Introduction
Oral squamous cell carcinoma (OSCC) accounts for
approximately 3% of all newly diagnosed cancer cases, and is the
most common head and neck cancer (1). Despite modern improvements in the
treatment modalities, the 5-year overall survival rate of OSCC
patients is still approximately 50% (2). In the United States, there are
approximately 32,000 newly diagnosed OSCC cases and OSCC is likely
responsible for an estimated 6,500 deaths in 2016 (3), while in China, the newly diagnosed and
death cases was 48,000 and 22,100, respectively in 2015 (4). Therefore, it is critical to improve
clinical outcomes of OSCC.
New therapeutic strategies targeting molecules
critical for OSCC have shown promise in recent years (5–7),
however, radiotherapy is still one of the most effective
non-surgical treatments for tumors (8,9). As an
important treatment for cancers, radiation inhibits tumor growth,
promotes tumor cell apoptosis, and prolongs patient survival
(10). However, radioresistance
remains a major impediment to radiotherapy (11,12).
Cancer cells resistant to radiotherapy can result in local
recurrence (13). Exposure of
cancer cells to radiation can activate several signal pathways that
lead to radiation resistance, including Nuclear factor-κB (NF-κB)
and the signal transducer and activator of transcription 3 (STAT3)
(14–16). DNA-dependent protein kinase
catalytic subunit (DNA-PKcs) and ataxia telangiectasia-mutated gene
(ATM) induce radioresistance by triggering reparation of
radiation-induced DNA damage (17,18).
Specificity factor 1 (Sp1) was also reported to be involved in
radioresistance (19–21), therefore, Sp1 may serve as a target
for increasing cancer radiosensitivity.
Sp1 plays multiple roles in several cellular
processes, including cell growth, differentiation and apoptosis
(22). Sp1 is overexpressed in many
types of tumors, such as in breast cancers, pancreatic tumors,
thyroid tumors, gastric tumors, liver cancers and gliomas (23–25),
and is a negative prognostic factor for survival. The
transcriptional activity of Sp1 is modulated by several
post-translational modifications, such as acetylation (26), phosphorylation (27), sumoylation (28), and O-GlcNAcylation (29). Post-translational modification can
regulate protein level, transactivation activity, or DNA binding
affinity of Sp1 (30). Sp1 is a
ubiquitous transcription factor and functions by binding to the
promoter of its target genes, including MMP3, MMP9, and cyclin D1.
We have previously demonstrated that Sp1 could downregulate PTEN
expression by binding to the specific site on PTEN promoter
(26), but whether Sp1/PTEN
participate in radiosensitization remains unknown.
PTEN (Phosphatase and tension homolog deleted on
chromosome ten) is an important tumor-suppressor gene (31) and a dual-specificity phosphatase
that removes phosphates from both proteins and lipids (31). PTEN antagonizes the PI3K-AKT
signaling pathway (32). Mutation,
deletion or dysfunction of PTEN was found in many types of tumors,
such as breast cancers, glioblastomas (33), prostate cancers, thyroid cancers and
endometrial carcinomas (33). PTEN
acts as a pivotal determinant in regulating radio-response of
cancer cells. Several genes, miRNAs or drugs regulate
radioresistance through PTEN. Activation of PTEN by COX-2
inhibitors could induce cancer cell radiosensitivity (34). miRNA29a, miRNA21, miRNA16b could
regulate radiosensitivity through targeting PTEN (35–37).
PTEN/PI3K/AKT pathways were demonstrated mediating radiosensitivity
(35). PTEN/Akt/HIF-1α feedback
loop modulates miRNA210- induced radiosensitivity (37). Ionizing radiation induces EMT
(epithelial-mesenchymal transition) through inhibiting PTEN and
correspondingly activating Akt/GSK-3β/Snail signaling (38). Thus, whether Sp1 could regulate
radiosensitivity by targeting PTEN needs further study. Betulinic
acid (3β, hydroxy-lup-20 (29)-en-28-oic acid; BetA) is a naturally
occurring pentacyclic triterpenoid found in many kinds of fruits,
vegetables and most abundant in the Sambucus williamsii
Hance tree (39,40). Betulinic acid has anti-malarial,
anti-HIV and anti-inflammatory activities (41). BetA has also been found to have
anticancer activities in various cancers through inducing apoptosis
and inhibition of cell proliferation in several cancers, including
pancreatic cancer, prostate cancer, leukemia and melanoma (42–47).
BetA could regulate the expression or post-translational
modification of lamin B1, Sp1, NF-κB and result in cell
proliferation inhibition and apoptosis (46–48).
Furthermore, BetA exerts anticancer activity through selectively
inhibiting the growth of cancer cells but without affecting the
normal cells (41,49). Therefore, BetA is a promising
anticancer candidate. However, whether BetA could be used
increasing radiosensitization in OSCC was unknown.
In this study, we examined i) whether BetA could
induce radiosensitization in OSCC, ii) whether Sp1 involved in
radioresistance in OSCC and iii) whether BetA induced
radiosensitization through Sp1 and the underlying mechanism.
Materials and methods
Cell culture and treatments
CAL-27 cells were incubated in Dulbecco's modified
Eagle's medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine
serum (FBS) at 37°C with 5% CO2. Tca-83 cells were
incubated in RPMI-1640 medium (Gibco) with 10% FBS at 37°C with 5%
CO2. Cells were exposed to radiation (Cobalt-60) at
different doses at the dose rate of 2.544 Gy/min and then cultured
for 24 h. Cells were also exposed to BetA alone for 24 h. In
another treatment, cells were exposed to a combination of radiation
and BetA; first, cells were treated with BetA and then with
radiation.
Plasmids, reagents and antibodies
Wild-type or mutant PTEN promoter-reporters were
constructed in our previous study (26). The pcDNA3.0-SUMO-1 plasmid (Plasmid
#21154) was obtained from Addgene. BetA was purchased from Selleck
Chemicals (Houston, TX, USA). Anti-PTEN (#9552) antibody,
anti-cleaved caspase 3 antibody (#9661) were purchased from Cell
Signaling Technology (Danvers, MA, USA). SUMO-1 siRNA (sc-29498),
anti-β-actin (I-19) antibody (sc-1616) and anti-SUMO-1 antibody
(sc-9060) were purchased from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). Anti-Sp1 antibody (07–645) was purchased from Merck
Millipore (Billerica, MA, USA).
