Introduction
Recently, was reported that mucin expression and its
glycosylation patterns are altered abnormally in inflammatory,
premalignant and malignant conditions (1–6).
Mucins are known as glycoproteins with O- and
N-oligosaccharides and contain multiple tandem repeats of
10–80 amino acid residues (5).
There are two categories of mucins; one type is membrane-bound, and
the other is secreted/gel forming. It is well known that MUC1 and
MUC4 are the most characterized transmembrane mucins which are
significant in cellular physiology. The structure and biochemical
composition of these mucins helps them to offer lubrication and
hydration to cell surfaces as well as provide protection from
pathogens and degradative enzymes (7). Not only the expression of MUC1 and
MUC4, but also the glycosylation pattern is subject to vary and it
has been demonstrated in studies of several malignant epithelial
tumors, such as pancreatic, lung, colon, breast and prostate cancer
(3,5,8,9).
Studies on MUC1 and MUC4 revealed that they play
pivotal roles in cellular signaling, tumor immune surveillance,
tumor growth, metastasis, tumor-stromal cell interactions and
chemotherapy resistance (3,7,8,10).
There are a few reagents which have high specificity (e.g.
monoclonal antibodies) which can even recognize modified glycoforms
available, suggesting that mucins can be useful targets enabling
the detection of malignant epithelial tumors at an early stage
(11,12). Studies in malignant ovarian
neoplasms provide casual evidence that the expression as well as
the localization pattern of MUC1 are modified during their
progression, nevertheless, in the case of MUC4, more information is
required (13). In light of the
fact that MUC1 and MUC4 participate in the lubrication of cell
surfaces and provide protection, it is critical to observe their
functions as well as the variation of their expression while
malignant ovarian tumors develop and progress.
Her2 is a receptor tyrosine kinase that is a member
of the transmembrane epidermal growth factor type II receptor
family and is also known as erbB-2/CD340. Her2 overexpression has
been recognized as a stable molecular abnormality, driven in
several of the most common solid tumors including prostate,
cervical, ovarian, breast, lung, endometrial and colon cancer
(14–17). It has also been proposed that there
is a significant connection between Her2 overexpression and a poor
prognosis in lymph node-positive/negative patients with breast
cancer. Furthermore, it can be a strong marker for the therapy and
diagnosis of other solid tumors, e.g. multiple gynecological
cancers (18). Recently, MUC4 was
revealed to be involved in the development of ovarian cancer
through the stabilization and activation of Her2 (19,20).
Thus, it may be worth studying the detailed signaling pathways
related to the MUC4/Her2 pathway that could be targeted as novel
therapeutic options to treat ovarian cancer.
Previously, we identified auranofin, a rheumatoid
arthritis therapeutic agent approved by the Food and Drug
Administration (FDA), as a FOXO3 activator and revealed that
auranofin induces apoptosis in SKOV3 cells via the regulation of
the IKKβ/FOXO3 pathway (21). In
the present study, we demonstrated that auranofin regulates Her2
protein expression and that the anticancer activity of auranofin in
SKOV3 cells could be enhanced by the attenuation of MUC4 through
the regulation of the Her2/Akt/FOXO3 pathway. The present study may
be helpful in the selection of a potential antitumor agent
considering the expression of MUC4 in ovarian cancer patients.
Materials and methods
Cell lines
SKOV3, OVACAR5, MDA-MB-231 and MDA-MB-361 cells
[from the American Tissue Culture Collection (ATCC) Manassas, VA,
USA] were maintained in Dulbeccos modified Eagles medium (DMEM)
supplemented with 10% fetal bovine serum, and 1%
streptomycin/penicillin at 37°C in a humidified incubator
containing 5% CO2 in air.
Antibodies and chemical reagents
Mouse anti-β-actin antibody, dimethyl sulfoxide
(DMSO), glycerol, glycine, sodium chloride, Trizma base and
Tween-20 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Mouse anti-PARP1, rabbit anti-FOXO3, mouse anti-LaminA/C antibodies
and auranofin were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Rabbit anti-Her3, rabbit anti-Her2, rabbit
anti-phospho-FOXO3, rabbit anti-phospho-Akt, rabbit anti-Akt,
rabbit anti-caspase-3, rabbit anti-Bax, rabbit anti-Bim and rabbit
anti-Bcl2 antibodies were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). The rabbit anti-MUC4 antibody was
purchased from Abcam (Cambridge, MA). Goat anti-mouse and goat
anti-rabbit horseradish peroxidase-conjugated IgG were obtained
from Jackson ImmunoResearch (West Grove, PA, USA). ECL Western
Blotting Detection Reagents were obtained from GenDEPOT (Barker,
TX, USA).
WST-1 cell viability assay
A 200 µl aliquot of SKOV3 cells (1×103
cells in media) was added to each well of a 96-well plate and
incubated for 18 h at 37°C in a humidified incubator containing 5%
CO2 in air. After incubation, control or MUC4-siRNA
(Santa Cruz Biotechnology) was transfected followed by the addition
of auranofin (0 or 25 nM) into each well for 48 h. After
incubation, a 20 µl WST-1 solution was added to each well and the
incubation continued for 2 h. The visible absorbance at 560 nm of
each well was quantified using a microplate reader.
Cell counting assay
SKOV3 cells (1×104) were seeded in 6-cm
dishes and incubated at 37°C in a humidified incubator containing
5% CO2 in air for 18 h. After incubation, control or
MUC4-siRNA was transfected followed by the addition of auranofin (0
or 25 nM) into each dish for 0, 24, 48 and 72 h. Τhe number of
cells were assessed daily using a hemocytometer.
Colony formation assay
SKOV3 cells (5×102) were seeded in 6-cm
dishes and incubated at 37°C in a humidified incubator containing
5% CO2 in air for 18 h. After incubation, control or
MUC4-siRNA was transfected followed by the addition of auranofin (0
or 25 nM) into each dish for 7 days. Subsequently, the colonies
were washed twice with phosphate-buffered saline (PBS), fixed with
3.7% paraformaldehyde, and stained with 1% crystal violet solution
in distilled water.
Western blot analysis
Cells were washed with PBS and lysed in lysis buffer
(50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS,
pH 8.0) with protease and phosphatase inhibitors. Cell lysates were
centrifuged (10,000 × g, 4°C, 10 min) and the supernatants were
separated on 6 or 10% SDS-PAGE and blotted onto nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes
were blocked in 3% non-fat dry milk for 1 h at room temperature,
and probed with appropriate antibodies. The membranes were then
probed with HRP-tagged anti-mouse or anti-rabbit IgG antibodies
diluted 1:5,000–1:15,000 in 3% non-fat dry milk for 1 h at room
temperature. Chemiluminescence was detected using enhanced ECL.
Cytoplasmic and nuclear protein
fractionation
Cells from each condition were trypsinized,
centrifuged, washed, re-suspended in a cytoplasmic fractional
buffer (10 mM HEPES, pH 8.0, 50 mM NaCl, 500 mM sucrose, 1 mM EDTA,
0.5 mM spermidine, 0.15 mM spermine, 0.2% Triton X-100, 1 mM DTT, 2
µM phenylmethylsulfonyl fluoride (PMSF) and 0.15 U/ml aprotinin,
and incubated at 4°C for 30 min on a rotator. After centrifuging
the cell suspension at 10,000 rpm for 30 min at 4°C, the
supernatant was collected for cytoplasmic fractioning. The pellet
was washed with washing buffer (10 mM HEPES pH 8.0, 50 mM NaCl, 25%
glycerol, 0.1 mM EDTA, 0.5 mM spermidine and 0.15 mM spermine)
twice. The remaining pellet was re-suspended with a nuclear
fractional buffer (10 mM HEPES pH 8, 350 mM NaCl, 25% glycerol, 0.1
mM EDTA, 0.5 mM spermidine and 0.15 mM spermine) and incubated at
4°C for 30 min on a rotator. After centrifuging the cell suspension
at 13,000 rpm for 30 min at 4°C, the supernatant was collected for
nuclear fractioning. Protein in each fraction was quantified using
the Bradford protein determination reagent (Bio-Rad Laboratories)
and BSA as a standard.
