Introduction
Lung cancer is one of the most common human cancers,
and with the highest worldwide incidence and mortality (1). Adenocarcinoma is one of the more
common pathological types (2).
Despite advances in surgery, radiotherapy and targeted treatment,
survival is far from satisfactory. Most patients are given
paclitaxel (PTX)-based combination chemotherapy as a standard
treatment (3). Paclitaxel is a
taxane, and its cytotoxic effect depends on microtubule
polymerization, and inhibition of microtubule depolymerization
leads to a cell cycle block in the G2/M phase resulting in tumor
cell apoptosis or necrosis (4).
However, eventual development of resistance to paclitaxel is
inevitable and leads to treatment failure. The paclitaxel mechanism
of resistance is multifactorial and complex, involving changes in
drug-efflux, increased drug metabolism, interference with DNA
repair and cell cycle regulation, and disorders of cell apoptosis
and autophagy (5,6). Despite our knowledge of its anticancer
effects, little is known concerning the development of paclitaxel
resistance. A broadening of our understanding is essential for
improving treatment outcomes.
Much of the human genome is transcribed as long
non-coding RNAs (lncRNAs), a type of ncRNA 200 nt in length that is
not translated, but does influence the regulation of gene
expression and chemotherapy resistance in various ways (7,8). For
example, lncRNA n375709 expression was significantly increased in
paclitaxel-resistant CNE-2 nasopharyngeal carcinoma compared with
parental CNE-2 cells (9). Knockdown
of AK126698 activated the canonical Wnt signaling pathway and
induced cisplatin resistance in A549 human lung cancer cells
(10). lncRNA MEG3 overexpression
in drug resistant A549/DDP cells increased their chemosensitivity
to cisplatin both in vitro and in vivo by inhibiting
cell proliferation and inducing apoptosis (11). Upregulation of lncRNA GAS5 overcame
resistance of human lung adenocarcinoma cells to epidermal growth
factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy
(12). Other lncRNAs, such as
HOTTIP, NEAT1, PVT1 and ODRUL have also been reported to modulate
chemosensitivity (13–16). It has thus been established that the
upregulation and downregulation of lncRNAs is implicated in
chemotherapy resistance.
To the best of our knowledge, there have been no
studies concerning the roles of lncRNAs in the acquisition of
paclitaxel resistance in lung adenocarcinoma. We investigated
differential expression of lncRNAs and mRNAs in
paclitaxel-sensitive A549 and paclitaxel-resistant A549/PTX cells
using microarray assays. Nine differentially expressed lncRNAs and
six mRNAs were randomly selected for validation by real-time
quantitative PCR (RT-qPCR). Subsequent bioinformatics evaluation by
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analyses identified classical biological regulatory
functions and pathways that were differentially expressed in these
cell lines. Our results add to the knowledge concerning the
involvement of lncRNAs in the resistance to paclitaxel-based
chemotherapy in human lung adenocarcinoma cells, and thus, may
provide novel molecular therapeutic targets.
Materials and methods
Cell lines and cell culture
The A549 human lung adenocarcinoma cell line was
obtained from the Cell Bank of the Shanghai Branch of the Chinese
Academy of Sciences. A549/PTX cells were established in our
laboratory in a stepwise manner by exposing drug-sensitive A549
cells to increasing doses of paclitaxel (PTX; Bristol-Myers Squibb,
New York, NY, USA). The cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (both from Gibco, Carlsbad, CA, USA) at
37°C in humidified incubator with 5% CO2. The A549/PTX
cell culture medium also contained 200 ng/ml PTX to maintain the
drug-resistant phenotype. Cells in the logarithmic phase of growth
were used in all experimental procedures.
In vitro drug sensitivity assay
To evaluate differences in chemoresistance, cells
were seeded into 96-well plates at 5×103 cells/well and
incubated with various concentrations of PTX for 48 h. At 48 h, the
number of viable cells was assayed with Cell Counting Kit-8 (CCK-8;
Dojindo Laboratories, Kumamoto, Japan). CCK-8 reagent (10 µl) was
added to each well and incubation followed for 1 h at 37°C. The
number of viable cells was estimated by assessment of the optical
density (OD) at 450 nm, and the PTX concentration that produced 50%
inhibition of growth (IC50) was estimated from the
relative survival curves. Three independent experiments were
performed in five duplicate wells.
RNA extraction and RNA quality
control
Total RNA was extracted using TRIzol reagent
(Takara, Otsu, Japan) following the manufacturer's protocol. RNA
quantity and quality were assessed using a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and RNA integrity was assessed by electrophoresis on a
denaturing agarose gel. Isolated RNAs were stored at −80°C prior to
lncRNA microarray analysis and RT-qPCR.
lncRNA microarray
The GeneChip Human Transcriptome Array 2.0
(Affymetrix, Santa Clara, CA, USA) contains >6.0 million
distinct probes of both coding and non-coding transcripts.
Approximately 25,000 lncRNAs and 24,500 mRNAs listed in databanks
including RefSeq, Ensembl, UCSC (known genes and lincRNA
transcripts), NONCODE, the Human Body Map lincRNA and TUCP catalog,
and lncRNAdb, were detected.
RNA labeling and microarray
hybridization
RNA labeling and microarray hybridization were
performed following an Affymetrix GeneChip protocol from GMINIX
BioTech (Shanghai, China). Briefly, total RNA was purified after
removal of rRNA and tRNA. Each sample was amplified and transcribed
into fluorescent cRNA along the entire length of the transcript,
without the 3 bias, using a random priming method. After
purification, the labeled cRNAs were hybridized at 45°C for 16 h in
an Affymetrix hybridization oven. After being washed, the
hybridized arrays were scanned using Affymetrix GeneChip Scanner
3000, and data were extracted using Transcriptome Analysis Console
Software.
