Introduction
Telomeres are specific sequences that are composed
of tandem repeats of the TTAGGG at the end of human chromosomes
(1). The possible functions of the
non-coding DNA include the prevention of chromosome degradation,
end-to-end fusion, rearrangements and chromosome loss (2). In normal cells, telomere shortening
occurs with each cellular division. Telomerase is a reverse
transcriptase enzyme that is composed of a catalytic component,
telomerase RNA and a human telomerase reverse transcriptase (hTERT)
component (3). Telomerase activity
is undetectable in the majority of normal human somatic cells.
However, telomerase activity is maintained in stem/progenitor cells
in self-renewing tissues (4). In
addition, telomerase activation is observed in almost 90% of human
cancers, indicating that telomerase plays an important role in
cancer development (5).
Various transcriptional targeting strategies using
different tissue-specific promoters have been tested. The hTERT
promoter has been well characterized and has been shown to promote
the cancer cell-specific gene expression in a broad range of
malignancies (6–9). The hTERT promoter is widely applied in
cancer-specific gene therapy and imaging (10,11).
However, the use of the hTERT promoter is often limited because its
driving ability as a specific promoter is weak. Consequently, the
expression levels of the reporter gene and therapeutic genes are
insufficient (12–14). Thus, the modification of the hTERT
promoter-driven gene expression cassette is necessary for enhancing
the cancer-specific gene expression.
Gene expression promoters, such as the CMV
(cytomegalovirus), RSV (Rous sarcoma virus) and SV40 (simian virus
40) promoters, have been used to increase the expression of genes
in a variety of normal and cancer cells, and have achieved good
transduction efficiency (15).
Since the minimal element of a viral promoter is often used as a
basic transcriptional unit (16),
we modified the hTERT promoter by adding the CMV minimized promoter
and constructed a series of chimeric promoter-driven gene
expression cassettes. In the present study, we evaluated the extent
to which the novel cassettes enhanced gene expression and the
degree of hTERT promoter-dependency in various human cells.
Materials and methods
Cell culture
The following human cell lines were obtained from
the American Type Culture Collection (ATCC, Rockville, MD, USA) if
not notified: HEK293 (human embryonic kidney cell line), HeLa
(cervical cancer), PC3 (prostate adenocarcinoma), HepG2
(hepatocellular carcinoma), Hep3B (hepatocellular carcinoma),
HCT116 (colon cancer), RPTEC (normal renal proximal tubule cells;
Lonza, Basel, Switzerland), UMUC1 (bladder cancer), UMUC3 (bladder
cancer), HT1197 (bladder cancer), RT4 (bladder cancer), MCF7
(mammary gland adenocarcinoma), OUMS-24 (normal human fibroblasts;
kindly provided by Dr Masayoshi Namba), NHK (normal human
keratinocytes; Kurabo Industries Ltd., Osaka, Japan) and human iPS
(induced pluripotent stem) cells (Riken BCR, Tsukuba, Japan). These
cell lines were cultivated in D/F medium (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% FBS or cultivated using the medium
recommended by the supplier. The human iPS cells were cultured and
maintained as previously described (17).
Construction of the plasmid
vectors
We constructed new plasmids in which the promoter
consisted of the hTERT core promoter and the CMV minimized promoter
(hT/Cm). The series of modifications of the hT/Cm promoter-driven
construct are shown in Fig. 1B.
They were performed in the pDNR-1r promoter-less vector (Takara
Clontech, Mountain View, CA, USA). We finally developed a
(hT/Cm-R-hT) plasmid construct in which the following gene
expression elements were located in tandem: [hTERT core promoter,
CMV minimized promoter, RU5′ sequence (R), an inserted gene, BGH
polyA, hTERT enhancer (hT)] (Fig.
1B). The R segment and part of the U5 sequence (RU5′), BGH
(bovine growth hormone) polyadenylation (polyA) signal and the
sequences of multiple cloning sites were the same as those of
previous reports (15,18). The hTERT promoter element [189 bp:
Accession no. DQ264729 (1618–1806)] was used and inserted at either
the 5′-side alone or at both the 5′- and 3′-side of the cDNA in the
constructs. The minimal CMV sequence that was used in the present
study was as follows: GGTAGGCGTGTACGGTGGGAGGCCTATA
TAAGCAGAGCTCGTTTAGTGAACCGTCAGATCGCCT
GGAGACGCCATCCACGCTGTTTTGACCTCCATAGAA
GACACCGGGACCGATCCAGCCTCCGCGGCCCCGCA TTCGAGCTCGGTACCCGG.
