Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide because of its high metastasis rate, and lack of
widely accepted prognostic marker (1). Cancer metastasis is a complicated
process involving the cell ability to initiate and maintain growth
at a distant site and increase morbility (2). Unfortunately, although diagnosis and
treatment of CRC have made great progress, available measures to
predict or prevent metastasis of CRC are absent, which leads to the
poor clinical outcome and prognosis of CRC patients with metastasis
who usually have genetic and epigenetic abnormalities, such as KRAS
mutation and microsatellite instability (3–7).
In recent years, we have focused on studying the
mechanisms of the novel gene FAM172A, the family with sequence
similarity 172. This functionally unknown gene was first identified
at the translational level through western blotting (8), and it was regulated by high glucose
levels in human THP-1-derived macrophages (9). Our previous study showed that FAM172A
expression in cancerous tissues was obviously lower compared with
adjacent tissues of CRC cells by using immunohistochemical staining
(10). In addition, our other past
results showed that FAM172A could promote apoptosis and inhibit
proliferation of CRC cell lines by flow cytometry and STAT1 could
upregulate the expression of FAM172A when it was combined with the
promoter region of FAM172A, indicating that FAM172A might be a
novel tumor-suppressor gene (11).
However, more molecular mechanisms of FAM172A underlying this
process are still to be elucidated.
The structure and functions of FAM172A was analyzed
recently by us using bioinformatics method and it was found that
FAM172A is a target of a variety of miRNAs, such as miR-27a,
miR-26b and miR-135. As small non-coding RNA molecules, microRNAs
(miRNAs) can affect extensive biological processes endogenously
(12–14). miRNAs can bind to the
3′-untranslated region (UTR) of targeting messenger RNA (mRNA),
thus regulating protein-coding gene expression by repressing
translation (15–18). There is plenty of evidence that
miRNAs are an important influence on human oncogenesis and
metastasis (19,20) by regulating the expression of a
variety of pivotal genes, such as miR-9, miR-153 and miR-145
(21–27). Approximately 30–60% of the
protein-coding human genome genes are regulated by miRNAs (28). In consequence, to identify
tumor-related miRNAs and their mediated novel cancer networks is a
key step to illustrate the molecular mechanisms of development of
human CRC. Among numerous miRNAs, miR-27a has attracted our
research interest. Recent reports have shown that miR-27a represses
the expression of MHC class I by downregulating calreticulin thus
affecting tumor progression in colorectal cancer, which was
considered an oncomiRNA (29). Zhou
et al confirmed that BTG2 was a direct target of miR-27a in
gastric cancer and miR-27a inhibition obviously upregulated the
expression of BTG2 (30). In light
of these findings, we propose the hypothesis that miR-27a functions
as an oncomiRNA and plays an important role in the development of
CRC, especially in the progress of CRC invasion and metastasis by
regulating FAM172A.
To test or hypothesis, we detected the expression of
miR-27a in CRC samples. We uncovered that miR-27a was upregulated
in colorectal cancer cell lines and tissues and that may act as an
important prognostic factor for overall survival (OS) of patients
with CRC. Then we studied the effects of miR-27a in proliferation,
migration and invasion of CRC cells. In the present study, we
showed that FAM172A was a direct target of miR-27a by using a
dual-luciferase assay and the expression of FAM172A was regulated
by miR-27a in CRC cell lines by using real-time PCR and western
blotting. These results demonstrated that miR-27a promotes
metastasis of CRC cell lines by inhibiting FAM172A expression,
which provides a better understanding of how miR-27a regulates
mechanisms of FAM172A in the development of CRC.
Materials and methods
Patient tissue samples
A total of 60 pairs of CRC and adjacent normal
colorectal tissue (ANCT) samples were obtained from patients who
underwent surgery between January 2010 and December 2015 at Nanfang
Hospital, Southern Medical University (Guangzhou, China). The
tissue specimens were stored in liquid nitrogen. None of the
patients had received chemotherapy or radiotherapy before the
surgery. Informed consent was obtained from all study participants.
The study protocol was approved by the Ethics Committee of Nanfang
Hospital, Southern Medical University, Guangzhou, China.
Cell cultures
Human colorectal cancer cell lines (LoVo, HT-29,
SW480, HCT116, DLD-1 and RKO) and normal human colonic epithelia
(NCM460) were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The cell lines were
cultured in RPMI-1640 (Hyclone, USA) supplemented with 10% fetal
bovine serum (FBS) and were incubated at 37°C with 5%
CO2.
Quantitative real-time RT-PCR
(RT-qPCR)
Total RNA was extracted using TRIzol (Beyotime,
China) from the cultured cells, and total miRNA was extracted using
RNAiso for small RNA (Takara, Japan) from the cultured cells and
human tissues according to the manufacturer's instructions. For
analyzing mRNA expression of genes, reverse transcription was
performed using the First Strand cDNA Synthesis ReverTra Ace kit
(Toyobo, Japan), and the SYBR Green qPCR Master Mix (Takara) was
used carring out qPCR with the ABI 7500 Real-Time PCR system
according to the manufacturer's instructions. For miRNA analysis,
real-time PCR was performed using PrimeScript® miRNA
RT-PCR kit (Takara) according to the manufacturer's instructions.
The PCR conditions used were 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec, and 60°C for 30 sec. U6 snRNA and GAPDH were
used for normalization for miRNA and mRNA, respectively. The
relative expression was analyzed by the 2−ΔΔCt method
(31). The sequences of the miRNAs
and mRNA used in the present study are shown in Table I.
 | Table I.Sequences of RNA or DNA
oligonucleotides. |
Table I.
Sequences of RNA or DNA
oligonucleotides.
| Name | Sense strand/sense
primer (5′-3′) | Antisense
strand/antisense primer (5′-3′) |
|---|
| Primers for
RT-PCR |
| miR-27a |
CTAATCGTGTTCACAGTGGCTAAG |
TATGGTTTTGACGACTGTGTGAT |
| FAM172A |
CAACGAGAAGCCGATGTA |
GATGTGTCTAATGGTTCTGAG |
| MMP-2 |
GGGAGATCATCGGGACAACTC |
GGGCCTGGTTGAAAAGCAT |
| MMP-9 |
CCTGGAGACCTGAGAACCAATC |
CCACCCGAGTGTAACCATAGC |
| NF-κB |
AGCACGACAACATCTCATT |
GGCACAACTCCTTCATCC |
| GAPDH |
ACCCACTCCTCCACCTTTG |
CACCACCCTGTTGCTGTAG |
Bioinformatics analysis
TargetScan (http://www.targetscan.org/) and microRNA.org (http://www.microrna.org/microrna/) algorithm were used
for identifying the potential targets of miR-27a.
