Introduction
Hepatocellular carcinoma (HCC) is one of the most
common human malignant tumors, with a high degree of malignancy and
rapid progression, posing a serious threat to human health
(1). The onset and progression of
HCC are complex processes involving multiple factors, levels and
genes. Uncontrollable cell proliferation caused by disorders of
cell cycle regulation is one of the important mechanisms
responsible for the occurrence of various tumors including HCC
(2). Human phosphatase and tension
homolog deleted on chromosome 10 (PTEN) is an anti-oncogene that
regulates the cell cycle mainly by inhibiting progression from the
G1 to the S phase, thus suppressing cell proliferation.
PTEN further regulates the changes of tumor cell proliferation and
the cell cycle by negatively regulating the PI3K/Akt signaling
pathway (3,4). Acetylation is another mechanism that
regulates the activity of PTEN, and inhibiting the expressions of
histone deacetylases (HDACs) upregulates that of PTEN (5). Pan et al found that
trichostatin A inhibited the expression of HDAC while upregulating
that of PTEN, indicating that histone acetylation is a crucial
mechanism in the regulation of the activity of PTEN (6).
Histone acetylation is mainly regulated by histone
acetylases (HATs) and HDACs simultaneously. The balance between
HATs and HDACs stabilizes chromatin structures and gene expression,
which, when broken, may lead to chromatin structural changes and
transcriptional imbalance of genes related to cell proliferation,
the cell cycle and apoptosis. This is a key molecular mechanism for
tumor onset and progression (7). To
date, HDACs have been found to be aberrantly expressed in various
malignancies such as HCC, gastric, pancreatic and bladder cancer
(8–10). HDAC2, as a member of the HDAC
family, can widely regulate gene transcription and silencing. Noh
et al reported that the HDAC2 gene was highly expressed in
human HCC tissues, with its level increasing upon aggravation
(11). Zhang et al found
that after targeted downregulation of HDAC2, the expression level
of PTEN was significantly upregulated, thereby inhibiting tumor
cell proliferation (12).
As a lipid-soluble vitamin closely associated with
human health, vitamin D functions physiologically through its in
vivo metabolite 1,25(OH)2D3. However, by
regulating in vivo calcium and phosphorus metabolisms,
1,25(OH)2D3 and its analogues can also
inhibit tumor cell proliferation, promote differentiation, induce
apoptosis and suppress tumor invasion and metastasis (13,14).
Toropainen et al reported that
1,25(OH)2D3 downregulated MYC gene
expression, which was significantly decreased after interference
with HDAC2 (15). We previously
found that 1,25(OH)2D3 upregulated the
expression of PTEN and inhibited the proliferation of HCC cells
(16). Therefore, we postulated
that 1,25(OH)2D3 inhibited the proliferation
of HCC cells and arrested the cell cycle in the
G0/G1 phase by downregulating HDAC2 and
regulating the PTEN/PI3K/Akt signaling pathway. Thereby motivated,
we evaluated the effects of aberrant HDAC2 expression on
1,25(OH)2D3-inhibited HepG2 cell
proliferation, and explored the possible mechanism.
Materials and methods
Cell line and main reagents
Human HCC HepG2 cell line was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). PCR
primers were designed and synthesized by Invitrogen (Shanghai,
China). Lentiviruses for HDAC2 interference and overexpression were
packaged by Shanghai GeneChem Co., Ltd. (Shanghai, China).
1,25(OH)2D3 was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Thiazolyl blue (MTT) was purchased from
Amresco LLC (Solon, OH, USA). The cell cycle detection kit was
obtained from BD Biosciences (San Jose, CA, USA). Rabbit anti-human
PTEN, PI3K and p-PI3K monoclonal antibodies were purchased from
Abcam (Cambridge, MA, USA). Rabbit anti-human Akt and p-Akt
monoclonal antibodies were purchased from Cell Signaling Technology
Inc. (CST; Danvers, MA, USA). Rabbit anti-human β-actin polyclonal
antibody was obtained from Bio-World (Dublin, OH, USA). Horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody was
purchased from Beijing Bioss Antibodies Co., Ltd. (Beijing, China).
BCA protein quantification kit was purchased from Shanghai Generay
Biotech Co., Ltd. (Shanghai, China). Pre-stained protein Rainbow
marker was obtained from Beijing Solarbio Life Sciences Co., Ltd.
(Beijing, China). Total RNA extraction, SYBR® Premix Ex
Taq™ and PrimeScript® RT reagent kits were obtained by
Takara (Shiga, Japan).
Cell culture
Human HCC HepG2 cells were cultured in high-glucose
Dulbeccos modified Eagles medium (DMEM) containing 5% fetal bovine
serum and 100 U/ml penicillin-streptomycin, and incubated in an
incubator at 37°C with 5% CO2.
1,25(OH)2D3 was dissolved in 100% ethanol and
stored at −80°C.
Cell proliferation assay
HepG2 cells in the logarithmic growth phase were
collected and inoculated into 96-well plates at the density of
5×104 cells/ml. After 24 h of adherent growth, the cells
were starved in serum-free DMEM for 24 h and
1,25(OH)2D3 was added at final concentrations
of 10, 100 and 1,000 nM. Meanwhile, the control and zero wells were
set, and five replicate wells were set up for each group. Then,
they were cultured in the incubator at 37°C with 5% CO2
for 24, 48 and 72 h. After 10 µl of MTT solution was added into
each well, they were further incubated for 4 h. Finally, the
culture medium was carefully pipetted, and 50 µl of dimethyl
sulfoxide (DMSO) was added into each well. The plates were then
shaken on a shaking table at low speed for 10 min to completely
dissolve the formed crystals. The optical density (OD) of each well
was measured at 490 nm by a microplate reader. The cell
proliferation rate (%) was calculated as follows: =
(ODexperimental -
ODblank)/(ODcontrol -
ODblank).