We previously developed the (CMV-CMV) plasmid
construct (Fig. 1A) by adding the
CMV enhancer to the end of the conventional (CMV) construct, which
enhanced CMV promoter-dependent transcription (18). The full-length cDNAs of green
fluorescence protein (GFP) from the vector pEGFP-N2 (Takara
Clontech), red fluorescence protein (DsRed) from the vector
pDsRed-Express (Takara Clontech), glutathione-S-transferase (GST)
from the vector pGEX6P1 (GE Healthcare Life Sciences, Tokyo,
Japan), and luciferase from the vector pGL4.14 (luc2/Hygro)
(Promega BioSciences, San Luis Obispo, CA, USA) were amplified and
inserted into the newly designed constructs. An advanced two-step
transcriptional amplification (ad-TSTA) system, which has been
previously reported, was able to enhance cancer-specific hTERT
promoter-driven transcription (13).
Cell transfections and assays
The cells were plated in a culture medium in 6-well
or 24-well plates. After 24 h, the transient transfection of the
GFP-, luciferase- or indicated gene encoded in the CMV, CMV-CMV,
hT/Cm, hT/Cm-R, hT/Cm-hT, or hT/Cm-R-hT plasmid was performed using
the Lipofectamine transfection reagent (Invitrogen). At 24 h after
transfection, the expression of GFP was analyzed by fluorescence
microscopy or western blotting. The luciferase expression assay was
performed as previously described (13,14).
Briefly, the effector plasmid was co-transfected with the reporter
plasmid derived from the dual-reporter luciferase assay kit
(Promega, Madison, WI, USA). After 48 h of incubation, the cells
were harvested and their luciferase activity was determined using a
luciferase assay kit and a luminescence microplate reader,
according to the manufacturer's instructions.
Western blotting
The cells were harvested at 24 h after transfection
with the expression vectors and were subjected to SDS-PAGE followed
by western blotting, as previously described (15). Goat anti-schistosomal GST antibody
(GE Healthcare Life Sciences), rabbit anti-GFP antibody (Takara
Clontech), rabbit anti-DsRed antibody (Takara Clontech), rabbit
anti-telomerase (Abcam Inc., Cambridge, MA, USA) and mouse
anti-tubulin (Sigma, St. Louis, MO, USA) were used as the primary
antibodies.
TERT activity assays
A real-time QRT-PCR for hTERT was performed as
previously described and the results were estimated as the cellular
endogenous TERT activity (13,14).
The primers used for the real-time PCR were TERT-F',
5′-CCATCAGAGCCAGTCTCACCTTC-3′, TERT-R', 5′-ACCGTCTGGAGGCTGTTCA-3′;
glyceraldehyde-3-phosphatedehydrogenase (GAPDH)-F',
5′-GCACCGTCAAGGCTGAGAAC-3′, GAPDH-R' and
5′-TGGTGAAGACGCCAGTGGA-3′.
In vitro detection of viable cancer
cells with the hT/Cm-R-hT plasmid
The experimental system for the in vitro
detection of GFP-expressing HeLa cancer cells is shown in Fig. 6A. The target HeLa cancer cells were
pretreated with CellTracker Orange (Invitrogen) as a tracer
according to the manufacturer's instructions. The cancer cells were
then trypsinized and mixed with human peripheral blood mononuclear
cells (PBMCs) at the indicated target frequency ratios (14). The mixed cells were transfected with
the hT/Cm- or hT/Cm-R-hT-GFP plasmid. After 24 h, the GFP
expression of the cells was examined and CellTracker Orange
staining was observed by fluorescence microscopy.
Statistical analysis
The data are presented as the mean ± standard
deviation. A regression analysis was performed to examine the
correlation between 2 parameters. P-values of <0.05 were
considered to indicate statistically significant differences.
