Introduction
Bladder cancer is the fourth most common malignant
tumor in men and the ninth most common malignant cancer overall. In
2015, 75,000 new cases of bladder cancer were diagnosed in the
United States. Of these cases, 56,320 occurred in men and 16,000
succumbed to their disease (1).
Bladder cancer is classified as either non-muscle-invasive bladder
cancer (NMIBC) or muscle-invasive bladder cancer (MIBC); NMIBC
accounts for 70–80% of new cases and is treated by transurethral
resection of the bladder tumor (TURBT). However, the recurrence
rate in patients with NMIBC is high after treatment (≤70%) and
requires lifelong monitoring, often resulting in multiple surgical
treatments. Cystoscope and urine cytology are still the gold
standard for the diagnosis of bladder cancer and detection of
recurrence (2). Approximately 30%
of recurrent tumors will develop into MIBC. Partial cystectomy and
radical cystectomy are performed routinely for disease management;
however, many patients cannot undergo surgery because of early
tumor metastasis. Thus, the 5-year survival rate in patients is
only 20–40% (3,4). Accordingly, comprehensive treatment of
bladder cancer involves the use of radiotherapy, chemotherapy,
biological therapy, and other approaches (5,6).
Despite therapeutic advancements, no effective treatment options
currently exist to prevent the progression from NMIBC to MIBC
(7). Accordingly, a clear
understanding of the molecular mechanisms mediating the bladder
cancer occurrence and progression are urgently needed in order to
develop methods for the early diagnosis and treatment of this
disease.
We previously reported on a genomic analysis of 149
transitional cell cancer (TCC) samples by whole-genome and
whole-exome sequencing. Of these, 16 samples harbored deletions or
truncating mutations in stromal antigen 2 (STAG2), including 12
cases of metastatic bladder cancer (8). These mutations abolished STAG2
expression in bladder cancer specimens, whereas STAG2 was still
expressed in normal tissues. STAG2 inactivation requires only a
single mutation event because the STAG2 gene is located on
the X chromosome. STAG2 comprises 39 exons and encodes the
SA2 subunit of cohesion (9,10). In 2011, Solomon et al
demonstrated that targeted inactivation of STAG2 caused
chromatid cohesion defects and aneuploidy (11). However, Balbás-Martínez et al
(18) and Djos et al
(12) confirmed that recurrent
inactivation of STAG2 is not associated with aneuploidy in
bladder cancer or neuroblastoma tumors. Subsequently, loss of STAG2
expression was demonstrated in gastric cancer, colorectal cancer,
and prostate cancer tissues, although no mutations have been found
in these cancers. In contrast, STAG2 somatic mutations have
been identified in acute leukemia (13,14),
and recurrent mutations and deletions are associated with the
development of myeloid neoplasms (15).
STAG2 is a subunit of cohesion - a highly conserved
protein complex that contains four core subunits (SMC1A, SMC3,
RAD21 and STAG1/2) - and plays an important role in the separation
of sister chromatids during mitosis and meiosis, DNA repair, gene
expression regulation, genomic imprinting, and the
epithelial-to-mesenchymal transition (EMT). Because EMT is a key
step in cancer cell metastasis, functional defects in cohesin may
be related to both the occurrence and metastasis of cancer.
Interestingly, STAG2 mutations are not related to
chromosomal aberrations in tumor cells in NMIBC (10,16–18);
however, STAG2 mutations are known to be associated with
chromosomal aneuploidy in MIBC and metastatic bladder cancer
(8,11). Moreover, frequent inactivating
mutations in STAG2 are associated with low tumor grade and stage
and are inversely related to chromosomal copy number changes
(10).
In this study, we aimed to explore the role of STAG2
in bladder cancer development and progression. Our findings provide
important insights into the role of this molecule in the
pathogenesis of bladder cancer.
Materials and methods
Patient tissue specimens
Fifty-eight pairs of bladder cancer and matched
adjacent normal tissues were obtained by radical cystectomy from
Zhujiang Hospital (Southern Medical University, Guangdong, China).
The tissues were fixed in RNAlater reagent (Sigma, St. Louis, MO,
USA) and stored in liquid nitrogen. All procedures conducted in
studies involving human participants were performed in accordance
with the ethical standards of the institutional and/or national
research committee and with the 1964 Declaration of Helsinki and
its later amendments or comparable ethical standards.
Cell lines and culture
The T24, 5637, J82, UM-UC-3, and SW780 human bladder
cancer and SV-HUC-1 normal bladder cell lines were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). All
cells were maintained in a humidified atmosphere of 5% carbon
dioxide at 37°C and cultured in medium supplemented with 10% fetal
bovine serum.
Lentivirus packaging and generation of
stable cell lines
The STAG2 coding sequence was amplified by
polymerase chain reaction (PCR) using specific primers (forward:
5′-GAGGATCCCCGGGTACCGGTCGCCACCATGATAGCAGCTCCAGAAATACCAAC-3′;
reverse: 5′-CACACATTCCACAGGCTAGTTAAAACATTGACACTCCAAGAACTG-3′)
containing restriction enzyme sites for BamHI and
NheI at the 5′ and 3′ ends, respectively. STAG2 shRNA was
synthesized using the following sequences: shRNA1,
5′-TTATGCTTCCAAGATCAA-3′ and 5′-CTATGTAATCCTTTGGCAA-3′; shRNA2,
5′-AACGTGAATACTACTGTTA-3′ and 5′-AACGTGAATACTACTGTTA-3′. The STAG2
cDNA and shRNA fragments were ligated into the
Ubi-MCS-SV40-EGFP-IRES-puromycin and
hU6-MCS-Ubiquitin-EGFP-IRES-puromycin plasmids, respectively, and
then transfected into 293-T cells. Lentivirus titers and
concentrations were determined using standard procedures. For
transfection, 2×105 UMUC-3 or T24 cells were seeded in
6-well culture plates and cultured for 24 h prior to transduction.
Cells were divided into the following four groups: UMUC-3 cells
transfected with a lentiviral construct expressing STAG2; UMUC-3
cells transfected with a negative control lentiviral construct
(UMUC-3-NC); T24 cells transfected with a lentiviral construct
expressing STAG2 shRNA (T24-shSTAG2); and T24 cells transfected
with a negative control lentiviral construct (T24-shNC). All cell
lines were cultured using medium supplemented with polybrene for 12
h, and the medium was then replaced with normal medium. EGFP
expression was used to confirm plasmid expression 3 days later.
Stable cell lines were screened using medium containing a certain
concentration of puromycin.
