Introduction
The morbidity and mortality of esophageal cancer
rank the eighth and sixth among all malignant tumors worldwide
(1). Esophageal cancer is
classified into esophageal squamous cell carcinoma (ESCC) and
adenocarcinoma (EAC) based on the histopathologic type. In Western
countries, EAC represents the dominant subtype and the incidence
has increased markedly over the past decades, whereas the northern
regions of Henan Province, China, have the highest incidence of
ESCC (2). Despite the great
advances in early diagnosis and traditional treatment options
(surgery, chemotherapy and radiotherapy), the prognosis of patients
with advanced esophageal cancer remains poor with the 5-year
survival rate ranging from 15 to 25% (3). During the past decade, the field of
drug development has been transformed with the identification of
and ability to direct treatment at specific molecular targets. The
overexpression and aberrant function of epidermal growth factor
receptor (EGFR) and insulin-like growth factor-1 receptor (IGF-1R)
in a number of solid tumors including esophageal cancer, and the
important roles in the development of tumors have provided a
rationale for targeting the two receptors.
EGFR/HER1 is a member of the ErbB receptor tyrosine
kinase family, and there are three other members, HER2, HER3 and
HER4, in this family. Ligand (EGF and TGF-α) binding to the
receptors results in receptor homodimerization and
heterodimerization, activation of the intrinsic kinase domain and
initiation of a cascade of downstream signaling that ultimately
promotes tumor cell survival, proliferation, invasion and
metastasis (4). EGFR overexpression
has been observed in many human tumors, such as lung, head and
neck, colorectal, breast and ovarian (5–9). In
esophageal cancer, ~50–71% of ESCC patients have EGFR
overexpression (10). The IGF
system is comprised of the IGF ligands (IGF-1 and IGF-2),
cell-surface receptors (IGF-1R and IGF-2R) and six IGF-binding
proteins (IGFBPs). IGF-1R is a tetrameric glycoprotein containing
two α subunits and two β subunits linked by disulfide bonds, and
there is a 60% homology between IGF-1R and the insulin receptor.
IGF-1R binds IGF-1 with high affinity and IGF-2 and insulin with a
lower affinity. Similarly, when the ligands bind to IGF-1R,
auto-phosphorylation of the receptor tyrosine kinase is induced,
leading to the activation of multiple downstream signaling
pathways, such as PI3K/AKT and RAS/MAPK, each of which plays an
important role in cell proliferation, migration and metabolism.
IGF-1R overexpression has also been found in many tumors, including
60% of esophageal cancer, and its overexpression was found to
correlate with advanced tumor stage, depth of invasion, metastasis
and recurrence (11,12). IGF-1R and EGFR families show
homology in their structure and both the receptors share
considerable crosstalk in their functions. Co-expression of the two
receptors was observed in resected non-small cell lung cancer
(NSCLC), colorectal cancer, adrenocortical carcinoma, pancreatic
ductal adenocarcinoma, and was found to be associated with poor
pathological stage and shorter disease-free survival (DFS)
(13–16). A number of EGFR-targeted drugs
(gefitinib, erlotinib, cetuximab and panitumumab) alone or in
combination with chemotherapeutics have been tested clinically for
the treatment of patients with esophageal cancer, but the results
are disappointing (17–21). Different from the monoclonal
antibodies and tyrosine kinase inhibitors, antibody-drug conjugates
or ligand-toxin fusion proteins taking advantage of the specificity
of antibodies or ligands and the potent cytotoxic activity of
toxins have shown elevated antitumor efficacy. Furthermore, they
are effective even for patients who are resistant to targeted
drugs. For example, trastuzumab emtansine (T-DM1) consisting of
trastuzumab coupled to a cytotoxic agent, emtansine (DM1), showed
significantly improved progression-free and overall survival with
less toxicity than lapatinib plus capecitabine in patients with
HER2-positive advanced breast cancer previously treated with
trastuzumab and a taxane (22).
EGF-LDP-IGF-AE is a bispecific and
enediyne-energized fusion protein targeting both EGFR and IGF-1R
that was previously constructed by us (23). It contains two ligands (EGF and
IGF-1) specific for EGFR and IGF-1R and an enediyne antibiotic
lidamycin (LDM) with potent cytotoxicity. The two ligands were
designed as targeting moiety, directing the fusion protein to
cancer cells with EGFR and IGF-1R overexpression. LDM is composed
of a noncovalently bound apoprotein (LDP) and an active enediyne
chromophore (AE) that act as a cytotoxic moiety as previous studies
have demonstrated the extremely potent cytotoxicity of LDM to
various tumor cells and marked inhibitory effects on a panel of
xenografts in athymic mice (24).
In the present study, we measured the in vitro and in
vivo antitumor activity of EGF-LDP-IGF-AE on esophageal cancer
and the efficacy was compared with corresponding mono-specific
fusion proteins to determine whether dual inhibition of EGFR and
IGF-1R is more efficacious. The underlying mechanisms of the
antitumor effect of EGF-LDP-IGF-AE were also evaluated.
Materials and methods
Ethics statement
All experiments involved in animals were performed
according to the Declaration of Helsinki and according to
international and national guidelines, and the procedures were
approved by the Ethics Committee of Xinxiang Medical
University.
Tissue microarray and
immunohistochemical staining
Tissue microarrays containing a total of 75 pairs of
human ESCC tumor and corresponding adjacent normal tissues
(HEso-Squ150CS-01), were purchased from Shanghai Outdo Biotech Co.
Ltd. (Shanghai, China), and the immunohistochemical (IHC) staining
was used to analyze EGFR and IGF-1R expression. Tissue microarray
slides were deparaffinized in xylene, rehydrated with graded
ethanol and immersed in water. For antigen retrieval, the slides
were heated at 95°C for 40 min and incubated with 3% hydrogen
peroxide at room temperature for 15 min. Mouse anti-EGFR or
anti-IGF-1R antibody (diluted 1:100; Lab Vision Corporation,
Fremont, CA, USA) was applied overnight at 4°C, followed by Polymer
Helper (ZSGB-Bio, Beijing, China) for 20 min. Subsequently, the
slides were incubated with polyperoxidase anti-mouse IgG (ZSGB-Bio)
at 37°C for 30 min. According to the manufacturer's instructions,
the slides were reacted with DAB liquid system (Dako, Glostrup,
Denmark) and counterstained with hematoxylin. The assessment of
EGFR or IGF-1R staining was performed by two pathologists
separately using H-score systems as previously described (25): scores ≥201, 101–200, 1–100, 0
represent strongly positive (3+), moderately positive (2+), weakly
positive (1+) and negative (−) staining, respectively.
Cell lines and culture conditions
Human ESCC cell lines EC9706, TE-1, KYSE450 and
KYSE510 were obtained from the Cell Center of Peking Union Medical
College (Beijing, China). Cells were cultured in RPMI-1640 medium
(Gibco; Life Technologies) containing 10% (v/v) fetal bovine serum
(FBS; Gibco, Life Technologies) and 100 µg/ml streptomycin, 100
U/ml penicillin at 37°C with 5% CO2.
Preparation of fusion proteins and
their enediyne-energized analogues
DNA sequences coding for fusion protein EGF-LDP-IGF,
which contains the gene of human EGF (169 bp),
(G4S)2-linker (30 bp), apoprotein of LDM
(ldp, 330 bp), (G4S)2-linker (30 bp) and
human IGF-1 (210 bp) from 5′ to 3′ end, were synthesized by Beijing
Sunbiotech Co. Ltd. (Beijing, China), and then, it was cloned into
the pET30a vector to generate the plasmid pET30-egf-ldp-igf.
EGF-LDP-IGF protein was expressed in the Escherichia coli
strain BL21(DE3) according to the pET System Manual (11th edition;
Novagen, Madison, WI, USA) and purified by Ni2+ affinity
chromatography (HisTrap HP column; GE Healthcare, Milwaukee, WI,
USA), since the His6-tag was introduced to the COOH
terminal of EGF-LDP-IGF protein. The active chromophore of LDM (AE)
was isolated using a C4 column (GE Healthcare) with 22%
acetonitrile in 0.05% trifluoroactic acid mobile phase, and then
the enediyne-energized analogue of fusion protein EGF-LDP-IGF-AE
was prepared by integrating the AE into EGF-LDP-IGF. The
corresponding mono-specific fusion proteins (EGF-LDP and LDP-IGF)
and their enediyne-energized analogues (EGF-LDP-AE and LDP-IGF-AE)
were constructed in the same way.
Binding affinity assay
The binding affinity of EGF-LDP-IGF protein to
esophageal cancer cells was analyzed by immunofluorescence staining
assay. Cells were grown on coverslips, cultured for 24 h and fixed
with 4% paraformaldehyde for 10 min at room temperature. After
washed three times with 0.05% Tween-20 in phosphate-buffered saline
(PBS) for 5 min each, the cells were blocked with 5% bovine serum
albumin (BSA; Genview, China) for 1 h and subsequently incubated
with EGF-LDP-IGF protein (50 µg/ml) for 2 h at room temperature.
Then, they were incubated with mouse anti-His-tag antibody (diluted
1:100; Tiangen Biotech, China) overnight at 4°C, washed for three
times, followed by Alexa Flour 488-labeled goat anti-mouse antibody
(diluted 1:50; Beyotime Biotechnology, Shanghai, China) for 1 h.