Results
hT/Cm-R-hT system achieves powerful
gene expression
We previously reported a novel gene expression
construct that enables the enhancement of the expression level of a
cargo gene with the structural modification of the vectors
(15,18). The key to the modification is the
insertion of a certain enhancer (such as a CMV or hTERT enhancer)
just behind the polyadenylation (polyA) signal sequence and the
insertion of the RU5′ sequence, which enhances the transcription
efficiency. Based on the findings, we constructed four hT/Cm
promoter-based plasmids (Fig. 1B)
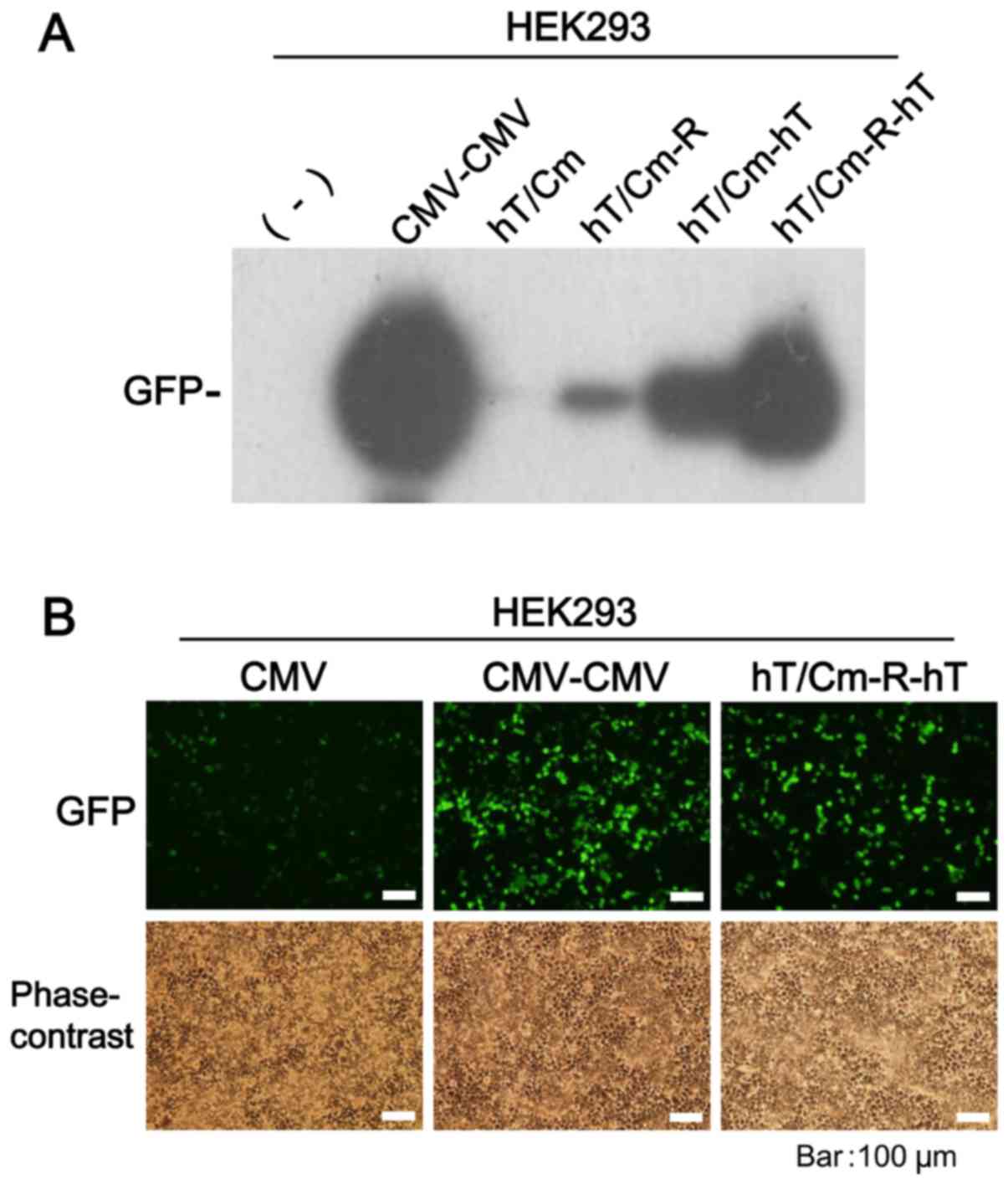
and compared them in vitro gene expression ability. The GFP
gene and HEK293 cells were used for this assessment. Western blot
analysis of the series of modifications revealed that the
expression of GFP was strongest in hT/Cm-R-hT plasmid (Fig. 2A). The expression of GFP by the
hT/Cm-hT plasmid was superior to that of the hT/Cm promoter alone
and the hT/Cm-R plasmid, indicating that the insertion of the hTERT
enhancer at the 3′-side of the cDNA markedly enhances the
expression of the cargo gene. Under fluorescence microscopy, the
hT/Cm-R-hT plasmid system showed GFP expression more strongly than
the conventional CMV construct (Fig.
