Introduction
Natural compounds from medicinal herbs compose a
significant part of first-line antitumor drugs, such as vinca
alkaloids, texans, podophyllotoxin and camptothecins (vincristine,
camptothecin and paclitaxel) (1).
Since malignant tumors tend to develop chemoresistance, there is
high demand for novel antitumor compounds in the clinical practice.
Natural herbal medicine has become a promising source for novel
discoveries, such as flavonoids (quercetin, silibinin) (2,3),
polyphenols (curcumin) (4),
alkaloids (berberine) (5), as well
as medicinal decoctions containing undefined bioactive ingredients
(6).
There are approximately 170 species of
amorphophallus worldwide (7), mainly distributed in tropical and
subtropical Asia and are used as a food source as well as in
traditional Chinese medicine (TCM). The tuber part from a major
species of amorphophallus, amorphophallus konjac,
locally produced in Zhejiang, China, has been used in the clinical
practice of TCM against cancer for decades (8). Reports on the antitumor efficacy of
tuber of amorphophallus konjac (hereafter referred to as TuAK) can
be dated back to the 1990s (9).
Recently, an Iranian group has also reported the antitumor effect
of amorphophallus konjac tuber using human hepatoma (10,11)
and colon cancer (12) models.
Thus, TuAK has become an acknowledged source of natural antitumor
compounds.
Many antitumor compounds affect apoptosis and
autophagy pathways, two major programmed cell death mechanisms
(13). Autophagy is featured by
delivery of intracellular contents to the lysosome for degradation.
On one hand, it serves as an alternative metabolic pathway to
provide extra nutrition and energy for cell survival; on the other
hand, over-activation of autophagy results in cell death by
self-digestion, which has been defined as type II programmed cell
death (14). Autophagy-targeting
drugs such as chloroquine (CQ) have been applied in clinical trials
for highly malignant and resistant tumors (www.clinicaltrials.gov). Thus, autophagy activation is
a double-edged sword in tumorigenesis and antitumor therapy,
facilitating either cell survival or cell death, depending on the
cellular context (15). Some TCM
herbs have been found to regulate the level of autophagy (16–18).
However, whether they are beneficial or detrimental for the tumor
cells is context-dependent and requires detailed investigation
under different conditions.
Our group has been investigating the efficacy of
TuAK as well as the underlying mechanism for over 10 years in both
clinical and laboratory circumstances (19). Recently, we reported the suppression
of hepatocellular carcinoma by TuAK extracts both in vitro
and in vivo via induction of apoptosis through survivin and
Bax (20). In this study, the
inhibitory effect of organic solvent-based TuAK extract (shortened
as TuAKe) against gastric cancer cells was studied, as well as the
underlying mechanisms associated with induction of cell cycle
arrest, apoptosis and autophagy. We also report data on a
retrospective study on life quality assessment in gastric cancer
patients who received TuAK-based decoction while undergoing
conventional chemotherapy.
Materials and methods
Reagents and antibodies
TuAK was purchased from Zhejiang Lin-An Medicinal
Herbs, Co., Ltd., (Zhejiang, China; Batch no. 061208) and was
authenticated by Zhejiang Chinese Medical University. A voucher
specimen is maintained in the university. 5-Fluorouracil (5-FU) was
from Nantong Jinghua Pharmaceutical Group, Co., Ltd., (Nantong,
China). Chloroquine (CQ) was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Other chemical reagents (grade AR) were from
Sigma-Aldrich, Amresco (Solon, OH, USA) or Aladdin Reagents Co.,
Ltd. (Shanghai, China). The following antibodies were used:
survivin (ZSGB-Bio, Beijing, China), Bax (ZSGB-Bio), Bcl-2 (Santa
Cruz Biotechnology, Santa Cruz, CA, USA), caspase-9 (Abcam,
Cambridge, MA, USA), LC3 (Sigma-Aldrich), Atg7 (Cell Signaling
Technology, Danvers, MA, USA) β-actin (Lianke Bio, Hangzhou, China)
and GAPDH (Lianke Bio). The secondary antibodies were conjugated to
HRP (Jackson Immunolab, West Grove, PA, USA).
Preparation of TuAK extracts
Dried TuAK was ground to fine particles, soaked in
8x volume of 95% ethanol overnight, and extracted twice by reverse
flow, 2 h each time. The ethanol collected was further extracted by
2x volume of ligarine twice. The ligarine extract was recovered and
concentrated by distillation in reduced pressure. The concentrated
extract was filtered to obtain the organic solvent-based extract.
The ethanol residuum was extracted by dH2O twice, 10x
and 8x volume respectively, and concentrated to obtain the aqueous
extract of TuAK. One mega-extraction using 15 kg of TuAK was
performed and the extracts obtained were stored at 4°C with
desiccants for the following experiments. The organic solvent-based
extract of TuAK is hereafter referred to as TuAKe.
Cell culture and cell viability
assay
SGC-7901, AGS and HEK293 cells were purchased from
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Science (Shanghai, China). Cells were cultured in Dulbeccos
modified Eagles medium (DMEM; Gibco, Carlsbad, CA, USA) containing
10% fetal bovine serum (FBS; Gibco) at 37°C with 5% CO2.
For the viability assays, cells were seeded in 96-well plates,
1×104 cells/well. Desired drugs, compounds and extracts
were added to the wells immediately afterwards and incubated with
the cells for 24 h. The cell viability was measured by spectrometry
(Multiscan MK3 spectrophotometer; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) using CCK-8 kit (Lianke Bio) at OD 450 nm.
Flow cytometric (FCM) analysis
Cells were seeded in 6-well culture plates and
treated by desired drugs or extracts, or by phosphate-buffered
saline (PBS) as control for 24 h, then trypsinized and washed by
PBS. Aliquots of resuspended cells (5×105/ml) were
stained by cell cycle and apoptosis analysis kit (Beckman Coulter,
Brea, CA, USA) based on the manufacturers instructions. FCM
analysis was performed immediately afterwards (FACSAria™; BD
Biosciences, San Jose, CA, USA).
