Introduction
Bladder cancer (BCa) is the fourth most common type
of cancer in the United States (1,2).
Approximately 75% of BCa patients are diagnosed with non-muscle
invasive bladder cancer (NMIBC) (3). Among the majority of NMIBC cases that
recur, roughly 15–20% progress to MIBC and lead to local invasion
and distant metastasis, representing the main cause of death
(4,5). Due to the high mortality rate
associated with BCa, novel treatment methods to fight the disease
are clearly warranted (6).
Currently, chemotherapy combined with gemcitabine and cisplatin
treatment is the most common treatment strategy for BCa, as well as
other carcinomas (7,8). However, in approximately 60–70% of
patients with metastatic BCa, relapse occurs in the first year and
drug resistance is becoming a major concern. The underlying
mechanisms of BCa remain unclear. Thus, it is of utmost importance
to gain a better understanding of the underlying mechanisms of
BCa.
Centromere protein U (CENPU) (9,10),
also known as KLIP1, MLF1IP (11,12),
CENP-50/PBIP1 and Plk1 (13,14) is
required for maintenance of sister chromatid adhesion during
recovery from spindle damage in chicken cells (10,15).
In human cells, loss of CENPU can cause mitotic defects in
chromosome attachment (16). As a
phosphorylation substrate of Polo-like kinase 1 (Plk1), a member of
the serine/threonine kinase family and regulator of a variety of
functions during the cell cycle, Plk1 recruitment to interphase and
mitosis kinetochore requires a phophorylation-dependent CENPU-Plk1
interaction (10,15). Moreover, CENPU plays a key role in
kinetochore-microtubule attachment by interacting with Hec1
(9).
Accumulating evidence has demonstrated that CENPU is
associated with tumorigenesis. One study showed that CENPU (MLF1IP)
was increased in various glioblastoma cell lines and plays an
important role in erythroleukemias (12). Moreover, CENPU overexpression showed
a positive correlation with progression and prognosis in luminal
breast cancer (17). Although CENPU
significantly promotes prostate cancer cell proliferation, a
difference in CENPU mRNA expression between CENPU upregulation and
downregulation was not found (18)
and to date the functional role of CENPU in human BCa has not yet
been established.
In this study, we evaluated the role of CENPU to
better determine its role in human bladder carcinogenesis.
Materials and methods
Human BCa specimens
In the present study, 100 patients with BCa who were
diagnosed at the First Affiliated Hospital of Bengbu Medical
College between January 2011 and December 2013 were enrolled. From
each patient, a tissue biopsy was obtained via surgical excision.
All samples were obtained after informed consent was provided and
used with approval from the Review Board of the First Affiliated
Hospital of Bengbu Medical College. At the time of diagnosis, the
ages of the patients ranged from 18 to 95 years, the median age
being 56 years. From all samples, 5-µm-thick formalin-fixed
paraffin-embedded slides were prepared in accordance with the
protocol of the Department of Pathology at the First Affiliated
Hospital of Bengbu Medical College. From all patients, data were
obtained including age at diagnosis, sex, tumor size, histological
grade, TNM stage and vital status of patients relative to
disease-specific survival at the 3-year follow-up appointment.
Immunohistochemistry
Immunohistochemical staining for CENPU was conducted
on 5-µm-thick formalin-fixed paraffin-embedded tissues. First, the
tissue was heated at 60°C, deparaffinized, and hydrated by
sequential washes in xylene, graded alcohol followed by
phosphate-buffered saline (PBS). The CENPU antigen was retrieved by
incubation with 0.1 mol/l citrate buffer (pH 6.0) for 30 min at
95°C. To quench endogenous peroxidase activity, tissues were washed
with PBS and incubated with 3% (v/v) H2O2 for
10 min at room temperature. Following three additional washes with
PBS, non-specific binding sites were blocked by incubation with 10%
(w/v) normal goat serum diluted in 1 part (w) bovine serum albumin
(BSA) and 99 parts (v) PBS for 30 min at room temperature. Tissues
were incubated overnight with 1 part anti-CENPU monoclonal antibody
(Abcam, Cambridge, MA, USA) and 999 parts blocking solution at 4°C.
Tissues were then washed three times with PBS, incubated in
blocking solution for 10 min at room temperature followed by
incubation with a biotinylated antibody and horseradish
peroxidase-labeled streptavidin for 1 h at room temperature. The
enzymatic reaction was developed with 3,3′-diaminobenzidine (DAB;
Sangon Biotechnology, Shanghai, China) for 5 min at room
temperature. Tissues were counterstained with hematoxylin (Sigma,
St. Louis, MO, USA) for 30 sec, and washed with PBS for 5 min.
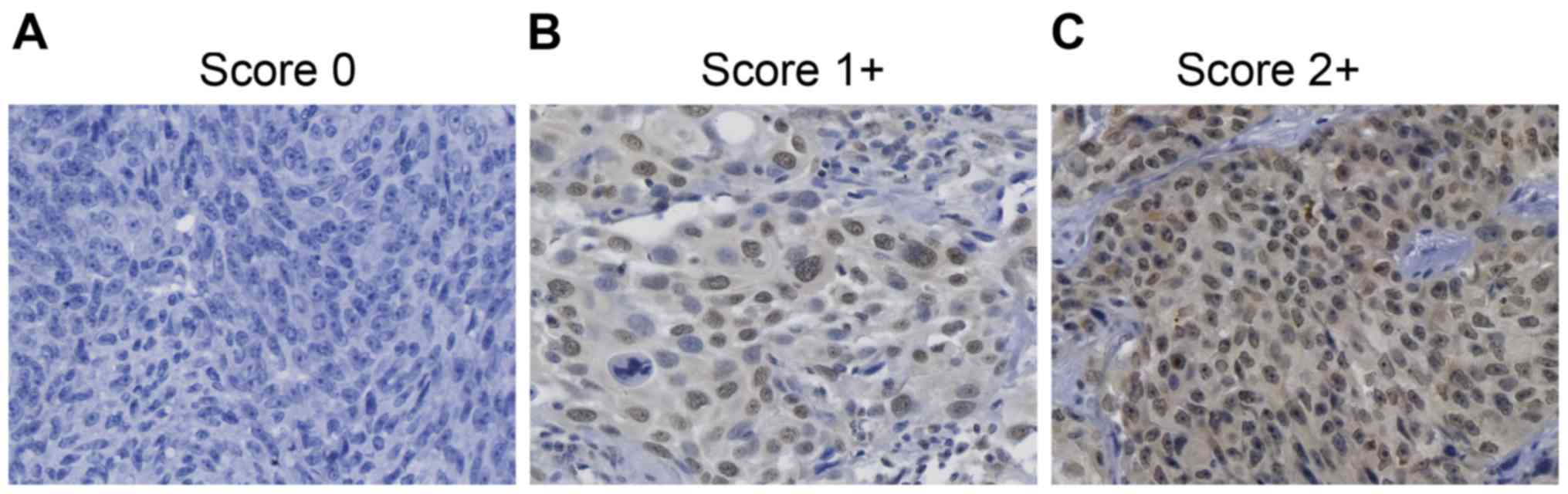
CENPU immunostaining was scored independently by two
pathologists. Scoring of CENPU expression was as previously
described (19) and based on two
variables: i) the percentage of positively stained tumor cells (0
to <5% positively stained cells = 0, 5–25% positively stained
cells = 1, or >50% positively stained cells =2) and ii) the
staining intensity (absent or low staining =0, moderate staining
=1, or high staining =2). Both scores were multiplied to obtain an
overall score for each specimen and was dichotomized into low
(scores of 0–1) or high (scores >2) classifications.
Cell lines
Human bladder cancer cell lines T24 and 5637 were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and cultured in Dulbecco's modified Eagles medium (DMEM)
supplemented with 10% fetal calf serum (both from Gibco, Shanghai,
China), 100 µg/ml streptomycin and 100 U/ml penicillin (both from
Sangon Biotech, Shanghai, China) in 5% CO2 at 37°C.
Media were replaced every other day and passaged upon reaching
70–80% confluency.
RNA isolation and quantitative
real-time PCR
Total RNA was extracted using a TRIzol kit
(Invitrogen, Shanghai, China) according to the manufacturer's
instructions. RNA quality was assessed using a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Shanghai, China). To
synthesize cDNA from total RNA, reverse transcription was performed
using the M-MLV-RTase kit (Promega, Shanghai, China). For
quantitative real-time PCR (qPCR; Applied Biosystems, Foster City,
CA, USA), SYBR-Green PCR Master Mix was used according to the
manufacturer's instructions. For each sample, 2.0 µg of total RNA
was used as a template for qPCR. The primer sequences are listed in
Table I. Results were normalized to
GAPDH (20) and data were analyzed
using the Pfaffl's method (21).
 | Table I.Primers used in this study. |
Table I.
