Introduction
Hepatocellular carcinoma (HCC) is a well known
primary liver cancer with very poor prognosis (1). According to a previous report, HCC is
the sixth most common cancer (2).
Furthermore, it is the third leading reason for cancer mortality
across the world (3). Each year,
>600,000 deaths are reported due to its difficult diagnosis and
ineffective treatments (4,5). The incidence rate of liver cancer is
increasing among both male and female, and the number of people
infected with HCC has doubled (6,7). Until
now, chemoprevention has been reported as the best treatment to
prevent liver cancer progression and development (8).
Presently, accumulating evidence suggests that a
variety of natural compounds isolated from plants are safe and
effective in many diseases, including cancer, such as liver cancer,
breast cancer and lung cancer (9).
Thus, we attempted to illustrate the role of fisetin
(3,3′,4′,7-tetrahydroxyflavone) (Fig.
1A) in HCC prevention or treatment. Fisetin is found in various
vegetables and fruits, including onions, cucumber, apples, grapes,
and strawberries (10). In
addition, fisetin is suggested to possess anti-oxidant,
anti-microbio, anti-inflammation and even anticancer properties in
a variety of animal models as well as cell cultures (11). Additionally, apoptosis in cancer
cells, such as cervical and breast, could be induced by fisetin,
contributing to inhibition of cancer progression (12). Therefore, we hypothesized that
fisetin could be of potential value in human liver cancer
inhibition.
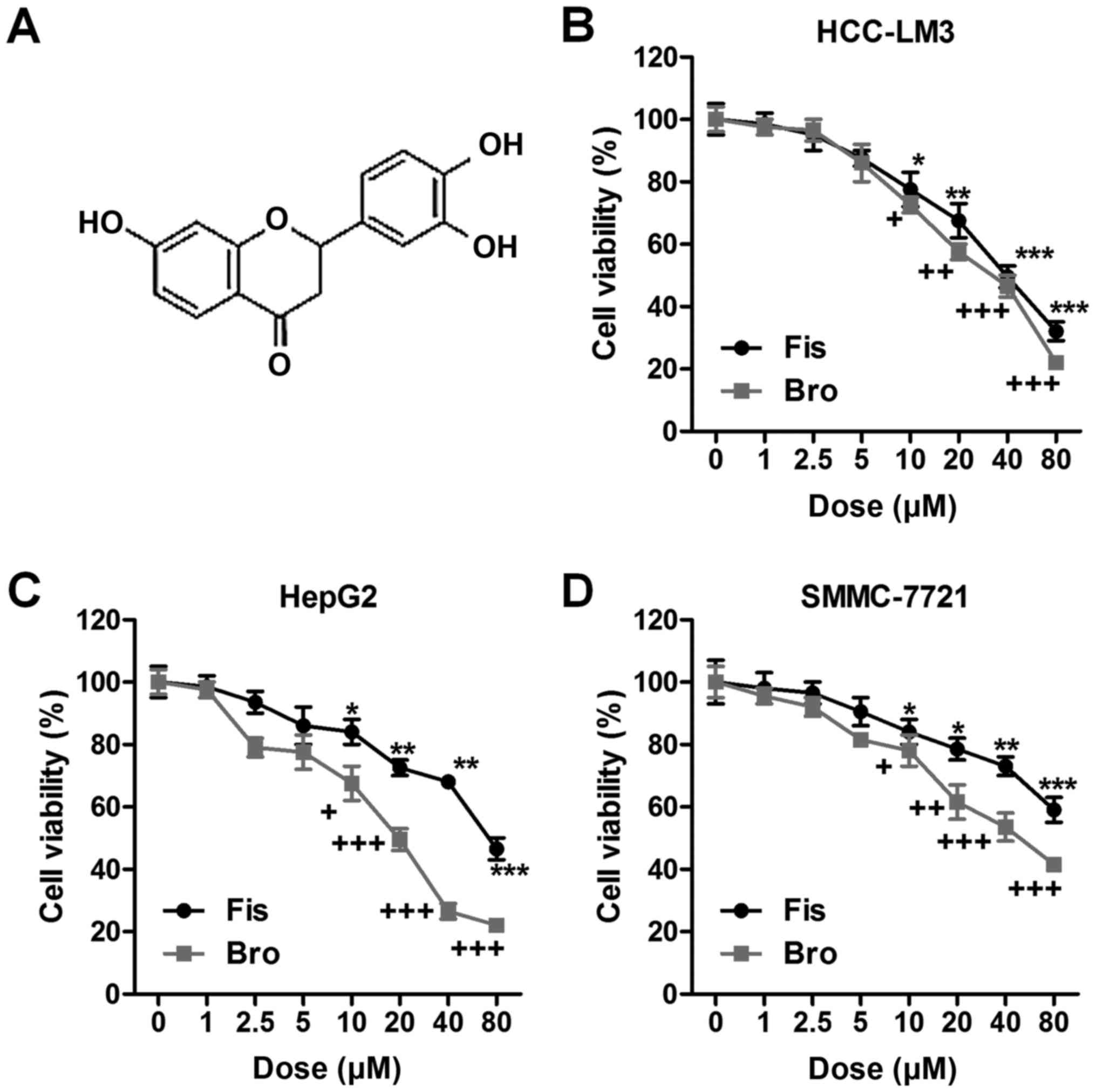 | Figure 1.Fisetin-suppresses liver cancer cells
proliferation related to death receptor 2 (DR2). (A) The chemical
structure of fisetin is shown. Liver cancer cells of (B) HCC-LM3,
(C) HepG2, and (D) SMMC-7721 were treated with different
concentrations of fisetin (0, 1, 2.5, 5, 10, 20, 40 and 80 µM) and
bromocriptine (0, 1, 2.5, 5, 10, 20, 40 and 80 µM) for 24 h, then
the cell proliferation and toxicity was determined via MTT
analysis. Data are presented as mean ± SEM. *P<0.05, **P<0.01
and ***P<0.001 versus Con group with Fis treatment;
+P<0.05, ++P<0.01 and
+++P<0.001 versus Con group with Bro treatment. Fis,
fisetin; Bro, bromocriptine. |
Dopamine (DA) is essential in sodium balance and
blood pressure modulation, which directly functions on lung, renal,
brain, and vascular bed ion transport (13). Five DA receptors have been reported,
indlucing DR1, DR2, DR3, DR4 an DR5 (14,15).
DR1 and DR5 are regarded as members of D1-like family, and DR2, DR3
and DR4 belong to D2-like family (16). DA has been reported to have
anticancer activities, such as lung cancer, gastric cancer and
cervical cancer, mainly via DR2 regulation (17). According to the possible role of DR2
in tumor suppression, it was also included in our study to explore
whether it was related to liver cancer progression. Thus, DR2
agonist in our study was used to explore how DRs perform in liver
cancer in vitro. Fisetin was used combined with DR2 agonist
to investigate underlying molecular mechanism related to HCC
progression.
Materials and methods
Cells and culture
Human liver caner cell lines, HCC-LM3 and SMMC-7721,
were purchased from American Type Culture Collection (ATCC, USA).