Western blotting
The RIPA buffer was used in lysing cells. The
Bradford protein assay (Bio-Rad, CA, USA) was used in quantifying
total protein. Protein samples were denatured at 100°C for 5 min.
For electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide
gels, 30 µg of total protein were loaded equally and then
transferred to polyvinylidene fluoride microporous membranes
(Millipore, MA, USA). Then samples were incubated with primary
antibody for 12 h at 4°C which were followed by incubation with
secondary antibody for 1 h at room temperature. Then, target
proteins were detected with enhanced chemiluminescence reagents
(Millipore). The primary antibodies including anti-FAM172A (1:700
dilution) (ab121364), anti-GAPDH (1:3,000 dilution) (ab8245),
anti-MMP-2 (1:1,000 dilution) (ab25483), anti-MMP-9 (1:1,000
dilution) (ab76003), anti-NF-κB (1:1,500 dilution) (ab32536) and
HRP-conjugated goat anti-rabbit igG (1:5,000 dilution) (ab6721)
were from Abcam Group (Abcam, UK).
Dual-luciferase reporter assay
The genome DNA was derived from HEK293T cells, and
then the 3′-untranslated region (UTR) of FAM172A was amplified by
PCR, which contained the putative miR-27a target sites and the
synthetic mutant 3′-UTR of FAM172A. Then products from PCR were
cloned into psiCHECK-2 vector (Promega, USA). All inserts were
sequenced to verify polymerase fidelity after digested by
XhoI and NotI. Subsequently HEK293T cells were
cultured in 24-well plates. The 50 nM of miRNA mimic and 200 ng of
psiCHECK-2 vector containing 3′-UTR of FAM172A were cotransfected
into each well of 24-well plates using Lipofectamine®
3000 (Invitrogen, USA). After 48 h, the analysis of results was
performed using the dual-luciferase reporter assay system (Promega)
accordance with the protocol. Firefly luciferase activity was used
normalizing Renilla luciferase activity. Experiments were
carried out at least in triplicate.
Lentivirus construction and
transfection
For transient overexpression and knockdown of
miR-27a, micrOFF™ hsa-miR-27a inhibitor, micrOFF™ hsa-miR-27a mimic
and micrON™ Negative Control (RiboBio, China) were at a final
concentration of 50 nM using Lipofectamine 3000 (Invitrogen). The
efficacy of transfection was tested by real-time reverse
transcription polymerase chain reaction (RT-PCR) analysis.
For FAM172A knockdown, three siRNAs (Sigma-Aldrich,
USA) were designed against FAM172A (GenBank accession no.
NM_032042.5). One control siRNA (Sigma-Aldrich) acted as a negative
control, which exhibited no significant sequence similarity to
human, mouse or rat gene sequence. On the basis of the
manufacturer's instructions, transfection was performed with
Lipofectamine 3000 (Invitrogen). The siRNA sequences are listed in
Table II. For FAM172A
overexpression, lentivirus was produced by transfecting HEK 293T
packaging cells in DMEM (Hyclone) with a 3-plasmid system. DNA used
for transfection was prepared by the mixture of pHelper 1.0,
pHelper 2.0 and pLVX-IRES-Neo-FAM172A. The empty vector
pLVX-IRES-Neo was purchased from Clontech Laboratories (Mountain
View, CA, USA), and the pLVX-IRES-Neo-FAM172A plasmid was generated
by inserting FAM172A sequence. MDA-MB-231 cells were transduced
with lentivirus in the presence of 6 µg/ml polybrene
(Sigma-Aldrich) for 24 h. Then cells were selected for 7 days in
2.5 mg/ml neomycin.
 | Table II.Sequences of RNA or DNA
oligonucleotides. |
Table II.
Sequences of RNA or DNA
oligonucleotides.
| Name | Sense strand/sense
primer (5′-3′) | Antisense
strand/antisense primer (5′-3′) |
|---|
| siRNA duplexes |
| FAM172A-siRNA1 |
GAUAUGGAGUAAUAGUACUTT |
AGUACUAUUACUCCAUAUCTT |
| FAM172A-siRNA2 |
GAAGCGACGUGAUUUCUAUTT |
AUAGAAAUCACGUCGCUUCTT |
| FAM172A-siRNA3 |
CAAUCUAUGUUUGGGAUCATT |
UGAUCCCAAACAUAGAUUGTT |
| FAM172A-siRNA4 |
GACAGACUCUGUUCACAAUTT |
AUUGUGAACAGAGUCUGUCTT |
| Control-siRNA |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) (Dojindo) was used
examining cell growth and viability. Cells were plated in 96-well
plates at 10,000 cells/well in DMEM medium supplemented with 10%
fetal bovine serum (FBS) and cultured at 24, 48, 72 and 96 h with 5
pmol miR-27a inhibitor, miR-con and miR-27a inhibitor + FAM172A
siRNA respectively. The DMEM medium containing 10% CCK-8 was added
to each well, which was a substitute for complete medium. Then the
plates were incubated for 1 h in the incubator. Absorbance was
measured at 490 nm using a Mithras LB 940 plate reader (Berthold,
Germany).
Cell migration and invasion assay
The 8-µm pore sized plain Transwell inserts (Costar,
UK) for migration and the 8-µm pore sized plain Transwell inserts
with the BD Matrigel (BD Biosciences, USA) for invasion were placed
in 24-well culture plates. Cells (3×105 per Transwell)
for migration and cells (1×105 per Transwell) for
invasion were seeded into the upper chamber, respectively, after
suspended in serum-free DMEM. DMEM containing 5% fetal bovine serum
for migration and DMEM containing 10% fetal bovine serum for
invasion served as a chemoattractant and were added to the bottom
chamber of 24-well plates, respectively. After incubation of 48 h,
cells on the upper surface were removed. Cells that migrated to the
lower surface were fixed and stained with 1% toluidine blue. Ten
fields per experimental condition were randomly selected as
previously described (32) and
micrographed with IX71 microscope (Olympus, Japan) to quantify
cells of migration and invasion. Images represent at least three
independent experiments.
FAM172A rescue experiments
miR-27a inhibitor was cotransfected with FAM172A
siRNA or the control siRNA. After transfection of 24 h, cells were
collected and used analyzing for proliferation, migration and
invasion as described above. The expression of FAM172A was verified
by western blotting.