2B). Notably, the robust expression that was observed in the
hT/Cm-R-hT construct was almost comparable to the strongest CMV-CMV
construct (Fig. 2A and B).
hT/Cm-R-hT system achieves robust
cancer-specific gene expression
To further confirm the sophistication of the
hT/Cm-R-hT construct in cancer-specific gene expression, we added
normal human cells for the in vitro analyses of gene
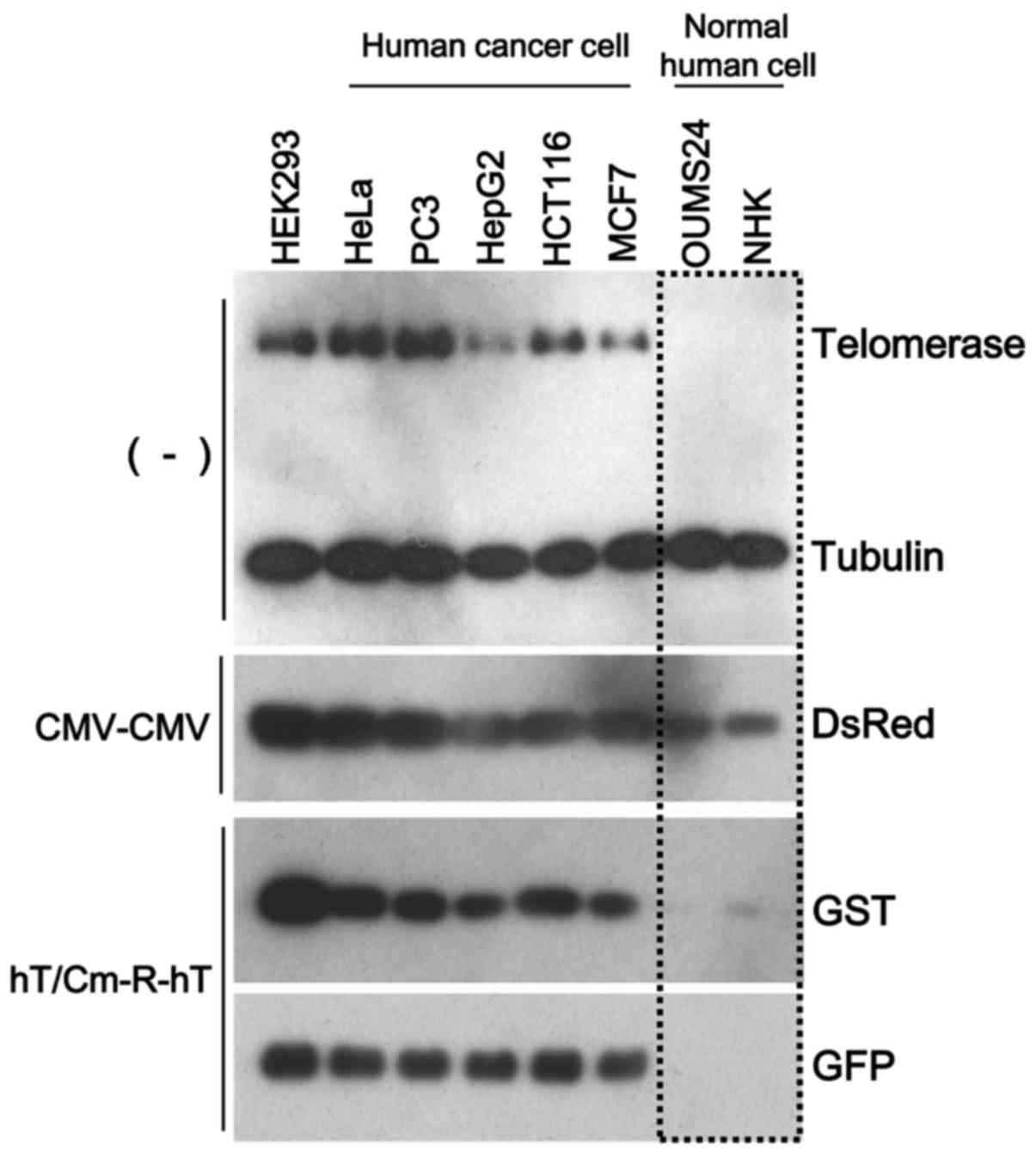
expression. We performed simultaneous triple transfection using
three types of reporter gene-expressing plasmids (CMV-CMV plasmid
encoding DsRed, hT/Cm-R-hT plasmids encoding GST or GFP). The
CMV-CMV plasmid, which works in a broad range of cell types,
including normal cells, was used for the negative control for the
cancer-specific gene expression. After the transfection of these
constructs to the indicated cells, we found that DsRed was
expressed in all cells, including the normal cells, while GST and
GFP were expressed at almost undetectable levels in the normal
cells, with the expression restricted to the cancer cells (Fig. 3). Thus, the hT/Cm-R-hT construct
possessed the ability of cancer-specific gene expression.
Consistent results were obtained in various normal and cancer cells
under fluorescence microscopy (Fig.
4A-C), showing that the plasmid enhanced the expression and
that the cancer-specific enhancement was not lost. In this
experimental condition, the expression of GFP was relatively strong
in human iPS cells, which are known for tumorigenicity, in