Human liver cancer cells of HepG2 were purchased from KeyGen
Biotech (Nanjing, China). SMMC-7721 and HepG2 cells were cultured
in DMEM (Gibco, USA) supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin. Human HCC-LM3 cells were
routinely cultured in RPMI-1640 medium (Gibco), containing 10%
fetal bovine serum (FBS) (Gibco), 1% penicillin/streptomycin. MRC-5
was cultured in DMEM (Gibco) supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin. All cells were cultured in
a humidified atmosphere with 5% CO2 and 95% humidity at
37°C in an incubator. All cells were cultured in a humidified
atmosphere with 5% CO2 and 95% humidity at 37°C in an
incubator. Fisetin (>98% purity), used for the treatment of lung
cancer, was purchased from Dayang Chem (Hangzhou, China), dissolved
in DMSO and stored at −20°C, and then diluted in medium for
experiments. The final DMSO concentration in our study was <0.1%
(v/v) in every treatment, and bromocriptine used was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyl-2H-tetrazolium bromide (MTT) assays
Cells of HepG2 (5×103), HCC-LM3 and
SMMC-7721 were seeded into a 96-well plate (Corning, USA) per well.
Bromocriptine (from 0 to 40 µM), fisetin ranging from 0–40 µM, was
added to the medium. The cells were then incubated at 37°C for 24
h, and the cell viability was detected by the colorimetric MTT
assay at 570 nm.
Cell migration and invasion analysis
for liver cancer cells
The liver cancer cells were seeded into the upper
chamber of a Transwell insert pre-coated with 5 µg/ml fibronectin
for migration or a BD™ Matrigel invasion chamber for invasion.
Medium with 10% serum was put in the lower chamber as a
chemo-attractant, and cells were then incubated for 4 h.
Non-migratory cells were removed from the upper chamber with a
cotton bud. The cells on the lower insert surface were stained with
Diff-Quick. Cells were counted as the number of cells observed in
three randomly different microscope fields of three independent
inserts.
Colony-forming analysis of liver
cancer cells
To explore the liver cancer cell proliferation, 60%
liver cancer cells of HCC-LM3 and HepG2 were treated with
bromocriptine and fisetin or the two drugs in combination for 24 h
in growth medium. The cells were then harvested in a separate tube
after incubation. Then, 500 cells/well were cultured in 6-well
plate (Corning) separately with complete growth medium and grown
for 2 weeks. The medium was replaced after 7 days. For incubation
of 14 days, the cells were washed with phosphate-buffered saline
(PBS) (Hyclone, USA). Then, cold methanol was used to fix cells for
10 min. Cells were stained with 0.5% crystal violet solution
(Chemcatch, USA) in 25% methanol at room temperature. The cells
were washed with water three times and air-dried for calculating
through an inverted microscope.
DA analysis
ELISA kits for DA determination in serum and tumor
tissue samples from mice were purchased from Nordhorn (Germany). DA
detection was performed following the manufacturer's
instructions.
Wesertn blot analysis
After treatments under different conditions, the
cells were harvested and the medium was removed. Then the cells
were washed with chilled PBS three times and lysed in ice-cold
lysis buffer with fresh protease inhibitor cocktail. The cell
lysates were centrifuged at 12,000 g for 20 min at 4°C to collect
the supernatant. BSA protein assay kit was used to detect the
protein concentrations following the manufacturer's instructions
(Thermo, USA). Protein extracts (40-ng) were separated by 10%
SDS-PAGE and were then transferred to polyvinylidene fluoride
membrane (Millipore, USA). The PVDF with proteins were blocked with
5% skim fat dry milk in 0.1% Tween-20 in Tris-buffered saline (TBS)
for 2 h to block the non-specific sites on blots. The primary
antibodies dissolved in blocking buffer were used to detect the
target protein blots at 4°C overnight for incubation. The bands on
PVDF were covered by chemiluminescence with Pierce ECL Western
Blotting Substrate reagents (Thermo Scientific, IL, USA). The
primary antibodies used in our study were: rabbit anti-GAPDH,
caspase-3, PARP, TGF-β1, VEGFR1, ERK1/2, p-ERK1/2, p38, pJNK,
E-cadherin, N-cadherin, and vimentin.
Flow cytometry assays
The Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (KeyGen) was used to determine apoptotic cells. Liver
cancer cells after different treatments were harvested and washed
with chilled PBS, then incubated in a darkroom for Annexin V-FITC
and PI for 15 min. Subsequently, the cells were analyzed by flow
cytometry (BD Biosciences, USA).
The hepatoma model
Sixty, 6-week old male, athymic BALB/C nude mice
were purchased from the Animal Center of Nanjing Medical University
(Nanjing, China) and cultured in a 25±2°C temperature and 50±10%
humidity-controlled environment with a standard 12-h light and dark
cycle with food and water in cages under germ-free conditions. All
processes were in line with the Institutional Animal Care and Use
Committee of the second Xiangya Hospital of Central South
University. Briefly, athymic BALB/C nude mice were implanted
orthotopically with liver cancer HCC-LM3 cells (3×106
cells). All tumor-bearing mice were divided into 4 groups randomly:
i) control; ii) Fis (20 mg/kg); iii) Fis (40 mg/kg); iv) Fis (80
mg/kg) daily through i.p. After 7 weeks of treatment, the mice were
sacrificed for the following experiments. The blood from mouse was
obtained through the eyeball, and then was centrifuged at 15,000 g
for 15 min. Fisetin was dissolved in DMSO and diluted in distilled
water for further use.
Immunohistochemical assays
The tissue in each group were fixed with 10%
buffered formalin, embedded in paraffin and sliced into 4–5-µm
thick sections. Tumor tissues also were subjected to
immunohistochemical (IHC) staining for the analysis of VEGFR1 and
Ki-67 expression. The immunfluorescent analysis was carried out as
described previously (18). The
cells or tissue samples were incubated with TGF-β1, as the primary
antibody, overnight at 4°C. The slides were then washed with cold
PBS and incubated with anti-rabbit secondary antibody (KeyGen). The
histological examination was performed following the standard
procedures described previously (19).
Statistical analysis
Data were expressed as mean ± SEM. Statistical
analyses were carried out with GraphPad PRISM (version 6.0; Graph
Pad Software). A P-value <0.05 was considered statistically
significant.
Results
Fisetin-suppresses liver cancer cell
proliferation related to death receptor 2 (DR2)
In order to explore whether fisetin could perform as
DR2 agonist showing important role in regulating liver cancer cell
prolifreration, the cell viability of liver cancer cells of
HCC-LM3, HepG2 and SMMC-7721 was calculated. As shown in Fig. 1B, fisetin at different
concentrations (0, 1, 2.5, 5, 10, 20, 40 and 80 µM) was
administered to HCC-LM3 cells. Fisetin showed significantly
inhibitory role in HCC-LM3 proliferation >10 µM. Similarly, the
death receptor 2 agonist of bromocriptine markedly suppressed
HCC-LM3 cell proliferation in a dose-dependent manner.
Consistently, in HepG2 cells, fisetin also displayed suppressive
effects on cell proliferation, which was in line with the role of
bromocriptine in HepG2 regulation (Fig.