Statistical analysis
The data are described as means ± SEM from at least
three independent experiments. The difference between two groups
was analyzed using an independent Student's t-test. When comparing
more than two groups, the difference was analyzed using one-way
ANOVA analysis of variance. The difference between the expression
level of miR-27a and the clinical variables was analyzed using
Spearman correlation test. Receiver-operating characteristic (ROC)
curves and the area under the ROC curve (AUC) were used for
assessing the feasibility of miRNA as a diagnostic factor to detect
CRC. The Kaplan-Meier analysis was used for analyzing the
progression of CRC. Statistical analysis was performed using SPSS
version 20.0 (SPSS, USA). P-values <0.05 were considered to
indicate a statistical significance.
Results
miR-27a is upregulated in tissues of
patients with CRC and CRC cell lines
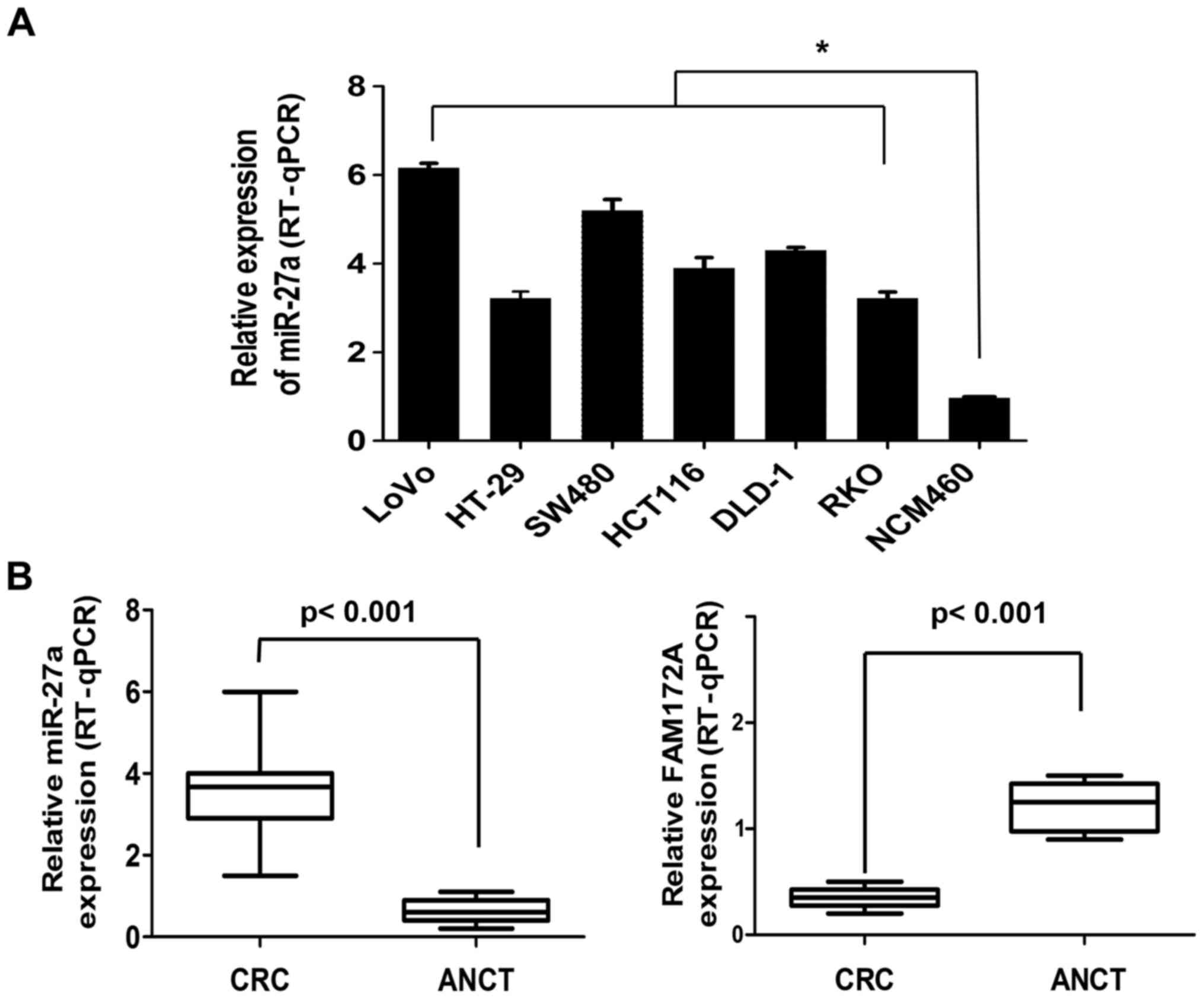
Respectively, we determined miR-27a expression
levels of six CRC cell lines (LoVo, HT-29, SW480, HCT116, DLD-1 and
RKO) and a normal colorectal cell line (NCM460) which acts as a
control. miR-27a expression levels in the six CRC lines were
significantly higher than the NCM460 cells (6.12-, 3.56-, 5.48-,
3.96-, 4.37- and 3.58-fold, respectively) (Fig. 1A). We collected 60 pairs of CRC
tissues and adjacent normal colorectal tissue (ANCT) to assess
miR-27a expression. The expression levels of miR-27a in CRC tissues
was higher than that of the ANCT samples (3.71-fold) (3.92±0.50 vs.
0.97±0.48, P<0.001). On the contrary, the expression levels of
FAM172A in CRC tissues was lower than that of the ANCT samples
(3.03-fold) (1.25±0.13 vs. 0.38±0.11, P<0.001) (Fig. 1B).