Primers used in this study.
| Gene | Accession no. | Primer
sequences | Size (bp) |
|---|
| CENPU | NM_024629 | Forward:
5′-ATGAACTGCTTCGGTTAGAGC-3′ | 246 |
|
|
| Reverse:
5′-TATTTCGCAGATGGCTTTCGG-3′ |
|
| CXCL8 | NM_000584 | Forward:
5′-TGGCAGCCTTCCTGATTT-3′ | 236 |
|
|
| Reverse:
5′-AACCCTCTGCACCCAGTT-3′ |
|
| RAC1 | NM_018890 | Forward:
5′-GTAGCAGCTCAGCTCTTTGGA-3′ | 228 |
|
|
| Reverse:
5′-TACCCGTGACACTTTCATTCC-3′ |
|
| CCND3 | NM_001287427 | Forward:
5′-ACCTGGCTGCTGTGATTGC-3′ | 158 |
|
|
| Reverse:
5′-GATCATGGATGGCGGGTAC-3′ |
|
| IL1A | NM_000575 | Forward:
5′-AGATGCCTGAGATACCCAAAACC-3′ | 147 |
|
|
| Reverse:
5′-CCAAGCACACCCAGTAGTCT-3′ |
|
| IL6 | NM_000600 | Forward:
5′-CAAATTCGGTACATCCTCG-3′ | 259 |
|
|
| Reverse:
5′-CTCTGGCTTGTTCCTCACTA-3′ |
|
| IL1B | NM_000576 | Forward:
5′-TCCTGTTGTCTACACCAATGCCCA-3′ | 190 |
|
|
| Reverse:
5′-GAACCAAATGTGGCCGTGGTTTCT-3′ |
|
| TNFRSF11B | NM_002546 | Forward:
5′-CCTTGCCCTGACCACTAC-3′ | 207 |
|
|
| Reverse:
5′-TTGCACCACTCCAAATCC-3′ |
|
| PTGS2 | NM_000963 | Forward:
5′-CTCCTGTGCCTGATGATTGC-3′ | 215 |
|
|
| Reverse:
5′-CAGCCCGTTGGTGAAAGC-3′ |
|
| MAD2L1 | NM_002358 | Forward:
5′-GAGTCGGGACCACAGTTTAT-3′ | 97 |
|
|
| Reverse:
5′-TTTTGTAGGCCACCATGCTA-3′ |
|
| FN1 | NM_212482 | Forward:
5′-GAGAATAAGCTGTACCATCGCAA-3′ | 200 |
|
|
| Reverse:
5′-CGACCACATAGGAAGTCCCAG-3′ |
|
| GAPDH |
| Forward:
5′-TGACTTCAACAGCGACACCCA-3′ | 121 |
|
|
| Reverse:
5′-CACCCTGTTGCTGTAGCCAAA-3′ |
|
Lentiviral transfection of T24
cells
Based on the gene expression profile in the human
BCa cell lines T24 and 5637, the BCa T24 cell line was selected for
the remaining experiments. When in the logarithmic phase, T24 cells
were digested with trypsin (Sangon Biotech) and resuspended in
DMEM. Cells were seeded in 6-well plates (5×104
cells/well) and incubated in 5% CO2 at 37°C until ~30%
confluency was reached. Next, two experimental groups were
constructed: i) an shCENPU group in which cells were transfected
with CENPU-siRNA GFP lentivirus and ii) a control (shCtrl) group in
which cells were transfected with a scramble sequence GFP
lentivirus (Genechem, Shanghai, China). An appropriate amount of
lentivirus was added according to the multiplicity of infection
(MOI). When, after 12 h, no cytotoxic effects were observed,
transfection was continued. After 24 h, the standard medium was
replaced. GFP fluorescence was used to evaluate transfection
efficiency 3 days post-transfection. When the transfection
efficiency was >80%, the cells were divided into two groups: one
group was transferred to a 12-well plate for future RNA extraction,
the other group was transferred to a 6-well plate for future
protein extraction.
Cell proliferation assays
ShCENPU and shCtrl cells were trypsinized and after
a logarithmic proliferation phase was reached, they were
resuspended in DMEM. Cells were plated in 96-wells at equal cell
density (2,000 cells/100 µl/well) followed by incubation at 37°C
with 5% CO2. After 24 h, a Cellomics ArrayScan VTI
imager (Thermo Fisher Scientific, Waltham, MA, USA) was used to
continuously measure GFP expression in each well over a 5-day time
period. The data were used for performing statistical data mapping
and construction of cell proliferation curves.
A BrdU assay for cell proliferation was conducted
according to the manufacturer's instructions (CST, Danvers, MA,
USA). Briefly, shCENPU and shCtrl cells were trypsinized and after
a logarithmic proliferation phase was reached, they were
resuspended in standard medium. The cells were plated in a 96-well
plate at an equal density (2,500 cell/100 µl/well) and incubated at
37°C in 5% CO2 for continuous detection over a 3-day
time period. Subsequently, the cells were collected,
fixing/denaturing solution (CST) was applied and the cells were
incubated for 30 min at room temperature. Detection antibody
solution was added (100 µl/well) and the cells were incubated for 1
h at room temperature. After removal of the medium, the cells were
washed 3 times with washing buffer. A HRP-conjugated secondary
antibody (100 µl/well) was added and the cells were incubated for 1
h at room temperature. After washing with washing buffer, 100 µl
TMB substrate was added to each well and incubated for 30 min at
25°C. One hundred microliters of STOP solution was added to each
well to terminate the reaction. The plates were oscillated for 10
min at room temperature. Cell proliferation was measured using a
microplate reader (Elx800; BioTek Instruments, Inc., Winooski, VT,
USA) at a wavelength of 450 nm.
Cell cycle assay
shCENPU and shCtrl cells were grown to ~80%
confluency, the supernatant was aspirated and after a single wash
with washing buffer, the cells were trypsinized. The cells were
washed with PBS and counted using a hemocytometer to ensure a
sufficient number of cells (>1×106/well, 3 wells per
experimental group). Cells were transferred to 5-ml centrifuge
tubes and centrifuged at 1,500 rpm for 5 min at 4°C. The
supernatant was discarded and the cell pellet was washed once with
ice cold PBS (pH 7.2–7.4). Cells were centrifuged for 5 min at
1,500 rpm at 4°C and fixed in ice cold 70% ethanol for 1 h.
Subsequently, the cells were centrifuged for 5 min at 1,500 rpm,
washed once with ice-cold PBS, and then centrifuged for 5 min at
1,500 rpm at 4°C. Based on the amount of cells, 1–1.5 ml
cell-staining solution [40X PI liquor (2 mg/ml, P4170; Sigma), 100X
RNase mother liquor (10 mg/ml, EN0531; Fermentas, USA) and 1X PBS
(Gibco, USA)] was used to resuspend the fixed cells. The cell
suspension was filtered through a 300-mesh nylon mesh prior to flow
cytometry on a FACSCalibur instrument (Becton-Dickinson, USA) at a
flow rate of 200–350 cells/sec.
Apoptosis assay
ShCENPU and shCtrl cells were trypsinized and
resuspended in standard medium after the logarithmic proliferation
phase was reached. After the cells were washed, Annexin V-APC
apoptosis detection kit and propidium iodide (PI, cat. no. 88-8007;
eBioscience, San Diego, CA, USA) were applied to determine
apoptotic cells according to the manufacturer's instructions.
Briefly, the cells were resuspended in 1X binding buffer at a
concentration of 1×106 cells/ml. Next, 100 µl of the
cell suspension was added to each of the following tubes: i) an
empty tube, ii) a tube containing Annexin V-FITC (5 µl); iii) a
tube containing PI (10 µl) and (iv) a tube containing both Annexin
V-FITC (5 µl) and PI (10 µl). After the tubes were gently mixed in
the dark for 15 min at room temperature, 1X binding buffer (400 µl)
was added to each tube and flow cytometry was conducted within 1
h.
Colony formation assay
ShCENPU and shcontrol cells were trypsinized and
resuspended in standard medium after the logarithmic proliferation
phase was reached. Cells were counted using a hemocytometer and
seeded at a density of 800 cells/well into a 6-well plate (3 wells
per experimental group). The cells were incubated at 37°C in 5%
CO2 and maintained for 14 days. Every three days, half
of the medium was replaced with fresh medium.
Images of cell colonies were captured under a
fluorescence microscope (MicroPublisher 3.3RTV; Olympus, Japan). In
brief, cells were washed with PBS and fixed with 4%
paraformaldehyde (1 ml/well; Shanghai Sangon, China) for 30–60 min
at room temperature. Next, the cells were washed with PBS and
stained with 500 µl Giemsa (Sigma-Aldrich, Shanghai, China) for 20
min at room temperature. Then, cells were washed with
ddH2O three times and left to dry. Under light
microscopy, a digital camera was used to capture images of each
slide and colony counts were obtained.
CENPU gene detection in human bladder
tissue
Gene expression of CENPU was determined in both 10
BCa tissue samples and cancer-adjacent normal tissues. The Ethics
Committee of the First Affiliated Hospital of Bengbu Medical
College approved this clinically oriented experiment. Prior to
sampling, written informed consent was obtained from all donors.
