Introduction
Thyroid cancer is the most common endocrine
malignancy, which affected ~63,000 persons in the USA, in 2014.
Studies between 1975 and 2009 revealed an annual increase in
incidence rate from 4.9 to 14.3 per 100,000 (1,2).
Thyroid cancer can be categorized according to histopathological
findings. Papillary thyroid carcinoma (PTC) is the most prevalent
subtype that accounts for ~70–80% of all thyroid cancer cases. One
report predicted that the incidence of PTC will double in the USA
by 2019 (3,4). The current protocol for treating PTC
involve the combination of thyroidectomy and radioiodine therapy.
However, these are often not curative for cases that exhibit
aggressive clinical characteristics. Hence, a better understanding
in molecular pathogenesis and mechanism in PTC would enable us to
develop more effective treatment targets and strategies.
WNT and its signaling pathways play a crucial role
in human organogenesis (5). In
human, WNT genes encode secretory signaling glycoproteins that are
cysteine rich and are 350–400 amino acids in length (6). Its corresponding signaling pathways
are classified according to the involvement of β-catenin: the
canonical (WNT-β-catenin pathway) (7) and the non-canonical pathways
(WNT/planar cell polarity (PCP) or WNT/Ca2+ pathway)
(8).
In the canonical pathway, WNT ligands bind to
Frizzled receptors that induce phosphoprotein Dishevelled for
activation. This activation would inhibit β-catenin phosphorylation
by glycogen synthase kinase-3β-adenomatous polyposis coli-axin
complex. Consequently, β-catenin accumulates in the cytoplasm and
eventually translocate into the nucleus, where it acts as a
transcriptional coactivator alongside the T-cell factor and
lymphoid enhancing factor (TCF/LEF). They form complexes that
initiate the expression of downstream genes such as cyclin D1 and
c-myc. Such activation seems to play an important role in cell
proliferation, differentiation, cell-cell adhesion and cell
migration (6,9,10).
Hence, any disruption in this pathway would contribute to
tumorigenesis.
WNT10A is one of the nineteen WNT signaling
glycoproteins and it is the focus of this study. The WNT10A genes
are encoded on human chromosome 17q21 (11). Previous studies indicated any
aberration of WNT10A in human can induce defects in tooth
morphogenesis, dentinogenesis, odontoblast differentiation, hair
follicle development, papillae of the tongue and sweat gland, nail
formation, and regeneration of the epidermis (12–14).
In addition, WNT10A mutation was demonstrated to play key roles in
carcinoma of esophageal, gastric, kidney and colorectal as well as
endometrioid carcinoma.
Further studies into cellular level revealed that
mutation promotes tumor cell proliferation and migration, which may
be linked to self-renewal of a subset of ESCC cells in esophageal
squamous cell carcinoma by regulating β-catenin (15–18).
In concurrence, the knockdown of WNT10A suppressed cell
proliferation. It also induces S-phase cell cycle arrest in mouse
embryonic palatal mesenchymal (MEPM) cells through WNT/β-catenin
signaling pathway (19). However,
the association of WNT ligands and its pathway in the pathogenesis
of PTC and progression have not yet been determined. In this study,
the WNT10A/β-catenin pathway was demonstrated to promote and play a
role in the PTC development.
Materials and methods
Specimens
A total of 35 cases of primary papillary thyroid
cancer and 35 cases of adjacent normal tissues were collected as
fresh frozen tissues from Shandong Provincial Hospital. All samples
were collected under the approved guidelines of Shandong Provincial
Hospital's institutional review board. The protocols were reviewed
and approved by the ethic committee of Shandong University (Jinan,
Shandong, China).
Microarray analysis
The microarray chip consisted of 27,326 different
human cDNAs (Angilent, Wilmington, DE, USA), in which house-keeping
gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the
internal control. The cDNAs from 5 cases of papillary thyroid
cancer were labeled with Cy5, and the cDNAs from 5 cases of
adjacent normal tissues were labeled with Cy3. The labeled cDNAs
were hybridized with microarray chip under standard conditions
according to the manufacturer's instructions. The data were
analyzed by Molecule annotation system 3.0.
Cell culture and transfection
Thyroid cancer cell lines GLAG-66 was used in this
study. All thyroid cancer cell lines were maintained in our
laboratory. GLAG-66 was maintained in Nutrient mixture F-12
(Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS) and penicillin/streptomycin (2%) at 37°C with 5%
CO2, as described previously (20). Cells were passaged 1:3 until 80%
confluence was reached in a 75-cm2 culture flask (Nest
Biotechnology, Shanghai, China). The ORF human WNT10A cDNA
expression plasmid and LEF1 cDNA expression plasmid were purchased
from Biosune Co. (Shanghai, China). FuGENE HD transfection reagent
(Roche Applied Science, Basel, Switzerland) was used for
transfection. All transfections were performed according to the
manufacturer's instructions.
SiRNA interference
Chemical modified Stealth siRNA (chemical modified
stealth siRNA) targeting WNT10AsiRNA and LEF1siRNA were from
RiboBio Co., Ltd. (Guangzhou, Guangdong, China). The sequence for
WNT10A siRNA was 5′-CCACGAATGCCAACACCAA-3′. The sequence for LEF1
siRNA was 5′-GCTACATATGCAGCTTTAT-3′. Cells were transfected with
siRNA by the Lipofectamine 3000 method (Life Technologies, CA,
USA).
RNA isolation and quantitative
real-time PCR
Total cellular or tissue RNA was extracted with
TRIzol (Life Technologies) according to the manufacturer's
instructions. First-strand cDNA was synthesized from 1 µg total
cellular or tissue RNA using PrimeScript™ RT Master Mix (Takara)
with random primers. Then cDNA was amplified for quantitative
real-time PCR, and the specific primers used were as follows: for
WNT10A, forward, 5′-TCCCATCTTCAGCAGAGGTTTC-3′ and reverse,
5′-CACTGCCTGCCTCCCAACT-3′; for β-actin, forward,
5′-AGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CACCTTCACCGTTCCAGTTTT-3′; for LEF1, forward,
5′-AGAGGAAGGCGATTTAGC-3′ and reverse, 5′-ACCACGGGCACTTTATTT-3′; for
CTNNB1, forward, 5′-GCAGCAACAGTCTTA CCT-3′ and reverse,
5′-ACAGGACTTGGGAGGTAT-3′; for CCND1 forward,
5′-GCGAGGAACAGAAGTGCG-3′ and reverse, 5′-TGGAGTTGTCGGTGTAGATGC-3′;
for MYC forward, 5′-TCCTGTCCGTCCAAGCAG-3′ and reverse,
5′-ACGCACAAGAGTTCCGTAG −3′. The real-time PCR reactions were
performed under the following conditions: denaturation at 95°C for
10 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30
sec, a total of 35 cycles. The real-time PCR reactions were
performed in the Roche 480 Real-Time PCR system with SYBR Premix Ex
Taq™ according to the manufacturer's instructions.
