Introduction
Cervical cancer, one of the most common
gynecological malignancies worldwide, threatens the health of women
(1). It has been reported that 80%
of cervical cancer cases occur in developing countries and that
persistent high-risk human papilloma virus (HPV) infection is
closely related to the occurrence of cervical cancer (2,3).
However, not all women infected with high-risk HPV progress to
cervical cancer, and few progress to squamous intraepithelial
lesions (SIL). Moreover, women who get infected with HIV and who
take immunosuppressive agents are more likely to develop SIL or
cervical cancer (4). Thus, immune
factors may be related to the pathogenesis of cervical cancer.
The B7 immunoregulatory family includes B7-1 (CD80),
B7-2 (CD86), B7-H1 (PD-L1), B7-H2 (ICOSL), B7-DC (PD-L2), B7-H3
(CD276) and B7-H4 (B7X) (5). B7s
play a crucial role in regulating adaptive immune response. The
B7:CD28 superfamily can promote T cell activation or inhibit the T
cell response (6). B7-H3 (or B7
homolog 3, CD276) was identified by database searches of a human
dendritic cell-derived cDNA library in 2001 (7). In addition, Zhang et al found
soluble B7-H3 in human serum/plasma in 2008 (8). B7-H3 has 2 forms: 2Ig- and 4Ig-B7-H3.
2Ig-B7-H3 has extracellular IgV-IgC domains and is present in both
mouse and human cells, whereas 4 Ig-B7-H3 consists of tandemly
duplicated IgV-IgC-IgV-IgC domains and is only expressed in human
cells (9,10). B7-H3 is widely expressed in various
organs such as lung, breast, liver, placenta, the prostate and in
dendritic cells. In addition, B7-H3 expression was elevated in
several cancer cell lines or tumor tissues, including prostate,
brain, pancreatic, breast, endometrial, liver, colorectal and oral
cancers, and osteosarcomas and hematologic malignancies (11–23).
B7-H3 is similar in molecular structure to B7-H1
(PD-L1), and PD-L1 is associated with PD-1 and restrains T cell
antitumor functions (24,25). However, the function of B7-H3 in the
immune response remains unclear. B7-H3 was originally discovered to
costimulate proliferation of CD4+ and CD8+ T
cells, enhance induction of cytotoxic T cells and selectively
stimulate interferon-γ (IFN-γ) production in the presence of T cell
receptor signaling (7). In sharp
contrast, B7-H3 downregulated T helper type 1-mediated immune
responses in a murine model (26).
Mouse and human B7-H3 inhibited CD4+ T cell activation
and downregulated cytokine production (10).
B7-H3 was proven to influence tumor cell biological
features in vitro and in vivo, but the role of B7-H3
in cancer is unclear. Immunohistochemical evidence suggests that
high expression of B7-H3 in cancer tissues may be associated with
disease spread and poor outcomes (14,17,27).
Recent functional studies revealed that high expression of B7-H3
may promote tumor cell metastatic ability (18,28,29).
The role of B7-H3 and its expression in cervical
cancer have not been extensively investigated. In the present
study, we aimed to investigate the relationship between B7-H3
expression and the prognosis of cervical cancer patients and its
potential mechanisms in cervical cancer in vitro.
Materials and methods
Patients and follow-up
The present study was approved by the Ethics
Committee of Qilu Hospital, Shandong University [ethics approval
code, KYLL-2014 (KS)-074]. Patient information was anonymized and
de-identified. All participants joined the present study after
providing informed consent.
Tissue and blood sample analysis
We identified 90 cervical cancer and 20 non-cervical
lesion patients with tissue available in paraffin-embedded blocks
who were treated at Qilu Hospital, Shandong University between 2009
and 2010. All cervical cancer patients were diagnosed with cervical
cancer for the first time and no patient accepted adjuvant therapy
prior to surgery. In addition, the exclusion criteria included: i)
patients who also had autoimmune diseases; ii) patients who had
received immunosuppressive drugs; iii) patients who were
HIV-positive; iv) the presence of synchronous tumors; v) patients
who had preoperative pelvic radiotherapy and vi) patients who had
preoperative chemotherapy. Follow-up was carried out according to
the National Comprehensive Cancer Network (NCCN) guidelines: every
month in the first year; every third month in the second year;
twice in the third, fourth and fifth years; and annually
thereafter. After treatment, all patients were followed up until
May 2016. Follow-up duration was calculated from the date of
surgery to the date of death and/or the last follow-up.
In order to measure the B7-H3 level in blood
samples, we identified 30 cervical cancer patients and 30 healthy
donors who were treated at Qilu Hospital, Shandong University
between 2015 and 2016. All cervical cancer patients were diagnosed
with cervical cancer for the first time and no patient accepted
adjuvant therapy prior to surgery. The inclusion criteria and
exclusion criteria of the cervical cancer patients were the same as
those described above. Patient blood samples were collected before
surgery and were stored at −80°C. We measured serum B7-H3 of 30
cervical cancer patients and 30 healthy donors (catalog no.
LS-F22755; LifeSpan Biosciences, Inc., Seattle, WA, USA).
Immunohistochemical (IHC)
staining
The antibody used was anti-B7-H3 [1:100; 14058P;
Cell Signaling Technology (CST) Danvers, MA, USA]. Staining was
performed as described in the manual of an ultrasensitive
streptavidin-peroxidase kit (PV-9001; ZSGB-Bio, Beijing, China).
IHC staining was evaluated by 2 gynecological pathologists blinded
to patient outcomes. The percent of tumor cells positive for B7-H3
were recorded in 25% intervals: 0, no positive cells; 1, 1–25%
positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells;
and 4, 76–100% positive cells; and IHC intensity was recorded as:
0, no staining; 1, weak staining; 2, moderate staining and 3,
strong staining. The 2 scores were then added to get the final
score as follows: 0–3, (−); 4–5, (+); 6–7, (++).
Enzyme-linked immunosorbent assay
(ELISA)
Patient blood samples were collected before surgery
and stored at −80°C. Serum B7-H3 was measured in cervical cancer
and healthy donors samples using ELISA (catalog no. LS-F22755;
LifeSpan Biosciences, Inc.).
Cell lines and cell culture
HeLa, SiHa and CaSki, human cervical cancer cell
lines, were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). Cells were cultured in Roswell Park
Memorial Institute (RPMI)-1640 (Gibco, Thermo Fisher Scientific,
Waltham, MA, USA) medium containing 10% fetal bovine serum (FBS)
(Clark, Houston, TX, USA), at 37°C in a 5% CO2
incubator.
Immunofluorescent staining
CaSki, SiHa and HeLa cells were cultured
(4×104 cells/well) in 24-well plates, and incubated at
37°C in a 5% CO2 incubator overnight. CaSki, SiHa and
HeLa cells were washed 3 times with phosphate-buffered saline
(PBS), fixed in 4% cold paraformaldehyde for 15 min, permeabilized
with 0.2% Triton X-100 for 10 min and blocked in 10% goat serum.