Immunofluorescence
Cells were seeded in 24-well culture plates on glass
coverslides, and treated at the desired conditions, then fixed and
observed under a fluorescence microscope (IX71-22FL/PH; Olympus,
Tokyo, Japan, or Axio A1; Carl Zeiss AG, Oberkochen, Germany).
Western blotting
Cells after desired treatment were scraped, spun
down, washed by PBS and resuspended in RIPA buffer with standard
protease and phosphatase inhibitors. After quantification of
protein concentration by BCA method (Lianke Bio), cell lysates were
mixed with loading buffer and subjected to SDS-PAGE with 20 µg
protein per lane. The proteins were later transferred to PVDF
membranes, which were incubated by primary and secondary
antibodies. The membranes were developed with an enhanced
chemiluminescence kit (Lianke Bio). Each experimental condition was
repeated for at least three times. Quantitative analysis of protein
levels determined by band intensities was carried out by ImageJ
(v1.48u).
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR)
Total RNA was extracted by TRIzol®
reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcripted to
cDNA by a commercial kit (Takara Bio, Shiga, Japan). Primers for
β-actin, BCL2 and BAX were synthesized by
Invitrogen (Shanghai, China) with the following sequences:
β-actin forward, (5-ACAACTTTGGTATCGTGGAAGGAC-3) and
β-actin reverse, (5-AGGTGGAGGAGTGGGTGTCG-3), BCL2
forward, (5-ATTGGGAAGTTTCAAATCAGC-3), BCL2 reverse,
(5-TGCATTCTTGGACGAGGG-3), BAX forward,
(5-GACACCTGAGCTGACCTTGG-3) and BAX reverse,
(5-GAGGAAGTCCAGTGTCCAGC-3). PCR was performed in three independent
experiments with multiple repeats (Applied Biosystems StepOnePlus;
Applied Biosystems, Foster City, CA, USA) and the products were
analyzed by agarose electrophoresis to compare mRNA levels.
Electron microscopy (EM)
Cells after treatment were scraped in PBS and spun
down. The pellets were fixed by 2.5% glutaraldehyde for one week,
then washed by 0.1 M PBS and fixed in 1% osmic acid for 1 h, washed
by ddH2O, dehydrated by serial concentrations of ethanol
from 50 to 100%. Then the pellets were dehydrated by 100% acetone,
permeated by acetone/resin (1:1, v/v) for 2 h and by pure resin
overnight. The resin was polymerized by serial heating at 37, 45
and 60°C, sectioned and stained by uranium acetate-lead citrate,
and then observed by EM equipped by the companion software (Tecnai;
Phillips, Amsterdam, the Netherlands).
Knocking-down the autophagy-related
gene ATG7
Cultured cells were infected with lentivirus
expressing shRNA targeting ATG7 gene and control scrambled
shRNA (Novobio, Shanghai, China) in the presence of polybrene.
Twenty-four hours after infection, knocking-down of Atg7 was
confirmed by western blotting, and cells were treated by TuAK for
another 24 h, with or without CQ for the last 6 h, followed by cell
viability assay.
Retrospective cohort study of gastric
cancer patients treated by TuAK-based decoction
The institutional ethics committee of Zhejiang
Chinese Medical University approved this study and waived the
necessity to obtain informed consent based on its nature as a
retrospective and observational study. A total of 30 inward gastric
cancer patients admitted to the First Affiliated Hospital of
Zhejiang Chinese Medical University were grouped into two. Ten
patients in the control group received conventional chemotherapy
using FAM regimen, namely combination of 5-FU, adriamycin and
mitomycin C. Twenty patients in the TuAK group received TuAK-based
decoction in addition to the FAM regimen. The evaluated Karnofsky
performance status (KPS) scores of all patients were over 60 upon
admission to the hospital, and showed no statistically signifcant
difference between the two groups. KPS scores of each patient were
re-evaluated after 8 weeks of treatment and compared.
Statistical analysis
Each experiment was performed at least three times
with multiple repeats, and the data were analyzed by SPSS (v13.0).
The statistical significance was calculated by one-way ANOVA to
compare between multiple groups, or by Students t-test when only
two groups were compared. P<0.05 was considered statistically
significant.