TUNEL assay
SKOV3 cells (1×104) were seeded in 6-cm
dishes and incubated at 37°C in a humidified incubator containing
5% CO2 in air for 18 h. After incubation, control or
MUC4-siRNA was transfected followed by the addition of auranofin (0
or 25 nM) into each dish for 2 h. Then, the cells were fixed with
4% paraformaldehyde solution and permeabilized with Triton X-100
(0.2%). For the TUNEL assay, cellular apoptosis was determined by
enzymatic labeling of DNA strand breaks with a TUNEL assay kit (the
DeadEnd Fluorometric TUNEL System; Promega, Madison, WI, USA)
according to the manufacturer's instructions. Nuclei were stained
with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI).
Annexin V staining analysis
The percentage of cells that underwent apoptosis was
determined using the FITC Annexin V apoptosis detection kit I (BD
Pharmingen, San Diego, CA, USA) with propiodium iodide (PI)
according to the manufacturer's instructions. Briefly, SKOV3 cells
(1×104) were seeded in 6-cm dishes and incubated at 37°C
in a humidified incubator containing 5% CO2 in air for
18 h. After incubation, control or MUC4-siRNA was transfected
followed by the addition of auranofin (0 or 25 nM) into each dish
for 2 h. Subsequently, the cells were washed in PBS, trypsinized
and resuspended in binding buffer. Then, the cells were aliquoted
into 5 ml culture tubes (1×105 cells/tube), and
incubated in binding buffer containing 5 µl of FITC Annexin V, and
5 µl of PI for 15 min at 25°C in the dark. The cells were then
analyzed using FACSCalibur (BD Biosciences, Franklin Lakes, NJ,
USA) and the data were analyzed by FlowJo (De Novo Software,
Glendale, CA, USA). Ten thousand events were collected in each
run.
Statistical analysis
The results are expressed as arithmetic mean ± SEM
(the standard error of the mean). To compare the statistical
meaning between the groups, two-sided unpaired Student's t-tests
were used. All experiments were repeated 3 times and the
representative data are shown. Statistical analyses were performed
using SPSS software (version 19.0; SPSS, Inc., Chicago, IL, USA).
Mean differences with P-values <0.05 were considered to be
statistically significant.
Results
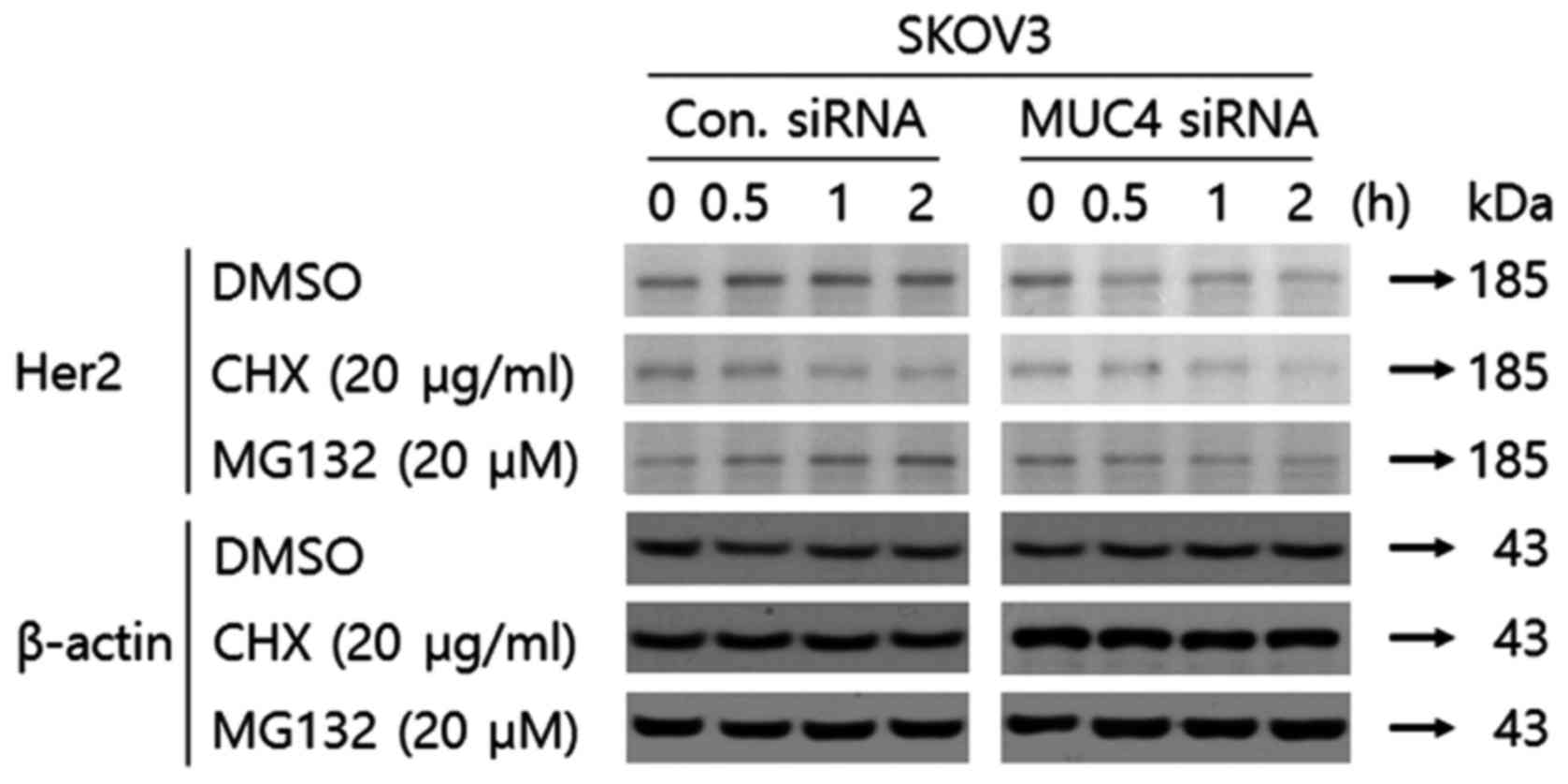
Transfection of MUC4-siRNA into SKOV3
cells decreases Her2 stability via a proteosomal pathway
According to Moorthy et al, Her2 protein
stability can be maintained by MUC4 in ovarian cancer cells
(19). We examined Her2 stability
in SKOV3 cells transfected with control-siRNA or siRNA against MUC4
with cycloheximide, an inhibitor of protein translation. As shown
in Fig. 1, the Her2 protein had
decreased stability since it disappeared earlier when transfected
with siRNA against MUC4 and cycloheximide as compared to the
control-siRNA and cycloheximide. To determine whether Her2
degradation upon attenuation of MUC4 by siRNA transfection was
proteosome-mediated, we adopted MG-132, a type of proteasome
inhibitor. While the expression level of Her2 was decreased at 0.5
h in SKOV3 cells transfected with MUC4-siRNA, the expression level
of Her2 was not decreased at over 4 h in SKOV3 cells transfected
with control-siRNA and MG-132 (Fig.
1) suggesting that degradation of Her2 by the transfection of
MUC4-siRNA is proteasome-dependent.
Transfection of MUC4-siRNA or
auranofin treatment into SKOV3 cells downregulates Her2 leading to
decreased phospho-Akt
Since Akt is a well-known downstream target of Her2,
we investigated whether the expression level of phospho-Akt was
regulated by transfection of MUC4-siRNA in SKOV3 cells by carrying
out western blot analysis. As shown in Fig. 2A, MUC4-siRNA transfection in SKOV3
cells decreased the expression level of phospho-Akt, but did not
affect the expression level of total Akt. Recently, we reported
that auranofin has anticancer activity via activation of FOXO3 in
SKOV3 cells. In a previous study, we demonstrated that auranofin
inhibited IKKβ leading to the promotion of FOXO3 translocation from
the cytoplasm into the nucleus. Wenhui et al reported that
IKKβ could be regulated by the PI3K/Akt/GSK3β pathway in colonic
smooth muscle (22). Thus, we
addressed whether auranofin treatment in SKOV3 cells affected MUC4,
phospho-Akt and Akt expression levels. As shown in Fig. 2B, auranofin treatment in SKOV3 cells
decreased the level of phospho-Akt, but did not alter the
expression levels of MUC4 or Akt. Notably, transfection of
MUC4-siRNA or auranofin treatment did not downregulate the protein
expression level of Her3. These results demonstrated that both
MUC4-siRNA transfection and auranofin treatment in SKOV3 cells not
only regulated Her2 specifically but downstream signaling targets
such as phospho-Akt as well.