comparison to normal cells (Fig. 4C and
D). The hTERT promoter-driven ad-TSTA plasmid, the gene
expression is also restricted in cancer cells (13,14),
was tested using iPS cells and similar results were obtained
(Fig. 4D).
hTERT-dependent gene expression of the
hT/Cm-R-hT system in human cancer cells
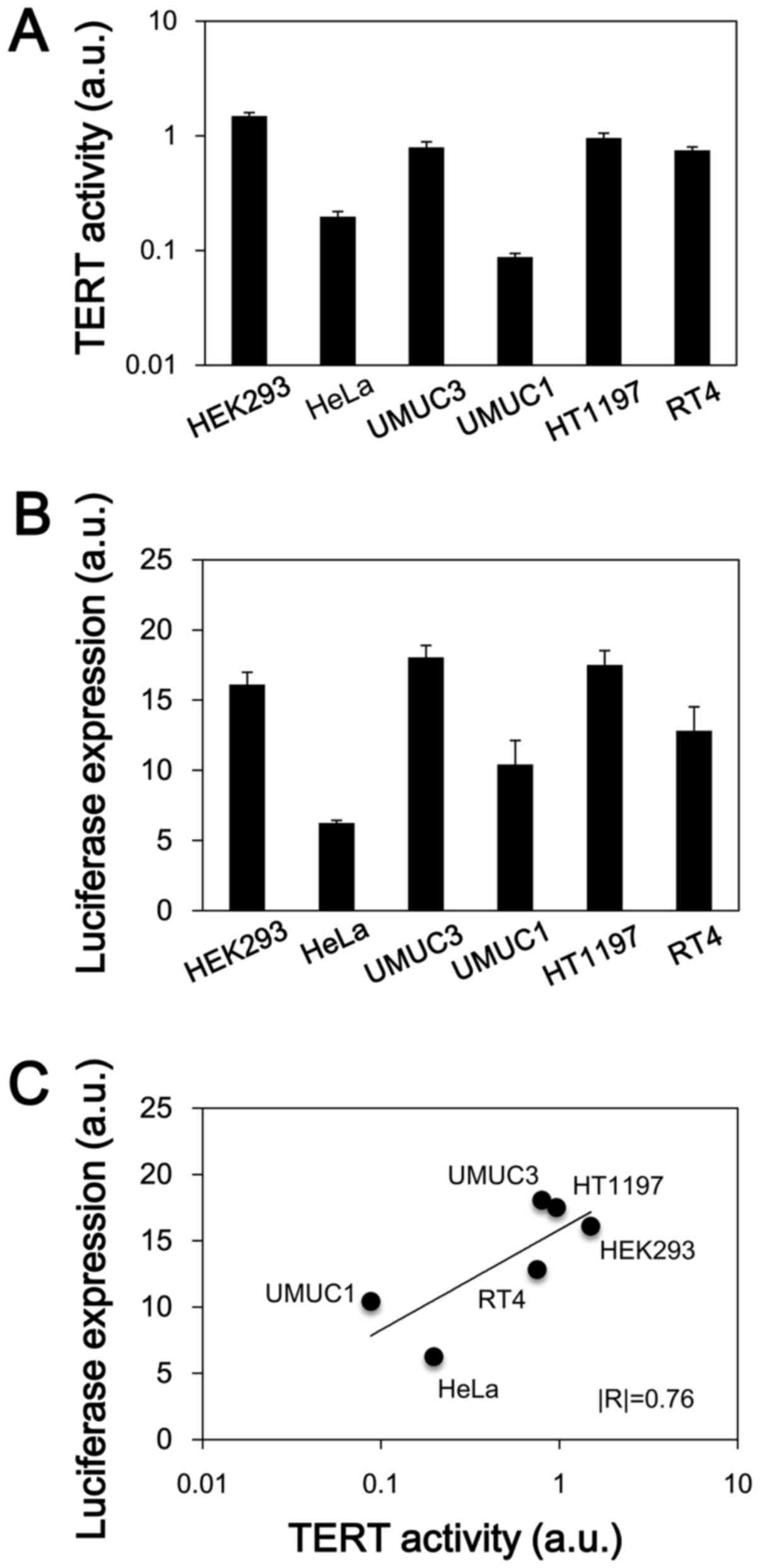
We examined whether the newly developed hT/Cm-R-hT
construct exhibits hTERT promoter-dependent gene expression. The
endogenous TERT activity was first determined in each of the cell
lines that was used in this assessment (Fig. 5A). HEK293, UMUC3, HT1197 and RT4
cells showed relatively higher activity levels in comparison to the
other cells. We next examined the expression of the luciferase
reporter gene in the hT/Cm-R-hT construct (Fig. 5B). The expression of luciferase
tended to be very similar to the TERT activity in the cell lines
(Fig. 5A and B). In a regression
analysis, a strong positive correlation (|R|=0.76) was confirmed
between the TERT activity and the luciferase expression level
(Fig. 5C); however, the result did
not reach statistical significance (P=0.08). This result reveals
that the gene expression of the hT/Cm-R-hT system is TERT
activity-dependent, which indicates that it is hTERT
promoter-dependent. These findings were consistent with the western
blot results, which revealed that the hT/Cm-R-hT plasmid achieved
cancer-specific gene expression depending on the endogenous
telomerase expression levels, indicating the levels of TERT
