Introduction
Ovarian cancer, the seventh most common cancer among
women, remains one of the leading causes of cancer-associated
morbidity and mortality. It has become the third most common cancer
of female genital organs, after cervical cancer and endometrial
cancer (1,2). cis-Dichlorodiammineplatinum
(II) (cisplatin) has been the standard of care against ovarian
cancer for several decades. However, its clinical efficacy is often
limited by extrinsic and intrinsic drug resistance as well as
nephrotoxicity (3). Hence, there is
an urgent need to identify new molecular targets in ovarian cancer.
Recent studies have shown that nucleolar stress plays a role in
cancer cell viability and apoptosis.
The nucleolus is a cellular organelle within the
nucleus where ribosomal DNA (rDNA) transcription and ribosome
biogenesis occur (4,5). It comprises distinct subcompartments,
and is divided into the granular compartment (GC), the dense
fibrillar component (DFC) and the fibrillar center (FC) (6). The structural stability of the three
subcompartments is important in their role in nucleolar function.
Nucleolar stress (also called ribosomal stress) is caused by
failures in ribosome assembly and ribosome biogenesis. This then
inhibits RNA polymerase I (Pol I) transcription that finally
results in the dysfunction of cellular homeostasis. Internal and
extrinsic stimuli, such as abnormal metabolic conditions,
ultraviolet irradiation and enhanced levels of reactive oxygen
species, can lead to nucleolar stress (7). Nucleolar stress inhibits viability and
induces apoptosis of cancer cells in vivo and in
vitro (6,7). Hence, targeting nucleolar stress as a
potential therapeutic strategy for cancer has already attracted
attention.
BMH-21, a planar tetracyclic small molecule,
preferentially binds GC-rich DNA sequences and inhibits RNA
polymerase I (Pol I) transcription, finally leading to nucleolar
stress and apoptosis. BMH-21 has a broad-spectrum anticancer effect
by potently and rapidly repressing Pol I independently of DNA
damage signaling. The aberrant Pol I transcription induces
nucleolar reorganization and activates p53 thus contributing to
apoptosis (8,9). Peltonen et al have reported
that BMH-21 has extensive and potent antitumor activity across
NCI60 cancer cell lines and inhibits the growth of tumors in
vivo (10). However, the
mechanism of action of BMH-21 in ovarian cancer cell death remains
poorly understood.
The aim of this study was to explore whether BMH-21
induces apoptosis in SKOV3 ovarian cancer cells and explore the
mechanism. Herein, we report that BMH-21 inhibited viability and
induced apoptosis in SKOV3 cells through a p53-dependent nucleolar
stress response pathway. These data suggest that induction of
nucleolar stress using an inducer such as BMH-21 may be a novel
strategy for ovarian cancer therapy.
Materials and methods
Cell culture
SKOV3 human ovarian cancer cells, Bel-7402 human
hepatic cancer cells and HeLa human cervical cancer cells were
obtained from the Chinese Academy of Medical Sciences. The cells
were cultured at 37°C in a 5% CO2 atmosphere at Roswell
Park Memorial Institute (RPMI)-1640 culture medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 U/ml
streptomycin. The experiment was divided into 4 groups: a control
group, 1 µM BMH-21 (SMl1183; Sigma, USA) group, 2 µM BMH-21 group
and 4 µM BMH-21 group.
MTT assay
Cell viability was determined by MTT assay. SKOV3,
Bel-7402 and HeLa cells in the exponential growth phase were seeded
into 96-well culture plates in 100 µl RPMI-1640 at a density of
8×103 cells/well. After 24-h incubation, the indicated
dose of BMH-21 was added to the 24-h incubation in four parallel
wells. MTT assays (Beyotime Institute of Biotechnology, Haimen,
China) were performed as follows: 20 µl MTT solution (5 mg/ml) in
phosphate-buffered saline (PBS; Beijing Zhongshan Golden Bridge
Biological Technology Co., Ltd., Beijing, China) was added to cells
for 4 h, after which 150 µl dimethyl sulfoxide (Beijing Chemical
Industry Co., Ltd., Beijing, China) was added to each well. Cells
were agitated for 10 min prior to absorbance measurements at 570 nm
using a Microplate Reader (Bio-Rad Laboratories, Hercules, CA,
USA). The growth inhibition rate was calculated as % inhibition = 1
- absorbance of experimental group / absorbance of control group ×
100. The mean value of the four replicate wells was calculated for
each treatment group.
Cell cycle analysis
Exponentially growing ovarian cancer cells were
seeded into 6-well culture plates at a density of 2×105
cells/well. After exposure to different experimental conditions,
the cells were trypsinized and resuspended in RPMI-1640 with 10%
FBS at a concentration of 1×106 cells/ml. For cell cycle
analysis, the cells were washed with phosphate-buffered saline
(PBS) and fixed with 70% ice-cold ethanol. Cells were then stained
with propidium iodide (PI). Cell cycle graphs were acquired using
the BD Accuri™ C6 flow cytometry system (BD Biosciences, USA) using
BD Accuri C6 software. For each sample, ≥1×104 cells
were recorded. ModFit Cell cycle analysis software was used to
analyze the percentage of cells in G0/G1, S and G2/M phases based
on DNA content.
