Introduction
Colorectal cancer (CRC) is the third most common
cancer in China and has become one of the leading causes of
cancer-related mortality in the developed countries (1–3). Every
year, there are approximately 1.2 million new cases diagnosed as
CRC worldwide (4).
Chemotherapy and radiotherapy are two important
methods for the treatment of CRC. However, radio- and
chemo-resistance leading to tumor recurrence and the corresponding
poor prognosis, is still a serious concern. Therefore, enhancing
radio- and chemo-sensitivity, overcoming radio- and
chemo-resistance, and improving the efficiency of radio- and
chemo-therapy have great practical significance in the clinical
treatment of CRC.
Low dose ionizing radiation (LDIR) is proved having
hormesis (5,6) and adoptive effect (7). LDIR generates distinct biological
effects in cancer cells from normal cells, e.g., it may affect the
growth of cancer cells via the activation of certain cell signaling
pathway, which does not exist in normal cells (8,9).
However, in which manner LDIR affects the proliferation of cancer
cells and the scope of application of LDIR in clinical carcinoma
therapy, are still in controversial and needed to be deeply
clarified.
In this study, we developed a differential LDIR
model. Instead of providing a single dose of 250 mGy, we divided
the LDIR radiation dose into a ten-time 25 mGy with 3-day intervals
in between. Then the intermittent LDIR was used jointly with 2 Gy
HDIR radiotherapy or 5-fluorouracil (5-FU) based chemotherapy; we
analyzed the cell growth of human colorectal adenocarcinoma cell
line HT-29 in these combination therapies and evaluated the
therapeutic effectivness of intermittent LDIR. We also investigated
the activation of cell signaling pathways after the combination
therapies. We hope this observation provides new insight into LDIR
and experimental evidence for the multimodal treatment of
colorectal cancer.
Materials and methods
Cell culture and treatments
Human colorectal adenocarcinoma cell line HT-29 was
purchased from American Type Culture Collection (ATCC, VA, USA).
HT-29 was maintained in MyCoy'5A media (Thermo Fisher Scientific,
Beijing, China) supplemented with 10% fetal bovine serum (FBS,
Hyclone, Beijing, China) and 1% antibiotics
(penicillin-streptomycin, Invitrogen, CA, USA). Cells were cultured
at 37°C in a humidified incubator with a constant air flow of 5%
CO2.
ATM inhibitor KU55933 (Selleck, Shanghai, China),
p53 inhibitor pifithrin-α (PFT-α, Beyotime Institute of
Biotechnology, Jiangsu, China), ERK inhibitor PD98059 (Selleck) and
p38MAPK inhibitor SB203580 (Selleck) were used to block the
function of ATM, p53 and MEK. The final concentration for all the
chemical inhibitors is 10 µM in the cell culture media.
LDIR strategy
HT-29 cells were ionizing irradiated at the dose
rate of 12.5 mGy/min (for LDIR) or 500 mGy/min (for HDIR) by X-RAD
320 (Precision X-RAD, North Branford, CT, USA). In order to
investigate the intermittent LDIR effect on colorectal cancer,
HT-29 cells were divided into three experimental groups. Group A,
cells were irradiated for 10 times with a dose of 25 mGy at each
time. The time interval between two irradiations was 3 days. The
total dose was 250 mGy and the time span was 30 days; group B,
cells were parallelly cultured for 30 days and accepted a dose of
250 mGy LDIR on day 30; group C, mock irradiation group as control.
After that, cell culture media were replaced and cells were
harvested immediately or continually cultured until the next step
of the experiment was carried out.
Treatment with chemotherapeutic drug
or HDIR
Chemotherapeutic drug 5-FU (Sigma, Shanghai, China)
was dissolved in PBS. For drug treatment, appropriate amount of
HT-29 colorectal cancer cells from experimental group A, B or C
were treated by 50 µg/ml 5-FU solution for 24 h. Then, cell culture
media were changed and cells were either harvested or used for the
proliferation assays. For radiotherapy, appropriate amount of HT-29
cells from group A, B or C were irradiated by 2 Gy HDIR (50
mGy/min), and cells were either harvested or used for the
proliferation assays.
Cell proliferation assay
WST-1 assay was performed to evaluate cell
proliferation. After the treatment by either anticancer drug or
HDIR, cell proliferation assays were determined using WST-1 cell
proliferation reagent (Beyotime Biotechnology). According to the
manufacturer's instructions, 10 µl WST-1 was added to 100 µl cell
culture medium and incubated at 37°C in the dark for 2.5 h. The
absorbance of 450 and 630 nm were measured by microplate reader
(Thermo Fisher Scientific). Final OD (optical density) was
designated as
OD450-OD630-ODblank.
Flow cytometry for cell cycle
distribution assay
Approximately 2×106 cells were collected
and washed by 1 ml cold PBS. Cells were centrifuged and resuspended
in 1 ml fixation solution (700 µl ethanol and 300 µl PBS). After
incubated at 4°C for 4 h, cells were washed twice with 1 ml PBS.
Cells were pelleted and suspended in 0.5 ml propidium iodide (PI,
Sigma) staining solution (50 µg/ml PI, 20 µg/ml RNase A and 0.2%
Triton X-100) and incubated in the dark at 37°C for 30 min. Cell
cycle distribution was analyzed by a BD FACSCalibur flow cytometer
(FACScan, BD Biosciences, CA, USA).
