Introduction
Breast cancer (BC) is the most common malignant
tumor and the highest cause of cancer-related deaths in women
world-wide (1). Because of the
development of adjuvant medication therapy such as chemotherapy,
endocrine therapy and anti-human epidermal growth factor 2 (HER2)
therapy, prognosis of BC patients has improved (2). The 5-, 10- and 15-year relative
survival rates are 89, 83 and 78%, respectively (3). However, recurrent BC after resection
is still difficult to cure (4).
Therefore, development of novel molecular targets is still
required. Several commercial multigene expression assays are
available (5). Although they can
help evaluate prognosis and appropriateness of adjuvant
chemotherapy, additional informative biomarkers may improve their
accuracy.
Derlin3 (DERL3) gene is located at 22q11.23;
its protein locates in the endoplasmic reticulum (EnRt). It belongs
to the Derlin family (DERL1, DERL2 and DERL3), which
mediates endoplasmic reticulum-associated degradation (ERAD) of
misfolded proteins, one of the EnRt stress responses (6–9).
Reportedly, EnRt stress responses contribute to cancer progression
(10,11). Although DERL1 overexpression
is reported in BC (12), colon
cancer (13) and non-small cell
lung cancer (NSCLC) (14), no
studies have addressed the role of DERL3 in BC. Referring to
EnRt stress responses in BC, previous studies demonstrate that
GRP78, an EnRt chaperone (15), is overexpressed in high-grade BC
(16) and XBP-1, a
UPR-related transcription factor, is overexpressed in BC (17). The relationship between DERL3
and these molecules remains elusive.
In searching for a new therapeutic target that is
overexpressed in BC, we chose DERL3 as a candidate. This
study therefore investigated DERL3 expression and functions
to determine whether DERL3 is a potential biomarker and
therapeutic target for BC.
Materials and methods
Sample collection
We obtained 13 human BC cell lines (BT-20, BT-474,
BT-549, HCC1419, HCC1954, Hs578T, MCF7, MDA-MB-231, MDA-MB-361,
MDA-MB-415, MDA-MB-468, SK-BR-3, and ZR-75-1) and two non-cancerous
breast epithelial cell lines (MCF-10A, and MCF-12A). BT-549,
HCC1419, HCC1954, and Hs578T were purchased from Japanese
Collection of Research Bioresources Cell Bank (Osaka, Japan),
BT-474, MCF-7, and MCF-12A were kindly provided by Dr David
Sidransky, the Director of the Department of Otolaryngology-Head
and Neck Surgery of Johns Hopkins University (Baltimore, MD, USA),
and others were from the American Type Culture Collection
(Manassas, VA, USA). Cells were stored at −80°C using
cell-preservative solution (Cell Banker; Mitsubishi Chemical
Medience Corporation, Tokyo, Japan) and cultured in RPMI-1640
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS), in an atmosphere containing 5% CO2
at 37°C (18,19).
We acquired 167 primary BC specimens from patients
who underwent breast surgery at Nagoya University Hospital between
March 2002 and November 2009. All specimens were diagnosed
histologically as BC, frozen immediately after resection, and
stored at −80°C. Adjacent non-cancerous specimens were resected
>3 cm from the tumor edges. BC specimens were classified
histologically using the Union for International Cancer Control
(UICC) staging system for BC (7th edition). Selected patients
received adjuvant chemotherapy, endocrine therapy and anti-HER2
therapy, according to their conditions, pathological factors and
physicians' discretion. Patient follow-up data and
clinicopathological parameters were collected from medical
records.
This study conforms to the ethical guidelines of the
Declaration of Helsinki. Enrollees granted written informed consent
for use of clinical samples and data, as required by our
institutional review board.
Quantitative real-time
reverse-transcription polymerase chain reaction (qRT-PCR)
DERL3 mRNA expression was determined by
qRT-PCR. We extracted RNA from cell lines (8.0×106 cells
per each cell line), 167 primary BCs, and adjacent normal tissues
using the RNeasy mini kit (Qiagen, Hilden, Germany) according to
the manufacturer's protocol. cDNA was synthesized from total RNAs
(1 µg) by M-MLV Reverse Transcriptase (Invitrogen, Frederick, MD,
USA) and Primer ‘random’ (Sigma-Aldrich). The qRT-PCR of
DERL3, GRP78 and XBP1 was performed using the
SYBR Green PCR Core Reagents kit (Applied Biosystems) as follows: 1
cycle of 95°C (10 min); 40 cycles of 95°C (5 sec), and 60°C (60
sec). The primers specific for each gene were as follows:
DERL3: forward 5′-CTCACTTTCCAGGCACCGT-3′and reverse
5′-TAGTAGATATGGCCCACCGC-3′, which generated a 110-bp product;
GRP78: forward 5′-GACATCAAGTTCTTGCCGTT-3′ and reverse
5′-CTCATAACATTTAGGCCAGC-3′, which generated a 260-bp product
(20); and XBP1: forward
5′-CAGACTACGTGCGCCTCTGC-3′ and reverse 5′-CTTCTGGGTAGACCTCTGGG-3′,
which generated a 208-bp product (21). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) mRNA levels were quantified to
normalize expression levels. Each sample was tested in triplicate;
data are shown as (DERL3 value)/(GAPDH value) (22,23).