1C). Fisetin also exhibited inhibitory role in SMMC-7721 cells.
Of note, bromocriptine, as DR2 agonist, reduced the liver cancer
cell viability (Fig. 1D). Taken
together, the results above indicated that fisetin shows similar
role with bromocriptine, reducing liver cancer cell lines
proliferation, which might be an essential therapeutic strategy for
liver cancer treatment and linked with the DR2 signal.
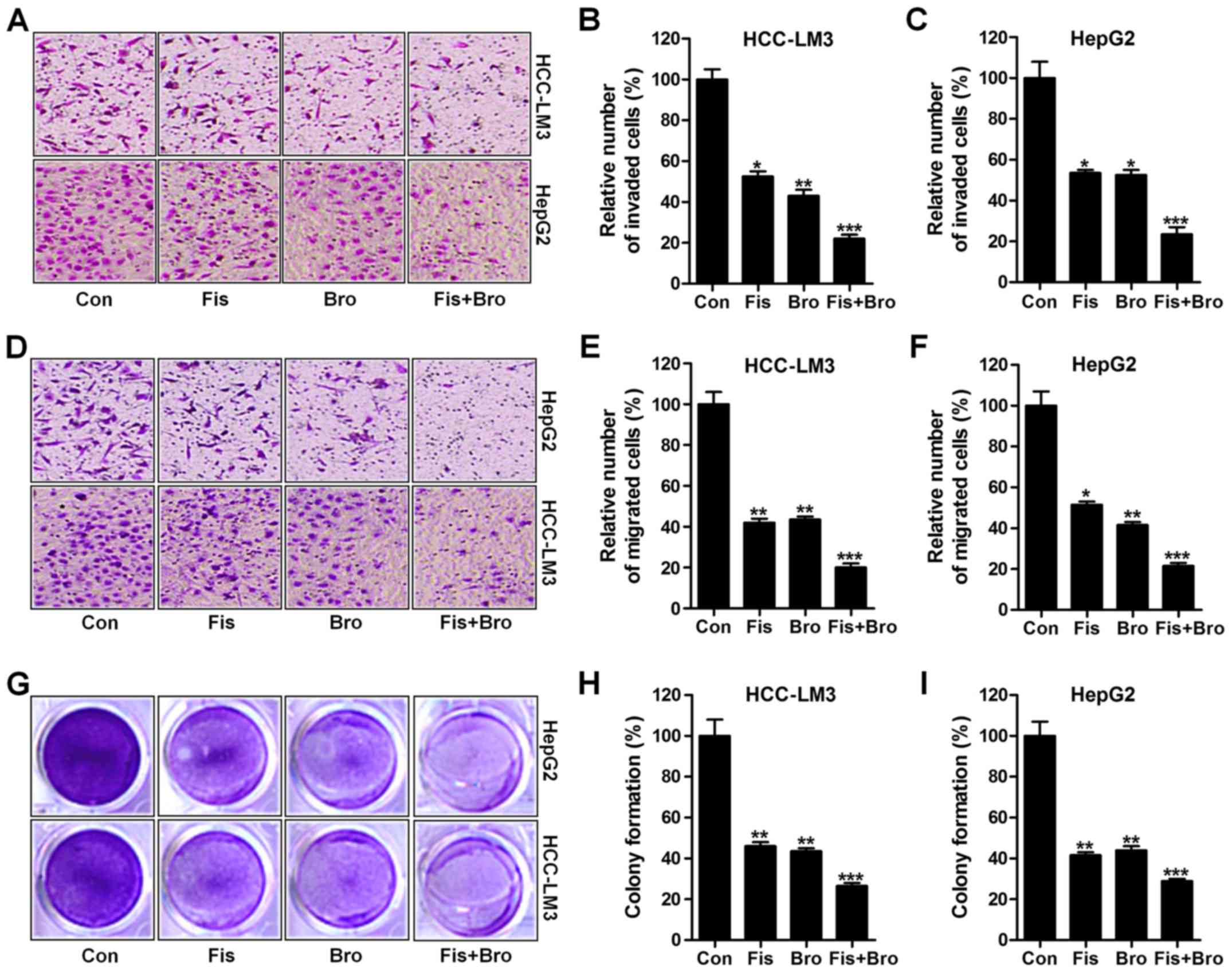
Effects of fisetin and bromocriptine
on liver cancer cell proliferation, migration and invasion
In this regard, we investigated the effects of
fisetin and bromocriptine treatment on liver cancer cell
proliferation, migration and invasion. Whether the treatment of
fisetin and bromocriptine influenced the clonogenic growth of
HCC-LM3 and HepG2, colony-forming analysis was also carried out. In
Fig. 2A and B, the invasion of
liver cancer cells were apparently reduced due to fisetin and
bromocriptine treatment in HCC-LM3 cells. Notably, fisetin and
bromocriptine co-treatment could further downregulate the number of
invaded cells, suggesting that fisetin might share similar
molecular mechanism with bromocriptine to regulate liver cancer
progression. Also, in HepG2 cells, fisetin and bromocriptine
reduced cell invasion, which was further enhanced for fisetin and
bromocriptine combination (Fig. 2A and
C). Next, fisetin and bromocriptine obviously reduced HCC-LM3
cell migration. Also, intensively suppressive role of fisetin and
bromocriptine co-treatment in cell migration inhibition was
observed (Fig. 2D and E). In
addition, fisetin and bromocriptine could considerably downregulate
HepG2 cell migration, which was promoted for fisetin and
bromocriptine in combination (Fig. 2D
and F). Finally, colony formation assays showed that fisetin
and bromocriptine monotherapy significantly reduced the colony
number of cancer cells compared to the control ones. Notably,
combination of fisetin and bromocriptine markedly decreased the
clonogenic growth of lung cancer cells of HCC-LM3 and HepG2
(Fig. 2G-I). The results illustrate
the capability of fisetin and bromocriptine to suppress liver
cancer cell invasion migration, and proliferation is apparently
stronger than the effect of fisetin and bromocriptine separately
performed in the present experiments, and fisetin, at least partly,
could perform as bromocriptine in controlling liver cancer
progression.