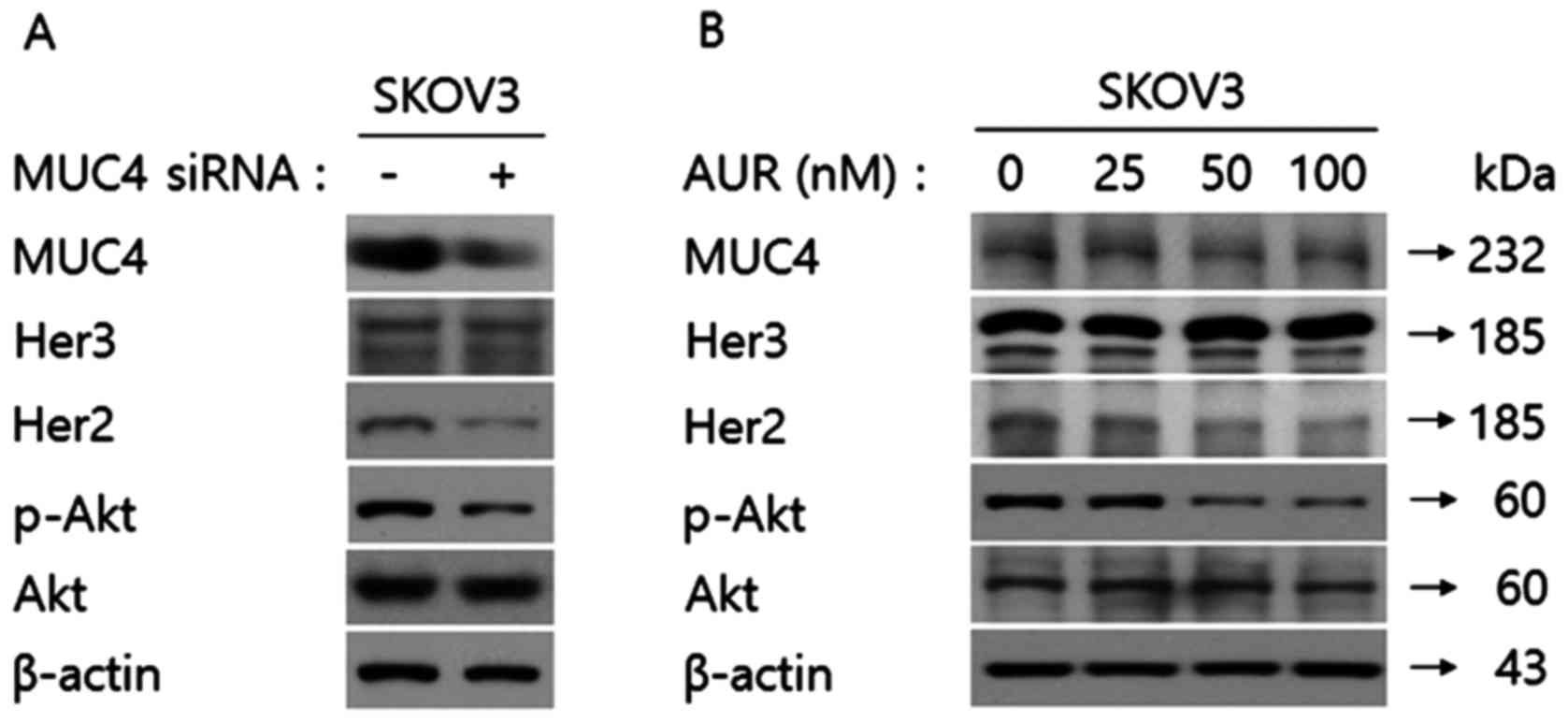 | Figure 2.Her2 is identified as the common
molecular target protein by siRNA transfection against MUC4 and
auranofin treatment in SKOV3 cells. (A) Transfection of MUC4-siRNA
into SKOV3 cells downregulates the expression level of Her2 leading
to a decreased phospho-Akt expression level. SKOV3 cells
(1×105) were seeded in 6-cm dishes and incubated at 37°C
in a humidified incubator containing 5% CO2 in air for
18 h. After incubation, the control or MUC4-siRNA was transfected.
The cell lysates were immune-blotted with anti-MUC4, anti-Her3,
anti-Her2, anti-phospho-Akt and anti-Akt antibodies. β-actin was
used as the loading control. (B) Auranofin treatment in SKOV3 cells
downregulates the expression level of Her2 leading to a decreased
phospho-Akt expression level. SKOV3 cells (1×105) were
seeded in 6-cm dishes and incubated at 37°C in a humidified
incubator containing 5% CO2 in air for 18 h. After
incubation, the cells were treated with auranofin (0, 25, 50 or 100
nM). The cell lysates were immune-blotted with anti-MUC4,
anti-Her3, anti-Her2, anti-phospho-Akt, and anti-Akt antibodies.
β-actin was used as the loading control. |
Attenuation of MUC4 combined with
auranofin treatment in SKOV3 cells synergistically activates FOXO3
translocation from the cytoplasm to the nucleus through the
regulation of the Her2/Akt/FOXO3 pathway
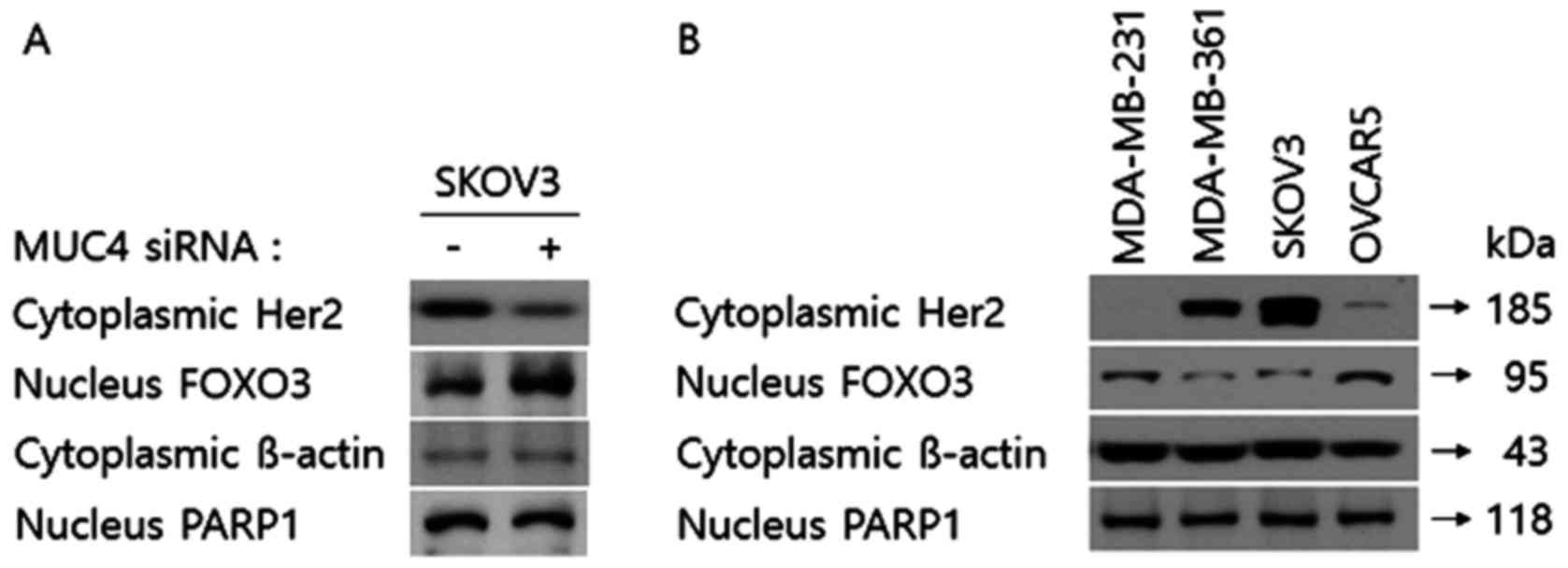
FOXO3 is a tumor-suppressive transcriptional factor
and known to be inactivated through translocation from the nucleus
into the cytoplasm. To date, 3 representative kinases, Akt, Erk and
IKKβ have been identified to induce this translocation of FOXO3 by
phosphorylation (23). We examined
the expression level of FOXO3 in both the cytoplasm and the nucleus
of SKOV3 cells transfected with the control or MUC4-siRNA. As shown
in Fig. 3A, the expression level of
Her2 of the cytoplasmic fraction in SKOV3 cells transfected with
MUC4-siRNA was downregulated compared to the control-siRNA.