Validation of aberrantly expressed
lncRNAs and mRNAs by RT-qPCR
Total RNA was extracted using TRIzol reagent, and
was then reverse-transcribed using a PrimeScript RT reagent kit
with gDNA Eraser (Perfect Real Time; Takara) following the
manufacturer's instructions. Reactants were incubated for 2 min at
42°C, 15 min at 37°C, 5 sec at 85°C, 7 min at 4°C, and then stored
at −20°C. The transcripts of nine differentially expressed lncRNAs
and six mRNAs were randomly selected for RT-qPCR using a SYBR-Green
assay (Takara, Dalian, China); GAPDH was used as an internal
control. Specific lncRNA and mRNA primers were designed using
Primer 5.0. The RT-qPCR reaction was set at an initial denaturation
step of 10 min at 95°C followed by 40 cycles of 95°C for 10 sec and
60°C for 1 min. All experiments were performed three times, and
fold changes of expression were calculated using the
2−ΔΔCt method.
Bioinformatics analysis
Affymetrix Transcriptome Analysis Console software
was used to analyze the acquired array images. Differentially
expressed lncRNAs and mRNAs were identified through fold-change
filtering. GO categories (www.geneontology.org) and KEGG pathway analyses
(http://www.genome.jp/kegg/) were
performed using the standard enrichment computation method.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 software (SPSS, Inc., Chicago, IL, USA). Results are
presented as the means ± standard deviation (SD) of three separate
assays. Differences between groups were assessed by two tailed
t-tests. P<0.05 was considered as statistically significant.
Results
CCK-8 assay of in vitro drug
sensitivity
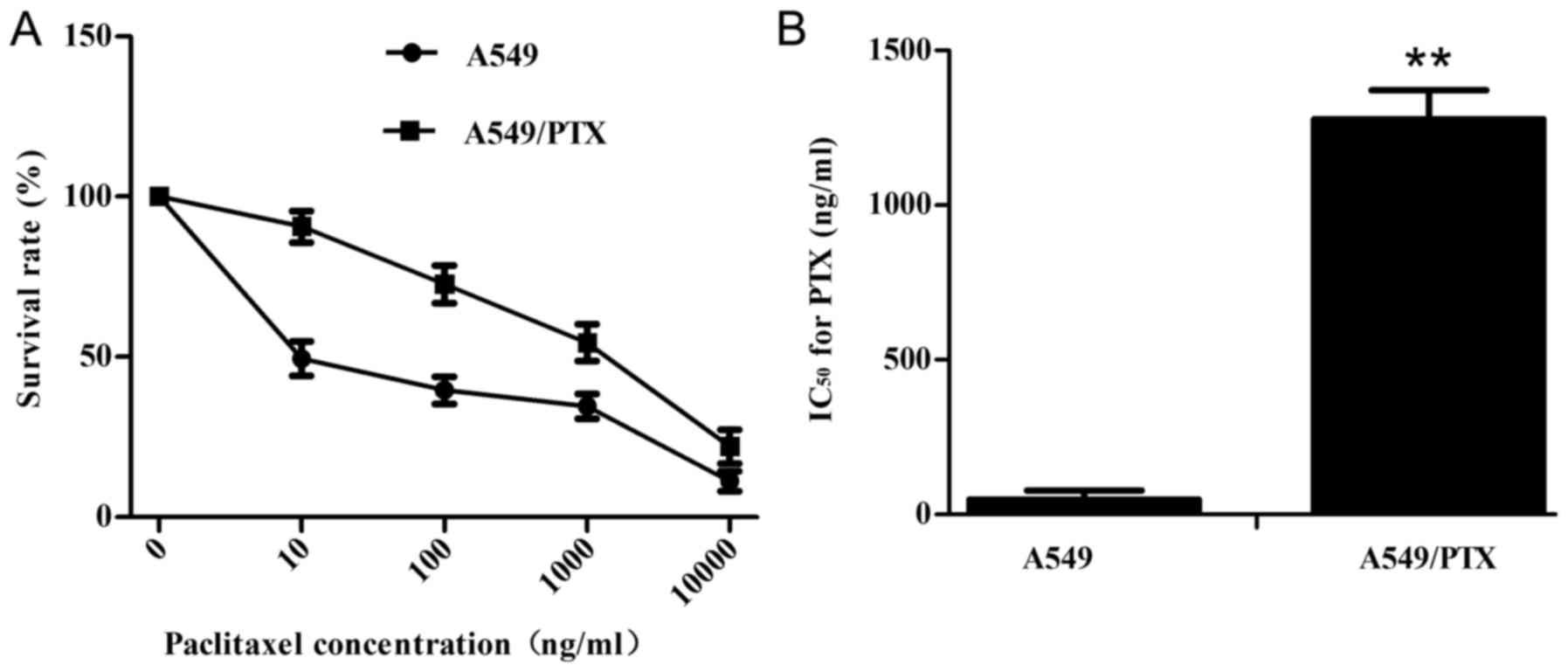
The paclitaxel-resistance of A549/PTX cells was
characterized by determining the IC50 value. After
treatment with various concentrations of paclitaxel for 48 h in
A549 and A549/PTX cells, the cell survival was assayed using CCK-8
(Fig. 1A). The paclitaxel
IC50 value for the drug-resistant A549/PTX cell line was
1240.12±6.13 and 18.21±0.84 ng/ml for the A549 cell line (Fig. 1B), with a drug-resistance index of
A549/PTX relative to A549 cells of 68 (P<0.01). A previously
reported criterion of high-resistance is an index >20 (17). The A549/PTX cells were revealed to
be more resistant to paclitaxel than the A549 cells.
RNA quality control
RNA quantification and quality were
spectrophotometrically assayed using the NanoDrop ND-1000. The OD
A260/A280 ratio of the total RNA was between 1.8 and 2.1. RNA
integrity was assessed by electrophoresis on a denaturing agarose
gel. The 28s and 18s ribosomal RNA bands were fairly sharp and
intense, and the 28s rRNA band was about twice as intense as the
18s rRNA band. The lncRNA and mRNA samples from the A549 and
A549/PTX cells were found to be suitable for microarray expression
profiling.