Protein extraction and western
blotting
Whole cell lysates were extracted with RIPA lysis
buffer (Applygen Technologies, Inc., Beijing, China). Protein
concentrations were measured with BCA protein assay (Thermo Fisher
Scientific, Waltham, MA, USA). Equal amounts of samples were
subjected to 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis (PAGE) and transferred onto a nitrocellulose
membrane (Millipore). The membrane was blocked with 5% fat-free
milk in TBS-T (Tris buffer saline-Tween-20) for 1 h. After
incubation with primary antibodies diluted, 1:1,000 in TBS-T
containing 1% milk overnight at 4°C, the membrane was extensively
washed with TBS-T thrice and then incubated with a secondary
antibody conjugated with fluorophore for 1 h at room temperature.
After extensively washed thrice with TBS-T, the membrane was
visualized with Odyssey infrared imaging system (Odyssey LI-COR).
For internal controls of equal loading, the blots were also
stripped and reprobed with β-actin antibody.
Luciferase assay
Luciferase assay was performed as previously
described (26). Briefly, PTEN
reporter plasmid (1 µg) was transfected with Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) into CAL-27 cells
in a 12-well plate. The transfected cells were lysed in a cell
lysis buffer 24 h after transfection. Luciferase activity was
measured with an LB960 microplate luminometer (Berthold) using
luciferin as the substrate, according to the manufacturer's
instructions (Promega Corp., Madison, WI, USA).
Assessment of cell apoptosis
Cells were washed with phosphate-buffered saline
(PBS) thrice, fixed with 10% formaldehyde for 5 min, and incubated
with 5 mg/ml 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI)
in the dark for 3 min at room temperature. After washed with PBS,
the cells were examined under a fluorescence microscope (Nikon
Corporation, Tokyo, Japan). The cells presenting features of
nuclear condensation and fragmentation were identified as apoptotic
cells and were counted within the six randomly selected fields. The
rate of apoptotic cells was presented as mean ± SD of at least
three independent experiments.
Immunoprecipitation
Whole-cell extracts (2.5 mg) were incubated in 500