Results
The in vitro antitumor activity of
TuAK extracts
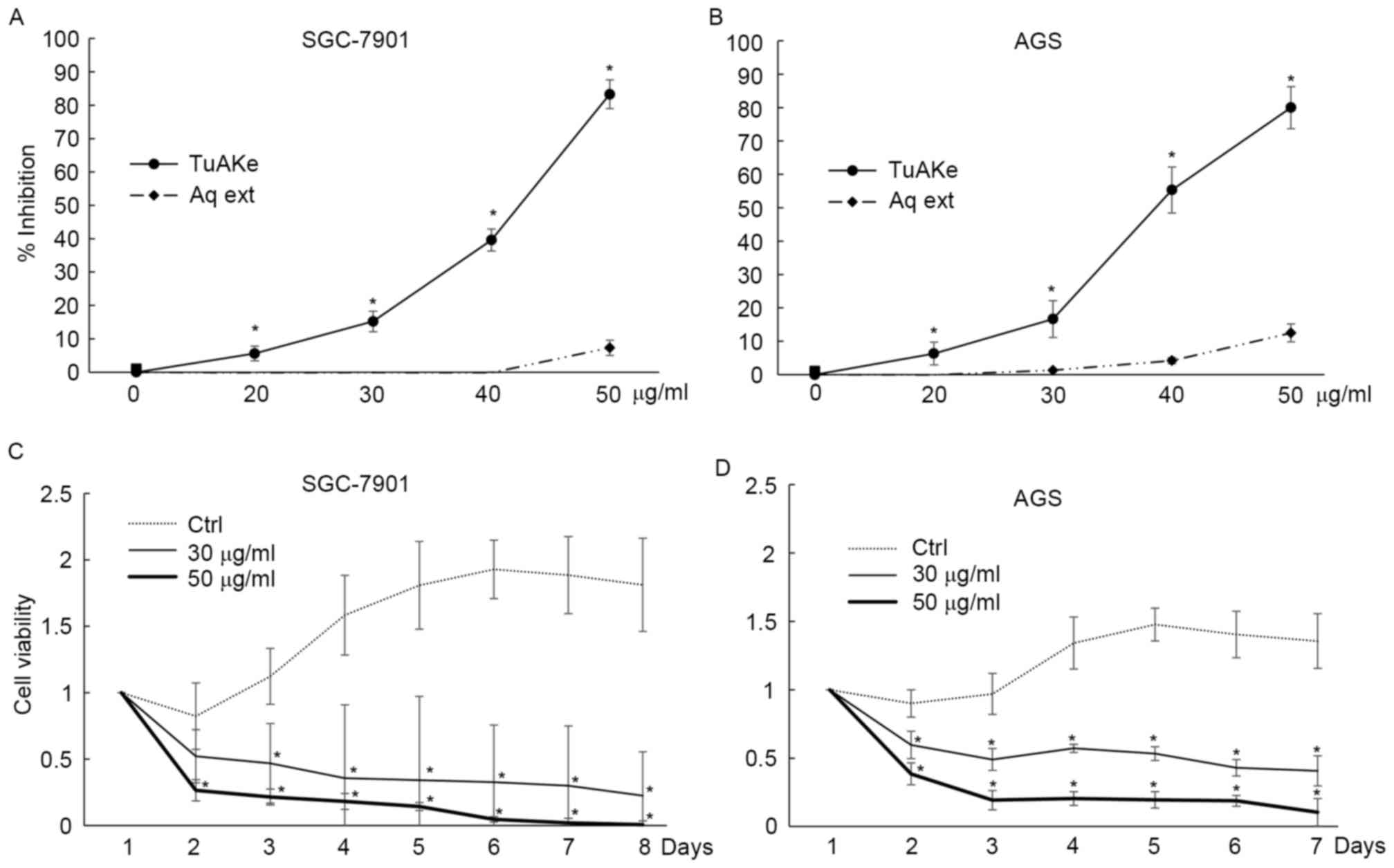
In cultured human gastric cancer cell lines SGC-7901
and AGS, within 24 h of treatment, the inhibitory effect of the
aqueous extract of TuAK was trivial compared with the organic
extract, TuAKe (Fig. 1A and B).
Based on the inhibition curves, in the two cell lines, the
IC50 of TuAKe was 35–45 µg/ml, while the aqueous extract
had little inhibitory effect. TuAKe, the more potent extract, was
used in the following experiments. In long-term experiments,
consecutive treatment of 50 µg/ml TuAKe daily killed ~100% SGC-7901
cells and ~80% of AGS cells within 7 days, while lower dose of
TuAKe (30 µg/ml) inhibited ~70% of SGC-7901 cells and 50% of AGS
cells (Fig. 1C and D).
TuAKe induces cell apoptosis and cell
cycle arrest
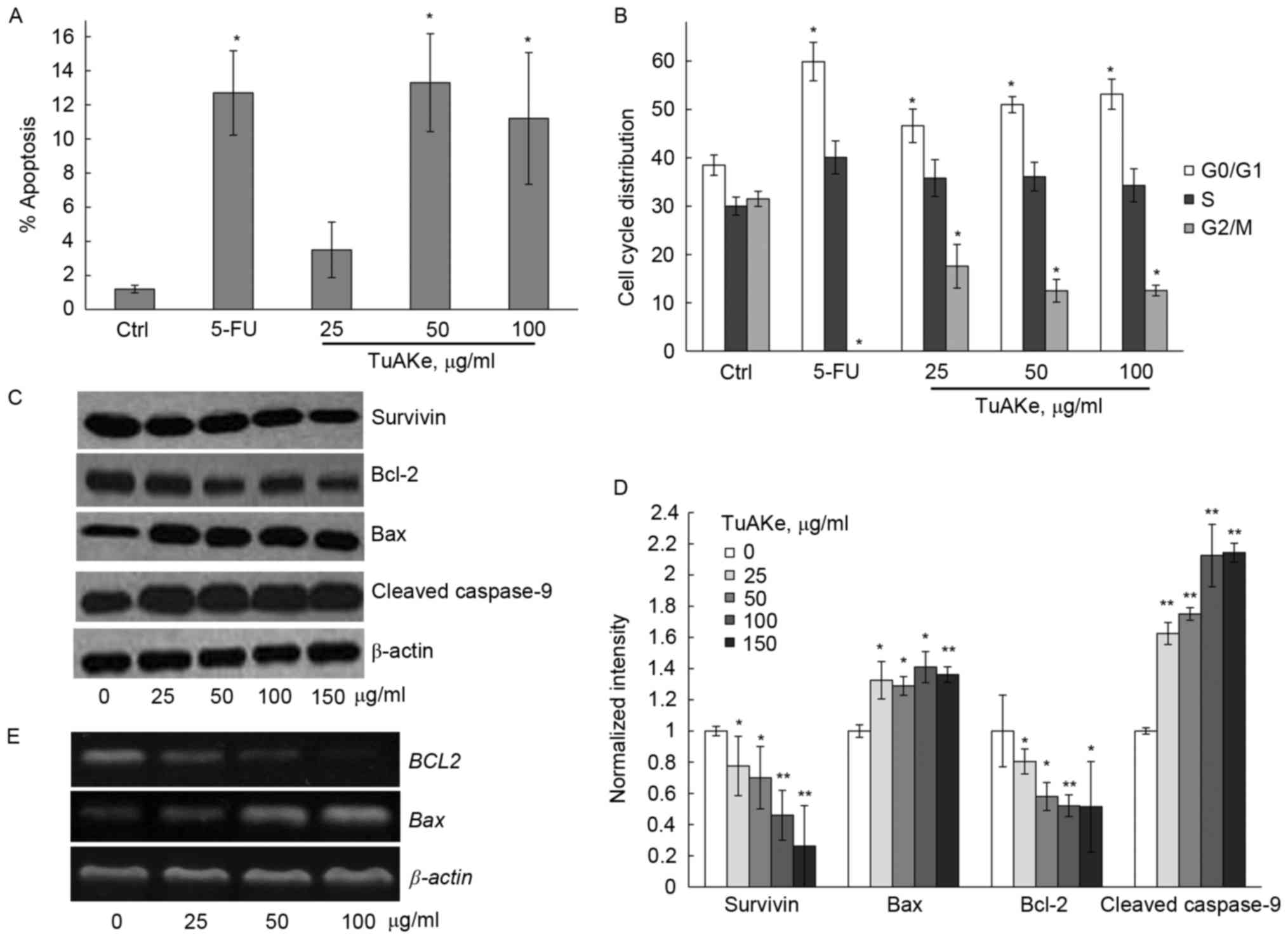
We analyzed the apoptotic rate in TuAKe-treated
SGC-7901 cells. In Fig. 2A, FCM was
carried out to detect the apoptotic cells induced by TuAKe after
co-staining of Annexin V-FITC and PI. After treatment of 24 h, 5-FU
induced significant increase of apoptosis; 25 µg/ml TuAKe increased
the apoptotic rate but without statistical significance. After
cells were treated by 50 or 100 µg/ml TuAKe, cell apoptosis rates
were significantly increased (13.3 and 11.2%, respectively) to
similar level as the 5-FU-treated group (12.7%).
Cell cycle distribution was analyzed by FCM in
SGC-7901 cells treated by TuAKe for 24 h (Fig. 2B). The percentage of cells in the
G0/G1 phase increased from 38.5% in the control (PBS) group to
46.6–63.9% in TuAKe-treated groups (25–100 µg/ml); on the other
hand, the percentage of cells in the G2/M phase decreased from
31.5% in the control group to 12.5–17.6% in TuAKe-treated groups.
Thus, TuAKe treatment resulted in cell cycle arrest in the G0/G1
phase.
TuAKe regulates the expression of
apoptosis-associated proteins
To further analyze the molecular evidence of
apoptosis, western blottings for several apoptosis-related markers
were carried out. Different concentrations of TuAKe could
significantly reduce the level of survivin; whereas, there was
significant decrease in Bcl-2 as well as increase in Bax and
cleaved caspase-9 levels (Fig. 2C and
D). By qRT-PCR, it was further demonstrated that TuAKe resulted