However, under the same conditions, the expression level of FOXO3
of the nuclear fraction (the active FOXO3) was upregulated. Then,
in order to confirm the expression patterns of cytoplasmic Her2 and
nuclear FOXO3 expression levels, we examined the cytoplasmic Her2
expression and the nuclear FOXO3 in the other cell lines with Her2
high (MDA-MB-361 and SKOV3) and low expression (MDA-MB-231 and
OVCAR5) levels. As shown in Fig.
3B, in MDA-MB-361 and SKOV3 cells the cytoplasmic the
expression level of Her2 was markedly increased while in MDA-MB-231
and OVCAR5 cells the nuclear FOXO3 expression was upregulated,
which was consistent with the results of Fig. 3A. Thus, it appears that Her2 may be
the common molecular target protein by siRNA transfection against
MUC4 and auranofin treatment in SKOV3 cells that activates FOXO3.
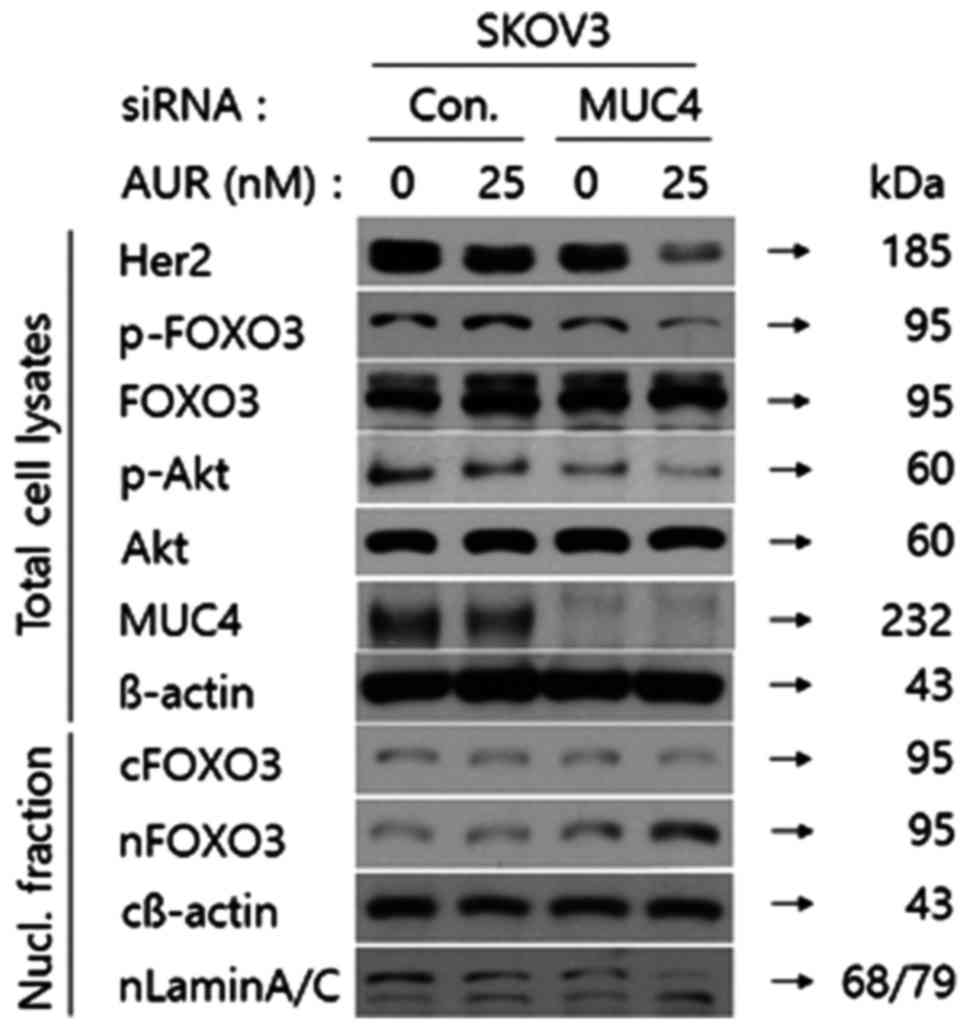
Therefore, we next assessed whether combination of MUC4 by siRNA
transfection with auranofin treatment in SKOV3 cells
synergistically promoted FOXO3 translocation from the cytoplasm to
the nucleus. As shown in Fig. 4,
Her2 and phospho-Akt expression levels were significantly decreased
in total cell lysates from SKOV3 cells transfected with MUC4-siRNA
followed by auranofin treatment. Furthermore, the phospho-FOXO3 by
Akt was downregulated under the same conditions compared to the
other conditions, too. However, we could not detect any difference
in the expression level of FOXO3 under any conditions. Thus, we
adopted nuclear fractional western blot analysis to assess the
expression level of FOXO3 in SKOV3 cells. As shown in Fig. 4. the expression level of FOXO3 was
decreased in the cytoplasmic fraction from SKOV3 cells transfected
with MUC4-siRNA followed by auranofin treatment but increased in
the nuclear fraction, which implied that FOXO3 was translocated
from the cytoplasm into the nucleus through the combination of MUC4
by siRNA transfection and auranofin treatment. These results
revealed that attenuation of MUC4 by siRNA transfection combined
with auranofin treatment in SKOV3 cells synergistically activated
FOXO3 translocation from the cytoplasm to the nucleus through the
regulation of the Her2/Akt/FOXO3 pathway.