Then the cells were stained with mouse anti-B7-H3 monoclonal
antibody (1:400; ab105922; Abcam, Cambridge, MA, USA) at 4°C
overnight. Cells were washed with PBS 3 times, and incubated with
secondary antibody (1:200; ZSGB-Bio) at room temperature in the
dark. After staining with 4,6-diamidino-2-phenylindole (DAPI)
(Beyotime Institute of Biotechnology, Shanghai, China) away from
light for 3 min, the cells were photographed under an Olympus IX51
inverted microscope (Olympus, Tokyo, Japan).
Generation of stable cell lines
The human B7-H3 (gene ID: 80381; NM_001024736)
targeting small hairpin (sh)RNA sequence and a negative
non-targeted control sequence were used to generate recombinant
lentiviral particles. Both sequences were designed by the
GenePharma Company (Shanghai, China). The targeting sequence of
B7-H3 was 5′-GUGCUGGAGAAAGAUCAAATTUUUGAUCUUUCUCCAGCACTT-3′. The
transfected cells included the B7-H3 shRNA (KD) and negative
non-targeted control (NC) group, and the non-transfected cells were
controls (CON). These 3 groups were used for the following
experiments.
We transfected HeLa and CaSki cells with the B7-H3
plasmid (GenePharma Company). The empty plasmid was also designed
by GenePharma Company, and used as a negative control. The
transfected cells included LV-B7-H3 (LV) and negative non-targeted
control (NC) group, and the non-transfected cells were the control
(CON) group.
This recombinant lentivirus was prepared and titered
for 1×109 TU/ml (transfection units). Cells were
cultured at 1×105 cells/well into 6-well tissue culture
plates overnight. The lentiviral supernatant was added into cells
and the multiplicity of infection (MOI) was 10. After transfection,
the puromycin dihydrochloride was used to generate stable cell
lines. Western blot analysis was performed to confirm the silencing
or overexpression of B7-H3.
Western blotting
Cells were lysed on ice and protein was extracted
using RIPA, PMSF and NaF (100:1:1; Beyotime Institute of
Biotechnology). Protein was measured using a BCA protein assay kit
(TianGen, Beijing, China). Then, 30 µg protein was separated with
SDS-PAGE and transferred onto polyvinylidene fluoride membranes.
Membranes were blocked with 5% non-fat dry milk and then incubated
with primary antibodies at 4°C overnight. Primary antibodies were
anti-B7-H3 (1:1,000; 14058P) and anti-GAPDH (1:1,000 dilution)
(both from CST). Membranes were washed 3 times with Tris-buffered
saline and Tween-20 (TBST) and incubated with secondary antibodies.
Signals were quantified using ImageQuant LAS 4000 (General Electric
Company, Fairfield, CT, USA). Data were analyzed with ImageJ
[National Institutes of Health (NIH), Bethesda, MD, USA].
Cell migration and invasion
assays
For the migration and invasion assays,
7×104 cells were resuspended in serum-free medium and
placed on the membrane of chambers (Corning, New York, NY, USA).
For the invasion assay, the membrane was covered with Matrigel (1:9
dilution; BD Biosciences, Franklin Lakes, NJ, USA). The lower
chambers contained medium with 20% FBS. Following 24 h of
incubation, the migrating cells in the lower chamber or invading
cells on the bottom of each well were stained with Giemsa
(Solarbio, China) followed by fixation in methyl alcohol for 30
min. Then, the number of cells in 6 randomly selected microscopic
fields (magnification, ×200) was counted with an Olympus IX51
inverted microscope.
Cell viability assay
Cell viability was assessed using the MTT assay.
Cells of each group were plated at 2,000 cells/well in a 96-well
plate for 24, 48 or 72 h. At each time point, 20 µl MTT was added
into each 96-well plate. After incubation at 37°C for 4 h the MTT
solution was removed. Dimethyl sulfoxide (100 µl) was added to each
well and evaluated at 490 nm with Infinite M200 PRO (Bio-Rad
Laboratories, Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corporation, Armonk, NY, USA). Results were presented
as means ± SD. Prognosis after surgery was estimated using the
Kaplan-Meier method. The association between expression of B7-H3
and tumor-node-metastasis (TNM) stage was analyzed using the
Spearman correlation method. Associations of B7-H3 with
cancer-specific survival were evaluated with Cox models.
Immunohistochemistry was assessed with the Chi-squared test.
Quantitative data comparisons were performed using a Student's
t-test and one-way analysis of variance (ANOVA). A p-value <0.05
was considered to indicate statistical significance.
Results
Clinical and pathological features and
patient outcomes
The mean age of the 90 cervical cancer patients was
44.66±10.11 years (range, 22–70 years). According to the
International Federation of Gynecology and Obstetrics (FIGO) stage,
12 patients were in stage Ia; 48 were in stage Ib; 23 were in stage
IIa; 3 were in stage IIb; and 4 were in stage IIIb. Median
follow-up time was 5.54±1.60 years. At the last follow-up, 13
patients had died from cervical cancer and the median survival
period of these 13 patients was 2.11±1.58 years.
Immunohistochemical staining
B7-H3 expression level in the cervical cancer
patient group was significantly higher than that in the normal
patient group (mean 72.22 vs. 15.00%; p<0.001).