Total RNA and cDNA synthesis
Total RNA from bladder cancer cells and normal
bladder cells was isolated using TRIzol reagent (Invitrogen,
Burlington, ON, USA) according to the manufacturer's protocol.
Then, cDNA was synthesized using a QuantiTect Reverse Transcription
kit (Qiagen GmbH, Hilden, Germany) with gDNA wipeout buffer in a
20-µl reaction containing 1 µg RNA. The reaction mixture was
incubated at 37°C for 5 min, heated at 95°C for 30 min, and then
cooled on ice.
Quantitative real-time reverse
transcription PCR (qRT-PCR)
qRT-PCR was performed using SYBR Premix Taq II
(Takara, Shiga, Japan) with β-actin (ACTB) as an internal
reference. Primer sequences were designed in Primer5 and are as
follows: STAG2 forward, 5′-ACGGAAAGTGGTTGAGGG-3′; reverse,
5′-GTGGAGGTGAGTTGTGGTGT-3′. ACTB forward,
5′-GAAATCGTGCGTGACATTAA-3′; reverse, 5′-AAGGAAGGCTGGAAGAGTG-3′.
Each 20-µl reaction mixture contained 0.5 µM of each primer, 10 µl
SYBR Premix, 7 µl RNase water, and 2 µl cDNA. Amplification was
carried out using a QuantStudio Dx System (Applied Biosystems,
Monza, Italy) with an initial denaturation at 95°C for 30 sec;
followed by 40 cycles of 95°C for 5 sec, 60°C for 34 sec, and 72°C
for 30 sec. All reactions were performed in duplicate. Gene
expression was quantified using the comparative threshold cycle
(CT) method.
Western blotting
Cell protein was extracted using RIPA lysis buffer
and a protease inhibitor mixture (Thermo Fisher Scientific,
Waltham, MA, USA) and the concentration was measured using a Piece
BCA Protein assay kit (Thermo Scientific). Protein samples (20 µg)
were subjected to SDS-PAGE on 10% gels and then transferred to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% non-fat skim milk in 50 mM Tris-HCl, 50 mM NaCl,
and 0.1% Tween-20 (TBST) for 1 h at 25°C, probed with rat
polyclonal anti-STAG2 antibodies (1:4,000; Abcam, Cambridge, UK) at
4°C overnight, and then incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG;
1:8,000; Abcam) in TBST for 1 h at room temperature.
Cell proliferation assays
Cell proliferation was measured with a Cell Counting
Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) and
5-ethynyl-20-deoxyuridine (Edu) assay kit (Ribobio, Wuhan, China)
according to the manufacturers' protocols. For CCK-8 assay, cell
lines were seeded at 4×103 cells/well in a 96-well
plate. After culturing for 0–4 days, CCK8 reagent was added to
plates at different time points and then incubated at 37°C for 2 h.
Proliferation was based on absorbance at 450 nm measured with a
microplate reader. For Edu assays, cells were seeded at
6×103 cells/well in a 96-well plate for 24 h, and then
incubated with 50 µM EdU for 3 h at 37°C, fixed with 4%
formaldehyde for 15 min, treated with 0.5% Triton X-100 for 20 min
at room temperature, and then washed with PBS three times before
incubating with 1X Apollo® reaction cocktail (Ribobio,
100 µl/well) for 30 min. The cells were then stained with Hoechst
33342 (5 µg/ml, 100 µl/well) for 30 min prior to imaging with a
fluorescent microscope (Olympus Corp., Tokyo, Japan).
Transwell cell migration and invasion
assays
Cell migration and invasion were evaluated using
transwell chambers with an 8-µm pore size (Costar, Corning, NY,
USA). For migration assays, 4×104 cells were seeded into
the upper chamber in serum-free medium, and 500 µl medium
supplemented with 10% FBS was added to the lower chamber. A similar
procedure was used for invasion assays, except that the upper
chamber was precoated with Matrigel basement membrane matrix (BD
Biosciences, Bedford, MA, USA). The cells were then incubated at
37°C for 24 or 48 h for migration and invasion studies,
respectively. The wells were then placed in 100% methanol to fix
the cells, and cells remaining on the upper side of the chamber
were then removed carefully using cotton swabs. Cells that had
migrated or invaded through the membrane were then stained using 5%
Giemsa staining, and the chambers were washed with PBS. Photographs
from five independent fields per well were captured using an
Olympus microscope (Hamburg, Germany). Each assay was carried out
in triplicate.
Cell cycle analysis
To determine the role of STAG2 in cell cycle
progression, transfected cells were harvested with trypsin and
centrifuged at 265 × g for 5 min. The cell pellet was washed in
pre-chilled Hanks D buffer (pH 7.2–7.4) and fixed with 75% ethanol
for at least 1 h before staining with 40X propidium iodide (PI)
solution (2 mg/ml), 100X RNase solution (10 mg/ml), and 1X Hanks D
buffer at 25:10:1,000 (v/v/v). The stained cells were then analyzed
with a Navi 105 flow cytometer (Beckman Coulter, Brea, CA, USA) at
a rate of 300–800 cells/sec.
Cell apoptosis assay
Apoptotic cells were identified by staining with an
Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(Invitrogen) and PI (Invitrogen). Briefly, transfected cells were
detached using trypsin, resuspended in complete medium, harvested
by centrifugation at 1,300 rpm for 5 min, and then incubated in 500
µl cold 1X binding buffer (Invitrogen) with 5 µl Annexin V-FITC in
the dark for 15 min at room temperature. Then, the stained cells
were collected by centrifugation and incubated in 500 µl cold
binding buffer with 3 µl PI for 15 min. Apoptosis was assessed
using a flow cytometer (Navi 105; Beckman Coulter).
Statistical analysis
Statistical analyses were performed using the SPSS
19.0 software package (SPSS, Chicago, IL, USA). Differential STAG2
expression in bladder cancer and paracarcinoma tissue was analyzed
using paired samples t-test, and the relationships between STAG2
expression and clinicopathological characteristics were analyzed
using Chi-square test. Other data were analyzed using unpaired
t-test. All values are expressed as means ± standard deviation (SD)
of at least three independent experiments. P<0.05 was considered
statistically significant.
Results
Analysis of STAG2 expression in
bladder cancer tissues and cell lines
STAG2 expression was examined in 58 pairs of bladder
cancer tissues and adjacent non-cancerous tissues, as well as
bladder cancer and normal cell lines. The patients' clinical
parameters are shown in Table I.