Inhibiting DERL3 expression by
DERL3-specific small interfering RNAs (siRNAs)
Four kinds of siRNAs specific for DERL3
(siDERL3) were used to transfect BT-549 cells. Their
sequences were siDERL3-1: 5′-GAUUCAGCUUCUUCUUCAATT-3′;
siDERL3-2: 5′-UUGAAGAAGAAGCUGAAUCCCAGGGTT-3′ (from a
previous study) (24);
siDERL3-3: 5′-UUGAAGUAGAGUUGAAAGGGG-3′; and
siDERL3-4: 5′-UGAAGAAGAAGCUGAAUCCCA-3′ (Hokkaido System
Science, Sapporo, Japan). AccuTarget Negative Control siRNA
Fluorescein-labeled (siControl; Cosmo Bio Co. Ltd., Tokyo, Japan)
served as control nontargeting siRNA. The BC cells were seeded into
10-cm dishes with 10 ml of antibiotic-free RPMI-1640 with 10% FBS,
and transfected with 400 pmol of siControl or siDERL3s in
the presence of 40-µl LipoTrust EX Oligo (Hokkaido System Science)
24 h after seeding. After transfection, cells were cultured in
antibiotic-free RPMI-1640 with 10% FBS for 72 h.
Western blotting
Cells were incubated in RIPA lysis buffer; lysates
were stored at −30°C. Total protein lysates (20 µg/well) were
electrophoretically transferred onto polyvinylidene difluoride
membranes that were blocked using 5% skim milk in 0.05% PBS-T, and
incubated at 4°C overnight with rabbit anti-DERL3 polyclonal
antibody (1:500; Abcam, Cambridge, UK). The membrane was then
washed and probed with an anti-rabbit secondary antibody conjugated
to horseradish peroxidase (Cell Signaling Technology, Beverly, MA,
USA). β-actin served as endogenous control (25).
Proliferation assay
Proliferation was evaluated using Cell Counting
Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). After transfection with siDERL3 (mixture of
siDERL3-1, −2, −3 and −4), BT-549 cells
(5×103/well) were seeded into 96-well plates with
RPMI-1640 containing 2% FBS. Optical density of each well was
measured 5 days after seeding, 2 h after adding 10 µl of CCK-8
solution.
Invasiveness assay
Invasiveness in Matrigel was determined using
BioCoat Matrigel Invasion Chambers (Corning Inc., Corning, NY, USA)
according to manufacturer's protocol. After transfection with mixed