µl extraction buffer with 4 µg anti-Sp1 antibody for 16 h at 4°C,
added with 40 µl protein A/G-agarose beads (Santa Cruz
Biotechnology), incubated again for 1 h at 4°C and then washed five
times with extraction buffer. The bound proteins were released by
boiling in a loading buffer and then subjected to immunoblot
analysis. Sumoylated Sp1 was detected using anti-SUMO-1 antibody.
The membranes were stripped for Sp1 detection.
Chromatin immunoprecipitation
assay
Chromatin immunoprecipitation (ChIP) assay was
performed using a ChIP assay kit (Upstate). Briefly, CAL-27 cells
were cross-linked with 1% formaldehyde. The chromatin was sonicated
into fragments ranging between 200 and 1000 bp and then was pulled
down by anti-Sp1 antibody for PCR amplification. The primers for
amplifying the fragments (−1138 to −606) containing Sp1-binding
site of the PTEN promoter are as follows: 5′-AGGCAGCTACACTGGGCAT-3′
(sense) and 5′-AGGAAGAGGCTGCACGGTTAGAAA-3′ (antisense). The PCR
products were analyzed on 1.5% agarose gel and then
photographed
Statistical analysis
Statistical analysis was performed using SPSS 11.5
for Windows. The data are presented as mean ± SD. Differences
between multiple groups were analyzed by one-way analysis of
variance. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
BetA enhances radiation-induced
inhibition of cell proliferation and apoptosis
To examine whether BetA could enhance
radiosensitization of OSCC, OSCC cells were treated with radiation
or BetA alone or a combination of radiation and BetA. First of all,
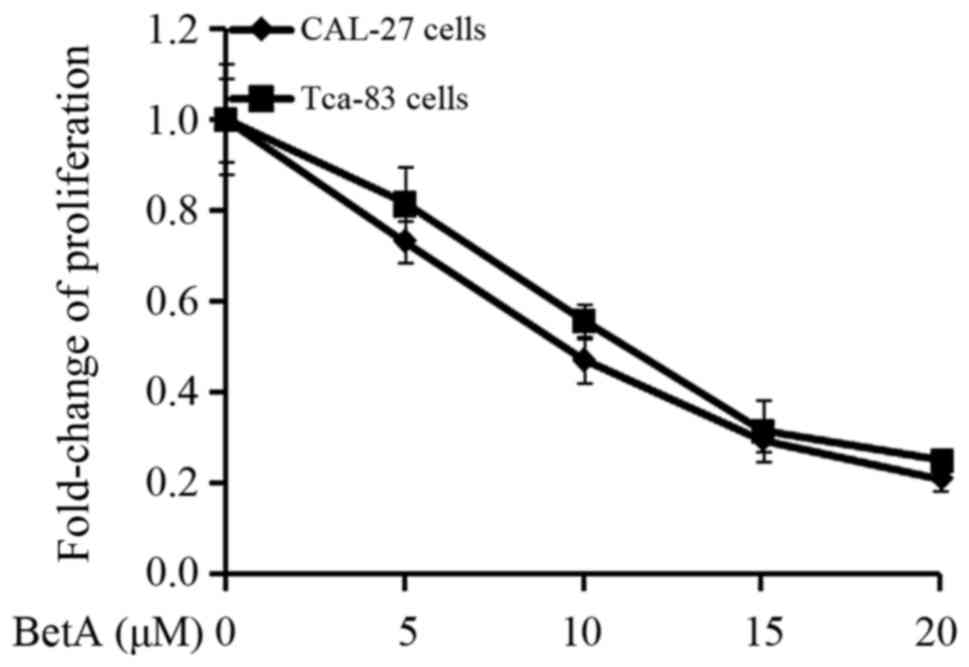
we determined the dose of BetA. We performed an MTT assay and found
that 10 µM of BetA could cause approximately 50% proliferation
inhibition of CAL-27 cells and Tca83 cells (Fig. 1); furthermore, we found that this
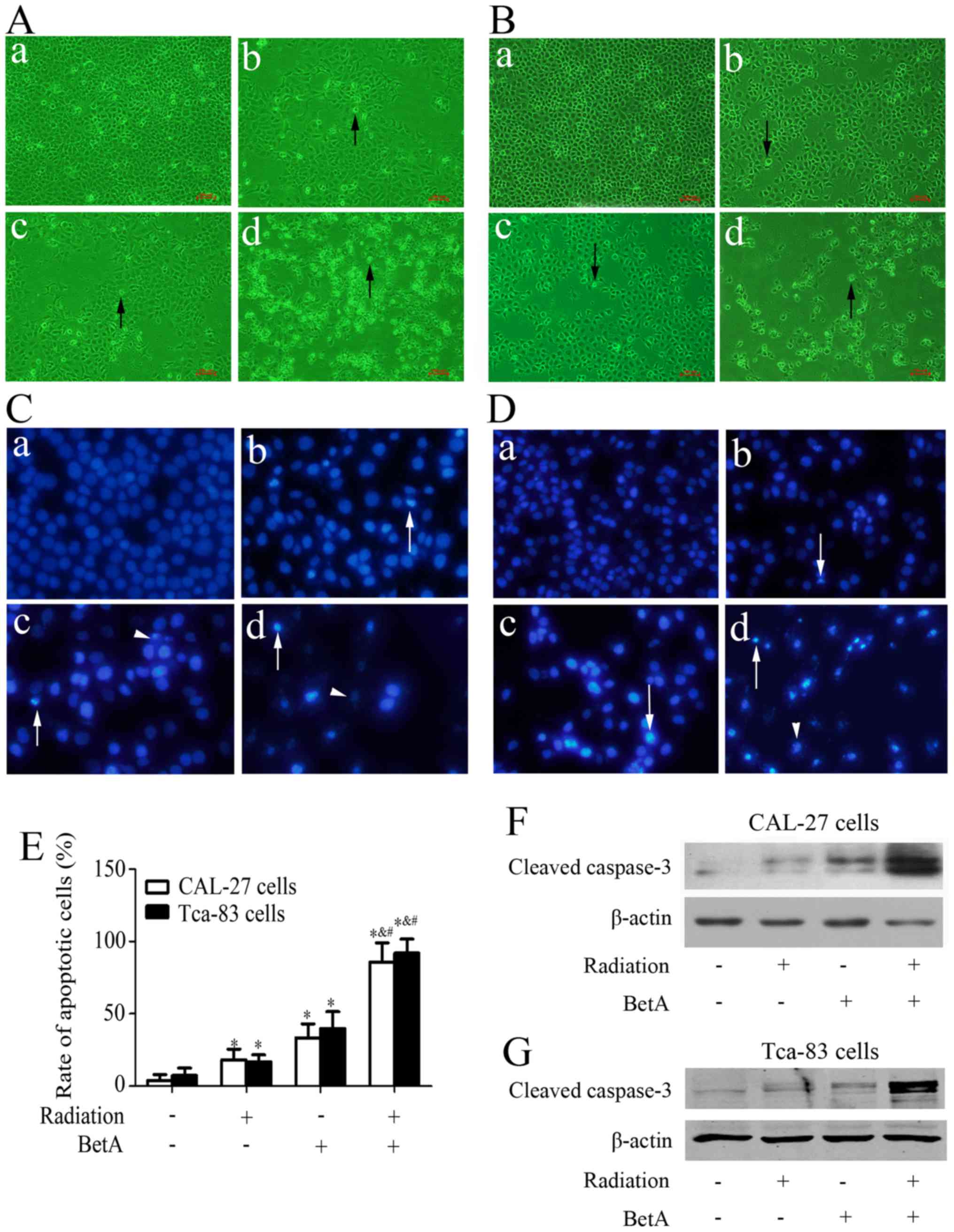
concentration of BetA only slightly promoted apoptosis of CAL-27
cells and Tca-83 cells, while combined with radiation, it
significantly induced apoptosis of these two cells (Fig. 2C-E). Actually, this concentration of
BetA was used also by some other teams in the study of BetA
inducing radiosensitization (50,51).