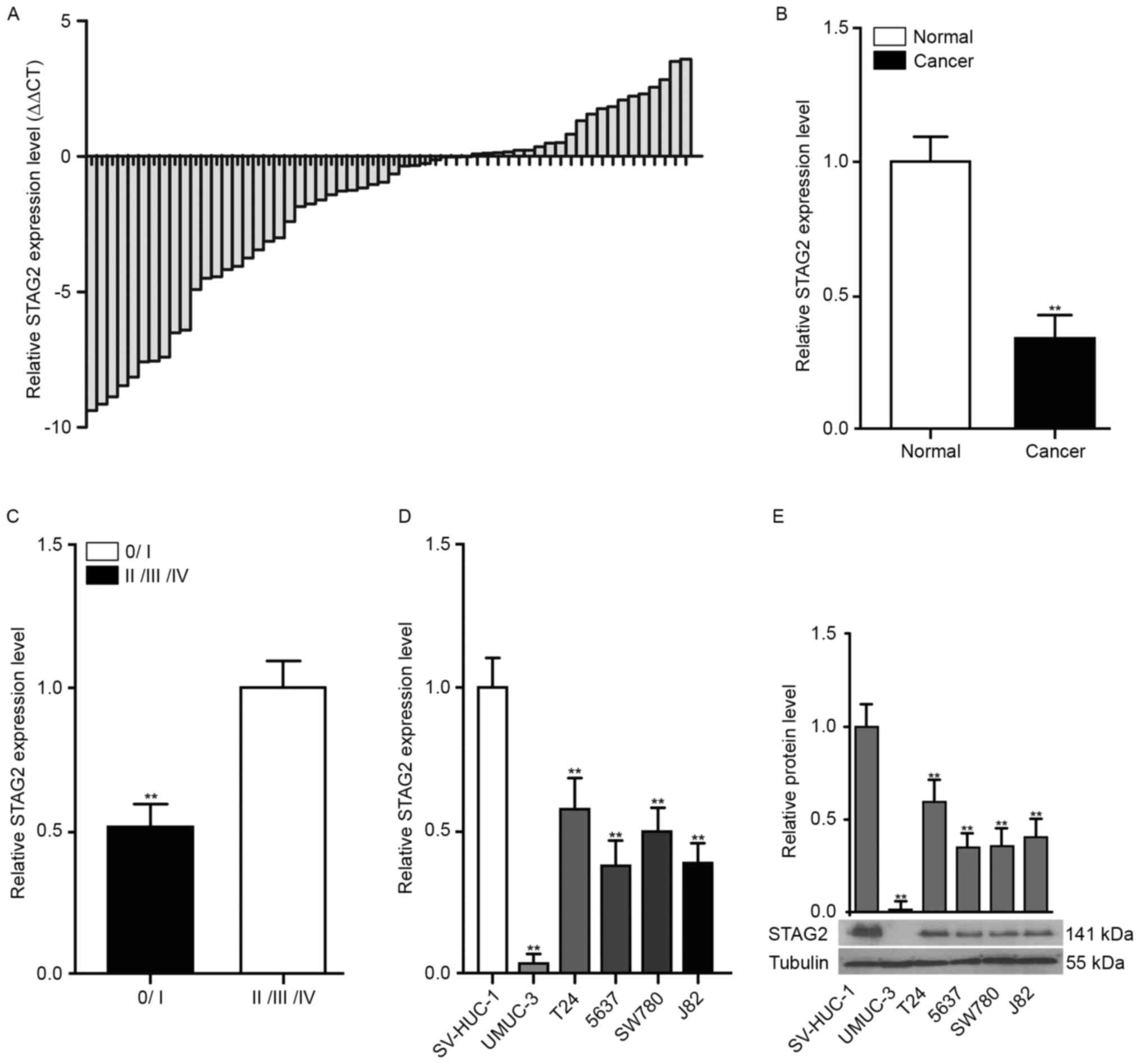
Notably, STAG2 expression was significantly decreased in 63.8% (37
of 58) of cancer tissues compared with that in adjacent normal
bladder tissue samples (Fig. 1A and
B) and significantly correlated with TNM stage (Fig. 1C). Similarly, STAG2 expression was
also markedly reduced in bladder cancer cell lines (Fig. 1D and E).
 | Table I.Correlation between STAG2 mRNA
expression and clinicopathological characteristics of the patients
with bladder cancer. |
Table I.
Correlation between STAG2 mRNA
expression and clinicopathological characteristics of the patients
with bladder cancer.
| Parameters | Patients, no.
(%) | STAG2 expression
high, no. (%) | STAG2 expression,
low no. (%) | P-value |
|---|
| All cases | 58 (100) | 21 (36.2) | 37 (63.8) |
|
| Sex |
|
Male | 39 (67.24) | 15 (38.46) | 24 (61.54) | 0.416 |
|
Female | 19 (32.76) | 6
(31.58) | 13 (68.42) |
|
| Age |
|
<65 | 31 (53.45) | 12 (38.71) | 19 (61.29) | 0.441 |
|
≥65 | 27 (46.55) | 9
(34.48) | 18 (65.52) |
|
| TNM stage |
|
0/I | 15 (25.86) | 10 (66.67) | 5 (33.33) | 0.006a |
|
II/III/IV | 43 (74.14) | 11 (25.58) | 32 (74.42) |
|
| Histological
grade |
| L | 35 (70) | 14 (40) | 21 (60) | 0.324 |
| H | 23 (30) | 7
(30.43) | 16 (69.57) |
|
| Lymph node
metastasis |
| N0 | 50 (86.21) | 17 (34) | 33 (66) | 0.569 |
| N1 or
above | 8
(13.79) | 3
(37.5) | 5
(62.5) |
|
Generation of stable cell lines
