Introduction
Pancreatic cancer is one of the most aggressive and
lethal malignant diseases worldwide. Due to the lack of early
detection techniques, the disease is usually diagnosed at an
advanced stage. Moreover, the highly aggressive and invasive nature
of this disease makes surgical resection difficult since at
diagnosis, it usually has metastasized to nearby and distant
organs. Thus, only a small portion of patients are indicated for
surgery, and even in this case, the 5-year survival rate remains
disappointedly steadily at ~7% (1,2).
Chemotherapy is an important alternative for the systematic
treatment of pancreatic cancer (3).
However, conventional radiotherapy and chemotherapies have limited
efficacy in improving the overall survival of patients diagnosed
with pancreatic cancer, since this disease is highly resistant to
almost all chemotherapeutic agents and traditional radiotherapies
(4). Gemcitabine
(2′,2′-difluorodeoxycytidine) is the standard first-line anticancer
agent for pancreatic cancer (5).
Although gemcitabine shows significant benefits in patients, the
response rate and prognosis remain disappointing and it shows a
modest benefit in terms of progression-free, disease-free and
overall survival (6). Therefore,
any strategy that enhances the sensitivity of pancreatic cancer to
gemcitabine may improve the prognosis of this fatal disease.
F-box and WD repeat domain-containing 7 (FBW7) is
the substrate recognition subunit of an Skp1-Cullin1-F-box (SCF)
ubiquitin ligase complex. FBW7 is deregulated in ~10% of all human
cancers, usually by point mutations or decreased expression by
epigenetic modifications (7). FBW7
possesses tumor-suppressive roles by targeting a series of
onco-proteins for degradation, including c-Myc, cyclin E, Jun,
Notch intracellular domain (NICD), hypoxia inducible factor-1α
(HIF-1α), Krüppel-like factor 5 (KLF5) and MCL-1 (8). Through mutations or epigenetic
silencing, decreased FBW7 expression drives proliferation,
attenuates apoptosis, induces genomic instability and maintains
stem-cell properties, which ultimately promotes tumorigenesis
(9,10). Our previous studies demonstrated
that in pancreatic cancer, the protein level of FBW7 was tightly
controlled by oncogenic Kras/ERK signaling. Decreased FBW7 caused
an increase in oncoprotein c-Myc, which promoted pancreatic cancer
proliferation and progression (11). Moreover, decreased FBW7 expression
was found to render metabolic advantages to pancreatic cancer cells
by inducing aerobic glycolysis through downregulation of
thioredoxin interacting protein (TXNIP) in a c-Myc-dependent manner
(12). Furthermore, we demonstrated
that decreased FBW7 expression induced cancer antigen 125 (CA125)
or Muc16 production in pancreatic cancer cells, which promoted the
metastatic capacity of pancreatic cancer cells (13). However, the contribution of FBW7 to
pancreatic cancer chemotherapy and the underlying mechanisms have
seldom been discussed.
Key determinants of gemcitabine cytotoxicity include
activities of the equilibrative nucleoside transporter 1 (ENT1),
deoxycytidine kinase (dCK) and ribonucleotide reductase subunit 1
(RRM1) (14). Gemcitabine is a
highly hydrophilic chemical, and passive diffusion through the
hydrophobic membrane is slow. Thus, it requires transporters to
facilitate its intake. ENT1 is the membrane transporter protein
that helps efficient permeation of gemcitabine into cells (15,16).
Gemcitabine is a prodrug, and it must be phosphorylated by dCK as
the rate limiting step for its cellular anabolism. dCK catalyzes
gemcitabine monophosphate to its active metabolites, gemcitabine
diphosphate and gemcitabine triphosphate. One mechanism of
gemcitabine cytotoxicity is blocking de novo DNA synthesis
through the inhibition of ribonucleotide reductase, thereby
blocking production of the deoxyribonucleotide precursors needed
for DNA synthesis. RRM1 and RRM2 are components that can be
inactivated by difluorodeoxycytidine-5′ phosphate. The
triphosphorylated form of gemcitabine can be incorporated into DNA
and leads to chain termination during DNA synthesis, promoting the
apoptosis of pancreatic cancer cells (17). Previous studies using large
multicenter cohorts of patients with resected pancreatic cancer
suggest that ENT, dCK and RRM1 levels predict the efficacy of
gemcitabine and patient prognosis (14).
In the present study, we analyzed the contribution
of FBW7 to gemcitabine resistance in pancreatic cancer, and
indicated that FBW7 increased the sensitivity to gemcitabine. We
demonstrated that anti-apoptotic player MCL-1 was not influenced by
FBW7 in pancreatic cancer cells. Thus, we examined the effect of
FBW7 on ENT, dCK and RRM1. We demonstrated that among these
determinants of gemcitabine efficacy, FBW7 regulated the ENT1
protein level. Moreover, membrane-bound ENT1 was increased in the
FBW7-overexpressing cells. Finally, we demonstrated that the ENT1
level was influenced by lysosome inhibition instead of proteosomal
inhibition, indicating a novel regulatory mechanism in ENT1
regulation. Collectively, our results provide novel targets for
improving gemcitabine resistance in pancreatic cancer.
Materials and methods
Cell culture
Human pancreatic cancer cell lines PANC-1 and Mia
PaCa-2 were obtained from the Shanghai Cell Bank (Shanghai, China).
PANC-1 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 0.1 mg/ml streptomycin. As for Mia PaCa-2 cells, an
additional 2.5% horse serum was used for its culture. The cells
were maintained in a humidified incubator at 37°C with 5%
CO2.
Establishment of cell lines stably
expressing FBW7
PANC-1 and Mia PaCa-2 cell lines that stably
expressed FBW7 were established by lentiviral-mediated
transfection. pCDH-CMV-MCS-EF1-Puro (System Biosciences, Palo Alto,
CA, USA) was used for generation of the lentiviral-expressing
constructs. Lentiviral particles were obtained by co-transfection
of lentiviral constructs of FBW7 with psPAX2 and pMD2.G vectors
into 293T cells in a ratio of 4:3:1. Stable cells lines were
obtained by infection and subsequent selection by puromycin.