After being washed five times with PBS, the cells were stained with
Hoechst 33258 (Beyotime Biotechnology) for 15 min at room
temperature. The images were observed under a Zeiss LSM 780
confocal laser scanning microscope (Carl Zeiss, Jena, Germany).
Cell viability assay
MTT assays were used to measure the cytotoxicity of
enediyne-energized fusion proteins to esophageal cancer cells in
vitro. Cells were seeded in 96-well plates (1,000–2,000
cells/well) and incubated for 24 h at 37°C with 5% CO2.
LDM and enediyne-energized fusion proteins (EGF-LDP-IGF-AE,
EGF-LDP-AE and LDP-IGF-AE) at different concentrations were added
to each well for 48 h of incubation. Then, 20 µl MTT (5 mg/ml;
Sigma) was subsequently added and incubated for another 4 h. The
supernatant was removed and 150 µl dimethyl sulfoxide (DMSO) was
added to each well. The absorbance at 570 nm was measured by an
ELISA reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Growth inhibition was calculated as the percentage of the untreated
controls and the IC50 values were calculated by GraphPad
Prism 5.
Cell cycle distribution analysis
Propidium iodide (PI) staining was used for
evaluating the effects of bispecific fusion protein EGF-LDP-IGF-AE
on cell cycle distribution. Cells (2×104) were plated in
60-mm dishes, cultured for 24 h and treated with 0.01, 0.05 and 0.1
nmol/l EGF-LDP-IGF-AE for 48 h. Subsequently, the cells were
digested by trypsin-EDTA and fixed with cold 70% ethanol. After
being washed three times with PBS, the cells were resuspended in
500 µl staining buffer containing PI (50 mg/ml, 25 µl) and RNase A
(100 mg/ml, 10 µl) and incubated at 37°C for 30 min according to
the manufacturer's instructions (Beyotime Biotechnology). Then,
cells were analyzed for fluorescence with a flow cytometer (BD
Biosciences, Heidelberg, Germany).
Cell apoptosis assay
The effect of EGF-LDP-IGF-AE on the apoptosis of
esophageal cancer cells was investigated by Annexin V-FITC/PI
staining. Cells were cultured in 6-well plates for 24 h and treated
with 0.1, 0.5, 1 and 2 nmol/l of EGF-LDP-IGF-AE for 48 h. Cells
were harvested, washed twice with PBS, resuspended in 500 µl
binding buffer containing 10 µl Annexin V-FITC and 5 µl PI
(Beyotime Biotechnology), incubated at room temperature for 10 min,
and analyzed for fluorescence with a flow cytometer (BD
Biosciences).
Western blot analysis
KYSE450 or EC9706 cells were seeded in 100-mm dishes
and grown to 70–80% confluence, after which the cells were washed
twice in PBS and cultured overnight in serum-free medium. Cells
were firstly exposed to EGF-LDP-IGF-AE (0.1 nmol/l), EGF-LDP-AE
(0.1 nmol/l) or LDP-IGF-AE (0.1 nmol/l) for 24, 48, 72 or 96 h,
followed by stimulation with human EGF (50 ng/ml), human IGF-1 (50
ng/ml) (both from Abcam, Cambridge, MA, USA), or both for 30 min at
37°C. Cells were then collected and lysed in cell lysis buffer
(Beyotime Biotechnology) containing 1 mmol/l phenylmethylsulfonyl
fluoride (PMSF) on ice for 30 min. Total proteins (30 µg) extracted
from the cells were applied on 10% SDS-PAGE and transferred to
polyvinylidenedifluoride membranes (PVDF; Millipore, Billerica, MA,
USA). After being blocked with 5% BSA for 1 h at room temperature,
the membranes were incubated with primary antibodies (diluted
1:1,000) overnight at 4°C and secondary HRP-conjugated antibodies
(diluted 1:4,000) (both from Cell Signaling Technology, Beverly,
MA, USA) for 1 h after being washed three times with 1X TBST
buffer. The specific bands were visualized with the Immobilon
Western Chemiluminescent HRP Substrate kit (Millipore) and captured
by Amersham Imager 600 system (GE Healthcare, Logan, UT, USA).
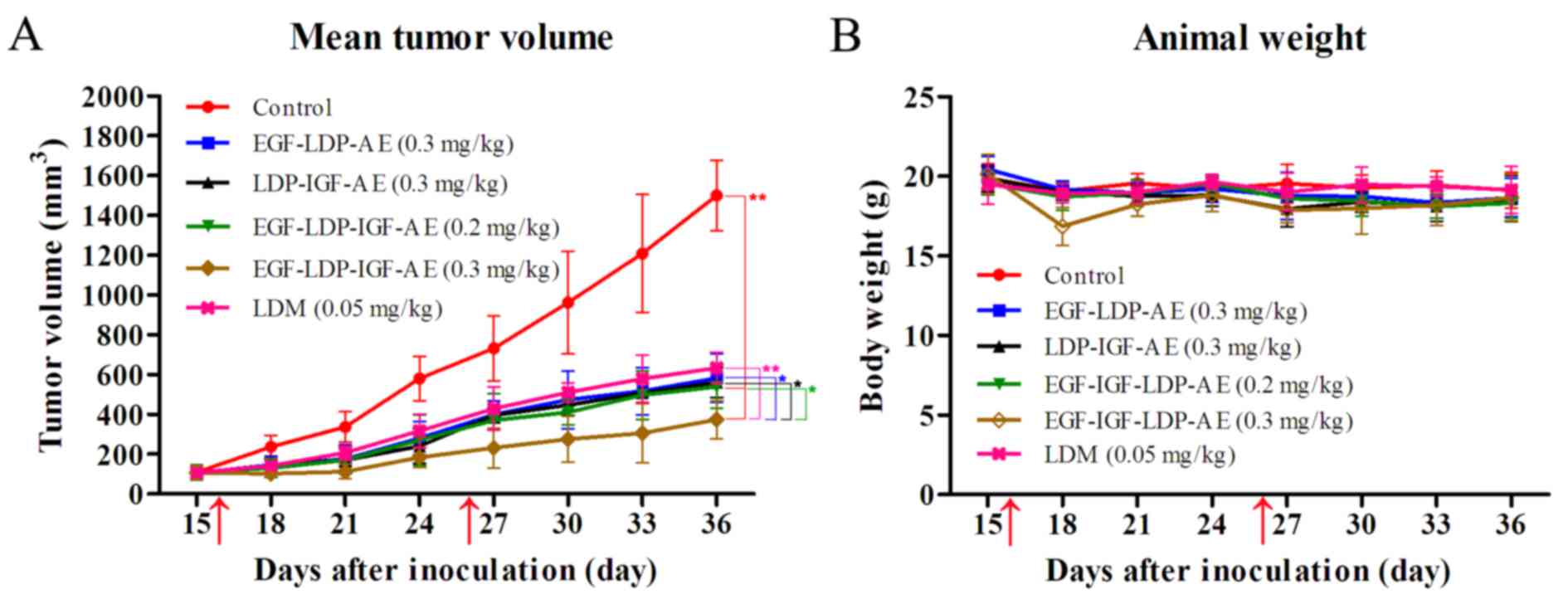
In vivo efficacy assay
Female BALB/c nude mice were purchased from Vital
River Laboratory Animal Technology Co. Ltd. (Beijing, China), and
the KYSE450 xenograft nude mouse model was performed to evaluate
the in vivo efficacy of fusion proteins. KYSE450 cells
(5×107) suspended in 200 µl PBS were inoculated s.c. in
the right armpit of nude mice. When the tumor size was >100
mm3, the nude mice were randomly divided into six groups
(n=6) and treated with EGF-LDP-AE (0.3 mg/kg), LDP-IGF-AE (0.3
mg/kg), EGF-LDP-IGF-AE (0.2 and 0.3 mg/kg) and LDM (0.05 mg/kg),
respectively. They received a 200 µl volume of PBS and injected
i.v. in the tail vein. Ten days after the first treatment,
tumor-bearing mice were injected with the fusion proteins again at
the same doses. Tumor size was measured every third day and tumor
volume (V) was determined using the formula: V = length ×
width2/2. The inhibition rates were calculated using the
formula: 1 - tumor volume (treated)/tumor volume (control) ×
100%.
Statistical analysis
Results of the present study were derived from three
independent experiments, analyzed by GraphPad Prism 5 software, and
are presented as mean ± SD. One-way ANOVA or two-way ANOVA and
Bonferroni post hoc analysis were used to compare the differences
between groups. P-values <0.05 were considered as statistically
significant. The densitometry analysis of the western blot results
was analyzed by ImageJ software.
Results
EGFR and IGF-1R are overexpressed in
esophageal cancer tissues
As shown in Fig. 1A and
B, there was strong and specific expression of both EGFR and
IGF-1R in the ESCC tissues. The negative and positive cases for
EGFR in ESCC tissues were 16 and 59, respectively, which were
significantly different from the paired adjacent normal tissues
(the negative and positive cases of 48 and 27, respectively;
Chi-square test, P<0.0001). The expression of IGF-1R in the ESCC
and paired adjacent normal tissues was also significantly different
(Chi-square test, P<0.0001; Table
I). The EGFR and IGF-1R expression results from the tissue
microarray are summarized in Table
I, and representative examples of negative and positive
staining with intensities of 1+, 2+ and 3+ are presented in
Fig. 1C. EGFR expression was
positive in 78.67% of the tumor tissues (59/75), and IGF-1R
expression was positive in 82.67% of the tumor tissues (62/75).