Wound healing assay
Confluent GLAG-66 cell monolayers on 6-well tissue
culture plastic dishes were transfected with pEnter-WNT10A,
pEnter-LEF1 and their control plasmid pEnter-MOCK, as well as
WNT10A and LEF1 siRNA. GLAG-66 cells were grown to confluent
monolayers on 6-well plates and a thin disposable tip was used to
create linear scratch wounds. Cultures were rinsed with PBS and
replaced with fresh quiescent medium containing 10% fetal bovine
serum. Wound images were taken with a digital camera mounted on a
light microscope at 0, 24, 36 and 72 h. The wound gap widths were
measured using ImageJ software.
Protein extraction and western blot
analysis
Total cellular or tissue protein extracts were
obtained using PMSF with RIPA (the ratio of PMSF to RIPA is 1:100).
Cells or tissues were washed twice with cold PBS and lysed in a
buffer containing 100 µl RIPA and 1 µl PMSF. Protein concentration
was determined by the BCA method using the BCA protein assay
(Thermo Scientific/Pierce, Courtaboeuf, France). Cellular or tissue
protein extracts were separated on 10% SDS-PAGE gel. Proteins were
transferred to polyvinylidene difluoride microporous membranes
(Millipore, MA, USA), and then blocked with 5% non-fat dry milk for
1 h at room temperature. The membranes were incubated overnight at
4°C with the primary antibody specific to WNT10A (rabbit
polyclonal, Abcam, 1:500, Abcam), β-catenin (rabbit monoclonal,
1:5,000, Abcam), LEF1 (rabbit monoclonal, 1:1,000, Abcam), MYC
(rabbit monoclonal, 1:10,000, Abcam), cyclin D1 (rabbit monoclonal,
1:10,000, Abcam) or GAPDH (rabbit polyclonal, 1:500 Boster Co.,
Ltd., Wuhan, China) as the internal control. The membranes were
washed and incubated with anti-rabbit antibody (rabbit polyclonal,
1:2,000 Boster) for 1 h at room temperature, which were
subsequently washed and shown using chemiluminescence reagent.
Cell proliferation assay
A total of 2×103 cells were cultured in
96-well plates. Cell proliferation was quantified using the Cell
Counting Kit-8 assay (Roche, Penzberg, Germany) according to the
manufacturer's instructions. Absorbance was measured on a
microplate reader (Tecan Sunrise, Mannedorf, Switzerland) at a
wavelength of 450 nm. Data represent the average values of three
independent experiments.
Apoptosis analysis
5×105 GLAG-66 cells in 6-well plates were
transfected with pEnter-WNT10A, pEnter-LEF1, control plasmid
pEnter-mock, WNT10AsiRNA and LEF1siRNA, and incubating for varied
time-points before the cells were digested and harvested by
centrifugation. Then the cells were separately fixed gently (drop
by drop) in 70% ethanol overnight at −20°C and then re-suspended in