in decreased BCL2 and increased BAX gene expression
(Fig. 2E).
Autophagy induction contributes to
TuAKe-induced cell death
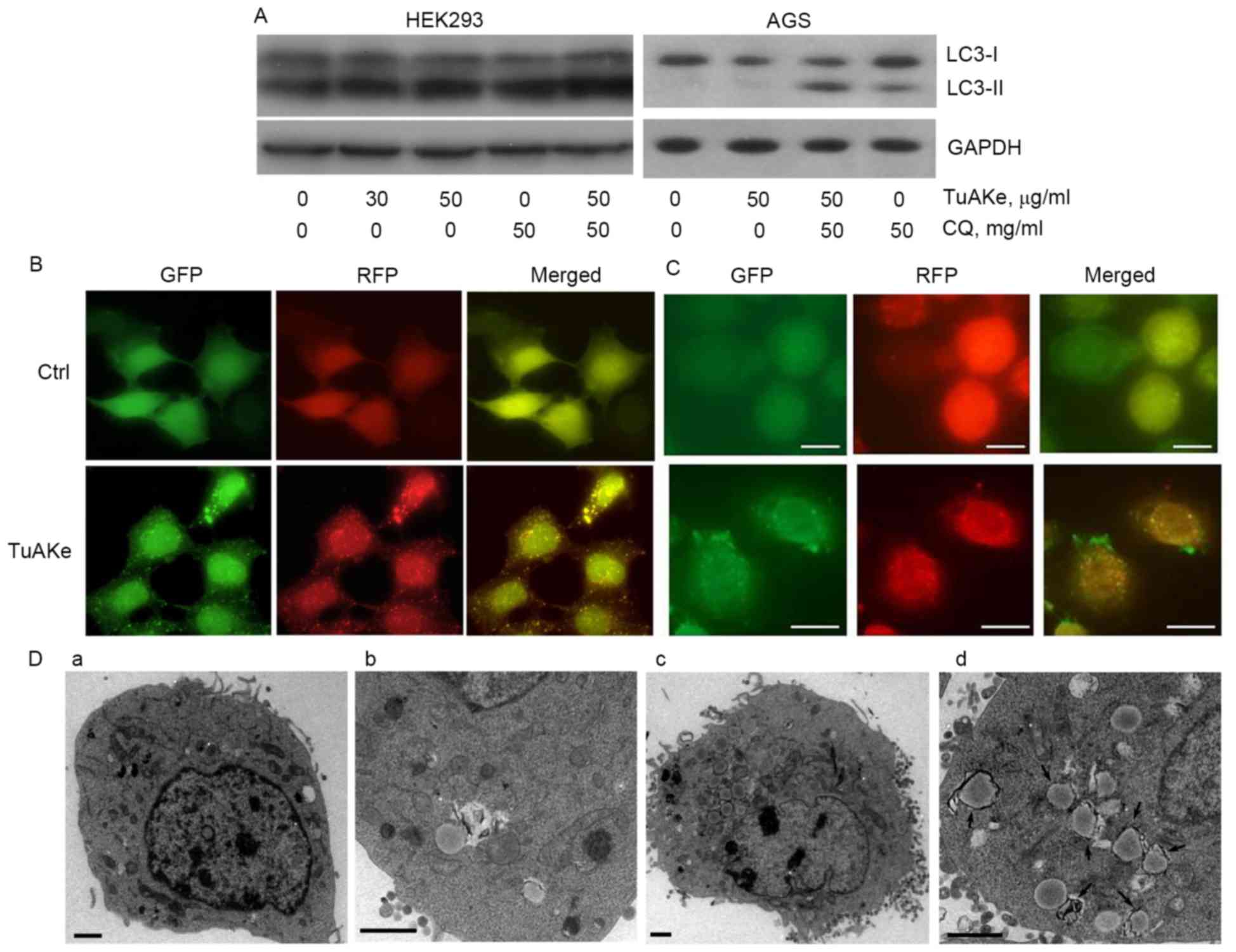
Autophagy flux analysis was carried out using HEK293
and AGS cells (Fig. 3A). HEK293, a
cell line commonly used in studies on autophagy as a tool, was less
sensitive to TuAKe compared with the two gastric cancer cell lines
used in this study. The IC50 of TuAKe on HEK293 was
higher than the maximum dose tested, 75 µg/ml (Fig. 4B), compared with 35–45 µg/ml in
tumor cells (Fig. 1A). As seen in
Fig. 3A, in both HEK293 and AGS
cells, TuAKe increased the level of LC3-II, the conjugate of
cytosolic autophagy-related protein LC3-I with
phosphatidylethanolamine (PE), an indicator of either autophagy
activation or blockage of autophagic degradation. In the autophagy
flux analysis, CQ, the lysosome inhibitor, increased the level of
LC3-II; combination of CQ and TuAKe further increased the level of
LC3-II, indicating the effect of TuAKe was to promote the formation
of LC3-II instead of blocking its degradation, thus, demonstrating
induction of autophagy by TuAKe.
The RFP-GFP-LC3 tag is a well-defined tool to
monitor the dynamic formation and degradation of LC3-II-positive
autophagosomes. Presence of yellow puncta (RFP-GFP-LC3-II) implies
autophagosomes. Moreover, because GFP is degraded faster than RFP
in the lysosomes, presence of red puncta (RFP only) implies
autolysosomes under degradation at the late stage. In both HEK293
and AGS cells stably expressing RFP-GFP-LC3-II, the control cells
were free of puncta (Fig. 3B and
C), while TuAKe induced significant increase in the number of
yellow puncta. Increase in the number of observable autophagosomes
further supported induction of autophagy by TuAKe demonstrated by
flux analysis (Fig. 3A).
Observed by EM (Fig.
3D), TuAKe induced significant increase in the number of
intracellular membranous organelles with high electron density,
typical of double-membraned autophagosomes (Fig. 3Dc-d). The size of autophagosome-like
structures observed under EM was comparable with the LC3-II
positive puncta from fluorescent microscopy.
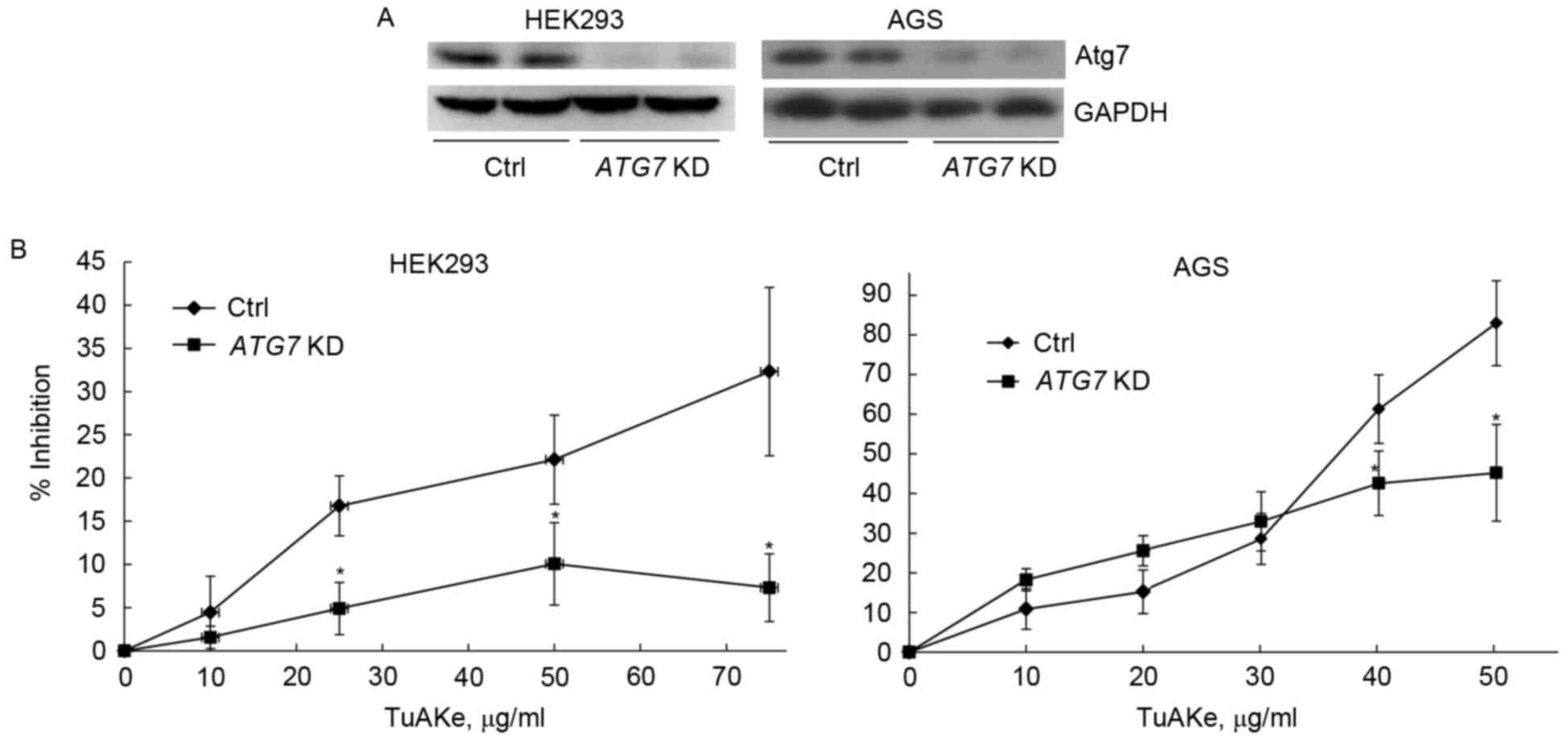
In both HEK293 and AGS cells, after suppression of
autophagy by silencing of ATG7 gene, decrease of Atg7 and
inhibition of autophagy was confirmed by western blotting (Fig. 4A). After targeted suppression of