Differentially expressed lncRNAs and
mRNAs
The Affymetrix GeneChip Human Transcriptome Array
2.0 was used for profiling both parental A549 and A549/PTX cells.
For microarray analysis, ~25,000 lncRNAs and 24,500 mRNAs were
detected. After quantile normalization and data filtering, we found
a >3-fold difference in the expression of 1,154 lncRNAs and
1,733 mRNAs in the A549/PTX cells compared with the A549 cells. Of
the 1,154 differentially expressed lncRNAs, 119 were upregulated
and 1,035 were downregulated (P<0.01); 28 of the mRNAs were
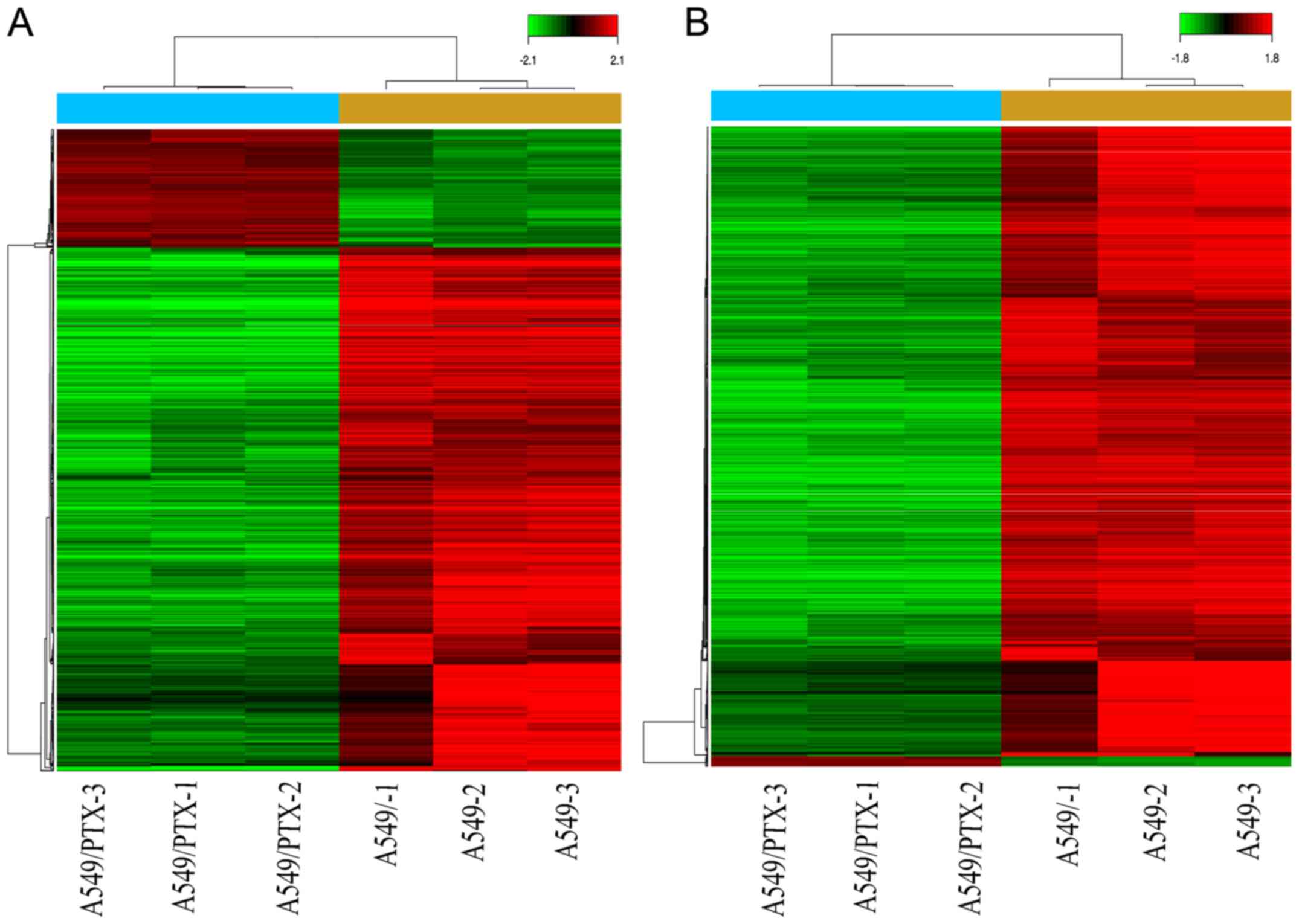
upregulated and 1,705 were downregulated (P<0.01). The
hierarchical cluster analysis of the expression of the lncRNAs and
mRNAs that were identified is shown in Fig. 2. The differentially expressed
lncRNAs and mRNAs are listed in Table
I, and the 10 lncRNAs and mRNAs with the greatest relative
upregulation or downregulation are shown in Tables II and III. lncRNA n334075 (fold change=8.77)
was the most significantly upregulated and lncRNA n335556 (fold
change=−308.61) was the most significantly downregulated. Norrin
cystine knot growth factor (NDP) mRNA (fold change=6.75) was the
most significantly upregulated and histone cluster 2 H2A family
member a4 (HIST2H2AA4) (fold change=−99.71) was the most
significantly downregulated. Downregulated lncRNAs and mRNAs were
more common than upregulated lncRNAs and mRNAs in our microarray
data.
 | Table I.Number of differentially expressed
lncRNAs and mRNAs. |
Table I.
Number of differentially expressed
lncRNAs and mRNAs.
| lncRNAs and
mRNAs | Fold change
>3 | Total |
|---|
| lncRNAs |
| 1,154 |
|
Upregulation | 119 |
|
|
Downregulation | 1,035 |
|
| mRNAs |
| 1,733 |
|
Upregulation |
28 |
|
|
Downregulation | 1,705 |
|
 | Table III.Ten most significantly upregulated
and downregulated mRNAs. |
Table III.