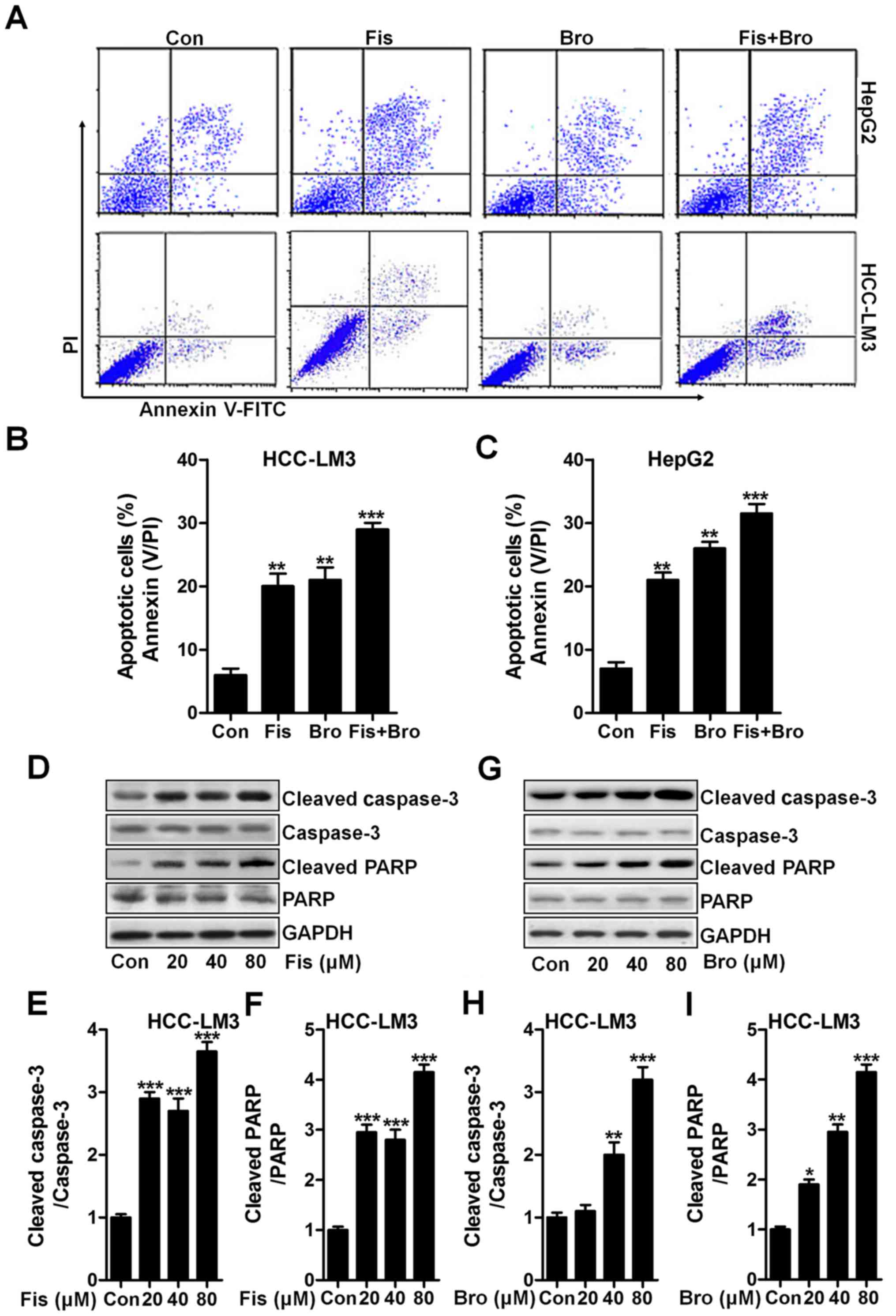
Fisetin and bromocriptine induce
apoptosis in liver cancer cells via caspase-3 activation
Apoptosis induction is known as a key mechanism,
contributing to cell death, which is widely used to explore and
find new therapeutic strategy (20). In this study, the flow cytometric
results exhibited that the fisetin and bromocriptine significantly
upregulated the apoptotic liver cancer cells HCC-LM3 and HepG2.
Interestingly, fisetin and bromocriptine combination caused an
obvious upregulation of apoptotic cells compared to the control
ones (Fig. 3A-C). Next, the caspase
activation of cancer cells after fisetin and bromocriptine and the
co-treatment were determined through western blot analysis. As
shown in Fig. 3D, fisetin markedly
induced high cleavage of caspase-3 in a dose-dependent manner.
Consequently, PARP was activated and apoptosis was induced.
Significantly, fisetin at the highest concentration resulted in an
obvious more intensive caspase-3 (Fig.
3E) and PARP (Fig. 3F) cleavage
in liver cancer cells of HCC-LM3. Significantly, HCC-LM3 treated by
bromocriptine at different concentrations could also stimulate
caspase-3 and PARP cleavage, which was performed in a
dose-dependent manner (Fig. 3G-I).
The results above show that caspase signaling pathway activation is
involved in fisetin- and bromocriptine-induced apoptosis, which
might be a possible molecular mechanism by which fisetin and
bromocriptine exhibits strong antitumor effects.
Fisetin and bromocriptine suppresses
EMT in vitro regulated by TGF-β1
EMT is known to play an important role in cancer
metastasis (21). Essentially,
TGF-β1 has been well reported to induce the process of EMT
(22). Fig. 4A shows western blot analysis of
TGF-β1 downregulated significantly compared to the control in
HCC-LM3 cells after fisetin treatment, dose-dependently (Fig. 4C). In addition, vimentin (Fig. 4A and D), E-cadherin (Fig. 4A and E) and N-cadherin (Fig. 4A and F) were apparently reduced due
to fisetin administration, which was in line with TGF-β1
expression. Importantly, bromocriptine indicated similar role in
suppressing TGF-β1 (Fig. 4B and G),
vimentin (Fig. 4B and H),
E-cadherin (Fig. 4B and I) and
N-cadherin (Fig. 4B and J) in
HCC-LM3 cells. Furthermore, immunofluorescence analysis proved that
TGF-β1 could be reduced in fisetin and bromocriptine treatment
(Fig. 4K and L). The data above
suggested that fisetin could ameliorate liver cancer progression
via EMT inhibition through TGF-β1 signaling pathway.