autophagy, the inhibition rate of TuAKe (10–75 µg/ml) on HEK293
cells was significantly decreased from 5–32% in the control group
to 2–10% in the siRNA ATG7 group (Fig. 4B). In AGS cells, decrease of
inhibition by TuAKe after ATG7 knockdown was only
significant when TuAKe was used in higher doses (40–50 µg/ml);
however, the inhibition rates between the two groups was highly
significant with the net difference of 30–40%.
TuAK improves the life quality of
gastric cancer patients
We evaluated the KPS scores in 30 gastric cancer
patients, 10 received conventional FAM chemotherapeutic regimen and
20 received FAM regimen plus TuAK-based decoction. As shown in
Table I, among the 20 patients in
the TuAK group, the KPS scores in 10 had improved, 7 were stable
and 3 were worsened; among the 10 patients from the FAM group, 1
had improved KPS score, 3 were stable and 6 were worsened. More
patients in the TuAK group obtained improved or stable KPS scores
(85%) compared with those in the FAM only group (40%), suggesting
TuAK-based medication could improve the life quality of gastric
cancer patients undergoing chemotherapy.
 | Table I.Life quality evaluation of gastric
cancer patients after amorphophallus konjac-based
therapies. |
Table I.
Life quality evaluation of gastric
cancer patients after amorphophallus konjac-based
therapies.
| Group | Improved KPS,
n | Stable KPS, n | Decreased KPS,
n | Improved or stable
KPS, % (n/n) |
|---|
| FAM | 1 | 3 | 6 | 40 (4/10) |
| FAM+TuAK | 10 | 7 | 3 | 85 (17/20) |
Discussion
Despite of its history in the clinical use and the
observable beneficial effects in the field of TCM, the tuber part
of amorphophallus konjac is not enlisted in the pharmacopeia
and its chemical composition is still poorly studied. As a whole
plant, Amorphophallus konjac is well tolerated as a food
source in some countries. It contains glucomannan (21,22),
monosaccharide (23),
oligosaccharide (24),
polysaccharide (14), aromatic
compounds including (+/−)-5,5′-dimethoxysesamin, erythrinasinate,
indole-3-carbaldehyde, serotonin, 3,4-dihydroxybenzoic acid,
3,4-dihydroxybenzaldehyde (13,16).
The glucomannans are the most studied bioactive components from the
powder of amorphophallus konjac, and have been registered in
clinical trials in adults (25) and