535 µl DDW containing 500 µl PI stain buffer, 10 µl RNaseA solution
(50X) and 25 µl PI. After 30 min at 37°C in the dark, the cells
were analyzed with flow cytometry equipped with an argon laser at
488 nm. Then cell cycle was determined and analyzed. Analysis of
apoptosis was quantified by Annexin V-FITC apoptosis detection kit
according to the manufacturer's instructions (BD Biosciences, USA)
(21,22). Apoptotic events were acquired and
analyzed by BD-FACS calibur instrument (BD Biosciences).
Results
WNT10A/β-catenin signaling pathway is
activated in papillary thyroid cancer
To investigate the molecular mechanisms involved in
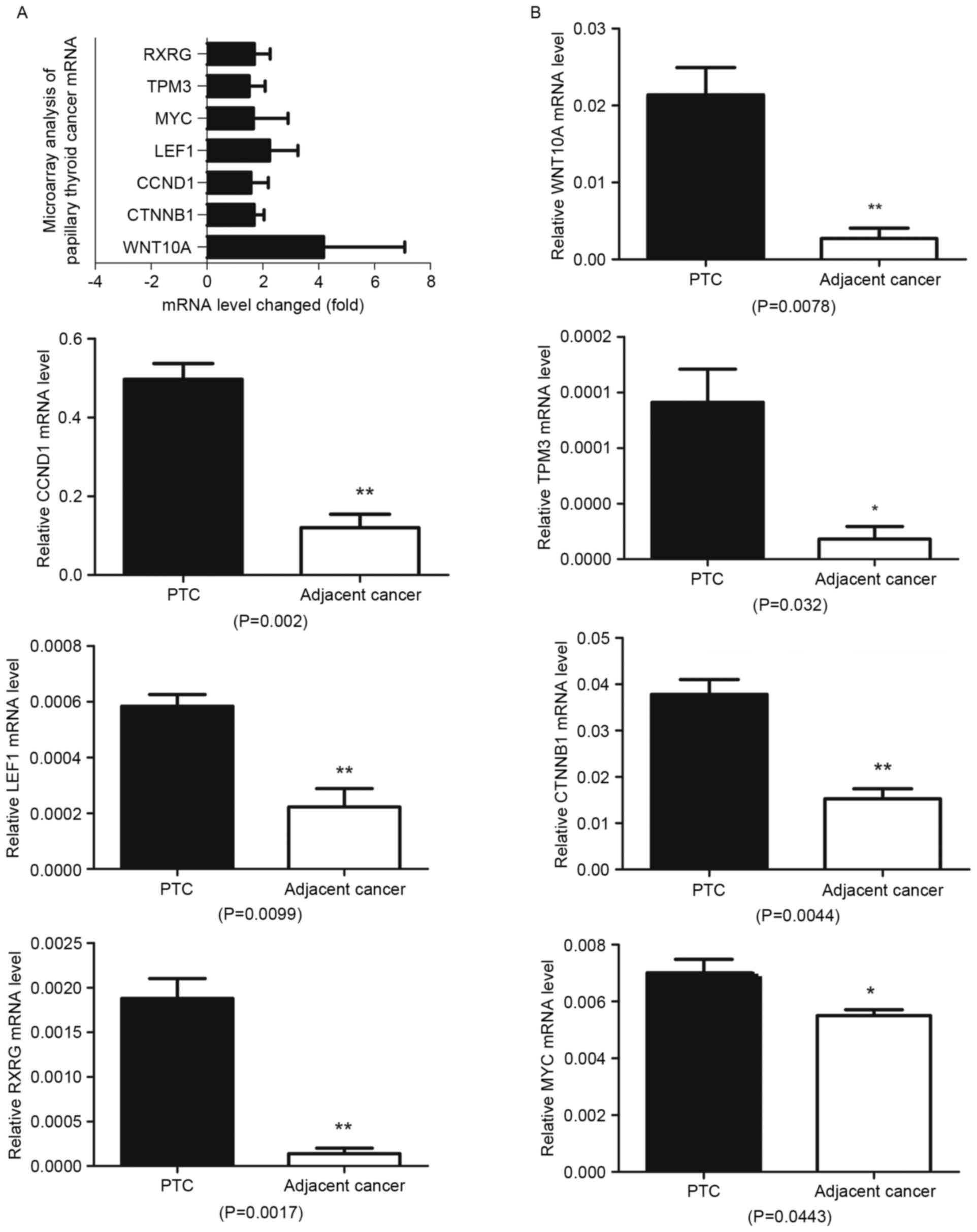
tumorigenesis of papillary thyroid cancer, microarray analysis was
performed to compare the variation of gene expressions within the
PCT cell populations and their adjacent normal tissues. Up- or
downregulation 1.5-fold was set as a cutoff value, and changes in
gene expression were analyzed using the Database for Annotation,
Visualization and Integrated Discovery (DAVID). In the DAVID
analysis, the Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway related to papillary thyroid cancer was hsa05216: thyroid
cancer, six genes (CCND1, RXRG, LEF1, MYC, CTNNB1 and TPM3) were
upregulated >1.5-fold in this pathway (Fig. 1A). All these genes were associated
with WNT/β-catenin signaling pathway, among them LEF1 and CTNNB1
play critical roles in WNT/β-catenin signaling pathway. Of the
signaling proteins, WNT10A was the only WNT ligand that was
upregulated >1.5-fold and its upregulation was found to be
>4-fold in the papillary thyroid cancer comparing to adjacent
normal tissues.
Molecules involved in WNT10A/β-catenin
signaling pathway are upregulated in papillary thyroid cancer
tissues
Next, the expression of WNT10A, LEF1, MYC, β-catenin
and cyclin D1 were verified by QRT-PCR in 15 cases of papillary
thyroid cancer (Fig. 1B). The
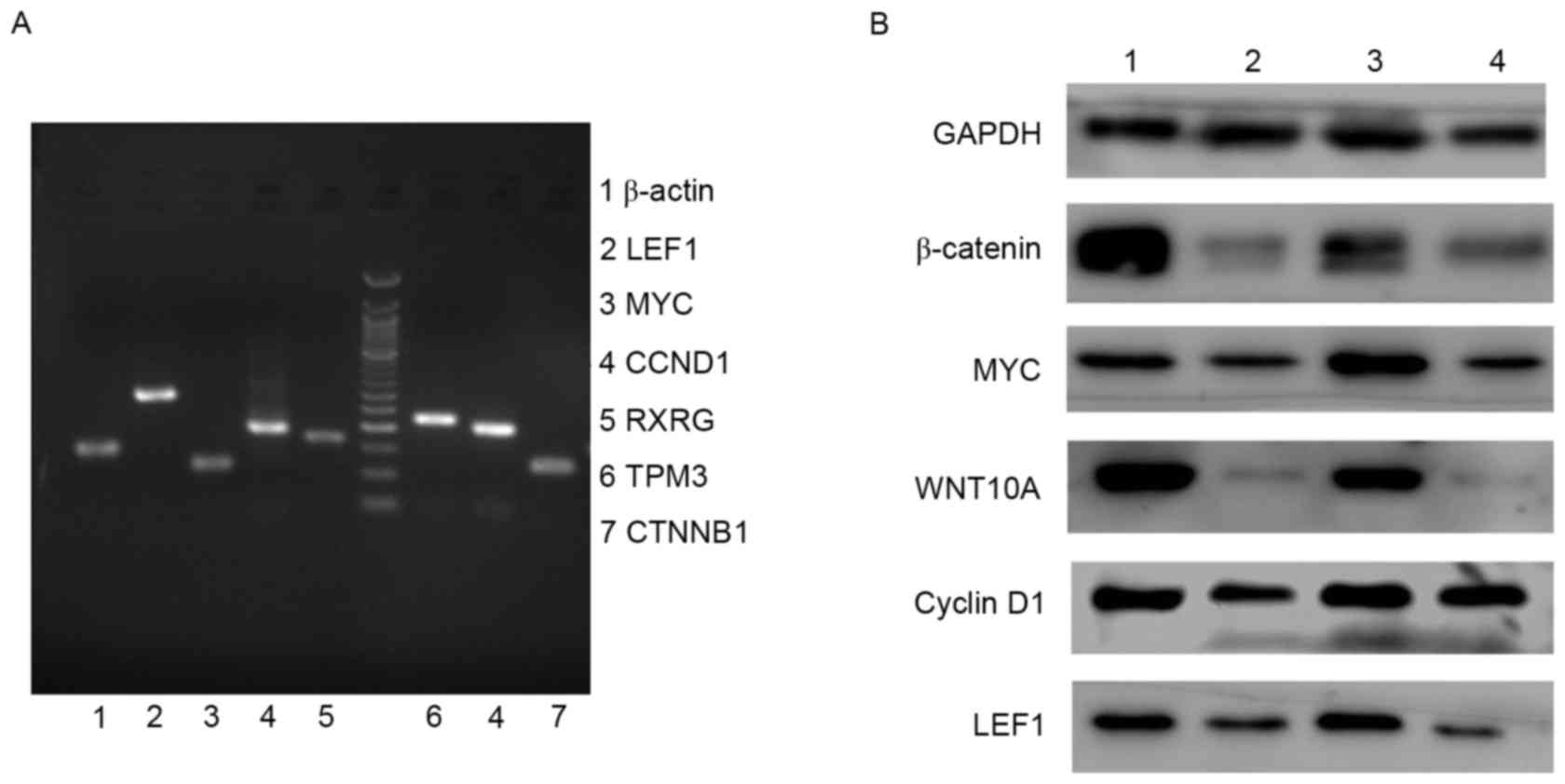
real-time PCR (QRT-PCR) products were further separated by agarose
gel electrophoresis and visualized to compare the initial levels of
WNT10A, LEF1, MYC, β-catenin and cyclin D1 mRNA in the tissues from
papillary thyroid cancer and adjacent normal tissues (Fig. 2A). To confirm the results at mRNA
level, WNT10A, LEF1, MYC, β-catenin and cyclin D1 expression was
further detected by western blot analysis in 10 cases of papillary
thyroid cancer and adjacent normal tissues, the results
demonstrated upregulated expression in papillary thyroid cancer
tissues comparing to adjacent normal ones (Fig. 2B). The results demonstrated that
WNT10A/β-catenin signaling pathway was activated in papillary
thyroid cancer tissues.