Therefore, we chose the concentration of 10 µM.
Two OSCC cell lines, CAL-27 and Tca-83 cells, were
exposed to radiation with 8 Gy at the dose rate of 2.544 Gy/min or
10 µM BetA alone or a combination of radiation and BetA for 24 h.
As shown in Fig. 2A-E, treatment
with radiation or BetA alone resulted in only a slight inhibition
of cell proliferation and a slight increase in apoptosis, whereas
treatment with radiation after BetA treatment significantly
enhanced radiation-induced cell proliferation inhibition and
apoptosis. Furthermore, we detected the level of cleaved caspase-3
and found that treatment with radiation or BetA alone resulted in
only a slight increase of cleaved caspase-3, while treatment with
radiation after BetA treatment significantly increased cleaved
caspase 3 (Fig. 2F and G).
Sp1 may be responsible for
radioresistance of OSCC
Sp1 is reported to play a key role in
radioresistance in several tumors. In order to reveal whether Sp1
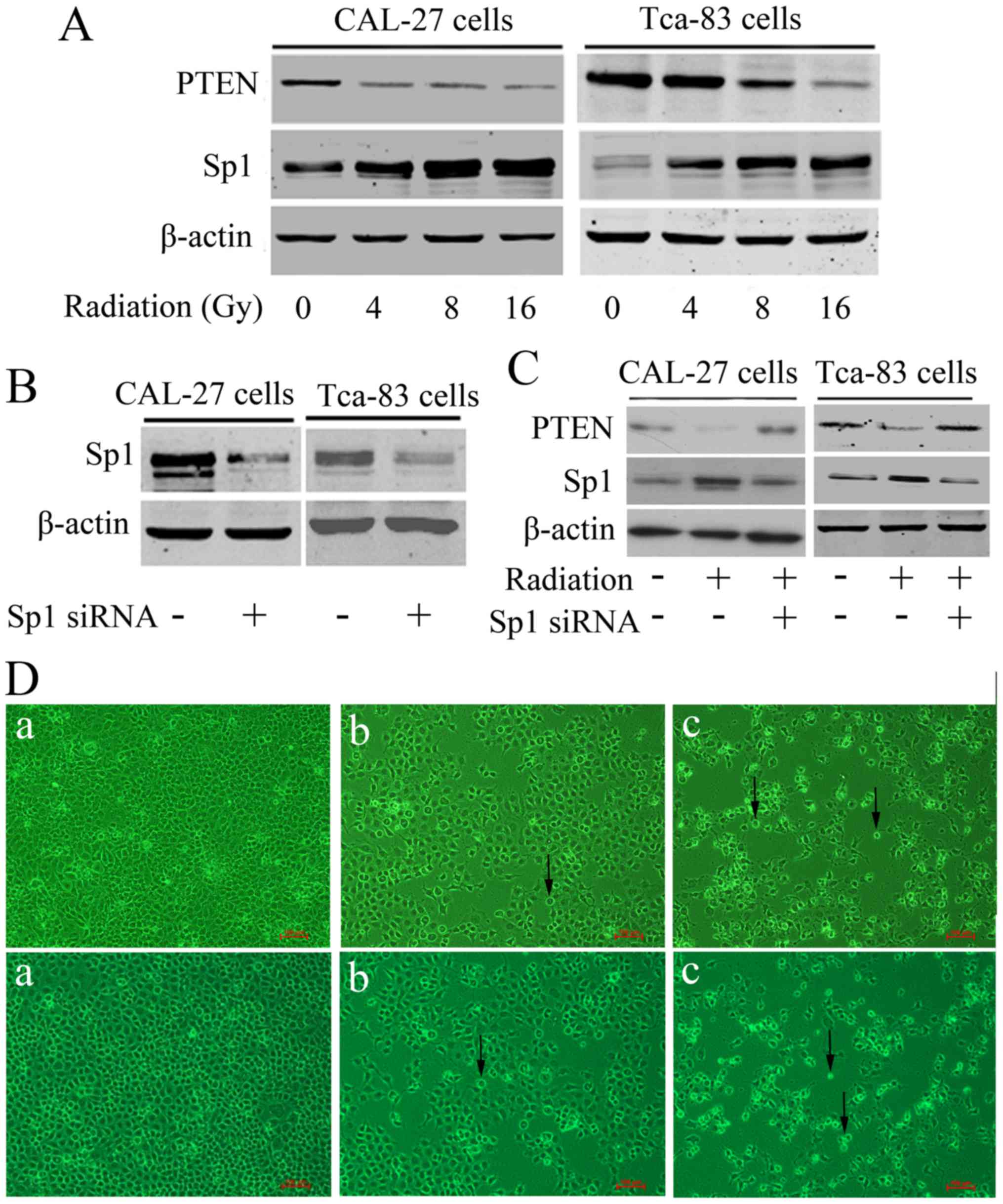
is also involved in OSCC radioresistance, we detected the
expression of Sp1 after radiation in CAL-27 cells and Tca-83 cells.
As shown in Fig. 3A, Sp1 was
dose-dependently upregulated by radiation in these cells, with a
corresponding downregulation of its downstream target, PTEN.
Furthermore, we knocked down Sp1 by transfecting Sp1 specific siRNA
into these two cells (Fig. 3B), and
we found that knockdown of Sp1 significantly antagonized
radiation-induced PTEN downregulation (Fig. 3C), as well as facilitated
radiation-induced inhibition of cell proliferation and apoptosis in
CAL-27 and Tca-83 cells (Fig. 3D).
The above suggested that Sp1 might be responsible for
radioresistance of OSCC.
BetA antagonizes radiation-induced Sp1
upregulation and rescued PTEN expression
As Sp1 may be responsible for radioresistance of
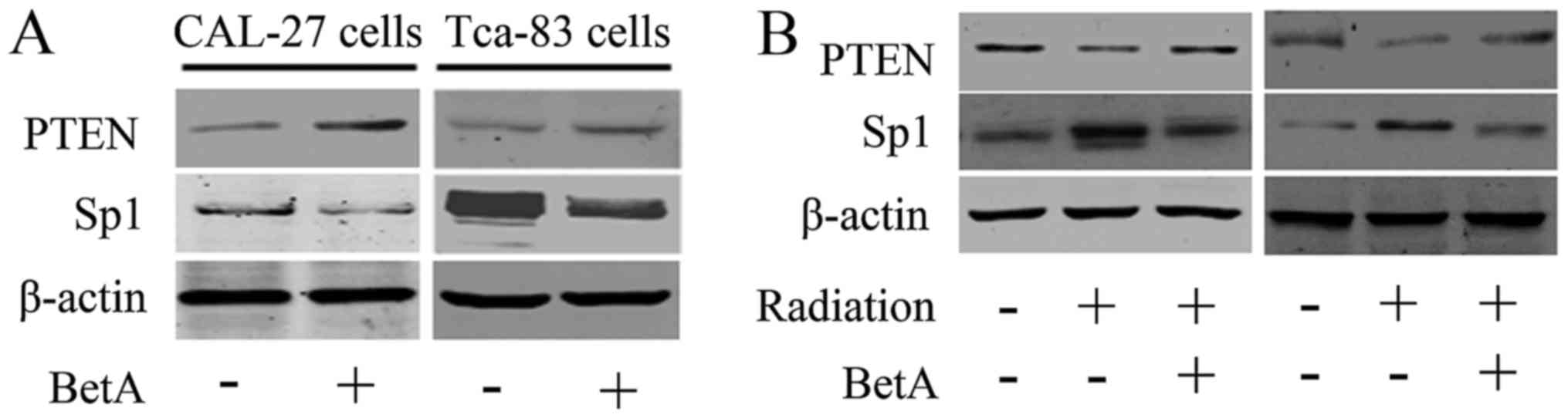
OSCC, we next detected whether BetA promoted radiosensitization
through Sp1. We treated CAL-27 and Tca-83 cells with BetA, as shown
in Fig. 4A, BetA downregulated Sp1
and upregulated PTEN in these cells. Moreover, we demonstrated that
BetA antagonizes radiation-induced Sp1 overexpression, with a
corresponding upregulation of PTEN (Fig. 4B).