Ten most significantly upregulated
and downregulated mRNAs.
| Upregulated in
A549/PTX | Downregulated in
A549/PTX |
|---|
|
|
|---|
| mRNAs | Fold change | mRNAs | Fold change |
|---|
| NDP | 6.75 | HIST2H2AA4 | −99.71 |
| TNF | 6.12 | ALDH1A1 | −92.66 |
| DDR2 | 5.76 | FTL | −77.03 |
| WNT6 | 5.69 | PTPLAD1 | −76.91 |
| NCAM1 | 4.58 | CYP24A1 | −73.79 |
| LOXL1 | 4.47 | AKR1C2 | −58.71 |
| KRTAP5-6 | 3.42 | NQO1 | −55.83 |
| IGLV7-43 | 3.39 | NETO2 | −46.82 |
| IGHV3-48 | 3.38 | RSL1D1 | −46.32 |
| SPPL2C | 3.34 | HIST1H1E | −41.36 |
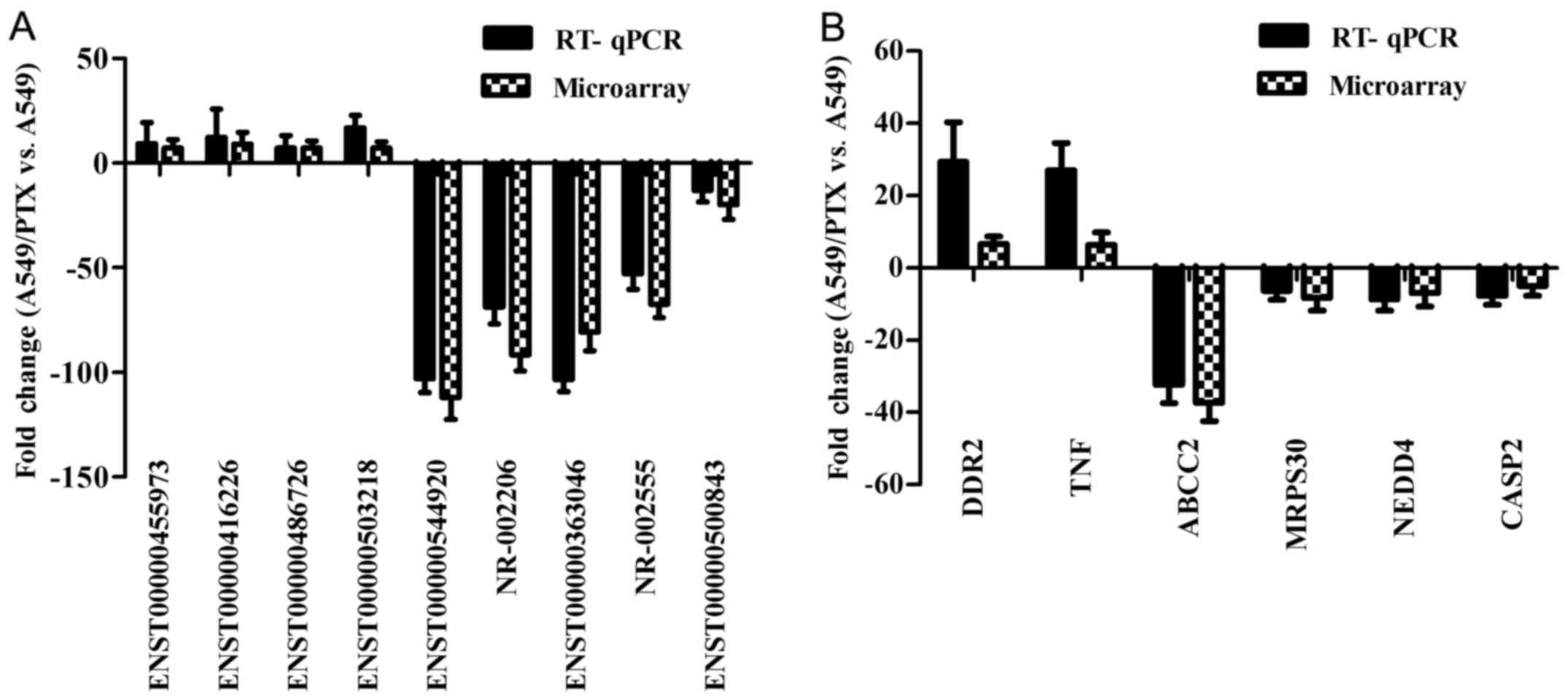
RT-qPCR validation of microarray
data
To validate the microarray data, nine differentially
expressed lncRNAs and six mRNAs were randomly selected for RT-qPCR
assay of RNA isolated from both resistant A549/PTX and sensitive
A549 cells. The primers used for PCR validation are listed in
Table IV. As shown in Fig. 3, the expression of lncRNA
ENST00000455973, ENST00000416226, ENST00000486726 and
ENST00000503218 was upregulated and the expression of
ENST00000544920, NR-002206, ENST00000363046, NR-002555 and
ENST00000500843 was downregulated. The expression of DDR2 and TNF
mRNA was upregulated and the expression of ABCC2, MRPS30, NEDD4,
and CASP2 was downregulated. The RT-qPCR results were thus,
consistent with the microarray data.
 | Table IV.Primers used for RT-qPCR
validation. |
Table IV.