Furthermore, 48 samples (64%) exhibited EGFR and IGF-1R
co-expression.
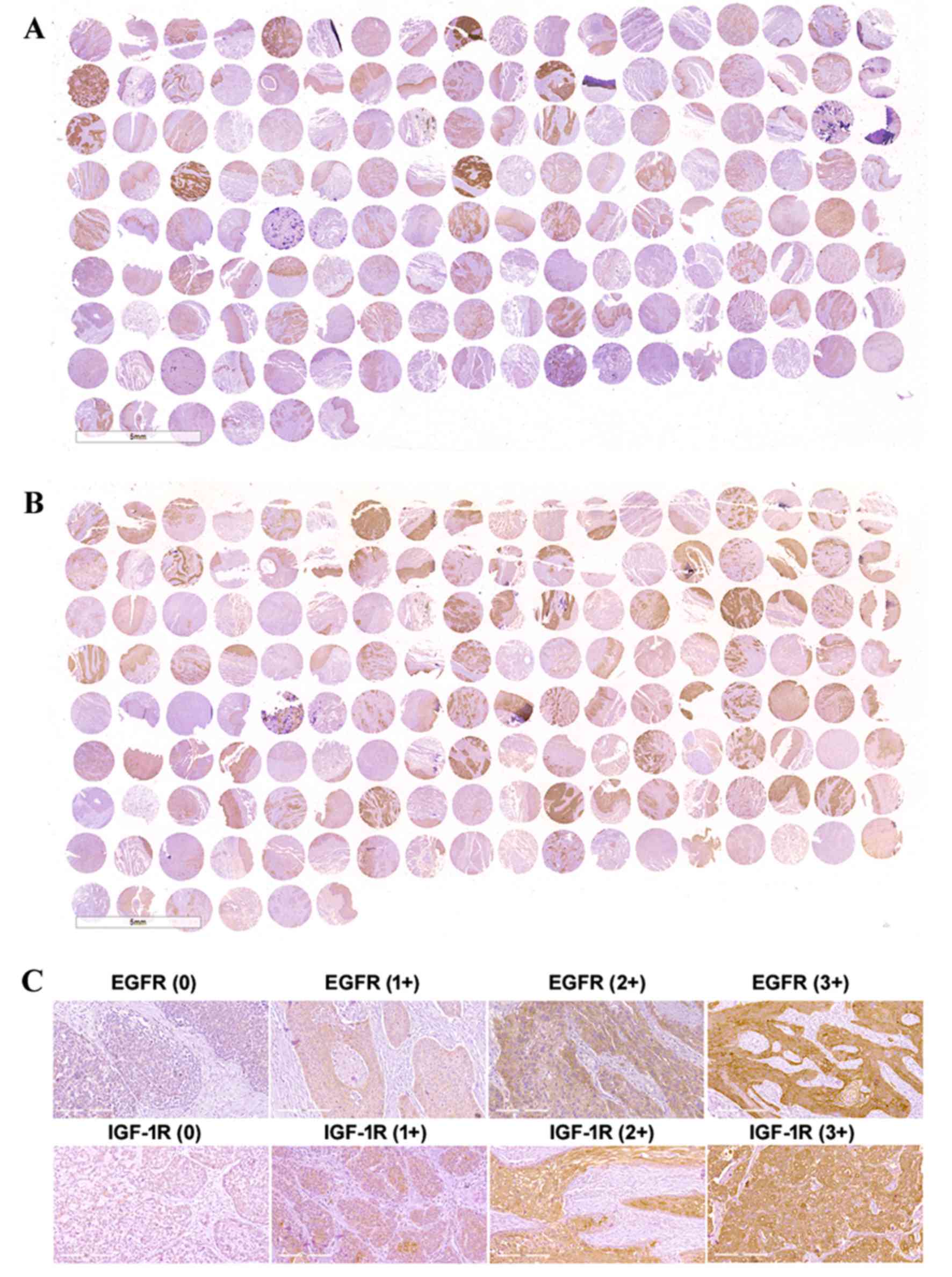 | Figure 1.Immunohistochemical analysis of EGFR
and IGF-1R expression in ESCC tissue microarrays. Overview of (A)
EGFR and (B) IGF-1R expression patterns in ESCC tissue microarrays.
Column 1, 3, 5, 7, 9, 11, 13, 15 and 17 include samples of ESCC
tissues. Column 2, 4, 6, 8, 10, 12, 14, 16 and 18 include samples
of paired adjacent normal tissues. (C) Representative examples of
negative and positive staining with intensities of 1+, 2+ and 3+
for EGFR and IGF-1R expression. The images were observed under a
microscope at a magnification of ×200. |
 | Table I.EGFR and IGF-1R expression in
esophageal squamous cell carcinoma and paired normal esophageal
tissues in a tissue microarray. |
Table I.
EGFR and IGF-1R expression in
esophageal squamous cell carcinoma and paired normal esophageal
tissues in a tissue microarray.
| Tissue | EGFR
expression | n (%) | IGF-1R
expression | n (%) |
|---|
| Esophageal squamous
cell carcinoma | Negative | 16
(21.3)a | Negative | 13
(17.3)b |
|
| Positive | 59
(78.7)a | Positive | 62
(82.7)b |
|
| Low (1+) | 47 (62.7) | Low (1+) | 39 (50.7) |
|
| Medium (2+) | 12 (16.0) | Medium (2+) | 17 (24.0) |
|
| High (3+) | 0 (0.0) | High (3+) | 6 (8.0) |
| Paired normal
esophageal epithelium | Negative | 48
(64)a | Negative | 38
(55.1)b |
|
| Positive | 27 (36)a | Positive | 31
(44.9)b |
|
| Low (1+) | 26 (33.4) | Low (1+) | 26 (37.7) |
|
| Medium (2+) | 1 (1.3) | Medium (2+) | 5 (7.2) |
|
| High (3+) | 0 (0.0) | High (3+) | 0 (0.0) |
Preparation of enediyne-energized
fusion proteins
The fusion protein EGF-LDP-IGF, EGF-LDP and LDP-IGF
were constructed, extracted and purified according to our previous
approach (26). The
enediyne-energized analogues of fusion proteins EGF-LDP-IGF-AE,
EGF-LDP-AE and LDP-IGF-AE were generated after the active
chromophore (AE) of LDM was assembled into fusion proteins. Four
enediyne-energized fusion proteins were successfully prepared as
measured by reverse-phase HPLC (23).
Binding affinity of the fusion protein
EGF-LDP-IGF to ESCC cells
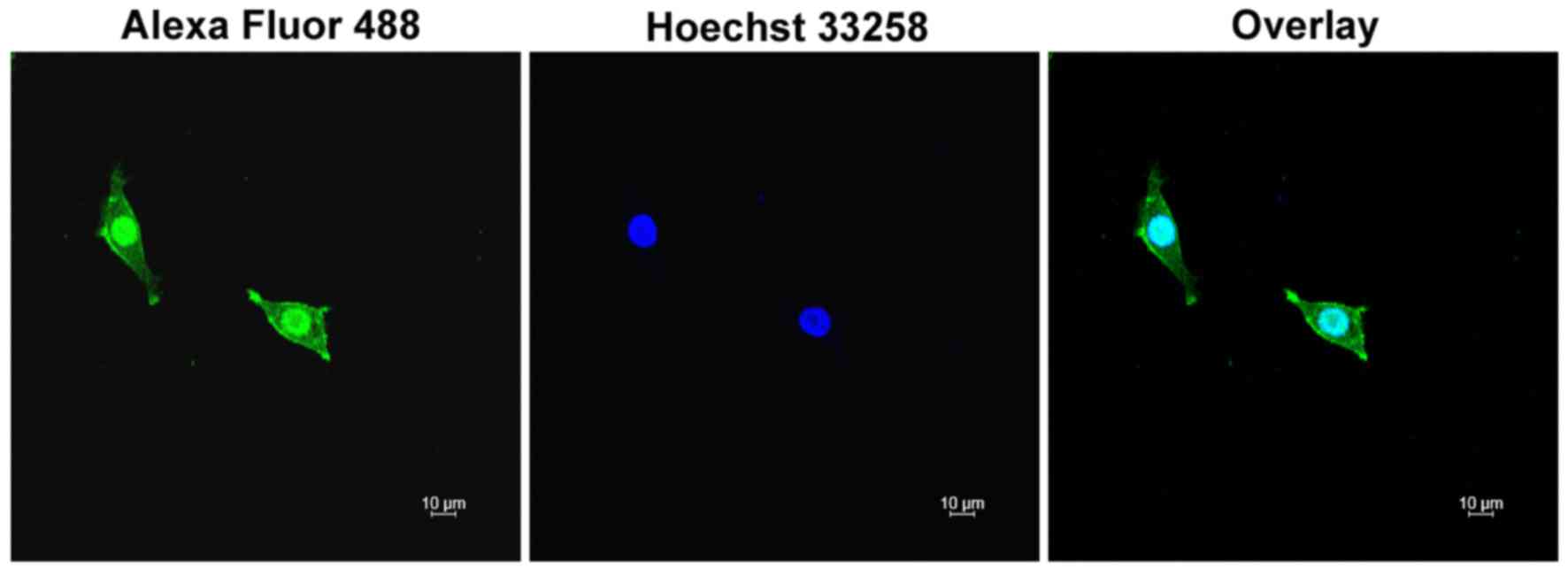
The binding affinity of EGF-LDP-IGF to ESCC cells
was analyzed by immunofluorescence staining. KYSE450 cells with
high EGFR and IGF-1R expression were incubated with EGF-LDP-IGF
protein. Following incubation with anti-His-tag antibody and Alexa
Flour 488-labeled antibody, the cells were observed under a
confocal laser scanning microscope. As shown in Fig. 2, there was green florescence located
on the membrane and cytoplasm of the KYSE450 cells which indicated
that the EGF-LDP-IGF protein was able to bind with the receptors on
the cell membrane, and then internalized into the cytoplasm through
receptor-mediated endocytosis.