The protocol for performing qPCR was as described above.
Microarray analysis
The genome-wide effects of CENPU knockdown were
assessed by a GeneChip® PrimeView™ Human Gene Expression
array (Affymetrix, Santa Clara, CA, USA), which represents 20,000
human transcripts. Three biological replicates of T24 cells
transfected with the shCENPU lentivirus (for 72 h) and shCtrl
lentivirus were included in the array experiment. RNA was isolated
via the TRIzol method (Invitrogen) and RNA quality was determined
by NanoDrop using a NanoDrop 2000 spectrophotometer and Agilent
Bioanalyzer 2100. For gene expression profiling purposes,
individual microarrays were used per sample. Briefly, 500 ng of
total RNA was reverse transcribed and labeled with biotin using the
GeneChip® 3 IVT labeling kit (Affymetrix) according to
the manufacturer's instructions. The labeled amplified RNA was
hybridized overnight to GeneChip® PrimeView™ Human Gene
Expression array (Affymetrix) at 60°C. Arrays were performed with
GeneChip® Hybridization Wash and Stain kit (Affymetrix)
using GeneChip® Fluidics Station 450 (Affymetrix),
according to the manufacturer's instructions. Data were normalized
using the GeneSpring normalization algorithms according to the
manufacturer's instructions. The normalized data were used to
generate lists of genes that were differentially expressed by at
least ±1.5-fold. Moreover, a differential score p-value <0.05
among the test samples was considered a differentially expressed
gene.
Ingenuity Pathway Analysis
Datasets derived from microarray analysis and
representing differentially expressed genes were imported into the
Ingenuity Pathway Analysis (IPA) tool (Ingenuity®
Systems, Redwood City, CA, USA; http://www.ingenuity.com). Differentially expressed
genes were mapped in the Ingenuity database to the genetic networks
available and then ranked by score according to the manufacturer's
instructions.
The IPA tool allows for the identification of
biological networks from a particular dataset, as well as its
global functions and functional pathways. In this context, a
network is a graphical representation of the relationships between
molecules. Molecules are represented as nodes and the biological
relationship between two nodes is represented as an edge (line).
All edges are supported by at least 1 reference, a textbook, or
from canonical information stored in the Ingenuity Pathways
Knowledge Base. The intensity of the node color indicates the
degree of up (red) or down (green) regulation. Nodes are displayed
using various shapes that represent the functional class of the
gene product. The IPA tool presents a significant value of genes;
it shows interaction with and how gene products act on each other,
directly or indirectly. This includes genes that are not part of
the microarray analysis. The networks created are ranked by the
number of significantly expressed genes and diseases that are most
significant.
Western blotting
At the protein level, the expression of target genes
was determined by immunostaining using specific antibodies.
Forty-eight hours post lentiviral transfection, the cells were
lysed using ice-cold RIPA lysis buffer (cat. no. P0013C; Beyotime,
Beijing, China). Lysates were centrifuged at 12,000 × g for 10 min
at 4°C, supernatants were collected and the protein concentration
was determined using a BCA protein assay kit (cat no. P0010S;
Beyotime). Per treatment, 20 µg of protein was separated by 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
to a polyvinylidene difluoride (PVDF) membrane. Membranes were
blocked in Tris-based buffered saline with 0.5% Tween-20 (TBST),
containing 5% skimmed milk for 1 h at room temperature. Membranes
were then incubated overnight at 4°C with the following antibodies:
mouse monoclonal anti-Flag® M2 antibody (cat no. F1804,
1:1,000 dilution; Sigma), rabbit polyclonal anti-IL1B antibody (cat
no. ab9722, 0.2 µg/ml), mouse monoclonal anti-CXCL8 antibody (cat
no. ab18672, 0.1 µg/ml), rabbit polyclonal anti-RAC1 antibody (cat
no. ab97568, 1:1,000 dilution), rabbit monoclonal anti-TNFRSF11B
antibody (cat no. ab73400, 1 µg/ml), and rabbit monoclonal
anti-IL1A antibody (cat no. ab9614, 0.2 µg/ml) (all from Abcam) and
mouse monoclonal anti-GAPDH antibody (cat no. sc-32233, 1:2,000
dilution; Santa Cruz Biotechnology). After incubation with primary
antibodies, membranes were incubated with secondary antibodies:
HRP-conjugated goat anti-mouse (cat no. sc-2005, 1:5,000 dilution)
or HRP-conjugated goat anti-rabbit IgG (cat no. sc-2004, 1:5,000
dilution) (both from Santa Cruz Biotechnology) for 1 h at 37°C.
Signals were visualized using the ECL-Plus kit (cat no. M3121;
Thermo Fisher Scientific) according to the manufacturer's
instructions. GAPDH was used as the loading control.
Statistical analysis
Statistical analyses were performed using SPSS
software version 20.0 (IBM, Armonk, NY, USA). Data are represented
as mean ± SD. Categorical data between groups were compared by
Chi-square test. Comparisons of data between groups were performed
using Student's t-test. Kaplan-Meier analysis was used for survival
analysis. A p-value of <0.05 was considered significant.
Results
CENPU expression and its association
with clinicopathological characteristics of non-muscle invasive BCa
tissues
Using immunohistochemical analysis, CENPU expression
was evaluated in 100 NMIBC tissues (Fig. 1). Sixty-seven patients showed high
levels of CENPU expression, whereas 33 patients showed low
expression of CENPU.
Table II shows the
association between CENPU expression and clinicopathological
characteristics. The results indicated that no significant
correlation was observed between CENPU expression and sex
(P=0.356), age (P=0.424), or histological grade (P=0.211). Instead,
high CENPU expression was associated with larger tumor size
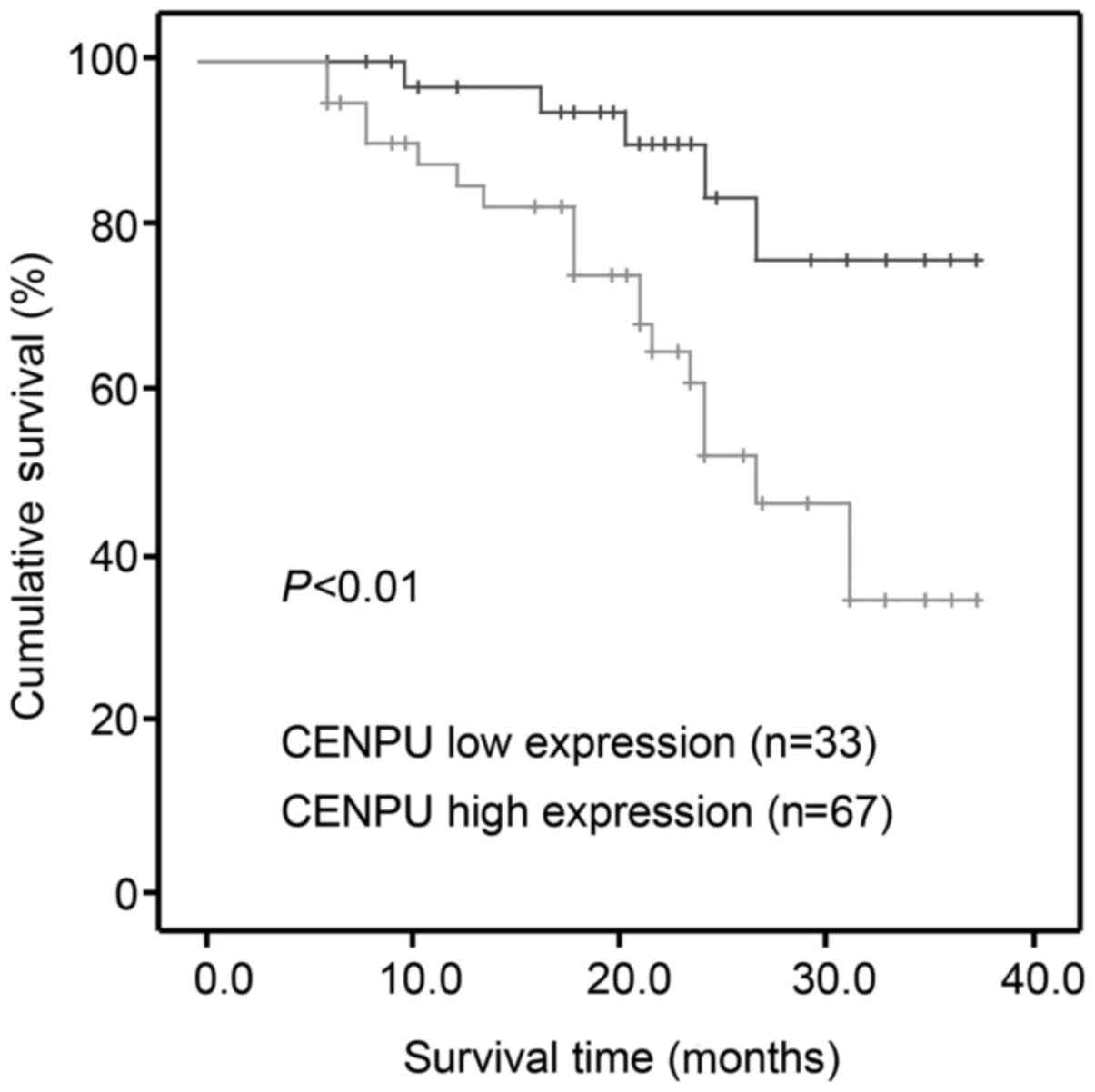
(P=0.032) and advanced TNM stage (P=0.029). Kaplan-Meier survival
analysis indicated that high CENPU expression was correlated with a
worse outcome compared to low CENPU expression (P<0.001;
Fig. 2).
 | Table II.Association between CENPU expression
and clinicopathological characteristics of the bladder cancer cases
(n=100). |
Table II.