Primers used for RT-qPCR
validation.
| lncRNAs and
mRNAs | Forward primer
sequence | Reverse primer
sequence |
|---|
|
ENST00000363046 |
GGACTCTGTTCCTCCCCTTTC |
GAGCCCCGTGTGGTTGG |
|
ENST00000500843 |
CCTGGCTGAGGTGAATAA |
TTGGACCCGAACATCTG |
|
ENST00000544920 |
AAAGATGAGGCAGAGGTCCAAG |
CGATCAGAGGGCGATGAAG |
| NR-002555
(LOC613037) |
GGGCAGAGGACTACCACAAATG |
TGTTGTTGAGTTGGAGGAGGTG |
| NR-002206
(GTF2IP1) |
GCTGTGTGGTGGTTGATGG |
CTCTTTTATTTCTTCTGTGGCTGGA |
|
ENST00000455973 |
GATGTGGGAAACAGTGGC |
GTAAGGCAGCAGGGAGG |
|
ENST00000416226 |
AGGAGAAACTCATCAGGC |
ATCTCTTCTACGGTGGCT |
|
ENST00000486726 |
CCTGTCTGGTGTCCTTGC |
CAGCAGGAGAGGCATCAG |
|
ENST00000503218 |
GCAAGTGAAGCCTGATACC |
AAAGCGTCTGTGAGCCTAA |
| TNF |
GTGACAAGCCTGTAGCCCATGTT |
TTATCTCTCAGCTCCACGCCATT |
| DDR2 |
CCCAGCTGTCAGATGAACAGGTTA |
TCAGGACAAATGGCTGGTTGAG |
| CASP2 |
TGGCATGCATCCTCATCATC |
TCTGGCTGAAACTGCCCACT |
| ABCC2 |
AGTGATCACCATCGCCCACA |
GTTCACATTCTCAATGCCAGCTTC |
| MRPS30 |
CGAACCCGAACCTGAACCT |
GATATGACCTCGCTCTCCTCGT |
| NEDD4 |
TGAAGCCCAATGGGTCAGAAATA |
GGACCCTGTTCACAAATCTCCAC |
| GAP |
TGCACCACCAACTGCTTAGC |
GGCATGGACTGTGGTCATGAG |
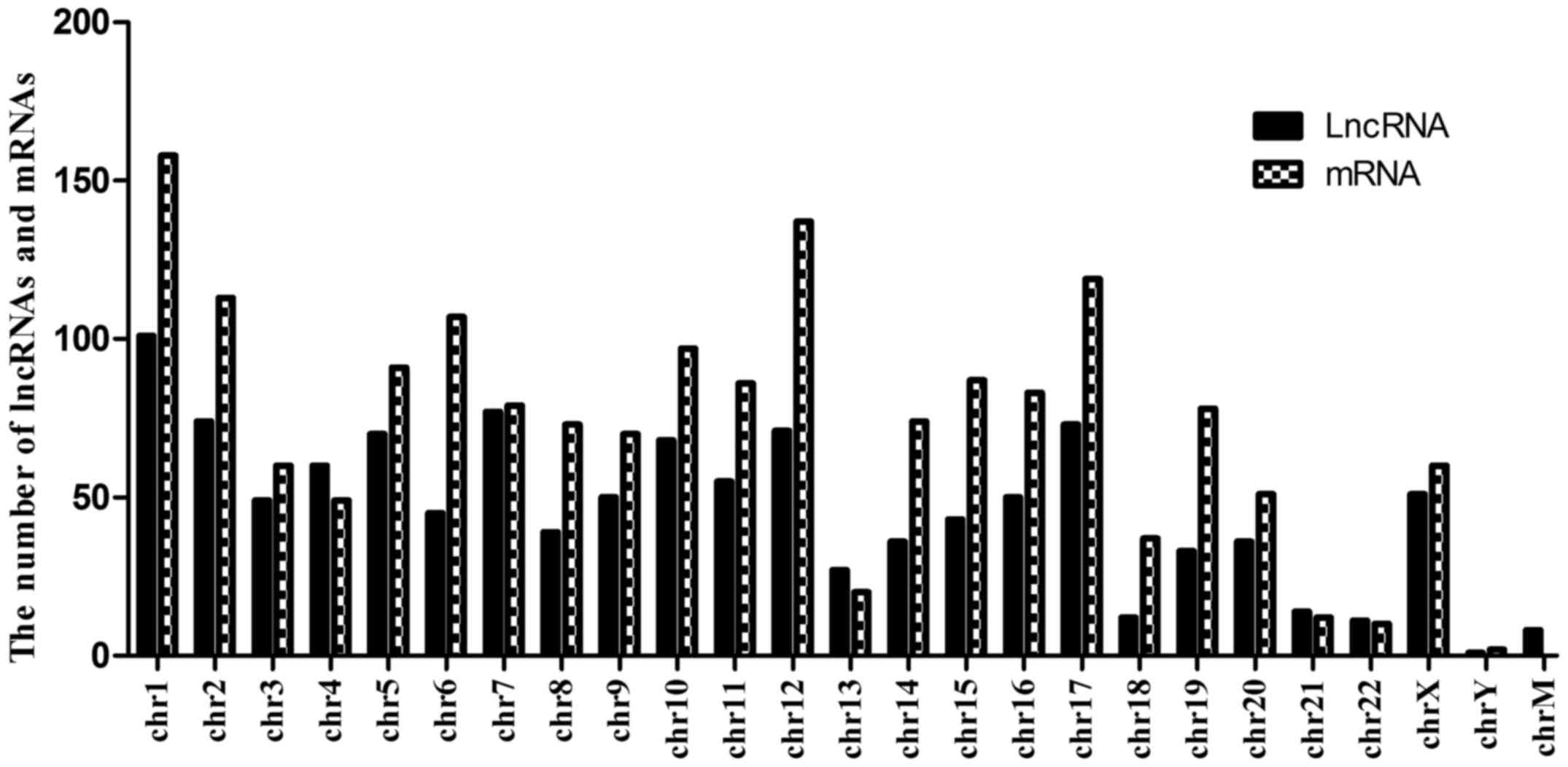
Chromosomal distribution of
differentially expressed lncRNAs and mRNAs
Since chromosomal imbalances have been associated
with drug resistance, a distribution plot of chromosomal location
was developed to show the chromosomal locations of the
differentially expressed lncRNAs and mRNAs. As shown in Fig. 4, the 1,154 lncRNAs and 1,733 mRNAs
were distributed throughout the genome, and were associated with
every chromosome. In the present study, aberrantly expressed
lncRNAs and mRNAs were most frequently found on chromosomes 1, 2,
6, 12 and 17, however, particularly on chromosome 1.
