Introduction
Head and neck cancer is the sixth most commonly
observed malignancy, and laryngocarcinoma is one of the most
commonly observed malignancies among the head and neck tumors. Its
morbidity ranks in the second place in respiratory tract neoplasms,
accounting for ~95% (1). With
industrialized development and aggravated air pollution, the
morbidity of laryngocarcinoma exhibits a gradual increasing trend
in China as well as worldwide (2).
As documented, the morbidity of laryngocarcinoma is increasing at a
rate of ~25% each year, and it is commonly observed in middle- and
old-aged men (2). According to the
latest research, though novel surgical treatments, chemotherapy
drugs, more advanced radiotherapy means and targeted drugs have
been applied in treating laryngocarcinoma in the last 30 years, the
overall survival of patients with laryngocarcinoma has not
improved, instead, it even shows a decreasing trend, which is ~50%;
and that of advanced stage laryngocarcinoma is even as low as
30–40%. The overall therapeutic effects are unsatisfying (3).
MicroRNA (miRNA), a type of non-coding RNA that
consists of 22 nucleotides and was discovered in recent years, has
an extensive gene expression regulation effect in vivo
(4). miRNAs are closely correlated
with the genesis and development of tumors, and they are attracting
more and more attention in regards to the early diagnosis,
metastasis monitoring, targeted therapy and prognosis of tumors
(5). An miRNA regulates the
biological characteristics of a tumor either by behaving as the
tumor-suppressor gene which downregulates the activity of the
proto-oncogene, or acting as the cancer-promoting gene to
downregulate the activity of the tumor-suppressor gene (6). In numerous diseases, the expression
profile of an miRNA exhibits certain characteristic alterations,
which are particularly obvious in tumors, in which the expression
profiles of the miRNA in the tumor tissue and the normal
paracarcinomatous tissue show marked differences (5). It has been determined that analyzing
the specific miRNA levels of various tumors contributes to the
early diagnosis and prognosis evaluation of the tumor, and miRNAs
show promise as novel molecular markers for the diagnosis,
recurrence, metastasis and prognosis of multiple malignancies
(7). Zhang et al reported
that miRNA-26a/b regulate DNA replication licensing, tumorigenesis,
and prognosis of lung cancer (8).
Zhang et al reported that miRNA-26b suppresses colon cancer
cell proliferation via LEF1 (9).
With in-depth research on the
chemoradiotherapy-resistance mechanism of tumor cells under a
hypoxic microenvironment, increasing importance has been bestowed
to a type of novel protein degradation process, which is called
autophagy. Autophagy is a type of lysosomal degradation process
that is essential to cellular survival, differentiation and
development as well as homeostasis. In addition, it recycles
nutrients, guarantees the dynamic balance between the synthesis and
degradation of the intracellular protein, regulates and defends
against cell stress, and thus maintains the normal function of
cells through the degradation of the intracellular macromolecular
substances and aging organelles (10,11).
The precise process of autophagy includes the
beginning of the autophagosome formation, extension, closure,
maturity and degradation, the molecular basis of which is the
activation of the protein sequence encoded by the
autophagy-associated gene (12).
The ATG gene was first discovered in yeast cells, and ATG1 is a
type of serine/threonine protein kinase, which is essential to the
initial period of autophagy (13).
ATG1 has five homologous proteins in mammals, which are Ulk1–4 and
STK36 (13). The major functional
molecules involved in the precise autophagy process can be roughly
divided into 4 groups: the first one is the ATG1/unc-51-like kinase
(Ulk) complex; apart from Ulk, the ATG13 and FIP200 scaffolds are
also included (it is considered as the autoploid of yeast ATG17)
(14).
PTEN is a type of tumor-suppressor gene, and
tumorsuppressor genes are types of growth regulatory genes or
negative regulatory genes, which can inhibit the transformation of
normal cells into malignant cells under normal conditions (15). When a gene mutation, a gene deletion
or gene inactivation develops and the gene loses its function, it
may induce the malignant transformation of normal cells (7). The protein encoded by PTEN, which
manifests the dual-specificity phosphatase activities of the lipid
phosphatase activity and the protein phosphatase activity in the
cytoplasm, is the first tumor-suppressor gene possessing
phosphatase activity that has been discovered to date, and the
lipid phosphatase activity is the main functional foundation for
PTEN to inhibit a tumor (16). It
plays a critical role in suppressing cell inhibition of apoptosis,
cell cycle arrest, cell migration and inhibiting angiogenesis
(17).
AKT is a type of protein kinase that plays an
important role in biological behaviors such as cell proliferation,
differentiation, apoptosis and migration, and it can phosphorylate
serine/threonine (18). The AKT
family members are comprised of three subtypes, namely, AKT1, AKT2
and AKT3, and they are also named PKBα, PKBβ and PKBγ (18). P-AKT influences the activation of
multiple downstream effector molecules through the phosphorylation
cascade reaction and the interaction with the target protein, thus
regulating multiple biological effects, such as cell growth and
proliferation, regulation of angiogenesis, cell apoptosis and
motion control (19). Current
research suggests that its out-of-control expression is closely
related to multiple tumors, and P-AKT is abundantly expressed in
tumor tissues (20). To gain a
better understanding of the association between miRNA-26b
expression and laryngeal cancer, we studied the effect of miRNA-26b
on the proliferation of laryngeal carcinoma and elucidated the
potential underlying mechanisms to provide new targets for
laryngeal carcinoma.
Materials and methods
Human tissue samples
Laryngeal carcinoma patients (n=59) and healthy
volunteers (n=8) and were obtained from the Huaihe Hospital of
Henan University. Whole blood was obtained and centrifuged at 2,000
× g for 10 min. Serum was obtained and saved at −80°C. Every two
months, we surveyed palindromia or survival rate of all laryngeal
carcinoma patients.
Quantification by real-time PCR
Total RNA was isolated from serum using RNAiso Plus
Reagent according to the manufacturer's instructions. cDNA was
generated following the SuperScript II RT Protocol (Invitrogen,
Carlsbad, CA, USA). qRT-PCR was performed using an Applied
Biosystems 7500 Fast Real-Time PCR System with the SYBR-Green PCR
Master Mix protocol (Applied Biosystems, Foster City, CA, USA). The
reactions were incubated at 95°C for 5 min, followed by 40 cycles
of 95°C for 20 sec, 60°C for 30 sec, and 72°C for 30 sec.
Cell lines and transfection
Squamous cell carcinoma of the larynx cell line
Hep-2 were cultured in RPMI-1640 medium, 10% fetal bovine serum
(FBS; Invitrogen, Shanghai, China) and penicillin/streptomycin at
37°C and 5% CO2. The Hep-2 cells were then transfected
with 100 nM anti-miR-26 and si-ULK2 or negative control mimics
(RiboBio, Guangzhou, China) using Lipofectamine 3000 (Invitrogen,
Guangzhou, China).
Cell proliferation assay
After transfection at 24, 48 and 72 h, the cells
were cultivated into 96-well plates and incubated with MTT solution
(0.5 mg/ml) for 4 h at 37°C. The reaction was stopped by lysing the
cells with 200 µl of dimethyl sulfoxide (DMSO) for 5 min. The
absorbance was recorded at 490 nm.
Apoptosis assay
Following transfection at 48 h, the cells were
cultivated into 6-well plates, and stained with an Annexin
V-FITC/propidium iodide (PI) staining kit (BD Biosciences, San
Jose, CA, USA) for 15 min at 4°C. Analysis was performed using a
FACScan flow cytometer (Becton-Dickinson, Bedford, MA, USA).
Caspase-3 and −9 assay
After transfection at 48 h, the cells were collected
and lysed with lysis buffer (CoWin Biotechnology, Beijing, China)
on ice for 30 min. Lysates were cleared by centrifugation at 10,000
× g for 15 min at 4°C. The protein concentrations were determined
using BCA protein assay kits (CoWin Biotechnology). Proteins
samples (10 µg) were incubated with caspase-3 and −9 assay kits for
1 h at 37°C. The absorbance was recorded at 405 nm.