Association between CENPU expression
and clinicopathological characteristics of the bladder cancer cases
(n=100).
|
|
| CENPU
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Total no. n
(%) | Low n (%) | High n (%) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 58 (58.0) | 17 (51.5) | 41 (61.2) | 0.356 |
|
Female | 42 (42.0) | 16 (48.5) | 26 (38.8) |
|
| Age (years) |
|
|
|
|
|
≤60 | 34 (34.0) | 13 (39.4) | 21 (31.3) | 0.424 |
|
>60 | 66 (66.0) | 20 (60.6) | 46 (68.7) |
|
| Tumor size
(cm) |
|
|
|
|
| ≤3 | 29 (29.0) | 5 (15.2) | 24 (35.8) | 0.032 |
|
>3 | 71 (71.0) | 28 (84.8) | 43 (64.2) |
|
| Histological
grade |
|
|
|
|
|
Urothelial | 61 (61.0) | 23 (69.7) | 38 (56.7) | 0.211 |
|
Other | 39 (39.0) | 10 (30.3) | 29 (43.3) |
|
| TNM stage |
|
|
|
|
|
0/I | 18 (18.0) | 2 (93.9) | 16 (23.9) | 0.029 |
|
II/III | 82 (82.0) | 31 (6.1) | 51 (76.1) |
|
CENPU gene expression in tissues and
cell lines
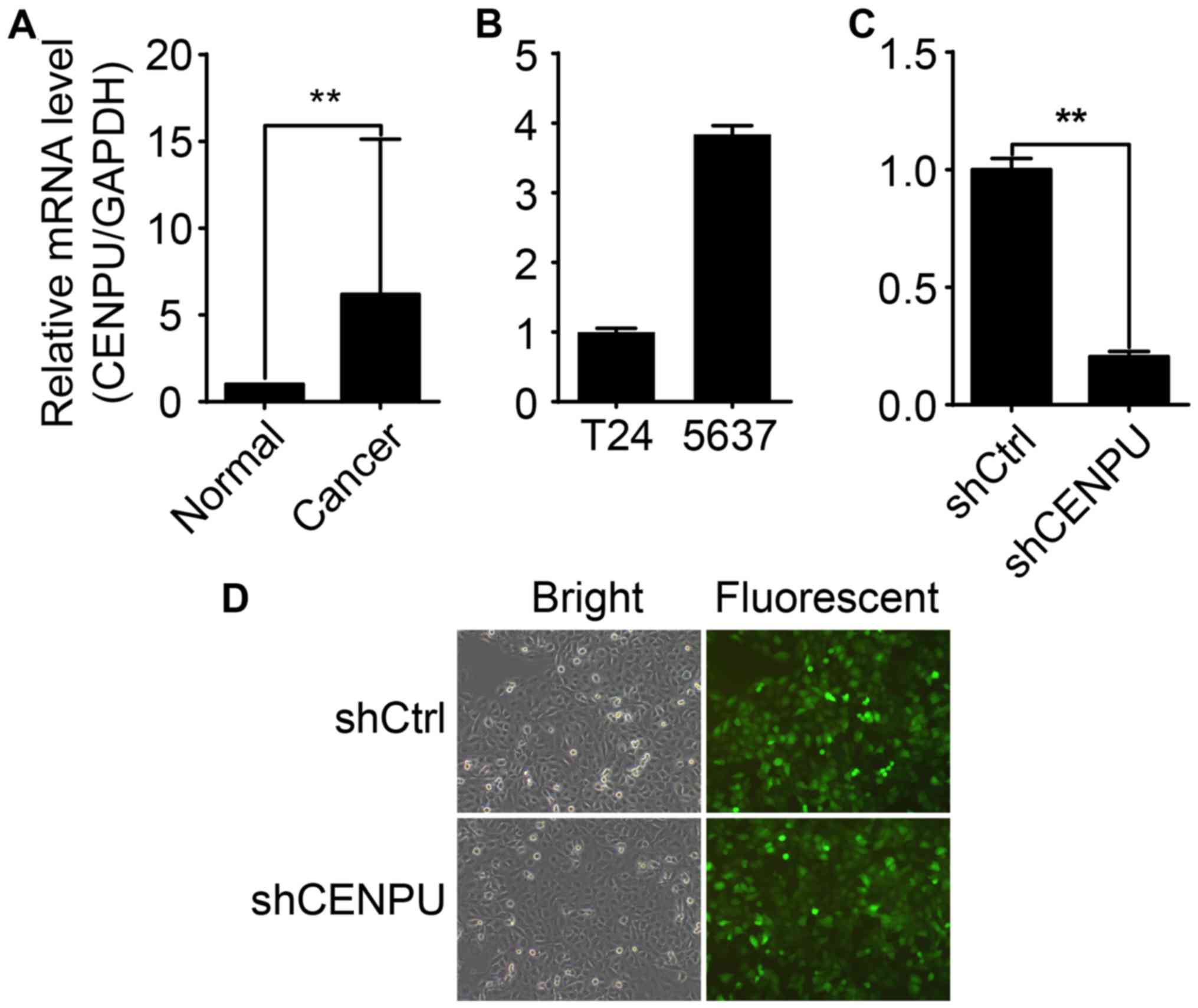
In this study, qPCR was performed to analyze the
CENPU gene expression profile in 10 BCa tissue samples and
cancer-adjacent normal tissue samples. Compared to CENPU mRNA
levels in normal tissues, the expression of CENPU in BCa tissues
was increased >6 fold (Fig. 3A).
CENPU mRNA expression varied between the T24 and 5637 cell lines
(Fig. 3B). T24 displayed the
highest endogenous CENPU expression and was therefore selected for
further investigation. Post-lentiviral transfection significantly
inhibited CENPU mRNA expression in the T24 shCENPU cells compared
to that noted in the control cells (Fig. 3D), indicating successful
transfection and CENPU gene expression knockdown (Fig. 3C).
CENPU knockdown inhibits T24 cell
proliferation
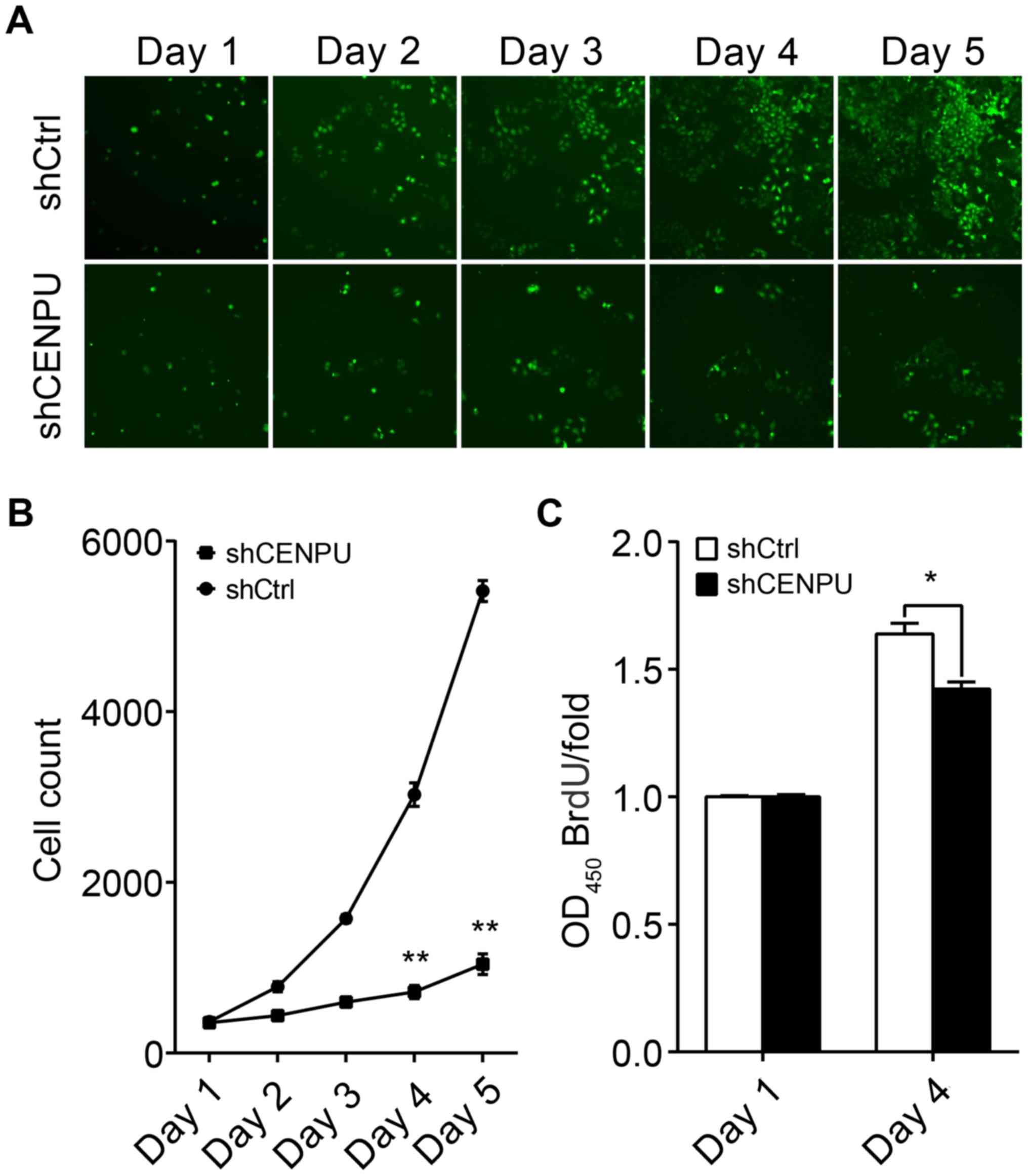
To evaluate the effect of CENPU expression on cell
proliferation, T24 cells transfected with either shCENPU or shCtrl
were plated into 96-well plates and analyzed for 5 days by
Cellomics. As illustrated in Fig.