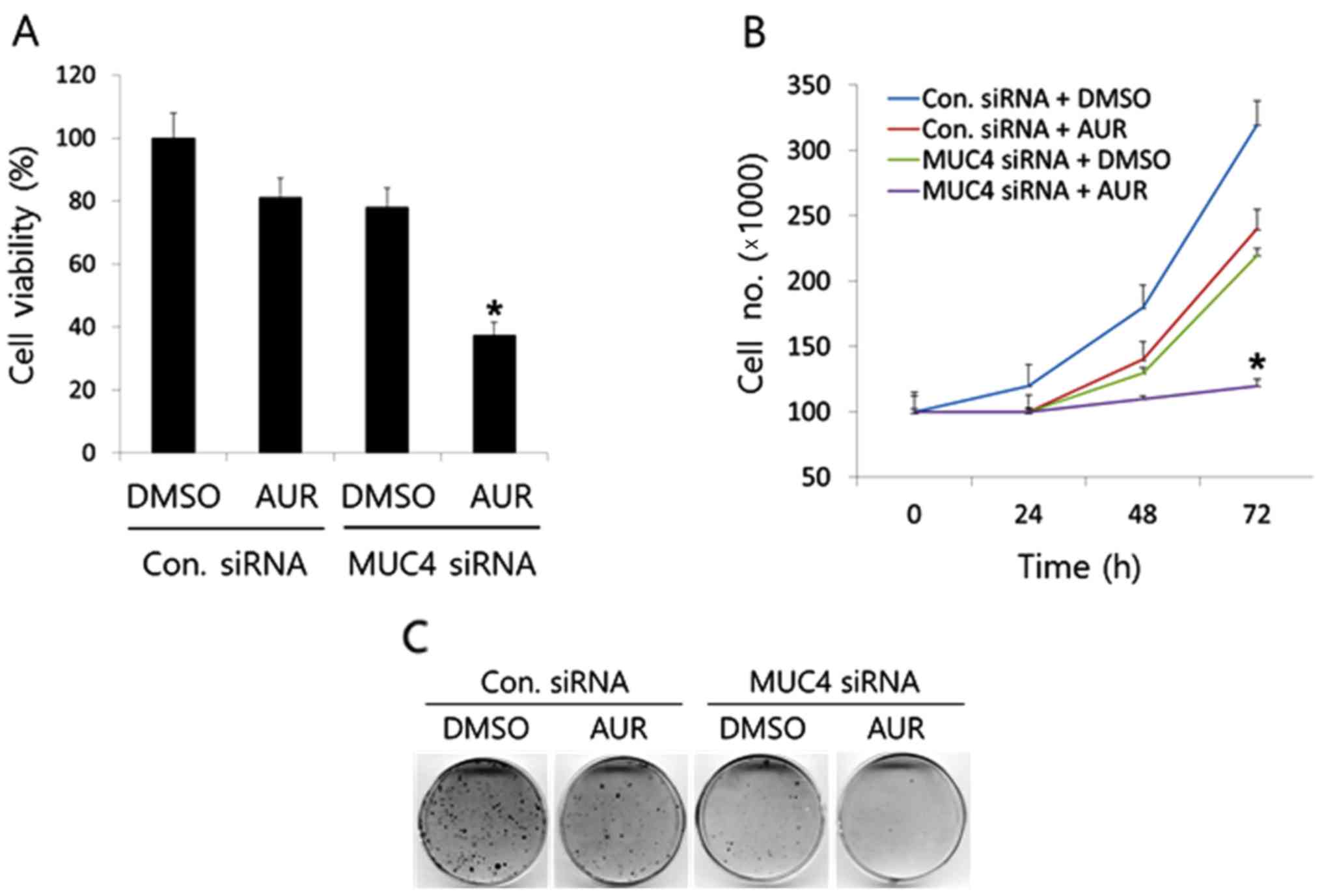
Attenuation of MUC4 potentiates the
anticancer activity of auranofin in SKOV3 cells
Then, we examined whether the anticancer activity of
auranofin was potentiated in SKOV3 cells after attenuating MUC4 by
siRNA transfection. We transfected SKOV3 cells with control or
MUC4-siRNA and treated cells with auranofin (0 or 25 nM), and then
assessed the survival and growth rate of SKOV3 cells using the
WST-1, cell counting, and colony formation assays. As shown in
Fig. 5A, although the sole
treatment of auranofin (25 nM) had a weak inhibitory effect (~20%
compared to the DMSO control) on SKOV3 cell survival, the
combination of MUC4-siRNA transfection with auranofin treatment
significantly enhanced the antitumor activity of auranofin. In
addition, the time-dependent cell counting assay results
demonstrated the synergistic potentiation of the anticancer
activity of auranofin (Fig. 5B),
which was confirmed by colony formation assay using the same
transfection and treatment conditions in SKOV3 cells (Fig. 5C). After 72 h of incubation, the
number of cells in SKOV3 cells transfected with MUC4-siRNA followed
by auranofin treatment (25 nM) was significantly lower (~3 times)
than that of sole MUC4-siRNA or auranofin treatment. Furthermore,
the 14-day results from the colony formation assay revealed that
combination of MUC4 by siRNA transfection and auranofin treatment
significantly inhibited the colony-forming ability of SKOV3 cells.
These results demonstrated that attenuation of MUC4 by siRNA
transfection potentiated the antitumor activity of auranofin on
cell survival and proliferation of SKOV3 cells.
Attenuation of MUC4 potentiates the
pro-apoptotic activity of auranofin in SKOV3 cells
To examine whether the pro-apoptotic activity of
auranofin was potentiated in SKOV3 cells after attenuation of MUC4
using MUC4-siRNA transfection or not, we performed a TUNEL assay,
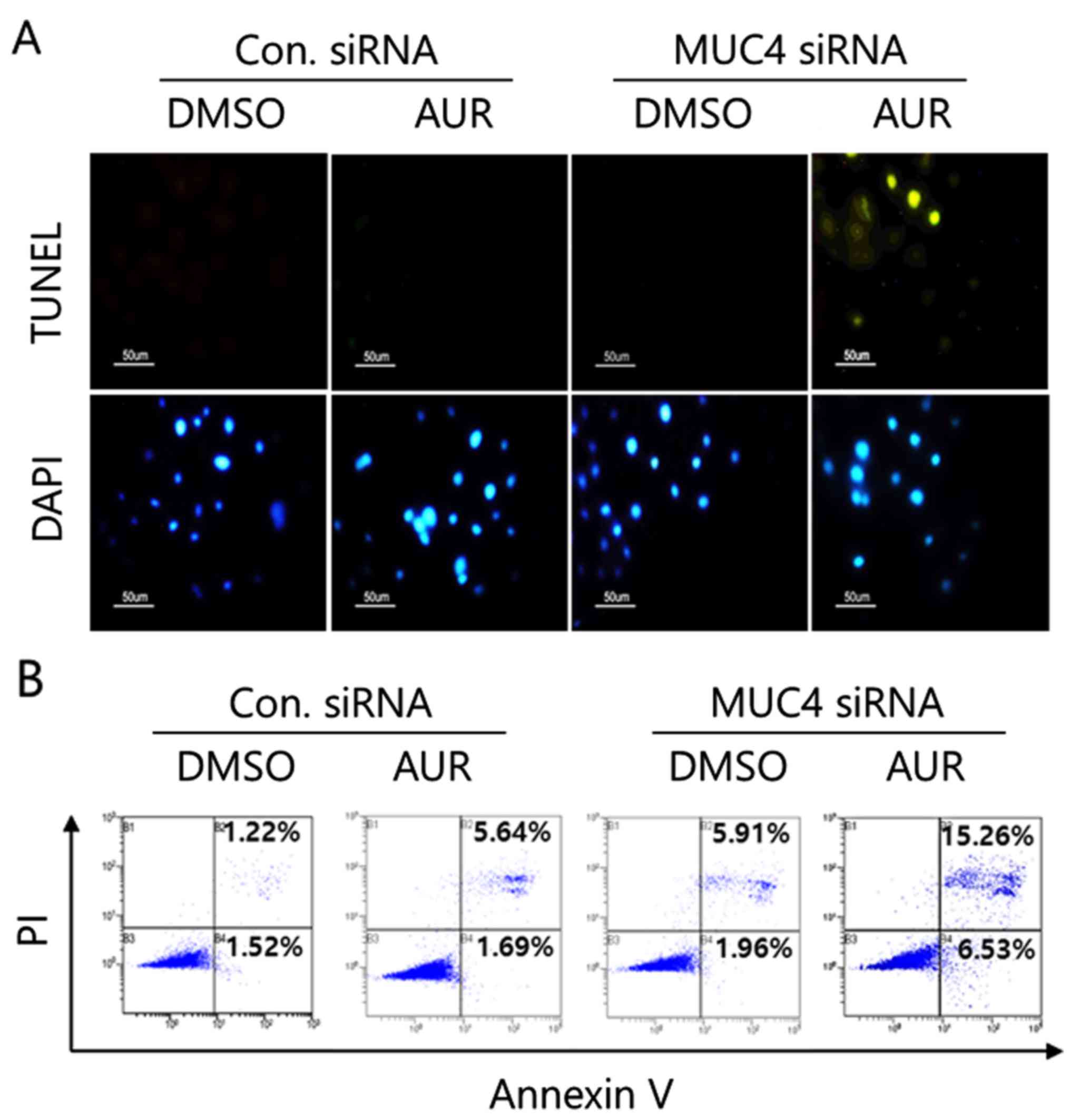
Annexin V staining analysis, and western blot analysis. As shown in
Fig. 6A, sole auranofin treatment
(25 nM for 2 h) did not induce the apoptotic nuclei but auranofin
treatment after MUC4-siRNA transfection resulted in the increased
apoptotic DNA degradation in SKOV3 cells, which was confirmed by
Annexin V staining analysis (Fig.