Cell viability assay
Cell viability was measured using the Cell Counting
Kit-8 (Dojindo, Tokyo, Japan). Briefly, 200 µl medium containing
cells (3,000/well) was seeded into 96-well plates. After culturing
for the indicated times, CCK-8 solution was added to each well at
37°C. After 2 h, the optical density (OD) values of each well were
measured using a microplate reader at a wavelength of 450 nm.
Cell apoptosis analysis
Cell apoptosis was assessed using flow cytometry.
For the cell apoptosis assay, PANC-1 and Mia PaCa-2 cells stably
transfected with FBW7 were incubated in the absence or presence of
gemcitabine for 24 or 48 h. The percentage of apoptotic cells was
analyzed by staining with fluorescein isothiocyanate-conjugated
Annexin V and propidium iodide (Invitrogen, Carlsbad, CA, USA),
followed by flow cytometric testing.
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). Takara PrimeScript RT reagent kit was used for
reverse transcription to obtain cDNA (Takara, Tokyo, Japan). The
expression status of the candidate genes and β-actin was determined
by quantitative real-time PCR using an ABI 7900HT Real-Time PCR
System (Applied Biosystems, Foster City, CA, USA). Primer sequences
are listed in Table I.
 | Table I.Primer sequences used in quantitative
real-time PCR. |
Table I.
Primer sequences used in quantitative
real-time PCR.
| ENT1 | F
5′-CTCCAACTCTCAGCCCACCAATGA-3′ |
|---|
|
| R
5′-GAAGTAACGTTCCCAGGTGCTGC-3′ |
| dCK | F
5′-CAAGACTGGCATGACTGGATGAA-3′ |
|
| R
5′-GGCACCTCTTGAAGATAATCGAAG-3′ |
| RRM1 | F
5′-TGGAGTACACCAGCAAAGATGAGG-3′ |
|
| R
5′-GGCGATGGCGTTTATTTGATAGGC-3′ |
| β-actin | F
5′-CTACGTCGCCCTGGACTTCGAGC-3′ |
|
| R
5′-GATGGAGCCGCCGATCCACACGG-3′ |
Western blotting
Cells were lysed in RIPA buffer (150 mM NaCl, 1%
NP-40, 50 mM Tris/HCl, pH 8.0 and 10% glycerol) containing protease
and phosphatase inhibitors purchased from Selleck (Houston, TX,
USA). Cell debris was removed by centrifugation at 12,000 rpm for
20 min at 4°C. Thermo Pierce® BCA Protein Assay kit was
used to test the protein concentrations. Equal amounts of protein
lysates were subjected to 10% SDS-PAGE and then transferred to
polyvinylidene difluoride (PVDF) membranes. The FBW7 antibody was
purchased from Bethyl Laboratories (Montgomery, TX, USA).
Antibodies against ENT1, RRM1 and β-actin were obtained from
Proteintech (Chicago, IL, USA). The dCK antibody was obtained from
Abcam (Cambridge, MA, USA).
Membrane protein extraction
In order to detect the distribution of proteins on
the cell membrane, Mem-PER™ Plus Membrane Protein Extraction kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used for the
extraction of membrane proteins. APT1A1 and TFR antibodies that
were purchased from Proteintech were used as loading controls.
Immunohistochemistry
Paraffin-embedded tissue slides were deparaffinized
in xylene, rehydrated through graded alcohol solutions, blocked in
methanol containing 3% hydrogen peroxide, and then incubated in the
FBW7 and ENT1 antibodies. Following rinsing with phosphate-buffered
saline (PBS) solution, the slides were incubated with secondary
antibodies and peroxidase reagent at room temperature. Finally, the
slides were incubated with 3,3′-diaminobenzidine solution at room
temperature for 10 min and counterstained with hematoxylin. The use
of human PDAC tissue specimens was evaluated and approved by the
Ethics Committee of Fudan University Shanghai Cancer Center, and
written informed consent was obtained from all participants.
Immunofluorescence
To observe the cellular distribution and endogenous
levels of the indicated proteins, immunofluorescence assay was
performed. In brief, the cells were fixed with 4% paraformaldehyde
at room temperature. Then, 0.5% Triton X-100/PBS solution was used
to permeabilize the cells. After washing the slides with
phosphate-buffered saline with Tween-20 (PBST)/bovine serum albumin
(BSA), the slides were blocked with PBST/BSA for 1 h at room
temperature. The cells on the slides were stained with primary
antibodies followed by PBST washing for five times, and then
secondary antibodies were applied. Vectashield® Mounting
Medium with 4,6-diamidino-2-phenylindole (DAPI) was used to label
DNA and prevent slow fading of the fluorescence. A laser scanning
confocal microscope (Leica Microsystems, Wetzlar, Germany) was used
to detect fluorescence.