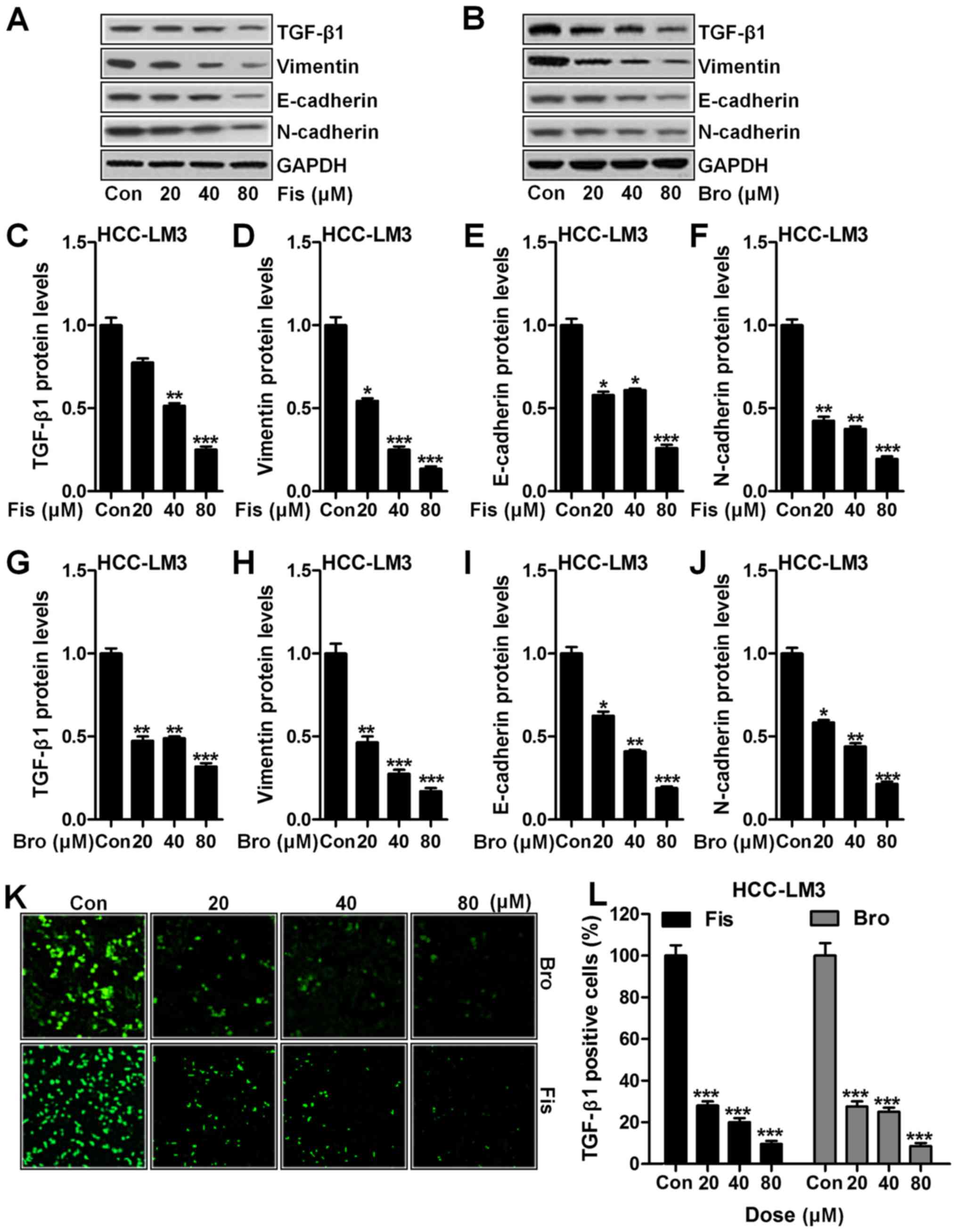 | Figure 4.Fisetin and bromocriptine suppressed
EMT in vitro regulated by TGF-β1 (A) Western blot analysis
of TGF-β1, vimentin, E-cadherin and N-cadherin in HCC-LM3 cells
after fisetin treatment at different concentrations. (B) Western
blot analysis was used to determine TGF-β1, vimentin, E-cadherin
and N-cadherin in HCC-LM3 cells after different concentrations of
treatment by bromocriptine. The quantification of (C) TGF-β1, (D)
vimentin, (E) E-cadherin, and (F) N-cadherin was evaluated after
immunoblot analysis in fisetin-treated liver cancer cells. (G)
TGF-β1, (H) vimentin, (I) E-cadherin, and (J) N-cadherin protein
expression levels were quantified following western blot assays in
bromocriptine-treated cells. (K) The representative images of
TGF-β1 in HCC-LM3 cells after fisetin and bromocriptine treatment
are shown. (L) The quantification of TGF-β1 immunofluorescent
intensity was exhibited. Data are presented as mean ± SEM.
*P<0.05, **P<0.01 and ***P<0.001 versus Con group without
any treatment. |
Fisetin and bromocriptine reduces the
VEGFR and MAPK signaling pathways
To explore the role of fisetin and bromocriptine on
VEGFR1 expression and to investigate the underlying molecular
mechanism, VEGFR1, ERK1/2, p38 and pJNK expression levels were
determined through western blot assays. As shown in Fig. 5A, we found that VEGFR1 (Fig. 5A and C), p-ERK1/2 (Fig. 5A and D), p38 (Fig. 5A and E) and Pjnk (Fig. 5A and F) expression levels in HCC-LM3
cells after fisetin treatment were downregulated markedly compared
to the control ones. Also, in bromocriptine-treated HCC-LM3 cells,
reduced protein levels of VEGFR1 (Fig.
5B and G), p-ERK1/2 (Fig. 5B and
H), p38 (Fig. 5B and I) and