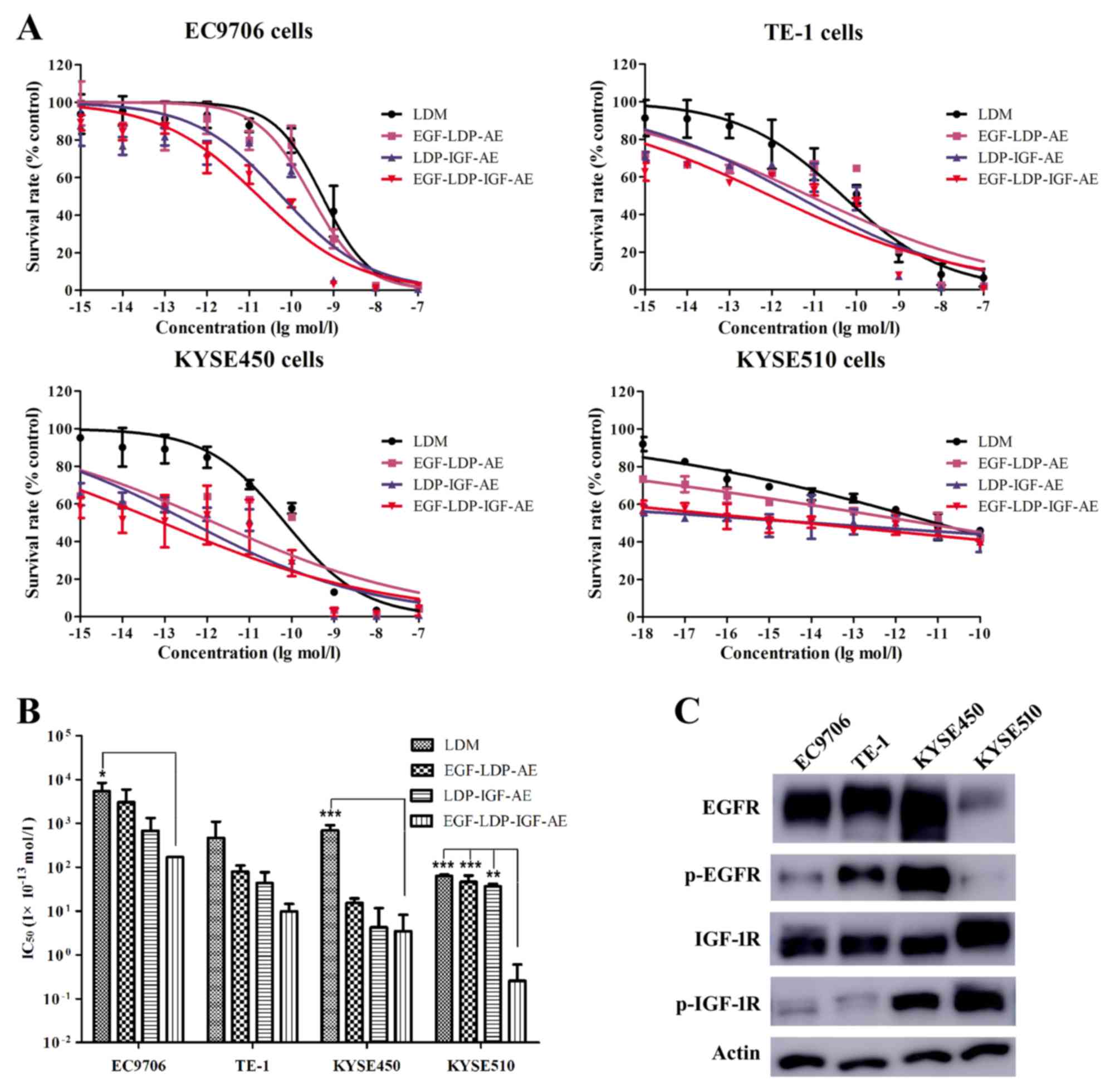
Cytotoxicity of enediyne-energized
fusion proteins in vitro
The cytotoxicity of the bispecific fusion protein
EGF-LDP-IGF-AE on four ESCC cell lines was assessed by MTT assays.
The naked LDM and corresponding mono-specific proteins (EGF-LDP-AE
and LDP-IGF-AE) were also tested for comparison. The bispecific
protein EGF-LDP-IGF-AE exhibited potent cytotoxic effect on the
different ESCC cell lines with IC50 values between
10−10 and 10−15 mol/l (Fig. 3A and B). LDM and mono-specific
enediyne-energized fusion proteins EGF-LDP-AE and LDP-IGF-AE also
showed strong cytotoxic activity against the four ESCC cell lines.
The IC50 values analyzed by one-way ANOVA and Dunnett's
multiple comparison tests revealed that there were significant
differences between EGF-LDP-IGF-AE and LDM in the KYSE450
(P<0.001) and EC9706 cells (P<0.05). In KYSE510 cells, the
differences were significant for EGF-LDP-IGF-AE vs. LDM
(P<0.001), EGF-LDP-IGF-AE vs. EGF-LDP-AE (P<0.001) and
EGF-LDP-IGF-AE vs. LDP-IGF-AE (P<0.01) (Fig. 3B).
To elucidate whether the phosphorylation and total
expression level of EGFR and IGF-1R in the ESCC cells was related
to the cytotoxicity of EGF-LDP-IGF-AE, we detected the levels of
phospho(p)-EGFR, p-IGF-1R and total EGFR, IGF-1R in the four
different ESCC cell lines using western blot assay, followed by
densitometry analysis of the band intensity by ImageJ software, and
the correlation analysis was carried out using Prism 5 software.
The results revealed that there was no significant correlation
between the IC50 values of EGF-LDP-IGF-AE and the
p-EGFR/IGF-1R or total-EGFR/IGF-1R expression levels (Fig. 3C; Table
II).
 | Table II.Phosphorylation and total expression
levels of EGFR and IGF-1R in ESCC cell lines and the
IC50 values for EGF-LDP-IGF-AE against different ESCC
cell lines. |
Table II.
Phosphorylation and total expression
levels of EGFR and IGF-1R in ESCC cell lines and the
IC50 values for EGF-LDP-IGF-AE against different ESCC
cell lines.