Results
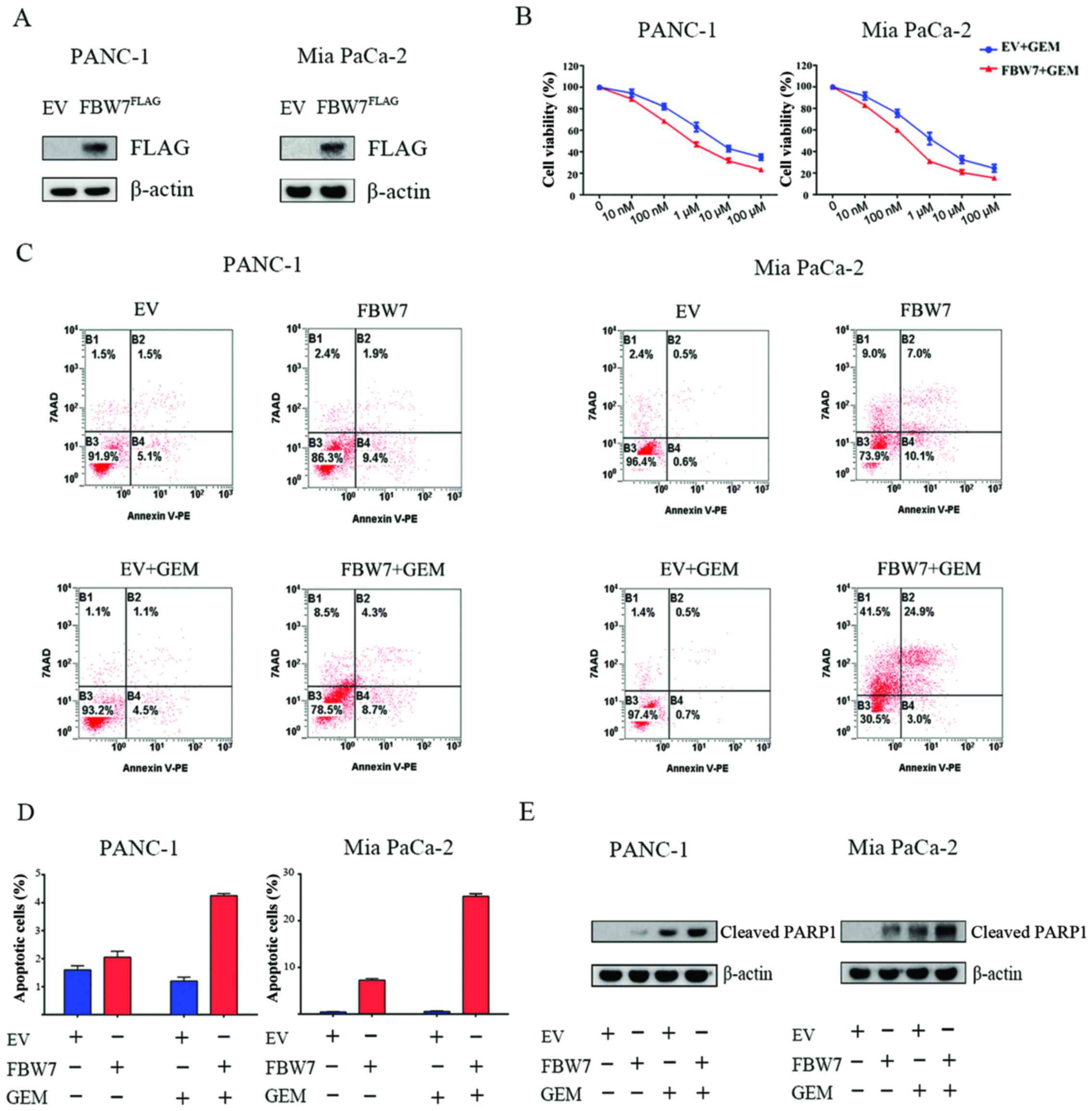
FBW7 increases sensitivity to
gemcitabine
To assess the role of FBW7 in the efficacy of
gemcitabine on pancreatic cancer cells, we overexpressed
FLAG-tagged FBW7 in PANC-1 and Mia PaCa-2-2 cells, and the effect
of overexpression was validated by immunoblotting with FLAG
antibody (Fig. 1A). We next studied
the chemosensitivity to gemcitabine in the FBW7-overexpressing
PANC-1 and Mia PaCa-2 cells. Empty vector (EV)-transfected and
FBW7-overexpressing (FBW7) pancreatic cancer cells were treated
with the indicated concentrations of gemcitabine for 72 h.
Significantly lower IC50 values were observed in the
FBW7-overexpressing PANC-1 and Mia PaCa-2 groups (Fig. 1B). Next, we performed cell apoptosis
assay, and our results demonstrated that introduction of FBW7
increased the apoptosis of PANC-1 and Mia PaCa-2 cells upon
gemcitabine treatment, compared to the relative control groups
(Fig. 1C and D). Moreover, we
observed an increase in the cleaved PARP1 level in the
FBW7-overexpressing cells treated with gemcitabine, indicating an
increase in apoptosis (Fig. 1E).
Taken together, the results indicated that FBW7 overexpression
increased sensitivity to gemcitabine in pancreatic cancer
cells.
FBW7 regulates the ENT1 protein
level
To ascertain the underlying molecular mechanism
accounting for the effect of FBW7 on gemcitabine sensitivity, we
first examined the protein level of MCL1, an anti-apoptotic protein
that accounts for drug resistance in many types of cancers. MCL-1
has been reported to be an FBW7 substrate, but we detected no
change in the MCL-1 protein level in the FBW7-overexpressing PANC-1
and Mia PaCa-2 cells, indicating that there may be other molecular
mechanisms (Fig. 2A). ENT1, dCK and
RRM1 have been reported to be key factors indicating gemcitabine
efficacy in pancreatic cancer (Fig.
2B). Thus, we detected the mRNA levels of these genes in the
FBW7-overexpressing PANC-1 and Mia PaCa-2 cells, but we detected no
obvious changes in the mRNA levels (Fig. 2C). Next, we examined the protein
level of these factors, and observed that FBW7 overexpression
significantly increased ENT1 at the protein level, but had slight
impact on the RRM1 protein level (Fig.
2D). Moreover, using two separate antibodies produced by
different manufacturers, we detected no dCK level in the PANC-1 and
Mia PaCa-2 cells. These results indicate that the regulation of
gemcitabine resistance by FBW7 may due to the impact of the
increase in ENT1 protein level.