Western blot analysis
After transfection at 48 h, the cells were collected
and lysed with lysis buffer (CoWin Biotechnology) on ice for 30
min. Lysates were cleared by centrifugation at 10,000 × g for 15
min at 4°C. The protein concentrations were determined using BCA
protein assay kits (CoWin Biotechnology). Proteins samples (50 µg)
were separated by 6–10% sodium dodecylsulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and then transferred onto
polyvinylidine fluoride (PVDF) membranes (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The membranes were incubated with Bax,
LC3, p62, PTEN, phosphorylated (p)-AKT, ULK2 (1:2,000; all from
Cell Signaling, Shanghai, China) and GAPDH (1:1,000; Beyotime,
Shanghai, China) overnight at 4°C. The membranes were then washed
with Tris-buffered saline and Tween-20 (TBST), and then incubated
with a horseradish peroxidase-conjugated secondary antibody
(1;5000; Cell Signaling) for 1 h at room temperature (RT). The
protein expression was detected with an electrochemiluminescence
(ECL) detection reagent (Beyotime) and quantified using Quantity
One software (Bio-Rad, Hercules, CA, USA).
Immunocytofluorescence
After transfection at 48 h, the cells were seeded
onto cell chamber slides and fixed with 4% formaldehyde at 4°C for
15 min. The cells were then stained with the ULK2 antibody (1:500;
Cell Signaling) at 4°C overnight. Subsequently, the cells were
incubated with a secondary antibody (donkey anti-rabbit FITC; 1:100
dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at
RT in the dark. Nuclei were counterstained with
4,6-diamidino-2-phenylindole (DAPI) at RT for 15 min, and images
were captured using an automated upright microscope system (Leica
DM4000B; Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Results are expressed as the mean ± SD. One-way
analysis of variance (ANOVA) was used to analyze the significance
between groups. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
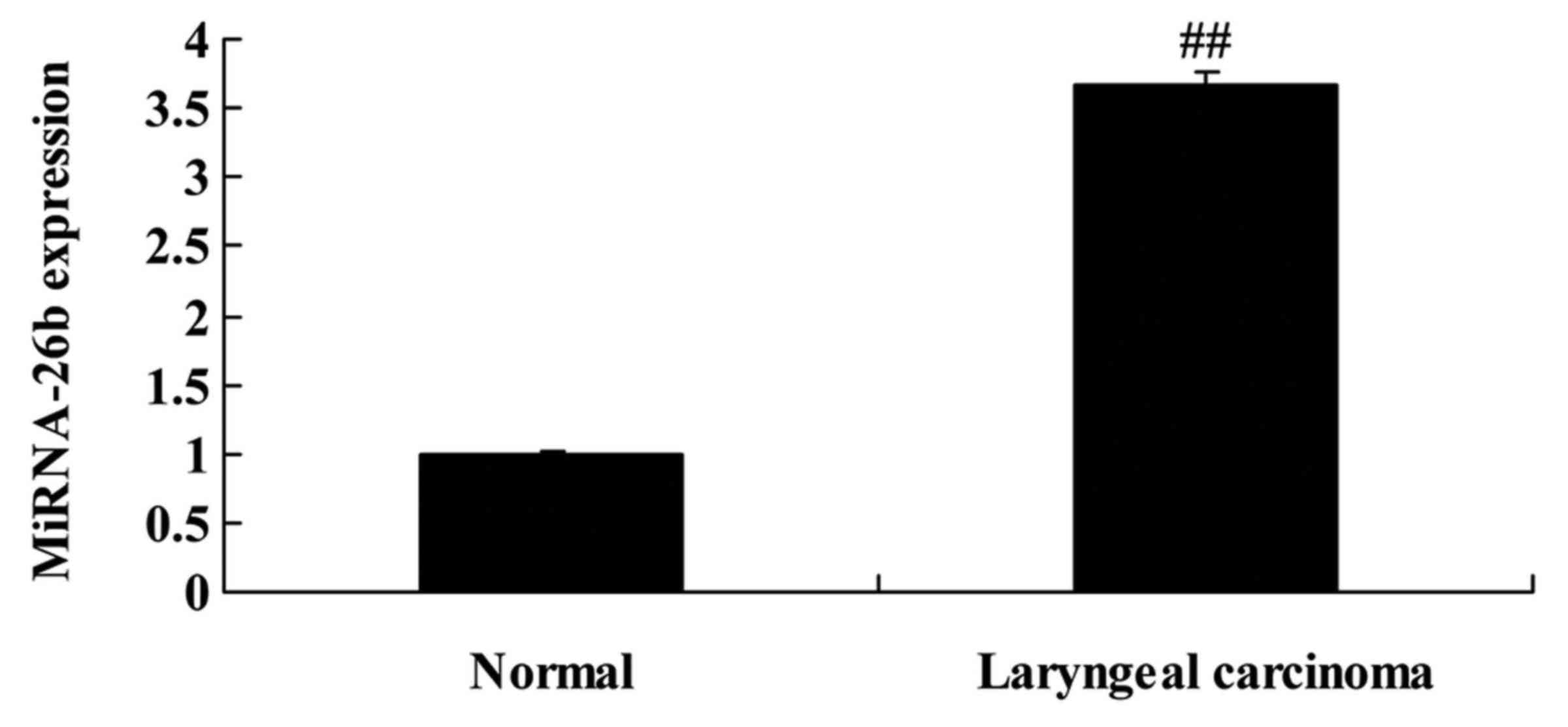
miRNA-26b expression in patients with
laryngeal carcinoma
Firstly, we analyzed miRNA-26b expression in
patients with laryngeal carcinoma and normal volunteers. We found
that miRNA-26b expression was significantly increased in patients
with laryngeal carcinoma, compared with normal volunteers (Fig. 1).
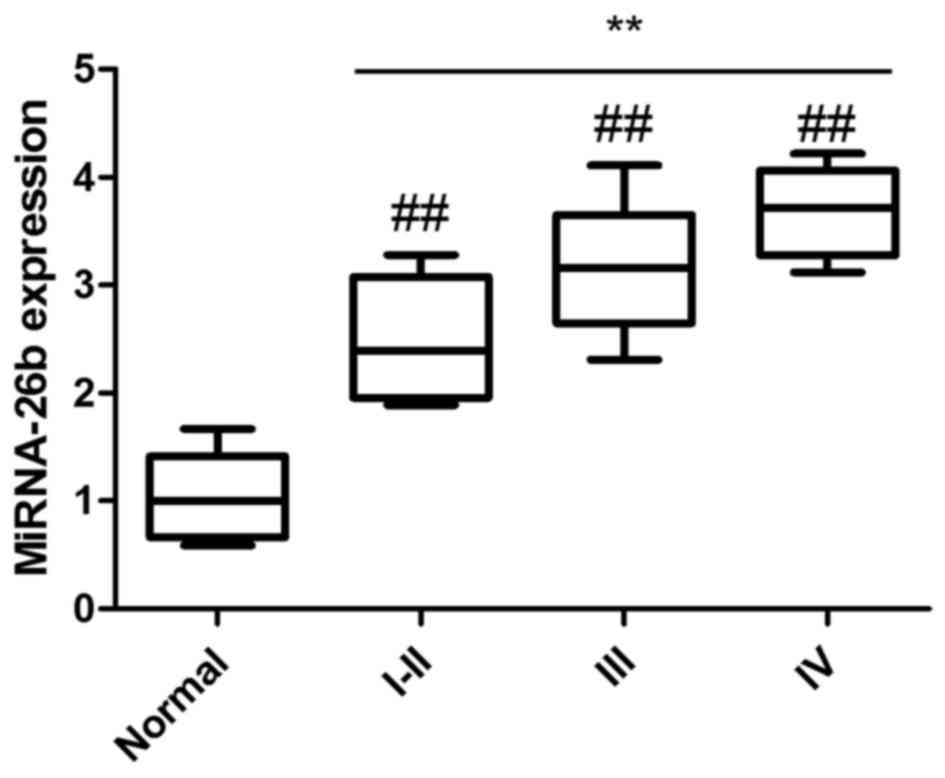
Relationship between miRNA-26b
expression and clinical TNM stage of patients with laryngeal
carcinoma
We analyzed the relationship between miRNA-26b
expression and the clinical tumor-node-metastasis (TNM) stage of
patients with laryngeal carcinoma to explore the effects of
miRNA-26b on laryngeal carcinoma. As shown in Fig. 2, the expression of miRNA-26b in
stages I–II, III or IV of patients with laryngeal carcinoma was
significantly increased compared with normal volunteers, and the
miRNA-26b expression of stage IV patients with laryngeal carcinoma
was higher than that of stage I–II patients with laryngeal
carcinoma. These results revealed that the expression of miRNA-26b
was increased in patients with laryngeal carcinoma as well as with
its progression.