miR-27a is a marker of diagnosis and
prognosis in patients with colorectal cancer
First, the expression levels of miR-27a in tissues
of 60 patients with CRC were evaluated by RT-qPCR. Also, we divided
the patients into a high expression and a low expression group
based on the median expression levels of miR-27a, which were used
to analyse the group clinicopathological characteristics. We
acquired data for age, sex, carcino-embryonic antigen (CEA),
carbohydrate antigen 19–9 (CA19-9), TNM stage, distant metastasis,
tumor size, and differentiation. Analysis showed that expression
levels of miR-27a are closely related to the TNM stage (P=0.026)
and distant metastasis (P=0.021) (Table III) which are all indicators of
poor prognosis. Then we assigned patients to low-miR-27a group
(expression Ct value <22.50) and high-miR-27a group (expression
Ct value ≥22.50) in order to determine correlation between the
levels of miR-27a in CRC tissues and the survival of the CRC
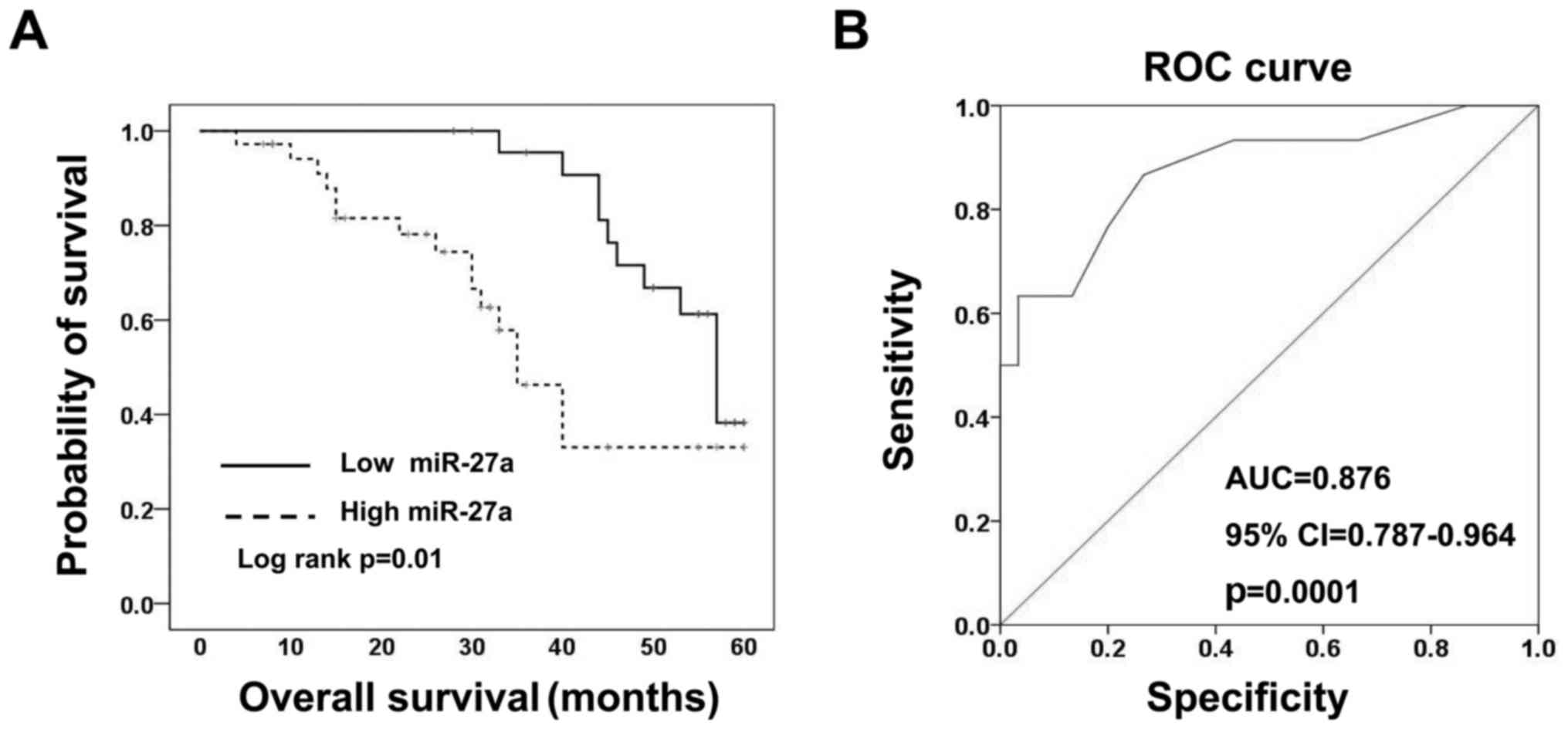
patients performed by a Kaplan-Meier survival analysis. The
Kaplan-Meier analysis indicated that the low-miR-27a group had even
better overall survival than the high-miR-27a group (Fig. 2A). These findings suggest that
miR-27a is a useful biomarker of poor prognosis for patients with
CRC. According to receiver-operating characteristic (ROC) curve
analyses, miR-27a had the best sensitivity and specificity when the
miR-27a Ct value was 22.50 (YI=0.633), and an AUC of 0.876
(P=0.0001; 95% CI, 0.787–0.964) (Fig.
2B) was obtained, which suggested that expression of miR-27a
could discriminate between CRC and adjacent non-tumor colorectal
tissues, thus may be used as a diagnostic marker for colorectal
cancer.
 | Table III.Correlation between expression status
of miR-27a and clinicopathological parameters. |
Table III.
Correlation between expression status
of miR-27a and clinicopathological parameters.
|
|
| miR-27a
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. of
patients | Low, n (%) | High, n (%) | P-value |
|---|
| Patient age
(years) |
|
≤60 | 41 | 22(54) | 19 (46) |
0.657 |
|
>60 | 19 | 9
(47) | 10 (53) |
|
| Sex |
|
Female | 32 | 14 (44) | 18 (56) |
0.839 |
|
Male | 28 | 13 (46) | 15 (54) |
|
| CEA |
|
Normal | 20 | 11 (55) | 9
(45) |
0.591 |
|
Elevated | 40 | 19 (48) | 21 (52) |
|
| CA19-9 |
|
Normal | 18 | 8
(45) | 10 (52) |
0.825 |
|
Elevated | 42 | 20 (48) | 22 (52) |
|
| TNM stage |
| I and
II | 27 | 15 (56) | 12 (44) |
0.026 |
| III and
IV | 33 | 9
(27) | 24 (73) |
|
| Distant
metastasis |
|
Absence | 36 | 24 (67) | 12 (33) |
0.021 |
|
Presence | 24 | 9
(38) | 15 (62) |
|
| Tumor size
(cm) |
| ≤4 | 26 | 12 (46) | 14 (54) |
0.610 |
|
>4 | 34 | 18 (53) | 16 (47) |
|
|
Differentiation |
| WD | 18 | 8
(44) | 10 (56) |
0.315 |
| MD | 23 | 11 (48) | 12 (52) |
|
| PD | 19 | 10 (53) | 9
(47) |
|
| FAM172A |
| ≥1 | 5 | 1
(20) | 4
(80) | <0.001 |
|
<1 | 55 | 6
(11) | 49 (89) |
|
FAM172A is a direct target of
miR-27a
In order to explore the regulation mechanism of
miR-27a in CRC, we used bioinformatic analysis to predict target
genes of miR-27a. As shown in Fig.
3A, there was a potential binding site of miR-27a predicted in
the 3′-UTR of FAM172A. To test the specific regulation of this
predicted binding site, we constructed a reporter vector which
consisted of the luciferase coding sequence from the 3′-UTR of
FAM172A (Luc-FAM172A 3′-UTR). The mutant was prepared which
consisted of the putative binding sites (Luc-FAM172A-mut 3′-UTR)
(Fig. 3A). Cotransfection
experiments indicated that miR-27a decreased the luciferase
activity of Luc-FAM172A 3′-UTR but had very little influence on
Luc-FAM172A-mut 3′-UTR (Fig. 3B),
which demonstrated that FAM172A is a potential target of miR-27a.
miR-27a was downregulated or upregulated in CRC cell lines
transfected with the miR-27a inhibitor or mimic, respectively
compared with the control-miR group (Fig. 3C).