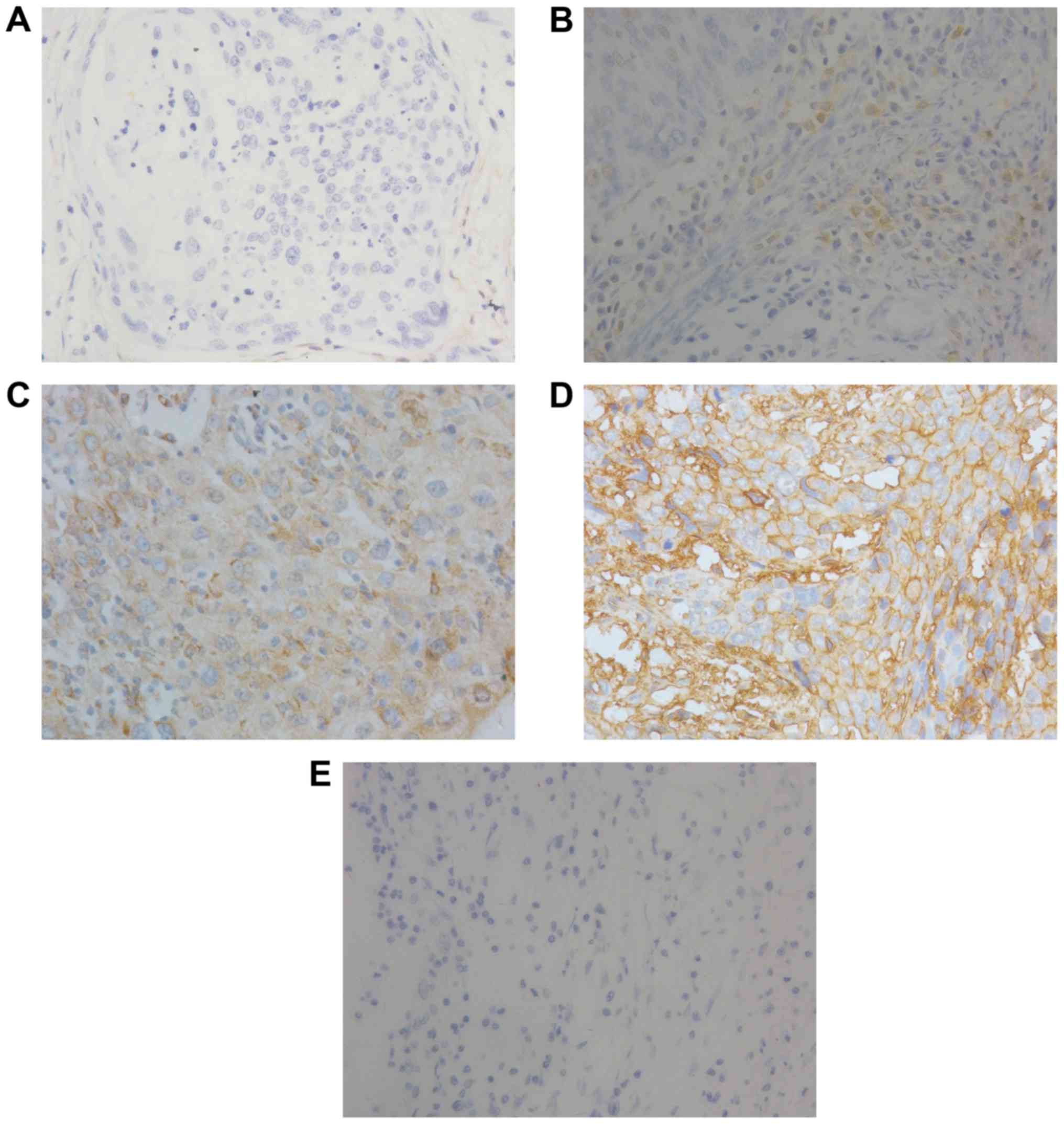
Immunohistochemical staining significantly revealed overexpressed
B7-H3 in the tumor tissues and positive staining for B7-H3
expression was detected in the cytoplasm of the cervical cancer
cells (Fig. 1). Strong intensity
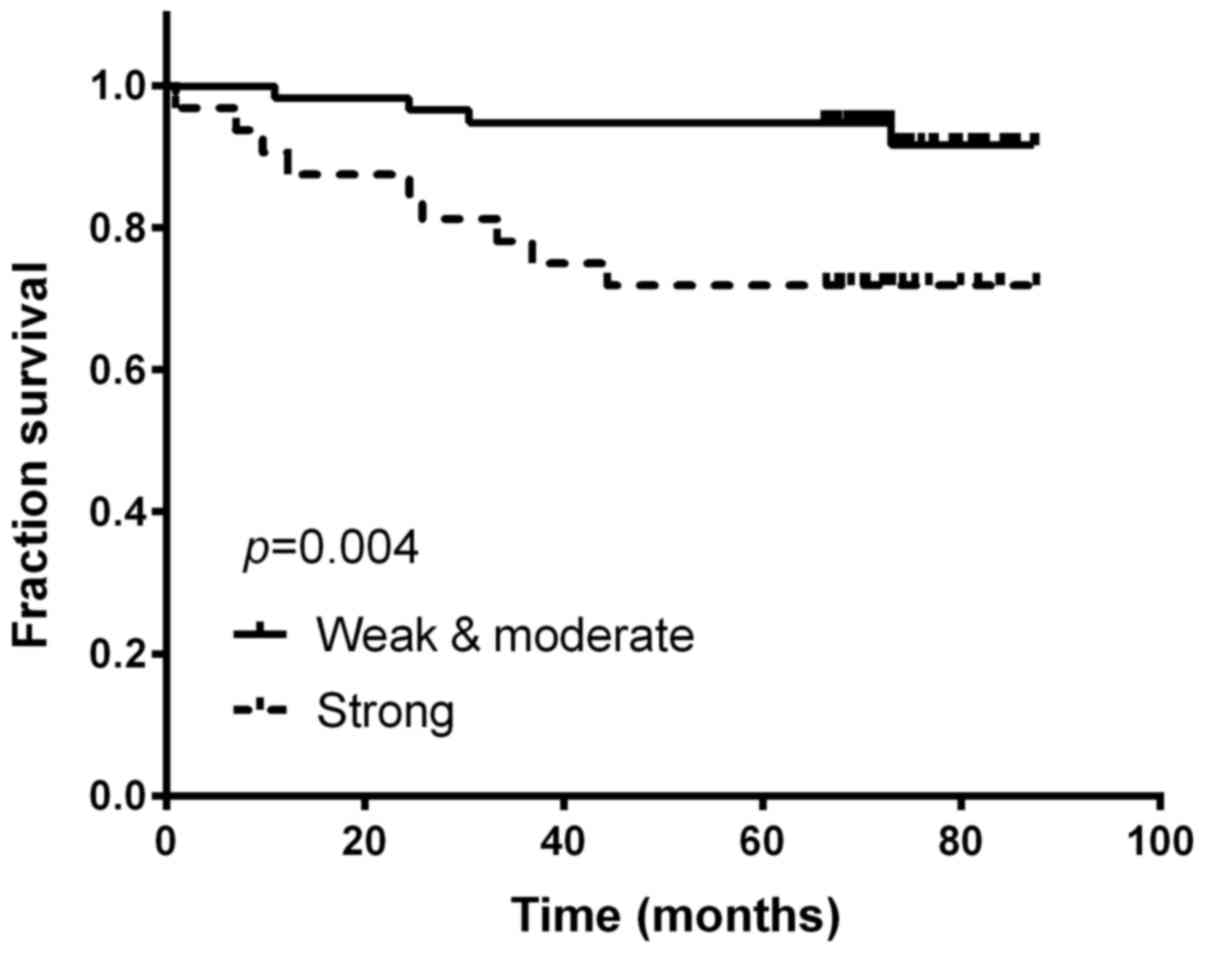
was found in 33 (36.67%) of the cervical cancer patients, and
patients with strong intensity staining were significantly more
likely to have worse prognosis (Kaplan-Meier method; p=0.004;
Fig. 2). As shown in Table I, the frequency of B7-H3 high
expression was significantly higher in patients with stage II/III
(21/30) compared to those with stage I (12/60; p<0.001). B7-H3
expression was correlated with TNM stage (r=0.509; p<0.001) and
the 5-year survival rate for patients with strong expression of
B7-H3 was significantly lower than those patients with negative to
moderate expression (94.8 vs. 71.9%; p=0.004). In a multivariable
Cox regression model, incorporation of age, histology,
differentiation, clinical stage and B7-H3 expression revealed that
patients with high expression of B7-H3 were significantly more
likely to die from cervical cancer [HR, 4.463; 95% confidence
interval (CI), 1.12–17.8; p=0.035] compared with those with
moderate or weak expression.
 | Table I.Correlation of B7-H3 expression with
clinicopathological characteristics of the cervical cancer
cases. |
Table I.