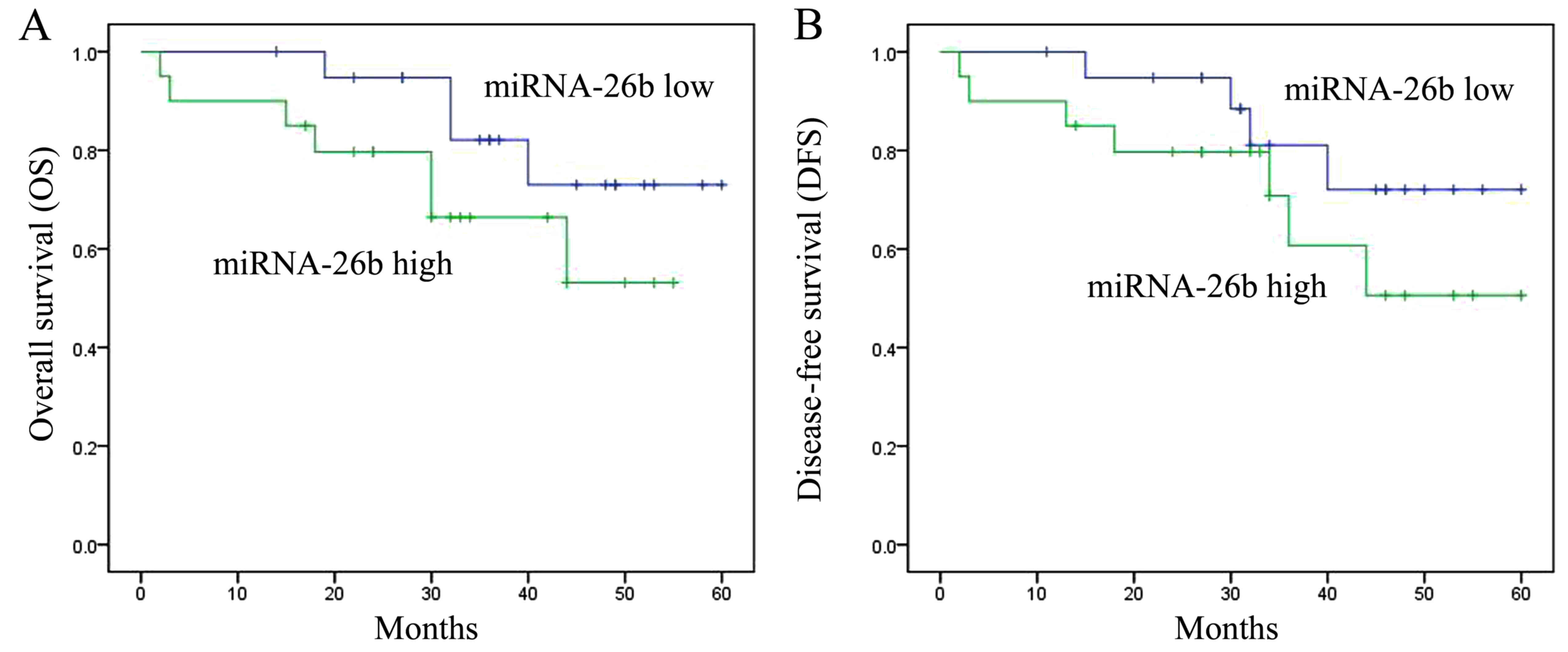
Relationship between the expression of
miRNA-26b and the survival rate of patients with laryngeal
carcinoma
Next, we also studied the relationship between the
expression of miRNA-26b and the survival rate of patients with
laryngeal carcinoma. The overall survival (OS) and disease-free
survival (DFS) of patients with laryngeal carcinoma with high
expression of miRNA-26b were lower than those of patients with low
expression of miRNA-26b (Fig.
3).
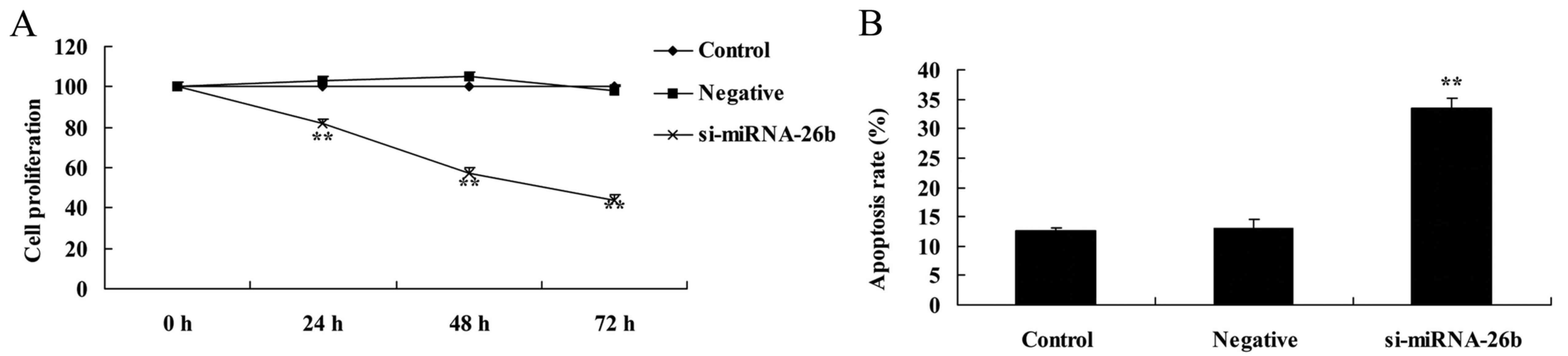
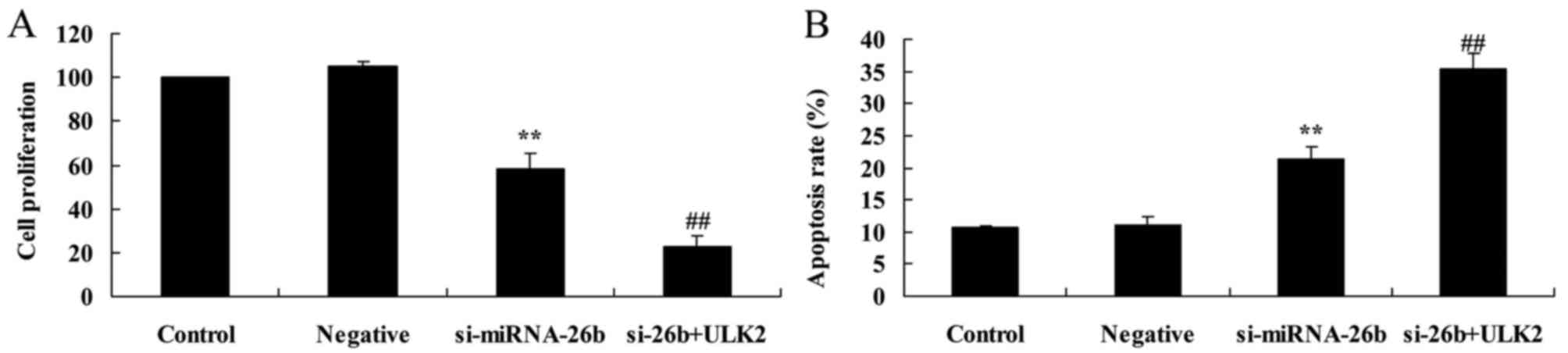
Downregulation of miRNA-26b inhibits
cell proliferation and induces apoptosis of laryngeal carcinoma
cells
The MTT assay and flow cytometric analysis with PI
staining revealed that downregulation of miRNA-26b inhibited cell
proliferation and induced apoptosis of laryngeal carcinoma cells,
compared with the negative control group (Fig. 4).