Flow cytometry for cell apoptosis
Annexin V-FITC and PI double staining flow cytometry
analyses were employed for the assay of cell apoptosis after
radiotherapy and chemotherapy. Approximately 1×106 HT-29
cells were plated in a 6-well plate containing 2 ml medium and
accepted the treatment of radio- or chemo-therapy. Fourty-eight
hours after the treatments, cells were collected in centrifuge
tubes, washed three times with cold PBS and binding buffer, and
then stained with Annexin V-FITC and PI (Annexin V-FITC Apoptosis
Detection kit, BD Bioscience) for apoptosis detection. Briefly,
HT-29 cells were first resuspended in binding buffer. Then, 5 µl of
Annexin V-FITC was added to the tubes and were incubated for 10 min
followed by the addition of 5 µl PI (Sigma). After 15-min
incubation in PI buffer, cells were immediately analysed using a
flow cytometer (BD Biosciences) with the FlowJo FACS analysis
software. The cells in the different portions represent the
different cell states as follows: the late-apoptotic cells are
present in the upper right portion, the viable cells are present in
the lower left portion, and the early apoptotic are the cells
present in the lower right portion.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cell total RNA was extracted using RNAzol (Sigma)
and the residual genomic DNA was removed by DNase I (Roche,
Shanghai, China). M-MLV Reverse Transcriptase (Invitrogen) was used
to synthesize cDNA. The mRNA expression level of caspase-3 and p21
was quantified by normalizing over β-actin. The sequences of the
primers used for RT-qPCR were as follows: p21-fwd,
5′-ATGTCCGTCAGAACCCATGC-3′; p21-rev, 5′-AGTCGAAGTTCCATCGCTCAC-3′;
caspase-3-fwd, 5′-GATGATGACATGGCGTGTCATA-3′; caspase-3-rev,
5′-AGCGACTGGATGAACCAGGA-3′; β-actin-fwd,
5′-ATCACCATTGGCAATGAGCG-3′; β-actin-rev,
5′-CGTCACACTTCATGATGGAGT-3′.
Western blot analysis
Cell total protein was extracted by protein
extraction kit (Thermo Fisher Scientific) and the protein
concentration was determined by BCA protein assay kit (Bio-Rad).
Appropriate quantity of protein was separated by SDS polyacrylamide
gel electrophoresis (SDS-PAGE) and then electrophoretically
transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). The blots were blocked with 5%
non-fat milk in TBST at 37°C for 1 h. Then, blots were probed with
monoclonal antibodies against ATM (1:500, Abcam, Shanghai, China),
p53 (1:1,500, DO7, Santa Cruz Biotechnology, Santa Cruz, CA, USA),
p21 (1:500, C-19, Santa Cruz Biotechnology), cyclin A (1:500, Santa
Cruz Biotechnology), CDK2 (1:500, Santa Cruz Biotechnology),
caspase-3 (1:1,000, Cell Signaling Technology, Beijing, China),
cleaved caspase-3 (1:1,000, Cell Signaling Technology),
ERK/phos-ERK (1:1,000, Cell Signaling Technology), p38/phos-p38
(1:1,000, Cell Signaling Technology) and β-actin (1:3,000, Santa
Cruz Biotechnology) at 4°C overnight. After washed for 3×5 min in
TBST, the blots were incubated with HRP conjugated goat anti-mouse
or goat anti-rabbit second antibodies (Santa Cruz) at room
temperature for 1 h. After washed for another 3×5 min in TBST, the
blots were visualized by the enhanced chemiluminescence system
(ECL; Thermo Fisher Scientific). Protein expression was determined
semiquantitatively by densitometric analysis with the Quantity One
software (Bio-Rad).
Statistical analysis
All experiments were repeated for at least three
times. Data and statistics are presented as means ± SD. The
significance was determined by Student's t-test using SPSS 17.0.
P<0.05 was considered to indicate a statistically significant
difference (*P<0.05; **P<0.01).