Cell cycle assay
HepG2 cells were seed into 6-well plates at the
density of 2×105 cells/ml. After 72 h of exposure at 100
nM of 1,25(OH)2D3, the cells were collected
by centrifugation after trypsin digestion, washed twice with
phosphate-buffered saline (PBS), fixed in 4°C 70% ethanol for 30
min, and centrifuged to discard the supernatant. The cells were
then washed twice with PBS, gently mixed with 150 µl of RNase and
propidium iodide (PI) in the dark, and then left still at room
temperature for 30 min and subjected to cell cycle detection using
a flow cytometer.
Real-time PCR
Total RNA was extracted using a Total RNA extraction
kit (Takara) according to the instructions. Then, the purity and
concentration of RNA were detected by a UV spectrometer. According
to the instructions of PrimeScript® RT reagent kit and
SYBR® Premix Ex Taq™ kit, reverse transcription and
target gene amplification were performed. The conditions for PCR
amplificationwere: 95°C for 30 sec, pre-denaturation for 30 sec, 1
cycle; 95°C for 5 sec, 60°C for 30 sec, 40 cycles. The relative
mRNA expression level was expressed as 2−ΔΔCt. All
experiments were performed in triplicate and repeated at least
three times. The primer sequences for HDAC2 and p21 and PTEN and
Akt were as follows: HDAC2 forward, 5′-ATAAAGCCACTGCCGAAGAA-3′ and
reverse, 5′-TCCTCCAGCCCAATTAACAG-3′; p21 forward,
5-CATGGGTTCTGACGGACAT-3 and reverse, 5-AGTCAGTTCCTTGTGGAGCC-3; PTEN
forward, 5′-GCTAGCCTCTGGATTTGACG-3′, and reverse,
5′-ACCAGGACCAGAGGAAACCT-3′; Akt forward, 5′-TGAAGGTGCCATCATTCTTG-3′
and reverse, 5′-ATGAGCGACGTGGCTATTGT-3′.
Western blotting
Total protein concentration was detected using the
BCA method. Protein (20 µg) was subjected to SDS-PAGE, and the gel
was then transferred to a nitrocellulose membrane that was blocked
in Tris-buffered saline and Tween-20 (TBST) containing 5% skimmed
milk for 2 h. Then incubation with primary antibodies against HDAC2
(1:1,000) and β-actin (1:2,000) overnight at 4°C followed and
subsequently with secondary antibodies for 2 h. Finally the
membranes were reacted with enhanced chemiluminescent (ECL) reagent
in dark for 1–3 min, and then exposed by X-ray film, developed and
scanned. The grey values of the target protein bands were analyzed
by a UVP gel imaging system.
HDAC2 RNA interference (RNAi) and
overexpression analysis
The targeted HDAC2 sequences were,
5′-GCTGGAGCTGTGAAGTTAAAC-3′ (forward) and
5′-GTTTAACTTCACAGCTCCAGC-3′ (reverse). HepG2 cells were transfected
with packaged lentiviruses for interference and overexpression, and
divided into an HDAC2 interference group, an HDAC2 overexpression
group, a blank control and a negative control group. The cells were
collected 48 h after transfection, and HDAC2 mRNA and protein
expression were detected by real-time PCR and western blotting,
respectively. HDAC2 RNAi or overexpression in combination with
1,25(OH)2D3 treatment were used to determine
cell proliferation, the cell cycle and related protein expression.
The HDAC2 interference and HDAC2 overexpression groups were
collected 48 h after transfection, inoculated into 96- or 6-well
plates, and treated with 100 nM 1,25(OH)2D3
for 72 h after adherent growth. Then, the cells were divided into a
blank control, a negative control, an HDAC2 interference, an HDAC2
overexpression and a 1,25(OH)2D3 group, and a
1,25(OH)2D3 in combination with HDAC2
overexpression group. The proliferation activity of each group was
detected using MTT assay, and the cell cycle was detected by flow
cytometry. PTEN, PI3K and Akt mRNA and protein expression were
detected by real-time PCR and western blotting, respectively.
Statistical analysis
All data were analyzed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA), and expressed as the mean ± standard deviation
(mean ± SD). Inter-group mean comparisons were performed by one-way
analysis of variance (ANOVA). P<0.05 was considered
statistically significant.