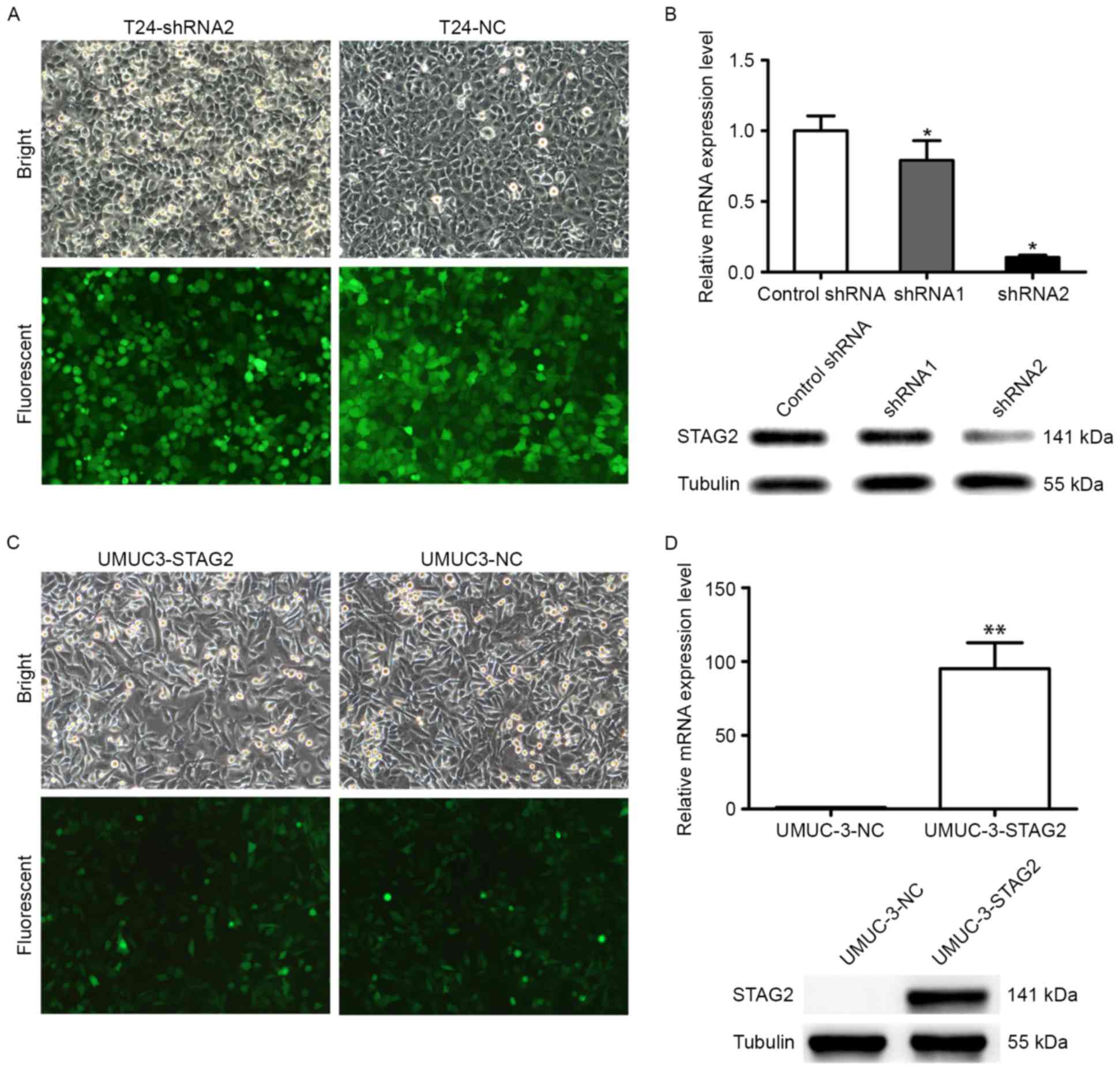
To explore its functional significance in bladder
cancer, STAG2 was overexpressed or knocked down in UMUC-3 and T24
cells with lentiviral vectors expressing STAG2 cDNA or shRNA,
respectively (Fig. 2A), resulting
in four stable cell lines (UMUC-3-NC, UMUC-3-STAG2, T24-shNC, and
T24-shSTAG2). As shown in Fig. 2,
STAG2 was successfully knocked down in T24 cells at both the RNA
and protein level, as shRNA2 knockdown effect is better, following
experiment to select shRNA2 cells (Fig.
2A and B). Furthermore, STAG2 was successfully overexpressed in
UMUC-3 cells at the RNA and protein levels (Fig. 2C and D). The infection efficiencies
in all four cell lines were >80%, which was considered to be
sufficient for subsequent experiments.
Effect of STAG2 expression on cell
proliferation
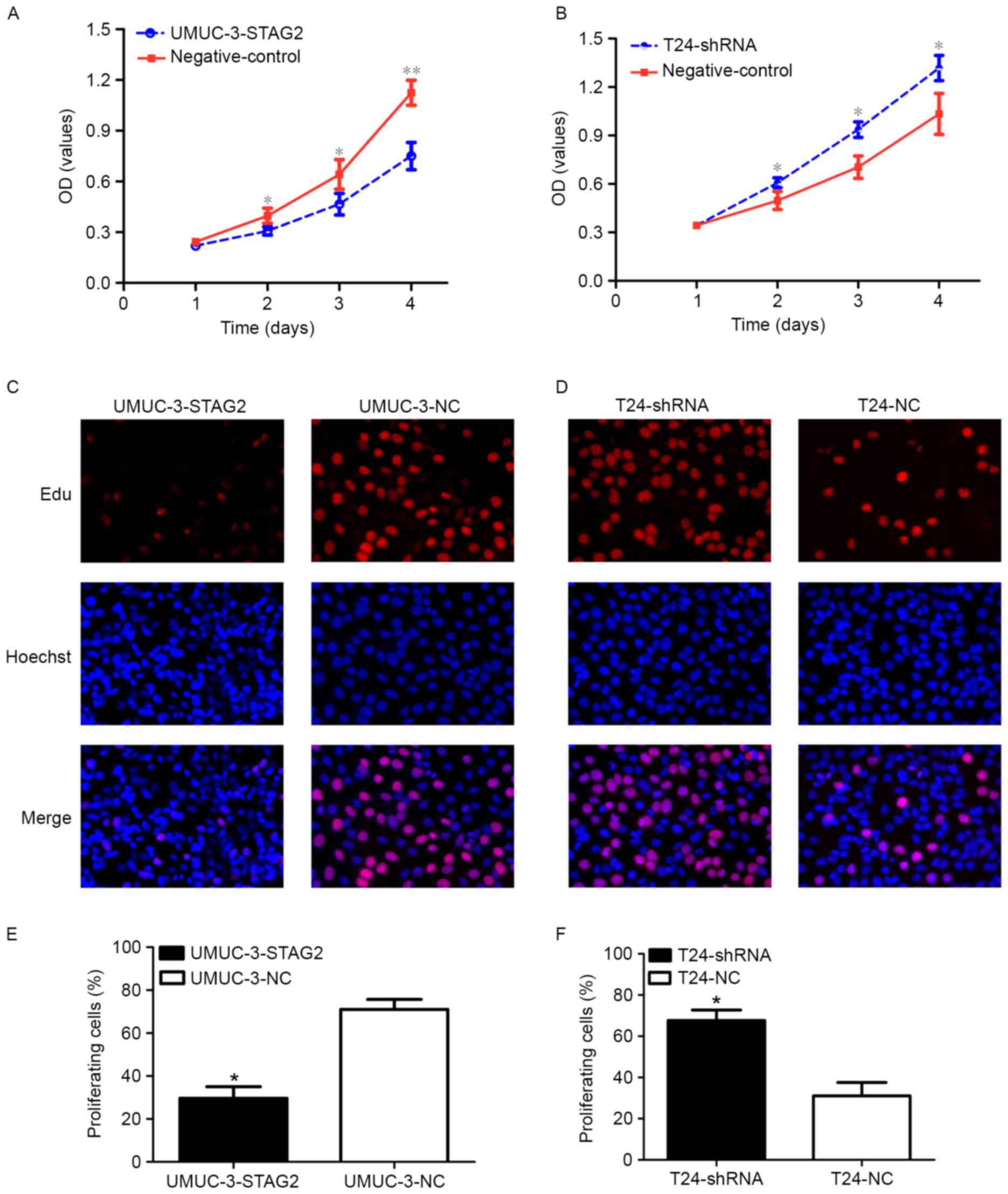
To investigate the impact of STAG2 on bladder cancer
cell proliferation, the effect of STAG2 knockdown or overexpression
on cell proliferation was examined in UMUC-3 and T24 cells with
CCK-8 and Edu assays. Notably, UMUC-3-STAG2 cells with higher STAG2
levels showed a marked delay in proliferation when compared to the
negative control group (Fig. 3A, C and
E), whereas T24-shRNA cells exhibited accelerated proliferation
(Fig. 3 B, D and F). Collectively,
these data suggest that STAG2 expression is negatively correlated
with bladder cancer cell proliferation.