Results
Single dose LDIR or intermittent LDIR
does not affect the cell growth of HT-29 colorectal cancer
cells
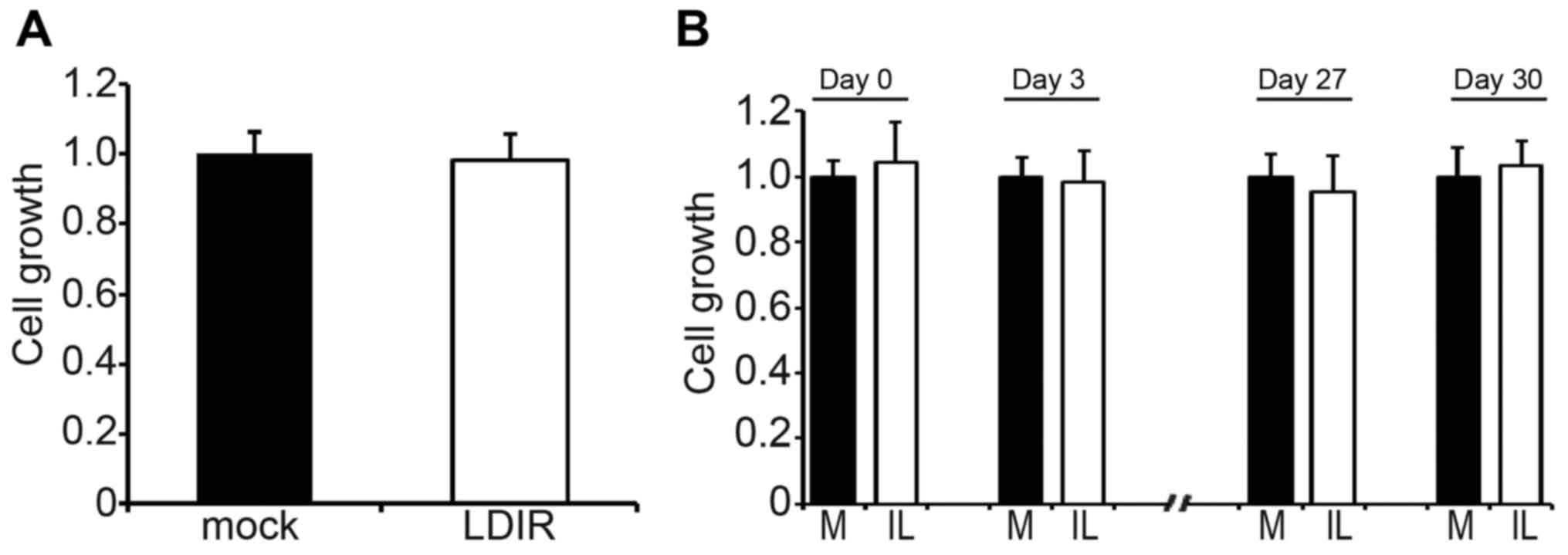
To illustrate the cell growth status after LDIR, we
used 250 mGy single dose LDIR or 25 mGy ten-times LDIR to irradiate
HT-29 colorectal cancer cells. The cell growth was determined by
WST-1 kit. We found that neither single dose LDIR (Fig. 1A) nor intermittent LDIR (Fig. 1B, only the results of days 0, 3, 27
and 30 are shown) changed the cell growth of HT-29 (P>0.05).
Intermittent LDIR promotes the
therapeutic effects of radiotherapy and chemotherapy
In order to investigate whether the LDIR affects the
therapeutic effect of radiotherapy or chemotherapy, HT-29
colorectal cancer cells were pretreated by either single dose LDIR
or intermittent LDIR before HDIR or 5-FU chemotherapy. The results
of cell growth assay are shown in Fig.
2A. Compared to the non-LDIR or single dose LDIR pretreatments,
the intermittent LDIR pretreatment obviously inhibited the cell
growth in radiotherapy (Fig. 2A)
and chemotherapy (Fig. 2B). The
cell morphology showed a similar result (Fig. 2C). We analyzed the cell cycle
distrubution by flow cytometry and found that the pretreatment of
intermittent LDIR induced a remarkable S phase arrest in both