Immunofluorescence staining and
confocal laser microscopy
Cells were seeded onto coverslips in 24-well plates
at a density of 5×104 cells/well for 24 h before
treatment, and then treated with increasing doses of BMH-21 (1, 2
and 4 µM) for 24 h. After treatment, cells were washed three times
with cold 0.1 M PBS and fixed in 4% (w/v) paraformaldehyde/PBS for
20–30 min, stained with the nuclear stain Hoechst
33342/H2O (2 µg/ml, Sigma) for 30 min, washed with 0.01
M PBS, and examined using Olympus FV1000 confocal laser microscopy
to reveal chromatin condensation. The expression and localization
of nucleolin (1:1,400 dilution, Abcam, Hong Kong, China),
nucleophosmin (1:1,400 dilution, Abcam) and fibrillarin (1:1,400
dilution, Abcam) were examined. Cells were cultured on coverslips
overnight, then treated with the indicated drugs, and rinsed with
0.1 M PBS three times. After incubation, the cells were fixed with
4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton
X-100 (Sigma-Aldrich) for 5 min, washed three times with 0.01 M
PBS, blocked for 30 min in 5% (w/v) non-immune animal serum (goat)
(Beyotime Biotechnology, Shanghai, China) PBS, and incubated with
primary antibody overnight at 4°C. The next day, the slides were
incubated with the Alexa Fluor-488/546-conjugated secondary
antibody (1:400 dilution; Invitrogen) for 1 h, then stained with
Hoechst 33342 (2 µg/ml) for 2 min and washed three times with PBS.
After mounting, the cells were examined by Olympus FV1000 confocal
laser microscopy.
Western blot analysis
Protein concentrations were measured using a Bio-Rad
Protein assay kit (Bio-Rad Laboratories). For western blot
analysis, protein lysates (30–50 µg) were separated on a 12%
SDS-PAGE gel and 15% SDS-PAGE gel and transferred onto an
Immobilon-P membrane (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% non-fat dry milk in buffer (10 mM
Tris-HCl, pH 7.6; 100 mM NaCl; and 0.1% Tween-20) for 2 h at room
temperature and then incubated with the appropriate primary
antibodies overnight at 4°C. Antibodies used were: anti-p53 and
anti-p-p53-Ser15 (1:1,000 dilution, SAB, College Park, MD, USA),
anti-MDM2 (1:1,000 dilution, SAB), anti-Bax (1:1,000 dilution,
Proteintech Group®, Chicago, IL, USA), anti-caspase-3
(1:1,000 dilution, Abcam) and anti-β-actin (1:1,000 dilution,
Proteintech Group). The following day, membranes were incubated
with horseradish peroxidase-conjugated secondary antibody (Thermo,
Waltham, MA, USA) at a 1:2,000 dilution for 2 h at room
temperature. Membranes were then incubated in ECL reagents and