Role of STAG2 in cell cycle
progression
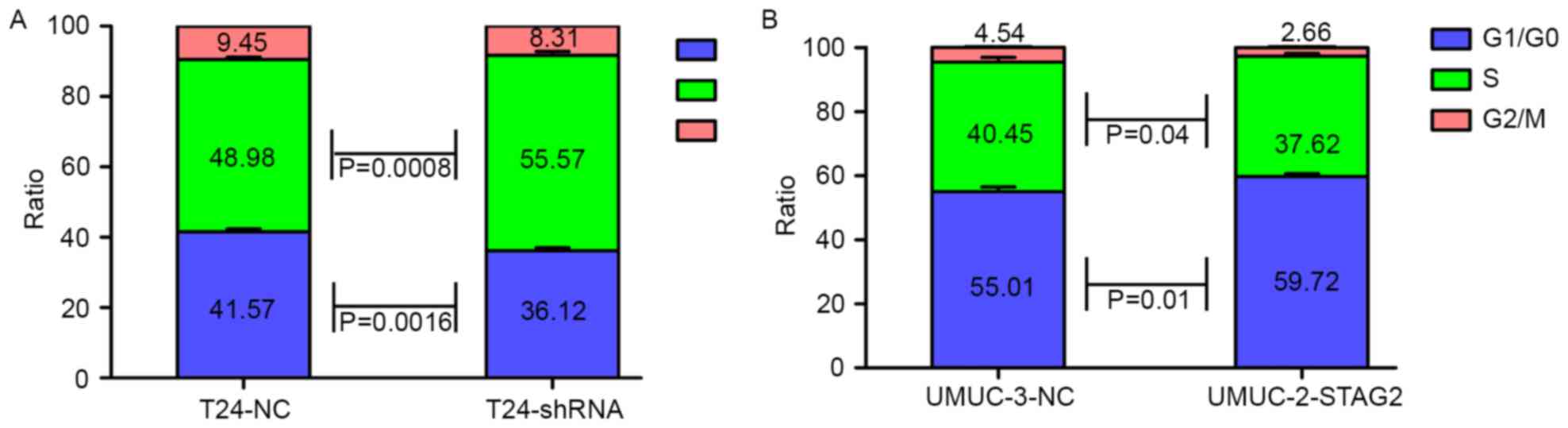
We next investigated whether STAG2 was necessary for
cell cycle progression in bladder cancer by PI staining. Consistent
with our observations on proliferation, flow cytometry analysis
demonstrated that STAG2 overexpression induced
G0/G1 arrest in UMUC-3 cells (Fig. 4B), whereas knockdown promoted
S-phase entry in T24 cells (Fig.
4A).
Participation of STAG2 in cell
migration and invasion
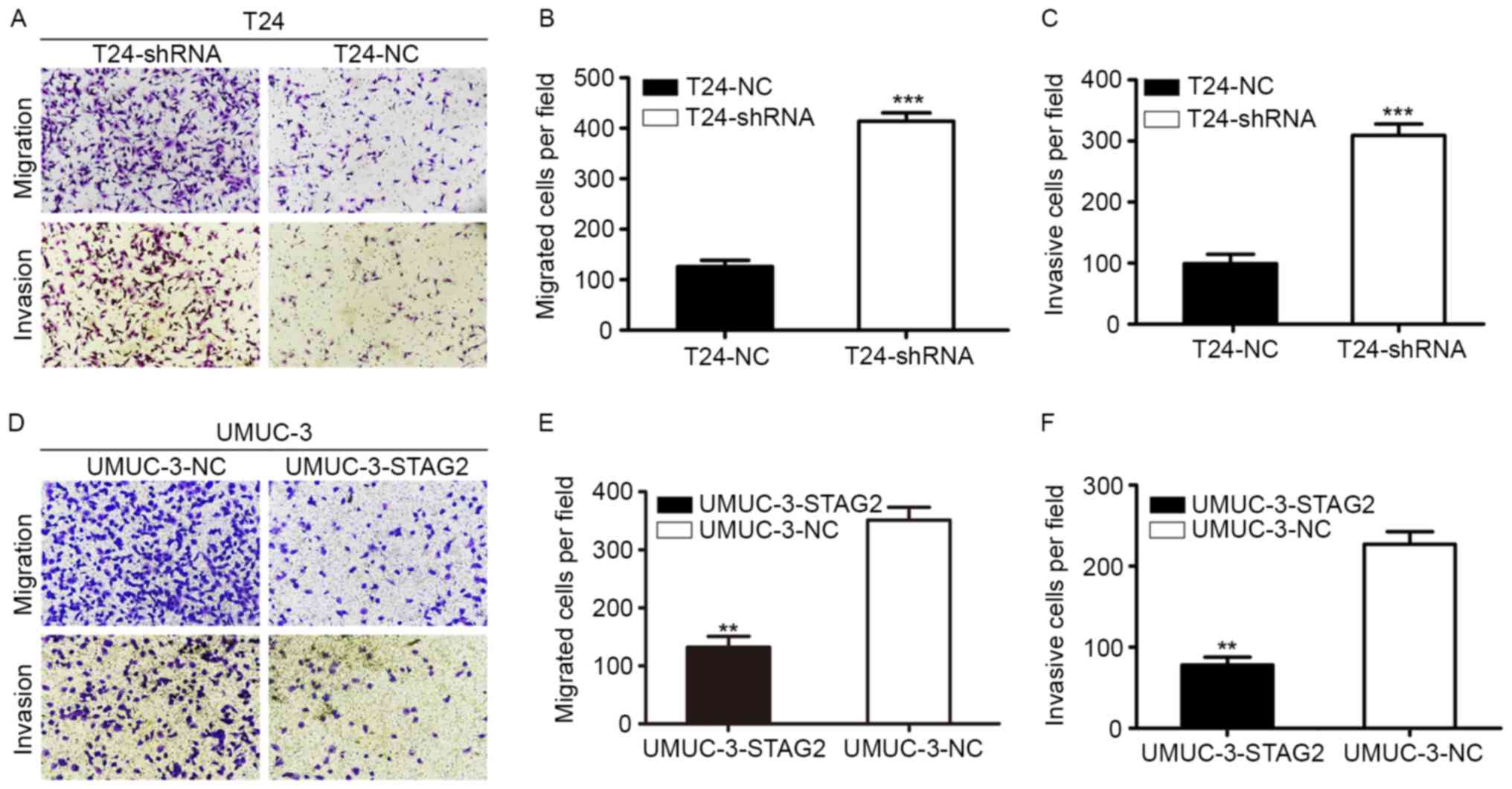
To further determine the relationship between STAG2
and bladder cancer cell invasion and migration, the role of STAG2
in cell migration and invasion was assessed with transwell
migration and invasion assays. In migration assays, significantly
more T24-shRNA cells migrated to the lower chamber than that
observed with T24-shNC cells (Fig. 5A