|
|
| Western blot
analysis Receptor expression (% actin) |
|---|
|
|
|
|
|---|
| Cell line | EGF-LDP-IGF-AE
IC50 (mol/l) ± SD | EGFR | p-EGFR | IGF-1R | p-IGF-1R |
|---|
| EC9706 | (7.44±0.07) ×
10−12 | 1.13 | 0.36 | 0.83 | 0.26 |
| TE-1 | (5.83±0.02) ×
10−13 | 1.08 | 1.07 | 1.09 | 0.22 |
| KYSE450 | (8.47±0.98) ×
10−14 | 0.91 | 1.18 | 1.22 | 1.17 |
| KYSE510 | (1.19±0.04) ×
10−15 | 0.56 | 0.18 | 1.36 | 1.20 |
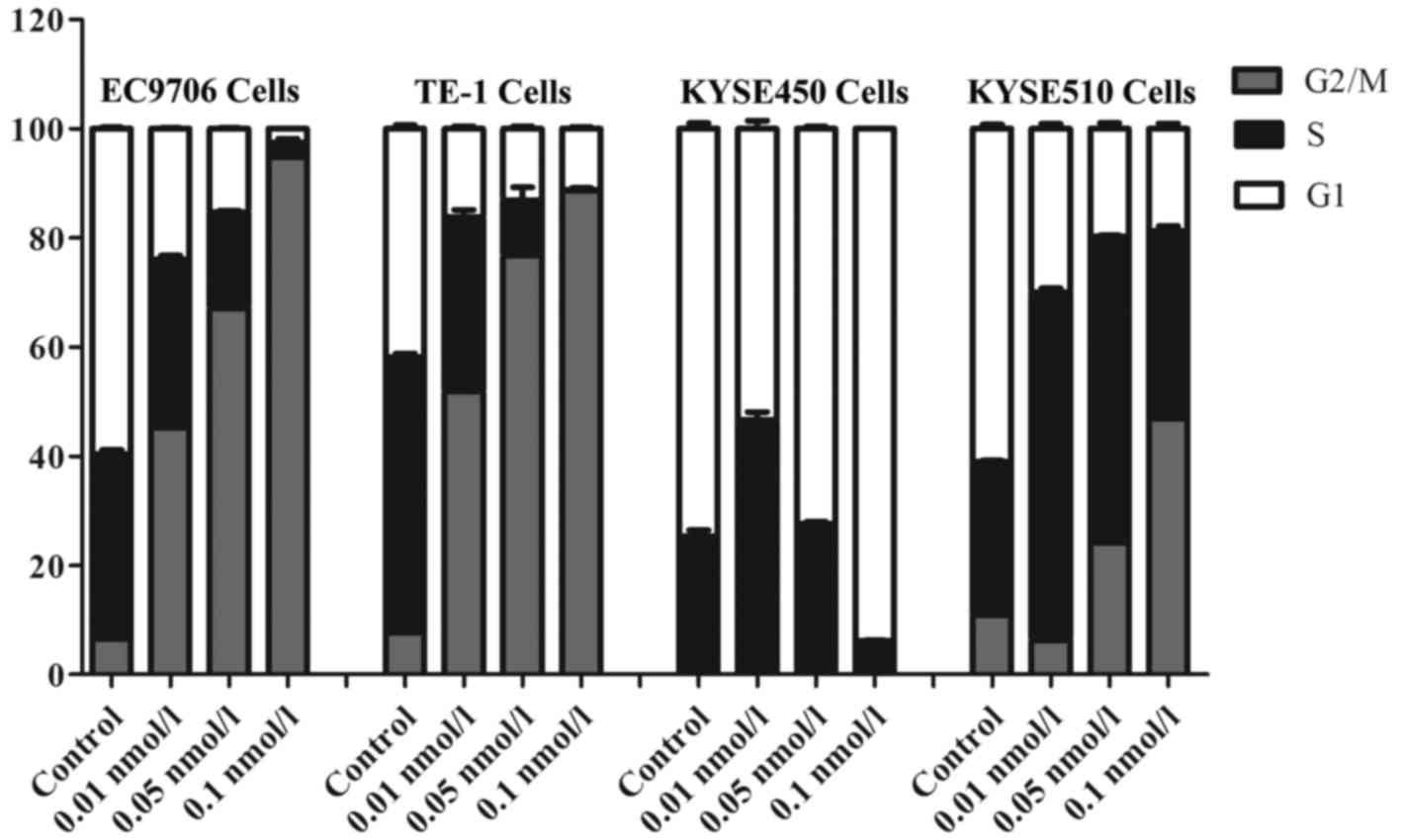
Effects of bispecific fusion protein
EGF-LDP-IGF-AE on cell cycle distribution
After treatment with 0.01, 0.05 and 0.1 nmol/l of
EGF-LDP-IGF-AE for 48 h, the ESCC cell lines were stained using PI
and the fluorescence was assessed by a flow cytometer. The changes
in cell cycle distribution are shown in Fig. 4. The percentages of control cells
(EC9706, TE-1 and KYSE510) distributed in the G2/M phase were
6.48±0.78, 7.70±0.16 and 10.87±0.68%, respectively, whereas the
percentages of the cells exposed to 0.1 nmol/l of EGF-LDP-IGF-AE
which distributed in the G2/M phase were 94.78±0.53, 88.73±0.42 and
46.82±2.28%, respectively. These data illustrated that a
significant G2/M arrest was caused by the EGF-LDP-IGF-AE treatment
in the three cell lines. However, data for the KYSE450 cells
indicated that an obvious G1 arrest resulted from 0.1 nmol/l of
EGF-LDP-IGF-AE treatment (93.85%, 0.1 nmol/l EGF-LDP-IGF-AE
treatment vs. 74.6%, control).
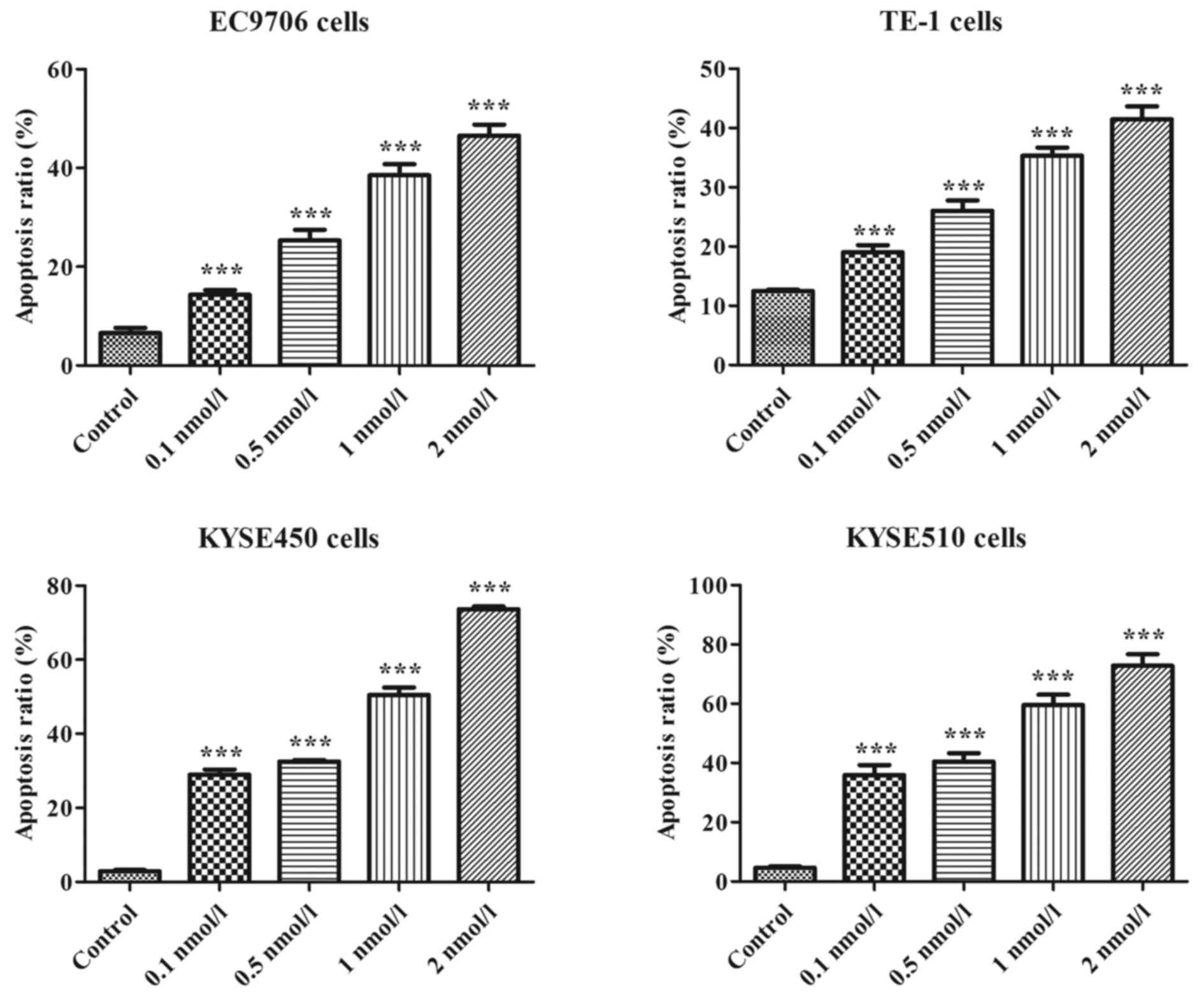
Effects of bispecific fusion protein
EGF-LDP-IGF-AE on cell apoptosis
The results of Annexin V-FITC/PI staining assays
revealed that the percentages of apoptotic cells (four ESCC cell
lines) increased significantly in a concentration-dependent manner
after treatment with EGF-LDP-IGF-AE for 48 h. As shown in Fig. 5, the percentages of apoptotic EC9706
cells after treatment with 0.1, 0.5, 1 and 2 nmol/l of
EGF-LDP-IGF-AE were 14.32±0.94, 25.35±2.12, 38.53±2.22 and
46.54±2.23%, respectively, which indicated a marked increase
compared with that of the control cells (6.56±1.08%; P<0.01).
Similar results were also obtained in the other ESCC cell lines
(TE-1, KYSE450 and KYSE510) after treatment with
EGF-LDP-IGF-AE.
Effects of bispecific fusion protein
EGF-LDP-IGF-AE on the activation of EGFR and IGF-1R signaling
pathways
EGFR and IGF-1R phosphorylation and downstream
signal transduction stimulated by EGF and IGF-1 was regulated by
treatment with the EGF-LDP-IGF-AE protein. In addition, the effect
of EGF-LDP-IGF-AE on the EGFR/IGF-1R signaling pathways was closely
related to the treatment time. As shown in Fig. 6A and C, in both the KYSE450 and
EC9706 cells, the phosphorylation of EGFR and IGF-1R and the two
key downstream signaling molecules, AKT and p44/42 MAPK (ERK), as
well as their total expression levels were not affected by the
EGF-LDP-IGF-AE treatment for 24 h. However, a significant decrease
in p-EGFR, p-AKT and p-ERK was observed with the extension of
exposure time (48, 72 and 96 h). Interestingly, treatment of
EGF-LDP-IGF-AE for 48, 72 and 96 h resulted in a marked increase in
p-IGF-1R. The total EGFR and IGF-1R expression levels were
decreased after exposure to EGF-LDP-IGF-AE for 48 and 72 h in the
KYSE450 cells, but their expression remained unchanged in the
EC9706 cells (except for the reduction of total IGF-1R when treated
with EGF-LDP-IGF-AE for 96 h). EGF-LDP-IGF-AE treatment for 48, 72
and 96 h also resulted in a marked decrease in total AKT and ERK in
both KYSE450 and EC9706 cells (Fig. 6A
and C).