WNT10A/β-catenin signaling pathway
activation promotes proliferation of thyroid cells
To investigate the role of WNT10A/β-catenin
signaling pathway in mediating migration and invasion of thyroid
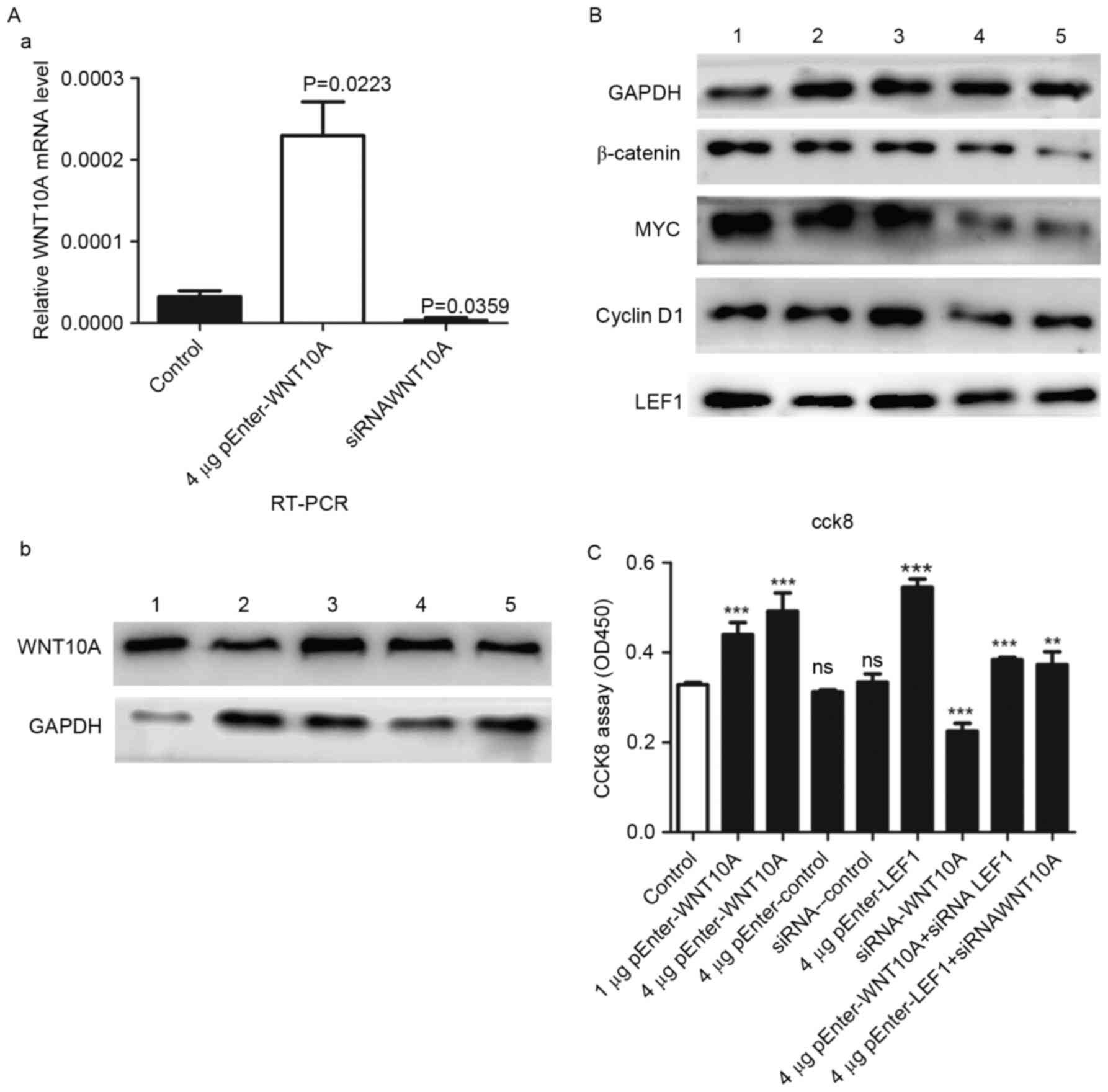
cancer cells, cultured GLAG-66 cells were transfected with
pEnter-WNT10A, pEnter-LEF1 and control plasmid pEnter-mock. WNT10A
siRNA and LEF1siRNA were employed to knockdown the expression of
the two genes, and the proliferations of the treated cells were
quantified with the Cell Counting Kit-8 assay. The expression of
WNT10A in the cell lines was confirmed by RT-PCR and western blot
analysis (Fig. 3A), the levels of
β-catenin, LEF1, MYC and cyclin D1 protein were induced (Fig. 3B), suggesting that WNT10A
effectively activates β-catenin signaling pathway in thyroid cancer
cells. As a response to the WNT/β-catenin signaling pathway
activation, the CCK-8 assay results showed that the WNT10A and LEF1
overexpressing cells exhibited significant higher level of
proliferation as compared with the empty vector controls and WNT10A
siRNA (Fig. 3C).