activity (Fig. 3).
In vitro detection of viable cancer
cells using hT/Cm-R-hT-GFP plasmid system
We next evaluated the utility of the hT/Cm-R-hT
plasmid system in the in vitro detection of viable cancer
cells (Fig. 6A). HeLa cancer cells
were mixed with PBMCs and the cells were transfected with the
hT/Cm-R-hT-GFP plasmid. The GFP expression of these cells was
examined under fluorescence microscopy. HeLa cancer cells were
pretreated with the CellTracker Orange as a tracer to ensure that
the cells expressing the GFP signals were indeed the target HeLa
cancer cells. Dual fluorescence microscopy confirmed that most of
GFP-positive cultured cells were HeLa cancer cells that had been
labeled with CellTracker Orange at different target frequency
ratios (1:10, 1:1000 and 1:10000) (Fig.
6B). The fact that HeLa cancer cells could be morphologically
distinguished from the other PBMCs in the bright field reinforced
the accuracy of the experiments.
Discussion
The success of gene therapies and imaging approaches
using the cancer-specific gene expression is influenced by the
expression levels of gene products. Although the hTERT promoter has
been well-characterized as a broad range cancer-specific promoter
(6,9), the gene expression driven by the hTERT
promoter is often weak due to its poor transcriptional activity
(13,14). On the other hand, the combination of
a promoter and an enhancer has been attempted in our previous
studies, with several showing improved gene transcription (15,18).