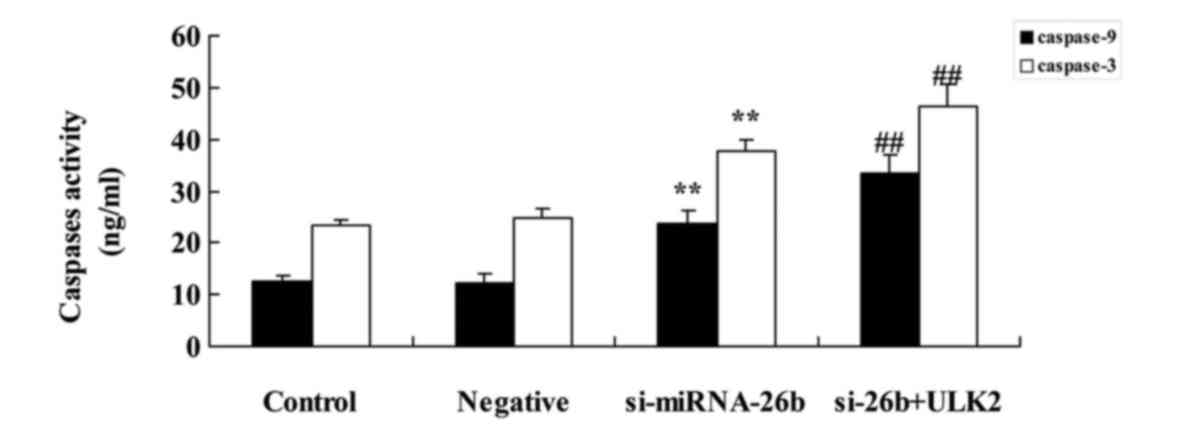
Downregulation of miRNA-26b promotes
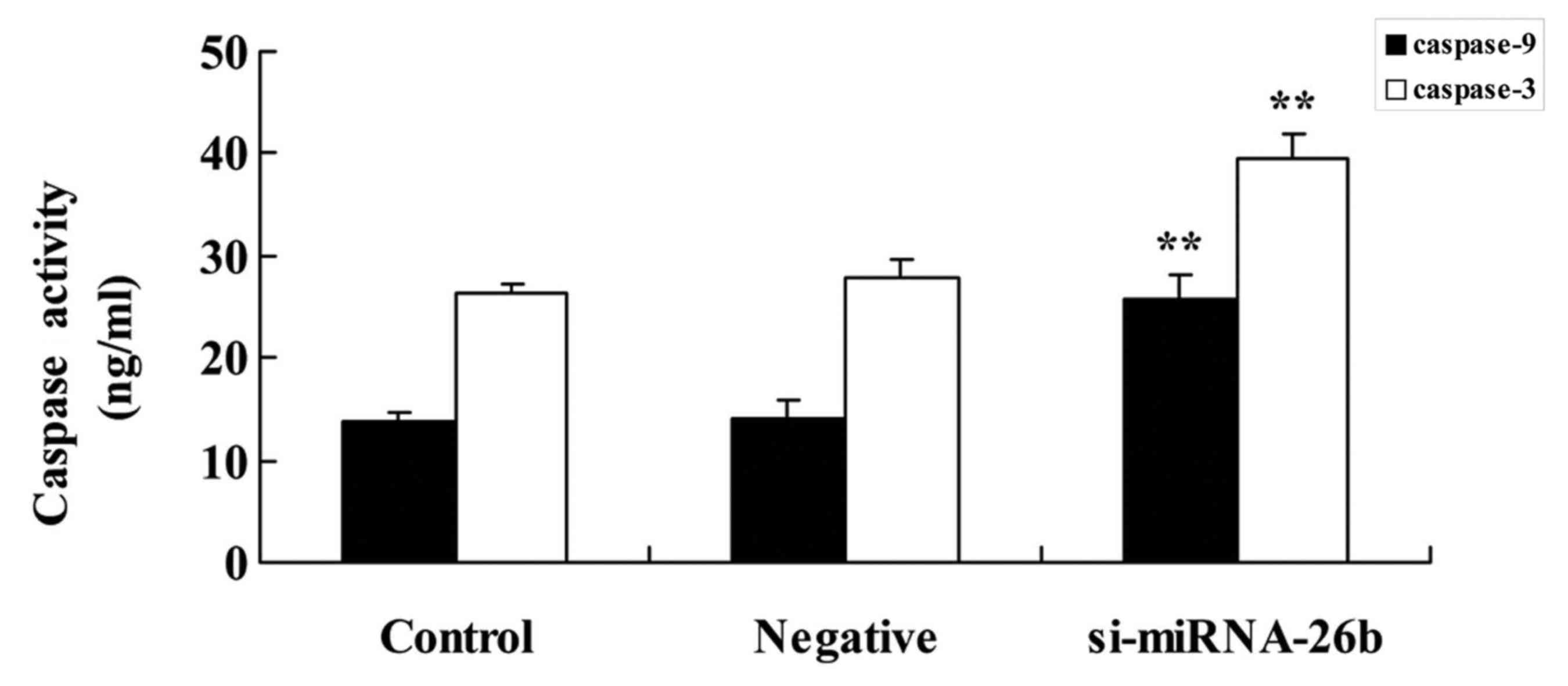
caspase-3/−9 activities of laryngeal carcinoma cells
We examined whether the expression of miRNA-26b
affects caspase-3/−9 activities of laryngeal carcinoma cells.
Downregulation of miRNA-26b significantly promoted caspase-3/−9
activities of laryngeal carcinoma cells compared with the negative
control group (Fig. 5).
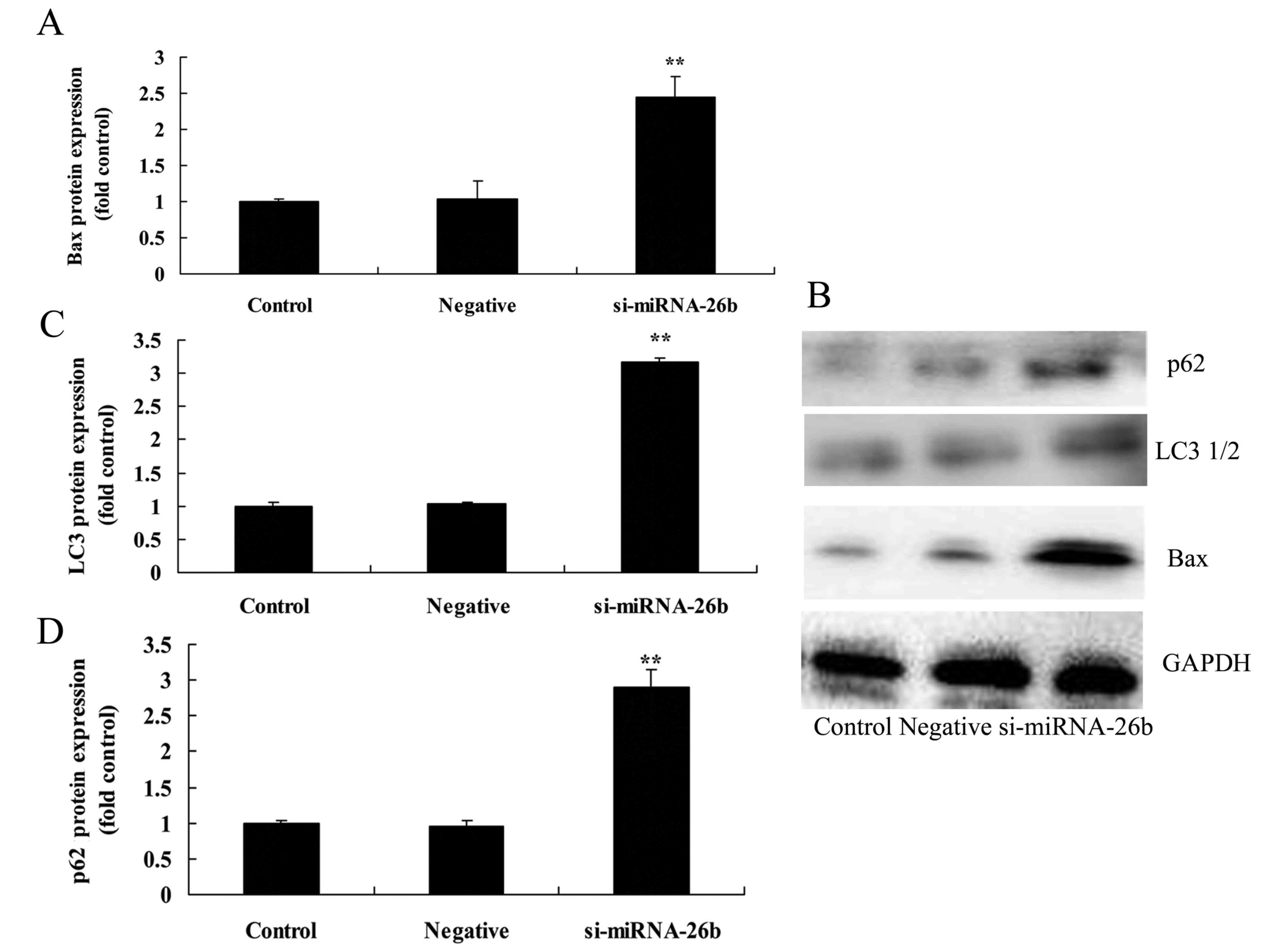
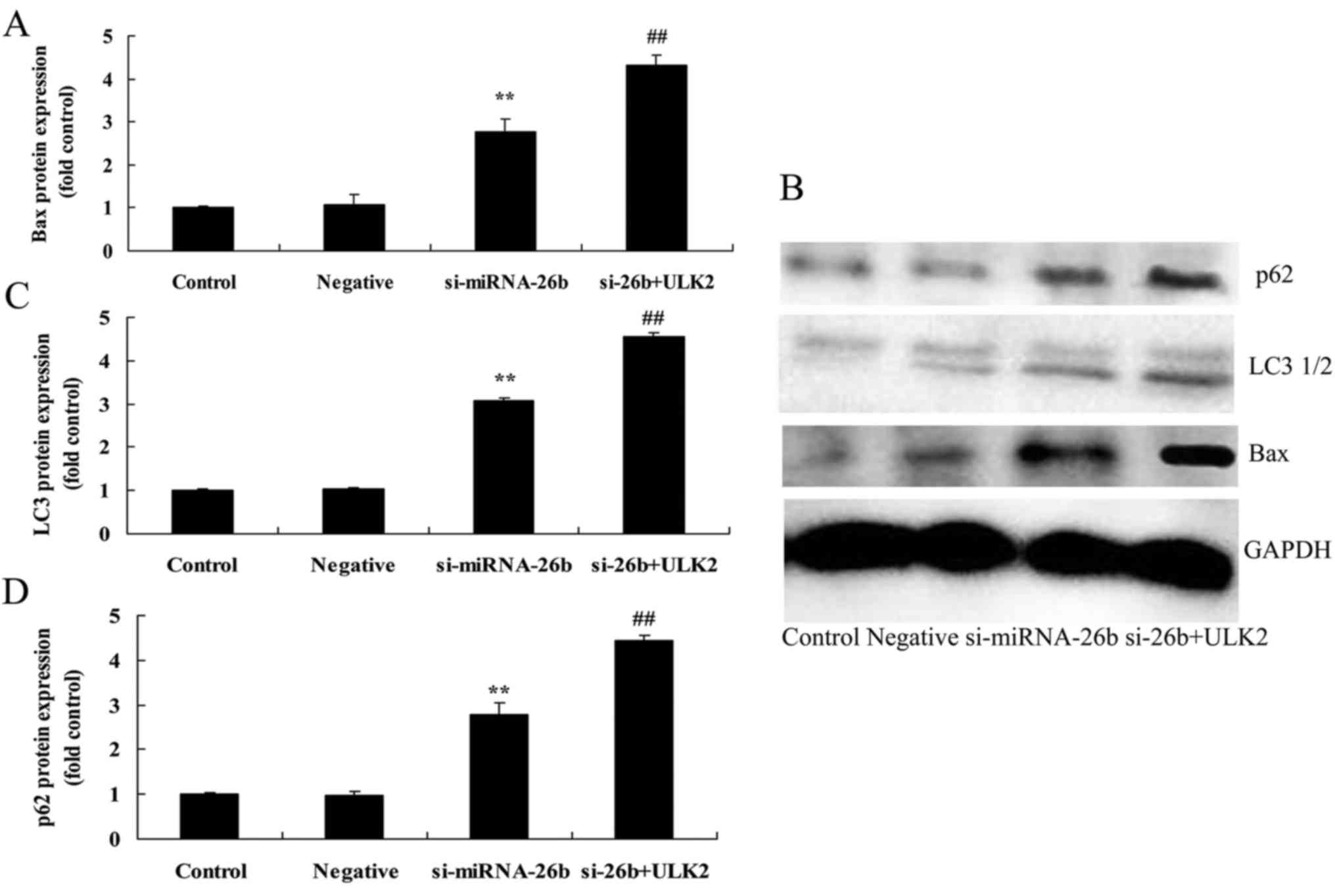
Downregulation of miRNA-26b promotes
Bax, LC3 and p62 protein expression of laryngeal carcinoma
cells
We next assessed the functional role of miRNA-26b on
autophagy of laryngeal carcinoma cells. Fig. 6 revealed that the downregulation of
miRNA-26b significantly promoted Bax, LC3 and p62 protein
expression of laryngeal carcinoma cells compared with the negative