BetA inhibits Sp1 expression through
inducing Sp1 sumoylation
Sp1 protein stability may be regulated by several
protein modifications. To explore the underlying mechanism of BetA
regulating Sp1, we detected whether Sumoylation regulated Sp1
expression in CAL-27 cells. We overexpressed SUMO-1, an enzyme
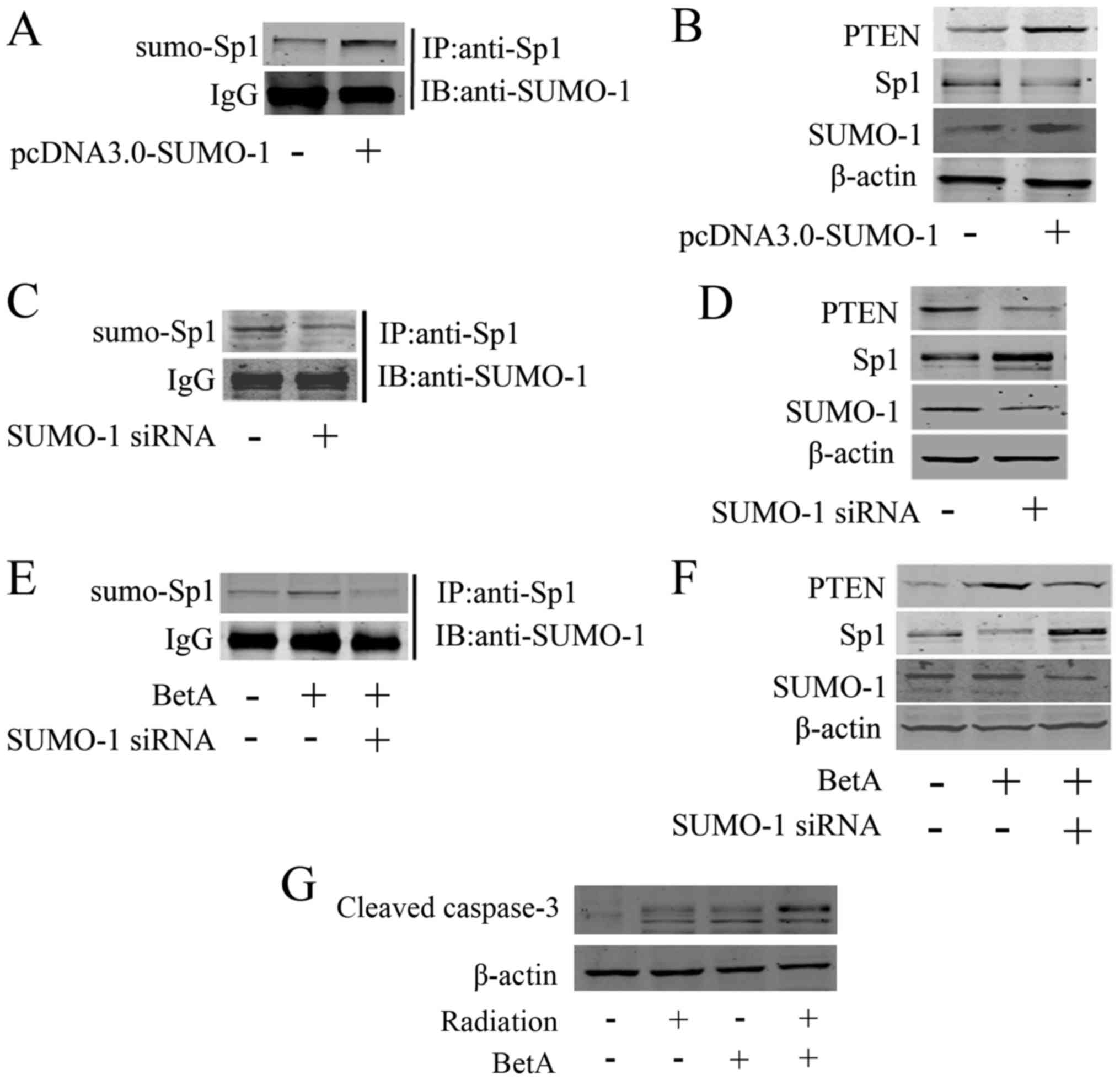
regulating sumoylation of several proteins. As shown in Fig. 5A, overexpression of SUMO-1 increased
Sp1 sumoylation, as well as decreased Sp1 expression and increased
PTEN expression (Fig. 5B). Whereas,
knockdown of SUMO-1 using its specific siRNA downregulated Sp1
sumoylation (Fig. 5C) and
correspondingly upregulated Sp1 and downregulated PTEN (Fig. 5D). We further investigated whether
knockdown of SUMO-1 antagonized BetA-induced Sp1 sumoylation. The
immunoprecipitation assay showed that BetA-induced Sp1 sumoylation,
indicating that BetA promoted the interaction between SUMO-1 and
Sp1, whereas knockdown of SUMO-1 could antagonize BetA-induced Sp1
sumoylation (Fig. 5E). Furthermore,
western blot analysis showed that knockdown of SUMO-1
correspondingly antagonized BetA-induced Sp1 downregulation and the
corresponding PTEN upregulation (Fig.
5F). Noteworthy, western blot analysis showed that BetA did not
regulate the expression of SUMO-1 (Fig.
5F), thus, BetA only regulates the affinity of SUMO-1 to Sp1
but not the expression of SUMO-1. In addition, we knocked down
SUMO-1 in CAL-27 cells, and detected whether knockdown of SUMO-1
impaired the BetA-induced radiosensitization. The results showed
that BetA only slightly induced radiosensitization in the condition
of SUMO-1 being knocked down, indicating that knockdown of SUMO-1
at least partially impaired the BetA-induced radiosensitization
(Fig. 5G).