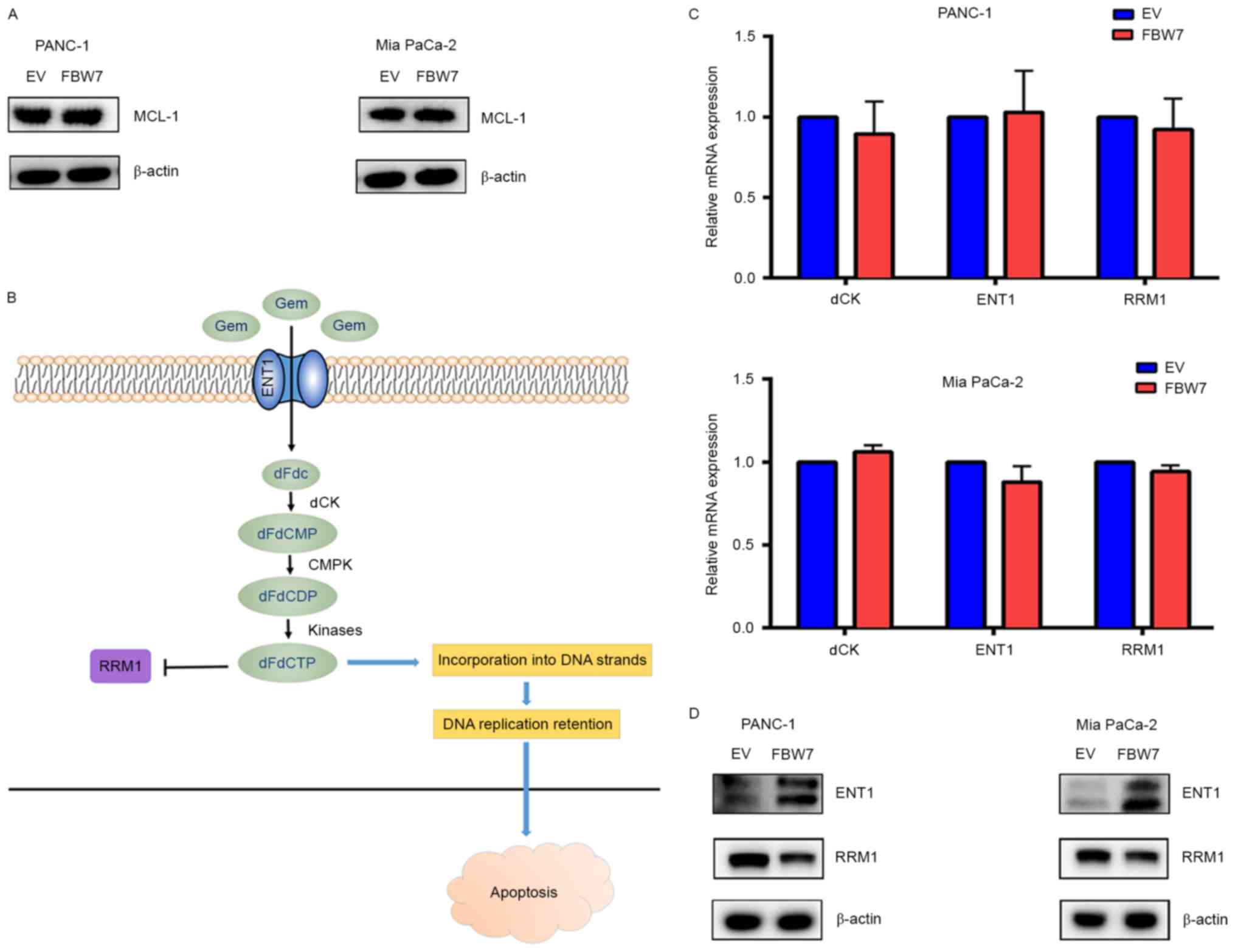 | Figure 2.FBW7 regulates the ENT1 protein
level. (A) Overexpression of the FBW7 substrate, anti-apoptotic
factor MCL-1, is commonly regarded as a factor that regulates drug
resistance. However, in the FBW7-overexpressing PANC-1 and Mia
PaCa-2 cells, we detected no significant change in MCL-1 level,
indicating that other molecular mechanisms underlie gemcitabine
resistance. (B) Schematic representation of gemcitabine activation.
ENT1 is responsible for gemcitabine transport, dCK catalyzes the
phosphorylation of gemcitabine for subsequent activation, and RRM1
is inhibited by activated gemcitabine. ENT1, dCK and RRM1 are
indicators of gemcitabine efficacy in pancreatic cancer. (C) FBW7
did not participate in the regulation of ENT1, dCK and RRM1 at the
transcriptional level. (D) FBW7 increased the protein levels of
ENT1 in PANC-1 and Mia PaCa-2 cells, while it had slight influence
on the RRM1 protein level. dCK was not detected in the present
study. GEM, gemcitabine. |
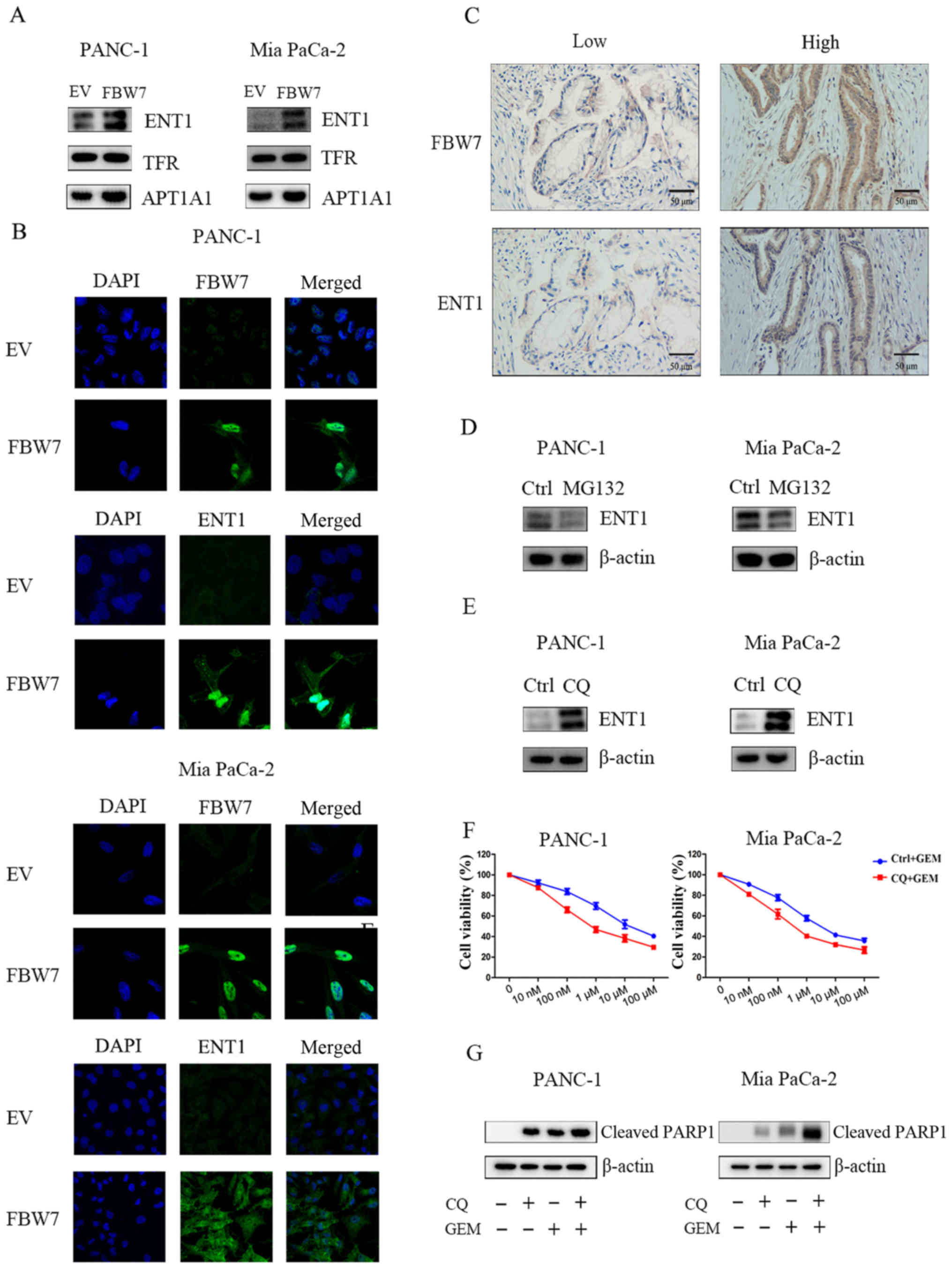
FBW7 regulates ENT1 membrane
distribution
ENT1 membrane localization is responsible for
gemcitabine efficient uptake. Thus, we examined the membrane
localization of ENT1 in FBW7-overexpressing PANC-1 and Mia PaCa-2
cells; loading controls were used by examining the protein levels
of TFR or ATP1A1. Our results revealed that ATP1A1 was markedly
altered, while no change in TFR was observed. Thus, we took TFR as
a membrane loading control. In addition, our results indicated that
overexpression of FBW7 increased the membrane distribution of ENT1
(Fig. 3A). Furthermore, our results
demonstrated that overexpression of FBW7 increased the total level
of ENT1 and membrane-bound ENT1 as reflected by the
immunofluorescence assay (Fig. 3B).
To further confirm the regulatory role of FBW7 on ENT1, we
performed IHC staining in patients with PDAC. As observed, the ENT1
level was significantly higher in patients that displayed higher
FBW7 expression, reflecting a positive correlation between FBW7 and
ENT1 in PDAC patients (Fig. 3C).
Factors that are involved in protein levels include
proteasome-mediated degradation and lysosomal degradation. Thus, we
treated PANC-1 and Mia PaCa-2 cells with proteasome inhibitor
MG132, but detected no obvious change in ENT1 levels in the PANC-1
and Mia PaCa-2 cells (Fig. 3D).
Then, we treated cells with one lysosome inhibitor chloroquine
(CQ), and observed a significant increase in the ENT1 protein
level. These results suggested a novel lysosome-mediated regulation
of ENT1 in pancreatic cancer cells (Fig. 3E). Further experiments demonstrated