Results
Effects of
1,25(OH)2D3 on HepG2 cell proliferation and
the cell cycle
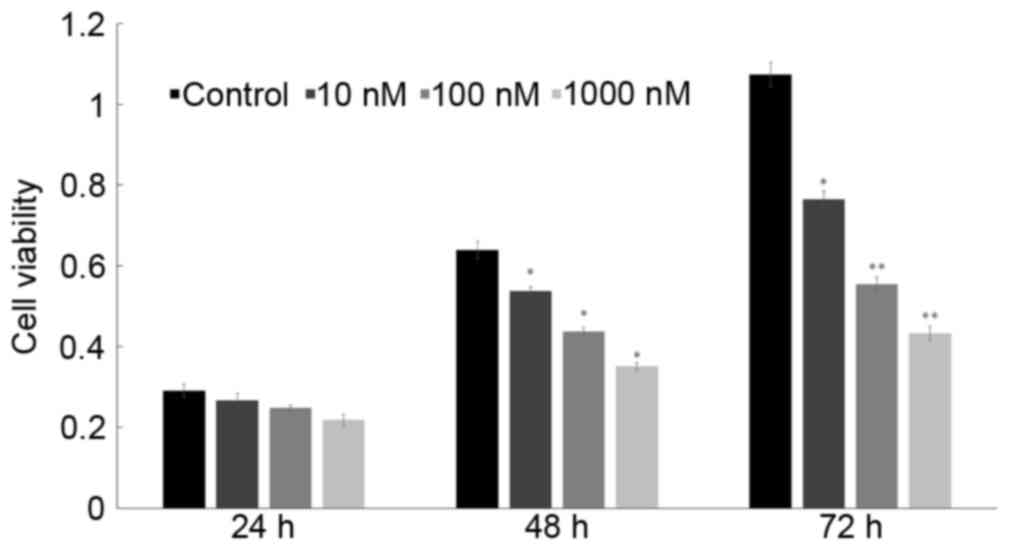
An MTT assay (Fig.
1) revealed that after treatment with 10, 100 and 1,000 nM of
1,25(OH)2D3, the 24-h inhibition rates of
HepG2 cells were 8.6, 14.8 and 25.1%, respectively, the 48-h ones
were 15.9, 31.5 and 45.1%, respectively, and the 72-h ones were
28.8, 48.3 and 59.7%, respectively. Therefore,
1,25(OH)2D3 markedly inhibited the
proliferation activity of HepG2 cells in a dose-dependent manner.
Since 72 h at 100 nM of 1,25(OH)2D3 treatment
significantly inhibited the proliferation, the dose and time were
selected thereafter.
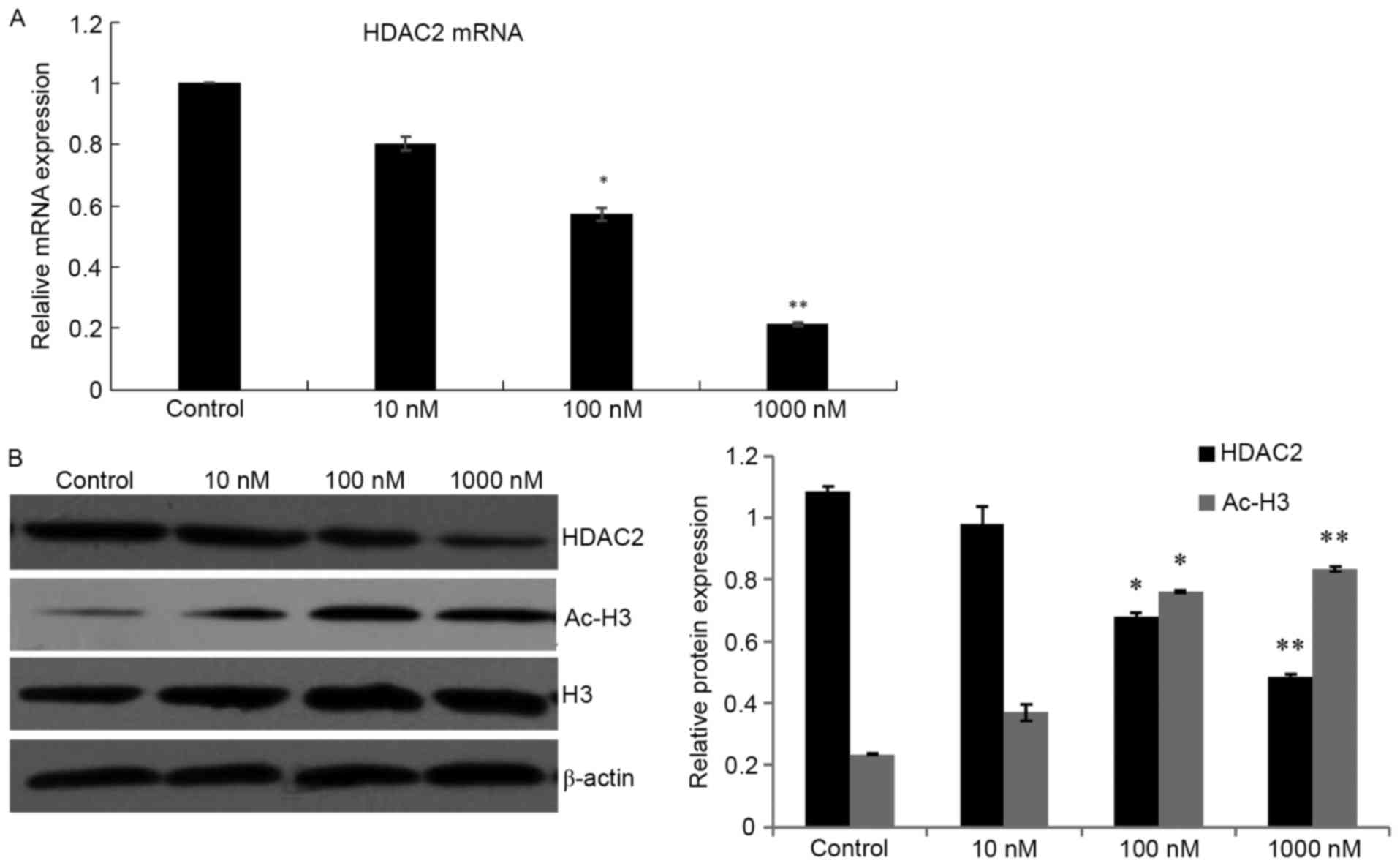
1,25(OH)2D3
downregulates HDAC2 expression in HepG2 cells
To investigate the inhibitory effect of
1,25(OH)2D3 in HDAC-dependant mechanisms,
HepG2 cells were treated with different concentrations of
1,25(OH)2D3. The results revealed that HDAC2
mRNA and protein expression levels in the HepG2 cells were
dose-dependently and significantly downregulated compared with
those in the control group (P<0.05). Therefore, to further
confirm the inhibition of HDAC by
1,25(OH)2D3, the acetylation status of
histone protein H3 was detected. The expression of the ac-H3
protein was significantly increased (P<0.05), however that of
the total H3 protein remained unchanged (Fig. 2).