images were captured by Syngene Bio Imaging (Synoptics, Cambridge,
UK). Densitometric quantitation of bands was performed using
Syngene Bio Imaging tools. Data are presented as the mean ±
standard deviation (SD) from three independent experiments.
Statistical analysis
Results are expressed as means ± standard deviation
(SD) or means ± standard error of mean (SEM), as indicated in the
figure legends. Data are representative of three independent
experiments performed in triplicate. Statistical analysis of the
data was performed using one-way ANOVA. The Tukey's post hoc test
was used to determine the significance for all pairwise comparisons
of interest. Differences were considered statistically significant
for values of P<0.05.
Results
BMH-21 inhibits the viability of
cancer cells
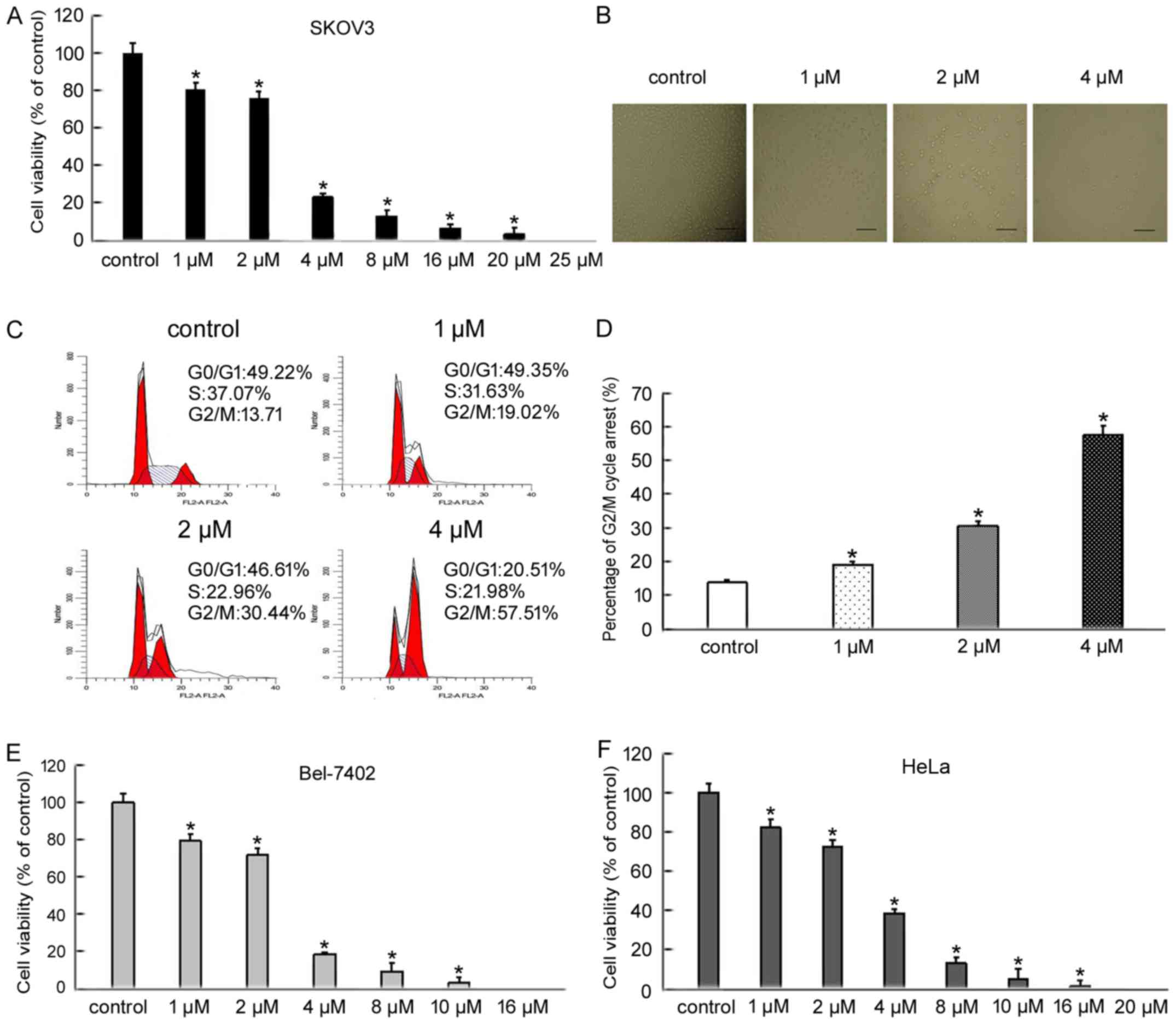
To evaluate the inhibitory effect of BMH-21 on
SKOV3, Bel-7402 and HeLa cell viability, cells were treated with
increasing doses of BMH-21 for 24 h and cell viability was
determined by an MTT assay. BMH-21 treatment decreased the
viability of SKOV3, Bel-7402 and HeLa cells in a dose-dependent
manner (Fig. 1A, E and F). Based on
MTT results, we treated SKOV3 cells with increasing doses of BMH-21
(1, 2 and 4 µM) for 24 h. We examined the changes in SKOV3 cell
morphology using an inverted optical microscope. The cells treated
with BMH-21 became fragmented and round when compared with control
cells (Fig. 1B). To examine the
distribution of cell cycle progression, we confirmed the effect of
BMH-21 in various cell cycle phases using flow cytometry. BMH-21
resulted in a marked increase in the percentage of cells blocked at
G2/M phase (Fig. 1C and D). These
findings indicated that BMH-21 effectively inhibited cancer cell
viability and suggested that BMH-21 induced cell death.
BMH-21 induces apoptosis in SKOV3
cells
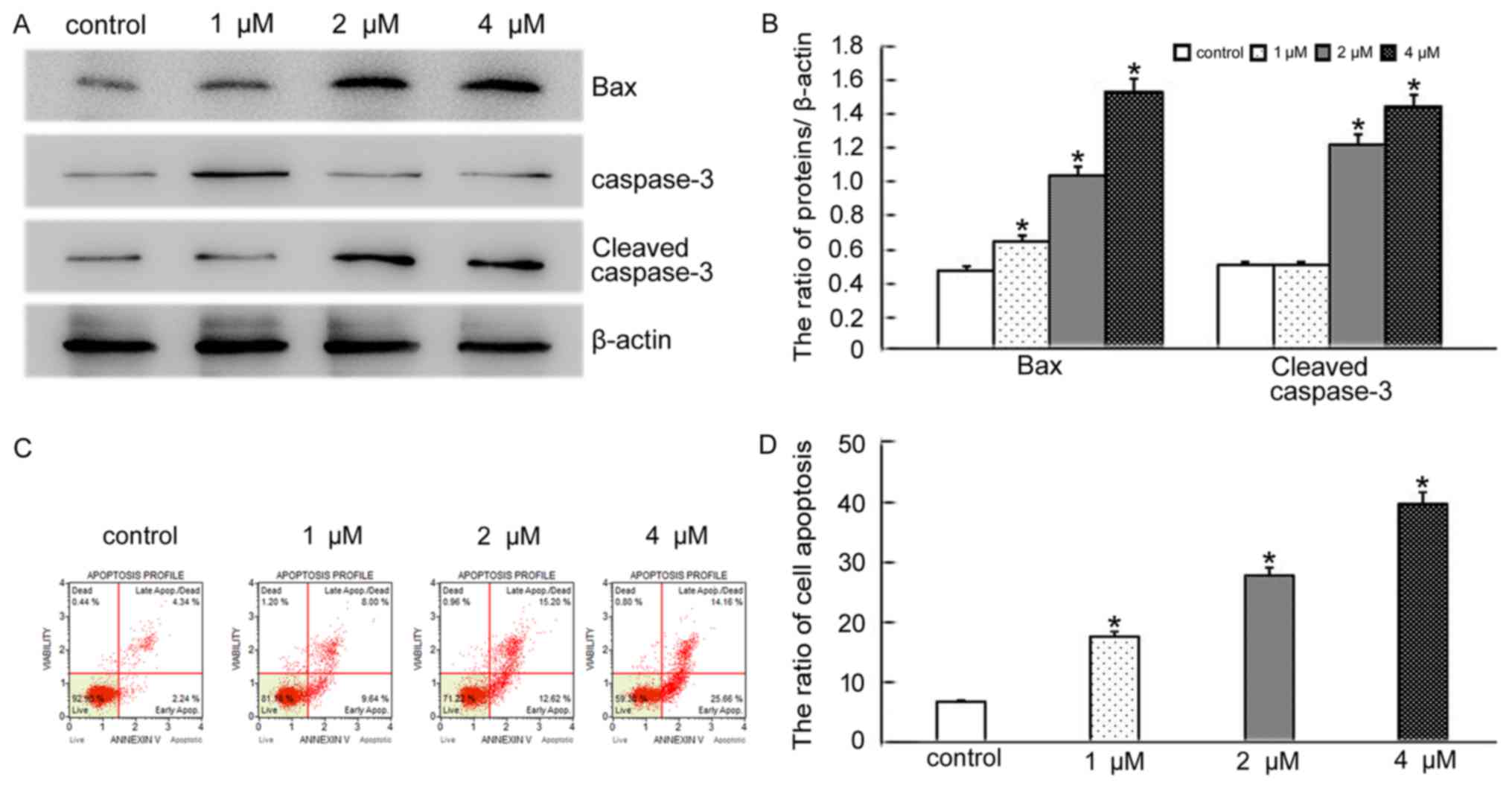
BCL2 associated X (BAX), a member of the BCL2
family, is a pro-apoptotic protein. Overexpression of BAX triggers
the release of mitochondrial proteins that cleave and thereby
activate caspase-3 resulting in apoptosis (11). We therefore decided to explore if
the morphological changes seen after BMH-21 treatment were a result
of apoptotic induction in SKOV3 cells. Based on the MTT results,
SKOV3 cells were treated with the same doses of BMH-21 for 24 h and
the levels of BAX and cleaved caspase-3 were detected by western
blotting. The results showed that the levels of BAX and cleaved
caspase-3 increased in BMH-21 treated cells, compared with the
control group (Fig. 2A and B).