and B), whereas fewer UMUC-3-STAG2 cells migrated to the lower
chamber than UMUC-3-NC counterparts (Fig. 5D and E). Similar results were
observed with invasion assays. Specifically, STAG2 knockdown
increased invasion in T24 cells (Fig.
5A and C), while overexpression blocked invasion in UMUC-3
cells (Fig. 5D and F). Therefore,
these results suggested that STAG2 inhibited cell migration and
invasion in bladder cancer.
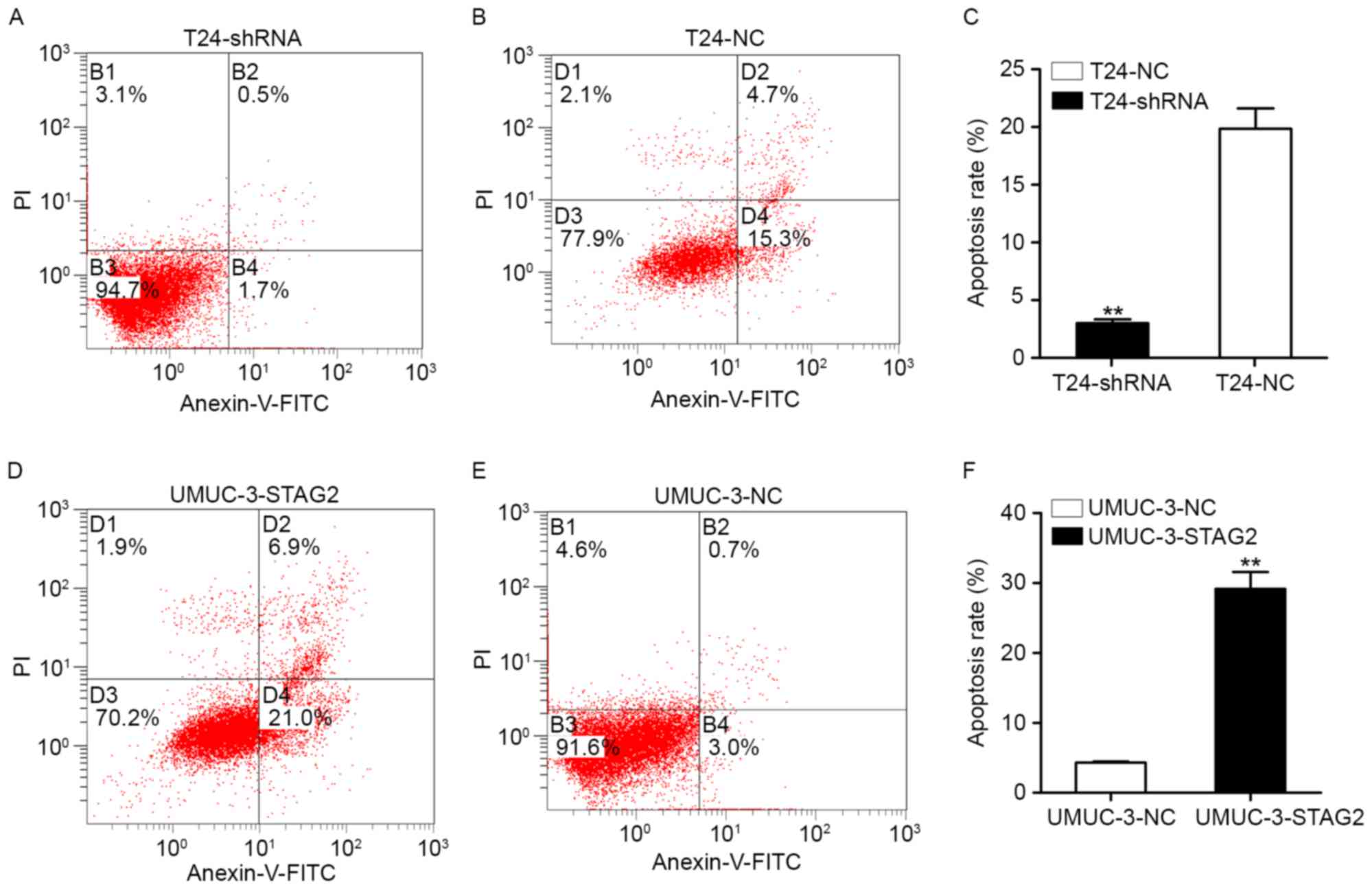
Role of STAG2 gene in cell
apoptosis
We examined whether STAG2 expression affected
apoptosis in bladder cancer cells. As shown in Fig. 5, apoptosis was significantly
decreased in T24-shRNA cells compared with that of T24-shNC cells
(Fig. 6A-C). Conversely, apoptosis
was significantly increased in UMUC-3-STAG2 cells compared with
that in UMUC-3-shNC cells (Fig.
6D-F). These results suggest that STAG2 induces apoptosis in
bladder cancer cells.