6B). Auranofin treatment after MUC4-siRNA transfection induced
significant Annexin V-positive cell populations (15.26% of
apoptotic and dead cells and 6.53% of apoptotic cells) compared to
the sole treatment (5.64% of apoptotic and dead cells and 1.69% of
apoptotic cells) in SKOV3 cells. In addition, auranofin treatment
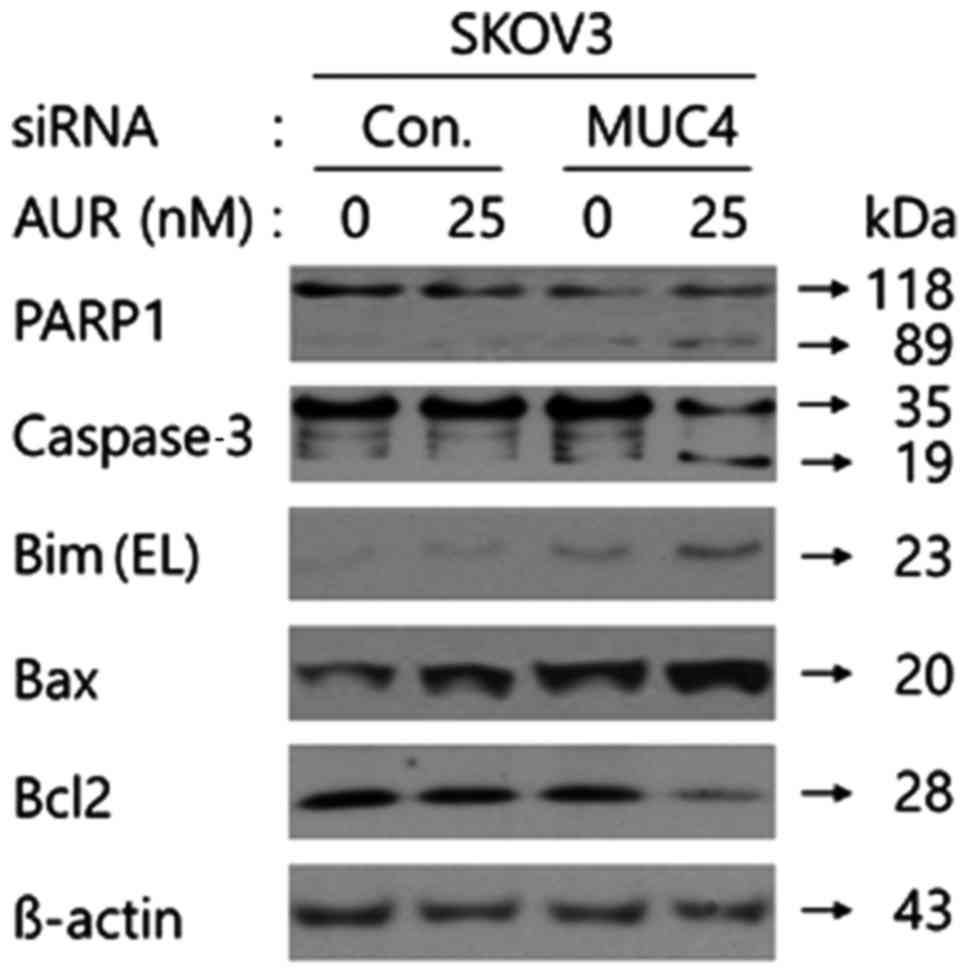
after MUC4-siRNA transfection induced the cleavage of PARP1 and
caspase-3 and upregulated the expression of Bax and Bim EL in SKOV3
cells compared to the sole treatment, whereas auranofin treatment
after MUC4-siRNA transfection decreased the Bcl2 expression level
under the same conditions (Fig. 7).
These results revealed that combination of MUC4 by siRNA
transfection with auranofin treatment may exhibit its apoptotic
effect through the caspase-3-mediated apoptosis mechanism in SKOV3
cells, the upregulation of the mitochondrial pro-apoptotic Bax and
Bim proteins, and the downregulation of the anti-apoptotic Bcl2
protein expression.
Discussion
Ovarian cancer is one of the major causes of
gynecological cancer-related deaths each year in the US. Although
ovarian cancer after initial cytoreductive surgery has been
generally shown to have a favorable response to combination
chemotherapy (first line), advanced ovarian cancer is responsible
for the worse prognosis of patients, due to acquired chemotherapy
resistance (24,25). While chemotherapies for ovarian
cancer are currently being developed, the overall survival has not
considerately increased since a significant number of these
patients develop a resistance to the therapies and the majority of
cancers susceptible to treatments in the beginning, become
refractory (26). Therefore, a new
targeted therapy needs to be developed for ovarian cancer
resistance to chemotherapy.
MUC4 is a type of mucin glycoproteins and known as
an activator of Her2 by inducing the dimerization of Her2 with
other ErbB receptors (27,28). There are lots of studies that have
identified the existence of the MUC4/Her2 complex in various tumors
and cancer cell lines (29,30). In addition, research has
demonstrated that downregulation of MUC4 destabilizes HER2
expression (19,29). Similar to previous findings, the
present study also revealed that silencing of MUC4 by siRNA
transfection into SKOV3 cells decreases Her2 stability via a
proteosomal pathway (Fig. 1).
Notably, auranofin treatment in SKOV3 cells downregulated Her2
expression (Fig. 2B). Thus, both
MUC4-siRNA transfection and auranofin treatment in SKOV3 cells
regulate Her2.
Since Her2 is known to activate many downstream
signaling targets including Akt (31), we investigated the effect of MUC4
knockdown by siRNA transfection in SKOV3 cells on Akt
phosphorylation. As shown in Fig.
2B, we observed decreased Akt phosphorylation in MUC4 knockdown
cells compared to control-siRNA transfected cells. Consistent with
our present study, Kaur et al recently revealed that MUC4
was involved in the regulation of lipocalin2 through the
Her2/Akt/NF-κB signaling pathway in pancreatic cancer cells
(6). Furthermore, auranofin
treatment in SKOV3 cells induced decreased Akt phosphorylation.
Therefore, we reasoned that the anticancer activity of auranofin
may be enhanced by attenuating MUC4 expression through the
regulation of the Her2/Akt pathway.
FOXO3 belongs to the human Forkhead-box (FOX) gene
family which is characterized by a distinct Fork head DNA-binding
domain (32). FOXO3 transcription
factors exert functions in various processes including cellular
differentiation, proliferation, cell cycle arrest, cell death,
resistance to environmental stress and metabolism (33,34). A
great number of clinical studies have recently shown that the
protein expression level of FOXO3 has far-reaching effects on
cancer patient survival rates (35,36).
These observations revealed that FOXO3 could function as a
prognostic marker in cancer, thereby FOXO3 regulation could be an
anticancer therapeutic strategy. FOXO3 is known to be inactivated
via a ubiquitin/proteasome system-mediated protein degradation
after translocation from the nucleus into the cytoplasm. To date, 3
representative kinases such as Akt, Erk and IKKβ have been
identified to induce this translocation of FOXO3 by phosphorylation
(23).
Although we confirmed that the Her2 and phospho-Akt
expression levels were significantly decreased in total cell
lysates of SKOV3 cells transfected with MUC4-siRNA followed by
auranofin treatment (Fig. 4), we
could not detect any difference in the expression level of FOXO3.
FOXO3 is a transcriptional factor and should be located in the
nucleus to perform its transcriptional activity. Thus, researchers
often try to perform the nuclear fractional western blotting by
separating the cytoplasmic and nuclear protein fraction from cells
to investigate the expression pattern of the specific
transcriptional factor. Based on our recent studies on the
activators for the FOXO3 transcriptional activity (21,37),
we adopted the nuclear fractional western blot analysis to assess
the expression level of FOXO3 in SKOV3 cells transfected with
MUC4-siRNA and auranofin treatment (Fig. 4) and observed that the expression
level of FOXO3 was increased in the nucleus fraction implying the
translocation of FOXO3 from the cytoplasm into the nucleus.