Additionally, flow cytometry analysis revealed that BMH-21 induced
SKOV3 cell apoptosis in concentration-dependent manner (Fig. 2C and D). The results suggested that
BMH-21 induces apoptosis in SKOV3 cells through a BAX-caspase-3
pathway.
BMH-21 induces nucleolar stress in
SKOV3 cells
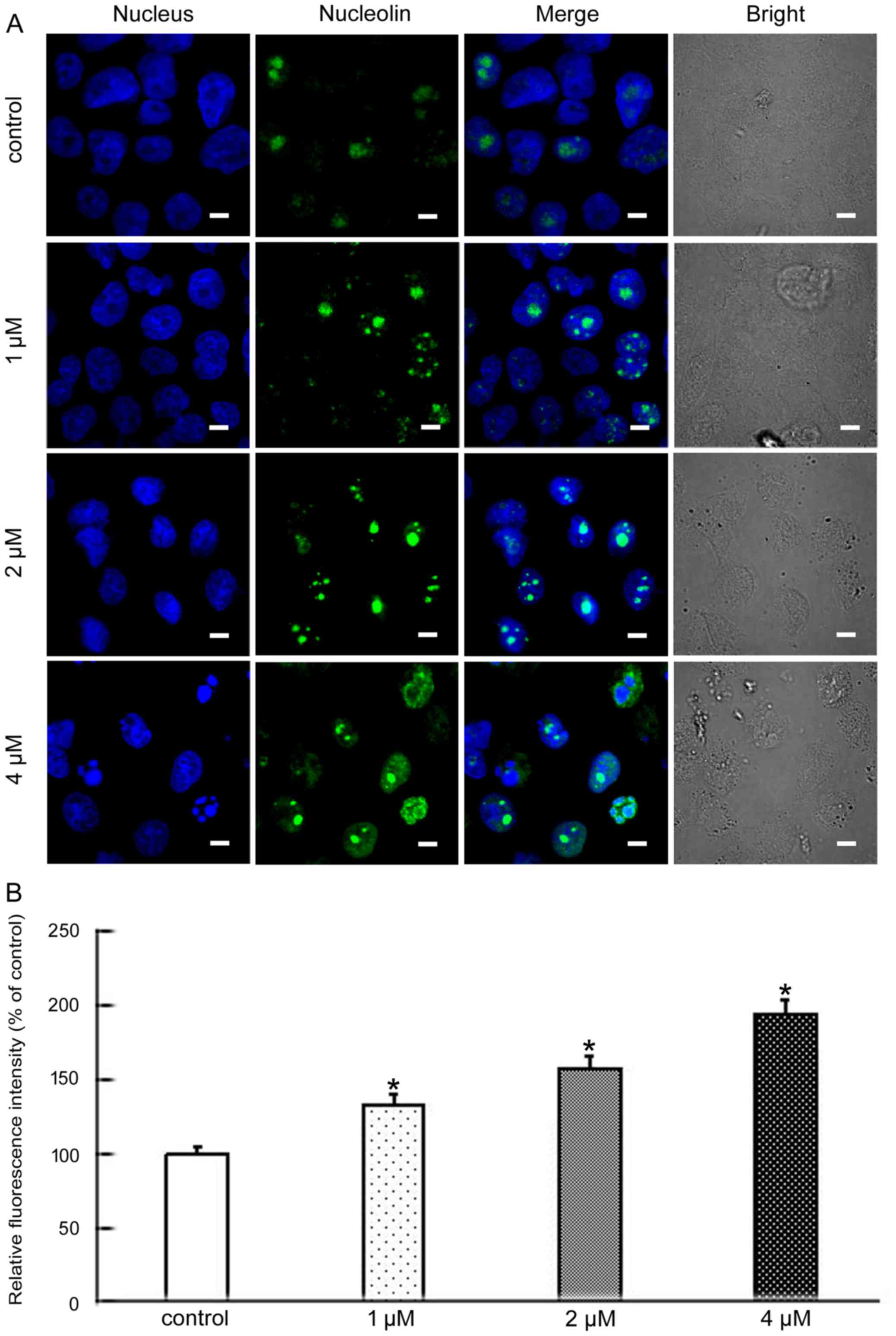
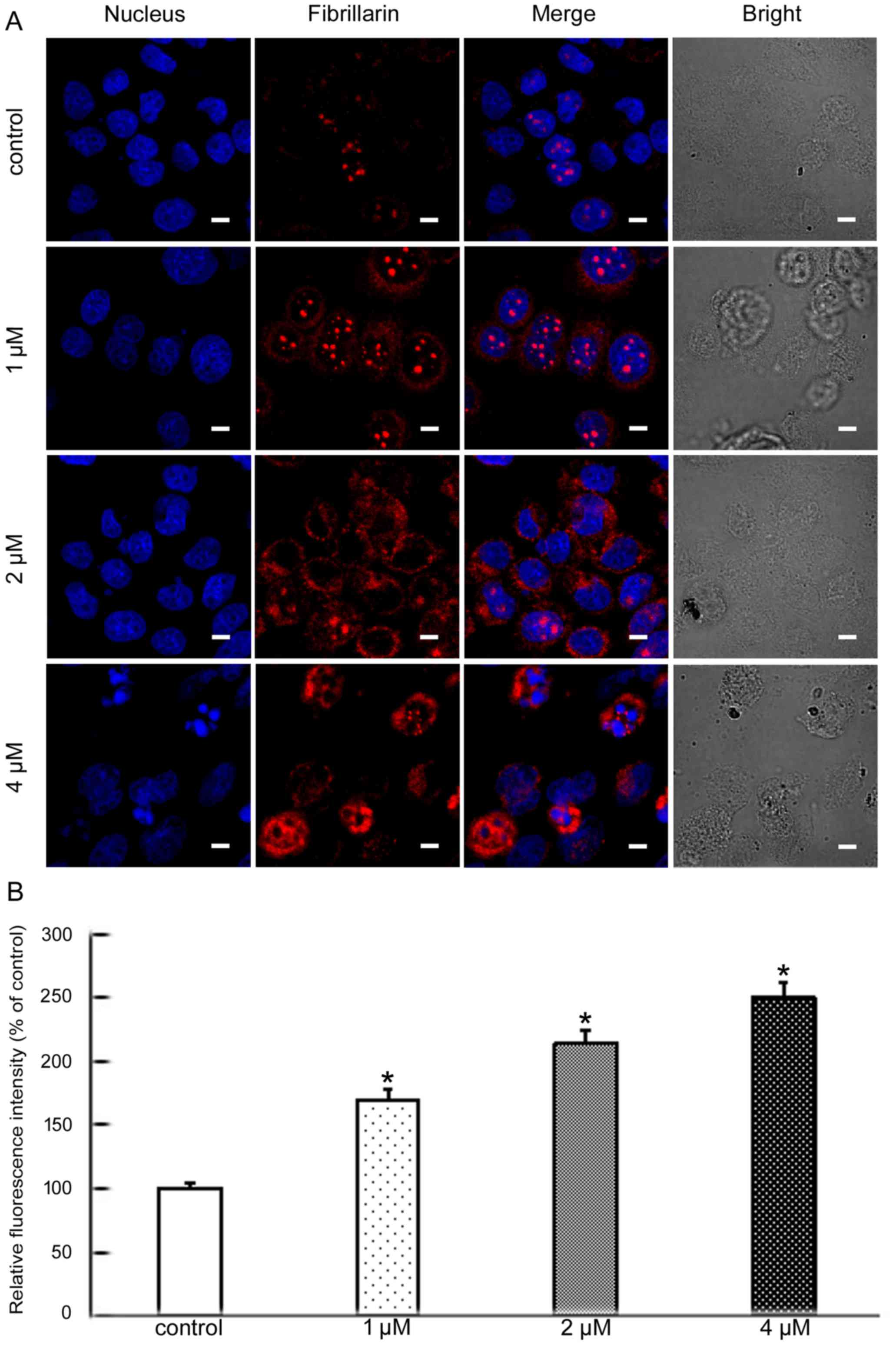
Nucleolin, nucleophosmin and fibrillarin, the major
nucleolar proteins of proliferating eukaryotic cells, play an
important role in nucleolar stress. When nucleolar stress occurs,
the levels of these three proteins increase and the proteins
translocate from the nucleus to the cytoplasm (12,13).
Nucleolin is the major nucleolar protein of exponentially growing
eukaryotic cells, and participates in many modulations including
rDNA transcription, RNA metabolism, and ribosome assembly (14). Nucleophosmin is a highly and
ubiquitously expressed protein, and plays crucial roles in ribosome
maturation and export, centrosome duplication and cell cycle
progression (15). Fibrillarin is
one of the most studied nucleolar proteins and also is an early
marker for the site of formation of the newly forming nucleolus.
Its main functions are methylation and processing of pre-rRNA
(16). To further analyze the
molecular mechanisms of apoptosis induced by BMH-21 we explored
whether nucleolar stress was involved. The level and cellular
localization of nucleolin, nucleophosmin and fibrillarin were
examined by confocal microscopy. These results showed that BMH-21
increased the levels of all three proteins and resulted in their
nuclear exclusion (Figs. 3–5). This suggested that BMH-21 induced
nucleolar stress in SKOV3 cells.