siDERL3s, BT-549 cells (2.5×104/well) were
suspended in serum-free RPMI-1640 and seeded in upper chambers.
After 60 h of incubation, cells on membrane surfaces were fixed,
stained, and counted in ten randomly selected microscope
fields.
Migration assay
Migration of BT-549 cells was determined using a
wound-healing assay. After transfection with mixed siDERL3s,
BT-549 cells (3×104/well) were seeded into each well of
a 35-mm dish with culture insert (Ibidi, Martinsried, Germany) in
RPMI-1640 containing 2% FBS. After 24 h, the insert was removed,
and wound widths were measured at 100-µm intervals (20
measurements/well, ×40 magnification) at cell-dependent time
intervals.
Statistical analysis
Differences in DERL3 mRNA expression between
two groups were evaluated using Mann-Whitney test. Correlations
between DERL3 mRNA levels and those of GRP78 and
XBP1 were analyzed using the Spearman's rank correlation
test. Significance of associations between DERL3 mRNA
expression and clinicopathological parameters was analyzed with
χ2 test. Overall survival (OS) and disease-free survival
(DFS) were calculated using Kaplan-Meier method; differences in
survival curves were evaluated by log-rank tests. Multivariate
regression analysis used Cox proportional hazards model to identify
prognostic factors; variables for which P<0.05 were entered into
the final model. All statistical analyses were performed on JMP 12
software (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
DERL3 mRNA expression in BC and
non-cancerous cell lines
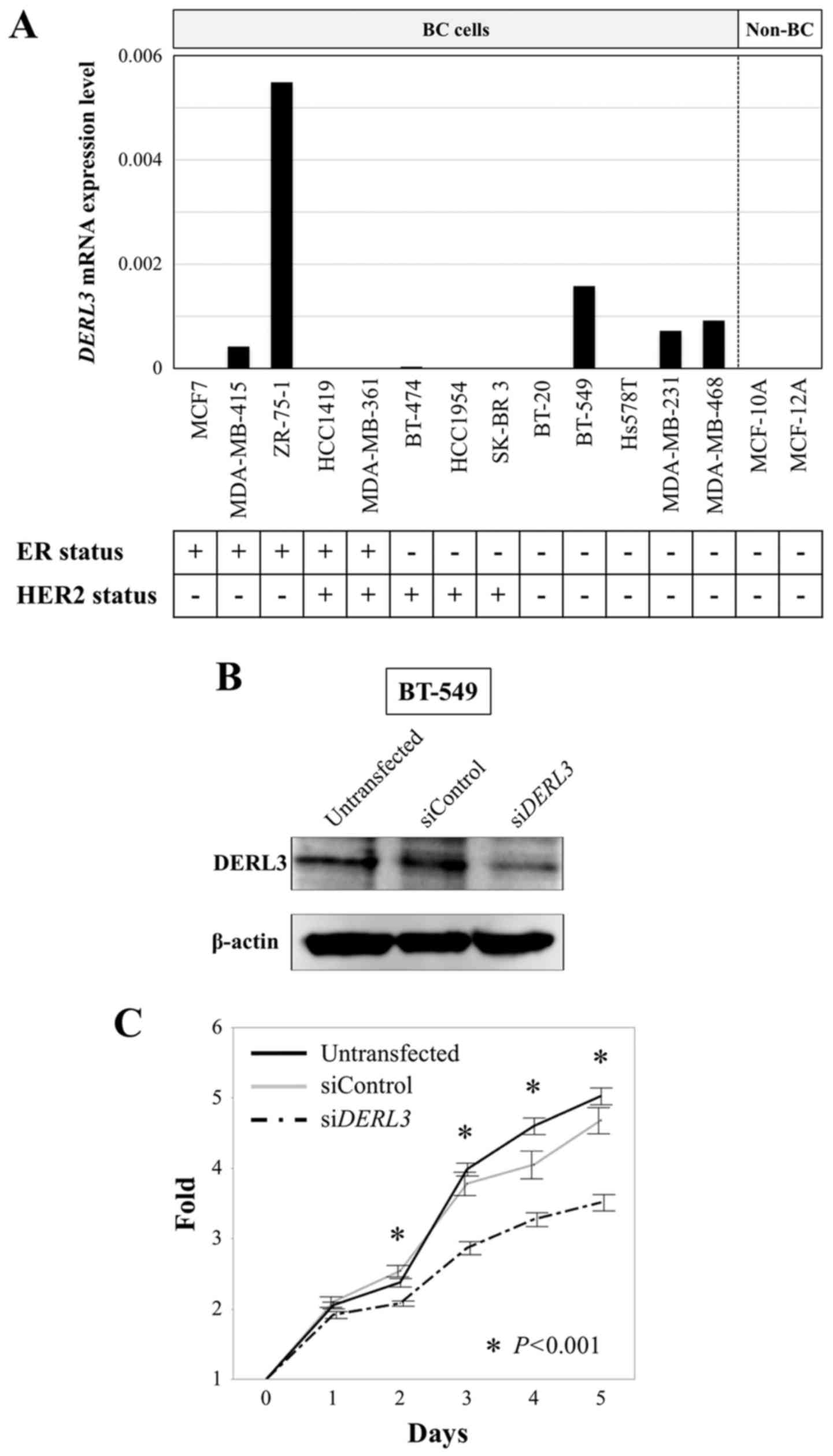
DERL3 mRNA expression was detectable in five
BC cell lines (38%), but not in other BC cell lines or
non-cancerous cell lines (Fig. 1A).
In BC cell lines, DERL3 mRNA expression did not differ
between estrogen receptor (ER) positive and ER negative, HER2
positive and HER2 negative, or triple-negative and other subtypes.
ER and HER2 status of the cell lines were evaluated in previous
studies (26,27).
Expression analysis of DERL3 and genes
encoding putative functional partners in BC cell lines