Correlation of B7-H3 expression with
clinicopathological characteristics of the cervical cancer
cases.
|
|
| B7-H3
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. of pts.
(n=90) | Low | High | P-value |
|---|
| Age (years) |
|
|
| 0.142 |
|
18–45 | 40 | 22 | 18 |
|
|
46–70 | 50 | 35 | 15 |
|
| FIGO stage |
|
|
| <0.001 |
| I | 60 | 48 | 12 |
|
|
II–III | 30 | 9 | 21 |
|
|
Differentiation |
|
|
| 0.491 |
|
Well | 17 | 12 | 5 |
|
|
Moderate or poor | 73 | 45 | 28 |
|
|
Histology |
|
|
| 0.152 |
|
Squamous cell carcinoma | 74 | 44 | 30 |
|
|
Adenocarcinoma and
adenosquamous carcinoma | 16 | 13 | 3 |
|
ELISA
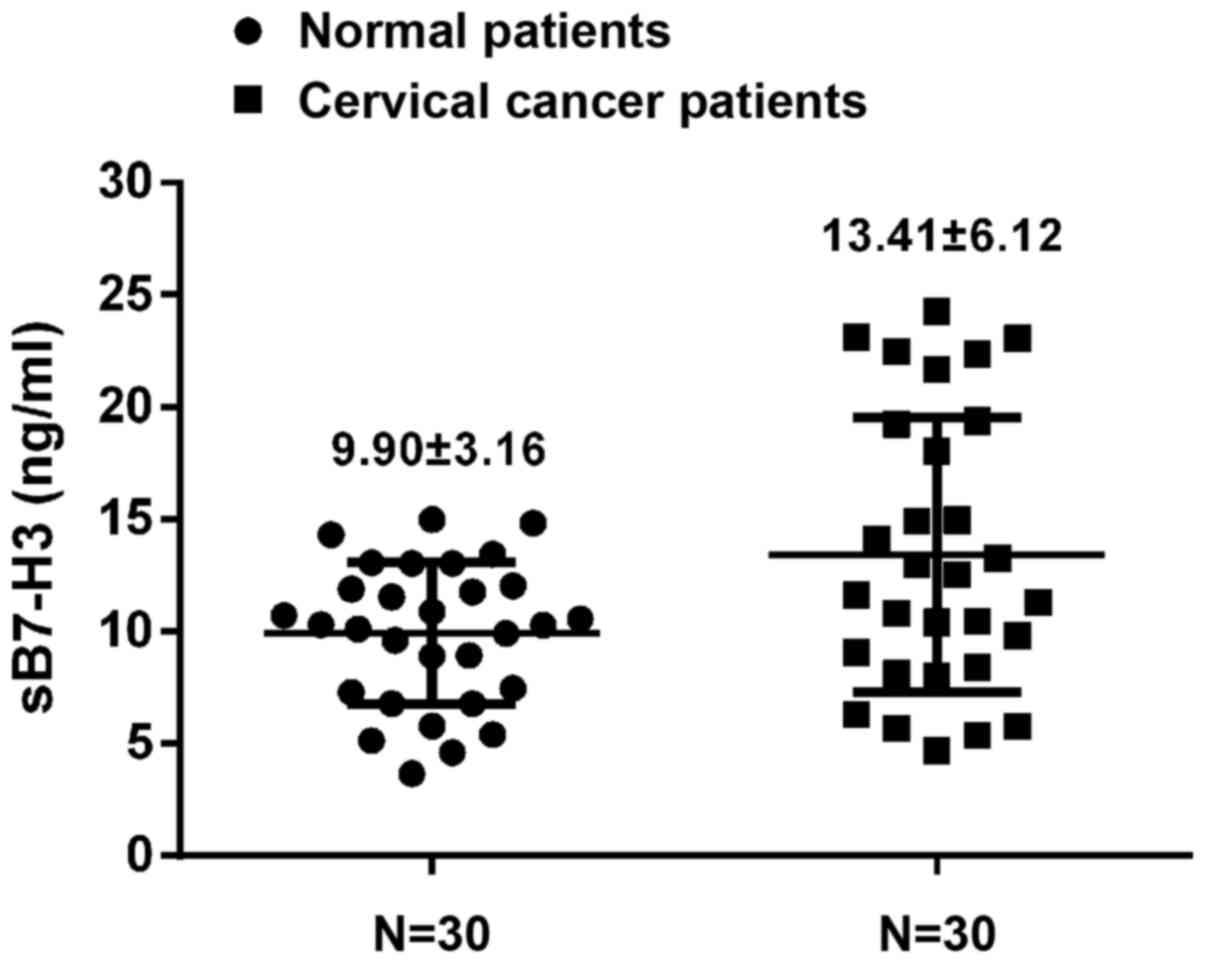
Serum B7-H3 was measured in normal donors and
cervical cancer patients and B7-H3 in cervical cancer patients was
significantly higher than that noted for the normal donors
(13.41±6.12 vs. 9.90±3.16 ng/ml; p=0.007) (Fig. 3).