radiotherapy (36.2 vs 27.3%, compared with the single dose LDIR
group; Fig. 2D) and chemotherapy
(40.4 vs 27.9%, compared with the single dose LDIR group; Fig. 2E). We also used the PI-Annexin V kit
to assay cell apoptosis. As can be seen in Fig. 2F, the pretreatment of intermittent
LDIR increased cell apoptosis to 46% in radiotherapy and 49% in
chemotherapy. The apoptosis percentages were markedly higher than
in the intermittent LDIR groups (Fig.
2G, P<0.01).
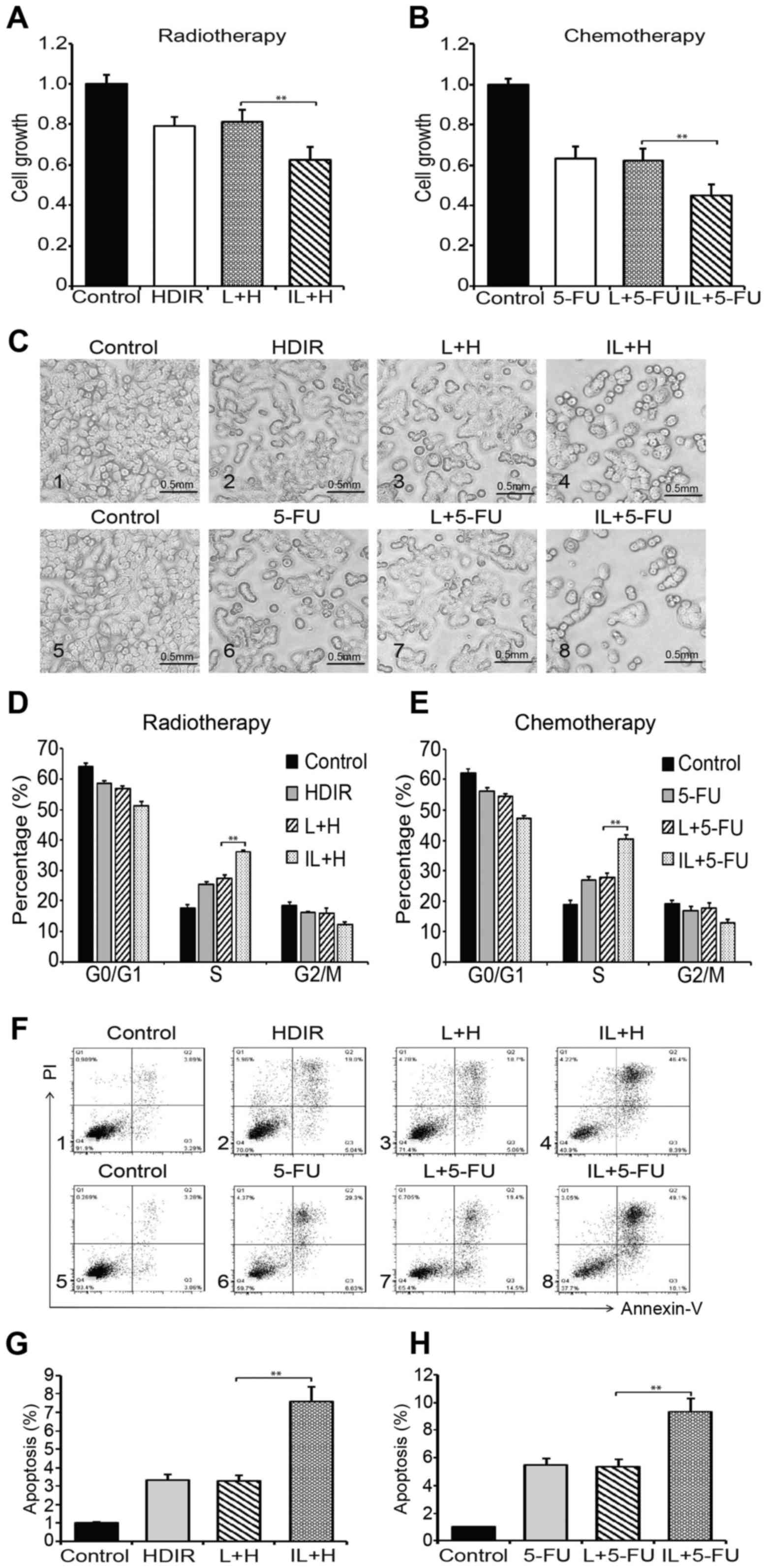 | Figure 2.Intermittent LDIR promotes the
therapeutic effects of radiotherapy and chemotherapy. (A) HT-29
cells were administered either HDIR, LDIR plus HDIR, or
intermittent LDIR plus HDIR. Cell growth was determined by WST-1
assays. (B) HT-29 cells were treated by either 5-FU, LDIR plus
5-FU, or intermittent LDIR plus 5-FU. Cell growth was determined by
WST-1 assays. (C) Cell morphology. Plot C1-C4, radiotherapy group.
Plot C5-C8, chemotherapy group. (D) Analysis of HT-29 cell cycle
distribution by flow cytometry after radiotherapy. (E) Analysis of
HT-29 cell cycle distribution by flow cytometry after chemotherapy.
(F) Analysis of HT-29 cell apoptosis by flow cytometry. Plot D1-D4,
radiotherapy group; Plot D5-D8, chemotherapy group. (G) Cell