We evaluated the correlation between DERL3
mRNA expression levels and the genes GRP78 and XBP1
that could potentially functionally interact with DERL3.
There were no correlations between DERL3 and GRP78
mRNA expression levels (correlation coefficient 0.253, P=0.405).
Although it was not statistically significant, we found a weak
correlation between DERL3 and XBP1 mRNA expression
levels (correlation coefficient 0.335, P=0.263).
Effects of DERL3 inhibition on BC cell
phenotypes
BT-549 cell line was transfected with
siDERL3s to determine DERL3 functions in BC. Western
blot analysis confirmed that DERL3 was decreased by the
transfections (Fig. 1B). We
evaluated proliferation, invasion, and migration. DERL3
inhibition significantly decreased proliferation over days 2–5
compared with the untransfected and siControl cells (P<0.001;
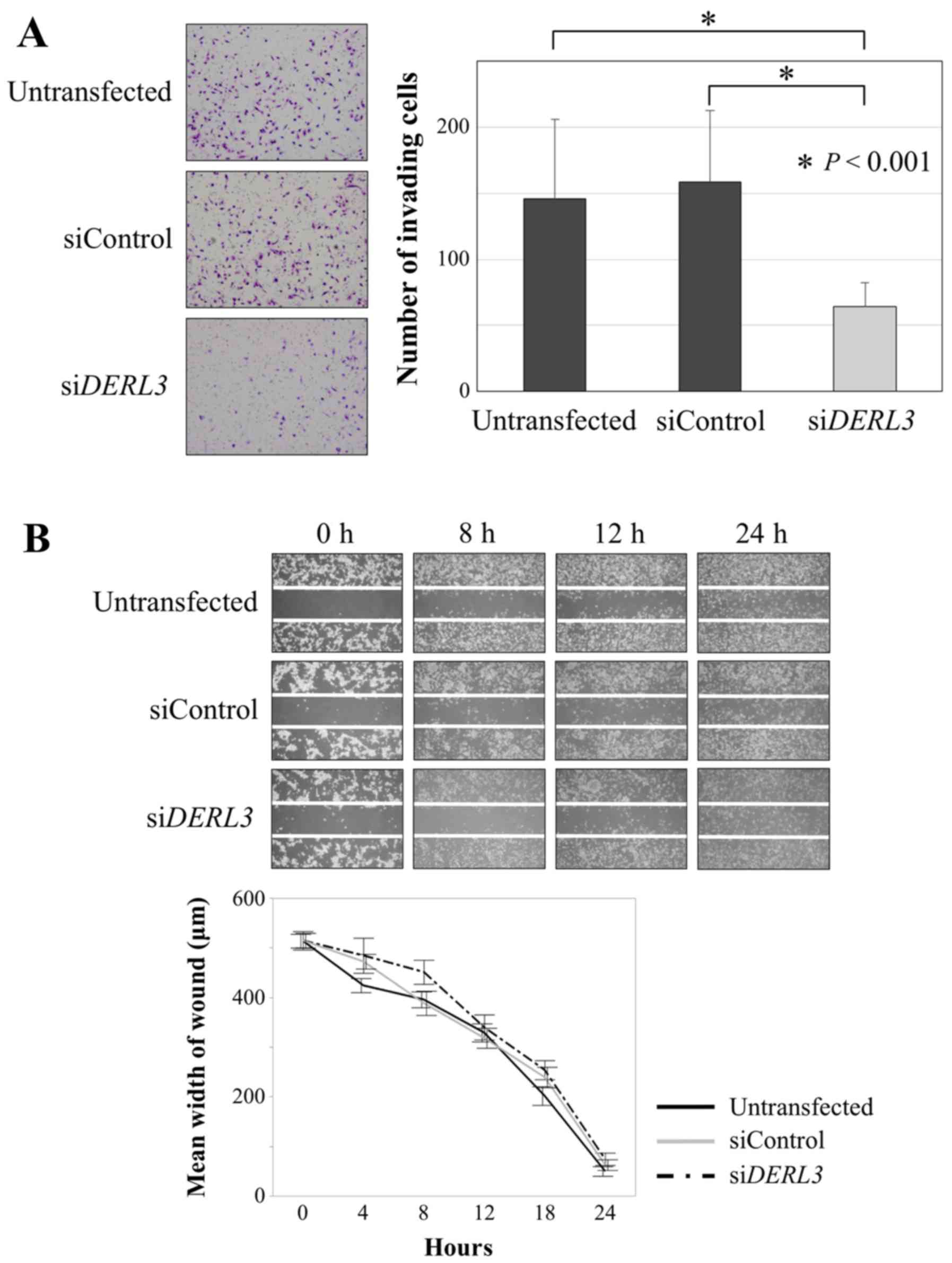
Fig. 1C). In the invasion assay,
significantly fewer DERL3-inhibited cells invaded the
Matrigel than did untransfected and siControl cells (P<0.001;
Fig. 2A). In contrast, there was no
difference in the migration assay (Fig.
2B).
Patient characteristics
All 167 patients were women, whose mean age was
54.4±11.6 years (range: 26–78 years); and whose disease stages were
stage 0: 7; stage I: 47; stage II: 78; stage III: 34; and stage IV:
1. Their median follow-up period was 100.0 months (range: 8–155
months) or until death.
DERL3 mRNA expression levels did not differ
significantly between BC tissues and adjacent non-cancerous tissues
(P=0.125). DERL3 mRNA expression levels were higher in BC
tissues than in adjacent non-cancerous tissues for 94 (56.3%) of
the 167 patients. Of note, DERL3 mRNA expression levels for
several BC specimens were as low as their non-cancerous
counterparts, whereas some BC specimens did express high
DERL3 mRNA levels.
Clinical and prognostic significance
of DERL3 mRNA expression in BC specimens
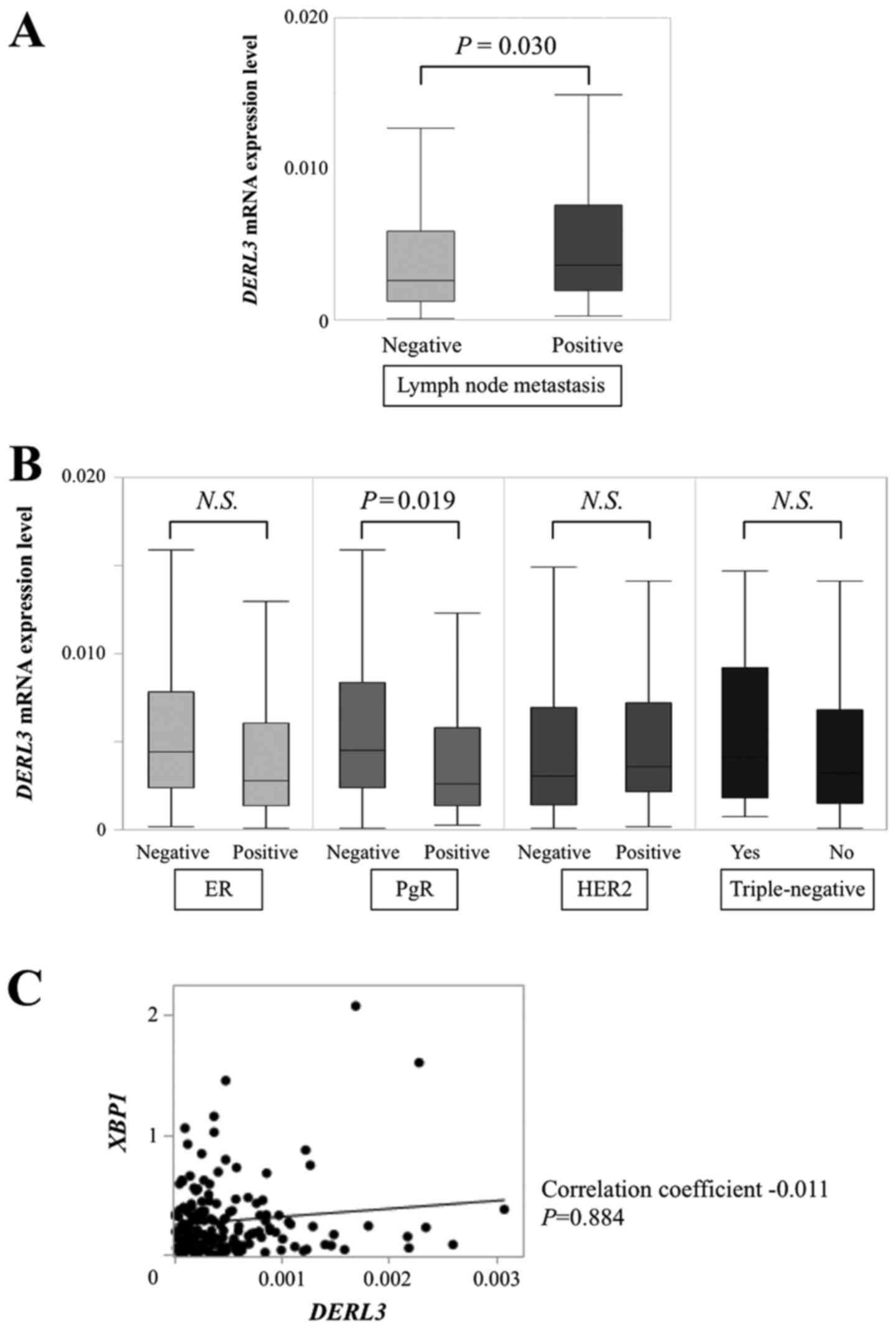
Among the 167 patients, we found DERL3 mRNA
expression to be significantly higher in those with lymph node (LN)
metastasis (n=82) than in those without LN metastasis (n=85;