in children (25,26) against obesity and diabetes through
regulating food absorption in the gut and subsequently decreasing
body weight. Glucomannans are typically extracted by water followed
by coagulation by ethanol, which produces much higher yields
(27) compared with the organic
solvents-based isolation methods used in this study.
Theoretically, aquatic extract contains sugar, amino
acids, protein and salts; ethanol could extract most active
components besides sugar and proteins; while ligarine extract
contains lipids, volatile oil, wax, as well as sterides and
terpenes (28). Previous reported
information on the chemical composition of the organic extract of
TuAK is limited; there is only one report in Chinese on 31
chemicals identified by gas chromatography-mass spectrometry
(GC-MS) using extraction method similar as ours (29). In our unpublished data, by high
pressure liquid chromatography-mass spectrometry (HPLC-MS), up to
71 different chemicals were identified from TuAKe, including
nitrogen and sulfur-containing compounds. From our data and of
others, most chemicals contained in the antitumor TuAKe fraction
are different species of organic compounds including nitrogen and
sulfur-containing compounds. Among the 94 organic compounds
identified from whole amorphophallus konjac powder, none was
found in the list of toxic compounds by the standards of USFDA
(30). From out data, the
cytoxicity of TuAKe was significantly lower in non-tumor cell line
HEK293 compared with gastric cancer cell lines, supporting the
previously reported safety of amorphophallus konjac. It is likely
that the TuAKe we obtained does not contain cytotoxic compounds;
however, further separation and identification of the chemical
compositions are to be carried out in future studies using more
sophisticated tools such as NMR.
Our data indicated that the organic solvent-based
extract, TuAKe, had much stronger antitumor potency compared with
the aqueous extract, suggesting ethanol and ligarine based
extraction methods to be more appropriate for the purpose of
antitumor treatments of TuAK instead of the traditional water-based
extraction. The bioactive chemicals are to be identified. However,
according to the holistic theory of TCM, it is also likely that the
efficacy is due to the combinational use of multiple
ingredients.