Immunofluorescent staining
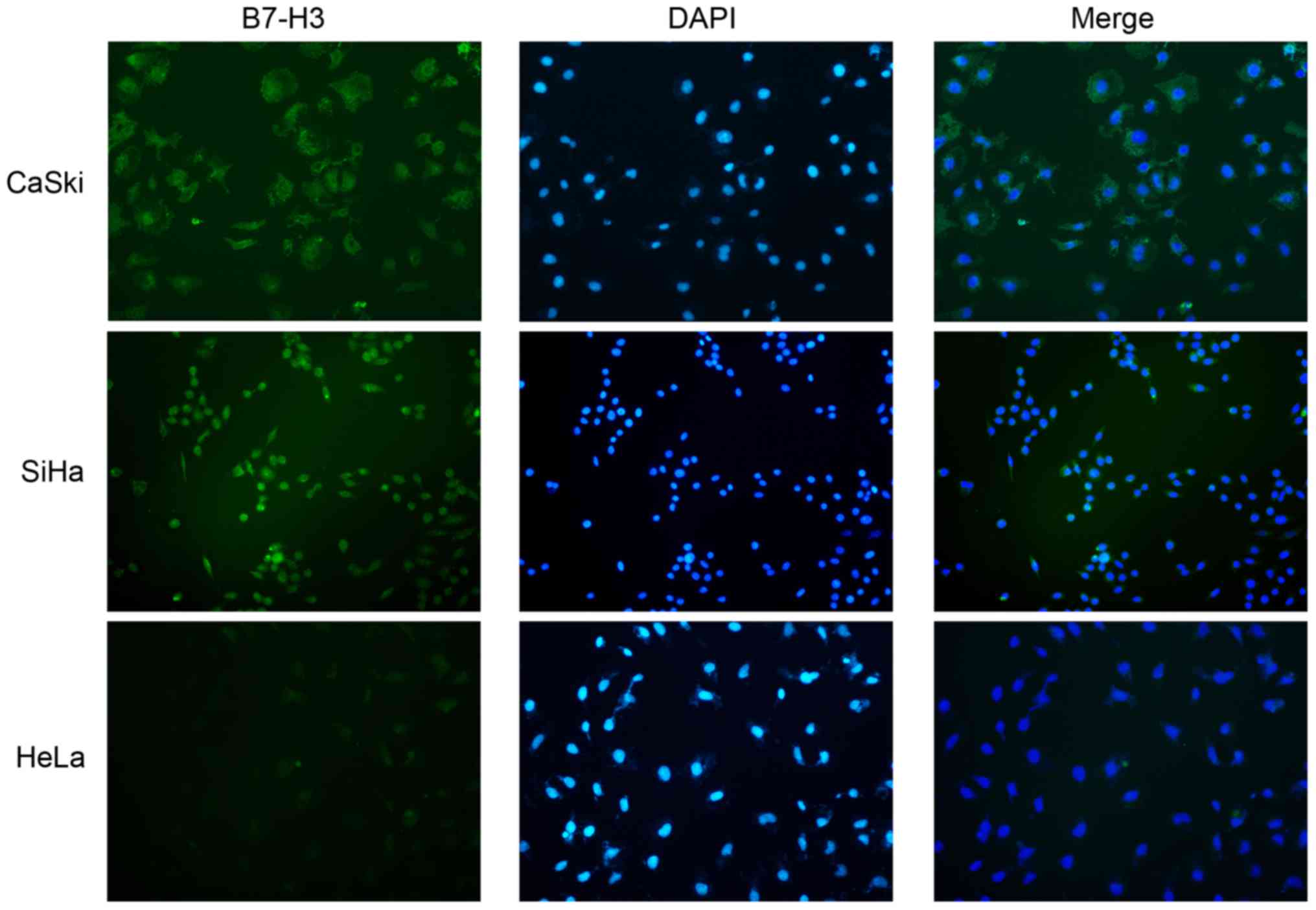
Cellular localization of B7-H3 in cervical cancer
cell lines was studied with immunofluorescence in CaSki, SiHa and
HeLa cell lines and B7-H3 was mostly distributed in the cytoplasm
(Fig. 4).
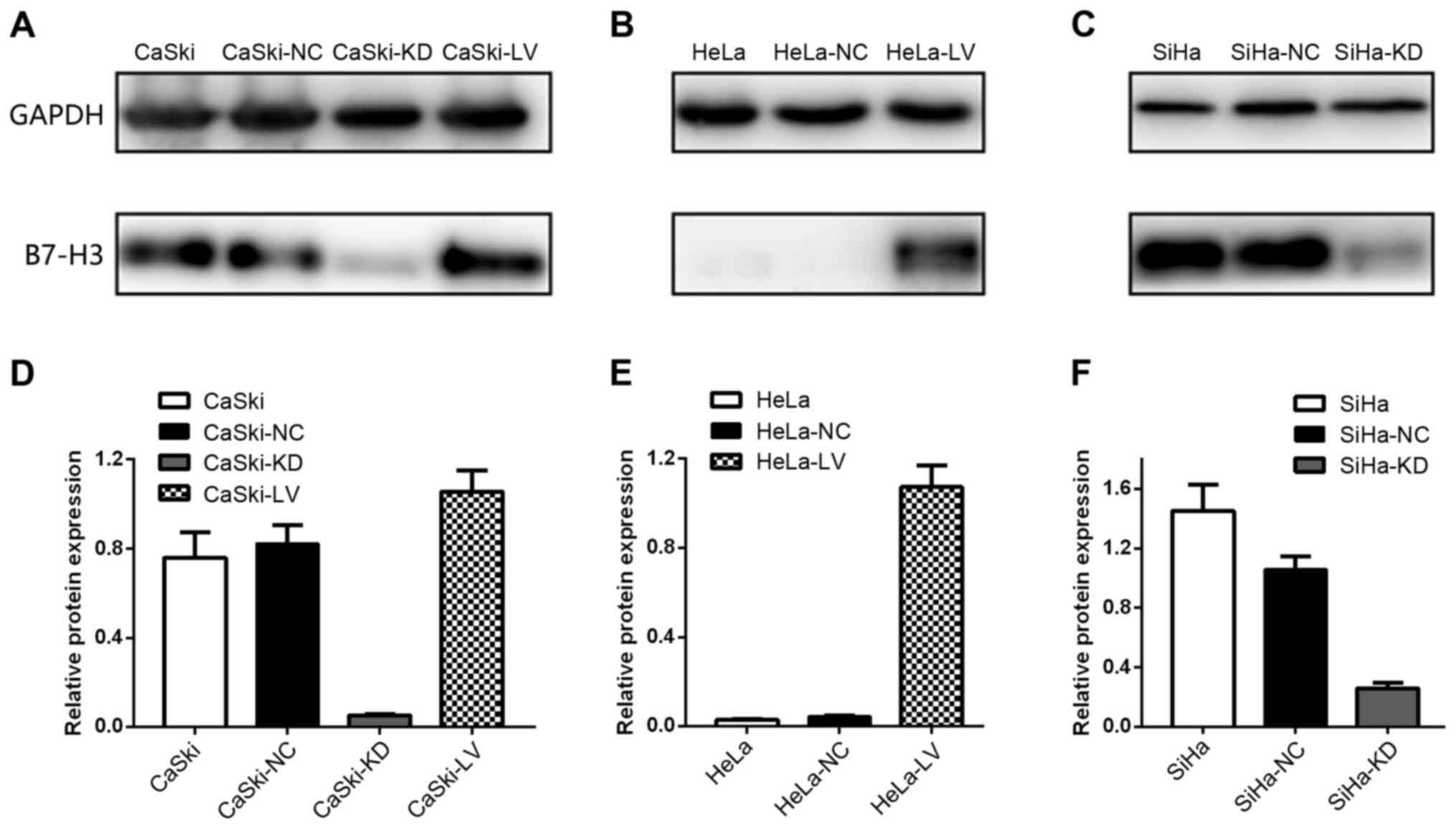
B7-H3 upregulation and downregulation
in cervical cancer cell lines
After infection with a lentiviral vector, HeLa,
CaSki and SiHa cells were assessed using western blotting (Fig. 5). Lentiviral infection was efficient
and the data revealed that upregulation and downregulation of the
B7-H3 gene was stable and efficient.