control group.
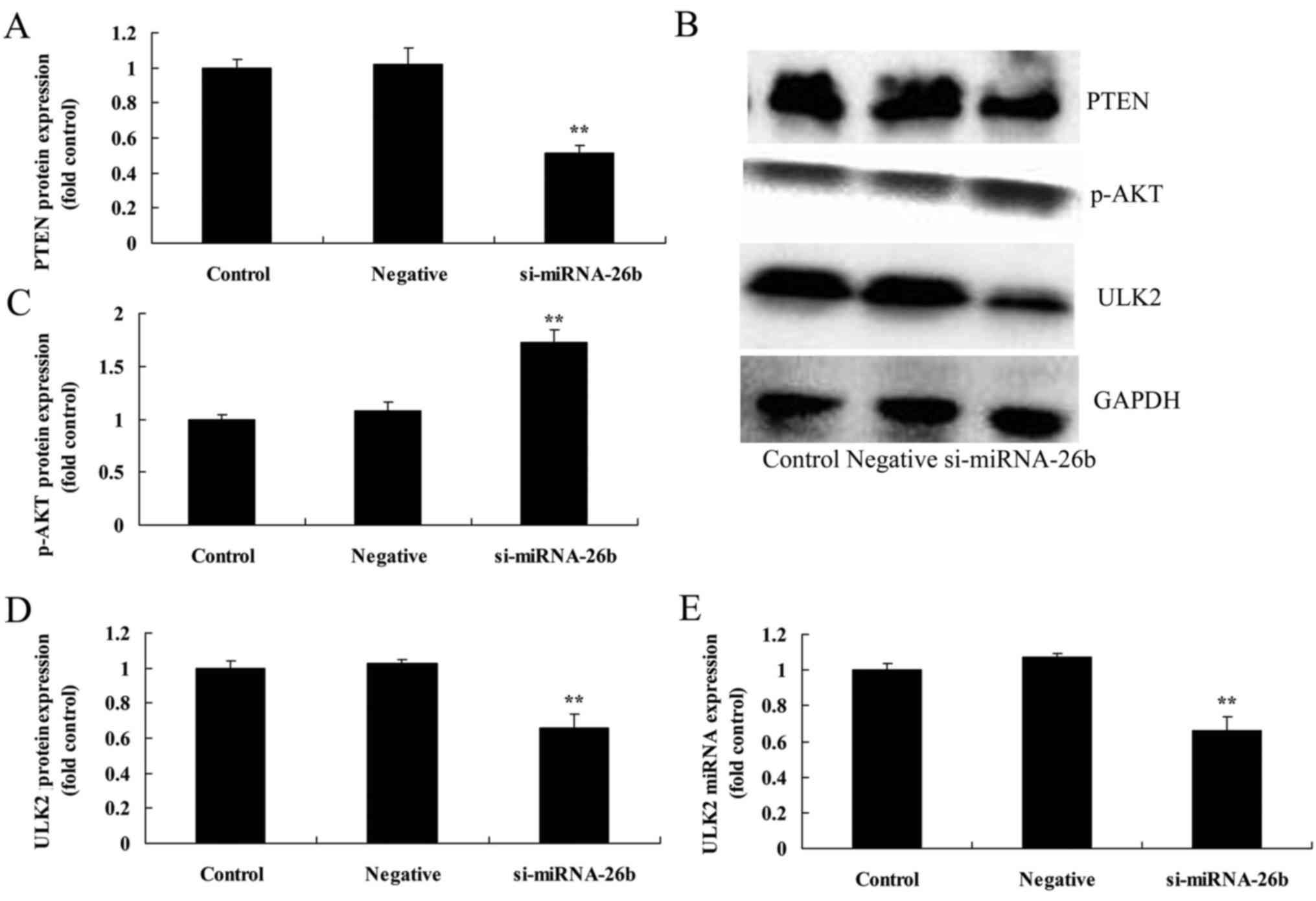
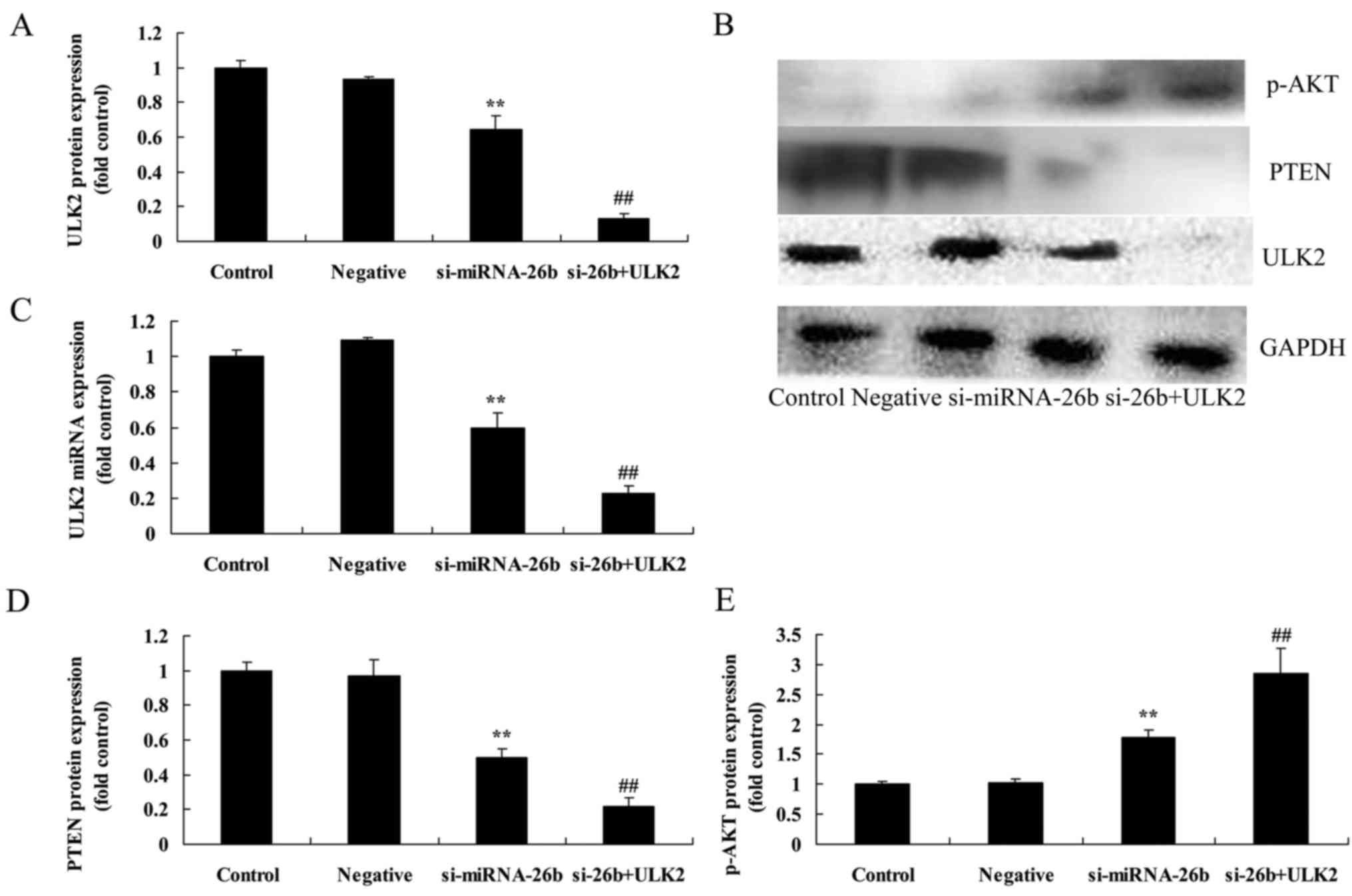
Downregulation of miRNA-26b decreases ULK2 mRNA and
protein expression as well as PTEN protein expression and increases
p-AKT protein expression of laryngeal carcinoma cells. We further
confirmed whether miRNA-26b affected ULK2 mRNA and protein
expression as well as PTEN and p-AKT protein expression of
laryngeal carcinoma cells. Fig. 7
revealed that the dowregulation of miRNA-26b decreased ULK2 mRNA
and protein expression as well as PTEN protein expression and
increased p-AKT protein expression of laryngeal carcinoma cells,
compared with the negative control group.
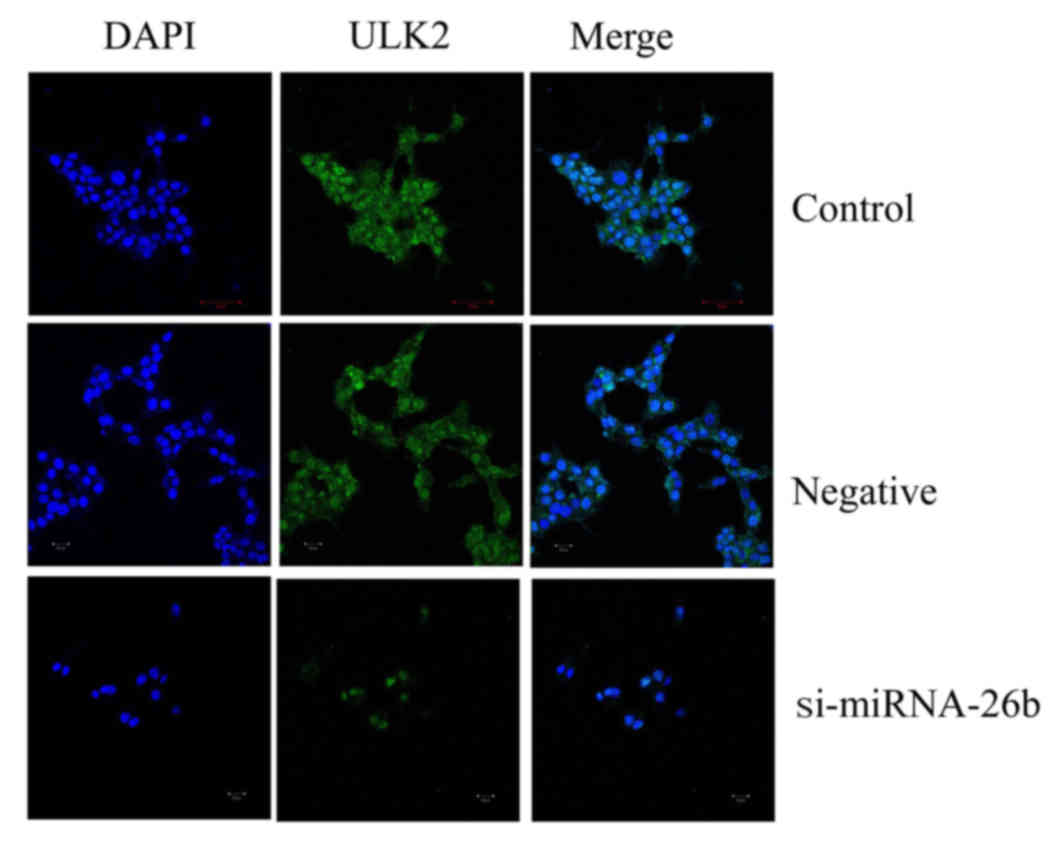
Downregulation of miRNA-26b affects
ULK2 protein expression of laryngeal carcinoma cells as determined
using immunocytofluorescence
We used immunocytofluorescence to observe ULK2
protein expression of laryngeal carcinoma cells. Fig. 8 revealed that the downregulation of
miRNA-26b promoted inhibition of ULK2 protein expression of
laryngeal carcinoma cells.
Concomitant downregulation of ULK2 and
miRNA-26b enhances the miRNA-26b-inhibited PTEN and
miRNA-26b-induced p-AKT protein expression of laryngeal carcinoma
cells
To address the potential involvement of ULK2 in the
effects of miRNA-26b on laryngeal carcinoma cells, si-ULK2 was used
to inhibit laryngeal carcinoma cells. As shown in Fig. 9, the concomitant downregulation of
ULK2 and miRNA-26b significantly enhanced the miRNA-26b-inhibited
ULK2 protein expression and mRNA expression, as well as the PTEN
protein expression and the miRNA-induced p-AKT protein expression
of laryngeal carcinoma cells, compared with the si-miRNA-26b group
alone.