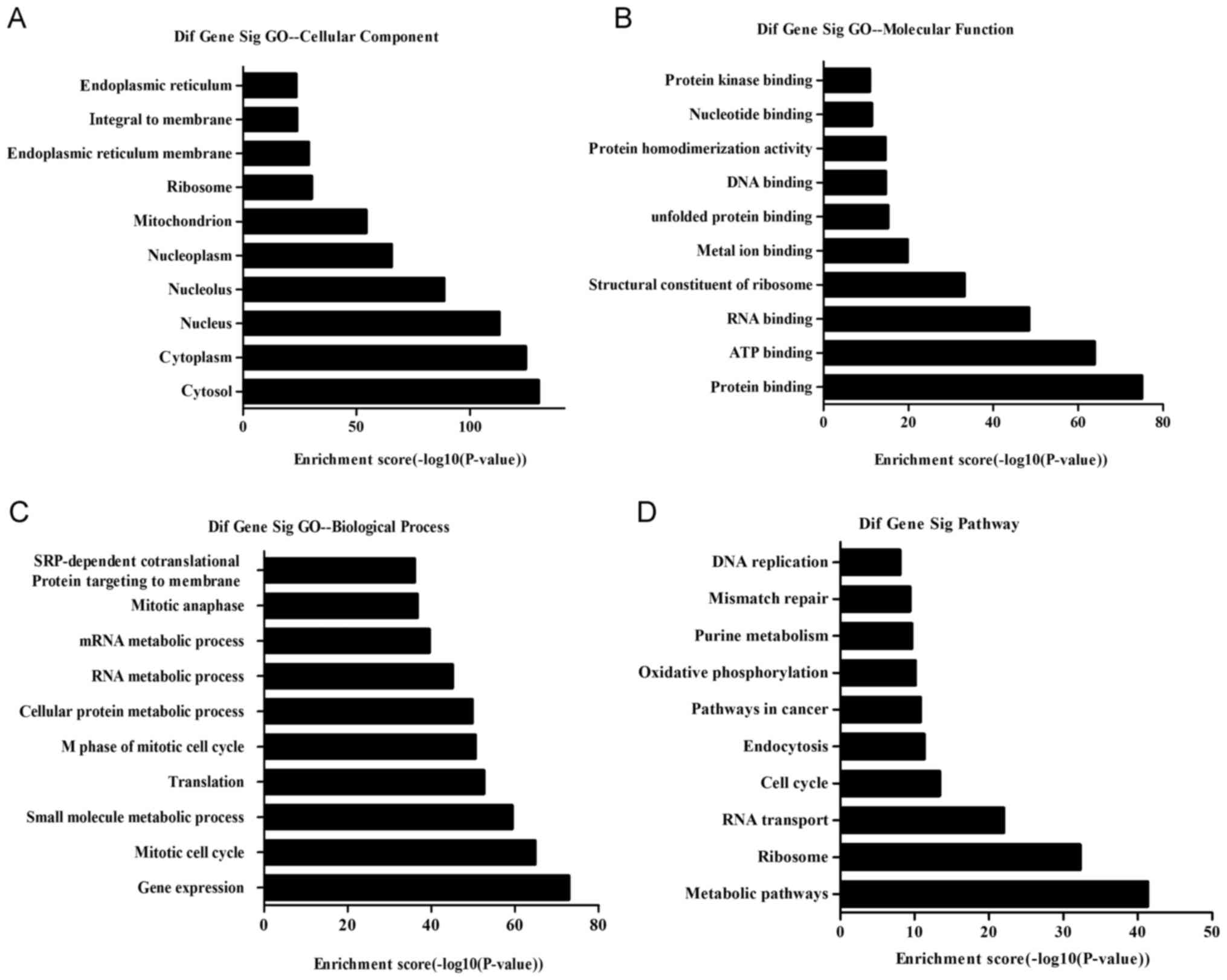
GO and KEGG pathway analyses
GO analysis was applied to identify the functions of
the differentially expressed mRNAs. The GO database includes the
primary functional classifications of the National Center for
Biotechnology Information (NCBI), and includes three categories,
i.e., cellular components, molecular functions and biological
processes. The microarray data obtained in the present study
revealed that the differentially expressed genes were enriched for
GO terms related to the cytosol, cytoplasm, nucleus and nucleolus
(cellular components); protein, ATP and RNA bindings, and
structural constituents of ribosomes (molecular functions); and
gene expression, mitotic cell cycle, small-molecule metabolic
process, and translation (biological processes). The top 10 GO
terms within each of the three categories, cellular components,
molecular functions and biological processes, are shown in Fig. 5A-C.
We conducted pathway analysis of the differentially
expressed mRNAs using the latest KEGG database, which identified
the biological pathways involved in paclitaxel resistance. We found
96 pathways corresponding to aberrant mRNAs, and the predominant
pathways are shown in Fig. 5D. The
top 10 pathways were metabolic, ribosomal, RNA transport, cell
cycle, endocytosis, pathways in cancer, oxidative phosphorylation,
purine metabolism, mismatch repair and DNA replication.
Discussion
Paclitaxel is the standard and most effective
chemotherapy drug used for lung adenocarcinoma, but drug resistance
is a major clinical obstacle that limits its clinical benefits
(18). Although some molecular
mechanisms of paclitaxel resistance in various types of cancers
have been reported in the past few decades (19), the precise cause of paclitaxel
resistance in lung adenocarcinoma remains unclear. Research is
urgently warranted to fully understand and overcome it. Evidence
that lncRNAs are involved in chemotherapy resistance is increasing
(20–22), but to the best of our knowledge
there is little data on the correlations between lncRNAs and
paclitaxel resistance in human lung adenocarcinoma.