pJNK (Fig. 5B and J) were observed.
The data above indicated that fisetin could perform as
bromocriptine, showing suppressive role in liver cancer progression
via VEGFR1 and EMT-related signaling pathway inhibition.
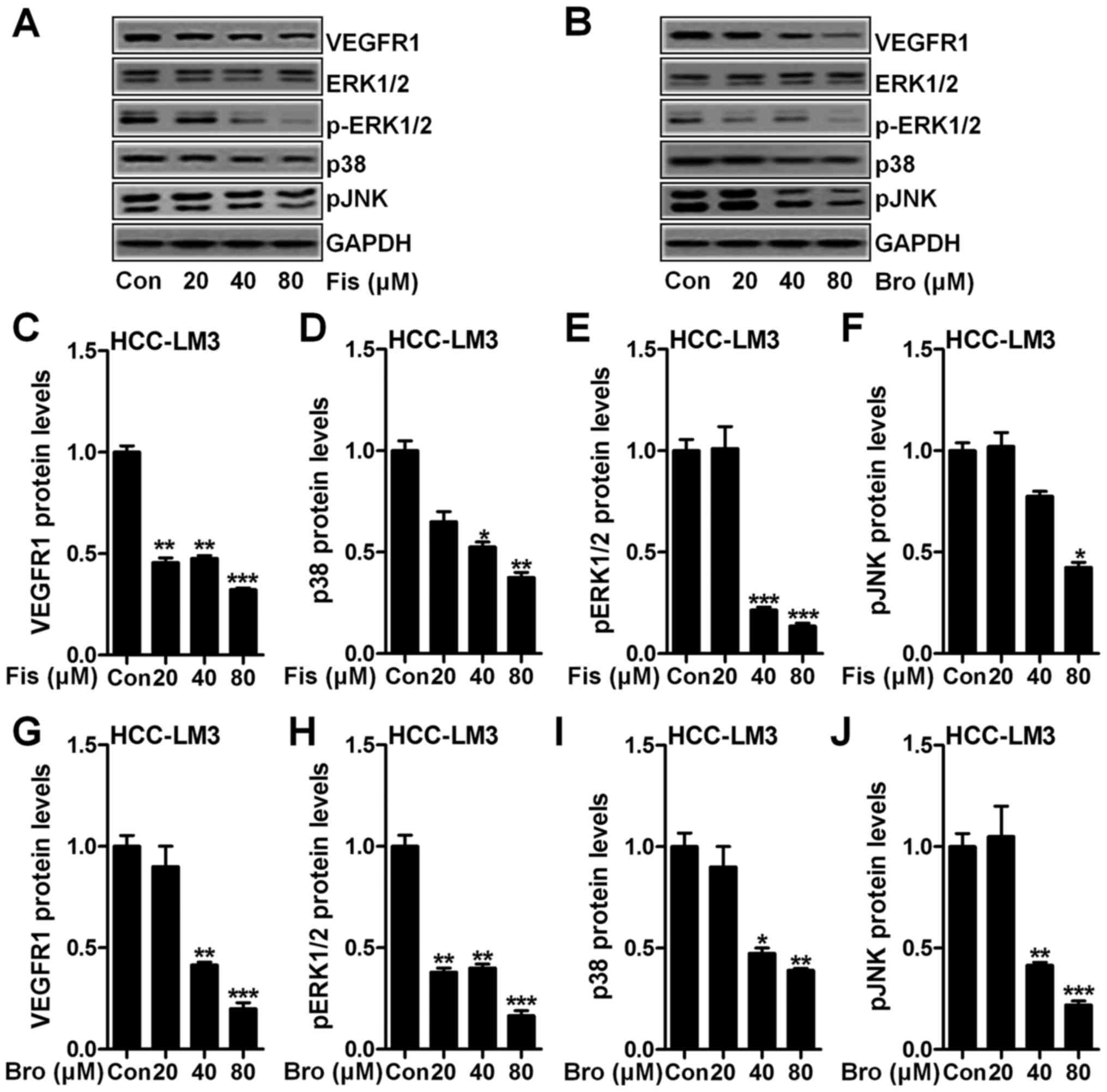 | Figure 5.Fisetin and bromocriptine reduces
VEGFR and MAPK signaling pathway. (A) Western blot assays of VEGFR,
p-ERK1/2, ERK1/2, p38 and pJNK in HCC-LM3 cells after fisetin
treatment at different concentrations. (B) Western blot assays were
performed to detect protein expression levels of VEGFR, p-ERK1/2,
ERK1/2, p38 and pJNK in HCC-LM3 cells after bromocriptine treatment
at different concentrations. The quantification of (C) VEGFR, (D)
p38, (E) p-ERK1/2, and (F) pJNK is displayed. (G) VEGFR, (H)
p-ERK1/2, (I) p38, and (J) pJNK expression levels at protein level
was calculated. Data are presented as mean ± SEM. *P<0.05,
**P<0.01 and ***P<0.001 versus Con group without any
treatment. |
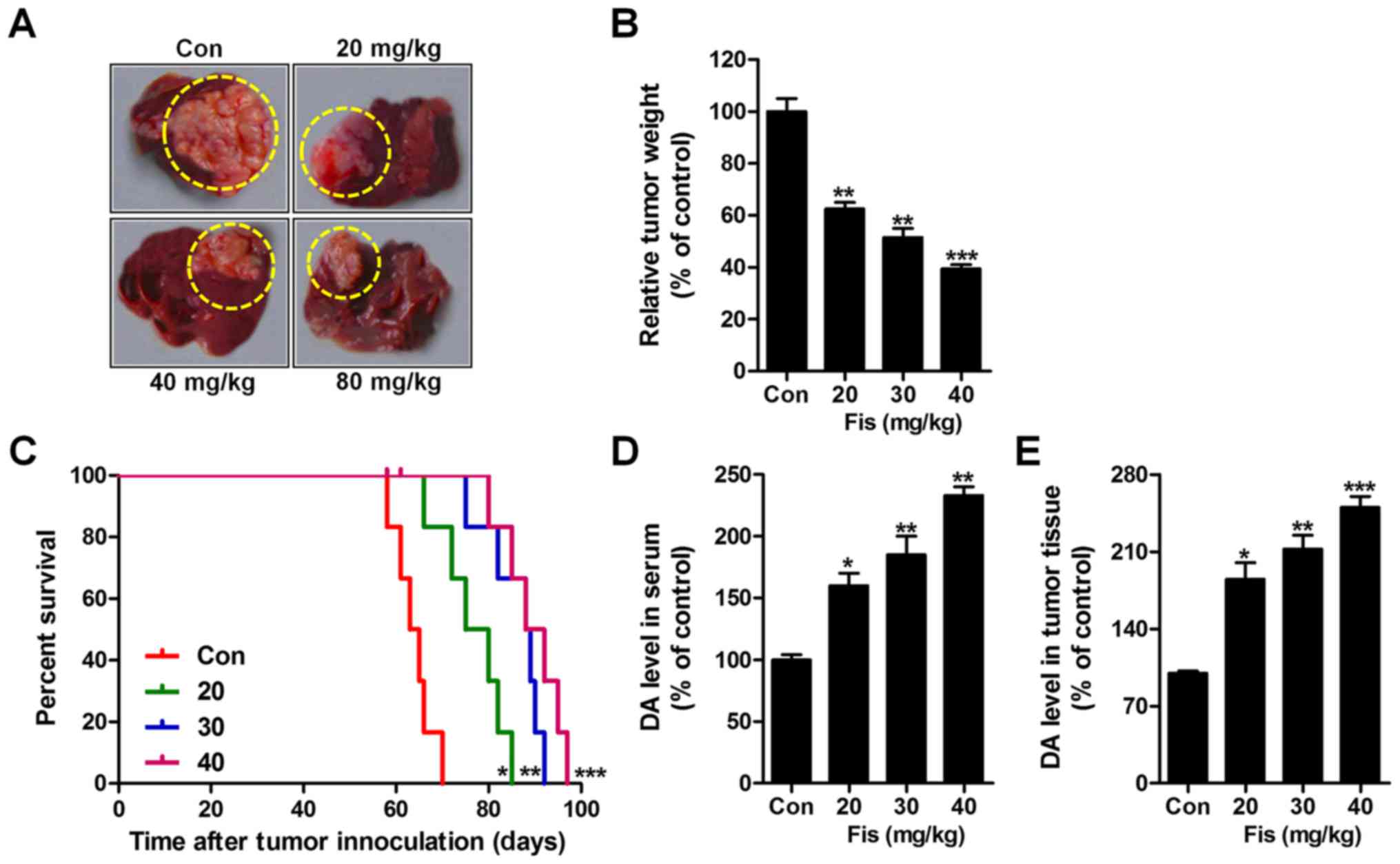
Fisetin inhibits liver cancer growth
of transplanted liver cancer cell line
In this regard, the role of fisetin i.p. injection
in vivo study was evaluated. As shown in Fig. 6A and B, fisetin administration
significantly reduced the weight of tumors, isolated from mice with
orthotopically implanted tumors. Also, survival analysis showed
that fisetin treatment significantly prolonged the survival time of
mice with orthotopically implanted tumors in comparison to the
control ones (Fig. 6C). Finally, DA
levels were measured via ELISA kits. In the serum (Fig. 6D) and tumor tissue (Fig. 6E) from mice with orthotopically
implanted tumors, DA was expressed highly, which was downregulated
significantly after fisetin treatments in a dose-dependent
manner.