For the purpose of overcoming the weakness of the hTERT promoter,
we herein developed a novel hT/Cm-R-hT construct. We demonstrated
that the plasmid robustly enhances the hTERT promoter-driven
cancer-specific gene expression. Based on the cancer-specificity,
we also confirmed the availability of this cassette for the in
vitro imaging and detection of human cancer cells mixed with
normal human cells.
In the current gene expression system, we used a
chimeric promoter element derived from the hTERT and CMV promoters
upstream of the cargo gene. When the hT/Cm promoter was combined
with the subsequent RU5′ sequence, an inserted gene, BGH polyA, and
the hTERT enhancer, the expression of GFP was significantly
enhanced to a level that was nearly comparable to the CMV-CMV
plasmid construct, which is one of the strongest expression systems
that we reported (18).
Furthermore, as the TERT activity was elevated in multiple cancer
cell lines, the luciferase gene expression of the hT/Cm-R-hT system
was stronger. These results indicate that this system is
advantageous in the cancer cells with higher TERT activity in terms
of the gene expression.
Importantly, we were able to use the hT/Cm-R-hT-GFP
plasmid to examine the fluorescence imaging of the promoter-driven
human cancer cells by microscopy. The hT/Cm-R-hT-GFP plasmid
successfully induced cancer-specific gene expression, showing the
robust expression of GFP in the target cancer cells, but no visible
expression of GFP in PBMCs. Viable human cancer cells were
selectively visualized in cultured cells containing a mixture of
10-, 1000-, and 10000-fold more PBMCs. An ideal diagnostic agent
for cancer would be able to selectively target tumor cells. The
present study indicates that the hT/Cm-R-hT system may be useful
for detection of target cancer cells. The current system can be
applied to the in vitro detection of cancer cells
disseminated in vivo in the blood and in other types of body
fluid.
We also tried to assess our vector in iPS cells. The
human iPS cells are invaluable for therapeutic approaches owing to
their pluripotency except for the high ability of tumor formation
in vivo. One of the reasons of tumorigenicity comes from
marked expression of hTERT in iPS cells (19). This leads to our expectation that
our newly developed vector is able to detect not only cancer cells
but also in vivo tumor-initiating cells, such as iPS cells.
As expected, the expression of GFP was relatively strong in human
iPS cells in comparison to normal cells. These results suggest that
our innovative vector has an advantage to distinguish
tumor-initiating cells from normal cells with significant
sensitivity. In addition to this, we also considered that the
vector is useful to assess stem cells and differentiated cells in
the iPS population.
Since the current system can also be applied to
other types of vectors, such as virus vectors, this approach is
widely expected to become a valuable tool for enhancing the hTERT
promoter-dependent cancer-specific gene expression. It would be of
interest to extend this strategy to clinical practice and to
confirm the efficacy of the system as a non-invasive method for
diagnosing and evaluating various types of cancer.
Acknowledgements
This work was supported by scientific research
grants (JSPS KAKENHI grant nos.: JP16K11004, JP15H04297,
JP15H04974) from the Ministry of Education, Culture, Sports,
Science and Technology of Japan and supported by a grant for
promotion of science and technology in Okayama prefecture (by MEXT)
‘Creation of nanobiotargeted therapy using REIC as a therapeutic
gene for cancer’. The authors would like to thank Ms. Fusaka Oonari
and Ms. Shun-Ai Li (Okayama University) for their valuable
assistance. Okayama University and Momotaro-Gene Inc. are applying
for patents on the gene expression systems of the CMV-CMV and
hT/Cm-based constructs that were described in the present study.
Drs M.S., Y.N., H.K., N.H.H. and M.W. own stock in Momotaro-Gene
Inc.
References
|
1
|
Greider CW: Telomere length regulation.
Annu Rev Biochem. 65:337–365. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greider CW: Chromosome first aid. Cell.
67:645–647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura TM and Cech TR: Reversing time:
Origin of telomerase. Cell. 92:587–590. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tahara H, Yasui W, Tahara E, Fujimoto J,
Ito K, Tamai K, Nakayama J, Ishikawa F, Tahara E and Ide T:
Immuno-histochemical detection of human telomerase catalytic
component, hTERT, in human colorectal tumor and non-tumor tissue
sections. Oncogene. 18:1561–1567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takakura M, Kyo S, Kanaya T, Hirano H,
Takeda J, Yutsudo M and Inoue M: Cloning of human telomerase
catalytic subunit (hTERT) gene promoter and identification of
proximal core promoter sequences essential for transcriptional
activation in immortalized and cancer cells. Cancer Res.