P=0.030; Fig. 3A). DERL3
expression did not differ with regard to T categories or UICC
stage. Among conventional biomarkers (Fig. 3B), DERL3 mRNA expression in
progesterone receptor (PgR)-negative specimens (n=52) was
significantly higher than in PgR-positive specimens (n=115;
P=0.019). There were not significant differences between
ER-positive (n=127) and -negative (n=40; P=0.070), HER2- positive
(n=39) and -negative (n=119; P=0.577; missing data for 9 patients),
or triple-negative (n=18) and non-triple-negative specimens (n=148;
P=0.467; missing data for 1 patient). We evaluated the correlation
between DERL3 mRNA levels and XBP1 mRNA levels in the
patients' BC specimens. There were no correlations between mRNA
expression levels of these genes (correlation coefficient −0.011,
P=0.884; Fig. 3C).
The patients with the highest quartile of
DERL3 expression were designated as ‘high DERL3
group’ (n=42), and the remaining patients were designated as
‘others’ (n=125). We found no significant differences with regard
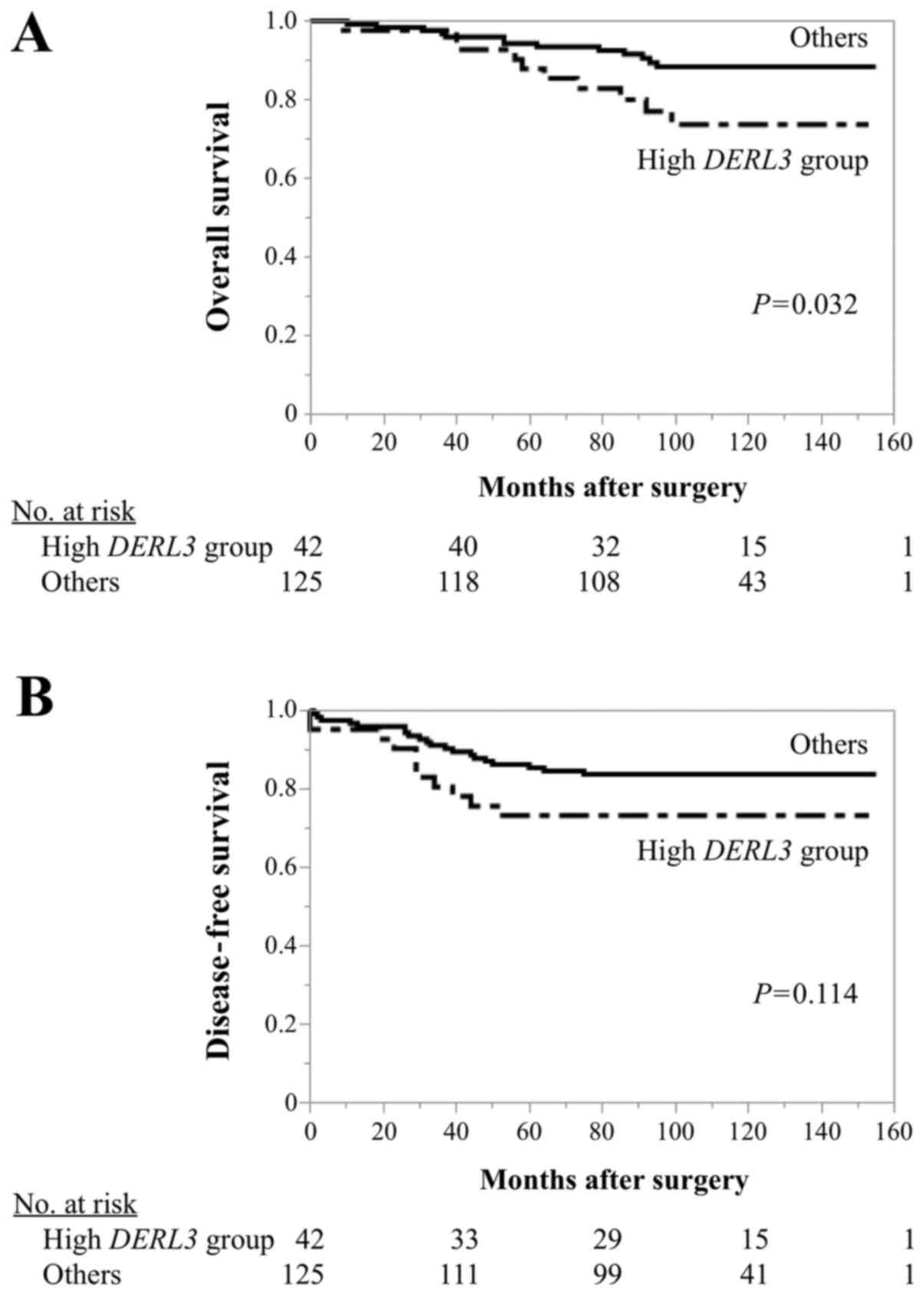
to any tested clinicopathological characteristics (Table I). The high DERL3 group
experienced significantly shorter OS than others (5-year OS, high
DERL3 group: 87.9%; others: 94.3%; P=0.032; Fig. 4A). Moreover, high DERL3 group
tended to have poorer DFS than others, although there were no
statistically significant differences (5-year DFS, high
DERL3 group: 73.3%; others: 85.4%; P=0.114; Fig. 4B).
 | Table I.Associations between DERL3
mRNA expression and clinicopathological characteristics of 167
patients with breast cancer. |
Table I.
Associations between DERL3
mRNA expression and clinicopathological characteristics of 167
patients with breast cancer.
| Clinicopathological
parameter | High DERL3
group (n=42) | Others (n=125) |
P-valuea |
|---|
| Age |
|
| 0.065 |
| ≤60
year | 32 | 76 |
|
| >60
year | 10 | 49 |
|
| Histology |
|
| 0.265 |
|
DCIS | 0 | 7 |
|
|
IDC | 39 | 109 |
|
|
ILC | 2 | 4 |
|
|
Others | 1 | 5 |
|
| UICC T factor |
|
| 0.207 |
|
Tis | 0 | 7 |
|