apoptosis percentage in radiotherapy. (H) Cell apoptosis percentage
in chemotherapy. H, HDIR; L, LDIR; IL, intermittent LDIR.
**P<0.01 as compared with the single dose LDIR group. |
Intermittent LDIR activates cell cycle
arrest and apoptosis pathway
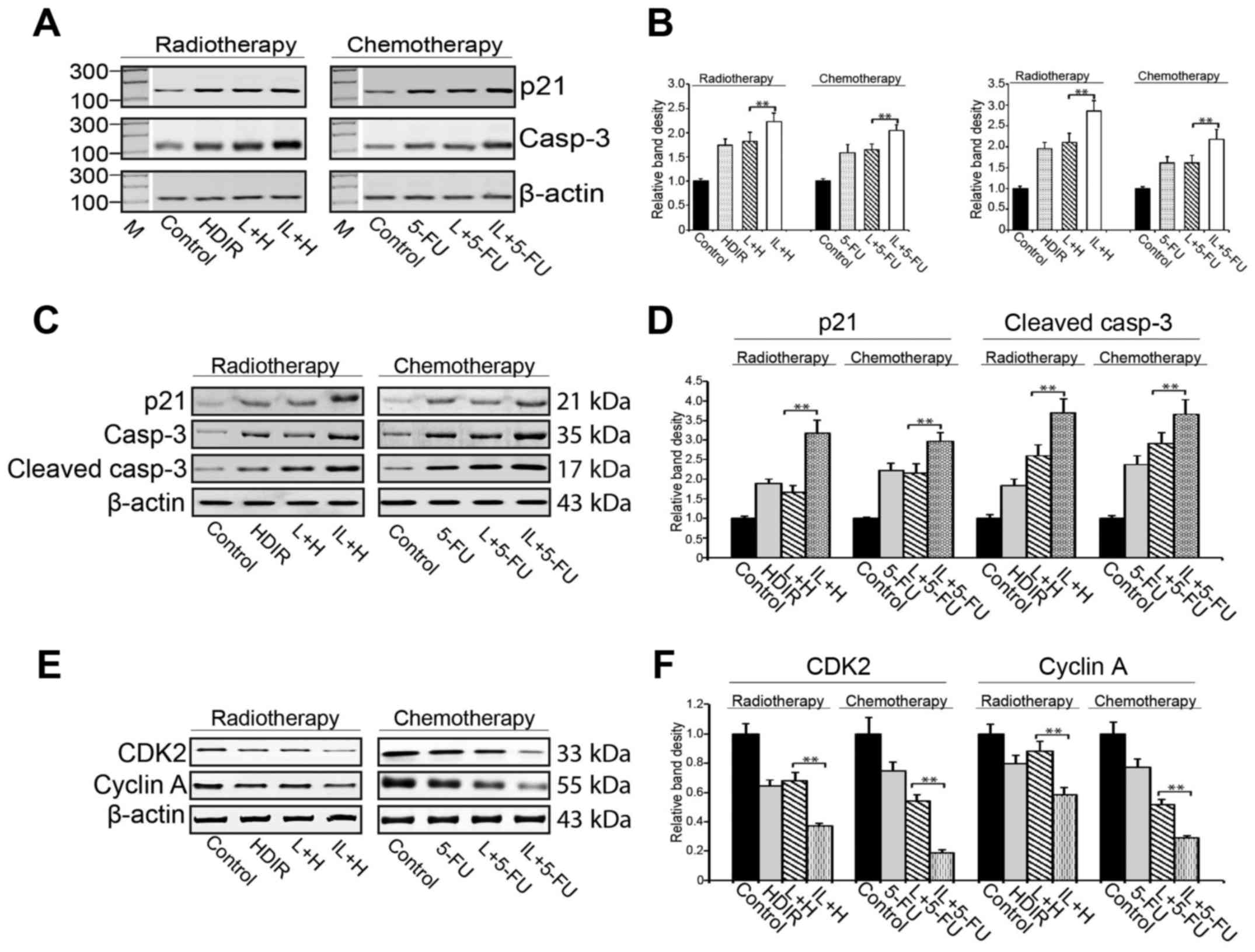
To delineate the mechanism underlying this enhanced
cell growth inhibition after intermittent LDIR, we used RT-PCR and
western blotting to examine the cell cycle arrest and apoptosis
pathway genes for their expression in the HDIR and 5-FU treated
cells. We observed that although both the HDIR and 5-FU treatments
upregulated the expression of p21 and caspase-3 genes, single dose
LDIR pretreatment did not enhance the killing effect of
radiotherapy or chemotherapy (P>0.05, Fig. 3A and B). Interestingly, the
intermittent LDIR pretreatment markedly increased the expression of
p21 and caspase-3 compared to single dose LDIR (P<0.01, Fig. 3A and B). The activation of the cell
cycle arrest and apoptosis pathways were also confirmed via the
detection of p21, caspase-3 (cleaved caspase-3), CDK2 and cyclin A
by western blotting (Fig.
3C-F).
Intermittent LDIR activates ATM/p53
pathway in HDIR radiotherapy
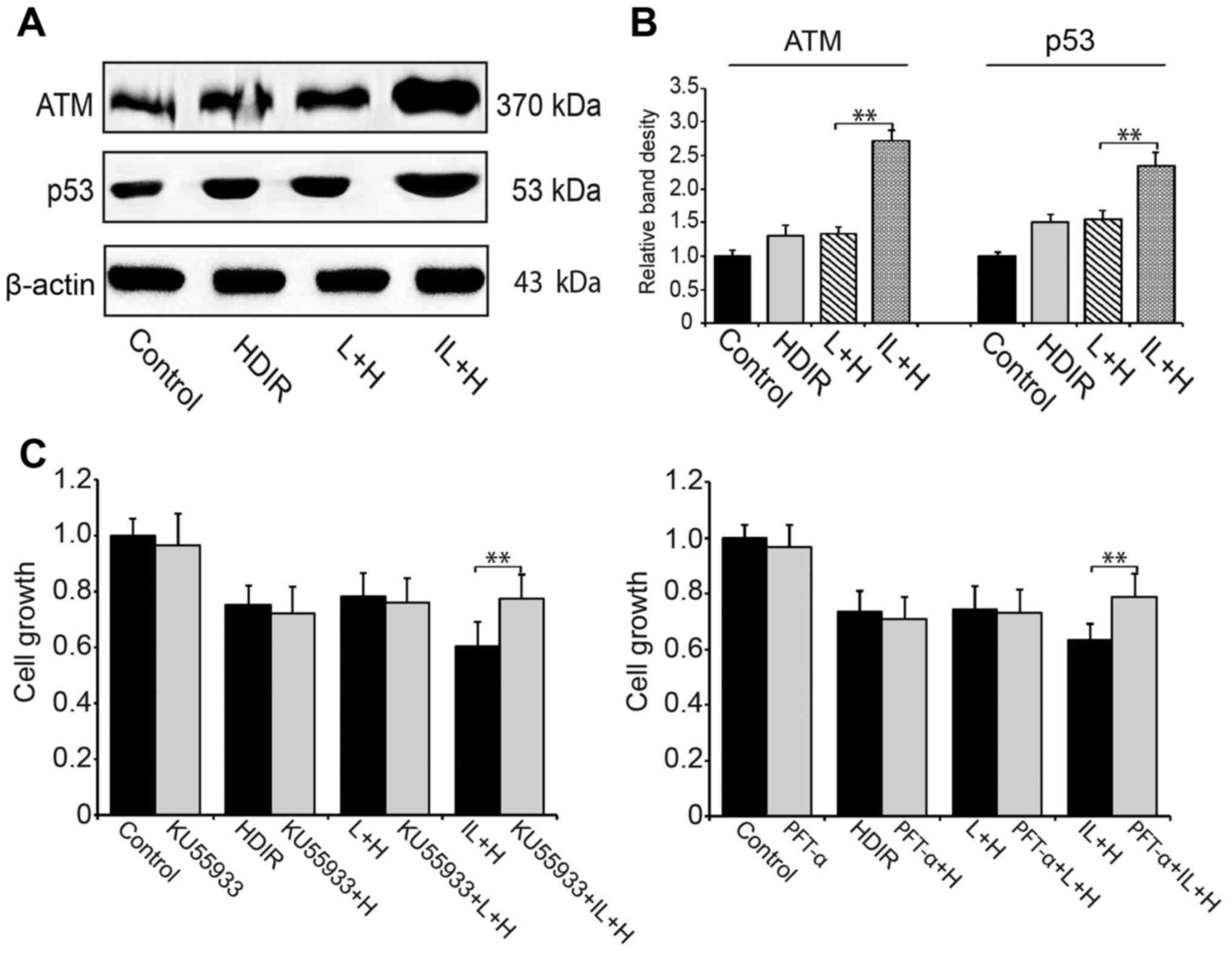