WNT10A/β-catenin signaling pathway
activation suppressed late apoptosis of thyroid cancer cells
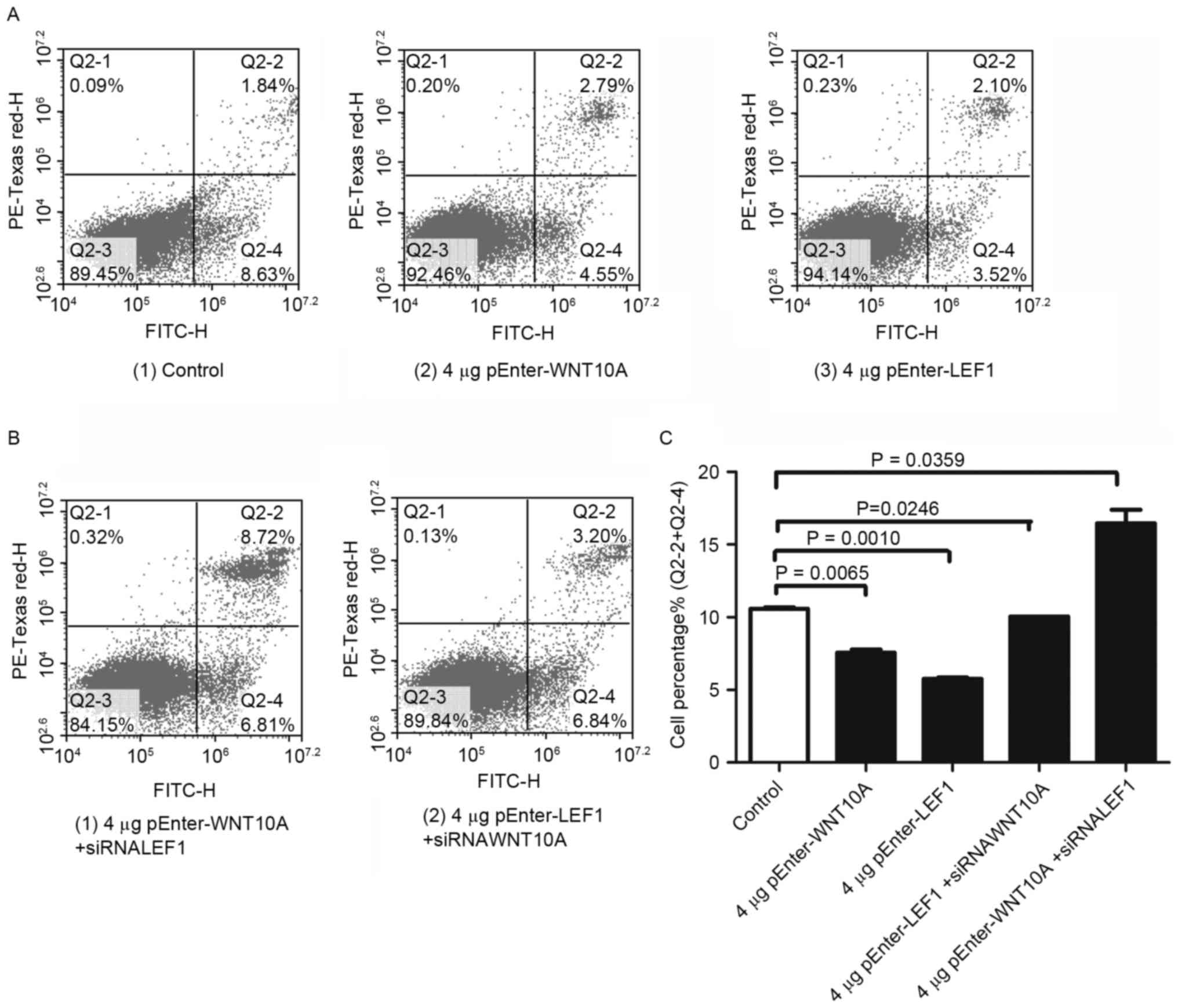
GLAG-66 cells were then examined by flow cytometry
to test whether cell cycle and cell apoptosis were modulated
concomitant with WNT10A and LEF1 administration. Following
transfection with the pEnter-WNT10A and pEnter-LEF1 plasmid,
respectively. The results indicated a lower percentage of late
apoptotic cells after WNT10A and LEF1 overexpression (Fig. 4A, Q2-4). As expected, higher
percentage of late apoptotic cells were detected after reduction of
WNT10A and LEF1 with siRNA (Fig.
4B, Q2-4). Quantification of the results confirmed the
experimental conclusion (Fig.
4C).
