetes mellitus stimulates pancreatic
cancer growth and epithelial-mesenchymal transition-mediated
metastasis via a p38 MAPK pathway. Oncotarget. 7:38539–38550.
2016.PubMed/NCBI
|
|
40
|
Su CC, Chen JY, Din ZH, Su JH, Yang ZY,
Chen YJ, Wang RY and Wu YJ: 13-acetoxysarcocrassolide induces
apoptosis on human gastric carcinoma cells through
mitochondria-related apoptotic pathways: p38/JNK activation and
PI3K/AKT suppression. Mar Drugs. 12:5295–5315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Benvenuto M, Mattera R, Masuelli L,
Taffera G, Andracchio O, Tresoldi I, Lido P, Giganti MG, Godos J,
Modesti A, et al: (±)-Gossypol induces apoptosis and autophagy in
head and neck carcinoma cell lines and inhibits the growth of
transplanted salivary gland cancer cells in BALB/c mice. Int
J Food Sci Nutr. 1–15. 2016.
|