We investigated the radiotherapy most related
ATM/p53 pathway in HDIR treated HT-29 cells. Using western
blotting, we showed that compared to single dose LDIR, intermittent
LDIR pretreatment could activate the ATM/p53 pathway more
efficiently and the expression of ATM and p53 proteins were
upregulated significantely (Fig. 4A and
B). To confirm that the ATM/p53 pathway was specifically
activated by intermittent LDIR, ATM and p53 inhibitors were added
to the cell culture media prior to the HDIR radiotherapy. Cell
growth assay showed that after the ATM/p53 activities were blocked,
the intermittent LDIR-induced cell growth inhibition in
radiotherapy was abolished (P<0.01, Fig. 4C).
Intermittent LDIR activates ERK and
p38MAPK pathways in 5-FU chemotherapy
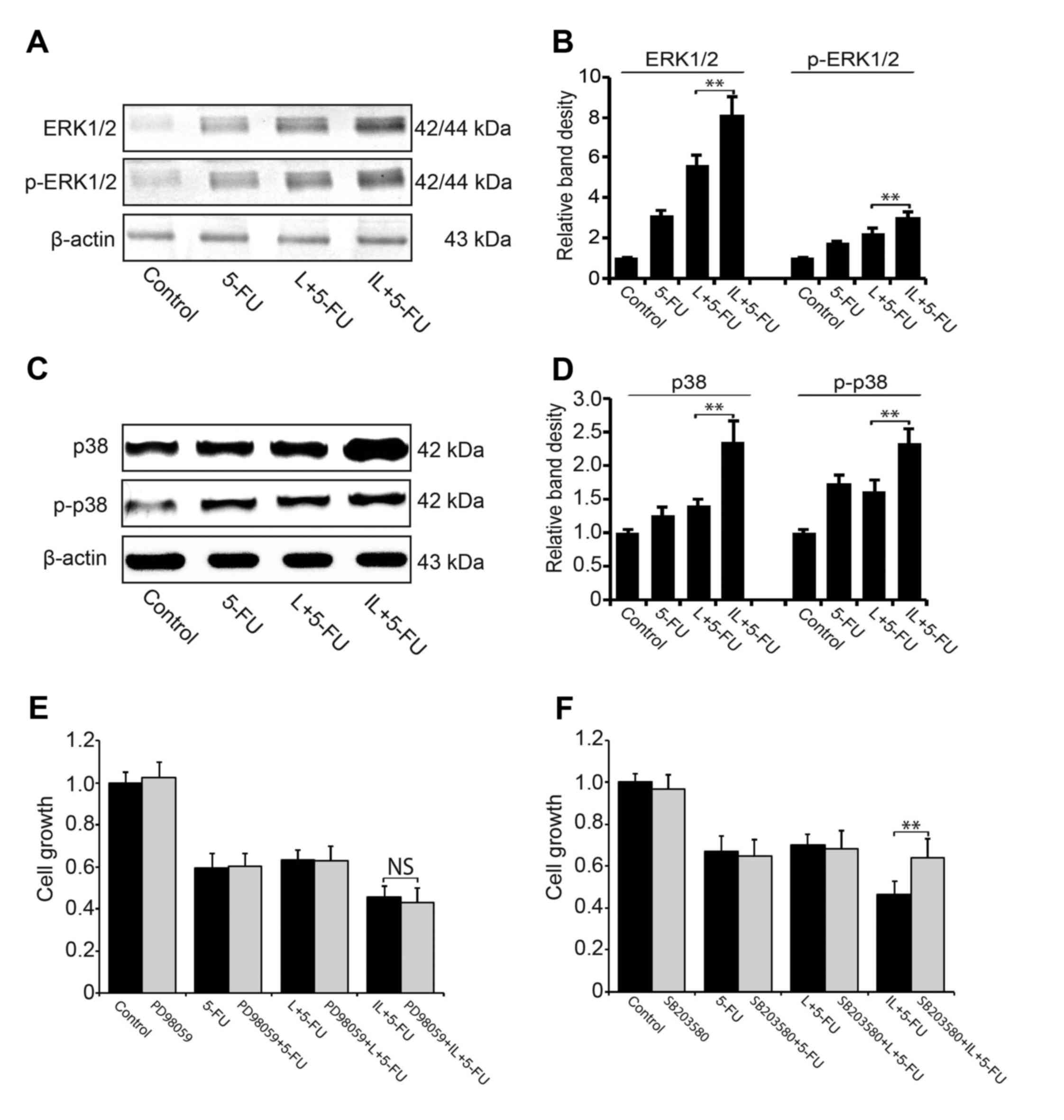
We also investigated the activity of ERK and p38MAPK
pathways in 5-FU chemotherapy. Using western blotting, we
demonstrated that compared to 5-FU or single dose LDIR plus 5-FU,
the pretreatment of intermittent LDIR increased the expression of
ERK/p-ERK (Fig. 5A and B) and
p38/p-p38 significantly (Fig. 5C and
D). When the p38MAPK pathway was blocked by SB203580,
intermittent LDIR-induced cell proliferation inhibition in 5-FU
chemotherapy was reversed (Fig.