| T1 | 18 | 52 |
|
| T2 | 19 | 56 |
|
| T3 | 4 | 5 |
|
| T4 | 1 | 5 |
|
| Node status |
|
| 0.054 |
|
Negative | 16 | 69 |
|
|
Positive | 26 | 56 |
|
| UICC pathological
stage |
|
| 0.211 |
|
0/I/II | 30 | 101 |
|
|
III/IV | 12 | 24 |
|
| ER status |
|
| 0.424 |
|
Positive | 30 | 97 |
|
|
Negative | 12 | 28 |
|
| PgR status |
|
| 0.062 |
|
Positive | 24 | 91 |
|
|
Negative | 18 | 34 |
|
| HER2 status |
|
| 0.792 |
|
Positive | 11 | 28 |
|
|
Negative | 31 | 88 |
|
|
Unknown | 0 | 9 |
|
|
Triple-negative |
|
| 0.800 |
|
True | 5 | 13 |
|
|
False | 37 | 111 |
|
|
Unknown | 0 | 1 |
|
| Adjuvant
therapy |
|
| 0.460 |
|
Endocrine therapy alone | 13 | 44 |
|
|
Chemotherapy alone | 11 | 19 |
|
|
Endocrine and
chemotherapy | 15 | 49 |
|
|
None | 3 | 13 |
|
Multivariate analysis of OS identified UICC
pathological stage III/IV disease (HR: 6.43; 95% CI: 2.26–20.3,
P<0.001) as an independent prognostic factor, but not high
DERL3 expression (HR: 2.27; 95% CI: 0.92–5.47, P=0.074;
Table II).
 | Table II.Prognostic factors for overall
survival in 167 patients with breast cancer. |
Table II.
Prognostic factors for overall
survival in 167 patients with breast cancer.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variables | n | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (≤60) | 108 | 1.17 | 0.50–3.03 | 0.733 |
|
|
|
| UICC pathological
stage (III/IV) | 36 | 7.65 | 3.35–18.4 | <0.001 | 6.43 | 2.26–20.3 |
<0.001a |
| ER status
(negative) | 40 | 2.43 | 1.01–5.54 | 0.047 | 2.34 | 0.52–17.5 | 0.279 |
| PgR status
(negative) | 52 | 2.32 | 1.01–5.30 | 0.048 | 0.95 | 0.14–3.74 | 0.951 |
| HER2 status
(positive) | 39 | 2.76 | 1.16–6.40 | 0.022 | 1.14 | 0.38–3.32 | 0.815 |
| Triple-negative
(yes) | 18 | 2.23 | 0.65–5.95 | 0.183 |
|
|
|
| Adjuvant
chemotherapy (yes) | 94 | 3.11 | 1.24–9.43 | 0.002 | 0.82 | 0.23–2.99 | 0.751 |
| High DERL3
expression | 42 | 2.40 | 1.02–5.45 | 0.044 | 2.27 | 0.92–5.47 | 0.074 |
Discussion
In this study, we showed that DERL3 promotes
BC cell proliferation and invasiveness, and that high DERL3
patients experienced poorer prognosis.