MicroRNA-27a plays a negative
regulation role in FAM172A expression
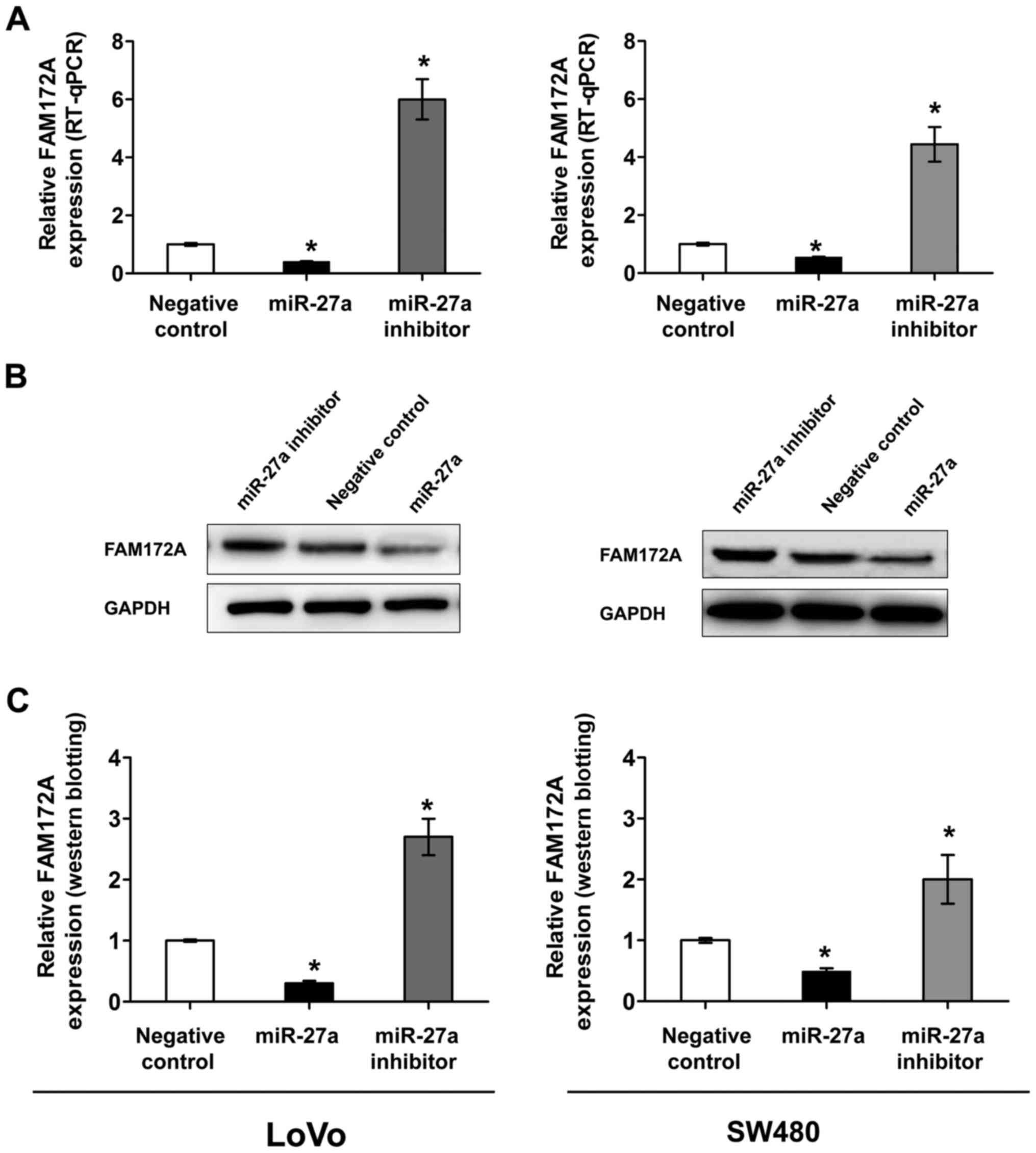
In order to determine whether miR-27a is a regulator
in FAM172A expression, we utilized LoVo and SW480 cells transfected
with control-miR, miR-27a mimic or miR-27a inhibitor, respectively.
Suppression of miR-27a in colorectal cancer cells resulted in a 4-
to 6-fold increase in FAM172A mRNA levels and overexpression of
miR-27a led to a 40–60% reduced FAM172A mRNA levels (Fig. 4A). As shown in Fig. 4B, an approximately 2- to 3-fold
increase in protein levels of FAM172A in colorectal cancer cells
transfected with inhibition of miR-27a, and overexpression of
miR-27a resulted in ~30–40% reduced protein levels in both LoVo and
SW480 cells.