4A and confirmed by quantification in Fig. 2B, the number of cells in the shCtrl
group significantly increased over the duration of 4 days; however
the number of cells in the shCENPU group did not change (Fig. 4B). In addition, BrdU assay
demonstrated that the BrdU ratio was significantly reduced in the
shCENPU-transfected cells as compared to that noted in the
shCtrl-transfected cells (Fig.
4C).
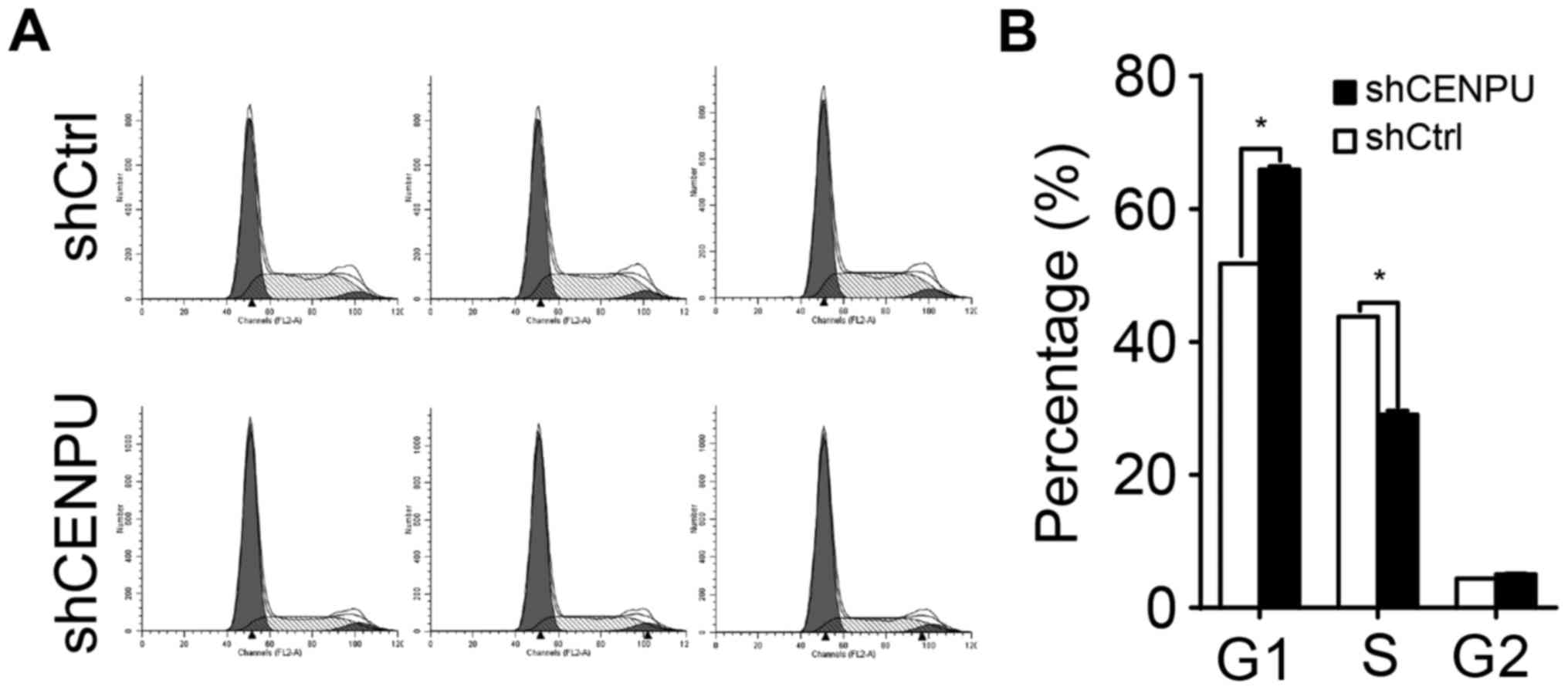
CENPU knockdown leads to cell cycle
arrest
To determine whether CENPU is required for cell
cycle progression, cell cycle distribution of the T24 cells was
evaluated by flow cytometry (Fig.
5A). As shown in Fig. 5B,
shCENPU-knockdown cells demonstrated a significant increase in the
percentage of cells in the G1 phase when compared to the percentage
in the control cells (P<0.05) and a decrease in the percentage
of cells in the S phase (P<0.05). Taken together, these data
suggest that CENPU is involved in cell proliferation regulation and
blocks cell cycle progression in the G1 phase.
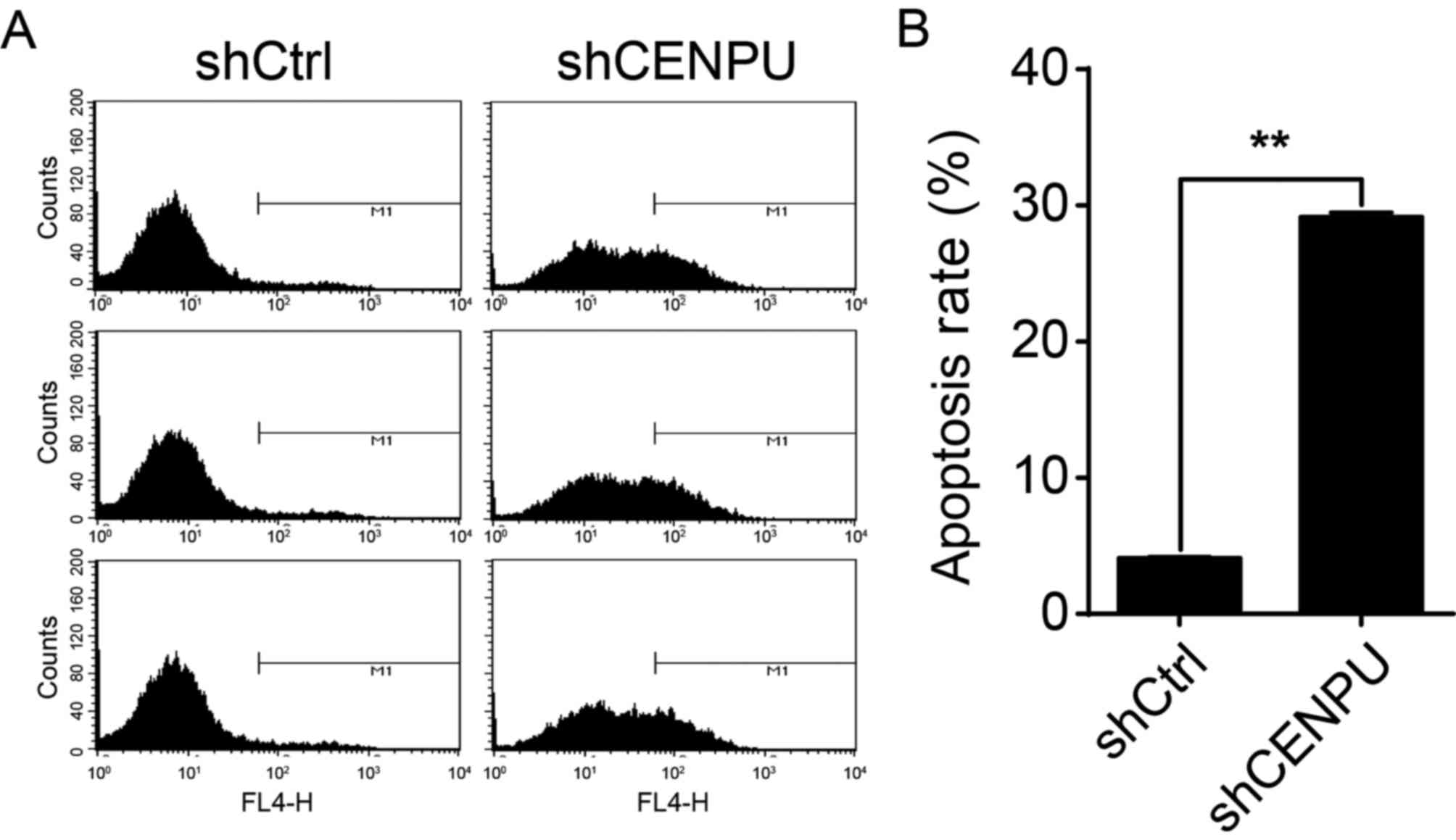
CENPU knockdown in T24 cells augments
cell apoptosis
To test whether or not CENPU expression affects T24
cell apoptosis, CENPU was knocked down in T24 cells and the
presence of apoptotic cells was determined by flow cytometry
(Fig. 6A). As shown in Fig. 6B, apoptosis was significantly
increased in shCENPU cells compared to that noted in the shCtrl
group (P<0.01). These results suggest that, in T24 cells, CENPU
expression is a regulator of apoptosis.