Sumoylation inhibits Sp1 binding to
PTEN promoter
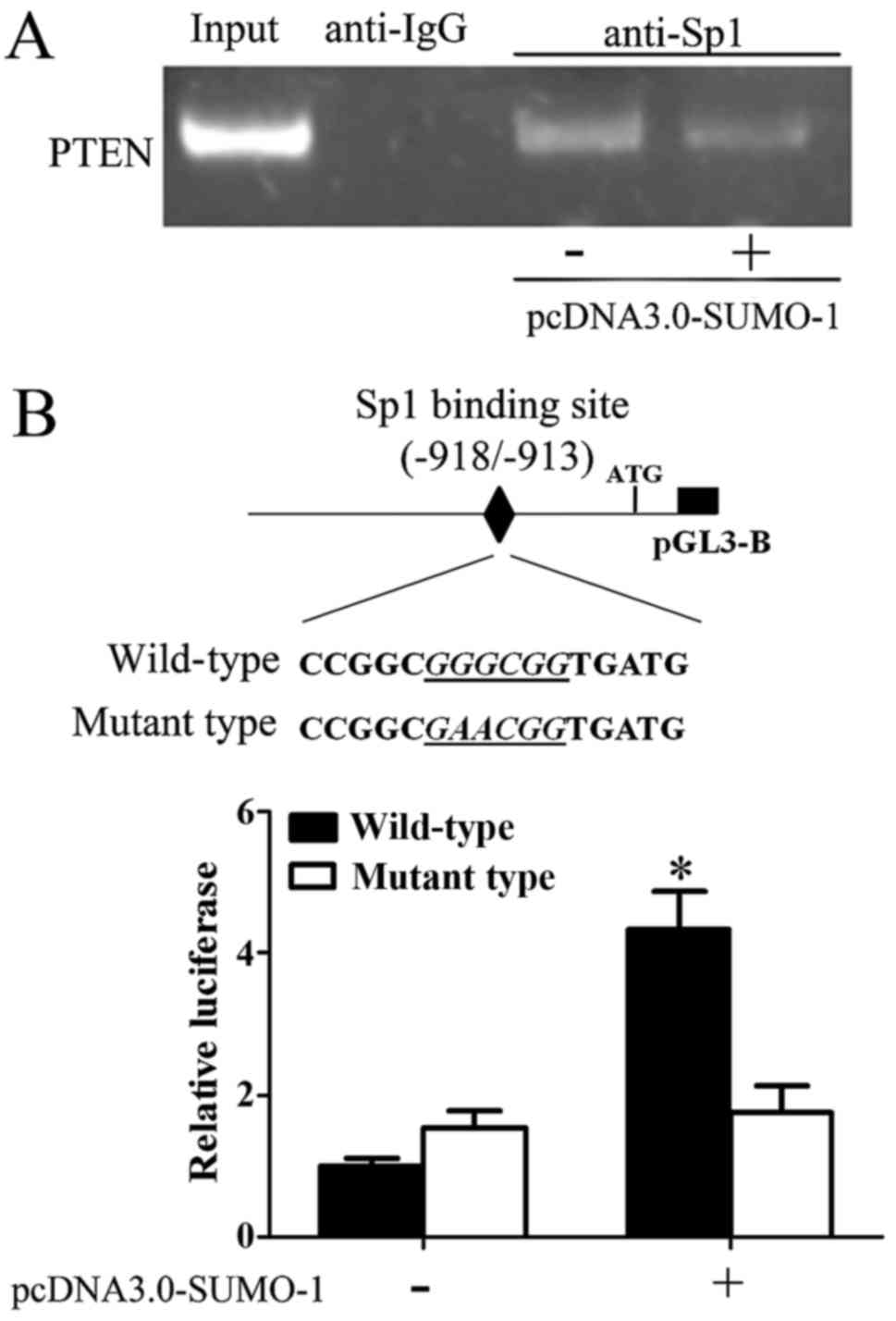
Since we demonstrated that BetA induced sumoylation
of Sp1 and correspondingly induced Sp1 downregulation and PTEN
overexpression, we investigated how sumoylation of Sp1 regulated
PTEN. We transfected SUMO-1 into CAL-27 cells to induce Sp1
sumoylation and then performed ChIP assay. As shown in Fig. 6A, increase of Sp1 sumoylation
inhibited Sp1 DNA-binding activity to PTEN promoter. Further, we
also detected PTEN promoter activity. We found that overexpression
of SUMO-1 decreased PTEN promoter activity, whereas, once we
mutated Sp1 specific binding site in PTEN promoter, overexpression
of SUMO-1 could not regulate the PTEN promoter (Fig. 6B), indicating that sumoylation
regulated Sp1 DNA binding activity.
Discussion
In the present study, we proved for the first time
that BetA induced radiosensitization in OSCC through inducing Sp1
sumoylation. First, BetA increased radiosensitization in OSCC.
Second, Sp1 was upregulated after radiation and responsible for
radioresistance, however, BetA could antagonize radiation-induced
Sp1 overexpression. Third, BetA inhibited Sp1 expression through
inducing Sp1 sumoylation, correspondingly inducing PTEN
expression.
Overexpression of Sp1 mediates radioresistance in
OSCC. Radioresistance is a huge impediment to radiotherapy
(52). Cancer cells resistant to
radiotherapy can result in therapy failure and the local recurrence
(53,54). Several pathways and genes have been
reported to be involved in radioresistance including nuclear
factor-κB (NF-κB), signal transducer and activator of transcription
3 (STAT3), DNA-dependent protein kinase catalytic subunit
(DNA-PKcs) and ataxia telangiectasia-mutated gene (ATM) (14–18).
Our finding of Sp1 involved in regulating radiosensitization
through regulating PTEN revealed a new mechanism of radioresistance
in OSCC. PTEN inhibited AKT activation thus it is involved in three
major radioresistance mechanisms: intrinsic radioresistance,
tumor-cell proliferation, and hypoxia (55). Actually, Sp1 could interact with
some other traditional molecules which could influence the outcome
of radiotherapy, for example, Sp1 could transcriptionally regulate
DNA-PKcs expression through binding to a GC-rich region in the
promoter (56). The interactions of
Sp1 with these molecules could be possible mechanisms for inducing
radioresistance, however, our finding of Sp1 regulating PTEN at
least partially involves in radioresistance. Our results suggested
that Sp1 could be a promising target for increasing
radiosensitization in OSCC radiotherapy.
BetA enhanced radiosensitization at least partially
by regulating Sp1/PTEN. We observed that BetA could significantly
enhance radiosensitization of the two types of cancer cells. We
also observed that BetA significantly inhibited Sp1 expression as
well as induced PTEN expression. Thereby BetA could rescue
radiation-induced Sp1 overexpression and PTEN inhibition.
Considering that PTEN plays a crucial role in radiosensitization
(34,57,58),
our results suggested that inhibition of Sp1 expression and
induction of PTEN expression by BetA contributed to the
radiosensitization of OSCC. Actually BetA and its derivatives have
already been demonstrated to regulate radiosensitization (50,51,59).
Bache et al used BetA at a concentration of 10 µM and
demonstrated that radiosensitization ability of BetA could be
enhanced in hypoxic condition (51); our study agreed with theirs, while
whether Sp1/PTEN pathway involves in hypoxic-induced
radiosensitization ability enhancement of BetA is still unknown.
Furthermore, there are also several mechanisms reveled underlying
BetA-induced radiosensitization (50,51,59).
However, our funding could be another important mechanism
underlying the BetA enhancement of radiosensitization besides the
other previously reported mechanisms (50,51,59),
and further supported the assumption that BetA could be a
clinically available and promising enhancer of tumor radiotherapy.
According to our results, the enhancement of radiosensitization by
BetA could be compromised in tumors with aberrant PTEN. Further
studies are needed to evaluate how much the enhancement on
radiosensitization by BetA would be compromised in PTEN-null cells.
Nevertheless, our results have clinical relevance because the
application of BetA as an enhancer for radiotherapy may be more
suitable for tumors whose PTEN was not mutated or deleted.
BetA upregulated PTEN protein expression partially
by inhibiting Sp1 expression, thereby activated PTEN transcription.
Several transcription factor regulating PTEN have been identified,
including NF-κB, Egr-1, p53 and Sp1 (26,60,61).