miR-27a inhibitor decreases
proliferation, migration and invasion of colon cancer cells, which
could be partially rescued by decreased FAM172A
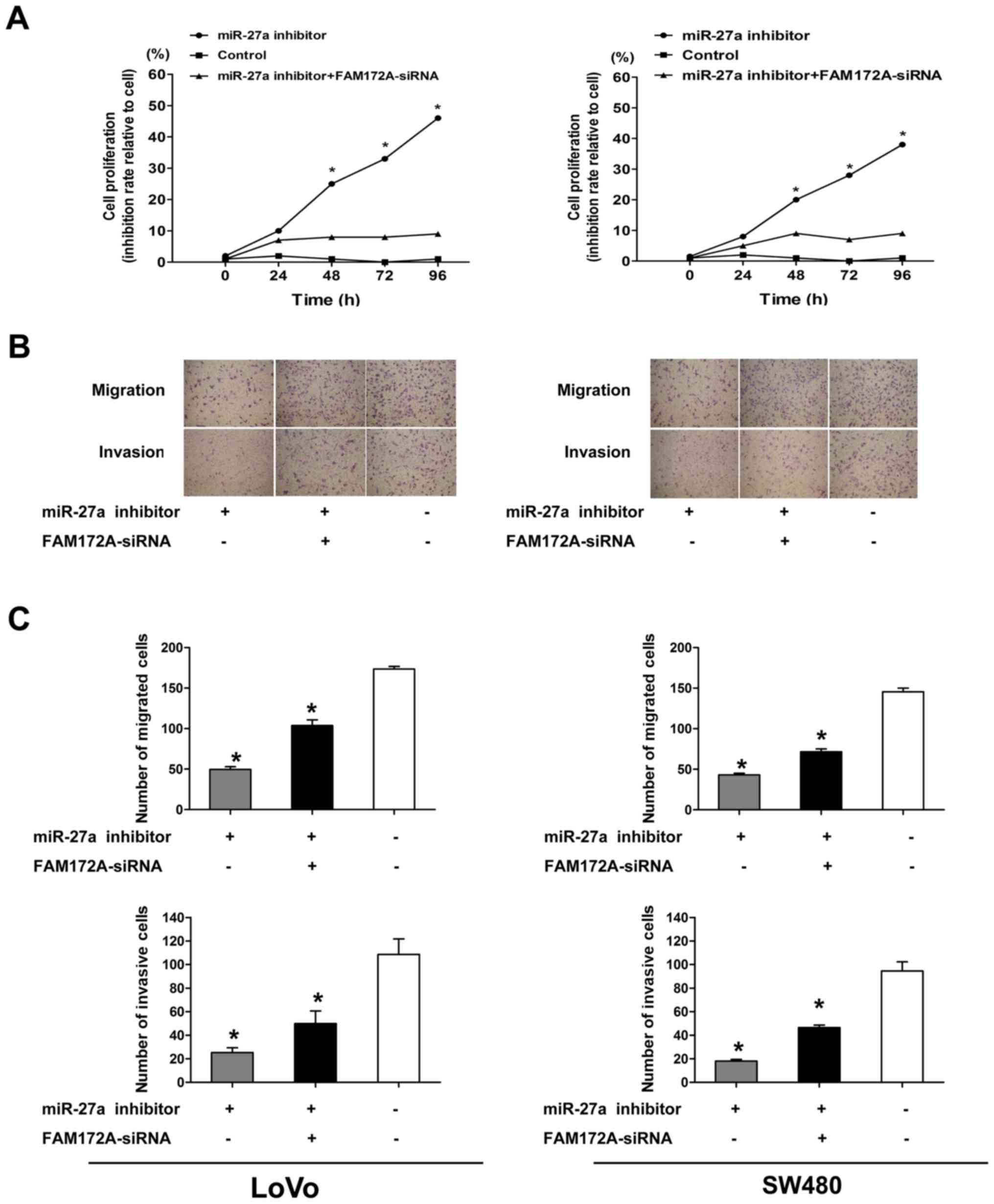
The role of miR-27a in the regulation of
proliferation, migration and invasion of CRC cells was further
investigated. We transfected the LoVo and SW480 CRC cell lines with
miR-27a inhibitor. As shown in Fig.
5A, we found that the cells transfected with the miR-27a
inhibitor obviously decreased proliferation compared with the cells
transfected with control-miR at 48, 72 and 96 h by cell
proliferation assay, but there was no obvious difference in
proliferation between CRC cells transfected with miR-27a inhibitor
and the control at 24 h. Compared with the negative control, both
the migration and invasion of CRC cells transfected with miR-27a
inhibitor were reduced at 48 h by Transwell migration and Matrigel
invasion assays (Fig. 5B and
C).
Moreover, we tested the levels of
NF-κB, MMP-2, MMP-9 and FAM172A mRNA and proteinin LoVo and SW480
cells transfected with miR-27a inhibitor and the negative control
respectively
The results indicated that the mRNA and protein
levels of these genes were decreased in CRC cell lines transfected
with miR-27a inhibitor, as compared with the negative control
(Fig. 6). In order to prove whether
FAM172A mediates the effects of miR-27a on migration and invasion,
we performed cell assays in LoVo and SW480 cell lines
co-transfected with miR-27a inhibitor and FAM172A-siRNA. As shown
in Fig. 5, the results suggested
that FAM172A-siRNA to some degree rescued the proliferation,
migration and invasion in LoVo and SW480 cell lines transfected
with miR-27a inhibitor, and decreased expression of FAM172A rescued
the mRNA and protein expression levels of NF-κB, MMP-2, MMP-9 and
FAM172A (Fig. 6).
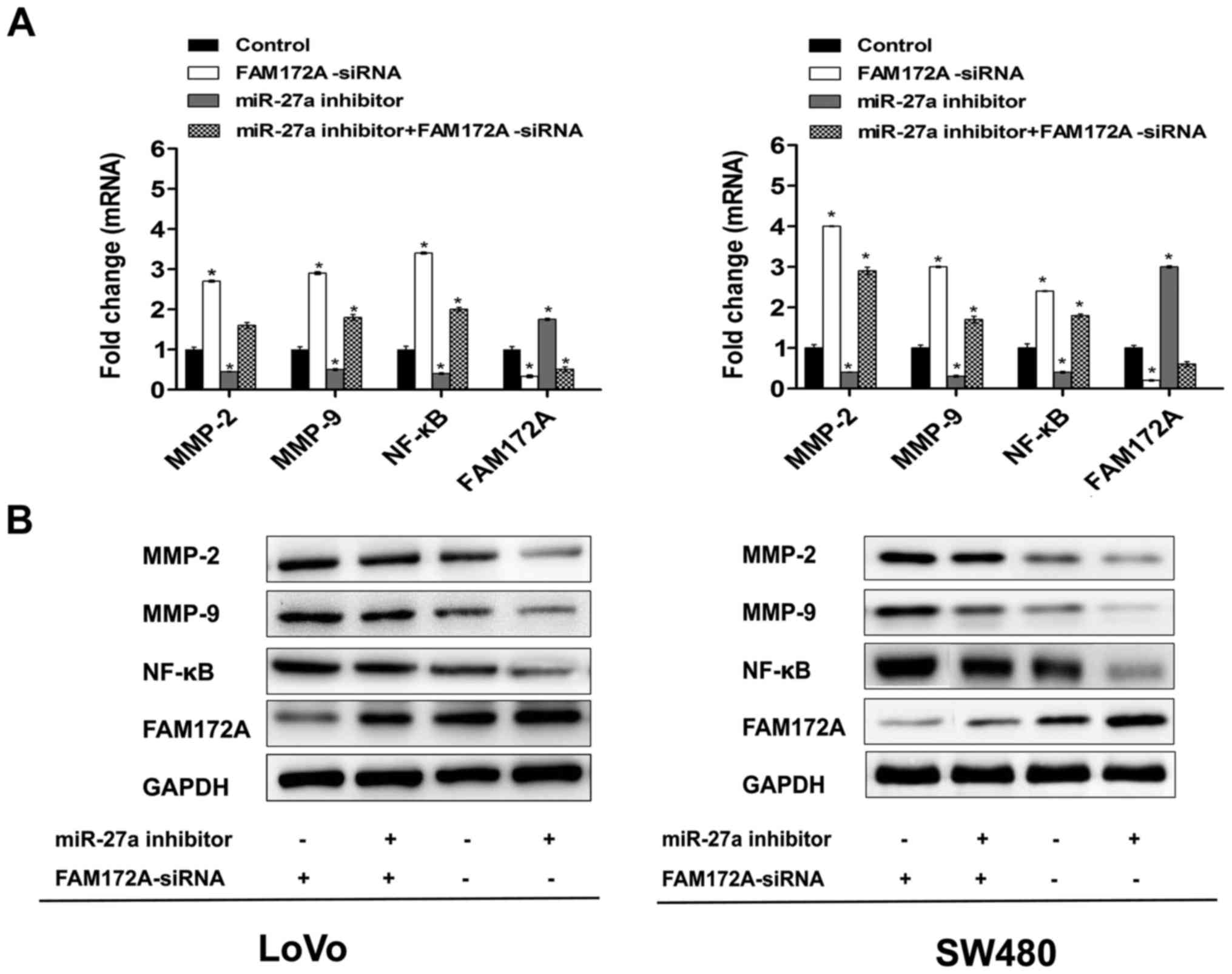 | Figure 6.miR-27a inhibitor suppresses levels
of NF-κB, MMP-2, MMP-9 and FAM172A expression. (A) CRC lines were
transfected with miR-27a inhibitor (50 nmol/l), miR-27a inhibitor +
FAM172A-siRNA or control, followed by RT-qPCR analysis of the
NF-κB, MMP-2, MMP-9 and FAM172A mRNA, *P<0.05. (B) CRC lines
were transfected with miR-27a inhibitor (50 nmol/l), miR-27a
inhibitor + FAM172A-siRNA or control, followed by western blot
analysis of the NF-κB, MMP-2, MMP-9 and FAM172A protein,
*P<0.05. |
Discussion
As a protein of 416 amino acid, FAM172A belongs to
the UPF0528 family and its protein-coding gene is located in human
chromosome 5 (9). Our previous
research showed that FAM172A expression had a lower expression in
CRC tissues of patients with metastasis compared with CRC tissues
of patients without metastasis (11). However, relevant studies of FAM172A
and its more unknown functions in CRC are still scarce.
There is increasing evidence that dysregulation of
miRNAs is involved in colorectal cancer. They bind to the
3′-untranslated region (UTR) of target genes, which promotes RNA
degradation and even regulate mRNA transcription (33), thus regulating the biological
function of cancer cells, including cell proliferation, migration
and invasion. Interestingly, our previous research showed that
STAT1 affected the expression of FAM172A via binding to the FAM172A
promoter. After co-transfection of STAT1 overexpression plasmid or
siRNA in LoVo cells, the transcriptional activity of FAM172A was
powerfully increased (12).
Therefore, FAM172A expression in colorectal cancer cells may depend
on its transcriptional regulation. Our present study found that the
miR-27a seed sequence has a complementarity to the 3′-UTR
nucleotide sequence of human FAM172A mRNA. miR-27a has been closely