Recently, we reported that auranofin, a
gold-combined drug used for rheumatoid arthritis in clinical
treatment, has the anticancer activity in SKOV3 cells via
regulation of the IKKβ/FOXO3 pathway (21). Including our previous studies, there
have been many studies dealing with the anticancer activity of
auranofin in various types of tumors (38–40).
According to Li et al, auranofin exerted anticancer activity
through the inhibition of the PI3K/AKT/mTOR signaling pathway in
non-small cell lung cancer cells (41). Similarly, we observed
auranofin-mediated downregulation of the phospho-Akt level in SKOV3
cells (Fig. 2B). Meanwhile, Tanaka
et al investigated the cross-talk between IKKβ/NF-κB and
PDK1/Akt pathways and demonstrated that PDK1 activated NF-κB
signaling through IKKβ phosphorylation (42). PDK1 is known to activate
(phosphorylate) Akt directly after being activated (phosphorylated)
by PI3K. Thus, we surmised that there are two signaling pathways
(Her2/PI3K/PDK1/Akt or Her2/PI3K/PDK1/IKKβ axis) downstream of
Her2. Currently, we are trying to examine whether auranofin
treatment or MUC4 expression status in cancer cells affects PDK1
activation.
In order to develop a novel therapeutic strategy, we
investigated the combinational effect of the attenuation of MUC4 by
siRNA transfection and auranofin treatment in ovarian cancer cells.
As shown in Fig. 5A and B, neither
silencing of MUC4 by siRNA or auranofin treatment (25 nM)
suppressed the growth or viability of SKOV3 cells compared to the
control siRNA or DMSO control. Colony formation assay results
revealed that either silencing of MUC4 by siRNA or auranofin
treatment (25 nM) apparently decreased the colony formation ability
of SKOV3 cells. We demonstrated that the synergistic anticancer
activity of the combination was due to the downregulation of Her2
expression and phosphorylated Akt, which translocated FOXO3 from
the cytosol into the nucleus and activated the transcriptional
activity of FOXO3, inducing caspase-3-mediated apoptosis as well as
Bim EL expression (Figs. 6 and
7). Recently, Lee et al
revealed that treatment of entinostat, a class I histone
deacetylase inhibitor, increased the anticancer activity of
lapatinib, a Her2/EGFR dual tyrosine kinase inhibitor, in
HER2-overexpressing breast cancer cells via FOXO3-mediated Bim
expression (43). To the best of
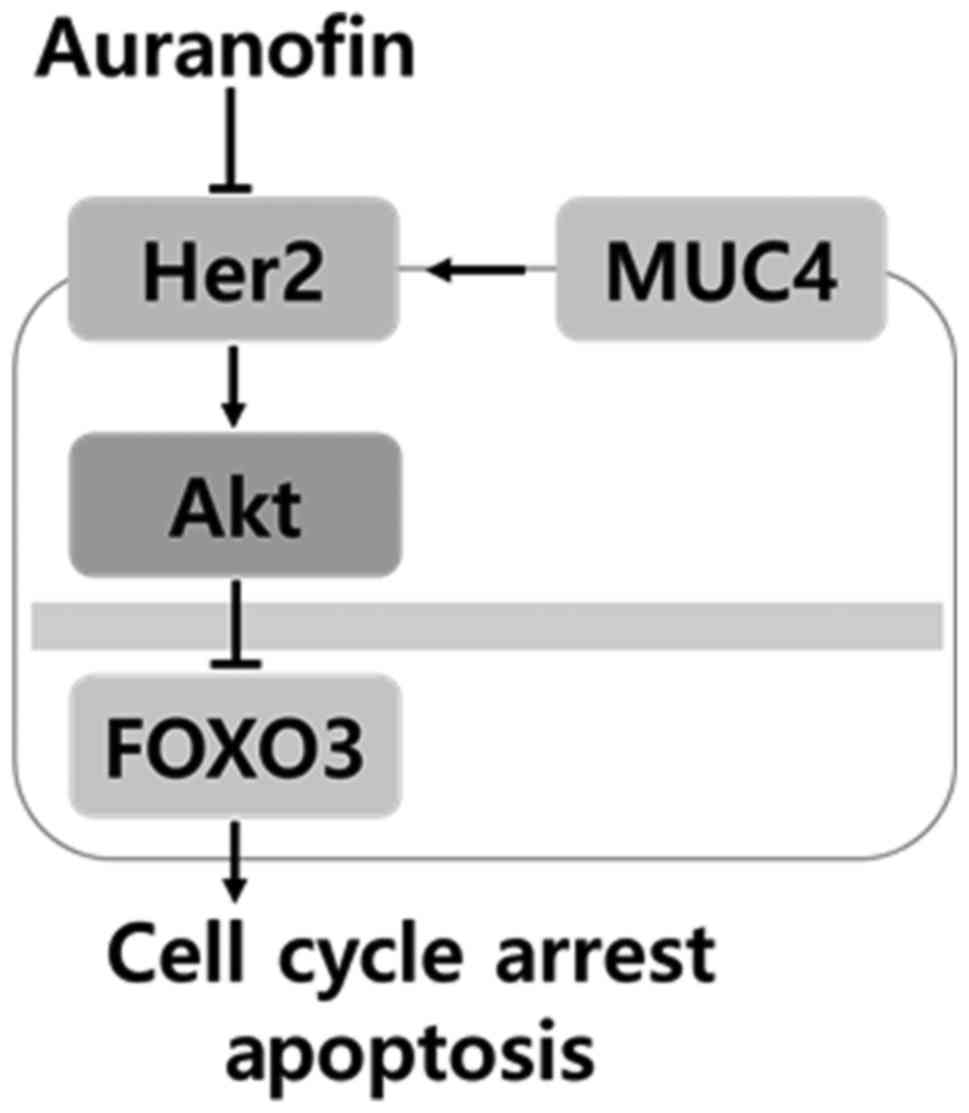
our knowledge, as described in Fig.
8, this is the first study demonstrating that the
MUC4/Her2/Akt/FOXO3 signaling pathway is involved in regulating
ovarian cancer cell development. Further studies analyzing
tissue-micro array (TMA) from patients with ovarian cancer may be
necessary to compare our current results to the clinical TMA data,
which may provide the molecular basis of a novel anticancer
therapeutic strategy. Collectively, auranofin regulated the
Her2/Akt/FOXO3 signaling pathway in SKOV3 cells and may be used as
a potential antitumor agent considering the expression of MUC4 in
ovarian cancer patients.
Acknowledgements
The present study was supported by the Basic Science
Research Program by the National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science, and Technology
(NRF-2014R1A6A3A04054307).
References
|
1
|
Hauber HP, Foley SC and Hamid Q: Mucin
overproduction in chronic inflammatory lung disease. Can Respir J.
13:327–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park HU, Kim JW, Kim GE, Bae HI, Crawley
SC, Yang SC, Gum JR Jr, Batra SK, Rousseau K, Swallow DM, et al:
Aberrant expression of MUC3 and MUC4 membrane-associated mucins and
sialyl Le(x) antigen in pancreatic intraepithelial neoplasia.
Pancreas. 26:e48–e54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh AP, Chauhan SC, Bafna S, Johansson
SL, Smith LM, Moniaux N, Lin MF and Batra SK: Aberrant expression
of transmembrane mucins, MUC1 and MUC4, in human prostate
carcinomas. Prostate. 66:421–429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voynow JA and Rubin BK: Mucins, mucus, and
sputum. Chest. 135:505–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rachagani S, Torres MP, Moniaux N and
Batra SK: Current status of mucins in the diagnosis and therapy of
cancer. Biofactors. 35:509–527. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur S, Sharma N, Krishn SR, Lakshmanan I,
Rachagani S, Baine MJ, Smith LM, Lele SM, Sasson AR, Guha S, et al:
MUC4-mediated regulation of acute phase protein lipocalin 2 through
HER2/AKT/NF-κB signaling in pancreatic cancer. Clin Cancer Res.