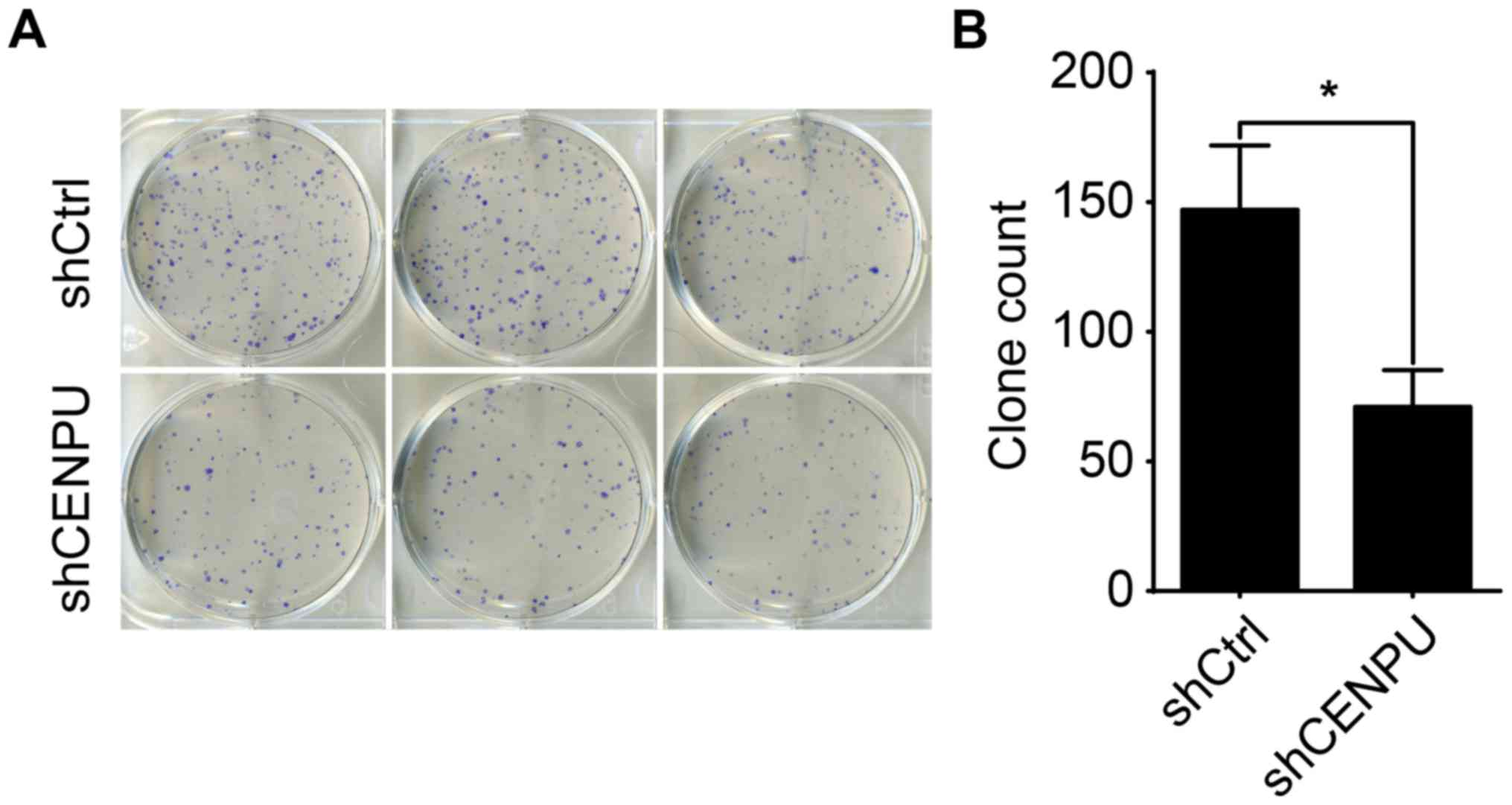
Knockdown of CENPU suppressed cell
colony formation
Next, we studied the colony-formation capacity of
T24 cells treated by the shCENPU lentivirus. Two groups of T24
cells (shCENPU and control cells) were grown for 14 days and
allowed to form colonies. As shown in Fig. 7, CENPU knockdown resulted in a
nearly 0.5-fold decrease in the number of colonies as compared with
the shCtrl group (P<0.05).
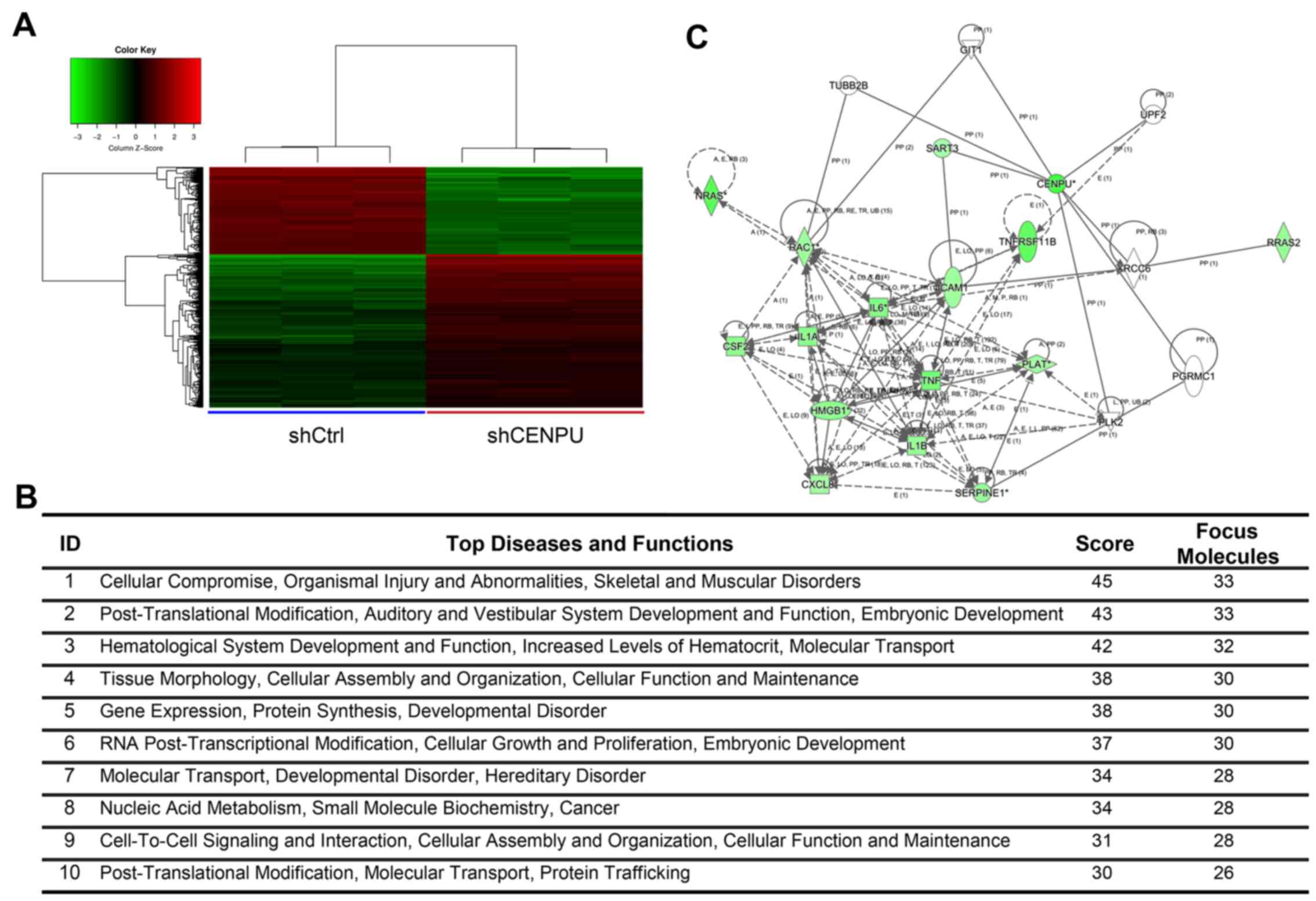
Genome-wide effects of CENPU knockdown
in T24 cells
The gene expression profiles of T24 cells knocked
down for CENPU or shCtrl cells were determined by
GeneChip® PrimeView™ Human Gene Expression array. Three
biological replicates were used and raw data were analyzed using
GeneSpring v11 software. Data were average normalized and filtered
by detection of a p-value <0.05 and differential p-value
<0.05. The gene expression array identified 1,274 differentially
expressed genes (809 genes were downregulated and 465 upregulated)
(Fig. 8A).