WNT10A/β-catenin signaling pathway
activation enhances migration of the thyroid cells
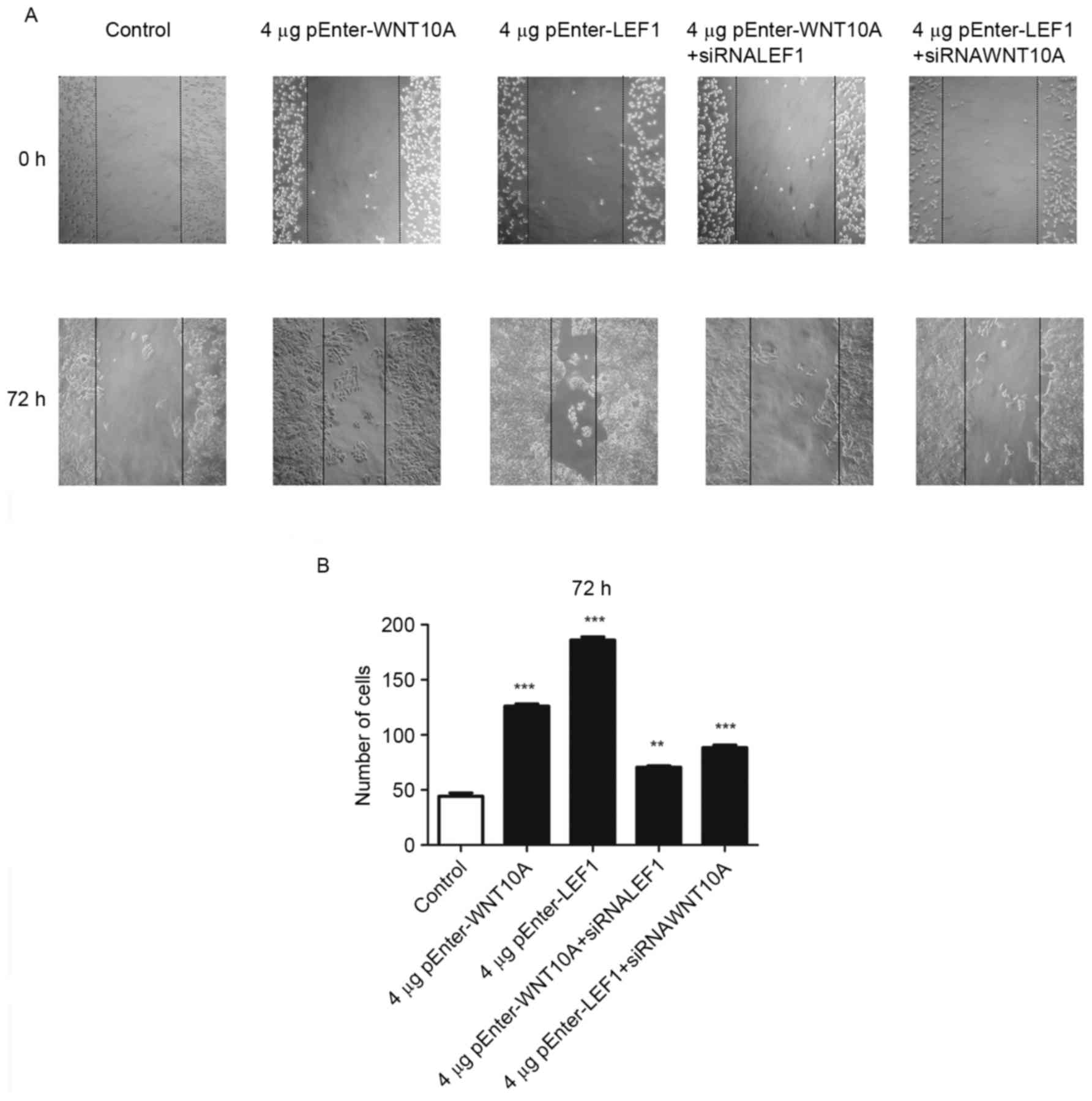
Next, the directional migration of GLAG-66 cells was
examined with the wound-healing assay under WNT10A/β-catenin
signaling pathway activation. The ‘wound’ was created in confluent
cell cultures (0 h), and migration of cells into the gap was
monitored after 72 h. As shown in Fig.
5A, GLAG-66 cells grew into the wound as time passed, and the
speeds of the WNT10A and LEF1 overexpressing cells migrating into
the wound were significantly increased compared to the control
cells. In line with this result, knockdown of WNT10A and LEF1
expression with their siRNA reduced the migration ability of
GLAG-66 cells, respectively. Quantification of wound healing assay
confirmed this conclusion (Fig.
5B). These data showed that upregulation of WNT10A and LEF1
expression significantly increased migration of the GLAG-66
cells.
Discussion
WNT/β-catenin signaling is known to be an important
factor in stem cell regulation of intestinal cancer (23,24),
breast cancer (25–27) and skin cancer (28). Activation of canonical WNT signaling
pathway is an important process both in the establishment and
maintenance of cancer stem cells (29), as well as promoting self-renewal
phenotype (18). Previous studies
have indicated that DACT2 (30),
PPFP (31), Dickkopf-1 (32), GPX3 (33), miR-146b-5p (34) and PROX1 (35) are methylated or disordered in
thyroid cancer, leading to growth, metastasis and EMT of thyroid
cancer cells by activating WNT signaling pathway. The WNT/β-catenin
pathway is a direct and forward enhancer of the TTF-1 expression
that can regulate serum thyroglobulin levels, which is intimately
linked to follow-up of PTC patients (5,36).
In previous studies, WNT10A was shown to play an
important role in the pathogenesis of idiopathic pulmonary fibrosis
(37), agenesis of the maxillary
permanent canines and dental agenesis (38,39),
hypohidrotic ectodermal dysplasia (40) and keratoconus (41). Specifically, the aberration of
WNT10A leads to malformation of ectodermal appendages during
development. During hair follicle morphogenesis, expression of
WNT10A is weakly upregulated in the placode compared with adjacent
epidermis (42). Actually, WNT10A
uniquely observed in basal and mammary stem cells may regulate the
canonical WNT signaling pathway to maintain basal and mammary stem
cell activity and was secreted by mammary stem cells (43). Consequently, it is believed that
WNT10A may be necessary for epithelial migration and proliferation
during normal development. However, the role of WNT10A in PTC is
still unknown.
By employing microarray analysis of PTC, we found
that WNT10A was upregulated in PTC tissues but limitedly expressed
in adjacent normal tissues. WNT10A and WNT6 may be considered to be
the key factors in human carcinogenesis through activation of
WNT/β-catenin pathway (44).
Previous studies have shown that WNT6 expression is not
significantly different in primary gastric cancer. However, another
report claimed that WNT10A was upregulated in primary gastric
cancer (45). It was further
verified that overexpression or knockdown of WNT10A had direct
influence on proliferation, migration and invasion of PTC cells by
modulating WNT/β-catenin pathway in thyroid papillary cancer. In
our study, it was found that overexpression of WNT10A led to
increase in β-catenin, upregulated cyclin D1 and c-myc expression
and it was the same with overexpression of LEF1. Forced WNT10A
expression in GLAG-66 cells resulted in increased cell
proliferation, migration, invasiveness, and cell transformation.