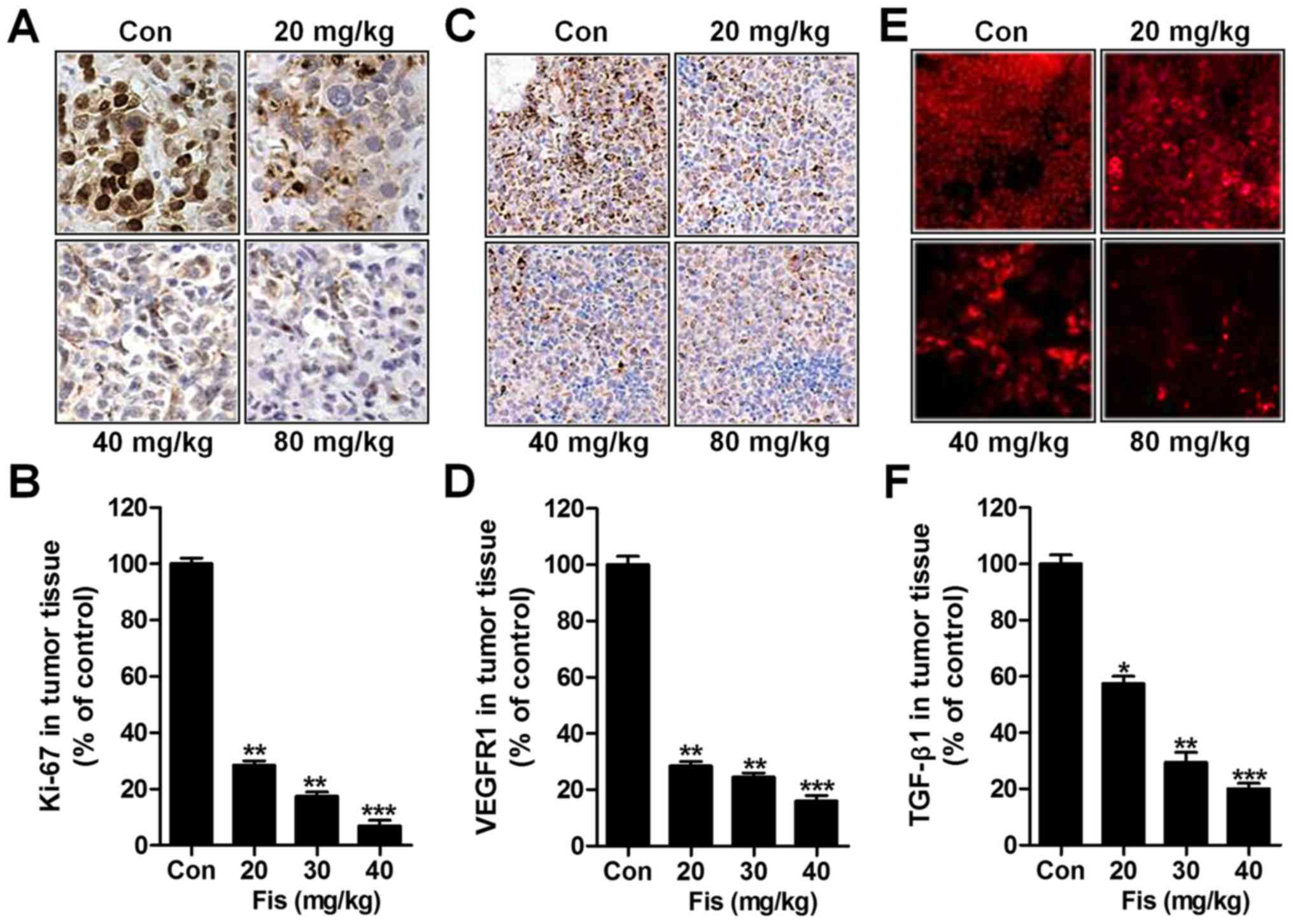
Next, immunohistochemical analysis was used to
detect Ki-67, and VEGFR1 levels from tumor specimens. As shown in
Fig. 7A and B, Ki-67 was
significantly downregulated due to fisetin administration, which
was dose-dependent. Similarly, VEGFR1 was also impeded in
fisetin-treated group, which was in line with the results in
vitro (Fig. 7C and D). TGF-β1
was reduced in tumor tissue samples isolated from fisetin-treated
mice through immunofluorescent analysis (Fig. 7E and F). The data above indicated
that fisetin could suppress liver cancer development, which was
related to death receptor signaling.
Discussion
Hepatocellular carcinoma (HCC) is reported as the
most common form of primary liver cancer. There is still no
effective therapies to prevent it (1–3,23).
Compounds with bioactivity extracted from various plants are
catching attention due to their less toxicity and more effective
activity in controlling disease progression (24). For instance, the isolated and
purified fatty acid from A. spinosus was discovered to
inhibit the HepG2 cell proliferation (25). In our study, similar role was found
by the use of fisetin. Fisetin changed DA levels in the serum of
mice as well as in the tumor tissue samples. In addition, fisetin
performs as DR2 agonist in regulating liver cancer cell
proliferation, suggesting that on the one hand, the nervous system
is involved in HCC development. Also, fisetin might be used as a
crucial and effective agent in HCC suppression.
First in this study, fisetin was found to inhibit
liver cancer cell proliferation, migration and invasion. It has
been suggested that apoptosis induction is a main molecular
mechanism by which cancer cells experience cell death (26). Also, presently, many drugs used for
apoptosis induction are explored in order to find effective
therapeutic strategies for cancer prevention (27). The caspase-related mechanism is
considered as a major signal pathway resulting in apoptosis
(28). Disorders of caspases are
related to various cancer cell immortality (29). In our study, we found that fisetin
could activate caspase-3 activity. The cleaved caspase-3 was
expressed highly with the increasing of fisetin treatment, which
was in line with the effects of bromocriptine on HCC-LM3 cells,
suggesting that fisetin, to some degree, shows similar role with
bromocriptine in regulating liver cancer. Subsequently, PARP
cleavage was improved, activating apoptosis eventually. Also, our
flow cytometry analysis indicated that apoptosis was induced for
fisetin, bromocriptine and the two combinations. Of note, fisetin
and bromocriptine co-treatment could further upregulate apoptosis,
which might be due to fisetin administration enhancing
bromocriptine role in liver cancer apoptosis induction and
promoting cell death.
Previous studies have suggested that dopamine has
close relationship with stress and movement (30). As a significant part of the nervous
system, dopamine plays important role in development of many
diseases, including cancer (31).
Dopamine has been indicated to possess anticancer properties
(32). In our study, we found that
dopamine was upregulated in the serum and tumor tissue samples of
mice with liver cancer for fisetin administration. The data
indicated that dopamine was also involved in liver cancer
progression, and fisetin could suppress liver cancer development,
which was related to dopamine mediation. However, further study is
needed to investigate the specific relationship between fisetin and
dopamine in liver cancer progression. TGF-β1 is well known in tumor
growth, which could be regulated for ERK signaling pathway
(33). ERK signaling pathway could
activate TGF-β1 expression, contributing to various tumor
metastasis (34). In our study,
TGF-β1 was highly expressed in liver cancer cells and tumors, which
was downregulated for fisetin administration, as well as
bromocriptine treatment. Also, the following signals of p38 and
pJNK were inhibited for fisetin and bromocriptine, accompanied with
vimentin, E-cadherin and N-cadherin, which are important members
associated with tumor growth (35,36).
In conclusion, this study indicated that fisetin
could influence the nervous system, at least partly, as dopamine
receptors function. Fisetin shows suppressive role in liver cancer
development, which could reduce the volume of liver cancer and
prolong the survival of mice. Fisetin could be considered as
dopamine agonist to inhibit liver cancer progression. Our study
indicated that fisetin was beneficial for liver cancer inhibition.