We found that TuAKe exhibited antitumor effect at
relatively low doses, especially in long-term treatment. Induction
of apoptosis and cell cycle distribution reached the saturation
point when the concentration of TuAKe was >50 µg/ml. Although
safe to consume, our data also suggest that TuAK-based medication
in relatively smaller doses would be efficient to achieve desired
effects.
A TCM decoction contains hundreds of different
chemical components and can modulate many molecular targets
simultaneously to result in complicated cascades of cellular
events. Qing-Yi-Hua-Ji formula, a TuAK-based decoction, was
reported to affect multiple downstream targets including Ski
(31), Notch 4 and Jagged 1
(32). We have studied how TuAKe
regulates cell death pathways. Firstly, TuAKe induced apoptosis by
regulation of apoptosis-related proteins including survivin, Bcl-2
and Bax. Survivin belongs to the family of inhibitors of apoptosis
proteins (IAP) that regulate cell death by inhibition of caspase
activation. It is expressed only in cells in the G2/M phase, but
absent in differentiated cells (33,34).
Survivin is highly expressed in most human tumors and fetal
tissues, and has thus become a biomarker and a therapeutic target
in cancer (35). The Bcl-2 family
of proteins contain anti-apoptotic proteins including Bcl-2, as
well as pro-apoptotic proteins Bax and Bid (36). Decrease of BCL2 and increase
of BAX expression was also detected at the mRNA level after
TuAKe treatment. Thus, TuAK could regulate apoptosis through the
anti-apoptotic IAP and Bcl-2 family proteins, although was not
necessarily dependent on them. Moreover, TuAKe could simultaneously
increase the levels of the pro-apoptotic proteins Bax and
caspase-9. Furthermore, TuAKe was regulatory on cell cycle
distribution. It increased the proportion of cells in the G0/G1
phases and decreased those in the G2/M phases. This result is
consistent with decrease of survivin, which is expressed in a cell
cycle-dependent manner (33,34).
Similar regulatory effect of survivin and Bax by TuAKe was also
found in hepatocarcinoma cells in our previous study (20).
In the present study, it was found that activation
of autophagy was an indispensable antitumor mechanism of TuAKe. By
flux analysis, TuAKe was shown to induce autophagy in both HEK293
and AGS cells. By fluorescent imaging of RFP-GFP-LC3 positive
puncta, TuAKe was shown to induce the formation of LC3-II-positive
puncta representing the maturing autophagosomes again in both cell
lines. Under EM, autophagosome-like double membraned structures
with high electron densities, a golden marker for autophagy
induction, could be induced by TuAKe (37). Judged by the similar scale in size
and numbers, the autophagosome-like structures under EM could be
the same structure as the RFP-GFP-LC3-II puncta under fluorescent
microscopy. To examine whether activation of autophagy contributed
to TuAKe-induced cell death, autophagy was specifically inhibited
by knocking-down the expression of ATG7, which functions as
an E1-like enzyme to form both LC3-II and the Atg5-Atg12 complex in
the autophagosome elongation step, and is required for the
canonical autophagy (38,39). Suppression of autophagy by
knocking-down of ATG7 significantly reduced the
anti-proliferative capacity of TuAKe in both HEK293 and AGS cells,
implying an essential role of autophagy activation in the
biological events induced by TuAKe.
By our retrospective analysis, TuAK-based decoction
exhibited beneficial effects in gastric cancer patients undergoing
chemotherapy marked by significantly improved or stable KPS scores.
The previously mentioned Qing-Yi-Hua-Ji formula, a decoction with
TuAK as one of the major ingredients, has also been reported to
increase the survival of late stage pancreatic cancer patients
(40). However, the decoction is
traditionally prepared on water-based extraction, while our data
suggest organic solvents could provide extracts with higher
antitumor potency.
In conclusion, based on this study, TuAKe inhibits
gastric cancer cells through induction of cell cycle arrest,
apoptosis, as well as autophagy. Autophagy is indispensable in the
antitumor mechanism of TuAKe. Our findings strongly support the use
of TuAK-based medication as an alternative or as a combinational
component in conventional treatment regimens against gastric
cancer, and support the feasibility to isolate active antitumor
compounds from TuAK. The detailed bioactive components and the
molecular targets are to be revealed by future studies.
Acknowledgements
The present study is funded by the National Science
Foundation of Zhejiang Province, China (no. LY15H160024 to X.C.,
no. LY16H270006 to L.P.) and by the National Science Foundation of
China (no. 81202021 to X.C.).
References
|
1
|
Safarzadeh E, Shotorbani Sandoghchian S
and Baradaran B: Herbal medicine as inducers of apoptosis in cancer
treatment. Adv Pharm Bull. 4:(Suppl 1). 421–427. 2014.PubMed/NCBI
|
|
2
|
Srivastava S, Somasagara RR, Hegde M,
Nishana M, Tadi SK, Srivastava M, Choudhary B and Raghavan SC:
Quercetin, a natural flavonoid interacts with DNA, arrests cell
cycle and causes tumor regression by activating mitochondrial
pathway of apoptosis. Sci Rep. 6:240492016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang K, Wang W, Jin X, Wang Z, Ji Z and
Meng G: Silibinin, a natural flavonoid, induces autophagy via
ROS-dependent mitochondrial dysfunction and loss of ATP involving
BNIP3 in human MCF7 breast cancer cells. Oncol Rep. 33:2711–2718.
2015.PubMed/NCBI
|
|
4
|
Kumar G, Mittal S, Sak K and Tuli HS:
Molecular mechanisms underlying chemopreventive potential of
curcumin: Current challenges and future perspectives. Life Sci.
148:313–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin P, Zhang C and Li N: Berberine
exhibits antitumor effects in human ovarian cancer cells.