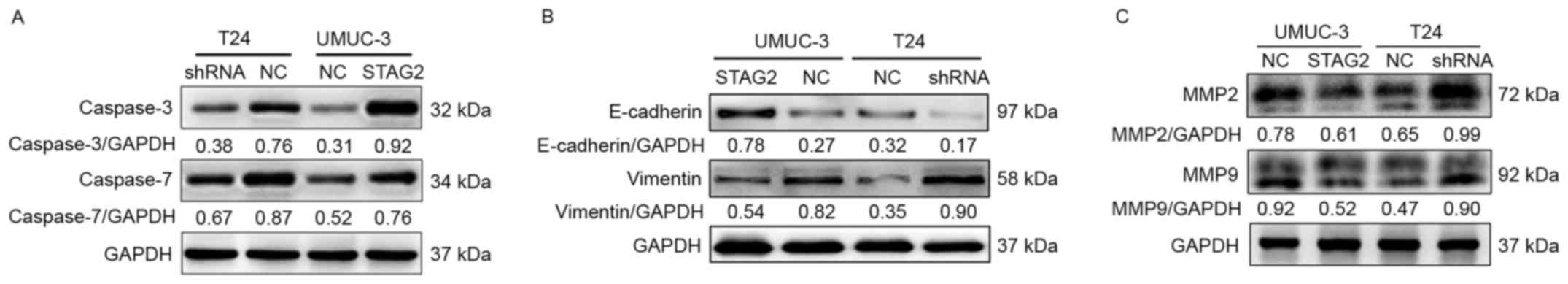
Effects of STAG2 on signaling
pathways
Finally, we analyzed the signaling pathways likely
to be affected by STAG2 expression by western blotting. As shown in
Fig. 7, E-cadherin, caspase-3, and
caspase-7 expression was significantly enhanced in UMUC-3-STAG2
cells compared with that in UMUC-3-shNC cells (Fig. 7A and B). In contrast, vimentin,
matrix metalloproteinase (MMP) 2, and MMP9 were markedy increased
in T24-shRNA cells compared with T24-shNC cells (Fig. 7B and C).
Discussion
Bladder cancer is one of the most common malignant
tumors worldwide (19). Although
early detection and treatment can improve survival rates, only ~50%
of patients are diagnosed and treated prior to the development of
invasive lesions (20). Therefore,
new tumor markers and therapeutic targets are urgently needed to
improve treatment outcomes. In this study, we evaluated the
expression and function of STAG2, an important subunit of cohesin
(16), in bladder cancer. Our
findings demonstrate that low STAG2 expression is associated with
TNM stage in bladder cancer, where it plays important roles in
mediating cell motility and cell cycle progression. STAG2 is
stably expressed by non-tumor tissues; however, truncating
mutations have been identified in 36% of non-invasive bladder
cancer samples and 16% of invasive bladder cancer samples. In
bladder cancer, STAG2 mutations have been observed as truncated
mutations, including nonsense, frameshift, and splice site
mutations, resulting in absence of the protein's C-terminal domain.
In some cases, STAG2 expression is completely abolished independent
of gene mutations, suggesting that other mechanisms may also cause
loss of STAG2 expression, such as CpG promoter methylation
(10,21,22).
Balbás-Martinez et al previously reported that STAG2
inactivating mutations are significantly associated with low tumor
stage and grade, multicentricity, and tumor size and inversely
related to chromosomal copy number changes (18). In addition, the absence of STAG2
expression was associated with lower tumor stage and grade and with
female sex (10). In the present
study, we concluded that loss of STAG2 mRNA expression was
closely related to low tumor grade in bladder cancer; however, no
significant correlations were found between STAG2 loss and other
clinical features, indicating that STAG2 inactivation was an early
event that did not affect cancer progression. Recent studies have
provided contradictory findings regarding the relationship between
the STAG2 expression and the prognosis in patients with bladder
cancer. For instance, the study by Balbás-Martinez et al
determined that STAG2 deletion associated with a better prognosis
in both NMIBC and MIBC (18);
however, Guo et al found that loss of STAG2 expression
correlated with poor prognosis (8).
Moreover, STAG2 deletion is associated with different prognoses in
patients with MIBC and NMIBC (22).
The variability amongst these studies may be attributed to the
surgical approach or postoperative treatment of patients, or
regional and ethnic differences. Notably, Balbás-Martinez et
al reported that STAG2 knockdown had no effect on aneuploidy,
whereas Solomon et al found that STAG2 inactivation resulted
in cohesin downregulation and aneuploidy (18,22),
for the chromosomal aneuploidy studies, the inconsistent results
were likely attributed to differences in the selected cell lines.
Specifically, 639V, RT112, and UMUC-11 were used in Balbás-Martínez
et al study, whereas Solomon et al used RT4.
Furthermore, STAG2 is reportedly downregulated in colorectal
cancer, gastric cancer, breast cancer, non-small cell lung cancer,
and prostate cancer, suggesting that STAG2 gene inactivation
is a common feature of solid cancers (13).
In this study, we found that STAG2 was downregulated
in bladder cancer tissues when compared to matched, normal bladder
tissues, suggesting that STAG2 may exhibit inhibitory effects on
bladder cancer development. Significantly, we found that STAG2
downregulation correlated with clinical stage and pathological
grade, and inhibited the migration and invasion of bladder cancer
cells. Previous studies have shown that tumor cell migration and
invasion are related to the EMT process, which is characterized by
loss of epithelial cadherin (E-cadherin). Notably, EMT is also
required for cancer progression and metastasis, wherein the
zinc-dependent endopeptidases MMP2 and MMP9 degrade extracellular
matrix components to mediate migration in tumor cells. Consistent
with this notion, both MMP2 and MMP9 have been shown to play
important roles in bladder cancer progression (23,24).
Similarly, our data showed that STAG2 knockdown caused the
downregulation of E-cadherin protein expression as well as the
upregulation of vimentin, MMP2, and MMP9, suggesting that STAG2
regulates invasion and migration via EMT signaling in bladder
cancer cells. However, additional studies are needed to determine
the specific mechanisms regulating these processes.
Interestingly, our findings demonstrated that STAG2
also regulated cell cycle progression in bladder cancer cells
independent of proliferation rate. Thus, further studies are
necessary to elucidate the mechanisms by which this occurs.
Interestingly, our findings indicated that caspase-7 - a critical
effector caspase in apoptosis - may be a target of STAG2. Caspase-7
is frequently downregulated in gastric cancer and colorectal
cancer, and inactivating mutations have been found in head/neck
cancer, esophageal cancer, and colon cancer. Such mutations can
result in loss of capacity for apoptosis, thereby promoting cancer.