To investigate the regulatory effects of lncRNAs in
paclitaxel resistance of lung adenocarcinoma, we established a
paclitaxel-resistant A549/PTX cell line and assayed its PTX
resistance. Microarray expression profiling of lncRNAs and mRNAs in
parental A549 and paclitaxel resistant A549/PTX cells identified a
total of 1,154 differentially expressed lncRNAs. We also found
aberrant expression of 1,733 mRNAs in A549/PTX cells compared with
the parental A549 cells. Various of the differentially expressed
lncRNAs and mRNAs including, MALAT1, TUG1, HOTAIR, ABCB1 (MDR1) and
Wnt6 have been previously reported in other chemoresistant cancers
(23–27). Whether the molecular mechanisms of
chemoresistance are the same as in the A549/PTX cells warrants
further investigation. However, in our screening results, the 10
predominantly upregulated and downregulated lncRNAs have not been
previously related to treatment resistance. Consequently, their
mechanism of resistance regulation and their function in resistant
cells are not clear. lncRNAs regulate neighboring protein-coding
genes (28), and we found that some
of the aberrantly expressed lncRNAs may play important roles in
paclitaxel resistance by regulating nearby coding genes. For
example, upregulated lncRNA ENST00000447028 was found to be located
near VEGF-B mRNA. VEGF-B mRNA was reported by Yang et al to
take part in the resistance of lung cancer cells to the
chemotherapeutic drug EGFR-TKI (29). We found that VEGF-B mRNA was
strongly upregulated, therefore, ENST00000447028 may influence
paclitaxel resistance by regulating VEGF-B mRNA. Another
upregulated lncRNA, ENST00000416226, was found to be located near
Wnt6 mRNA, which was upregulated in our results, and is involved in
both oncogenesis and chemoresistance in human cancers (30). We thus, speculate that lncRNAs may
influence paclitaxel resistance in human lung adenocarcinoma by
regulating the expression of their nearby coding genes. Further
studies of the effect of overexpression or knockdown of lncRNAs and
western blot analyses should be performed to investigate the
precise relationships.
We found that the aberrantly expressed lncRNAs and
mRNAs were distributed throughout the genome and could be found in
every chromosome, particularly on chromosome 1. Perhaps, all the
chromosomes participate in paclitaxel resistance. The importance of
chromosome 1 in the occurrence of paclitaxel resistance in lung
adenocarcinoma warrants investigation.
The biological functions and signaling pathways
associated with the lncRNAs and mRNAs identified in the present
study, were evaluated by GO and KEGG pathway analyses. The most
enriched GOs were cytosol (cellular components), protein binding
(molecular function) and gene expression (biological processes). In
addition, many of the identified GO terms in our results have been
reported in other cancer chemoresistance studies. For example,
protein and nucleotide binding, and metabolic processes have been
reported to be involved in the chemoresistance of lung and
colorectal cancer (31). This
suggests that lncRNAs among those we identified may have regulated
chemoresistance of the A549/PTX cells by influencing the expression
of these GO database genes. Pathway analysis revealed a total of 96
pathways corresponding to all the differentially expressed mRNAs.
Some of the top 10 pathways that we identified have previously been
associated with chemoresistance. For example, Wu et al using
genome-wide microarrays found that the metabolic pathway
participated in EGFR-TKI resistance of lung adenocarcinoma
(32). Zhu et al reported
that the cell cycle pathway was associated with the development of
doxorubicin resistance in the MG63/DXR human osteosarcoma cell line
(33). Zhou et al found that
the DNA replication pathway contributed to the occurrence of
gemcitabine resistance in SW1990 pancreatic cancer cells (34). These data revealed that the
differentially expressed lncRNAs may regulate paclitaxel resistance
in lung adenocarcinoma through these classical pathways.
In conclusion, the present study reveals, for the
first time, numerous differentially expressed lncRNAs and mRNAs in
A549 and A549/PTX cells which may play an important role in
regulating paclitaxel resistance through various biological
functions and signaling pathways. We described a novel approach to
clarify the molecular mechanisms of paclitaxel resistance in human
lung adenocarcinoma. The results provide a novel rationale for
studies on reversal of paclitaxel resistance and for identifying
patients who can benefit the most from chemotherapy. Evaluation of
clinical samples for in vivo validation is warranted.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31571198), and the
Outstanding Scientific Fund of Shengjing Hospital (no. 201601).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A National Cancer Database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun QL, Sha HF, Yang XH, Bao GL, Lu J and
Xie YY: Comparative proteomic analysis of paclitaxel sensitive A549
lung adenocarcinoma cell line and its resistant counterpart
A549-Taxol. J Cancer Res Clin Oncol. 137:521–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khongkow P, Gomes AR, Gong C, Man EP,
Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US, et
al: Paclitaxel targets FOXM1 to regulate KIF20A in mitotic
catastrophe and breast cancer paclitaxel resistance. Oncogene.
35:990–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SH, Seong MA and Lee HY: p38
MAPK-induced MDM2 degradation confers paclitaxel resistance through
p53-mediated regulation of EGFR in human lung cancer cells.
Oncotarget. 7:8184–8199. 2016.PubMed/NCBI
|
|
6
|
Holleman A, Chung I, Olsen RR, Kwak B,
Mizokami A, Saijo N, Parissenti A, Duan Z, Voest EE and Zetter BR:
miR-135a contributes to paclitaxel resistance in tumor cells both
in vitro and in vivo. Oncogene. 30:4386–4398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirano T, Yoshikawa R, Harada H, Harada Y,
Ishida A and Yamazaki T: Long noncoding RNACCDC26, controls myeloid
leukemia cell growth through regulation of KIT expression. Mol
Cancer. 14:902015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Wu K, Yang Z, Zhao Q and Fan D, Xu
P, Nie Y and Fan D: Multidrug-resistance related long non-coding
RNA expression profile analysis of gastric cancer. PLoS One.
10:e01354612015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren S, Li G, Liu C, Cai T, Su Z, Wei M,
She L, Tian Y, Qiu Y, Zhang X, et al: Next generation deep
sequencing identified a novel lncRNA n375709 associated with
paclitaxel resistance in nasopharyngeal carcinoma. Oncol Rep.
36:1861–1867. 2016.PubMed/NCBI
|
|
10
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P,
Lu B, Liu G and Wang Z: The long noncoding RNA MEG3 contributes to
cisplatin resistance of human lung adenocarcinoma. PLoS One.