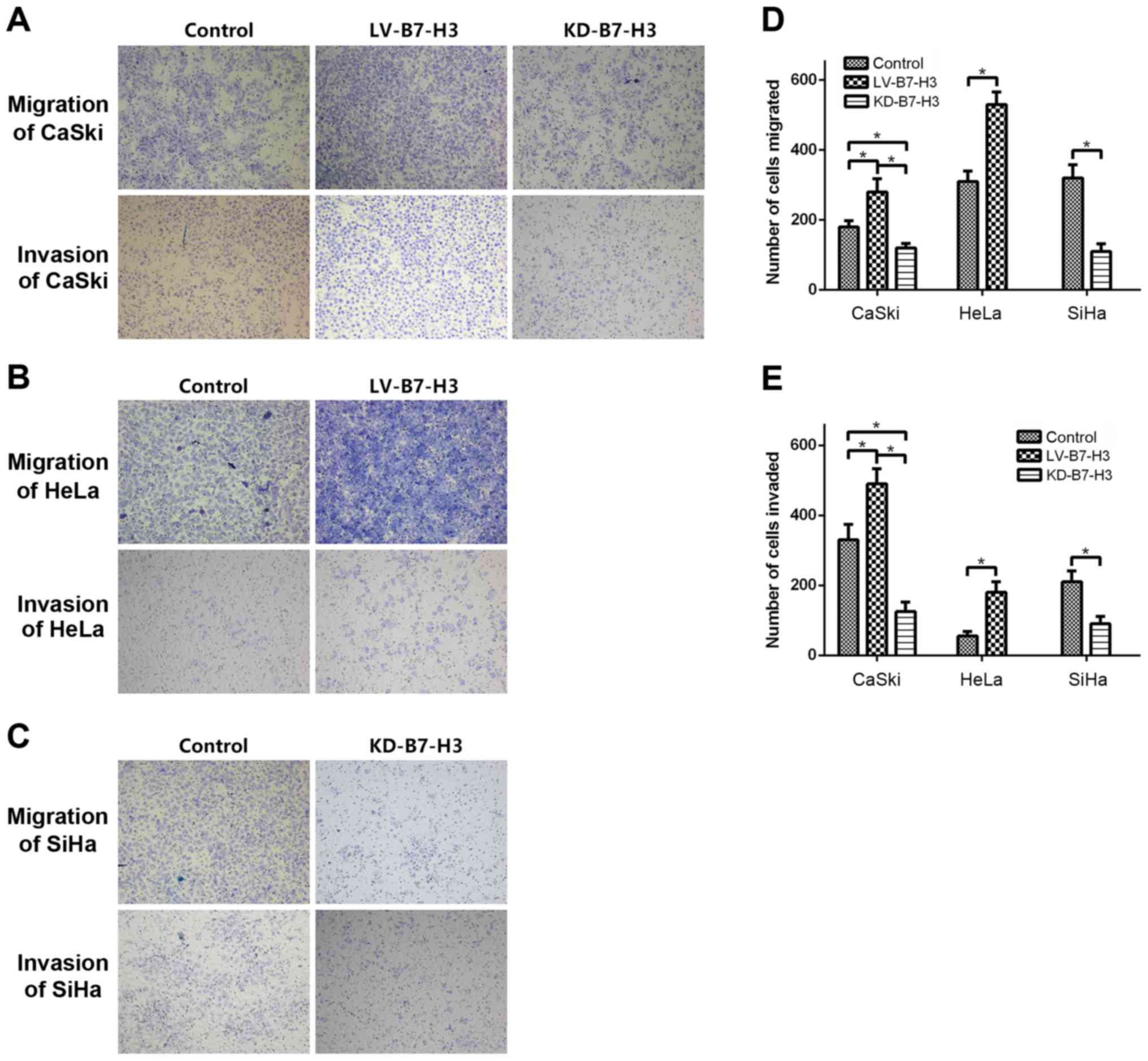
Cell migration and invasion assay
To investigate whether B7-H3 influences tumor
migration and invasion, Transwell migration and invasion assays
were used to compare cell migration and invasiveness in each group,
and Fig. 6 shows that KD-B7-H3 cell
migration to the lower chamber was significantly reduced after 24 h
of incubation compared to the controls (CaSki, p=0.001; SiHa,
p=0.005). In contrast, migration of LV-B7-H3 cells was increased
(CaSki, p=0.003; HeLa, p=0.03).
Cell viability assay
MTT assay was used to measure whether B7-H3
influenced cervical cancer cell viability, and data showed that
cell viability declined in the KD-B7-H3 groups at 24, 48 and 72 h
(Fig. 7) compared to the normal
controls (CaSki, p=0.026; SiHa, p<0.001). LV-B7-H3 group
viability was significantly increased compared to the normal
controls (CaSki, p=0.009; HeLa, p<0.001).
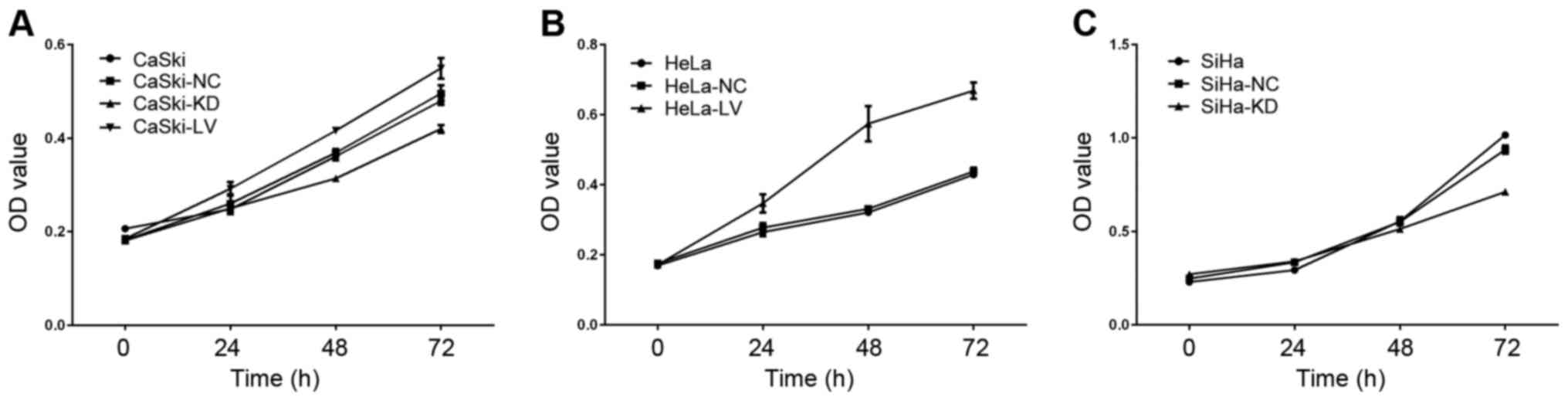 | Figure 7.The role of B7-H3 in cell viability.