However, further study is still needed to clarify the molecular
mechanism by which fisetin influences dopamine in the nervous
system.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y and
Miao Y: Meta-analysis of surgical resection and radiofrequency
ablation for early hepatocellular carcinoma. World J Surg Oncol.
10:1632012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DuBray BJ Jr, Chapman WC and Anderson CD:
Hepatocellular carcinoma: A review of the surgical approaches to
management. Mo Med. 108:195–198. 2011.PubMed/NCBI
|
|
6
|
Salhab M and Canelo R: An overview of
evidence-based management of hepatocellular carcinoma: A
meta-analysis. J Cancer Res Ther. 7:463–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Minicis S, Candelaresi C, Marzioni M,
Saccomano S, Roskams T, Casini A, Risaliti A, Salzano R, Cautero N,
di Francesco F, et al: Role of endogenous opioids in modulating HSC
activity in vitro and liver fibrosis in vivo. Gut. 57:352–364.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohno H, Sakai T, Saito S, Okano K and
Kitahara K: Treatment of experimental autoimmune uveoretinitis with
atorvastatin and lovastatin. Exp Eye Res. 84:569–576. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Byun MW: Schizonepeta tenuifolia ethanol
extract exerts anti-inflammatory activity through the inhibition of
TLR4 signaling in lipopolysaccharide-stimulated macrophage cells. J
Med Food. 17:350–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H
and Tian B: Fisetin, a dietary flavonoid, induces cell cycle arrest
and apoptosis through activation of p53 and inhibition of NF-kappa
B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol.
108:84–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang
YC, Bau DT and Hsieh YH: Fisetin induces apoptosis in human
cervical cancer HeLa cells through ERK1/2-mediated activation of
caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 86:263–273.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Contreras F, Fouillioux C, Bolívar A,
Simonovis N, Hernández-Hernández R, Armas-Hernandez MJ and Velasco
M: Dopamine, hypertension and obesity. J Hum Hypertens. 16 Suppl
1:S13–S17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hazelwood LA, Free RB, Cabrera DM,
Skinbjerg M and Sibley DR: Reciprocal modulation of function
between the D1 and D2 dopamine receptors and the Na+,K+-ATPase. J
Biol Chem. 283:36441–36453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salyer S, Lesousky N, Weinman EJ, Clark
BJ, Lederer ED and Khundmiri SJ: Dopamine regulation of
Na+-K+-ATPase requires the PDZ-2 domain of sodium hydrogen
regulatory factor-1 (NHERF-1) in opossum kidney cells. Am J Physiol
Cell Physiol. 300:C425–C434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YR and Yuan ZY: Dopamine-mediated
inhibition of renal Na+/K+-ATPase in HK-2 cells is reduced by
ouabain. Clin Exp Pharmacol Physiol. 37:613–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calarge CA, Ellingrod VL, Acion L, Miller
DD, Moline J, Tansey MJ and Schlechte JA: Variants of the dopamine
D2 receptor gene and risperidone-induced hyperprolactinemia in
children and adolescents. Pharmacogenet Genomics. 19:373–382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trussardi-Regnier A, Lavenus S, Gorisse MC
and Dufer J: Thalidomide alters nuclear architecture without ABCB1
gene modulation in drug-resistant myeloma cells. Int J Oncol.
35:641–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cagin YF, Parlakpinar H, Vardi N, Polat A,
Atayan Y, Erdogan MA and Tanbek K: Effects of dexpanthenol on
acetic acid-induced colitis in rats. Exp Ther Med. 12:2958–2964.
2016.PubMed/NCBI
|
|
20
|
Yang PM, Tseng HH, Peng CW, Chen WS and
Chiu SJ: Dietary flavonoid fisetin targets caspase-3-deficient
human breast cancer MCF-7 cells by induction of
caspase-7-associated apoptosis and inhibition of autophagy. Int J
Oncol. 40:469–478. 2012.PubMed/NCBI
|
|
21
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyazono K, Ehata S and Koinuma D:
Tumor-promoting functions of transforming growth factor-β in
progression of cancer. Ups J Med Sci. 117:143–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wheelhouse NM, Lai PB, Wigmore SJ, Ross JA
and Harrison DJ: Mitochondrial D-loop mutations and deletion
profiles of cancerous and noncancerous liver tissue in hepatitis B
virus-infected liver. Br J Cancer. 92:1268–1272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Zijl F, Zulehner G, Petz M, Schneller
D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H and Mikulits
W: Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin Z, McDonald ER III, Dicker DT and
El-Deiry WS: Deficient tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) death receptor transport to the
cell surface in human colon cancer cells selected for resistance to
TRAIL-induced apoptosis. J Biol Chem. 279:35829–35839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HS, Lee JW, Soung YH, Park WS, Kim SY,
Lee JH, Park JY, Cho YG, Kim CJ, Jeong SW, et al: Inactivating
mutations of caspase-8 gene in colorectal carcinomas.
Gastroenterology. 125:708–715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Ong CN and Shen HM: Involvement
of proapoptotic Bcl-2 family members in parthenolide-induced
mitochondrial dysfunction and apoptosis. Cancer Lett. 211:175–188.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pennarun B, Meijer A, De Vries EG,
Kleibeuker JH, Kruyt F and de Jong S: Playing the DISC: Turning on
TRAIL death receptor-mediated apoptosis in cancer. Biochim Biophys
Acta. 1805:123–140. 2010.PubMed/NCBI
|
|
29
|
Jung YH, Heo J, Lee YJ, Kwon TK and Kim
YH: Quercetin enhances TRAIL-induced apoptosis in prostate cancer
cells via increased protein stability of death receptor 5. Life
Sci. 86:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia L and Zhang MH: Comparison of
probiotics and lactulose in the treatment of minimal hepatic
encephalopathy in rats. World J Gastroenterol. 11:908–911. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamada M, Chiba T, Sasabe J, Nawa M,
Tajima H, Niikura T, Terashita K, Aiso S, Kita Y, Matsuoka M, et
al: Implanted cannula-mediated repetitive administration of
Abeta25-35 into the mouse cerebral ventricle effectively impairs
spatial working memory. Behav Brain Res. 164:139–146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seamans JK and Yang CR: The principal
features and mechanisms of dopamine modulation in the prefrontal
cortex. Prog Neurobiol. 74:1–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu Q: Transforming growth factor-beta1
protects against pulmonary artery endothelial cell apoptosis via
ALK5. Am J Physiol Lung Cell Mol Physiol. 295:L123–L133. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baarsma HA, Spanjer AI, Haitsma G,
Engelbertink LH, Meurs H, Jonker MR, Timens W, Postma DS, Kerstjens
HA and Gosens R: Activation of WNT/β-catenin signaling in pulmonary
fibroblasts by TGF-β1 is increased in chronic obstructive pulmonary
disease. PLoS One. 6:e254502011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu GL, Yang HJ, Liu T and Lin YZ:
Expression and significance of E-cadherin, N-cadherin, transforming
growth factor-β1 and Twist in prostate cancer. Asian Pac J Trop
Med. 7:76–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|