Sp1 downregulates PTEN expression through binding to a specific
site (26). Our study showed that
BetA inhibited Sp1 sumoylation and correspondingly upregulated PTEN
expression through inhibiting Sp1 expression and DNA-binding
activity. Considering that we have previously demonstrated that
only acetylated Sp1 could regulated PTEN expression (26), so whether the two post-translational
modifications, sumoylation and acetylation, could be
inter-regulated by each other or have competition needs further
study. There are already several reports on interaction of
sumoylation and acetylation, as Kim et al reported that
acetylation of FXR at site K217 inhibited the sumoylation of FXR at
K227 (62). Therefore, it is
possible that there is a mutual regulation of Sp1 sumoylation and
acetylation. Furthermore, BetA was reported to affect Sp1
sumoylation through sentrin-specific protease 1. Our study
demonstrated BetA regulating Sp1 sumoylation while revealing a new
mechanism. Although, which mechanism is more important remains
unknown, these two studies do suggest BetA could be a promising
anticancer drug. Sumoylation of Sp1 has already been reported to be
affected by BetA (63), our study
agreed their conclusion and further illustrated that BetA regulated
PTEN through Sp1 sumoylation and correspondingly induced
radiosensitization. In addition, Sp1 was reported to have two
sumoylation sites, K16 and K683 (28), but in treatment with BetA, which one
was sumoylated remains unknown, so further study to confirm which
one was involved in BetA-induced Sp1 sumoylation is required.
There are several limitation as some questions are
still unclear: 1) sumoylation regulates Sp1 binding to PTEN
promoter, but the underlying mechanism needs to be revealed; 2)
radiosensitization ability of BetA could be enhanced in hypoxic
condition (51); however, whether
Sp1/PTEN pathway is involved in this process is unknown. These are
very interesting questions, and we will continue their
clarification. Further animal study and preclinical studies are
needed to support our conclusion.
In conclusion, we showed that BetA blocked
radiation-induced Sp1 overexpression and PTEN downregulation
through inducing Sp1 sumoylation and thereby contributed to
radiosensitization of OSCC.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant no. 81272958 and 81472530).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao L: Oral squamous cell carcinoma -
progresses from risk assessment to treatment. Chin J Dent Res.
15:83–88. 2012.PubMed/NCBI
|
|
6
|
Wolchok JD and Chan TA: Cancer: Antitumour
immunity gets a boost. Nature. 515:496–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumann M, Krause M, Overgaard J, Debus J,
Bentzen SM, Daartz J, Richter C, Zips D and Bortfeld T: Radiation
oncology in the era of precision medicine. Nat Rev Cancer.
16:234–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin NE and D'Amico AV: Progress and
controversies: Radiation therapy for prostate cancer. CA Cancer J
Clin. 64:389–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones RM, Sloane VM, Wu H, Luo L, Kumar A,
Kumar MV, Gewirtz AT and Neish AS: Flagellin administration
protects gut mucosal tissue from irradiation-induced apoptosis via
MKP-7 activity. Gut. 60:648–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jun S, Jung YS, Suh HN, Wang W, Kim MJ, Oh
YS, Lien EM, Shen X, Matsumoto Y, McCrea PD, et al: LIG4 mediates
Wnt signalling-induced radioresistance. Nat Commun. 7:109942016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Advani SJ, Camargo MF, Seguin L, Mielgo A,
Anand S, Hicks AM, Aguilera J, Franovic A, Weis SM and Cheresh DA:
Kinase-independent role for CRAF-driving tumour radioresistance via
CHK2. Nat Commun. 6:81542015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu HC, Yang YC, Chen YJ, Lin H, Ou YC,
Chien CC, Huang EY, Huang HY, Lan J, Chi HP, et al: Increased
expression of SKP2 is an independent predictor of locoregional
recurrence in cervical cancer via promoting DNA-damage response
after irradiation. Oncotarget. 7:44047–44061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai M, Ma X, Li X, Wang X, Mei Q, Li X, Wu
Z and Han W: The accomplices of NF-κB lead to radioresistance. Curr
Protein Pept Sci. 16:279–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed KM, Zhang H and Park CC: NF-κB
regulates radioresistance mediated by β1-integrin in
three-dimensional culture of breast cancer cells. Cancer Res.
73:3737–3748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aggarwal BB and Sung B: NF-κB in cancer: A
matter of life and death. Cancer Discov. 1:469–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y,
Zhang J, Yuan J, Wang M, Chen D, Sun Y, et al: ATM-mediated
stabilization of ZEB1 promotes DNA damage response and
radioresistance through CHK1. Nat Cell Biol. 16:864–875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Yang H, Palmbos PL, Ney G, Detzler
TA, Coleman D, Leflein J, Davis M, Zhang M, Tang W, et al:
ATDC/TRIM29 phosphorylation by ATM/MAPKAP kinase 2 mediates
radioresistance in pancreatic cancer cells. Cancer Res.
74:1778–1788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Kang M, Qin YT, Wei ZX, Xiao JJ
and Wang RS: Sp1 is over-expressed in nasopharyngeal cancer and is
a poor prognostic indicator for patients receiving radiotherapy.
Int J Clin Exp Pathol. 8:6936–6943. 2015.PubMed/NCBI
|
|
20
|
Kang M, Xiao J, Wang J, Zhou P, Wei T,
Zhao T and Wang R: MiR-24 enhances radiosensitivity in
nasopharyngeal carcinoma by targeting SP1. Cancer Med. 5:1163–1173.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enomoto A, Fukasawa T, Takamatsu N, Ito M,
Morita A, Hosoi Y and Miyagawa K: The HSP90 inhibitor
17-allylamino-17-demethoxygeldanamycin modulates radiosensitivity
by downregulating serine/threonine kinase 38 via Sp1 inhibition.
Eur J Cancer. 49:3547–3558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cawley S, Bekiranov S, Ng HH, Kapranov P,
Sekinger EA, Kampa D, Piccolboni A, Sementchenko V, Cheng J,
Williams AJ, et al: Unbiased mapping of transcription factor
binding sites along human chromosomes 21 and 22 points to
widespread regulation of noncoding RNAs. Cell. 116:499–509. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zannetti A, Del Vecchio S, Carriero MV,
Fonti R, Franco P, Botti G, D'Aiuto G, Stoppelli MP and Salvatore
M: Coordinate up-regulation of Sp1 DNA-binding activity and
urokinase receptor expression in breast carcinoma. Cancer Res.