(A) CaSki, CaSki-NC, CaSki-KD and CaSki-LV groups were cultured for
0, 24, 48 and 72 h, and cell viability was measured via MTT assay.
(B) HeLa, HeLa-NC and HeLa-LV groups were cultured for 0, 24, 48
and 72 h, and cell viability was measured via MTT assay. (C) SiHa,
SiHa-NC and SiHa-KD groups were cultured for 0, 24, 48 and 72 h,
and cell viability was measured using MTT assay. |
Discussion
B7-H3 is a B7 ligand family member discovered in
2001 (7). Recent studies indicate
that B7-H3 is linked to tumor malignancy, but its clinical and
functional significance in cervical cancer is unclear. To the best
of our knowledge, we offered the first study of the role of B7-H3
in cervical cancer proliferation and metastasis in vitro.
Using immunohistochemistry, we measured B7-H3 expression and noted
that B7-H3 was significantly higher in cervical cancer than that
noted in normal cervical tissues (p<0.001), and patients with
strong intensity staining were significantly more likely to have
worse prognosis (p=0.004). We also demonstrated that B7-H3 promoted
cervical cancer cell proliferation, migration and invasiveness
in vitro.
The immune system is key to preventing HPV viral
oncogenesis and tumor progression (30). In an adaptive immune response,
activation of T cells requires 2 signals: first T cell receptors
recognize peptides presented by major histocompatibility complexes
(MHCs) on antigen presenting cells; secondly, co-stimulatory
molecules provide the second signal (6). The B7:CD28 superfamily is crucial for
regulating adaptive cellular immunity, not only elevating T cell
activation, but also inhibiting T cell responses by providing
positive or negative second signals (6,31). The
B7-1:CD28 pathway delivers positive signals for T cell activation
and survival; however, the B7-2:CTLA4 (cytotoxic T
lymphocyte-associated antigen 4) pathway inhibits the T cell
response (32). Moreover, B7s may
contribute to tumor cell proliferation, migration and invasion
(33–36). B7-H3 is a B7 ligand family member
first discovered to play a stimulatory role in antitumor immune
response in gastric carcinoma and in a lymphoma model, B7-H3
inhibited tumor growth and induced an antitumor response (37,38).
However, in other human tumors, such as renal clear cell carcinoma
and prostate cancer, high expression of B7-H3 was related to
adverse clinicopathological features and poor outcomes and B7-H3
was thought to promote tumor progression (39,40).
Reasons for such discrepancies are not clear and screening the
B7-H3 receptor may help clarify the confusion. Other than
immunological functions, B7-H3 can promote tumor cell proliferation
and invasion in vitro and downregulation of B7-H3 expression
may enhance tumor cell sensitivity to chemotherapeutic drugs
according to recent functional studies (16,29,41,42).
In the present study, we investigated the relationship between
B7-H3 and the progression of cervical cancer.
We have shown that B7-H3 was aberrantly expressed in
cervical cancer tissues and higher expression of B7-H3 in cervical
cancer patients may be associated with poor prognosis. ELISA data
indicated that sB7-H3 was significantly higher in the cervical
cancer patients blood samples compared to healthy donors. Our
results were consistent with the studies in context of other
tumors. Arigami et al revealed that high B7-H3 expression in
breast cancer cells was a tumor progression factor and was
associated with the extent of regional nodal metastasis (14). Increased B7-H3 in prostate cancer
represented an independent predictor of disease spread and poor
outcomes (11,27). Crispen et al suggested that
B7-H3 could be an independent prognostic factor for clear cell
renal cancer (39). High B7-H3
expression was also associated with lymph node metastasis and TNM
stage in non-small cell lung cancer (43). Wang et al found that
osteosarcoma patients with higher B7-H3 expression had shorter
survival and time to tumor recurrence (18). The results of the present study
indicate that B7-H3 may be a new prognostic factor for cervical
cancer.
To further explore whether B7-H3 is associated with
tumor metastasis, invasion and migration assays in vitro
were performed, and data showed that high expression of B7-H3
promoted cervical cell migration and invasion. B7-H3 had also been
approved to play a role in the metastasis of other tumors. Xie
et al demonstrated that soluble B7-H3 significantly promoted
migration and invasion of pancreatic cancer cells (41). B7-H3 overexpression in a prostate
cancer cell line (PC-3) significantly increased migration and
invasiveness of PC-3 cells (44).
Downregulation of B7-H3 inhibited the migratory potential and
Matrigel invasiveness of melanoma cells and B7-H3 could be a
potential therapeutic target for antimetastatic therapy (29).
Previous functional studies indicated that B7-H3 had
a critical role in tumor cell line proliferation. Zhang et
al showed that B7-H3 silencing significantly inhibited U937
cell growth (42). In mantle cell
lymphoma, high expression of B7-H3 promoted tumor proliferation
in vitro and in a mouse model (45). In the present study, our results
revealed that downregulation of B7-H3 significantly inhibited
cervical cancer cell growth in vitro, conversely,
upregulation of B7-H3 significantly promoted cervical cancer cell
growth. These results indicated that B7-H3 could enhance tumor
progression and B7-H3 might be a potential therapeutic target for
cervical cancer.
In summary, to the best of our knowledge, this is
the first study to suggest a clinical and functional role of B7-H3
in cervical cancer. Our results indicated that aberrant B7-H3
expression might be associated with poor prognosis and B7-H3
affected cervical cancer cell proliferation and metastasis in
vitro. Study limitations include a small sample size and a lack
of data validation in vivo. The molecular biological
mechanisms of B7-H3 in metastasis and progression of cervical
cancer warrant further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572559).
References
|
1
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akhtar-Danesh N, Lytwyn A and Elit L:
Five-year trends in mortality indices among gynecological cancer
patients in Canada. Gynecol Oncol. 127:620–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crow JM: HPV: The global burden. Nature.
488:S2–S3. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devi Prabha K and Priya Bindhu N:
Conventional Pap smear screening in HIV seropositive women in South
India. J Obstet Gynaecol India. 63:55–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofmeyer KA, Ray A and Zang X: The
contrasting role of B7-H3. Proc Natl Acad Sci USA. 105:10277–10278.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung J and Suh WK: The CD28-B7 family in
anti-tumor immunity: Emerging concepts in cancer immunotherapy.
Immune Netw. 14:265–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al: B7-H3: A
costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang G, Hou J, Shi J, Yu G, Lu B and
Zhang X: Soluble CD276 (B7-H3) is released from monocytes,
dendritic cells and activated T cells and is detectable in normal
human serum. Immunology. 123:538–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun M, Richards S, Prasad DVR, Mai XM,
Rudensky A and Dong C: Characterization of mouse and human B7-H3
genes. J Immunol. 168:6294–6297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling V, Wu PW, Spaulding V, Kieleczawa J,
Luxenberg D, Carreno BM and Collins M: Duplication of primate and
rodent B7-H3 immunoglobulin V- and C-like domains: Divergent
history of functional redundancy and exon loss. Genomics.