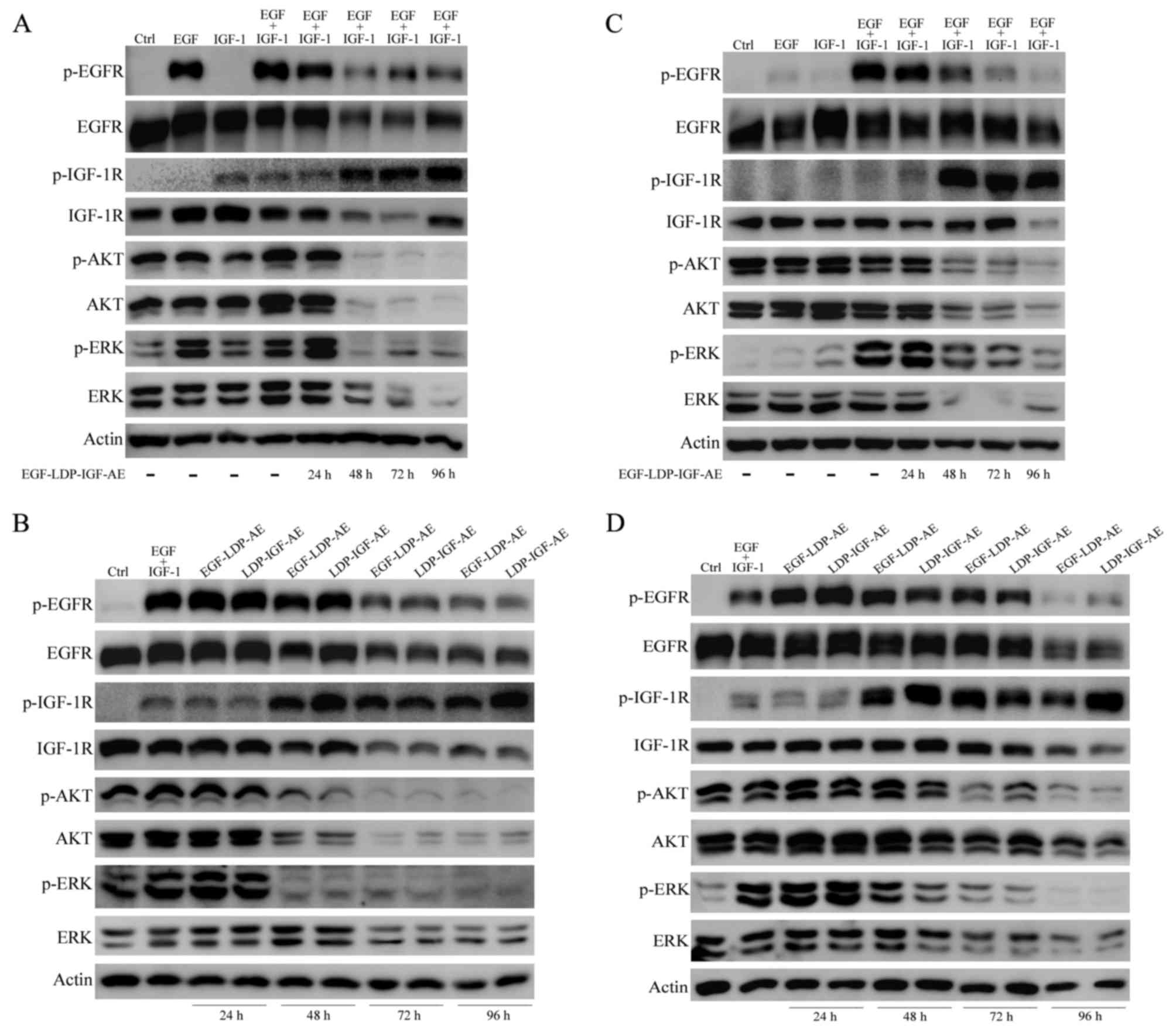 | Figure 6.Effects of EGF-LDP-IGF-AE on the EGFR
and IGF-1R signaling pathways. (A and C) KYSE450 or EC9706 cells
were treated with 0.1 nmol/l EGF-LDP-IGF-AE for 24, 48, 72 and 96
h. (B and C) KYSE450 or EC9706 cells were treated with EGF-LDP-AE
or LDP-IGF-AE for 24, 48, 72 and 96 h. Then, the cells were
stimulated with human EGF, IGF-1 or both for 30 min. The expression
level of key molecules in the EGFR/IGF-1R signaling pathways (e.g.
phosphorylated(p)-EGFR, p-IGF-1R, p-ERK and p-AKT) and total EGFR,
IGF-1R, ERK and AKT were determined using western blot analysis.
β-actin was regarded as a loading control. |
The effects of mono-specific fusion proteins
EGF-LDP-AE and LDP-IGF-AE on EGFR/IGF-1R signaling were also
assessed in the KYSE450 and EC9706 cells. Similar to the bispecific
fusion protein, EGF-LDP-AE or LDP-IGF-AE treatment for 24 h did not
exhibit effects on the EGFR/IGF-1R signaling pathways. In KYSE450
cells, activation of EGFR was inhibited after exposure to
EGF-LDP-AE or LDP-IGF-AE for 72 and 96 h whereas p-IGF-1R was
upregulated after treatment for 48, 72 and 96 h. The
phosphorylation of two downstream molecules AKT and ERK was
significantly reduced after treatment for 48, 72 and 96 h. Total
expression level of EGFR, IGF-1R, AKT and ERK was decreased when
the exposure time was extended to 72 and 96 h (Fig. 6B). In EC9706 cells, p-EGFR and total
EGFR was downregulated only after treatment for 96 h. Levels of
p-IGF-1R, p-AKT and p-ERK were significantly altered after
treatment for 48, 72 and 96 h, in which p-IGF-1R was increased and
p-AKT and -ERK were decreased (Fig.
6D).
Efficacy of enediyne-energized fusion
proteins in vivo
In vivo antitumor efficacy of both bispecific
and mono-specific enediyne-energized fusion proteins was
investigated in a human esophageal cancer KYSE450 xenograft nude
mouse model. As shown in Fig. 7A,
LDM, EGF-LDP-IGF-AE, EGF-LDP-AE and LDP-IGF-AE significantly
suppressed the growth of KYSE450 xenografts. The bispecific fusion
protein EGF-LDP-IGF-AE at dosages of 0.2 and 0.3 mg/kg inhibited
the growth of xenografts by 64.1 and 75.1%, respectively (P<0.01
compared with the PBS-treated group; P<0.05 between the two
EGF-LDP-IGF-AE-treatment groups at different dosages). Furthermore,
the EGF-LDP-IGF-AE-treated group at the dosage 0.3 mg/kg showed
statistically significant differences (P<0.01) compared with the
LDM-treated group at the maximum tolerated dosage (0.05 mg/kg,
inhibition rate, 57.8%). Mono-specific fusion proteins EGF-LDP-AE
and LDP-IGF-AE at a dosage of 0.3 mg/kg demonstrated similar tumor
growth inhibition to bispecific EGF-LDP-IGF-AE protein at a dosage
of 0.2 mg/kg (inhibition rates of 61.2 and 62.6% for EGF-LDP-AE and
LDP-IGF-AE respectively). However, when given at the same dosage
(0.3 mg/kg), the EGF-LDP-IGF-AE-treated group showed more
significant tumor growth inhibition compared with the mono-specific
counterparts (P<0.05). No animals died in all groups, and body
weight curves showed that the animals tolerated well the
administered dosage of the fusion proteins (Fig. 7B).
Discussion
Results from the human ESCC tissue microarray
detection in the present study and other previous studies, have
revealed that EGFR and IGF-1R are highly co-expressed in ESCC. In
addition, the abnormal expression of these receptors is associated
with reduced survival, increased risk of relapse and poor prognosis
(10,12). Therefore, various EGFR-targeted
drugs including monoclonal antibodies (mAbs, cetuximab and
panitumumab) and tyrosine kinase inhibitors (TKIs; gefitinib and
erlotinib) have been examined in the clinical for esophageal cancer
patients. However, the efficacy was far from satisfactory (17–21).
Since the crosstalk between EGFR and IGF-1R pathways exist,
strategies of the dual-inhibition of both pathways have been
pursued for enhanced antitumor efficacy. Strategies include: i) the
combination of mAbs or TKIs against different growth factors or
receptor tyrosine kinases (RTKs); and ii) bispecific drugs
targeting two molecules. Improved antitumor efficacy has been
achieved by the combination of mono-specific therapeutic compounds
involved in the inhibition of tumor growth, metastasis and
anti-angiogenesis (27–30). However, the combination therapy
requires the development of individual or combinatorial drugs,
which requires a significant investment for production, preclinical
and clinical studies. Moreover, some combinations may increase the
toxicity and shorten progression-free survival compared to
administration of single drugs, such as bevacizumab with
panitumumab or cetuximab in advanced colorectal cancer (31,32).