BMH-21 activates p53 signaling pathway
in SKOV3 cells
There is increasing evidence that suggests nucleolar
stress can lead to induction of the p53 pathway in cells. Under
normal conditions, MDM2 proto-oncogene (MDM2) binds directly to
p53, promoting the degradation of p53 and inhibiting its activity
(17). Nucleolar stress causes
disruption of MDM2-p53 binding, leading to decreased MDM2 levels
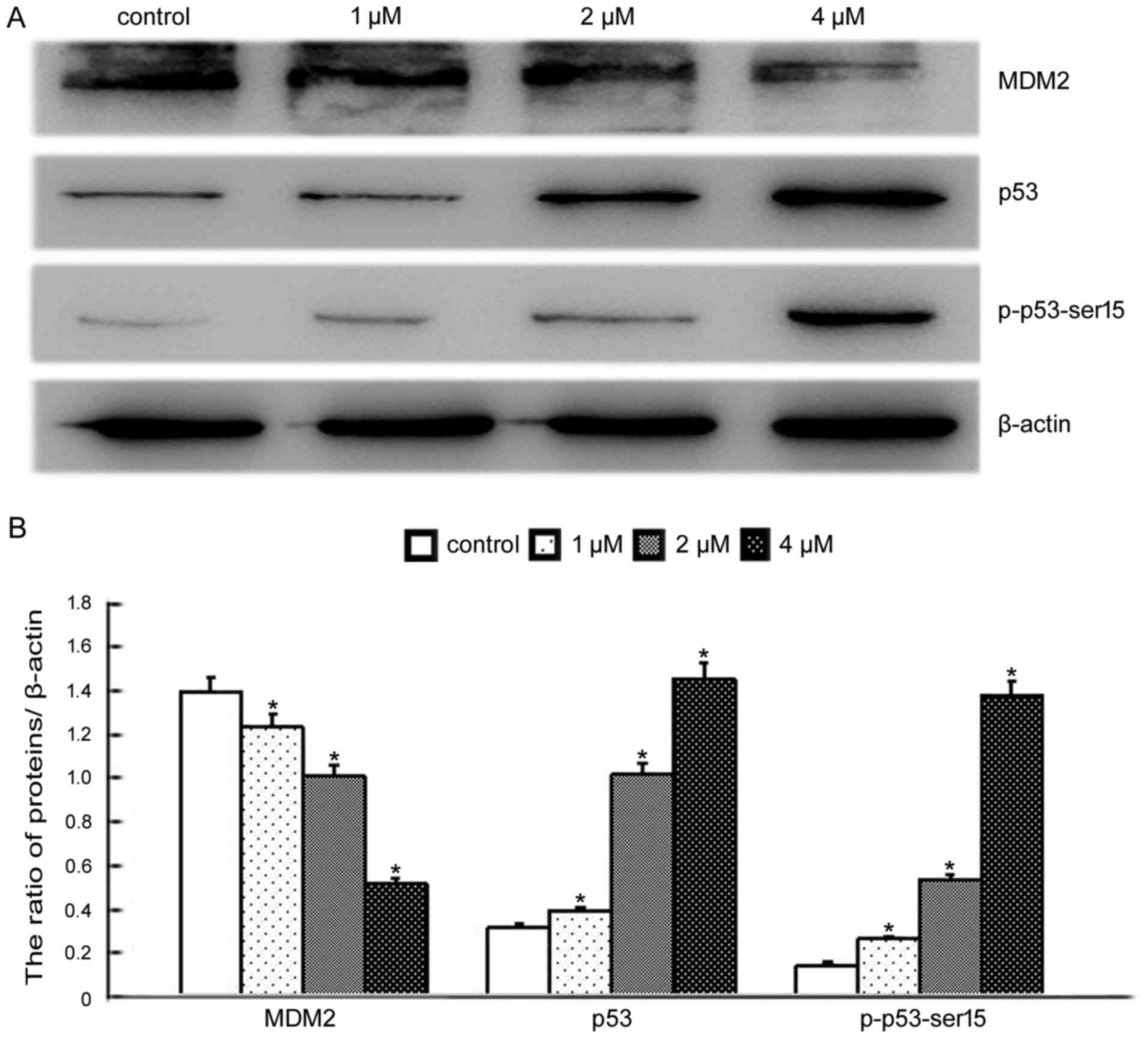
and increased p53 and p-p53-Ser15 levels (18). We therefore investigated whether
BMH-21-induced nucleolar stress had an impact on the p53 pathway.
Western blotting was conducted to determine the levels of MDM2, p53
and p-p53-Ser15. These results indicated that BMH-21 treatment
decreased MDM2 levels, increased p53 and p-p53-Ser15 levels in a
dose-dependent manner (Fig. 6),
supporting the involvement of the p53 pathway in the response to
BMH-21 in SKOV3 cells.
Discussion
Ovarian cancer remains the most common gynecologic
tumor of the genital tract. Cisplatin is the most commonly used and
effective chemotherapeutic drug for ovarian cancer. Patients with
ovarian cancer usually respond well to initial chemotherapy with
cisplatin. However, most patients develop resistance to cisplatin
during the course of their treatment (1,19).
Hence, there is an urgent need to identify new therapeutic targets
in ovarian cancer and to develop novel, potent and specific drugs.
Negi et al reported that BMH-21 selectively killed cancer
cells by nucleolar stress while sparing normal cells, suggesting
that the nucleolus is a potential new target area in tumor
treatment (20). In our study, we
found that BMH-21 inhibited viability and induced apoptosis in a
dose-dependent manner (Figs. 1 and
2).