82:365–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM,
Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML,
et al: B7-H3 ligand expression by prostate cancer: A novel marker
of prognosis and potential target for therapy. Cancer Res.
67:7893–7900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu H, Cheung IY, Guo HF and Cheung NKV:
MicroRNA miR-29 modulates expression of immunoinhibitory molecule
B7-H3: Potential implications for immune based therapy of human
solid tumors. Cancer Res. 69:6275–6281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arigami T, Narita N, Mizuno R, Nguyen L,
Ye X, Chung A, Giuliano AE and Hoon DSB: B7-h3 ligand expression by
primary breast cancer and associated with regional nodal
metastasis. Ann Surg. 252:1044–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brunner A, Hinterholzer S, Riss P, Heinze
G and Brustmann H: Immunoexpression of B7-H3 in endometrial cancer:
Relation to tumor T-cell infiltration and prognosis. Gynecol Oncol.
124:105–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi
Y, Shi JY, Xu YF, Shi YH, Song K, et al: B7-H3 is expressed in
human hepatocellular carcinoma and is associated with tumor
aggressiveness and postoperative recurrence. Cancer Immunol
Immunother. 61:2171–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ingebrigtsen VA, Boye K, Tekle C, Nesland
JM, Flatmark K and Fodstad O: B7-H3 expression in colorectal
cancer: Nuclear localization strongly predicts poor outcome in
colon cancer. Int J Cancer. 131:2528–2536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Zhang Q, Chen W, Shan B, Ding Y,
Zhang G, Cao N, Liu L and Zhang Y: B7-H3 is overexpressed in
patients suffering osteosarcoma and associated with tumor
aggressiveness and metastasis. PLoS One. 8:e706892013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong
F, Zhang ZX, Zhang GB, Zhang XG and Zhao H: B7-H3 overexpression in
pancreatic cancer promotes tumor progression. Int J Mol Med.
31:283–291. 2013.PubMed/NCBI
|
|
20
|
Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG
and Huang JA: Induced expression of B7-H3 on the lung cancer cells
and macrophages suppresses T-cell mediating anti-tumor immune
response. Exp Cell Res. 319:96–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Lv X, Wu Y, Xu J, Wang L, Chen W,
Zhang W, Li J, Zhang S and Qiu H: Expression of costimulatory
molecule B7-H3 and its prognostic implications in human acute
leukemia. Hematology. 20:187–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JT, Chen CH, Ku KL, Hsiao M, Chiang
CP, Hsu TL, Chen MH and Wong CH: Glycoprotein B7-H3 overexpression
and aberrant glycosylation in oral cancer and immune response. Proc
Natl Acad Sci USA. 112:13057–13062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guery T, Roumier C, Berthon C, Renneville
A, Preudhomme C and Quesnel B: B7-H3 protein expression in acute
myeloid leukemia. Cancer Med. 4:1879–1883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suh WK, Gajewska BU, Okada H, Gronski MA,
Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et
al: The B7 family member B7-H3 preferentially down-regulates T
helper type 1-mediated immune responses. Nat Immunol. 4:899–906.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemke D, Pfenning PN, Sahm F, Klein AC,
Kempf T, Warnken U, Schnölzer M, Tudoran R, Weller M, Platten M, et
al: Costimulatory protein 4IgB7H3 drives the malignant phenotype of
glioblastoma by mediating immune escape and invasiveness. Clin
Cancer Res. 18:105–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tekle C, Nygren MK, Chen YW, Dybsjord I,
Nesland JM, Maelandsmo GM and Fodstad O: B7-H3 contributes to the
metastatic capacity of melanoma cells by modulation of known
metastasis-associated genes. Int J Cancer. 130:2282–2290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tindle RW: Immune evasion in human
papillomavirus-associated cervical cancer. Nat Rev Cancer. 2:59–65.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen X, Wang L, Wang W, Zhao L and Shan B:
B7-H4 facilitates proliferation of esophageal squamous cell
carcinoma cells through promoting interleukin-6/signal transducer
and activator of transcription 3 pathway activation. Cancer Sci.
107:944–954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng L, Jiang J, Gao R, Wei S, Nan F, Li
S and Kong B: B7-H4 expression promotes tumorigenesis in ovarian
cancer. Int J Gynecol Cancer. 19:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abadi YM, Jeon H, Ohaegbulam KC,
Scandiuzzi L, Ghosh K, Hofmeyer KA, Lee JS, Ray A, Gravekamp C and
Zang X: Host b7x promotes pulmonary metastasis of breast cancer. J
Immunol. 190:3806–3814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun X, Vale M, Leung E, Kanwar JR, Gupta R
and Krissansen GW: Mouse B7-H3 induces antitumor immunity. Gene
Ther. 10:1728–1734. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu
KF, Zhao JM, Zhang GB and Zhang XG: Relationship between
co-stimulatory molecule B7-H3 expression and gastric carcinoma
histology and prognosis. World J Gastroenterol. 12:457–459. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crispen PL, Sheinin Y, Roth TJ, Lohse CM,
Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich
BC, et al: Tumor cell and tumor vasculature expression of B7-H3
predict survival in clear cell renal cell carcinoma. Clin Cancer
Res. 14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Benzon B, Zhao SG, Haffner MC, Takhar M,
Erho N, Yousefi K, Hurley P, Bishop JL, Tosoian J, et al:
Correlation of B7-H3 with androgen receptor, immune pathways and
poor outcome in prostate cancer: An expression-based analysis.
Prostate Cancer Prostatic Dis. 20:28–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie C, Liu D, Chen Q, Yang C, Wang B and
Wu H: Soluble B7-H3 promotes the invasion and metastasis of
pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep.
6:275282016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang W, Wang J, Wang Y, Dong F, Zhu M,
Wan W, Li H, Wu F, Yan X and Ke X: B7-H3 silencing by RNAi inhibits
tumor progression and enhances chemosensitivity in U937 cells. Onco
Targets Ther. 8:1721–1733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M,
Duan W, Zhou X, Liang R and Tao M: B7-H1 and B7-H3 are independent
predictors of poor prognosis in patients with non-small cell lung
cancer. Oncotarget. 6:3452–3461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan H, Wei X, Zhang G, Li C, Zhang X and
Hou J: B7-H3 over expression in prostate cancer promotes tumor cell
progression. J Urol. 186:1093–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Wang Y, Wang J, Dong F, Zhu M,
Wan W, Li H, Wu F, Yan X and Ke X: B7-H3 silencing inhibits tumor
progression of mantle cell lymphoma and enhances chemosensitivity.
Int J Oncol. 46:2562–2572. 2015.PubMed/NCBI
|