20:688–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaturvedi P, Singh AP, Moniaux N,
Senapati S, Chakraborty S, Meza JL and Batra SK: MUC4 mucin
potentiates pancreatic tumor cell proliferation, survival, and
invasive properties and interferes with its interaction to
extracellular matrix proteins. Mol Cancer Res. 5:309–320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bafna S, Kaur S and Batra SK:
Membrane-bound mucins: The mechanistic basis for alterations in the
growth and survival of cancer cells. Oncogene. 29:2893–2904. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inata J, Hattori N, Yokoyama A, Ohshimo S,
Doi M, Ishikawa N, Hamada H and Kohno N: Circulating KL-6/MUC1
mucin carrying sialyl Lewisa oligosaccharide is an
independent prognostic factor in patients with lung adenocarcinoma.
Int J Cancer. 120:2643–2649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schroeder JA, Masri AA, Adriance MC,
Tessier JC, Kotlarczyk KL, Thompson MC and Gendler SJ: MUC1
overexpression results in mammary gland tumorigenesis and prolonged
alveolar differentiation. Oncogene. 23:5739–5747. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elazeez Abd TA, El-Balshy A-L, Khalil MM,
El-Tabye MM and Abdul-Halim H: Prognostic significance of P27 (Kip
1) and MUC1 in papillary transitional cell carcinoma of the urinary
bladder. Urol Ann. 3:8–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patriarca C, Colombo P, Taronna Pio A,
Wesseling J, Franchi G, Guddo F, Naspro R, Macchi RM, Giunta P, Di
Pasquale M, et al: Cell discohesion and multifocality of carcinoma
in situ of the bladder: New insight from the adhesion molecule
profile (e-cadherin, Ep-CAM, and MUC1). Int J Surg Pathol.
17:99–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du L, Qian X, Dai C, Wang L, Huang D, Wang
S and Shen X: Screening the molecular targets of ovarian cancer
based on bioinformatics analysis. Tumori. 101:384–389. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slichenmyer WJ and Fry DW: Anticancer
therapy targeting the erbB family of receptor tyrosine kinases.
Semin Oncol. 28 Suppl 16:S67–S79. 2001. View Article : Google Scholar
|
|
15
|
Schmidt M, Lewark B, Kohlschmidt N,
Glawatz C, Steiner E, Tanner B, Pilch H, Weikel W, Kölbl H and Lehr
HA: Long-term prognostic significance of HER-2/neu in
untreated node-negative breast cancer depends on the method of
testing. Breast Cancer Res. 7:R256–R266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ross JS and Fletcher JA: The
HER-2/neu oncogene in breast cancer: Prognostic factor,
predictive factor, and target for therapy. Oncologist. 3:237–252.
1998.PubMed/NCBI
|
|
17
|
Ladjemi MZ, Jacot W, Chardès T, Pèlegrin A
and Navarro-Teulon I: Anti-HER2 vaccines: New prospects for breast
cancer therapy. Cancer Immunol Immunother. 59:1295–1312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hynes NE and Stern DF: The biology of
erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta.
1198:165–184. 1994.PubMed/NCBI
|
|
19
|
Ponnusamy MP, Seshacharyulu P, Vaz A, Dey
P and Batra SK: MUC4 stabilizes HER2 expression and maintains the
cancer stem cell population in ovarian cancer cells. J Ovarian Res.
4:72011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponnusamy MP, Singh AP, Jain M,
Chakraborty S, Moniaux N and Batra SK: MUC4 activates HER2
signalling and enhances the motility of human ovarian cancer cells.
Br J Cancer. 99:520–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SH, Lee JH, Berek JS and Hu MC:
Auranofin displays anticancer activity against ovarian cancer cells
through FOXO3 activation independent of p53. Int J Oncol.
45:1691–1698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu W, Li F, Mahavadi S and Murthy KS:
Upregulation of RGS4 expression by IL-1beta in colonic smooth
muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the
PI3K/Akt/GSK3beta pathway. Am J Physiol Cell Physiol.
296:C1310–C1320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee S and Kaye SB: New strategies in
the treatment of ovarian cancer: Current clinical perspectives and
future potential. Clin Cancer Res. 19:961–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shigetomi H, Higashiura Y, Kajihara H and
Kobayashi H: Targeted molecular therapies for ovarian cancer: An
update and future perspectives (Review). Oncol Rep. 28:395–408.
2012.PubMed/NCBI
|
|
27
|
Singh AP, Moniaux N, Chauhan SC, Meza JL
and Batra SK: Inhibition of MUC4 expression suppresses
pancreatic tumor cell growth and metastasis. Cancer Res.
64:622–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carraway KL, Perez A, Idris N, Jepson S,
Arango M, Komatsu M, Haq B, Price-Schiavi SA, Zhang J and Carraway
CA: Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in
cancer and epithelia: To protect and to survive. Prog Nucleic Acid
Res Mol Biol. 71:149–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaturvedi P, Singh AP, Chakraborty S,
Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP
and Batra SK: MUC4 mucin interacts with and stabilizes the HER2
oncoprotein in human pancreatic cancer cells. Cancer Res.
68:2065–2070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramsauer VP, Pino V, Farooq A, Carraway
Carothers CA, Salas PJ and Carraway KL: Muc4-ErbB2 complex
formation and signaling in polarized CACO-2 epithelial cells
indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol
Cell. 17:2931–2941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holbro T and Hynes NE: ErbB receptors:
Directing key signaling networks throughout life. Annu Rev
Pharmacol Toxicol. 44:195–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
33
|
Alvarez B, Martínez-A C, Burgering BM and
Carrera AC: Forkhead transcription factors contribute to execution
of the mitotic programme in mammals. Nature. 413:744–747. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furukawa-Hibi Y, Kobayashi Y, Chen C and
Motoyama N: FOXO transcription factors in cell-cycle regulation and
the response to oxidative stress. Antioxid Redox Signal. 7:752–760.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu M, Zhao Y, Xu F, Wang Y, Xiang J and
Chen D: The expression and prognosis of FOXO3a and Skp2 in human
ovarian cancer. Med Oncol. 29:3409–3415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fei M, Zhao Y, Wang Y, Lu M, Cheng C,
Huang X, Zhang D, Lu J, He S and Shen A: Low expression of Foxo3a
is associated with poor prognosis in ovarian cancer patients.
Cancer Invest. 27:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park SH, Chung YM, Ma J, Yang Q, Berek JS
and Hu MC: Pharmacological activation of FOXO3 suppresses
triple-negative breast cancer in vitro and in vivo. Oncotarget.
7:42110–42125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gandin V, Fernandes AP, Rigobello MP, Dani
B, Sorrentino F, Tisato F, Björnstedt M, Bindoli A, Sturaro A,
Rella R, et al: Cancer cell death induced by phosphine gold(I)
compounds targeting thioredoxin reductase. Biochem Pharmacol.
79:90–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schuh E, Pflüger C, Citta A, Folda A,
Rigobello MP, Bindoli A, Casini A and Mohr F: Gold(I) carbene
complexes causing thioredoxin 1 and thioredoxin 2 oxidation as
potential anticancer agents. J Med Chem. 55:5518–5528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pessetto ZY, Weir SJ, Sethi G, Broward MA
and Godwin AK: Drug repurposing for gastrointestinal stromal tumor.
Mol Cancer Ther. 12:1299–1309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Hu J, Wu S, Wang L, Cao X, Zhang X,
Dai B, Cao M, Shao R, Zhang R, et al: Auranofin-mediated inhibition
of PI3K/AKT/mTOR axis and anticancer activity in non-small cell
lung cancer cells. Oncotarget. 7:3548–3558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tanaka H, Fujita N and Tsuruo T:
3-Phosphoinositide-dependent protein kinase-1-mediated IkappaB
kinase beta (IkkB) phosphorylation activates NF-kappaB signaling. J
Biol Chem. 280:40965–40973. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee J, Bartholomeusz C, Mansour O,
Humphries J, Hortobagyi GN, Ordentlich P and Ueno NT: A class I
histone deacetylase inhibitor, entinostat, enhances lapatinib
efficacy in HER2-overexpressing breast cancer cells through
FOXO3-mediated Bim1 expression. Breast Cancer Res Treat.
146:259–272. 2014. View Article : Google Scholar : PubMed/NCBI
|