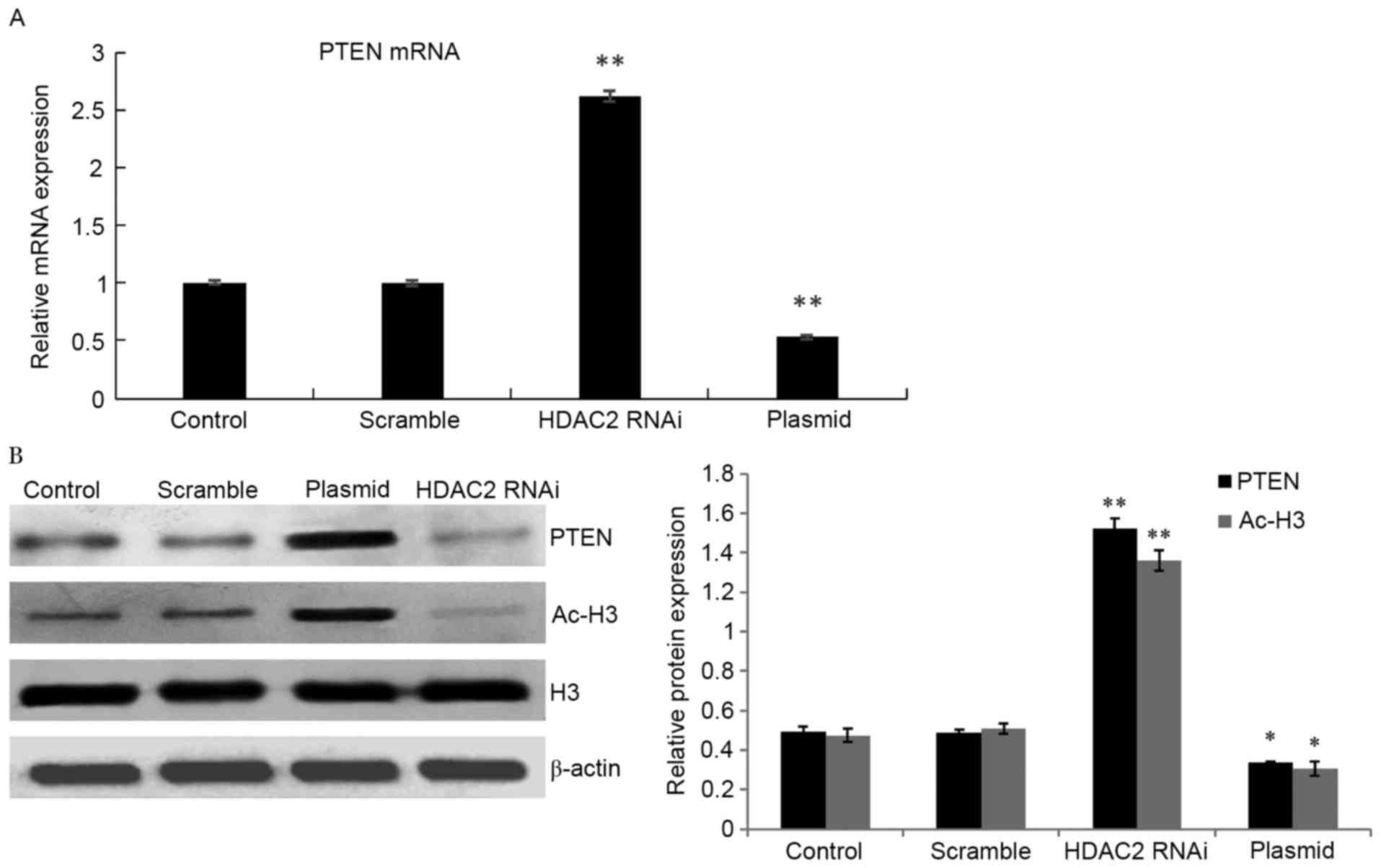
Expression of PTEN is regulated by
HDAC2 in HepG2 cells
Real-time PCR and western blotting revealed that
compared with the negative control group, PTEN gene expression in
the HDAC2-interference group was significantly upregulated. While
PTEN expression was obviously decreased in the HepG2 cells
transfected with the pEGFP-LV2-HDAC2 plasmid in comparison to the
low-level expression of endogenous PTEN in HepG2 cells. The
expression of ac-H3 was substantially increased when HDAC2 was
blocked (Fig. 3).