Concomitant downregulation of ULK2 and
miRNA-26b enhances the miRNA-26b-inhibited cell proliferation and
the miRNA-26b-induced apoptosis of laryngeal carcinoma cells
In order to investigate the potential involvement of
ULK2 in the effects of miRNA-26b on laryngeal carcinoma cells, the
cell proliferation and induced apoptosis of laryngeal carcinoma
cells were assessed. As shown in Fig.
10, the concomitant downregulation of ULK2 and miRNA-26b
significantly enhanced the miRNA-26b-inhibited cell proliferation
and the miRNA-26b-induced apoptosis of laryngeal carcinoma cells,
compared with the si-miRNA-26b group alone.
Concomitant downregulation of ULK2 and
miRNA-26b enhances the miRNA-26b-induced caspase-3/−9 activities of
laryngeal carcinoma cells
We next investigated the caspase-3/−9 activities of
laryngeal carcinoma cells after ULK2 downregulation. As shown in
Fig. 11, the concomitant
downregulation of ULK2 and miRNA-26b significantly increased the
miRNA-26b-induced caspase-3/−9 activities of laryngeal carcinoma
cells compared with the si-miRNA-26b group alone.
Concomitant downregulation of ULK2
enhances miRNA-26b-induced autophagy, Bax, LC3 and p62 protein
expression of laryngeal carcinoma cells
In order to investigate the potential involvement of
ULK2 in the miRNA-26b-induced autophagy of laryngeal carcinoma
cells, Bax, LC3 and p62 protein expression of laryngeal carcinoma
cells were assessed. As shown in Fig.
12, the concomitant downregulation of ULK2 and miRNA-26b
significantly enhanced miRNA-26b-induced autophagy, Bax, LC3 and
p62 protein expression of laryngeal carcinoma cells compared with
the si-miRNA-26b group alone.
Discussion
Laryngocarcinoma is a type of malignancy with high
morbidity, which accounts for ~2.4% of newly-diagnosed malignancies
worldwide each year (21). With the
increasing development of the industrialization in China, the
morbidity rate exhibits a gradual increasing trend. Approximately
2–4/100,000 cases of laryngocarcinoma belong to the second most
commonly observed head and neck tumor, which appears mostly in
middle- and old-aged men (22). It
is generally considered that the genesis of laryngocarcinoma may be
related to bad habits such as smoking, air pollution and
occupational factors (22).
Research in recent years revealed that a majority of tumor patients
have improved survival. However, the survival rate of
laryngocarcinoma has gradually decreased from 57.1 to 51.9%
(23). Determining the survival of
laryngocarcinoma at present may contribute to further assessing its
trend. We found that miRNA-26b expression was significantly
increased in patients with laryngeal carcinoma compared with normal
volunteers.
Research on miRNA has provided a promising research
direction for exploring the invasion and metastasis mechanism of
laryngocarcinoma, searching for novel diagnostic markers and
treatment targets (24). The
in-depth research on the regulatory mechanism of miRNA is of great
theoretical and practical significance in order to eventually
reveal the mechanism of cell proliferation, differentiation and
carcinogenesis of laryngocarcinoma, search for new diagnostic
markers and treatment targets, and realize the effective and
individualized treatment of tumors (25). In the present study we found that
downregulation of miRNA-26b inhibited cell proliferation and
induced apoptosis of Hep-2 cells.
It was discovered through in-depth research that
autophagy cannot only inhibit the genesis of tumors, but can also
promote the genesis of tumors (10). The various roles that autophagy
plays in tumors may be related to the developmental process of a
tumor. In the early stage of carcinogenesis, the inhibition of
autophagy promotes the sustainable development of the primary
cancer cell, thus exerting a distinct antitumor effect (10,26).
When the tumor lies in the growth and proliferative stages, the
body improves the excessive growth of tumor cell-induced
malnutrition and hypoxia through upregulation of autophagy and
inhibition of the excessive proliferation of the tumor cells, and
in this case, autophagy plays a tumor-promoting role (26). These results indicate that
downregulation of miRNA-26b promoted LC3 and p62 protein expression
in Hep-2 cells. Yuan et al revealed that
HIF-2α-MALAT1-miR-216b regulates autophagy in hepatocellular
carcinoma cells (27).
The beginning of autophagy is controlled by the
Ulk1/2 kinase complex. They sense the signals from mTOR1, and when
the autophagy begins, the aforementioned PI3K and Beclinl may form
a complex, and the Ptlns3p it produces is necessary for the
recruitment of other ATG proteins that are closely related to
autophagy formation (28). Next,
the formation of the ATG5-ATG12 complex plays an important role in
the extension of the tunica media during the autophagosome
formation process (29). This
complex may recruit the cytosol-associated LC3, and transform it
into LC3II which can combine with the autophagosome (30). LC3II binds with the membrane of the
autophagosome under the action of ATG-3, and inserts itself between
the inner and outer membrane of the autophagosome (30). After the autophagosome encapsulates
the substances to be degraded, all types of ATG proteins return to
the cell. Subsequently, the autophagosome binds with the lysosome
and degrades the encapsulated substances (13). Furthermore, in the present study we
found that downregulation of miRNA-26b decreased ULK2 mRNA and
protein expression in Hep-2 cells. Clotaire et al indicated
that miRNA-26b inhibits autophagy through targeting ULK2 in
prostate cancer cells (29).
The normal expression of the PTEN gene can inhibit
the malignant transformation of the cell, and the mutation,
deletion or inactivation of the gene may induce decreased
expression of the protein, thus losing the antitumor effect, which
frequently manifests as a mutation, a deletion or inactivation in
multiple tumors (31). PTEN
dephosphorylates the PI(3,4,5)P3 in
the 3′ carboxyl locus and forms PI(4,5)P2,
antagonizes the activity of PI3K, and promotes the progressive
growth of a tumor (16,32). These studies demonstrated that
downregulation of miRNA-26b inhibited PTEN and induced p-AKT
protein expression in Hep-2 cells in vitro, suggesting that
miRNA-26b could be the target in cancer treatment via the PTEN/AKT
signaling pathway. Palumbo et al suggested that the
suppression of miRNA-26b regulates pituitary tumors through the
regulation of the PTEN/AKT pathway (33).
The PI3K/Akt pathway is an important growth factor
pathway in vivo, which can activate the anti-apoptotic
mechanism, promote glucose metabolism and protein synthesis, thus
promoting cell growth and proliferation (19). Abnormity in the signal transduction
pathway may result in the abnormally increased cell growth,
proliferation, metabolism and anti-apoptotic effects, which
participate in the genesis and development of most tumors (34). Therefore, the PI3K/Akt pathway also
named the anti-apoptotic pathway is considered as the primary
pathway for cancer cell survival. Furthermore, we found that
concomitant downregulation of ULK2 and miRNA-26 further enhanced
miRNA-26b-induced inactivation of the PTEN/AKT pathway.
Collectively, we demonstrated that miRNA-26b
regulates ULK2 and the PTEN/AKT pathway in laryngeal carcinoma
autophagy and apoptosis. Based on our findings and those from
others, miRNA-26b may play a key role in cell growth and death of
laryngeal carcinoma and thus may be a new target for gene therapy
in laryngeal carcinoma.
References
|
1
|
Miszczyk L, Maciejewski B, Tukiendorf A,
Woźniak G, Jochymek B, Gawryszuk A and Szweda M: Split-course
accelerated hyperfractionated irradiation (CHA-CHA) as a sole
treatment for advanced head and neck cancer patients-final results
of a randomized clinical trial. Br J Radiol. 87:201402122014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghai B, Jain K, Bansal D and Bhatia N:
End-tidal sevoflurane concentration for ProSeal™ versus Classic™
laryngeal mask airway insertion in unpremedicated anaesthetised
adult females. Anaesth Intensive Care. 44:221–226. 2016.PubMed/NCBI
|
|
3
|
Takácsi-Nagy Z, Hitre E, Remenár É, Oberna
F, Polgár C, Major T, Gödény M, Fodor J and Kásler M: Docetaxel,
cisplatin and 5-fluorouracil induction chemotherapy followed by
chemoradiotherapy or chemoradiotherapy alone in stage III–IV
unresectable head and neck cancer: Results of a randomized phase II
study. Strahlenther Onkol. 191:635–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janoray G, Pointreau Y, Garaud P, Chapet
S, Alfonsi M, Sire C, Jadaud E and Calais G: Long-term results of a
multicenter randomized phase III trial of induction chemotherapy
with cisplatin, 5-fluorouracil, ± docetaxel for larynx
preservation. J Natl Cancer Inst. 108:pii: djv368. 2015.PubMed/NCBI
|
|
5
|
Yu X and Li Z: The role of microRNAs
expression in laryngeal cancer. Oncotarget. 6:23297–23305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CX, Zhu Y, Duan GL, Yao JF, Li ZY,
Li D and Wang QQ: Screening for miRNAs related to laryngeal
squamous carcinoma stem cell radiation. Asian Pac J Cancer Prev.