Elucidation of pathways and
interactions among differentially expressed genes
To determine possible biological interactions of the
differently regulated genes, datasets representing genes with an
altered expression profile derived from microarray analyses were
imported in the IPA tool.
IPA analysis identified 25 significant networks
(data not shown). Fig. 8B
represents the list of the top 10 networks identified by IPA. Of
these networks, ‘Cellular compromise, organismal injury and
abnormalities, skeletal and muscular disorders’ was the highest
rated network with 25 focus molecules and had a significance score
of 45 and implicated 33 genes (Fig.
8B). Additional IPA analysis showed that CENPU knockdown was
involved in the regulation of numerous genes that are involved in
the HMGB1 signaling pathway. These include CXCL8, IL1A, NRAS,
ICAM1, RAC1, IL6, HMGB1, RRAS2, IL1B, CSF2, SERPINE1, TNF,
TNFRSF11B, and PLAT. The Ingenuity Pathway of CENPU and the HMGB1
signaling pathway is shown in Fig.
8C. Results of qPCR analysis demonstrated that CENPU knockdown
significantly downregulated the expression of CCND3, CXCL8, FN1,
IL1A, IL1B, IL6, MAD2L1, RAC1, and TNFRSF11B. In addition, CENPU
knockdown upregulated PTGS2 expression (Fig. 9A). Western blot analysis further
confirmed that CENPU knockdown affected the downregulation of IL1B,
CXCL8, RAC1 and IL1A expression (Fig.
9B and C).
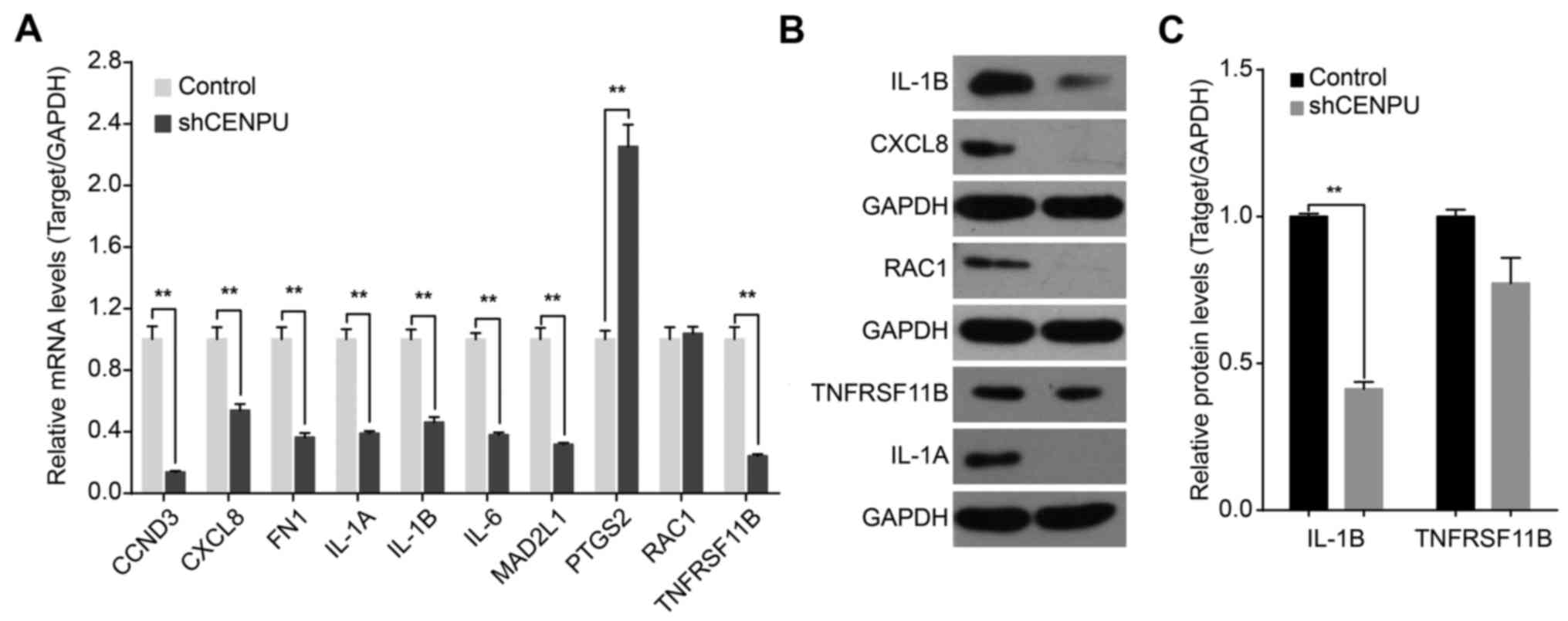 | Figure 9.CENPU knockdown affects the
expression levels of downstream genes. (A) Quantitative PCR
confirmation of mRNA expression of CCND3, CXCL8, FN1, IL-1A, IL-1B,
IL-6, MAD2L1, PTGS2, RAC1 and TNFRSF11B in T24 cells transfected
with either a control or shCENPU lentivirus. Data represent mean ±
SD of relative mRNA quantity normalized to GAPDH mRNA expression.
(B) Western blot analysis of protein expression of IL-1B, CXCL8,
RAC1, TNFRSF11B and IL-1A. (C) Densitometric analysis of target
proteins in T24 cells transfected with control or shCENPU
lentivirus. **P<0.01. CENPU, centromere protein U. |
Discussion
To the best of our knowledge, our study is the first
to demonstrate the expression, clinical value, and the potential
functional mechanism of action of CENPU in BCa. As expected, our
qPCR data demonstrated that CENPU was abundantly expressed in
cancer-related tissues, whereas in cancer-adjacent normal tissues
CENPU expression was weak. Immunohistochemical studies showed that,
in BCa, high CENPU expression was significantly correlated with
unfavorable clinicopathological characteristics such as tumor size
and TNM stage. After CENPU expression in normal and BCa tissue was
confirmed, we used BCa cell lines for further experiments to better
establish the mechanistic role of CENPU in human bladder
carcinogenesis.
Our in vitro studies indicated a variable
CENPU expression in two BCa cell lines that represent different
molecular features. The 5637 cell line is an in vitro model
for high-risk NMIBC (22,23), whereas the T24 cell line is an
invasive BC cell line (24). The
finding that the T24 cell line which has a higher invasive
potential displayed higher CENPU expression as determined by qPCR
is consistent with the immunohistochemical analysis. These results
confirmed CENPU expression according to the invasive potential of
the T24 cells. We also found that T24 cell proliferation and cell
colony formation were significantly reduced in the
shCENPU-transfected cells. Apoptosis was significantly increased in
the CENPU-silenced BCa cells. CENPU-silenced T24 cells showed
significant cell cycle arrest at the G1 phase. To date, only one
study has demonstrated that CENPU knockdown in prostate cancer cell
line PC-3 inhibited cell proliferation, colony formation, increased
apoptosis. However, in the study, the cell cycle did not appear to
be affected (18).
The CENPU gene (also known as MLF1IP, Cenp-50/PBIP1
and KLIP1) encodes a 46-kDa nuclear-localizing transcription
suppressor protein that has been associated with malignancy in
previous research (25). In
addition to its transcription suppressor activity, CENPU is
required for stable kinetochore-microtubule attachment, proper
chromosome segregation and recovery from spindle damage during
mitosis (9,10,14).
CENPU was initially identified in 2004 by Hanissian et al,
who implied a possible role for CENPU deregulation in the
pathogenesis of erythroleukemias (11). Subsequently, the same group found
that CENPU upregulation was associated with enhanced neuropoiesis
and glioblastoma tumor development in both humans and rodents
(12). More recently, CENPU
upregulation has been identified in human breast cancer (17) and familial colorectal cancer (Lynch
syndrome) patients (26). However,
no obvious alterations of CENPU expression were observed in human
prostate cancer tissue (18).