that combination of CQ application increased the sensitivity to
gemcitabine in the PANC-1 and Mia PaCa-2 cells (Fig. 3F). Moreover, our results
demonstrated that treatment with the combination of CQ and
gemcitabine increased the level of cleaved PARP1, further
supporting the hypothesis that lysosome-induced ENT1 degradation
may participate in the acquisition of gemcitabine resistance
(Fig. 3G).
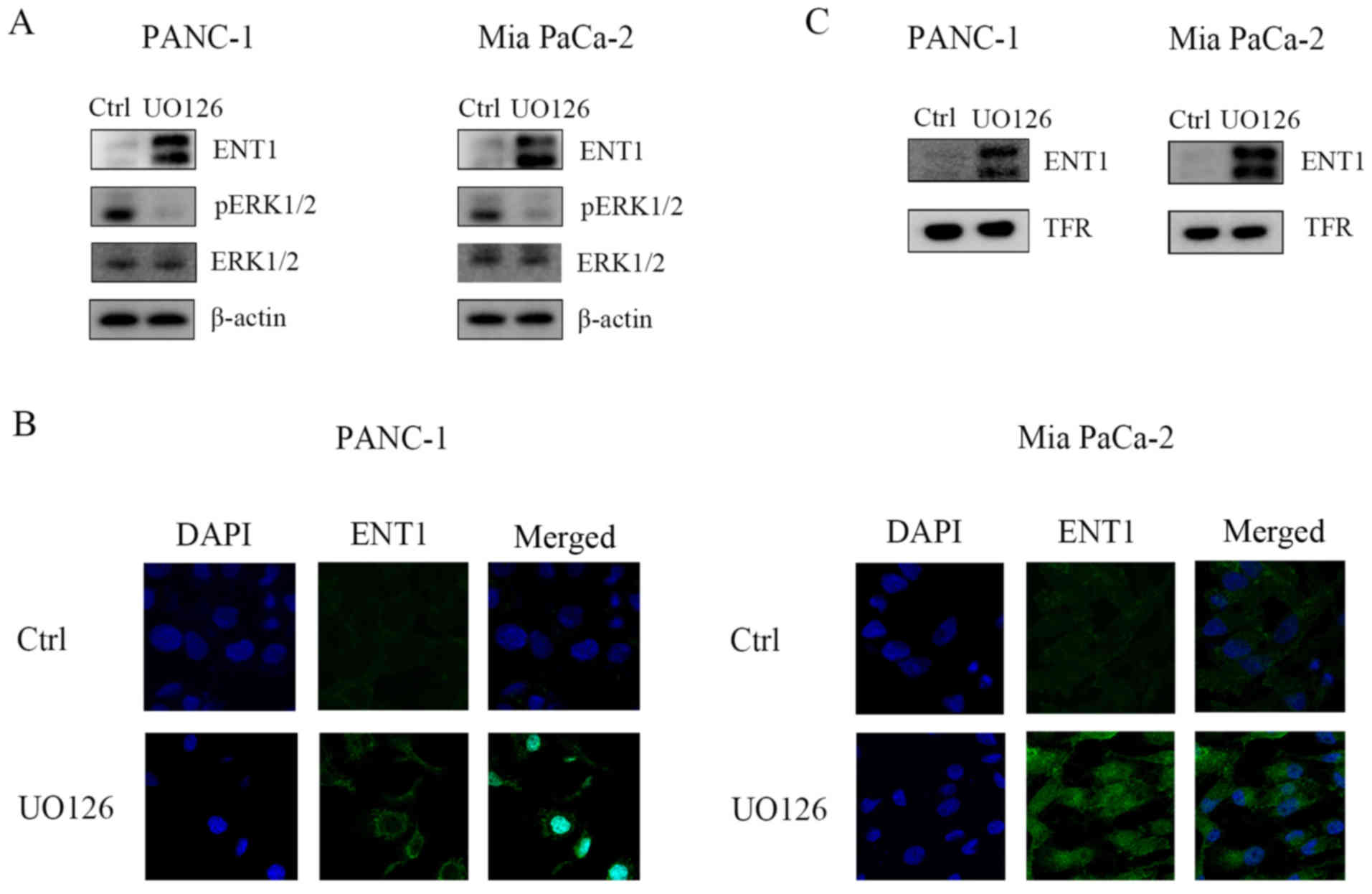
FBW7 upstream regulator ERK regulates
ENT1 abundance
Our previous studies (11) demonstrated that ERK phosphorylates
and destabilizes FBW7 in pancreatic cancer cells. To ascertain
whether ERK regulates ENT1 abundance in pancreatic cancer cells, we
first treated pancreatic cancer cells with MEK inhibitor UO126, and
our results indicated that ERK inhibition upregulated the protein
levels of ENT1 (Fig. 4A).
Furthermore, immunofluorescence results also demonstrated that
UO126 treatment elevated ENT1 levels (Fig. 4B). Next, we performed membrane
protein purification, and observed an increase in the ENT1 level in
the cell membrane (Fig. 4C). These
results suggest that ERK kinase activity may be responsible for the
decrease in ENT1, which subsequently renders resistance to
chemotherapy. Moreover, the ERK/FBW7 axis may function as a novel
target for improving chemotherapy sensitivity.
Discussion
Pancreatic cancer is a lethal disease, and its death
rate is almost equal to its incidence. Despite significant progress
in the diagnosis and treatment of the disease, its 5-year survival
rate remains desperately low at ~6%. Surgical resection,
chemotherapy and radiotherapy are the primary options for the
treatment of pancreatic cancer. However, due to early metastasis,
only a small proportion of patients are suitable for surgery. Thus,
gemcitabine-based chemotherapy has a pivotal role in the treatment
of locally advanced and metastatic pancreatic cancer. Yet, the
response rate is still unsatisfactory and gemcitabine resistance
remains a hurdle for the improvement of overall survival. Thus,
understanding the biological mechanisms involved in gemcitabine
resistance may help to improve the efficacy of gemcitabine-based
chemotherapy (18).
Our previous studies uncovered novel functions of
FBW7 as a novel tumor-suppressor in pancreatic cancer via
suppressing c-Myc, and c-Myc-induced malignancies such as aerobic
glycolysis, increased CA125 production and the resultant metastasis
(11–13). However, its role in the resistance
to chemotherapy in pancreatic cancer has seldom been discussed. In
the present study, we analyzed the contribution of FBW7 in
gemcitabine resistance and observed that introduction of FBW7
renders sensitivity to gemcitabine in PANC-1 and Mia PaCa-2 cells.