14:4533–4537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei
WI, Ho WK and Wong TS: MicroRNA 744-3p promotes MMP-9-mediated
metastasis by simultaneously suppressing PDCD4 and PTEN in
laryngeal squamous cell carcinoma. Oncotarget. 7:58218–58233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Xiao D, Wang Z, Zou Y, Huang L,
Lin W, Deng Q, Pan H, Zhou J, Liang C, et al: MicroRNA-26a/b
regulate DNA replication licensing, tumorigenesis, and prognosis by
targeting CDC6 in lung cancer. Mol Cancer Res. 12:1535–1546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Kim K, Li X, Moreno M, Sharp T,
Goodheart MJ, Safe S, Dupuy AJ and Amendt BA: MicroRNA-26b
represses colon cancer cell proliferation by inhibiting lymphoid
enhancer factor 1 expression. Mol Cancer Ther. 13:1942–1951. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin C, Wang Z, Li L, He Y, Fan J, Liu Z,
Zhao S and Ju D: The role of autophagy in the cytotoxicity induced
by recombinant human arginase in laryngeal squamous cell carcinoma.
Appl Microbiol Biotechnol. 99:8487–8494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pereira DL, Dos Santos Ferreira AC, de
Faria GP and Kwee JK: Autophagy interplays with apoptosis and cell
cycle regulation in the growth inhibiting effect of Trisenox in
HEP-2, a laryngeal squamous cancer. Pathol Oncol Res. 21:103–111.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li R, Tan S, Yu M, Jundt MC, Zhang S and
Wu M: Annexin A2 regulates autophagy in Pseudomonas
aeruginosa infection through the Akt1-mTOR-ULK1/2 signaling
pathway. J Immunol. 195:3901–3911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miki Y, Tanji K, Mori F, Utsumi J, Sasaki
H, Kakita A, Takahashi H and Wakabayashi K: Alteration of upstream
autophagy-related proteins (ULK1, ULK2, Beclin1, VPS34 and AMBRA1)
in Lewy body disease. Brain Pathol. 26:359–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shukla S, Patric IR, Patil V, Shwetha SD,
Hegde AS, Chandramouli BA, Arivazhagan A, Santosh V and
Somasundaram K: Methylation silencing of ULK2, an autophagy gene,
is essential for astrocyte transformation and tumor growth. J Biol
Chem. 289:22306–22318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Snietura M, Jaworska M, Mlynarczyk-Liszka
J, Goraj-Zajac A, Piglowski W, Lange D, Wozniak G, Nowara E and
Suwinski R: PTEN as a prognostic and predictive marker in
postoperative radiotherapy for squamous cell cancer of the head and
neck. PLoS One. 7:e333962012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JQ, Liang Z, Wu M, Sun YM and Liu HX:
Expression of p27 and PTEN and clinical characteristics in early
laryngeal squamous cell carcinoma and their correlation with
recurrence. Int J Clin Exp Pathol. 8:5715–5720. 2015.PubMed/NCBI
|
|
17
|
Guo Q and Li Y: Research on the expression
of Survivin and PTEN in laryngeal squamous cell carcinoma
transplanted on the back sides of nude mice treated by gold throat
tablets. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
26:1134–1137, 1143. 2012.(In Chinese). PubMed/NCBI
|
|
18
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
SOX2 promotes the migration and invasion of laryngeal cancer cells
by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep.
31:2651–2659. 2014.PubMed/NCBI
|
|
19
|
García-Carracedo D, Villaronga MA,
Álvarez-Teijeiro S, Hermida-Prado F, Santamaría I, Allonca E,
Suárez-Fernández L, Gonzalez MV, Balbín M, Astudillo A, et al:
Impact of PI3K/AKT/mTOR pathway activation on the prognosis of
patients with head and neck squamous cell carcinomas. Oncotarget.
7:29780–29793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bao YY, Zhou SH, Lu ZJ, Fan J and Huang
YP: Inhibiting GLUT-1 expression and PI3K/Akt signaling using
apigenin improves the radiosensitivity of laryngeal carcinoma in
vivo. Oncol Rep. 34:1805–1814. 2015.PubMed/NCBI
|
|
21
|
Wang J, Zhao X, Pan X, Zhao L, Zhou J and
Ji M: The role of primary surgical treatment in young patients with
squamous cell carcinoma of the larynx: A 20-year review of 34
cases. World J Surg Oncol. 13:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong JT and Zhou SH: Warburg effect,
hexokinase-II, and radioresistance of laryngeal carcinoma.
Oncotarget. 8:14133–14146. 2017.PubMed/NCBI
|
|
23
|
Yoo GH, Moon J, Leblanc M, Lonardo F, Urba
S, Kim H, Hanna E, Tsue T, Valentino J, Ensley J, et al: A phase 2
trial of surgery with perioperative INGN 201 (Ad5CMV-p53) gene
therapy followed by chemoradiotherapy for advanced, resectable
squamous cell carcinoma of the oral cavity, oropharynx,
hypopharynx, and larynx: Report of the Southwest Oncology Group.
Arch Otolaryngol Head Neck Surg. 135:869–874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirisola V, Mora R, Esposito AI, Guastini
L, Tabacchiera F, Paleari L, Amaro A, Angelini G, Dellepiane M,
Pfeffer U, et al: A prognostic multigene classifier for squamous
cell carcinomas of the larynx. Cancer Lett. 307:37–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao S, Wang J, Xie J, Zhang T and Dong P:
Role of miR-138 in the regulation of larynx carcinoma cell
metastases. Tumour Biol. 2015.
|
|
26
|
Espinoza-Mellado MR, Reyes-Picaso C,
Garcés-Pérez MS, Jardón-Serrano CV, López-Villegas EO and
Giono-Cerezo S: Haemophilus influenzae triggers autophagy in
HEp-2 cells. Arch Microbiol. 198:199–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan P, Cao W, Zang Q, Li G, Guo X and Fan
J: The HIF-2α-MALAT1-miR-216b axis regulates multi-drug resistance
of hepatocellular carcinoma cells via modulating autophagy. Biochem
Biophys Res Commun. 478:1067–1073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan EY, Kir S and Tooze SA: siRNA
screening of the kinome identifies ULK1 as a multidomain modulator
of autophagy. J Biol Chem. 282:25464–25474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clotaire DZ John, Zhang B, Wei N, Gao R,
Zhao F, Wang Y, Lei M and Huang W: MiR-26b inhibits autophagy by
targeting ULK2 in prostate cancer cells. Biochem Biophys Res
Commun. 472:194–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM,
Cao J, Kundu M and Kim DH: ULK-Atg13-FIP200 complexes mediate mTOR
signaling to the autophagy machinery. Mol Biol Cell. 20:1992–2003.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Phadngam S, Castiglioni A, Ferraresi A,
Morani F, Follo C and Isidoro C: PTEN dephosphorylates AKT to
prevent the expression of GLUT1 on plasmamembrane and to limit
glucose consumption in cancer cells. Oncotarget. 7:84999–85020.
2016.PubMed/NCBI
|
|
32
|
Kan X, Sun Y, Lu J, Li M, Wang Y, Li Q,
Liu Y, Liu M and Tian L: Co-inhibition of miRNA-21 and miRNA-221
induces apoptosis by enhancing the p53-mediated expression of
pro-apoptotic miRNAs in laryngeal squamous cell carcinoma. Mol Med
Rep. 13:4315–4320. 2016.PubMed/NCBI
|
|
33
|
Palumbo T, Faucz FR, Azevedo M, Xekouki P,
Iliopoulos D and Stratakis CA: Functional screen analysis reveals
miR-26b and miR-128 as central regulators of pituitary
somatomammotrophic tumor growth through activation of the PTEN-AKT
pathway. Oncogene. 32:1651–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang XJ and Jia SS: Fisetin inhibits
laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2
signaling pathways. Biomed Pharmacother. 83:1164–1174. 2016.
View Article : Google Scholar : PubMed/NCBI
|