When cells undergo stress through hypoxia and
nutrient deprivation, misfolded proteins increase, and activate the
unfolded protein response (UPR) (11,28),
which upregulates ERAD, and contributes to progression in cancer
cells (10,11). Reportedly, GRP78, an EnRt
chaperone (15), is overexpressed
in high-grade BC (16).
Furthermore, XBP-1, a UPR-related transcription factor, is
overexpressed in BC, but is hardly detectable in non-cancerous
breast tissues (17). These
findings suggest that UPR promotes a malignant BC phenotype.
DERL1 is the most widely studied derlin
(6), and regulated by the IRE-XBP1
pathway (8,29). Its overexpression reportedly
prevents induction of apoptosis in stressed BC cells and correlates
with advanced pathological tumor grade and node-positive status in
BC (12). It also contributes to
malignant phenotype in colon cancer and in NSCLC by activating the
PI3K/AKT and EGRF-EPK pathways, respectively (13,14).
There have been only a few studies on DERL3.
Although DERL3 is assumed to be regulated by IRE1-XBP1
pathway such as DERL1 (8),
no significant correlations were found between DERL3 and
XBP1 mRNA expression levels. There might be another pathway
that regulates the expression of DERL3 in BC cells.
According to previous studies, DERL3 has a protective
function for cardiomyocytes through the ERAD mechanism (30), and low DERL3 expression has
been associated with a more malignant phenotype and poorer
prognosis in colorectal cancer (31). We have shown that DERL3 mRNA
is scarcely expressed not only in non-cancerous breast cell lines
but also in several BC cell lines along with a considerable
proportion of BC specimens. These findings suggest that both
non-cancerous breast cells and BC cells express little DERL3
mRNA under slight EnRt stress.
DERL3 inhibition significantly decreased BC
cell proliferation and invasion, and patients with high
DERL3 group tended to be LN metastasis-positive (P=0.054;
Table I). These results indicate
that DERL3 does promote malignant phenotype when
overexpressed in BC. Noteworthy, DERL1 mRNA overexpression
also correlates with positive LN metastasis in BC, colon cancer and
NSCLC by the inhibition of apoptosis and the activation of
PI3K/AKT, EGRF-EPK pathways, respectively (12–14).
DERL3 might regulate such mechanisms in BC, and it promotes
LN metastasis, leading to more malignant phenotype. Further studies
are required to determine the underlying mechanisms. We found that
the high DERL3 group experienced shorter OS, independently
of ER, PgR and HER2 status, which implies its potential as a
prognostic biomarker for all BC subtypes.
These findings have several possible clinical
applications. DERL3 levels in resected samples can help
predict prognosis. Patients with high DERL3 specimens may
require more aggressive adjuvant therapy; and if DERL3 mRNA
expression levels can be determined from pre-surgical biopsies,
they might help indicate whether neoadjuvant therapy is
appropriate. Although further studies are warranted, our study
suggests the possibility of developing new therapies for BC that
target DERL3.
This study has some limitations. First, the roles of
DERL3 in the ERAD pathway of cancer cells, and of ERAD in
BC, are unclear. Pathway analyses might elucidate the role of
DERL3 in UPR. Second, interventions such as adjuvant
medication might have affected relationships between patient
DERL3 mRNA levels and prognoses. Finally, these results were
obtained from in vitro data and should be verified by in
vivo studies.
In conclusion, we found DERL3 to promote a
malignant BC phenotype. High DERL3 mRNA expression in the BC
tissue resulted in poor prognosis. DERL3 mRNA expression is
a potential prognostic marker and DERL3 protein could be a
candidate therapeutic target for BC.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giordano SH, Buzdar AU, Smith TL, Kau SW,
Yang Y and Hortobagyi GN: Is breast cancer survival improving?
Cancer. 100:44–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwata H: Future treatment strategies for
metastatic breast cancer: Curable or incurable? Breast Cancer.
19:200–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ, et al: Panel
Members: Tailoring therapies - improving the management of early
breast cancer: St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenblatt EJ, Olzmann JA and Kopito RR:
Derlin-1 is a rhomboid pseudoprotease required for the dislocation
of mutant α-1 antitrypsin from the endoplasmic reticulum. Nat
Struct Mol Biol. 18:1147–1152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lilley BN and Ploegh HL: A membrane
protein required for dislocation of misfolded proteins from the ER.
Nature. 429:834–840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oda Y, Okada T, Yoshida H, Kaufman RJ,
Nagata K and Mori K: Derlin-2 and Derlin-3 are regulated by the
mammalian unfolded protein response and are required for
ER-associated degradation. J Cell Biol. 172:383–393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye Y, Shibata Y, Yun C, Ron D and Rapoport
TA: A membrane protein complex mediates retro-translocation from
the ER lumen into the cytosol. Nature. 429:841–847. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cox JS, Shamu CE and Walter P:
Transcriptional induction of genes encoding endoplasmic reticulum
resident proteins requires a transmembrane protein kinase. Cell.