The nucleolus is a specialized sub-nuclear
compartment of eukaryotic cells where rRNA synthesis and ribosome
assembly take place. Recent studies have shown that, apart from its
traditional function, the nucleolus plays a vital role in viral
replication, control of aging, cell cycle regulation, and apoptosis
(21–24). The perturbation of ribosome
synthesis, induced by various stimuli, can lead to nucleolar stress
(23). In recent years, research
has focused on the relationship between nucleolar stress and cancer
(25). As proliferating tumor cells
have enhanced ribosome biogenesis compared with normal somatic
cells, the tumor cells are more sensitive to nucleolar stress and
this provides a therapeutic window (26). Interestingly, Quin et al
proposed that inhibition of nucleolar stress represented an
emerging hallmark of cancer and thus the nucleolus would be a
potential target for cancer treatment (6). Nucleophosmin, nucleolin and
fibrillarin are ubiquitously expressed nucleolar multifunctional
proteins, and can act as markers for nucleolar stress (27). Nucleolar stress not only induces
expression of these proteins, but also contributes to their
translocation from the nucleus to the cytoplasm, thus activating
downstream death signaling processes. In the present study, we
found that BMH-21 induced the translocation of nucleophosmin,
nucleolin and fibrillarin to the cytoplasm (Figs. 3–5),
suggesting that nucleolar stress had occurred.
The tumor suppressor protein p53 is a downstream
effector of nucleolar stress. Under normal circumstances, the E3
ubiquitin ligase MDM2 binds to p53 and is involved in the
degradation of p53 by the ubiquitin-proteasome system leading to
very low levels of p53 (28–31).
After nucleolar stress, a number of ribosomal proteins bind to MDM2
disrupting its interaction with p53. MDM2 expression is then
decreased thus leading to p53 stabilization and activation
(32). Activation of p53 is
reported to be controlled by phosphorylation of p53 at critical
serine residues in the N terminus. When activated, p-p53-Ser15
induces cell cycle arrest or apoptosis via induction of a series of
target genes (including BAX, PMAIP1 and BBC3)
(33,34). Further evidence has been provided by
Lin et al who showed that tubeimoside-1 (TBMS1) induced
nucleolar stress and apoptosis in the human lung cancer cell line
NCI-H460. The nucleolar stress was dependent on a p53/MDM2
mechanism, suggesting that p53-dependent nucleolar stress may be
responsible for the death effect of TBMS1 (35). Consistent with the above reports, we
found that BMH-21 decreased MDM2 expression, increased p53 and
p-p53-Ser15 expression (Fig.
6).
In conclusion, the present study demonstrated that
BMH-21 induced nucleolar stress responses, and resulted in the
accumulation of the tumor suppressor protein p53 and p-p53-Ser15,
thus inhibiting the viability and inducing apoptosis in SKOV3
cells. Our data provide new insights into the mechanism of
nucleolar stress and suggest that nucleolar stress is a potential
target for ovarian cancer therapy.
Acknowledgements
This study was supported by the National Nature and
Science Foundation of China (81372793 and 81671041). The Department
of Education of Jilin Province Project (no. 2016237), and
Scientific Research Foundation of Jilin Province for University
Students. The authors would like to thank Director Dominic James
from Liwen Bianji (Edanz Group China) for the language editing of
this manuscript.
References
|
1
|
Xie S, Zheng H, Wen X, Sun J, Wang Y, Gao
X, Guo L and Lu R: MUS81 is associated with cell proliferation and
cisplatin sensitivity in serous ovarian cancer. Biochem Biophys Res
Commun. 476:493–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gershenson DM and Frazier AL: Conundrums
in the management of malignant ovarian germ cell tumors: Toward
lessening acute morbidity and late effects of treatment. Gynecol
Oncol. 143:428–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wijdeven RH, Pang B, Assaraf YG and
Neefjes J: Old drugs, novel ways out: Drug resistance toward
cytotoxic chemotherapeutics. Drug Resist Updat. 28:65–81. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L and Chen J: SirT1 and rRNA in the
nucleolus: Regulating the regulator. Oncoscience. 1:111–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hariharan N and Sussman MA: Stressing on
the nucleolus in cardiovascular disease. Biochim Biophys Acta.
1842:798–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quin JE, Devlin JR, Cameron D, Hannan KM,
Pearson RB and Hannan RD: Targeting the nucleolus for cancer
intervention. Biochim Biophys Acta. 1842:802–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hein N, Hannan KM, George AJ, Sanij E and
Hannan RD: The nucleolus: An emerging target for cancer therapy.
Trends Mol Med. 19:643–654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colis L, Ernst G, Sanders S, Liu H,
Sirajuddin P, Peltonen K, DePasquale M, Barrow JC and Laiho M:
Design, synthesis, and structure-activity relationships of
pyridoquinazolinecarboxamides as RNA polymerase I inhibitors. J Med
Chem. 57:4950–4961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peltonen K, Colis L, Liu H, Jäämaa S,
Moore HM, Enbäck J, Laakkonen P, Vaahtokari A, Jones RJ, af
Hällström TM, et al: Identification of novel p53 pathway activating
small-molecule compounds reveals unexpected similarities with known
therapeutic agents. PLoS One. 5:e129962010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peltonen K, Colis L, Liu H, Trivedi R,
Moubarek MS, Moore HM, Bai B, Rudek MA, Bieberich CJ and Laiho M: A
targeting modality for destruction of RNA polymerase I that
possesses anticancer activity. Cancer Cell. 25:77–90. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X and Yu H: Matrine inhibits
diethylnitrosamine-induced HCC proliferation in rats through
inducing apoptosis via p53, Bax-dependent caspase-3 activation
pathway and down-regulating MLCK overexpression. Iran J Pharm Res.
15:491–499. 2016.PubMed/NCBI
|
|
12
|
Colis L, Peltonen K, Sirajuddin P, Liu H,
Sanders S, Ernst G, Barrow JC and Laiho M: DNA intercalator BMH-21
inhibits RNA polymerase I independent of DNA damage response.