Dual targeting strategies with bispecific agents have been
classified into two types: i) those that directly act on target
molecules, such as bispecific antibodies and ii) those that depend
on targets for delivering an active moiety to killing tumor cells,
such as bispecific immunotoxins or fusion proteins. A number of
bispecific antibodies targeting both EGFR and IGF-1R (EI-04 and
XGFR) have demonstrated superior antitumor activity in preclinical
models (33,34), and bispecific immunotoxins/fusion
proteins developed by Vallera et al also demonstrated either
enhanced antitumor activity or broader spectrum of reactivity than
the mono-specific molecules (35–37).
EGF-LDP-IGF-AE is a bispecific enediyne-energized fusion protein
that was constructed by fusing the natural ligands of EGFR and
IGF-1R (EGF and IGF-1) to an enediyne antibiotic lidamycin (LDM;
C1027) with potent antitumor activity. There are two advantages of
EGF-LDP-IGF-AE over the mono-specific fusion proteins. Firstly, the
two ligands were designed for receptor binding and subsequent
intracellular delivery of the ‘warhead’, and the LDM acts as toxic
moiety for killing tumor cells. The dual-targeting characteristics
and the inclusion of the potent cytotoxic payload provide the
EGF-LDP-IGF-AE with improved tumor selectivity and enhanced
cytotoxicity. Secondly, due to the presence of small targeting
ligands (EGF, 6.2 kDa and IGF, 7.6 kDa) and small cytotoxin (LDM,
15 kDa), the EGF-LDP-IGF-AE protein is composed of 253 amino acids
with a molecular weight of 27.1 kDa, and the smaller size provides
it with enhanced solid tumor penetration, increased tumor uptake
and lower immunogenicity. As a result, the bispecific fusion
protein EGF-LDP-IGF-AE exhibited potent antitumor efficacy against
esophageal cancer.
Binding with EGFR and IGF-1R and internalization
were the prerequisites for EGF-LDP-IGF-AE to exhibit its tumor
cell-selective cytotoxicity. The results from the immunofluorescent
staining assay showed that green fluorescence was located in the
membrane and cytoplasm of the ESCC cells, which indicated that the
EGF-LDP-IGF protein could bind with the receptors on the cell
membrane and then internalize into the cytoplasm through
receptor-mediated endocytosis. The bispecific fusion protein
EGF-LDP-IGF-AE showed extremely potent cytotoxicity to ESCC cells
in vitro. However, the correlation analysis revealed that
there was no significant correlation between the IC50
values of EGF-LDP-IGF-AE and the p-EGFR, p-IGF-1R and total EGFR
and IGF-1R expression levels. Similar results were also reported by
other studies concerning targeted drugs, such as erlotinib and
lapatinib (38,39). We speculated that the mechanisms
underlying the internalization of EGF-LDP-IGF-AE into the tumor
cells was mainly dependent on receptor-mediated endocytosis. Yet,
the AE molecules may dissociate from the EGF-LDP-IGF protein
outside the cells, and then the small naked AE molecules enter the
cells in receptor-independent mechanisms. This assumption will be
further investigated, and the identification of the key molecules
to predict the responsiveness to EGF-LDP-IGF-AE may be another
focus of further research. This may allow identification of
patients who may benefit from the EGF-LDP-IGF-AE-targeted
therapy.
In vitro, two-way ANOVA analysis revealed
that bispecific EGF-LDP-IGF-AE had stronger cytotoxicity than
mono-specific fusion protein EGF-LDP-AE in four ESCC cell lines,
but the differences between EGF-LDP-IGF-AE and another
mono-specific fusion protein LDP-IGF-AE in KYSE450 and KYSE510
cells were not significant. Actually, LDP-IGF-AE protein was more
cytotoxic than EGF-LDP-AE in all ESCC cell lines (P<0.05). The
cytotoxicity of fusion proteins depended on the presence of the
active enediyne chromophore (AE); therefore, the reconstitution
efficiency of AE to fusion protein EGF-LDP or LDP-IGF was closely
related to their cytotoxicity. The protein structure of EGF-LDP may
affect its reconstitution efficiency, resulting in the lower
cytotoxicity. Results from the in vivo experiments also
revealed a more significant tumor growth inhibition following the
EGF-LDP-IGF-AE treatment. EGF-LDP-IGF-AE at a dosage of 0.3 mg/kg
yielded tumor growth inhibition of 75.1%, which showed a
statistically significant difference compared with the LDM-treated
group (P<0.01) and mono-specific fusion protein-treated groups
(P<0.05). Furthermore, no mice died in the
EGF-LDP-IGF-AE-treated group and weight loss in the mice at the
termination of the experiment did not exceed 10% of the
pretreatment weight, which indicated that nude mice tolerated well
the EGF-LDP-IGF-AE at a dosage of 0.3 mg/kg. This dosage was six
times the maximum tolerated dose of LDM. These results revealed
that bispecific EGF-LDP-IGF-AE protein was less toxic to normal
tissues than naked LDM in vivo, and this may be due to the
capacity of binding the two receptors of the bispecific protein.
Therefore, it preferably bound to the tumor cells highly expressing
both receptors instead of binding to normal cells with low
expression of one or both receptors. In addition, bispecific fusion
proteins may extend the patient coverage which is economically
advantageous, as a portion of patients may have EGFR overexpression
whereas IGF-1R overexpression may be present in another portion of
patients.
To illuminate the mechanisms underlying the
cytotoxic effects of EGF-LDP-IGF-AE on ESCC cells, PI and Annexin
V-FITC/PI staining assays were used to determine cell cycle arrest
and cell apoptosis, and the effects on EGFR/IGF-1R signaling was
analyzed by western blotting. The data from cell cycle analysis
indicated that EGF-LDP-IGF-AE caused a significant G2/M arrest in
the EC9706, TE-1 and KYSE510 cells and a G1 arrest in the KYSE450
cells following 0.1 nmol/l EGF-LDP-IGF-AE treatment. Additionally,
EGF-LDP-IGF-AE also induced significant apoptosis in the ESCC cells
in a concentration-dependent manner. After treatment with
EGF-LDP-IGF-AE for 48, 72 and 96 h, activation of EGFR and the two
key downstream signaling molecules AKT and ERK was inhibited and
the signal transduction was blocked. However, the level of p-IGF-1R
was significantly increased after exposure to EGF-LDP-IGF-AE for
48, 72 and 96 h. This phenomenon could be explained by the fact
that EGF-LDP-IGF-AE treatment activated IGF-1R by phosphorylation.
Then the activated IGF-1R internalized into the cytoplasm and was
transported to the lysosome for degradation. As a result, the cell
surface IGF-1R was greatly reduced which resulted in a significant
decrease in the interactions between IGF ligands and IGF-1R.
Therefore, the signaling pathways mediated by IGF-1R were
inhibited. The total IGF-1R expression was reduced after treatment
with EGF-LDP-IGF-AE which confirmed our speculation.
In summary, bispecific fusion protein EGF-LDP-IGF-AE
demonstrated potent cytotoxicity to ESCC cells and caused
significant cell cycle arrest and apoptosis in vitro. It
also showed high efficacy in suppressing the growth of human
esophageal cancer xenografts in vivo. These findings suggest
that EGF-LDP-IGF-AE may be a potential candidate for esophageal
cancer therapy, which may be developed further for clinical
application.
Acknowledgements
The present study was supported by a grant from the
National Science Foundation of China (no. 81202447), and the
Support Project for Talents of Science and Technology Innovation in
Universities of Henan (no. 15HASTIT040).
References
|
1
|
Homs MY, Voest EE and Siersema PD:
Emerging drugs for esophageal cancer. Expert Opin Emerg Drugs.
14:329–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang LD, Zhou FY, Li XM, Sun LD, Song X,
Jin Y, Li JM, Kong GQ, Qi H, Cui J, et al: Genome-wide association
study of esophageal squamous cell carcinoma in Chinese subjects
identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet.
42:759–763. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rowinsky EK: The erbB family: Targets for
therapeutic development against cancer and therapeutic strategies
using monoclonal antibodies and tyrosine kinase inhibitors. Annu
Rev Med. 55:433–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirsch FR, Varella-Garcia M, Bunn PA Jr,
Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C and Franklin WA:
Epidermal growth factor receptor in non-small-cell lung carcinomas:
Correlation between gene copy number and protein expression and
impact on prognosis. J Clin Oncol. 21:3798–3807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliveira-Silva RJ, de Carvalho A Carolina,
de Souza Viana L, Carvalho AL and Reis RM: Anti-EGFR therapy:
Strategies in head and neck squamous cell carcinoma. Recent Patents
Anticancer Drug Discov. 11:170–183. 2016. View Article : Google Scholar
|
|
7
|
Ooi A, Takehana T, Li X, Suzuki S,
Kunitomo K, Iino H, Fujii H, Takeda Y and Dobashi Y: Protein
overexpression and gene amplification of HER-2 and EGFR in
colorectal cancers: An immunohistochemical and fluorescent in situ
hybridization study. Mod Pathol. 17:895–904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DiGiovanna MP, Stern DF, Edgerton SM,
Whalen SG, Moore D II and Thor AD: Relationship of epidermal growth
factor receptor expression to ErbB-2 signaling activity and
prognosis in breast cancer patients. J Clin Oncol. 23:1152–1160.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheng Q and Liu J: The therapeutic
potential of targeting the EGFR family in epithelial ovarian
cancer. Br J Cancer. 104:1241–1245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moorcraft SY and Chau I: Investigational
therapies targeting the ErbB family in oesophagogastric cancer.