related to the tumor process, and it has been shown to regulate the
biological function of cancer cells, including cell proliferation,
migration and invasion by affecting target genes (30,31).
We provided evidence that miR-27a decreased the luciferase energy
of Luc-FAM172A 3′-UTR but barely had an effect on Luc-FAM172A-mut
3′-UTR, indicating that FAM172A was a direct target of miR-27a by
luciferase reporter assay. Furthermore, both mRNA and protein
levels of FAM172A was downregulated in LoVo and SW480 cells
transfected with miR-27a. The silencing of miR-27a can upregulate
the mRNA level and protein levels of FAM172A, indicating that
miR-27a participates in regulating FAM172A expression. Therefore
FAM172A is a downstream target gene of miR-27a.
Our prior studies showed that overexpression of
FAM172A inhibited the migrational capability and cell invasiveness
of CRC cell lines by suppressing the expression of MMPs (11). To further explore the biological
functions of FAM172A regulated by miR-27a in LoVo and SW480 cell
lines, we determine the effect of miR-27a on cell poliferation,
migration and invasion. In this study, we first confirmed that cell
migration and invasion become weaken in CRC cells transfected with
miR-27a inhibitor, which can be significantly rescued by FAM172A
siRNA. Interestingly, mRNA and protein levels of MMP-2, MMP-9 and
FAM172A are decreased in CRC cells transfected with miR-27a
inhibitor, which also can be significantly rescued by FAM172A
siRNA. Accordingly, miR-27a-FAM172A axis is linked to the migration
and invasion of LoVo and SW480 cell lines. Cell migration and
invasion occur in the tumorigenic process. Therefore, migration and
invasion can resulted in metastasis, thus causing deaths of ~90% of
cancer patients (34). MMPs are
critical players in tumor migration and invasion through
degradation of ECM components. Other studies have shown that MMP-2
expression is significantly relevant for the tumor metastasis
(35) and expression of MMP-9 is
closely related to poor prognosis and metastasis of CRC (36). MMP-2 and MMP-9 are frequently
upregulated in cancer cells (37,38).
Thus miR-27a-mediated novel targets contribute to the metastatic
pathways.
Our previous results manifested that upregulation of
FAM172A inhibited cell proliferation in LoVo and SW480 cell lines.
Instead, proliferation of these cells treated with FAM172A siRNA
was induced significantly (12). In
the present study, we found that FAM172A overexpression decreased
the expression of NF-κB. In addition, significantly decreased
proliferation and expression of NF-κB are observed in the miR-27a
inhibition group, which can be significantly rescued by FAM172A
siRNA. Accordingly, miR-27a-FAM172A axis is linked to the
proliferation of CRC cells. Some research has shown that NF-κB are
also related to cell migration and invasion (39,40).
The NF-κB signaling pathway has some effect on human health and
disease, particularly in cancers. Activation of NF-κB is frequently
involved in a variety of tumors, thus promoting the development of
tumors, which may lead to different etiology and pathogenesis, like
mutations or deletions of IκBα in Hodgkin's lymphoma,
amplifications or translocations of NIK in multiple myeloma
(41,42). Deregulation of the miRNAs also leads
to the abnormal activation of the NF-κB pathway by regulating the
NF-κB pathway except the genomic aberrations of protein-coding
genes. Thus, a deeper understanding of NF-κB signaling may
contribute to progress for determination of the molecular pathology
of a variety of cancer. miRNAs may provide novel targets for
anticancer therapy by regulating the NF-κB activity.