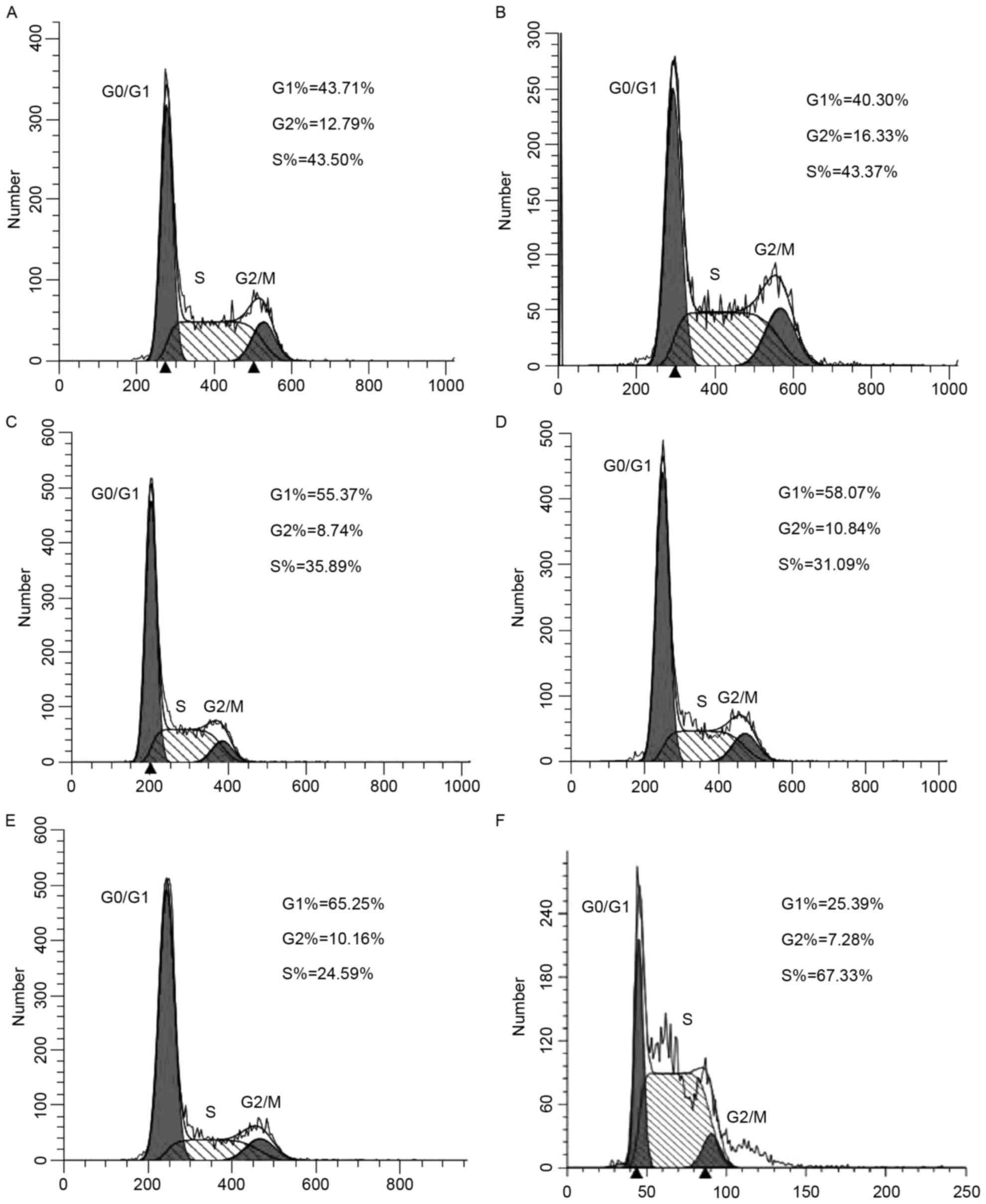
Effects of HDAC2 interference and
overexpression in the HepG2 cell cycle
After transfection with interference and
overexpression plasmids and 72 h of
1,25(OH)2D3 (100 nM) treatment, significantly
more HepG2 cells in the HDAC2 gene interference group were arrested
in the G0/G1 phase, but fewer cells were in
the S phase than those in the negative control group (P<0.05)
(Fig. 4). The HDAC2 gene
overexpression group had exactly the opposite results (P<0.05).
Compared with the 1,25(OH)2D3 group, the
1,25(OH)2D3-treated HDAC2 gene overexpression
group had significantly fewer cells in the
G0/G1 phase, but significantly more cells in
the S phase (P<0.05). The blank control and negative control
groups had similar results (P>0.05).
1,25(OH)2D3
promotes HDAC-mediated PTEN activation through downregulation of
the Akt signaling pathway
To determine whether
1,25(OH)2D3-inhibited cell proliferation is
closely related to an Akt signal, we examined the mRNA and protein
levels of Akt in cells following 1,25(OH)2D3
treatment for 72 h. Real-time PCR (Fig.