59:551–557. 1999.PubMed/NCBI
|
|
7
|
Kishimoto H, Kojima T, Watanabe Y, Kagawa
S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y,
et al: In vivo imaging of lymph node metastasis with
telomerase-specific replication-selective adenovirus. Nat Med.
12:1213–1219. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maida Y, Kyo S, Sakaguchi J, Mizumoto Y,
Hashimoto M, Mori N, Ikoma T, Nakamura M, Takakura M, Urata Y, et
al: Diagnostic potential and limitation of imaging cancer cells in
cytological samples using telomerase-specific replicative
adenovirus. Int J Oncol. 34:1549–1556. 2009.PubMed/NCBI
|
|
9
|
Cong YS, Wen J and Bacchetti S: The human
telomerase catalytic subunit hTERT: Organization of the gene and
characterization of the promoter. Hum Mol Genet. 8:137–142. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang P, Kaku H, Chen J, Kashiwakura Y,
Saika T, Nasu Y, Urata Y, Fujiwara T, Watanabe M and Kumon H:
Potent antitumor effects of combined therapy with a
telomerase-specific, replication-competent adenovirus (OBP-301) and
IL-2 in a mouse model of renal cell carcinoma. Cancer Gene Ther.
17:484–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kojima T, Hashimoto Y, Watanabe Y, Kagawa
S, Uno F, Kuroda S, Tazawa H, Kyo S, Mizuguchi H, Urata Y, et al: A
simple biological imaging system for detecting viable human
circulating tumor cells. J Clin Invest. 119:3172–3181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iyer M, Wu L, Carey M, Wang Y, Smallwood A
and Gambhir SS: Two-step transcriptional amplification as a method
for imaging reporter gene expression using weak promoters. Proc
Natl Acad Sci USA. 98:14595–14600. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Ueki H, Ochiai K, Huang P,
Kobayashi Y, Nasu Y, Sasaki K, Kaku H, Kashiwakura Y and Kumon H:
Advanced two-step transcriptional amplification as a novel method
for cancer-specific gene expression and imaging. Oncol Rep.
26:769–775. 2011.PubMed/NCBI
|
|
14
|
Ueki H, Watanabe M, Kaku H, Huang P, Li
SA, Ochiai K, Hirata T, Noguchi H, Yamada H, Takei K, et al: A
novel gene expression system for detecting viable bladder cancer
cells. Int J Oncol. 41:135–140. 2012.PubMed/NCBI
|
|
15
|
Watanabe M, Sakaguchi M, Kinoshita R, Kaku
H, Ariyoshi Y, Ueki H, Tanimoto R, Ebara S, Ochiai K, Futami J, et
al: A novel gene expression system strongly enhances the anticancer
effects of a REIC/Dkk-3-encoding adenoviral vector. Oncol Rep.
31:1089–1095. 2014.PubMed/NCBI
|
|
16
|
Agha-Mohammadi S, O'Malley M, Etemad A,
Wang Z, Xiao X and Lotze MT: Second-generation
tetracycline-regulatable promoter: Repositioned tet operator
elements optimize transactivator synergy while shorter minimal
promoter offers tight basal leakiness. J Gene Med. 6:817–828. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Senju S, Haruta M, Matsumura K, Matsunaga
Y, Fukushima S, Ikeda T, Takamatsu K, Irie A and Nishimura Y:
Generation of dendritic cells and macrophages from human induced
pluripotent stem cells aiming at cell therapy. Gene Ther.
18:874–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakaguchi M, Watanabe M, Kinoshita R, Kaku
H, Ueki H, Futami J, Murata H, Inoue Y, Li SA, Huang P, et al:
Dramatic increase in expression of a transgene by insertion of
promoters downstream of the cargo gene. Mol Biotechnol. 56:621–630.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Wang F, Okuka M, Liu N, Ji G, Ye
X, Zuo B, Li M, Liang P, Ge WW, et al: Association of telomere
length with authentic pluripotency of ES/iPS cells. Cell Res.
21:779–792. 2011. View Article : Google Scholar : PubMed/NCBI
|