In addition, caspase-7 is highly associated with caspase-3, a major
executioner in apoptosis frequently downregulated in solid tumors
and associated with a poor prognosis (25). Our findings also identified
caspase-3 as a target of STAG2, further supporting the
pro-apoptotic role of STAG2 in bladder cancer. In view of the
impact of STAG2 on cell apoptosis, cell invasion, and migration, we
examined cells by western blotting and found that STAG2 likely
regulates various signaling pathways associated with these
processes. However, this was a preliminary analysis, and further
research is needed to clarify the mechanisms by which this
occurs.
In conclusion, our findings demonstrated that STAG2
downregulation, deletion, or loss of function is a common event in
bladder cancer and may be associated with cancer metastasis through
the regulation of MMP2, MMP9, and EMT. Additionally, STAG2 may be
involved in apoptosis by regulating caspase-3 and caspase-7
expression. Thus, these data suggest that STAG2 may be a potential
therapeutic target in patients with bladder cancer.
Acknowledgements
This study was supported by funding from the
National Natural Science Foundation of China (grant no. 81270740),
the Shenzhen Science and Technology Project (grant nos. JCYJ
20140416180323426 and JSGG 20160301162913683) and the High Level
University's Medical Discipline Construction (grant no.
2016031638).
Glossary
Abbreviations
Abbreviations:
|
STAG2
|
stromal antigen 2
|
|
BC
|
bladder cancer
|
|
MMP
|
matrix metalloproteinase
|
|
NMIBC
|
non-muscle-invasive bladder cancer
|
|
MIBC
|
muscle-invasive bladder cancer
|
|
TURBT
|
transurethral resection of the bladder
tumor
|
|
TCC
|
transitional cancer cells
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
CCK-8
|
Cell Counting Kit −8
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karakiewicz PI, Benayoun S, Zippe C,
Lüdecke G, Boman H, Sanchez-Carbayo M, Casella R, Mian C, Friedrich
MG, Eissa S, et al: Institutional variability in the accuracy of
urinary cytology for predicting recurrence of transitional cell
carcinoma of the bladder. BJU Int. 97:997–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobs BL, Lee CT and Montie JE: Bladder
cancer in 2010: How far have we come? CA Cancer J Clin. 60:244–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark PE, Agarwal N, Biagioli MC,
Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel
TM, Lele SM, et al: National Comprehensive Cancer Network (NCCN):
Bladder cancer. J Natl Compr Canc Netw. 11:446–475. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolenz C and Lotan Y: Molecular biomarkers
for urothelial carcinoma of the bladder: Challenges in clinical
use. Nat Clin Pract Urol. 5:676–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo G, Sun X, Chen C, Wu S, Huang P, Li Z,
Dean M, Huang Y, Jia W, Zhou Q, et al: Whole-genome and whole-exome
sequencing of bladder cancer identifies frequent alterations in
genes involved in sister chromatid cohesion and segregation. Nat
Genet. 45:1459–1463. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darwiche N, Freeman LA and Strunnikov A:
Characterization of the components of the putative mammalian sister
chromatid cohesion complex. Gene. 233:39–47. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor CF, Platt FM, Hurst CD, Thygesen HH
and Knowles MA: Frequent inactivating mutations of STAG2 in bladder
cancer are associated with low tumour grade and stage and inversely
related to chromosomal copy number changes. Hum Mol Genet.
23:1964–1974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solomon DA, Kim T, Diaz-Martinez LA, Fair
J, Elkahloun AG, Harris BT, Toretsky JA, Rosenberg SA, Shukla N,
Ladanyi M, et al: Mutational inactivation of STAG2 causes
aneuploidy in human cancer. Science. 333:1039–1043. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Djos A, Fransson S, Kogner P and
Martinsson T: Aneuploidy in neuroblastoma tumors is not associated
with inactivating point mutations in the STAG2 gene. BMC Med Genet.
14:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MS, Kim SS, Je EM, Yoo NJ and Lee SH:
Mutational and expressional analyses of STAG2 gene in solid
cancers. Neoplasma. 59:524–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung NG, Kim MS, Yoo NJ and Lee SH:
Somatic mutation of STAG2, an aneuploidy-related gene, is rare in
acute leukemias. Leuk Lymphoma. 53:1234–1235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kon A, Shih LY, Minamino M, Sanada M,
Shiraishi Y, Nagata Y, Yoshida K, Okuno Y, Bando M, Nakato R, et
al: Recurrent mutations in multiple components of the cohesin
complex in myeloid neoplasms. Nat Genet. 45:1232–1237. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Losada A, Yokochi T, Kobayashi R and
Hirano T: Identification and characterization of SA/Scc3p subunits
in the Xenopus and human cohesin complexes. J Cell Biol.
150:405–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jessberger R: Cohesin's dual role in the
DNA damage response: Repair and checkpoint activation. EMBO J.
28:2491–2493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balbás-Martínez C, Sagrera A,
Carrillo-de-Santa-Pau E, Earl J, Márquez M, Vazquez M, Lapi E,
Castro-Giner F, Beltran S, Bayés M, et al: Recurrent inactivation
of STAG2 in bladder cancer is not associated with aneuploidy. Nat
Genet. 45:1464–1469. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cha K, Hadjiiski L, Chan HP, Caoili EM,
Cohan RH and Zhou C: CT urography: Segmentation of urinary bladder
using CLASS with local contour refinement. Phys Med Biol.
59:2767–2785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao T, Wallace J and Felsenfeld G:
Specific sites in the C terminus of CTCF interact with the SA2
subunit of the cohesin complex and are required for
cohesin-dependent insulation activity. Mol Cell Biol. 31:2174–2183.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Solomon DA, Kim JS, Bondaruk J, Shariat
SF, Wang ZF, Elkahloun AG, Ozawa T, Gerard J, Zhuang D, Zhang S, et
al: Frequent truncating mutations of STAG2 in bladder cancer. Nat
Genet. 45:1428–1430. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cormio L, Sanguedolce F, Massenio P, Di
Fino G, Selvaggio O, Bufo P and Carrieri G: Osseous metaplasia
within a urothelial bladder cancer nodal metastasis: A case report.
Anal Quant Cytopathol Histpathol. 36:117–119. 2014.PubMed/NCBI
|
|
24
|
Zhao H, Yuan X, Jiang J, Wang P, Sun X,
Wang D and Zheng Q: Antimetastatic effects of licochalcone B on
human bladder carcinoma T24 by inhibition of matrix
metalloproteinases-9 and NF-кB activity. Basic Clin Pharmacol
Toxicol. 115:527–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|