5A) revealed that compared with the negative control group, the
1,25(OH)2D3 and HDAC2-interference groups had
significantly higher PTEN mRNA expression levels but significantly
lower PI3K and Akt mRNA expression levels (P<0.05). However,
compared with the 1,25(OH)2D3 group, the PTEN
mRNA expression of the 1,25(OH)2D3-treated
HDAC2 gene overexpression group was significantly decreased,
whereas the PI3K and Akt mRNA expression levels were significantly
increased (P<0.05). Western blotting (Fig. 5B) revealed that the
1,25(OH)2D3 group did not affect the total
Akt protein levels. However, Akt phosphorylation decreased in
comparison to the control after treatment with
1,25(OH)2D3 for 72 h. The PTEN expression in
the HDAC2-knockdown group was upregulated more significantly than
that in the scrambled-shRNA group. The expression of phosphorylated
Akt and PI3K was markedly decreased in the HDAC2-knockdown group
compared with the cells transfected with scrambled shRNA. To
further analyze the interactions between
1,25(OH)2D3 and HDAC2 in regulating the
activation of the PI3K/Akt signaling pathway, we overexpressed
HDAC2 in HepG2 cells and treated them
1,25(OH)2D3. The phosphorylation levels of
PI3K and Akt were significantly increased, but that of PTEN was
markedly decreased, with unchanged total PI3K and Akt protein
expression. Nevertheless, the phosphorylation levels of PI3K and
Akt, which decreased after 1,25(OH)2D3
treatment compared with the cells treated with the pEGFP-LV2-HDAC2
plasmid, still exceeded those of control and vector groups
(Fig. 5B). Therefore, the PTEN gene
may undergo deacetylation which enhances PI3K/Akt activation.
Meanwhile, 1,25(OH)2D3 inhibited the
activation of Akt and downregulated the expression of
phosphorylated Akt.
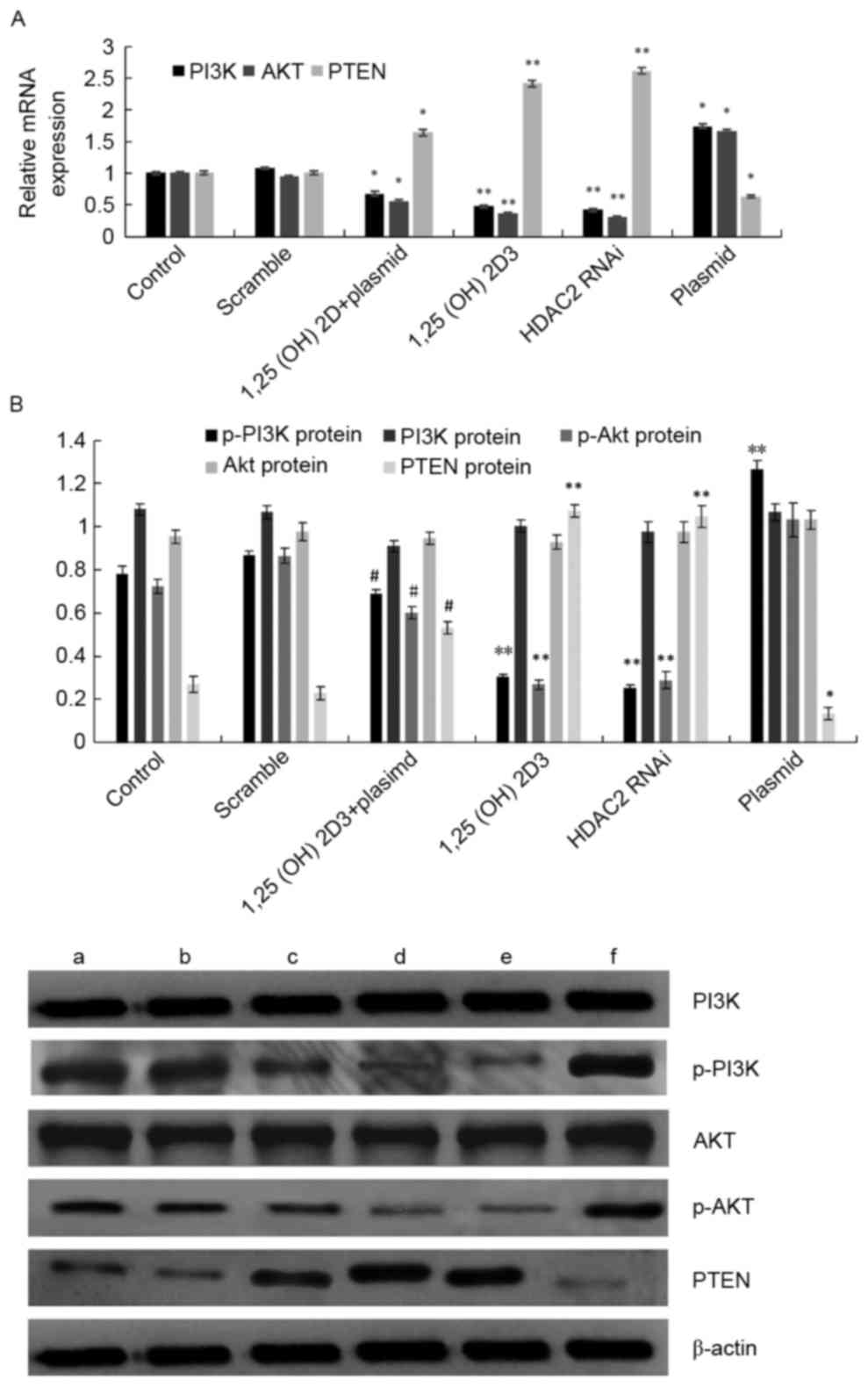 | Figure 5.Effects of downregulation of the
HDAC2 gene by 1,25(OH)2D3 on PTEN, PI3K and
Akt expression in HepG2 cells. HepG2 cells were treated with
pEGFP-LV2-HDAC2 plasmid, pEGFP-LV2-HDAC2 plasmid and
1,25(OH)2D3, 100 nM
1,25(OH)2D3, HDAC2 RNAi and PEGFP-LV2-HDAC2
plasmid for 48 h. (A) PI3K, Akt and PTEN mRNA expression levels
were analyzed by real-time PCR. (B) Phosphorylation of Akt and PI3k
were detected by western blotting. a, Control; b, scramble; c,
1,25(OH)2D3 + plasmid; d, 100 nM
1,25(OH)2D3; e, HDAC2 RNAi; f, plasmid;
**P<0.01 vs. the control and vector groups;
#P<0.05 vs. pEGFP-LV2-HDAC2 plasmid group. |
Discussion
1,25(OH)2D3 inhibits the
proliferation of many types of cells, induces differentiation,
promotes apoptosis and regulates various tumor and immune cells
(17). In the present study,
1,25(OH)2D3 effectively inhibited the
proliferation activity of HCC cells in dose- and time-dependent
manners, i.e. the inhibitory effects became more apparent with
increasing drug concentration and treatment time. Possibly,
1,25(OH)2D3 activated signal transduction
molecules such as protein kinase C, mitogen-activated protein
kinase, phospholipase A, protein kinase A and PI3K in
VDR-independent manners. As a result, intracellular Ca2+
was rapidly changed, and proteins such as Bcl-2 and c-jun were
activated or deactivated, ultimately affecting cell proliferation,
differentiation and apoptosis (18,19).