73:1197–1206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Hua H, Ran Y, Zhang H, Liu W, Yang
Z and Jiang Y: Derlin-1 is overexpressed in human breast carcinoma
and protects cancer cells from endoplasmic reticulum stress-induced
apoptosis. Breast Cancer Res. 10:R72008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan X, He X, Jiang Z, Wang X, Ma L, Liu L,
Wang X, Fan Z and Su D: Derlin-1 is overexpressed in human colon
cancer and promotes cancer cell proliferation. Mol Cell Biochem.
408:205–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong QZ, Wang Y, Tang ZP, Fu L, Li QC,
Wang ED and Wang EH: Derlin-1 is overexpressed in non-small cell
lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated
up-regulation of MMP-2 and MMP-9. Am J Pathol. 182:954–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J and Lee AS: Stress induction of
GRP78/BiP and its role in cancer. Curr Mol Med. 6:45–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez PM, Tabbara SO, Jacobs LK,
Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA and Patierno SR:
Overexpression of the glucose-regulated stress gene GRP78 in
malignant but not benign human breast lesions. Breast Cancer Res
Treat. 59:15–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujimoto T, Onda M, Nagai H, Nagahata T,
Ogawa K and Emi M: Upregulation and overexpression of human X-box
binding protein 1 (hXBP-1) gene in primary breast cancers. Breast
Cancer. 10:301–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda M, Shimizu D, Fujii T, Tanaka H,
Shibata M, Iwata N, Hayashi M, Kobayashi D, Tanaka C, Yamada S, et
al: Protein arginine methyltransferase 5 is associated with
malignant phenotype and peritoneal metastasis in gastric cancer.
Int J Oncol. 49:1195–1202. 2016.PubMed/NCBI
|
|
19
|
Kanda M, Shimizu D, Nomoto S, Takami H,
Hibino S, Oya H, Hashimoto R, Suenaga M, Inokawa Y, Kobayashi D, et
al: Prognostic impact of expression and methylation status of
DENN/MADD domain-containing protein 2D in gastric cancer. Gastric
Cancer. 18:288–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing X, Li Y, Liu H, Wang L and Sun L:
Glucose regulated protein 78 (GRP78) is overexpressed in colorectal
carcinoma and regulates colorectal carcinoma cell growth and
apoptosis. Acta Histochem. 113:777–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szegezdi E, Duffy A, O'Mahoney ME, Logue
SE, Mylotte LA, O'brien T and Samali A: ER stress contributes to
ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res
Commun. 349:1406–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda M, Nomoto S, Oya H, Takami H,
Shimizu D, Hibino S, Hashimoto R, Kobayashi D, Tanaka C, Yamada S,
et al: The expression of melanoma-associated antigen D2 both in
surgically resected and serum samples serves as clinically relevant
biomarker of gastric cancer progression. Ann Surg Oncol. 23:(Suppl
2). S214–S221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanda M, Shimizu D, Fujii T, Sueoka S,
Tanaka Y, Ezaka K, Takami H, Tanaka H, Hashimoto R, Iwata N, et al:
Function and diagnostic value of Anosmin-1 in gastric cancer
progression. Int J Cancer. 138:721–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kadowaki H, Nagai A, Maruyama T, Takami Y,
Satrimafitrah P, Kato H, Honda A, Hatta T, Natsume T, Sato T, et
al: Pre-emptive quality control protects the ER from protein
overload via the proximity of ERAD components and SRP. Cell Rep.
13:944–956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oya H, Kanda M, Sugimoto H, Shimizu D,
Takami H, Hibino S, Hashimoto R, Okamura Y, Yamada S, Fujii T, et
al: Dihydropyrimidinase-like 3 is a putative hepatocellular
carcinoma tumor suppressor. J Gastroenterol. 50:590–600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, et
al: The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and
AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
28
|
Hochachka PW, Buck LT, Doll CJ and Land
SC: Unifying theory of hypoxia tolerance: Molecular/metabolic
defense and rescue mechanisms for surviving oxygen lack. Proc Natl
Acad Sci USA. 93:9493–9498. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lilley BN and Ploegh HL: Multiprotein
complexes that link dislocation, ubiquitination, and extraction of
misfolded proteins from the endoplasmic reticulum membrane. Proc
Natl Acad Sci USA. 102:14296–14301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belmont PJ, Chen WJ, Pedro MN San,
Thuerauf DJ, Lowe N Gellings, Gude N, Hilton B, Wolkowicz R,
Sussman MA and Glembotski CC: Roles for endoplasmic
reticulum-associated degradation and the novel endoplasmic
reticulum stress response gene Derlin-3 in the ischemic heart. Circ
Res. 106:307–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lopez-Serra P, Marcilla M, Villanueva A,
Ramos-Fernandez A, Palau A, Leal L, Wahi JE, Setien-Baranda F,
Szczesna K, Moutinho C, et al: A DERL3-associated defect in the
degradation of SLC2A1 mediates the Warburg effect. Nat Commun.
5:36082014. View Article : Google Scholar : PubMed/NCBI
|