To uncover the underlying molecular mechanism, we firstly examined
the level of anti-apoptotic factor MCL-1 in FBW7-overexpressing
PANC-1 and Mia PaCa-2 cells. MCL-1 is a FBW7 substrate and
possesses anti-apoptotic functions. Previous studies have
demonstrated that MCL1 is overexpressed in many types of cancers,
and its upregulation renders cytotoxic resistance (19–21).
However, in the present study, we observed no variations in MCL-1
in the FBW7-overexpressing cells. Thus, we investigated changes in
the expression of three genes associated with gemcitabine uptake
and metabolism, including ENT1, dCK and RRM1. Decreased expression
of these genes was found to predict worse prognosis of pancreatic
cancer patients and are associated with innate and acquired
gemcitabine resistance. Our results demonstrated that FBW7
regulated proteins levels of ENT1 but not at the transcriptional
level. To ascertain the possible mechanism, we treated cells with
MG132, a proteasome inhibitor. The results indicated that MG132
exerted no significant impact on the protein levels of ENT1.
Lysosomes are dynamic organelles that receive and degrade
macromolecules from the secretory, endocytic, autophagic and
phagocytic membrane-trafficking pathways (22). The importance of lysosomes in
oncogenesis, cancer progression, metastasis and apoptosis have been
revealed in recent years (23).
Thus, we treated the cells with the lysosome inhibitor,
chloroquinone, and observed a significant increase in the protein
level of ENT1. These results suggest that lysosome-mediated
degradation of ENT1 may play a novel role in the acquisition of
gemcitabine resistance. However, the direct molecular mechanisms
underlying the membrane endocytosis and lysosome degradation need
further investigation. Membrane endocytosis and subsequent
autophagosome and lysosome degradation is a multi-step process
(24). The membrane intake by
vesicular traffic is the first step, and ADP-ribosylation factor
(ARF) small GTPases regulate vesicular traffic and organelle
structure by recruiting coat proteins to form cargos for
intracellular traffic (25). Among
these ARF proteins, ARF1 and ARF6, are two of the best
characterized ARF proteins that function in biological processes
such as secretion, endocytosis, phagocytosis, cell adhesion and
tumor cell invasion (26). Membrane
proteins that are sorted for intracellular transporting are
transported to destinations such as the endoplasmic reticulum (ER),
Glogi apparatus, autophagosomes and lysosomes. Recent years have
witnessed the importance of membrane protein intracellular
trafficking in the progression of cancer cells (27–29).