As one of the important physiological functions of
cells, proliferation, which is regulated by the cell cycle,
proceeds by division. There are two key stages in the cell cycle:
G1 to S and G2 to M. Regulating the two
stages is thus of great significance to the in-depth understanding
of cell development and growth as well as the control of tumor
growth (20). In the present study,
the effects of 1,25(OH)2D3 on the cell cycle
of HepG2 cells were evaluated by flow cytometry. After being
treated with 1,25(OH)2D3, the cells were
arrested in the G0/G1 phase, accompanied by
fewer cells in the S phase. Thus, 1,25(OH)2D3
affected the cell cycle progression of HCC cells, which may partly
contribute to the resistance to proliferation.
HDAC2 is a member of the HDACs protein family.
Highly expressed in most malignant tumors, it can influence the
onset and progression of tumors by regulating genes related to
proliferation, cell cycle and apoptosis as well as transcription of
oncogenes and anti-oncogenes, as is therefore a popular target for
anticancer drug design (21). In
the present study, the effects of the HDAC2 gene interference and
overexpression on cell proliferation were assessed by MTT assay.
Compared with the control group, the proliferation ability of HepG2
cells was significantly decreased after HDAC2 gene interference.
After overexpression of HDAC2 gene, the proliferation of HepG2
cells was significantly enhanced, being consistent with the results
of Lee et al (22). Thus,
HDAC2 played a vital role in regulating the proliferation of HCC
cells. In addition, 1,25(OH)2D3 herein
downregulated the expression of HDAC2, whereas it enhanced the
acetylation level of histone H3, thus we postulated that
1,25(OH)2D3 inhibited the proliferation of
HepG2 cells and induced their apoptosis possibly by downregulating
HDAC2 gene expression. To confirm this hypothesis, HepG2 cells
overexpressing the HDAC2 gene were treated with
1,25(OH)2D3, and the resulting proliferation
was detected by MTT assay. Compared with the
1,25(OH)2D3-treated normal HepG2 cells, HDAC2
overexpression significantly weakened the inhibitory effects of
1,25(OH)2D3.
PTEN, is one of the crucial antitumor genes in the
post-p53 era. Upregulating PTEN can block the cell cycle and induce
apoptosis. Furthermore, PTEN can also inhibit cell proliferation
and induce apoptosis by negatively regulating the cell growth
signaling pathway PI3K/Akt (4).
Acetylation is another mechanism involved in the regulation of PTEN
activity, and inhibiting the expression of HDACs can upregulate
that of PTEN (23). In the present
study, after targeted interference of the HDAC2 gene, the
expression levels of both PTEN mRNA and protein were significantly
upregulated while those of p-PI3K and p-Akt were downregulated,
accompanied by a significantly increased acetylation level of
histone H3. Hence, downregulating HDAC2 suppressed the
proliferation of HCC cells by effectively inhibiting HDACs,
boosting histone acetylation, upregulating the expression of PTEN
and inhibiting activation of the downstream Akt signaling pathway,
as reported by Zhang et al (12). In the present study,
1,25(OH)2D3 increased the PTEN level via the
PI3K/Akt signaling pathway, probably being linked to the
downregulation of HDAC2. As suggest by our findings, the expression
of HDAC2 was negatively correlated with that of PTEN.
In conclusion, we have demonstrated that
1,25(OH)2D3 may have inhibitory effects in
HepG2 cell cycle progression by HDAC2-mediated PTEN upregulation
and inhibition of the PI3K/Akt signaling pathways. The present
study may provide an attractive therapeutic modality for liver
cancer.
Acknowledgements
This study was financially supported by the Science
and Technology Project of Health and Family Planning Commission of
Huizhou Province no. gzwjkj2016-1-013) and the Doctoral Scientific
Research Foundation of Guizhou Medical University.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
4
|
Jang HD, Noh JY, Shin JH, Lin JJ and Lee
SY: PTEN regulation by the Akt/GSK-3β axis during RANKL signaling.