10:e01145862015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong S, Qu X, Li W, Zhong X, Li P, Yang S,
Chen X, Shao M and Zhang L: The long non-coding RNA GAS5, enhances
gefitinib-induced cell death in innate EGFR tyrosine kinase
inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR
via downregulation of the the via downregulation of the IGF-1R
expression. J Hematol Oncol. 8:432015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H,
Wang Y, Zeng J, Song Y, Gao W, et al: The long non-coding RNA
HOTTIP promotes progression and gemcitabine resistance by
regulating HOXA13 in pancreatic cancer. J Transl Med. 13:842015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao C, Zhang J, Wang Q and Ren C:
Overexpression of lncRNA NEAT1 mitigates multidrug resistance by
inhibiting ABCG2 in leukemia. Oncol Lett. 12:1051–1057.
2016.PubMed/NCBI
|
|
15
|
Ding J, Li D, Gong M, Wang J, Huang X, Wu
T and Wang C: Expression and clinical significance of the long
non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther.
7:1625–1630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CL, Zhu KP, Shen GQ and Zhu ZS: A
long non-coding RNA contributes to doxorubicin resistance of
osteosarcoma. Tumour Biol. 37:2737–2748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Snow K and Judd W: Characterisation of
adriamycin- and amsacrine-resistant human leukaemic T cell lines.
Br J Cancer. 63:17–28. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu R, Liu X, Zheng Y, Gu J, Xiong S,
Jiang P, Jiang X, Huang E, Yang Y, Ge D, et al: MicroRNA-7
sensitizes non-small cell lung cancer cells to paclitaxel. Oncol
Lett. 8:2193–2200. 2014.PubMed/NCBI
|
|
19
|
Chatterjee A, Chattopadhyay D and
Chakrabarti G: miR-17-5p downregulation contributes to paclitaxel
resistance of lung cancer cells through altering beclin1
expression. PLoS One. 9:e957162014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee H, Kim C, Ku JL, Kim W, Yoon SK, Kuh
HJ, Lee JH, Nam SW and Lee EK: A long non-coding RNA snaR
contributes to 5-fluorouracil resistance in human colon cancer
cells. Mol Cells. 37:540–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You L, Chang D, Du HZ and Zhao YP:
Genome-wide screen identifies PVT1 as a regulator of Gemcitabine
sensitivity in human pancreatic cancer cells. Biochem Biophys Res
Commun. 407:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang S, Gao H, Tong Y, Yang J, Tang R, Niu
Y, Li M and Guo L: Long noncoding RNA-HOTAIR affects
chemoresistance by regulating HOXA1 methylation in small cell lung
cancer cells. Lab Invest. 96:60–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiao F, Hu H, Han T, Yuan C and Wang L,
Jin Z, Guo Z and Wang L: Long noncoding RNA MALAT-1 enhances stem
cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci.
16:6677–6693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Zhang M, An G and Ma Q: LncRNA TUG1
acts as a tumor suppressor in human glioma by promoting cell
apoptosis. EExp Biol Med. 241:644–649. 2016. View Article : Google Scholar
|
|
25
|
Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W,
De W, Wang Z and Wang R: The long noncoding RNA HOTAIR contributes
to cisplatin resistance of human lung adenocarcinoma cells via
downregualtion of p21WAF1/CIP1 expression. PLoS One. 8:e772932013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aldonza MB, Hong JY, Bae SY, Song J, Kim
WK, Oh J, Shin Y, Lee SH and Lee SK: Correction: Suppression of
MAPK signaling and reversal of mTOR-dependent MDR1-associated
multidrug resistance by 21α-methylmelianodiol in lung cancer cells.
PLoS One. 10:e01278412015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng N, Li X, Zhao C, Ren S, Chen X, Cai
W, Zhao M, Zhang Y, Li J, Wang Q, et al: Microarray expression
profile of long non-coding RNAs in EGFR-TKIs resistance of human
non-small cell lung cancer. Oncol Rep. 33:833–839. 2015.PubMed/NCBI
|
|
29
|
Yang X, Zhang Y, Hosaka K, Andersson P,
Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y, et al:
VEGF-B promotes cancer metastasis through a VEGF-A-independent
mechanism and serves as a marker of poor prognosis for cancer
patients. Proc Natl Acad Sci USA. 112:E2900–E2909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su HY, Lai HC, Lin YW, Liu CY, Chen CK,
Chou YC, Lin SP, Lin WC, Lee HY and Yu MH: Epigenetic silencing of
SFRP5 is related to malignant phenotype and chemoresistance of
ovarian cancer through Wnt signaling pathway. Int J Cancer.
127:555–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiong W, Jiang YX, Ai YQ, Liu S, Wu XR,
Cui JG, Qin JY, Liu Y, Xia YX, Ju YH, et al: Microarray analysis of
long non-coding RNA expression profile associated with
5-fluorouracil-based chemoradiation resistance in colorectal cancer
cells. Asian Pac J Cancer Prev. 16:3395–3402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Y, Yu DD, Hu Y, Yan D, Chen X, Cao HX,
Yu SR, Wang Z and Feng JF: Genome-wide profiling of long non-coding
RNA expression patterns in the EGFR-TKI resistance of lung
adenocarcinoma by microarray. Oncol Rep. 35:3371–3386.
2016.PubMed/NCBI
|
|
33
|
Zhu KP, Zhang CL, Shen GQ and Zhu ZS: Long
noncoding RNA expression profiles of the doxorubicin-resistant
human osteosarcoma cell line MG63/DXR and its parental cell line
MG63 as ascertained by microarray analysis. Int J Clin Exp Pathol.
8:8754–8773. 2015.PubMed/NCBI
|
|
34
|
Zhou M, Ye Z, Gu Y, Tian B, Wu B and Li J:
Genomic analysis of drug resistant pancreatic cancer cell line by
combining long non-coding RNA and mRNA expression profling. Int J
Clin Exp Pathol. 8:38–52. 2015.PubMed/NCBI
|