Expert Opin Investig Drugs. 23:1349–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin M, Guan X, Liao Z and Wei Q:
Insulin-like growth factor-1 receptor-targeted therapy for
non-small cell lung cancer: A mini review. Am J Transl Res.
1:101–114. 2009.PubMed/NCBI
|
|
12
|
Adachi Y, Ohashi H, Imsumran A, Yamamoto
H, Matsunaga Y, Taniguchi H, Nosho K, Suzuki H, Sasaki Y, Arimura
Y, et al: The effect of IGF-I receptor blockade for human
esophageal squamous cell carcinoma and adenocarcinoma. Tumour Biol.
35:973–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ludovini V, Bellezza G, Pistola L,
Bianconi F, Di Carlo L, Sidoni A, Semeraro A, Del Sordo R,
Tofanetti FR, Mameli MG, et al: High coexpression of both
insulin-like growth factor receptor-1 (IGFR-1) and epidermal growth
factor receptor (EGFR) is associated with shorter disease-free
survival in resected non-small-cell lung cancer patients. Ann
Oncol. 20:842–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahari D, Yamada Y, Okita NT, Honda T,
Hirashima Y, Matsubara J, Takashima A, Kato K, Hamaguchi T, Shirao
K, et al: Relationships of insulin-like growth factor-1 receptor
and epidermal growth factor receptor expression to clinical
outcomes in patients with colorectal cancer. Oncology. 76:42–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu L, Qi Y, Xu Y, Lian J, Wang X, Ning G,
Wang W and Zhu Y: Co-inhibition of EGFR and IGF1R synergistically
impacts therapeutically on adrenocortical carcinoma. Oncotarget.
7:36235–36246. 2016.PubMed/NCBI
|
|
16
|
Valsecchi ME, McDonald M, Brody JR, Hyslop
T, Freydin B, Yeo CJ, Solomides C, Peiper SC and Witkiewicz AK:
Epidermal growth factor receptor and insulinlike growth factor 1
receptor expression predict poor survival in pancreatic ductal
adenocarcinoma. Cancer. 118:3484–3493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dutton SJ, Ferry DR, Blazeby JM, Abbas H,
Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A,
Falk S, et al: Gefitinib for oesophageal cancer progressing after
chemotherapy (COG): A phase 3, multicentre, double-blind,
placebo-controlled randomised trial. Lancet Oncol. 15:894–904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan JA, Blaszkowsky LS, Enzinger PC, Ryan
DP, Abrams TA, Zhu AX, Temel JS, Schrag D, Bhargava P, Meyerhardt
JA, et al: A multicenter phase II trial of single-agent cetuximab
in advanced esophageal and gastric adenocarcinoma. Ann Oncol.
22:1367–1373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorenzen S, Schuster T, Porschen R,
Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P,
Arnold D, Greten T, et al: Cetuximab plus cisplatin-5-fluorouracil
versus cisplatin-5-fluorouracil alone in first-line metastatic
squamous cell carcinoma of the esophagus: A randomized phase II
study of the Arbeitsgemeinschaft Internistische Onkologie. Ann
Oncol. 20:1667–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bendell JC, Meluch A, Peyton J, Rubin M,
Waterhouse D, Webb C, Burris HA III and Hainsworth JD: A phase II
trial of preoperative concurrent chemotherapy/radiation therapy
plus bevacizumab/erlotinib in the treatment of localized esophageal
cancer. Clin Adv Hematol Oncol. 10:430–437. 2012.PubMed/NCBI
|
|
21
|
Tebbutt NC, Price TJ, Ferraro DA, Wong N,
Veillard AS, Hall M, Sjoquist KM, Pavlakis N, Strickland A, Varma
SC, et al: Panitumumab added to docetaxel, cisplatin and
fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial.
Br J Cancer. 114:505–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krop IE, Lin NU, Blackwell K, Guardino E,
Huober J, Lu M, Miles D, Samant M, Welslau M and Diéras V:
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in
patients with HER2-positive metastatic breast cancer and central
nervous system metastases: A retrospective, exploratory analysis in
EMILIA. Ann Oncol. 26:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo XF, Zhu XF, Cao HY, Zhong GS, Li L,
Deng BG, Chen P, Wang PZ, Miao QF and Zhen YS: A bispecific
enediyne-energized fusion protein targeting both epidermal growth
factor receptor and insulin-like growth factor 1 receptor showing
enhanced antitumor efficacy against non-small cell lung cancer.
Oncotarget. 8:27286–27299. 2017.PubMed/NCBI
|
|
24
|
Shao RG and Zhen YS: Enediyne anticancer
antibiotic lidamycin: Chemistry, biology and pharmacology.
Anticancer Agents Med Chem. 8:123–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budwit-Novotny DA, McCarty KS, Cox EB,
Soper JT, Mutch DG, Creasman WT, Flowers JL and McCarty KS Jr:
Immunohistochemical analyses of estrogen receptor in endometrial
adenocarcinoma using a monoclonal antibody. Cancer Res.
46:5419–5425. 1986.PubMed/NCBI
|
|
26
|
Guo XF, Zhu XF, Shang Y, Zhang SH and Zhen
YS: A bispecific enediyne-energized fusion protein containing
ligand-based and antibody-based oligopeptides against epidermal
growth factor receptor and human epidermal growth factor receptor 2
shows potent antitumor activity. Clin Cancer Res. 16:2085–2094.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okita R, Shimizu K, Nojima Y, Yukawa T,
Maeda A, Saisho S and Nakata M: Lapatinib enhances
trastuzumab-mediated antibody-dependent cellular cytotoxicity via
upregulation of HER2 in malignant mesothelioma cells. Oncol Rep.
34:2864–2870. 2015.PubMed/NCBI
|
|
28
|
Wang CJ, Tong PJ and Zhu MY: The
combinational therapy of trastuzumab and cetuximab inhibits tumor
growth in a patient-derived tumor xenograft model of gastric
cancer. Clin Transl Oncol. 18:507–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nahta R, Hung MCC and Esteva FJ: The
HER-2-targeting antibodies trastuzumab and pertuzumab
synergistically inhibit the survival of breast cancer cells. Cancer
Res. 64:2343–2346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hecht JR, Mitchell E, Chidiac T, Scroggin
C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P,
et al: A randomized phase IIIB trial of chemotherapy, bevacizumab,
and panitumumab compared with chemotherapy and bevacizumab alone
for metastatic colorectal cancer. J Clin Oncol. 27:672–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stopeck AT, Unger JM, Rimsza LM, LeBlanc
M, Farnsworth B, Iannone M, Glenn MJ, Fisher RI and Miller TP: A
phase 2 trial of standard-dose cyclophosphamide, doxorubicin,
vincristine, prednisone (CHOP) and rituximab plus bevacizumab for
patients with newly diagnosed diffuse large B-cell non-Hodgkin
lymphoma: SWOG 0515. Blood. 120:1210–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong J, Sereno A, Aivazian D, Langley E,
Miller BR, Snyder WB, Chan E, Cantele M, Morena R, Joseph IB, et
al: A stable IgG-like bispecific antibody targeting the epidermal
growth factor receptor and the type I insulin-like growth factor
receptor demonstrates superior anti-tumor activity. MAbs.
3:273–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schanzer JM, Wartha K, Moessner E, Hosse
RJ, Moser S, Croasdale R, Trochanowska H, Shao C, Wang P, Shi L, et
al: XGFR*, a novel affinity-matured bispecific antibody targeting
IGF-1R and EGFR with combined signaling inhibition and enhanced
immune activation for the treatment of pancreatic cancer. MAbs.
8:811–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsai AK, Oh S, Chen H, Shu Y, Ohlfest JR
and Vallera DA: A novel bispecific ligand-directed toxin designed
to simultaneously target EGFR on human glioblastoma cells and uPAR
on tumor neovasculature. J Neurooncol. 103:255–266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang J, Li YM, Massague J, Sicheneder A,
Vallera DA and Hall WA: Intracerebral infusion of the bispecific
targeted toxin DTATEGF in a mouse xenograft model of a human
metastatic non-small cell lung cancer. J Neurooncol. 109:229–238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oh S, Stish BJ, Sachdev D, Chen H, Dudek
AZ and Vallera DA: A novel reduced immunogenicity bispecific
targeted toxin simultaneously recognizing human epidermal growth
factor and interleukin-4 receptors in a mouse model of metastatic
breast carcinoma. Clin Cancer Res. 15:6137–6147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
López-Ayllón BD, de Castro-Carpeño J,
Rodriguez C, Pernía O, Ibañez de Cáceres I, Belda-Iniesta C, Perona
R and Sastre L: Biomarkers of erlotinib response in non-small cell
lung cancer tumors that do not harbor the more common epidermal
growth factor receptor mutations. Int J Clin Exp Pathol.
8:2888–2898. 2015.PubMed/NCBI
|
|
39
|
Rusnak DW, Alligood KJ, Mullin RJ, Spehar
GM, Arenas-Elliott C, Martin AM, Degenhardt Y, Rudolph SK, Haws TF
Jr, Hudson-Curtis BL, et al: Assessment of epidermal growth factor
receptor (EGFR, ErbB1) and HER2 (ErbB2) protein expression levels
and response to lapatinib (Tykerb, GW572016) in an expanded panel
of human normal and tumour cell lines. Cell Prolif. 40:580–594.
2007. View Article : Google Scholar : PubMed/NCBI
|