Consistently, knockdown of WNT10A in GLAG-66 can decrease
intracellular β-catenin accumulation, downregulate cyclin D1 and
c-myc expression, and thus suppress cell proliferation, migration,
invasiveness, and cell transformation. Combined with the designs of
cell line models, our results suggested that WNT10A might exert an
autocrine effect on PTC, resulting in activation of WNT/β-catenin
signaling and promoting cell proliferation and migration.
These data indicated that WNT10A plays a crucial
role in carcinogenesis and aggressiveness in papillary thyroid
cancer by activating β-catenin-dependent pathway. This in turn
would also increase β-catenin, LEF1, MYC and cyclin D1 expression.
Similar results were obtained from esophageal squamous cell
carcinoma, the upregulation of WNT10A has been proven to induce
proliferation and migration and to promote a self-renewal phenotype
of esophageal carcinoma cell lines (18). Several molecular components in
Wnt/β-catenin signaling have been proposed as potential therapeutic
targets of cancer treatment (46).
Wnt/β-catenin signaling is associated with lymph node metastasis of
PTC by regulating the expression of cyclin D1 (47). Dickkopf-1, a Wnt/β-catenin pathway
inhibitor, suppressed proliferation and migration of PTC cells by
modulating the Wnt/β-catenin pathway (48). Our study demonstrated the role of
WNT10A/β-catenin pathway in thyroid tumorigenesis for the first
time. WNT10A/β-catenin shows potential as a future therapeutic
target for papillary thyroid cancer.
Acknowledgments
We gratefully acknowledge the financial support from
The National Natural Science Foundation of China (81272351), The
Fundamental Research Funds of Shandong University (2015JC010), The
Project of Medical and Health Technology Development Program in
Shandong Province (grant no. 2014ws0349), The Open Research Fund of
State Key Laboratory of Environmental Chemistry and Ecotoxicology
(KF2014-08) and The Primary Research and Development Plan of
Shandong Province (2016GSF201137).
References
|
1
|
Liu S, Semenciw R, Ugnat AM and Mao Y:
Increasing thyroid cancer incidence in Canada, 1970–1996: Time
trends and age-period-cohort effects. Br J Cancer. 85:1335–1339.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aschebrook-Kilfoy B, Schechter RB, Shih
YC, Kaplan EL, Chiu BC, Angelos P and Grogan RH: The clinical and
economic burden of a sustained increase in thyroid cancer
incidence. Cancer Epidemiol Biomarkers Prev. 22:1252–1259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Behrens J and Lustig B: The Wnt connection
to tumorigenesis. Int J Dev Biol. 48:477–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sastre-Perona A and Santisteban P: Role of
the wnt pathway in thyroid cancer. Front Endocrinol (Lausanne).
3:312012.PubMed/NCBI
|
|
7
|
Xing Y, Clements WK, Kimelman D and Xu W:
Crystal structure of a beta-catenin/axin complex suggests a
mechanism for the beta-catenin destruction complex. Genes Dev.
17:2753–2764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
James RG, Conrad WH and Moon RT:
Beta-catenin-independent Wnt pathways: Signals, core proteins, and
effectors. Methods Mol Biol. 468:131–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roberts DM, Slep KC and Peifer M: It takes
more than two to tango: Dishevelled polymerization and Wnt
signaling. Nat Struct Mol Biol. 14:463–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh M: WNT and FGF gene clusters
(Review). Int J Oncol. 21:1269–1273. 2002.PubMed/NCBI
|
|
12
|
Bohring A, Stamm T, Spaich C, Haase C,
Spree K, Hehr U, Hoffmann M, Ledig S, Sel S, Wieacker P, et al:
WNT10A mutations are a frequent cause of a broad spectrum of
ectodermal dysplasias with sex-biased manifestation pattern in
heterozygotes. Am J Hum Genet. 85:97–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cluzeau C, Hadj-Rabia S, Jambou M, Mansour
S, Guigue P, Masmoudi S, Bal E, Chassaing N, Vincent MC, Viot G, et
al: Only four genes (EDA1, EDAR, EDARADD, and WNT10A) account for
90% of hypohidrotic/anhidrotic ectodermal dysplasia cases. Hum
Mutat. 32:70–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nawaz S, Klar J, Wajid M, Aslam M, Tariq
M, Schuster J, Baig SM and Dahl N: WNT10A missense mutation
associated with a complete odonto-onycho-dermal dysplasia syndrome.
Eur J Hum Genet. 17:1600–1605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Wang Y and Xue F: Expression and
the clinical significance of Wnt10a and Wnt10b in endometrial
cancer are associated with the Wnt/β-catenin pathway. Oncol Rep.
29:507–514. 2013.PubMed/NCBI
|
|
16
|
Hsu RJ, Ho JY, Cha TL, Yu DS, Wu CL, Huang
WP, Chu P, Chen YH, Chen JT and Yu CP: WNT10A plays an oncogenic
role in renal cell carcinoma by activating WNT/β-catenin pathway.
PLoS One. 7:e476492012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kirikoshi H, Inoue S, Sekihara H and Katoh
M: Expression of WNT10A in human cancer. Int J Oncol. 19:997–1001.
2001.PubMed/NCBI
|
|
18
|
Long A, Giroux V, Whelan KA, Hamilton KE,
Tétreault MP, Tanaka K, Lee JS, Klein-Szanto AJ, Nakagawa H and
Rustgi AK: WNT10A promotes an invasive and self-renewing phenotype
in esophageal squamous cell carcinoma. Carcinogenesis. 36:598–606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng C, Xu Z, Li Z, Zhang D, Liu Q and Lu
L: Down-regulation of Wnt10a by RNA interference inhibits
proliferation and promotes apoptosis in mouse embryonic palatal
mesenchymal cells through Wnt/β-catenin signaling pathway. J
Physiol Biochem. 69:855–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saiselet M, Floor S, Tarabichi M, Dom G,
Hébrant A, van Staveren WC and Maenhaut C: Thyroid cancer cell
lines: An overview. Front Endocrinol (Lausanne).