In conclusion, higher expression of miR-27a was
observed in CRC tissues compared with ANCT. There was a positive
correlation between the expression of miR-27a and some
clinicopathological characteristics and overall survival of
patients with CRC. We proposed a novel regulatory mechanism
miR-27a-FAM172A axis for cell proliferation, migration and invasion
in CRC based on our observations. miR-27a is an oncogenic miRNA and
a direct regulator of FAM172A in CRC. The identification of this
novel molecular pathway mediated by the miR-27a-FAM172A axis and
its role may lead to a deeper knowledge on CRC tumorigenesis, thus
providing guiding reference for future research and patient
stratification according to miR-27a/FAM172A expression levels,
which ultimately designs novel individual treatments.
Acknowledgements
This study was supported by the Guangdong Natural
Science Foundation (2014A030313324) and the Guangdong Natural
Science Foundation (2015) and the Key Clinical Specialty Discipline
Construction Program and Undergraduate Training Program for
Innovation and Entrepreneurship (201512121116).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Metastasis suppressors alter the
signal transduction of cancer cells. Nat Rev Cancer. 3:55–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peltomäki P: Role of DNA mismatch repair
defects in the pathogenesis of human cancer. J Clin Oncol.
21:1174–1179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yun J, Rago C, Cheong I, Pagliarini R,
Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S,
Zhou S, et al: Glucose deprivation contributes to the development
of KRAS pathway mutations in tumor cells. Science. 325:1555–1559.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim TM, Laird PW and Park PJ: The
landscape of microsatellite instability in colorectal and
endometrial cancer genomes. Cell. 155:858–868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bostick M, Kim JK, Estève PO, Clark A,
Pradhan S and Jacobsen SE: UHRF1 plays a role in maintaining DNA
methylation in mammalian cells. Science. 317:1760–1764. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Dong X, Leong MC, Zhou W, Yang Z,
Chen F, Bao Y, Jia W and Hu R: Identification of the novel protein
FAM172A, and its up-regulation by high glucose in human aortic
smooth muscle cells. Int J Mol Med. 26:483–490. 2010.PubMed/NCBI
|
|
9
|
Li LX, Tao Z, Dong XH, Liang WC, Yang ZH,
Mou B, Bao YQ, Wang C, Jia WP and Hu RM: Molecular cloning of a
novel gene, C5orf21 gene and its roles in diabetic macroangiopathy.
Zhonghua Yi Xue Za Zhi. 89:2574–2577. 2009.(In Chinese). PubMed/NCBI
|
|
10
|
Cui C, Ye L, Huang Z, Huang S, Liu H and
Yu J: FAM172A is a tumor suppressor in colorectal carcinoma. Tumour
Biol. 37:6501–6510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian K, Zhang J, Lu J, Liu W, Yao X, Chen
Q, Lu S, Xiang G and Liu H: FAM172A modulates apoptosis and
proliferation of colon cancer cells via STAT1 binding to its
promoter. Oncol Rep. 35:1273–1280. 2016.PubMed/NCBI
|
|
12
|
Babashah S and Soleimani M: The oncogenic
and tumour suppressive roles of microRNAs in cancer and apoptosis.
Eur J Cancer. 47:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Place RF, Li LC, Pookot D, Noonan EJ and
Dahiya R: MicroRNA-373 induces expression of genes with
complementary promoter sequences. Proc Natl Acad Sci USA. 105:pp.
1608–1613. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu M and Chen H: The role of microRNAs in
colorectal cancer. J Genet Genomics. 37:347–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Michael MZ, O'Connor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
24
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:pp. 2257–2261.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cekaite L, Rantala JK, Bruun J, Guriby M,
Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe
RA, et al: MiR-9, −31, and −182 deregulation promote proliferation
and tumor cell survival in colon cancer. Neoplasia. 14:868–879.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valeri N, Braconi C, Gasparini P, Murgia
C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat
F, et al: MicroRNA-135b promotes cancer progression by acting as a
downstream effector of oncogenic pathways in colon cancer. Cancer
Cell. 25:469–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang F, Yang YZ, Shi CZ, Zhang P, Moyer
MP, Zhang HZ, Zou Y and Qin HL: UHRF1 promotes cell growth and
metastasis through repression of p16(ink4a) in colorectal cancer.
Ann Surg Oncol. 19:2753–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colangelo T, Polcaro G, Ziccardi P, Pucci
B, Muccillo L, Galgani M, Fucci A, Milone MR, Budillon A,
Santopaolo M, et al: Proteomic screening identifies calreticulin as
a miR-27a direct target repressing MHC class I cell surface
exposure in colorectal cancer. Cell Death Dis. 7:e21202016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, Liang X, Zhang L, Yang L, Nagao N,
Wu H, Liu C, Lin S, Cai G and Liu J: MiR-27a-3p functions as an
oncogene in gastric cancer by targeting BTG2. Oncotarget.
7:51943–51954. 2016.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Bacco F, Luraghi P, Medico E, Reato G,
Girolami F, Perera T, Gabriele P, Comoglio PM and Boccaccio C:
Induction of MET by ionizing radiation and its role in
radioresistance and invasive growth of cancer. J Natl Cancer Inst.
103:645–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spizzo R, Nicoloso MS, Croce CM and Calin
GA: SnapShot: MicroRNAs in cancer. Cell. 137:586–586.e1. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HJ, Song IC, Yun HJ, Jo DY and Kim S:
CXC chemokines and chemokine receptors in gastric cancer: From
basic findings towards therapeutic targeting. World J
Gastroenterol. 20:1681–1693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen W, Xi H, Wei B and Chen L: The
prognostic role of matrix metalloproteinase 2 in gastric cancer: A
systematic review with meta-analysis. J Cancer Res Clin Oncol.
140:1003–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Liu H, Luo X, Deng J, Pan Y and
Liang H: Overexpression of SMYD3 and matrix metalloproteinase-9 are
associated with poor prognosis of patients with gastric cancer.
Tumour Biol. 36:4377–4386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adiseshaiah P, Vaz M, Machireddy N,
Kalvakolanu DV and Reddy SP: A Fra-1-dependent, matrix
metalloproteinase driven EGFR activation promotes human lung
epithelial cell motility and invasion. J Cell Physiol. 216:405–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Belguise K, Kersual N, Galtier F and
Chalbos D: FRA-1 expression level regulates proliferation and
invasiveness of breast cancer cells. Oncogene. 24:1434–1444. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
41
|
Staudt LM: Oncogenic activation of
NF-kappaB. Cold Spring Harb Perspect Biol. 2:a0001092010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ben-Neriah Y and Karin M: Inflammation
meets cancer, with NF-κB as the matchmaker. Nat Immunol.
12:715–723. 2011. View Article : Google Scholar : PubMed/NCBI
|