Bone. 55:126–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang G, Jiang X, Pu H, Zhang W, An C, Hu
X, Liou AK, Leak RK, Gao Y and Chen J: Scriptaid, a novel histone
deacetylase inhibitor, protects against traumatic brain injury via
modulation of PTEN and AKT pathway: Scriptaid protects against TBI
via AKT. Neurotherapeutics. 10:124–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan L, Lu J, Wang X, Han L, Zhang Y, Han S
and Huang B: Histone deacetylase inhibitor trichostatin A
potentiates doxorubicin-induced apoptosis by up-regulating PTEN
expression. Cancer. 109:1676–1688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peserico A and Simone C: Physical and
functional HAT/HDAC interplay regulates protein acetylation
balance. J Biomed Biotechnol. 2011:3718322011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noh JH, Jung KH, Kim JK, Eun JW, Bae HJ,
Xie HJ, Chang YG, Kim MG, Park WS, Lee JY, et al: Aberrant
regulation of HDAC2 mediates proliferation of hepatocellular
carcinoma cells by deregulating expression of G1/S cell cycle
proteins. PLoS One. 6:e281032011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poyet C, Jentsch B, Hermanns T,
Schweckendiek D, Seifert HH, Schmidtpeter M, Sulser T, Moch H, Wild
PJ and Kristiansen G: Expression of histone deacetylases 1, 2 and 3
in urothelial bladder cancer. BMC Clin Pathol. 14:102014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giaginis C, Damaskos C, Koutsounas I,
Zizi-Serbetzoglou A, Tsoukalas N, Patsouris E, Kouraklis G and
Theocharis S: Histone deacetylase (HDAC)-1, −2, −4 and −6
expression in human pancreatic adenocarcinoma: Associations with
clinicopathological parameters, tumor proliferative capacity and
patients' survival. BMC Gastroenterol. 15:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh JH, Bae HJ, Eun JW, Shen Q, Park SJ,
Kim HS, Nam B, Shin WC, Lee EK, Lee K, et al: HDAC2 provides a
critical support to malignant progression of hepatocellular
carcinoma through feedback control of mTORC1 and AKT. Cancer Res.
74:1728–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Zhao B, Huang C, Meng XM, Bian EB
and Li J: Melittin restores PTEN expression by down-regulating
HDAC2 in human hepatocelluar carcinoma HepG2 cells. PLoS One.
9:e955202014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krishnan AV and Feldman D: Mechanisms of
the anti-cancer and anti-inflammatory actions of vitamin D. Annu
Rev Pharmacol Toxicol. 51:311–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feldman D, Krishnan AV, Swami S,
Giovannucci E and Feldman BJ: The role of vitamin D in reducing
cancer risk and progression. Nat Rev Cancer. 14:342–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toropainen S, Väisänen S, Heikkinen S and
Carlberg C: The down-regulation of the human MYC gene by the
nuclear hormone 1α,25-dihydroxyvitamin D3 is associated
with cycling of corepressors and histone deacetylases. J Mol Biol.
400:284–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang J, Yang G, Huang Y, Kong W and Zhang
S: 1,25(OH)2D3 inhibits the progression of
hepatocellular carcinoma via downregulating HDAC2 and upregulating
P21(WAFI/CIP1). Mol Med Rep. 13:1373–1380. 2016.PubMed/NCBI
|
|
17
|
Nibbelink KA, Tishkoff DX, Hershey SD,
Rahman A and Simpson RU: 1,25(OH)2-vitamin D3
actions on cell proliferation, size, gene expression, and receptor
localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol
Biol. 103:533–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jozilan HN, Horvath P, Kosa JP, Lakatos P,
Nemeth D, Wölfling J, Kovacs D, Bodnar B, Matyus P, Horvath E, et
al: P0321: Increased anti-tumor effect of vitamin D after CYP24A1
inhibition on HCC cell lines. J Hepatol. 62:(Suppl 2). S4292015.
View Article : Google Scholar
|
|
19
|
Fingas CD, Altinbas A, Schlattjan M,
Beilfuss A, Sowa JP, Sydor S, Bechmann LP, Ertle J, Akkiz H, Herzer
K, et al: Expression of apoptosis- and vitamin D pathway-related
genes in hepatocellular carcinoma. Digestion. 87:176–181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruijtenberg S and van den Heuvel S:
Coordinating cell proliferation and differentiation: Antagonism
between cell cycle regulators and cell type-specific gene
expression. Cell Cycle. 15:196–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JK, Noh JH, Eun JW, Jung KH, Bae HJ,
Shen Q, Kim MG, Chang YG, Kim SJ, Park WS, et al: Targeted
inactivation of HDAC2 restores p16INK4a activity and
exerts antitumor effects on human gastric cancer. Mol Cancer Res.
11:62–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee YH, Seo D, Choi KJ, Andersen JB, Won
MA, Kitade M, Gómez-Quiroz LE, Judge AD, Marquardt JU, Raggi C, et
al: Antitumor effects in hepatocarcinoma of isoform-selective
inhibition of HDAC2. Cancer Res. 74:4752–4761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang WJ, Lin CW, Lee CY, Chi LL, Chao YC,
Wang HN, Chiou BL, Chen TJ, Huang CY and Chen CN: NBM-HD-3, a novel
histone deacetylase inhibitor with anticancer activity through
modulation of PTEN and AKT in brain cancer cells. J Ethnopharmacol.
136:156–167. 2011. View Article : Google Scholar : PubMed/NCBI
|