3:1332012.PubMed/NCBI
|
|
21
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: A
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barker N, Ridgway RA, van Es JH, van de
Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR,
Sansom OJ and Clevers H: Crypt stem cells as the cells-of-origin of
intestinal cancer. Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teissedre B, Pinderhughes A, Incassati A,
Hatsell SJ, Hiremath M and Cowin P: MMTV-Wnt1 and
-DeltaN89beta-catenin induce canonical signaling in distinct
progenitors and differentially activate Hedgehog signaling within
mammary tumors. PLoS One. 4:e45372009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vaillant F, Asselin-Labat ML, Shackleton
M, Forrest NC, Lindeman GJ and Visvader JE: The mammary progenitor
marker CD61/beta3 integrin identifies cancer stem cells in mouse
models of mammary tumorigenesis. Cancer Res. 68:7711–7717. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shackleton M, Vaillant F, Simpson KJ,
Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ and
Visvader JE: Generation of a functional mammary gland from a single
stem cell. Nature. 439:84–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malanchi I, Santamaria-Martínez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morgan RG, Ridsdale J, Tonks A and Darley
RL: Factors affecting the nuclear localization of β-catenin in
normal and malignant tissue. J Cell Biochem. 115:1351–1361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Z, Herman JG, Brock MV, Sheng J,
Zhang M, Liu B and Guo M: Methylation of DACT2 promotes papillary
thyroid cancer metastasis by activating Wnt signaling. PLoS One.
9:e1123362014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vu-Phan D, Grachtchouk V, Yu J, Colby LA,
Wicha MS and Koenig RJ: The thyroid cancer PAX8-PPARG fusion
protein activates Wnt/TCF-responsive cells that have a transformed
phenotype. Endocr Relat Cancer. 20:725–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho SW, Kim YA, Sun HJ, Ahn HY, Lee EK, Yi
KH, Oh BC, Park DJ, Cho BY and Park YJ: Therapeutic potential of
Dickkopf-1 in wild-type BRAF papillary thyroid cancer via
regulation of β-catenin/E-cadherin signaling. J Clin Endocrinol
Metab. 99:E1641–E1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao H, Li J, Li X, Han C, Zhang Y, Zheng
L and Guo M: Silencing GPX3 expression promotes tumor metastasis in
human thyroid cancer. Curr Protein Pept Sci. 16:316–321. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang
X and Fan Y: MiR-146b-5p promotes metastasis and induces
epithelial-mesenchymal transition in thyroid cancer by targeting
ZNRF3. Cell Physiol Biochem. 35:71–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi D, Ramu S, Park E, Jung E, Yang S,
Jung W, Choi I, Lee S, Kim KE, Seong YJ, et al: Aberrant activation
of Notch signaling inhibits PROX1 activity to enhance the malignant
behavior of thyroid cancer cells. Cancer Res. 76:582–593. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gilbert-Sirieix M, Makoukji J, Kimura S,
Talbot M, Caillou B, Massaad C and Massaad-Massade L: Wnt/β-catenin
signaling pathway is a direct enhancer of thyroid transcription
factor-1 in human papillary thyroid carcinoma cells. PLoS One.
6:e222802011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oda K, Yatera K, Izumi H, Ishimoto H,
Yamada S, Nakao H, Hanaka T, Ogoshi T, Noguchi S and Mukae H:
Profibrotic role of WNT10A via TGF-β signaling in idiopathic
pulmonary fibrosis. Respir Res. 17:392016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kantaputra P, Kaewgahya M and Kantaputra
W: WNT10A mutations also associated with agenesis of the maxillary
permanent canines, a separate entity. Am J Med Genet A.
164A:360–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mostowska A, Biedziak B, Zadurska M,
Matuszewska-Trojan S and Jagodziński PP: WNT10A coding variants and
maxillary lateral incisor agenesis with associated dental
anomalies. Eur J Oral Sci. 123:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng B, Xiao X, Li S, Lu H, Lu J, Zhu L,
Yu D and Zhao W: Eight mutations of three genes (EDA, EDAR, and
WNT10A) identified in seven hypohidrotic ectodermal dysplasia
patients. Genes (Basel). 7:72016.
|
|
41
|
Cuellar-Partida G, Springelkamp H, Lucas
SE, Yazar S, Hewitt AW, Iglesias AI, Montgomery GW, Martin NG,
Pennell CE, van Leeuwen EM, et al: WNT10A exonic variant increases
the risk of keratoconus by decreasing corneal thickness. Hum Mol
Genet. 24:5060–5068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reddy S, Andl T, Bagasra A, Lu MM, Epstein
DJ, Morrisey EE and Millar SE: Characterization of Wnt gene
expression in developing and postnatal hair follicles and
identification of Wnt5a as a target of Sonic hedgehog in hair
follicle morphogenesis. Mech Dev. 107:69–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ji H, Goode RJ, Vaillant F, Mathivanan S,
Kapp EA, Mathias RA, Lindeman GJ, Visvader JE and Simpson RJ:
Proteomic profiling of secretome and adherent plasma membranes from
distinct mammary epithelial cell subpopulations. Proteomics.
11:4029–4039. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kirikoshi H, Sekihara H and Katoh M:
Up-regulation of WNT10A by tumor necrosis factor alpha and
Helicobacter pylori in gastric cancer. Int J Oncol.
19:533–536. 2001.PubMed/NCBI
|
|
45
|
Kirikoshi H, Sekihara H and Katoh M:
WNT10A and WNT6, clustered in human chromosome 2q35 region with
head-to-tail manner, are strongly coexpressed in SW480 cells.
Biochem Biophys Res Commun. 283:798–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Gill AJ, Issacs JD, Atmore B,
Johns A, Delbridge LW, Lai R and McMullen TP: The Wnt/β-catenin
pathway drives increased cyclin D1 levels in lymph node metastasis
in papillary thyroid cancer. Hum Pathol. 43:1044–1050. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Glinka A, Wu W, Delius H, Monaghan AP,
Blumenstock C and Niehrs C: Dickkopf-1 is a member of a new family
of secreted proteins and functions in head induction. Nature.
391:357–362. 1998. View
Article : Google Scholar : PubMed/NCBI
|