Little is known concerning the role of CENPU in
human BCa. Therefore, we evaluated the gene profiling in
shCENPU-transfected T24 cells to identify the mechanism of action
of CENPU knockdown. A total of 1,274 differentially expressed genes
were identified, including 809 downregulated genes and 465
upregulated genes. In this study, IPA was used to visualize the
co-deregulated genes affected by CENPU knockdown. Network analysis
identified 25 distinct signaling pathways, including the top-ranked
network ‘Cellular Compromise, organismal injury and abnormalities,
skeletal and muscular disorders’ (Fig.
8B). In-depth IPA analysis revealed that CENPU plays a role in
HMGB1 signaling (Fig. 8C).
Overexpression of HMGB1 is associated with progression and poor
prognosis in human BCa (27,28),
however downregulation of HMGB1 is known to inhibit the bioactivity
of BCa cell lines (29). The qPCR
results obtained in this study demonstrated that CENPU knockdown
downregulated the expression of members involved in HMGB1
signaling, including CCND3, CXCL8, FN1, IL1A, IL1B, IL6, MAD2L1,
RAC1, and TNFRSF11B, and upregulated PTGS2 expression. Western blot
analysis of key players in the HMGB1 pathway, including IL1B,
CXCL8, RAC1, and IL1A, demonstrated that all of these molecules
were significantly downregulated. Taken together, knockdown of
CENPU in T24 cells deregulated cell proliferation, colony
formation, cell cycle arrest and apoptosis via the HMGB1 signaling
pathway and therefore, high expression of CENPU causes an increased
risk for BCa tumorigenesis.
In conclusion, in T24 cells, CENPU knockdown
significantly inhibited cell proliferation, colony formation and
cell cycle arrest at the G1 stage and significantly promoted
apoptosis. The mechanism underlying the effect of CENPU knockdown
in BCa cells may involve the HMGB1 signaling pathway.
Acknowledgements
This study was supported by Key Projects of Science
Research for Colleges and Universities in Anhui Province
(KJ2015A280).
References
|
1
|
Gandaglia G, Popa I, Abdollah F,
Schiffmann J, Shariat SF, Briganti A, Montorsi F, Trinh QD,
Karakiewicz PI and Sun M: The effect of neoadjuvant chemotherapy on
perioperative outcomes in patients who have bladder cancer treated
with radical cystectomy: A population-based study. Eur Urol.
66:561–568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu HJ, Chang YH and Pan CC: Prognostic
significance of heat shock proteins in urothelial carcinoma of the
urinary bladder. Histopathology. 62:788–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun L, Lu J, Niu Z, Ding K, Bi D, Liu S,
Li J, Wu F, Zhang H, Zhao Z, et al: A potent chemotherapeutic
strategy with Eg5 inhibitor against gemcitabine resistant bladder
cancer. PLoS One. 10:e01444842015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang HW, Yoon HY, Ha YS, Kim WT, Kim YJ,
Yun SJ, Lee SC and Kim WJ: FAM70B as a novel prognostic marker for
cancer progression and cancer-specific death in muscle-invasive
bladder cancer. Korean J Urol. 53:598–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allory Y, Beukers W, Sagrera A, Flández M,
Marqués M, Márquez M, van der Keur KA, Dyrskjot L, Lurkin I,
Vermeij M, et al: Telomerase reverse transcriptase promoter
mutations in bladder cancer: High frequency across stages,
detection in urine, and lack of association with outcome. Eur Urol.
65:360–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenberg JE: Current status of
neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder
cancer. Expert Rev Anticancer Ther. 7:1729–1736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodge LS, Taub ME and Tracy TS: Effect of
its deaminated metabolite, 2,2-difluorodeoxyuridine, on the
transport and toxicity of gemcitabine in HeLa cells. Biochem
Pharmacol. 81:950–956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leijen S, Veltkamp SA, Huitema AD, van
Werkhoven E, Beijnen JH and Schellens JH: Phase I dose-escalation
study and population pharmacokinetic analysis of fixed dose rate
gemcitabine plus carboplatin as second-line therapy in patients
with ovarian cancer. Gynecol Oncol. 130:511–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hua S, Wang Z, Jiang K, Huang Y, Ward T,
Zhao L, Dou Z and Yao X: CENP-U cooperates with Hec1 to orchestrate
kinetochore-microtubule attachment. J Biol Chem. 286:1627–1638.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minoshima Y, Hori T, Okada M, Kimura H,
Haraguchi T, Hiraoka Y, Bao YC, Kawashima T, Kitamura T and
Fukagawa T: The constitutive centromere component CENP-50 is
required for recovery from spindle damage. Mol Cell Biol.
25:10315–10328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanissian SH, Akbar U, Teng B, Janjetovic
Z, Hoffmann A, Hitzler JK, Iscove N, Hamre K, Du X, Tong Y, et al:
cDNA cloning and characterization of a novel gene encoding the
MLF1-interacting protein MLF1IP. Oncogene. 23:3700–3707. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanissian SH, Teng B, Akbar U, Janjetovic
Z, Zhou Q, Duntsch C and Robertson JH: Regulation of myeloid
leukemia factor-1 interacting protein (MLF1IP) expression in
glioblastoma. Brain Res. 1047:56–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai W and Wang X: Grabbing Plk1 by the
PBD. Mol Cell. 24:489–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KS, Oh DY, Kang YH and Park JE:
Self-regulated mechanism of Plk1 localization to kinetochores:
Lessons from the Plk1-PBIP1 interaction. Cell Div. 3:42008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hori T, Okada M, Maenaka K and Fukagawa T:
CENP-O class proteins form a stable complex and are required for
proper kinetochore function. Mol Biol Cell. 19:843–854. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foltz DR, Jansen LE, Black BE, Bailey AO,
Yates JR III and Cleveland DW: The human CENP-A centromeric
nucleosome-associated complex. Nat Cell Biol. 8:458–469. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang DP and Luo RC: MLF1IP is correlated
with progression and prognosis in luminal breast cancer. Biochem
Biophys Res Commun. 477:923–926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Ji G, Shao Y, Qiao S, Jing Y, Qin
R, Sun H and Shao C: MLF1 interacting protein: A potential gene
therapy target for human prostate cancer? Med Oncol.
32:4542015.PubMed/NCBI
|
|
19
|
Radhika K and Prayaga AK: Estrogen and
progesterone hormone receptor status in breast carcinoma:
Comparison of immunocytochemistry and immunohistochemistry. Indian
J Cancer. 47:148–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vasconcelos-Nóbrega C, Pinto-Leite R,
Arantes-Rodrigues R, Ferreira R, Brochado P, Cardoso ML, Palmeira
C, Salvador A, Guedes-Teixeira CI, Colaço A, et al: In vivo and in
vitro effects of RAD001 on bladder cancer. Urol Oncol.
31:1212–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gazzaniga P, Silvestri I, Gradilone A,
Scarpa S, Morrone S, Gandini O, Gianni W, Frati L and Aglianò AM:
Gemcitabine-induced apoptosis in 5637 cell line: An in-vitro model
for high-risk superficial bladder cancer. Anticancer Drugs.
18:179–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YG, Macoska JA, Korenchuk S and Pienta
KJ: MIM, a potential metastasis suppressor gene in bladder cancer.
Neoplasia. 4:291–294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki H, Arakawa Y, Ito M, Saito S,
Takeda N, Yamada H and Horiguchi-Yamada J: MLF1-interacting protein
is mainly localized in nucleolus through N-terminal bipartite
nuclear localization signal. Anticancer Res. 27:1423–1430.
2007.PubMed/NCBI
|
|
26
|
Dominguez-Valentin M, Therkildsen C,
Veerla S, Jönsson M, Bernstein I, Borg A and Nilbert M: Distinct
gene expression signatures in lynch syndrome and familial
colorectal cancer type x. PLoS One. 8:e717552013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Jiang H, Zhu H, Zhang H, Gong J,
Zhang L and Ding Q: Overexpression of high mobility group box 1 and
2 is associated with the progression and angiogenesis of human
bladder carcinoma. Oncol Lett. 5:884–888. 2013.PubMed/NCBI
|
|
28
|
Yang GL, Zhang LH, Bo JJ, Huo XJ, Chen HG,
Cao M, Liu DM and Huang YR: Increased expression of HMGB1 is
associated with poor prognosis in human bladder cancer. J Surg
Oncol. 106:57–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Z, Zhong Z, Zhang L, Wang X, Xu R,
Zhu L, Wang Z, Hu S and Zhao X: Down-regulation of HMGB1 expression
by shRNA constructs inhibits the bioactivity of urothelial
carcinoma cell lines via the NF-κB pathway. Sci Rep. 5:128072015.
View Article : Google Scholar : PubMed/NCBI
|