5E). However, ERK pathway inhibitor PD98059 could not reverse
the proliferation inhibition induced by intermittent LDIR in
chemotherapy (Fig. 5F).
Discussion
Although surgery and chemotherapy are two preferred
options in clinical treatment of CRC patients, multimodal treatment
approaches are advocated for the therapy of CRC patients in
advanced stage. 5-FU is still the mainstay therapy for patients
with advanced CRC, but only <25% of patients with advanced CRC
show major responses after 5-FU-based chemotherapy. Consequently,
resistance to this drug is a major obstacle in CRC chemotherapy
(10). Radiotherapy is commonly
used to treat multi-tumors to attenuate the risk of recurrence.
Growing evidence from clinical trial has proved that an addition of
radiotherapy to exclusively surgical treatment of rectal cancer can
improve the patient prognosis (11). However, despite impressive initial
clinical responses, a large proportion of patients experience
resistance to radiotherapy.
In this study, in order to overcome radio- and
chemo-resistance in the treatment of CRC, a novel combination use
of intermittent LDIR with HDIR radiotherapy or 5-FU based
chemotherapy was tentatively investigated, and the effectiveness of
the pretreatment of LDIR was evaluated. Interestingly, our findings
suggest that intermittent LDIR could significantly enhance the
sensitivity of the following radiotherapy and chemotherapy. Cell
growth assays showed that the pretreatment of intermittent LDIR
obviously increased the killing ability of HDIR as well as
anticancer drug via a strong induction of cell apoptosis. Then we
investigated the involved mechanism on molecular level. Both
western blotting and RT-PCR results revealed that intermittent LDIR
pretreatment stimulated the expression of cell cycle arrest and
cell apoptotic genes, and it activated ATM/p53 pathway in
radiotherapy and p38MAPK pathway in chemotherapy more efficiently
than single dose LDIR. When we treated HT-29 cells with chemical
inhibitors of ATM/p53 and p38MAPK pathway to block the cell
signaling, the cell growth inhibition induced by the combination
therapy strategy was abolished.
5-FU is widely used as the first-line systemic
chemotherapy drug. It is an analog of uracil and exerts its
anticancer effects through blocking the normal synthesis of DNA and
disrupting RNA processing, and eventually lead to apoptosis in the
cancer cells (12,13). So far, various causes have been
found to contribute to 5-FU resistance, such as activation of the
JNK pathway (14), overexpression
of Bcl-2 and Bcl-X (15),
therapy-induced autophagy (16),
and MAPK pathway (17). de la
Cruz-Morcillo et al demonstrated that inhibition of p38MAPK
correlates with a decrease in the 5-FU-associated apoptosis and
chemical resistance in colorectal cancer cells (17). Our study is in agreement with de la
Cruz-Morcillo et al; both the two studies proved that it is
the p38MAPK rather than the other signaling pathways, e.g., ERK
pathway, that controls the cellular response to 5-FU.
ATM and p53 are two of the most important genes that
are involed in cellular response to radiotherapy and chemotherapy.
Yang et al found that the activation of ATM was the
initiating event in LDR induced hormesis and adaptive response, and
the distinct activation of ATM/AKT/GSK-3β signaling pathway may
explain the differential biological effects between lung cancer
cells and normal lung epithelial cells (18). Brazina et al showed that the
interplay between ATM, p53 and DAXX plays a key role in the
regulation of ionizing radiation or genotoxic drug-induced DNA
damage (19). Lin et al
found that in chronic lymphocytic leukemia, ATM/p53/p21 pathway
defects are strongly associated with chemoresistance or early
relapse (20). In our study, a
marked upregulation of ATM and p53 was observed after the combined
use of intermittent LDIR and HDIR radiotherapy, and we confirmed
that the activation of ATM/p53 pathway is a crucial event in the
regulation of cell apoptosis during the radiotherapy of HT-29
cells.