For example, epidermal growth factor receptor (EGFR) intracellular
transporting and recycling have been observed in many cancers,
promoting uncontrolled proliferation and metastasis of cancer cells
(27,30,31).
Another example is the activated hepatocyte growth factor (HGF)
receptor (Met) which undergoes rapid endocytosis and
uniquitin-dependent sorting to the lysosomal degradation pathway
(32,33). Recent studies have demonstrated that
this mode of downregulation can be circumvented by mutant receptors
bearing kinase-activating mutations that instead recycle to the
plasma membrane. These mutant receptors can elicit enhanced
signaling from endosomes, which is critical for cell motility and
tumorigenesis (34). Therefore,
strategies to target membrane endocytosis and lysosomal degradation
may provide novel therapeutic opportunities for inhibition of
malignant behaviors such as proliferation, invasion and metastasis
(35–37).
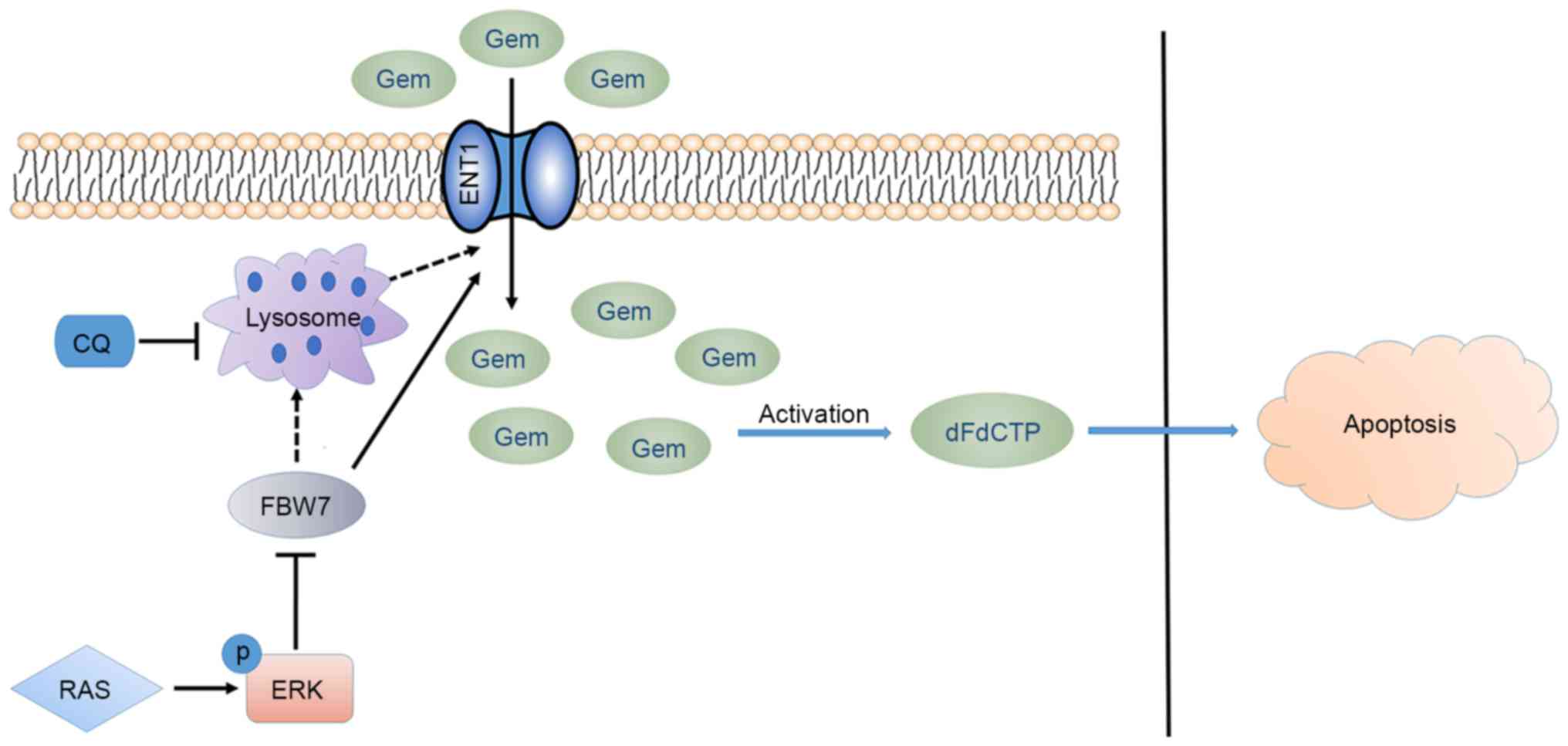
In the present study, we demonstrated that the
tumor-suppressor FBW7 promoted sensitivity to gemcitabine in
pancreatic cancer cells. Mechanistic studies demonstrated that FBW7
increased the protein levels of gemcitabine transporter ENT1 in
PANC-1 and Mia PaCa-2 cells. Further studies demonstrated that
lysosomal inhibition by chloroquinone also increased the protein
levels of ENT1. These observations indicated the novel functions of
FBW7 in chemotherapy resistance and lysosome or autophagosome
function (Fig. 5), and uncovered
novel aspects for improving drug resistance in pancreatic
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (nos. 81372651 and 81502031), the
Sino-German Center (GZ857), Ph.D. Programs Foundation of Ministry
of Education of China (20120071120104), and the Program of Science
and Technology Commission of Shanghai (nos. 13431900105 and
13DZ1942802).