Oncotarget. 5:4361–4369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stepiński D: Immunodetection of nucleolar
proteins and ultrastructure of nucleoli of soybean root
meristematic cells treated with chilling stress and after recovery.
Protoplasma. 235:77–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z and Xu X: Roles of nucleolin. Focus
on cancer and anti-cancer therapy. Saudi Med J. 37:1312–1318. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chopra A, Soni S, Pati H, Kumar D, Diwedi
R, Verma D, Vishwakama G, Bakhshi S, Kumar S, Gogia A, et al:
Nucleophosmin mutation analysis in acute myeloid leukaemia:
Immunohistochemistry as a surrogate for molecular techniques.
Indian J Med Res. 143:763–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shubina MY, Musinova YR and Sheval EV:
Nucleolar methyltransferase fibrillarin: Evolution of structure and
functions. Biochemistry (Mosc). 81:941–950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olausson Holmberg K, Nistér M and
Lindström MS: p53 -dependent and -independent nucleolar stress
responses. Cells. 1:774–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trino S, Iacobucci I, Erriquez D,
Laurenzana I, De Luca L, Ferrari A, Ghelli Luserna, Di Rorà A,
Papayannidis C, Derenzini E, Simonetti G, et al: Targeting the
p53-MDM2 interaction by the small-molecule MDM2 antagonist
Nutlin-3a: A new challenged target therapy in adult Philadelphia
positive acute lymphoblastic leukemia patients. Oncotarget.
7:12951–12961. 2016.PubMed/NCBI
|
|
19
|
Sun Y, Jin L, Liu JH, Sui YX, Han LL and
Shen XL: Interfering EZH2 expression reverses the cisplatin
resistance in human ovarian cancer by inhibiting autophagy. Cancer
Biother Radiopharm. 31:246–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Negi SS and Brown P: rRNA synthesis
inhibitor, CX-5461, activates ATM/ATR pathway in acute
lymphoblastic leukemia, arrests cells in G2 phase and induces
apoptosis. Oncotarget. 6:18094–18104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicolas E, Parisot P, Pinto-Monteiro C, de
Walque R, De Vleeschouwer C and Lafontaine DL: Involvement of human
ribosomal proteins in nucleolar structure and p53-dependent
nucleolar stress. Nat Commun. 7:113902016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eliopoulos AG and Volarevic S:
TPL2-NPM-p53 pathway monitors nucleolar stress. Oncoscience.
2:892–893. 2015.PubMed/NCBI
|
|
23
|
Boulon S, Westman BJ, Hutten S, Boisvert
FM and Lamond AI: The nucleolus under stress. Mol Cell. 40:216–227.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sloan KE, Bohnsack MT and Watkins NJ: The
5S RNP couples p53 homeostasis to ribosome biogenesis and nucleolar
stress. Cell Rep. 5:237–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang M, Whang P, Lewicki P and Mitchell
BS: Cyclopentenyl cytosine induces senescence in breast cancer
cells through the nucleolar stress response and activation of p53.
Mol Pharmacol. 80:40–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Russo A, Pagliara V, Albano F, Esposito D,
Sagar V, Loreni F, Irace C, Santamaria R and Russo G: Regulatory
role of rpL3 in cell response to nucleolar stress induced by Act D
in tumor cells lacking functional p53. Cell Cycle. 15:41–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin R, Jiang W and Liu D: Aluminum can
induce alterations in the cellular localization and expression of
three major nucleolar proteins in root tip cells of Allium
cepa var. agrogarum L. Chemosphere. 90:827–834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parkosadze G, Burkadze G, Mizandari M,
Sulakvelidze M and Sanikidze T: Role of proapoptotic p-53 factor in
pathogenesis of nonalcoholic hepatosteatosis. Georgian Med News.
2:55–60. 2013.(In Russian).
|
|
29
|
James A, Wang Y, Raje H, Rosby R and
DiMario P: Nucleolar stress with and without p53. Nucleus.
5:402–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu J, Han M, Shen J, Guan Q, Bai Z, Lang
B, Zhang H, Li Z, Zuo D, Zhang W, et al:
2-Methoxy-5((3,4,5-trimethosyphenyl)seleninyl) phenol inhibits MDM2
and induces apoptosis in breast cancer cells through a
p53-independent pathway. Cancer Lett. 383:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sriraman A, Li Y and Dobbelstein M:
Fortifying p53 - beyond Mdm2 inhibitors. Aging (Albany, NY).
8:1836–1837. 2016. View Article : Google Scholar
|
|
32
|
Barone G, Tweddle DA, Shohet JM, Chesler
L, Moreno L, Pearson AD and Van Maerken T: MDM2-p53 interaction in
paediatric solid tumours: Preclinical rationale, biomarkers and
resistance. Curr Drug Targets. 15:114–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang L and Zhang Z: Gambogic acid
inhibits malignant melanoma cell proliferation through
mitochondrial p66shc/ROS-p53/Bax-mediated apoptosis. Cell Physiol
Biochem. 38:1618–1630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alshatwi AA, Subash-Babu P and Antonisamy
P: Violacein induces apoptosis in human breast cancer cells through
up regulation of BAX, p53 and down regulation of MDM2. Exp Toxicol
Pathol. 68:89–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin Y, Xie G, Xia J, Su D, Liu J, Jiang F
and Xu Y: TBMS1 exerts its cytotoxicity in NCI-H460 lung cancer
cells through nucleolar stress-induced p53/MDM2-dependent
mechanism, a quantitative proteomics study. Biochim Biophys Acta.
1864:204–210. 2016. View Article : Google Scholar : PubMed/NCBI
|