In conclusion, this study demonstrates that a
combination use of intermittent LDIR and HDIR or 5-FU is a valuable
method in promoting the therapeutic effectiveness of radiotherapy
and chemotherapy, and this effect is dependent on the activation of
ATM/p53 and p38MAPK pathways on molecular level, which controls the
cell cycle progression and cell apoptosis. Of importance, since
tumor cells are more likely to be genetically heterogeneous,
different cells with different genetic or epigenetic backgrounds
may respond distinctly to the same kind of radiation even at the
same dose level.
References
|
1
|
Su XL, Wang YF, Li SJ, Zhang F and Cui HW:
High methylation of the SEPT9 gene in Chinese colorectal cancer
patients. Genet Mol Res. 13:2513–2520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang XD, Xu XH, Zhang SY, Wu Y, Xing CG,
Ru G, Xu HT and Cao JP: Role of miR-100 in the radioresistance of
colorectal cancer cells. Am J Cancer Res. 5:545–559.
2015.PubMed/NCBI
|
|
3
|
Tang FR and Loke WK: Molecular mechanisms
of low dose ionizing radiation-induced hormesis, adaptive
responses, radioresistance, bystander effects, and genomic
instability. Int J Radiat Biol. 91:13–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo M and Dou J: Advances and perspectives
of colorectal cancer stem cell vaccine. Biomed Pharmacother.
76:107–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luckey TD: Physiological benefits from low
levels of ionizing radiation. Health Phys. 43:771–789. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feinendegen LE: Evidence for beneficial
low level radiation effects and radiation hormesis. Br J Radiol.
78:3–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olivieri G, Bodycote J and Wolff S:
Adaptive response of human lymphocytes to low concentrations of
radioactive thymidine. Science. 223:594–597. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang G, Li W, Jiang H, Liang X, Zhao Y, Yu
D, Zhou L, Wang G, Tian H, Han F, et al: Low-dose radiation may be
a novel approach to enhance the effectiveness of cancer
therapeutics. Int J Cancer. 139:2157–2168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang X, Gu J, Yu D, Wang G, Zhou L, Zhang
X, Zhao Y, Chen X, Zheng S, Liu Q, et al: Low-dose radiation
induces cell proliferation in human embryonic lung fibroblasts but
not in lung cancer cells: Importance of ERK1/2 and AKT signaling
pathways. Dose Response. 14:15593258156221742016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin YK, Yoo BC, Hong YS, Chang HJ, Jung
KH, Jeong SY and Park JG: Upregulation of glycolytic enzymes in
proteins secreted from human colon cancer cells with 5-fluorouracil
resistance. Electrophoresis. 30:2182–2192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hafner MF and Debus J: Radiotherapy for
colorectal cancer: Current standards and future perspectives. Visc
Med. 32:172–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grem JL: 5-Fluorouracil: Forty-plus and
still ticking. A review of its preclinical and clinical
development. Invest New Drugs. 18:299–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui X, Kong N, Wang X, Fang Y, Hu X, Xu Y,
Chen W, Wang K, Li D, Jin W, et al: JNK confers 5-fluorouracil
resistance in p53-deficient and mutant p53-expressing colon cancer
cells by inducing survival autophagy. Sci Rep. 4:46942014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Violette S, Poulain L, Dussaulx E, Pepin
D, Faussat AM, Chambaz J, Lacorte JM, Staedel C and Lesuffleur T:
Resistance of colon cancer cells to long-term 5-fluorouracil
exposure is correlated to the relative level of Bcl-2 and Bcl-X(L)
in addition to Bax and p53 status. Int J Cancer. 98:498–504. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY,
Hung SC, Hsiao M, Yao CJ and Shieh MJ: Autophagy promotes
resistance to photodynamic therapy-induced apoptosis selectively in
colorectal cancer stem-like cells. Autophagy. 10:1179–1192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de la Cruz-Morcillo MA, Valero ML,
Callejas-Valera JL, Arias-González L, Melgar-Rojas P, Galán-Moya
EM, García-Gil E, García-Cano J and Sánchez-Prieto R: P38MAPK is a
major determinant of the balance between apoptosis and autophagy
triggered by 5-fluorouracil: Implication in resistance. Oncogene.
31:1073–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang G, Yu D, Li W, Zhao Y, Wen X, Liang
X, Zhang X, Zhou L, Hu J, Niu C, et al: Distinct biological effects
of low-dose radiation on normal and cancerous human lung cells are
mediated by ATM signaling. Oncotarget. 7:71856–71872.
2016.PubMed/NCBI
|
|
19
|
Brazina J, Svadlenka J, Macurek L, Andera
L, Hodny Z, Bartek J and Hanzlikova H: DNA damage-induced
regulatory interplay between DAXX, p53, ATM kinase and Wip1
phosphatase. Cell Cycle. 14:375–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin K, Adamson J, Johnson GG, Carter A,
Oates M, Wade R, Richards S, Gonzalez D, Matutes E, Dearden C, et
al: Functional analysis of the ATM-p53-p21 pathway in the LRF CLL4
trial: Blockade at the level of p21 is associated with short
response duration. Clin Cancer Res. 18:4191–4200. 2012. View Article : Google Scholar : PubMed/NCBI
|