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saif MW: Advanced stage pancreatic cancer:
Novel therapeutic options. Expert Rev Clin Pharmacol. 7:487–498.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cid-Arregui A and Juarez V: Perspectives
in the treatment of pancreatic adenocarcinoma. World J
Gastroenterol. 21:9297–9316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Inuzuka H, Zhong J, Wan L,
Fukushima H, Sarkar FH and Wei W: Tumor suppressor functions of
FBW7 in cancer development and progression. FEBS Lett.
586:1409–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min SH, Lau AW, Lee TH, Inuzuka H, Wei S,
Huang P, Shaik S, Lee DY, Finn G, Balastik M, et al: Negative
regulation of the stability and tumor suppressor function of Fbw7
by the Pin1 prolyl isomerase. Mol Cell. 46:771–783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhoondi S, Sun D, von der Lehr N,
Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D,
Marth C, et al: FBXW7/hCDC4 is a general tumor suppressor in
human cancer. Cancer Res. 67:9006–9012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhoondi S, Lindström L, Widschwendter M,
Corcoran M, Bergh J, Spruck C, Grandér D and Sangfelt O:
Inactivation of FBXW7/hCDC4-β expression by promoter
hypermethylation is associated with favorable prognosis in primary
breast cancer. Breast Cancer Res. 12:R1052010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H,
Gao J, Zhang B, Xu W, Liu J, et al: ERK kinase phosphorylates and
destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell
Res. 25:561–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji S, Qin Y, Liang C, Huang R, Shi S, Liu
J, Jin K, Liang D, Xu W, Zhang B, et al: FBW7 (F-box and WD repeat
domain- containing 7) negatively regulates glucose metabolism by
targeting the c-Myc/TXNIP (thioredoxin-binding protein) axis in
pancreatic cancer. Clin Cancer Res. 22:3950–3960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu
W, Liu J, Xiang J, Liang D, Hu Q, et al: Oncogenic KRAS targets
MUC16/CA125 in pancreatic ductal adenocarcinoma. Mol Cancer Res.
15:201–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maréchal R, Bachet JB, Mackey JR, Dalban
C, Demetter P, Graham K, Couvelard A, Svrcek M, Bardier-Dupas A,
Hammel P, et al: Levels of gemcitabine transport and metabolism
proteins predict survival times of patients treated with
gemcitabine for pancreatic adenocarcinoma. Gastroenterology.
143:664–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nordh S, Ansari D and Andersson R: hENT1
expression is predictive of gemcitabine outcome in pancreatic
cancer: A systematic review. World J Gastroenterol. 20:8482–8490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spratlin J, Sangha R, Glubrecht D, Dabbagh
L, Young JD, Dumontet C, Cass C, Lai R and Mackey JR: The absence
of human equilibrative nucleoside transporter 1 is associated with
reduced survival in patients with gemcitabine-treated pancreas
adenocarcinoma. Clin Cancer Res. 10:6956–6961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Plunkett W, Huang P, Searcy CE and Gandhi
V: Gemcitabine: Preclinical pharmacology and mechanisms of action.
Semin Oncol. 23 Suppl 10:S3–S15. 1996.
|
|
18
|
Kourie HR, Gharios J, Elkarak F, Antoun J
and Ghosn M: Is metastatic pancreatic cancer an untargetable
malignancy? World J Gastrointest Oncol. 8:297–304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ertel F, Nguyen M, Roulston A and Shore
GC: Programming cancer cells for high expression levels of Mcl1.
EMBO Rep. 14:328–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lucchetti C, Rizzolio F, Castronovo M and
Toffoli G: Research highlights. MCL1 and FBW7 as new predictive
candidate biomarkers of anti-tubulin agents. Pharmacogenomics.
12:1379–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirkegaard T and Jäättelä M: Lysosomal
involvement in cell death and cancer. Biochim Biophys Acta.
1793:746–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guicciardi ME, Leist M and Gores GJ:
Lysosomes in cell death. Oncogene. 23:2881–2890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maxfield FR and McGraw TE: Endocytic
recycling. Nat Rev Mol Cell Biol. 5:121–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seixas E, Barros M, Seabra MC and Barral
DC: Rab and Arf proteins in genetic diseases. Traffic. 14:871–885.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Souza-Schorey C and Chavrier P: ARF
proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell
Biol. 7:347–358. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Roy C and Wrana JL: Clathrin- and
non-clathrin-mediated endocytic regulation of cell signalling. Nat
Rev Mol Cell Biol. 6:112–126. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rainero E: Extracellular matrix
endocytosis in controlling matrix turnover and beyond: Emerging
roles in cancer. Biochem Soc Trans. 44:1347–1354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
White E, Mehnert JM and Chan CS:
Autophagy, metabolism, and cancer. Clin Cancer Res. 21:5037–5046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng K, Dai Q, Wei J, Shao G, Sun A, Yang
W and Lin Q: Stress-induced endocytosis and degradation of
epidermal growth factor receptor are two independent processes.
Cancer Cell Int. 16:252016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sorkin A: Internalization of the epidermal
growth factor receptor: Role in signalling. Biochem Soc Trans.
29:480–484. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joffre C, Barrow R, Ménard L, Calleja V,
Hart IR and Kermorgant S: A direct role for Met endocytosis in
tumorigenesis. Nat Cell Biol. 13:827–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seton-Rogers S: Signalling: Location,
location, location. Nat Rev Cancer. 11:462–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clague MJ: Met receptor: A moving target.
Sci Signal. 4:pe402011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mosesson Y, Mills GB and Yarden Y:
Derailed endocytosis: An emerging feature of cancer. Nat Rev
Cancer. 8:835–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tardy C, Codogno P, Autefage H, Levade T
and Andrieu-Abadie N: Lysosomes and lysosomal proteins in cancer
cell death (new players of an old struggle). Biochim Biophys Acta.
1765:101–125. 2006.PubMed/NCBI
|
|
37
|
Appelqvist H, Wäster P, Kågedal K and
Öllinger K: The lysosome: From waste bag to potential therapeutic
target. J Mol Cell Biol. 5:214–226. 2013. View Article : Google Scholar : PubMed/NCBI
|