Anticancer Agents Med Chem. 15:511–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang LH, Wang RP, Hu SY, Teng YH and Xie
WB: The effect of tou nong san on transplanted tumor growth in nude
mice. Evid Based Complement Alternat Med. 2015:5184542015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chua M, Baldwin TC, Hocking TJ and Chan K:
Traditional uses and potential health benefits of Amorphophallus
konjac K. Koch ex N.E.Br. J Ethnopharmacol. 128:268–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Committee CP: Pharmacopoeia of the
People's Republic of China. 1st. Beijing: China Chemical Industry
Press; 2010
|
|
9
|
Luo DY: Inhibitory effect of refined
Amorphophallus konjac on MNNG-induced lung cancers in mice.
Zhonghua Zhong Liu Za Zhi. 14:48–50. 1992.(In Chinese). PubMed/NCBI
|
|
10
|
Ansil PN, Nitha A, Prabha SP and Latha MS:
Curative effect of Amorphophallus campanulatus (Roxb.)
Blume. tuber on N-nitrosodiethylamine-induced hepatocellular
carcinoma in rats. J Environ Pathol Toxicol Oncol. 33:205–218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ansil PN, Wills PJ, Varun R and Latha MS:
Cytotoxic and apoptotic activities of Amorphophallus
campanulatus tuber extracts against human hepatoma cell line.
Res Pharm Sci. 9:269–277. 2014.PubMed/NCBI
|
|
12
|
Ansil PN, Prabha SP, Nitha A and Latha MS:
Chemopreventive effect of Amorphophallus campanulatus
(Roxb.) blume tuber against aberrant crypt foci and cell
proliferation in 1, 2-dimethylhydrazine induced colon
carcinogenesis. Asian Pac J Cancer Prev. 14:5331–5339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Tang ZH, Xu WS, Wu GS, Wang YF,
Chang LL, Zhu H, Chen XP, Wang YT, Chen Y, et al: Platycodin D
triggers autophagy through activation of extracellular
signal-regulated kinase in hepatocellular carcinoma HepG2 cells.
Eur J Pharmacol. 749:81–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen LL, Song JX, Lu JH, Yuan ZW, Liu LF,
Durairajan SS and Li M: Corynoxine, a natural autophagy enhancer,
promotes the clearance of alpha-synuclein via Akt/mTOR pathway. J
Neuroimmune Pharmacol. 9:380–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu D, Lao Y, Xu N, Hu H, Fu W, Tan H, Gu
Y, Song Z, Cao P and Xu H: Identification and characterization of
anticancer compounds targeting apoptosis and autophagy from Chinese
native Garcinia species. Planta Med. 81:79–89.
2015.PubMed/NCBI
|
|
19
|
Chen PF and Liu LM: Observation on the
anti-tumor effects and induction carcinoma cell's apoptosis of She
Liu Gu. Chinese J Bas Med TCM. 6:30–32. 2000.(In Chinese).
|
|
20
|
Pan L and Chen PF: In vitro and
in vivo suppression of hepatocellular carcinoma by
amorphophallus konjac tuber through regulation of survivin and bax.
Oncol Lett. (In press).
|
|
21
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Xia J, Wang Y and Xie B: Grain-size
effect on the structure and antiobesity activity of konjac flour. J
Agric Food Chem. 53:7404–7407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan YJ and Zong WX: The cellular decision
between apoptosis and autophagy. Chin J Cancer. 32:121–129.
2013.PubMed/NCBI
|
|
24
|
Rubinstein AD and Kimchi A: Life in the
balance - a mechanistic view of the crosstalk between autophagy and
apoptosis. J Cell Sci. 125:5259–5268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zalewski BM, Chmielewska A and Szajewska
H: The effect of glucomannan on body weight in overweight or obese
children and adults: A systematic review of randomized controlled
trials. Nutrition. 31:437–442e432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zalewski BM and Szajewska H: Effect of
glucomannan supplementation on body weight in overweight and obese
children: Protocol of a randomised controlled trial. BMJ Open.
5:e0072442015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harmayani E, Aprilia V and Marsono Y:
Characterization of glucomannan from Amorphophallus
oncophyllus and its prebiotic activity in vivo. Carbohydr
Polym. 112:475–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
XH K: Chemistry of Chinese Materia Medica.
China Press of Traditional Chinese Medicine Beijing: 2003
|
|
29
|
Li JHX: Study on Chemical Components of
Petroleum Ether Fraction of Alcohol Extract Obtained from
Amorphophallus Blume. J Xihua University-Natural Sci.
28:68–69. 2009.
|
|
30
|
Jy Y: The cultivation and application of
Amorphophallus Konjac. Hangzhou: Hangzhou University Press;
1991
|
|
31
|
Wang P, Chen Z, Meng ZQ, Luo JM, Lin JH,
Zhou ZH, Chen H, Wang K, Shen YH and Liu LM: Ski acts as
therapeutic target of qingyihuaji formula in the treatment of
SW1990 pancreatic cancer. Integr Cancer Ther. 9:50–58. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Y, Zhu F, Xu S and Liu L: Anti-tumor
effect of the extract from qingyihuaji formula on pancreatic cancer
by down-regulating Notch-4 and Jagged-1. J Tradit Chin Med.
35:77–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
Lett. 244:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chao DT and Korsmeyer SJ: BCL-2 family:
Regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Komatsu M, Waguri S, Ueno T, Iwata J,
Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et
al: Impairment of starvation-induced and constitutive autophagy in
Atg7-deficient mice. J Cell Biol. 169:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakatogawa H: Two ubiquitin-like
conjugation systems that mediate membrane formation during
autophagy. Essays Biochem. 55:39–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu LWL and Lin S: Therapeutic evaluation
on advanced pancreatic cancer treated by integrative Chinese and
western medicine: Clinical analysis of 56 cases. Chin J Integr Med.
10:236–237. 2004. View Article : Google Scholar
|