60:1546–1551. 2000.PubMed/NCBI
|
|
24
|
Vizcaíno C, Mansilla S and Portugal J: Sp1
transcription factor: A long-standing target in cancer
chemotherapy. Pharmacol Ther. 152:111–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Wei D, Huang S, Peng Z, Le X, Wu
TT, Yao J, Ajani J and Xie K: Transcription factor Sp1 expression
is a significant predictor of survival in human gastric cancer.
Clin Cancer Res. 9:6371–6380. 2003.PubMed/NCBI
|
|
26
|
Kou XX, Hao T, Meng Z, Zhou YH and Gan YH:
Acetylated Sp1 inhibits PTEN expression through binding to PTEN
core promoter and recruitment of HDAC1 and promotes cancer cell
migration and invasion. Carcinogenesis. 34:58–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang HC, Chuang JY, Jeng WY, Liu CI, Wang
AH, Lu PJ, Chang WC and Hung JJ: Pin1-mediated Sp1 phosphorylation
by CDK1 increases Sp1 stability and decreases its DNA-binding
activity during mitosis. Nucleic Acids Res. 42:13573–13587. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong L, Ji WK, Hu XH, Hu WF, Tang XC,
Huang ZX, Li L, Liu M, Xiang SH, Wu E, et al: Sumoylation
differentially regulates Sp1 to control cell differentiation. Proc
Natl Acad Sci USA. 111:5574–5579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HJ, Ryu JM, Jung YH, Lee KH, Kim DI
and Han HJ: Glycerol-3-phosphate acyltransferase-1 upregulation by
O-GlcNAcylation of Sp1 protects against hypoxia-induced mouse
embryonic stem cell apoptosis via mTOR activation. Cell Death Dis.
7:e21582016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang WC and Hung JJ: Functional role of
post-translational modifications of Sp1 in tumorigenesis. J Biomed
Sci. 19:942012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maehama T and Dixon JE: The tumor
suppressor, PTEN/MMAC1, dephosphorylates the lipid second
messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem.
273:13375–13378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng Z and Gan YH: Activating PTEN by
COX-2 inhibitors antagonizes radiation-induced AKT activation
contributing to radiosensitization. Biochem Biophys Res Commun.
460:198–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng L, Zhang Y, Liu Y, Zhou M, Lu Y,
Yuan L, Zhang C, Hong M, Wang S and Li X: MiR-106b induces cell
radioresistance via the PTEN/PI3K/AKT pathways and p21 in
colorectal cancer. J Transl Med. 13:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bardia A, Keenan TE, Ebbert JO, Lazovich
D, Wang AH, Vierkant RA, Olson JE, Vachon CM, Limburg PJ, Anderson
KE, et al: Personalizing aspirin use for targeted breast cancer
chemoprevention in postmenopausal women. Mayo Clin Proc. 91:71–80.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nimptsch K, Zhang X, Cassidy A, Song M,
O'Reilly ÉJ, Lin JH, Pischon T, Rimm EB, Willett WC, Fuchs CS, et
al: Habitual intake of flavonoid subclasses and risk of colorectal
cancer in 2 large prospective cohorts. Am J Clin Nutr. 103:184–191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He E, Pan F, Li G and Li J: Fractionated
ionizing radiation promotes epithelial-mesenchymal transition in
human esophageal cancer cells through PTEN deficiency-mediated Akt
activation. PLoS One. 10:e01261492015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lo YC, Chang YH, Wei BL, Huang YL and
Chiou WF: Betulinic acid stimulates the differentiation and
mineralization of osteoblastic MC3T3-E1 cells: Involvement of
BMP/Runx2 and beta-catenin signals. J Agric Food Chem.
58:6643–6649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lingaraju MC, Pathak NN, Begum J,
Balaganur V, Bhat RA, Ram M, Kumar D, Kumar D and Tandan SK:
Betulinic acid negates oxidative lung injury in surgical sepsis
model. J Surg Res. 193:856–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alakurtti S, Mäkelä T, Koskimies S and
Yli-Kauhaluoma J: Pharmacological properties of the ubiquitous
natural product betulin. Eur J Pharm Sci. 29:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ehrhardt H, Fulda S, Führer M, Debatin KM
and Jeremias I: Betulinic acid-induced apoptosis in leukemia cells.
Leukemia. 18:1406–1412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan Y, Yu R and Pezzuto JM: Betulinic
acid-induced programmed cell death in human melanoma cells involves
mitogen-activated protein kinase activation. Clin Cancer Res.
9:2866–2875. 2003.PubMed/NCBI
|
|
44
|
Pisha E, Chai H, Lee IS, Chagwedera TE,
Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown
DM, et al: Discovery of betulinic acid as a selective inhibitor of
human melanoma that functions by induction of apoptosis. Nat Med.
1:1046–1051. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang DM, Xu HG, Wang L, Li YJ, Sun PH, Wu
XM, Wang GJ, Chen WM and Ye WC: Betulinic acid and its derivatives
as potential antitumor agents. Med Res Rev. 35:1127–1155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li L, Du Y, Kong X, Li Z, Jia Z, Cui J,
Gao J, Wang G and Xie K: Lamin B1 is a novel therapeutic target of
betulinic acid in pancreatic cancer. Clin Cancer Res. 19:4651–4661.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao Y, Jia Z, Kong X, Li Q, Chang DZ, Wei
D, Le X, Suyun H, Huang S, Wang L, et al: Combining betulinic acid
and mithramycin a effectively suppresses pancreatic cancer by
inhibiting proliferation, invasion, and angiogenesis. Cancer Res.
71:5182–5193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kasperczyk H, La Ferla-Brühl K, Westhoff
MA, Behrend L, Zwacka RM, Debatin KM and Fulda S: Betulinic acid as
new activator of NF-kappaB: Molecular mechanisms and implications
for cancer therapy. Oncogene. 24:6945–6956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zuco V, Supino R, Righetti SC, Cleris L,
Marchesi E, Gambacorti-Passerini C and Formelli F: Selective
cytotoxicity of betulinic acid on tumor cell lines, but not on
normal cells. Cancer Lett. 175:17–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bache M, Bernhardt S, Passin S, Wichmann
H, Hein A, Zschornak M, Kappler M, Taubert H, Paschke R and
Vordermark D: Betulinic acid derivatives NVX-207 and B10 for
treatment of glioblastoma - an in vitro study of cytotoxicity and
radiosensitization. Int J Mol Sci. 15:19777–19790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bache M, Zschornak MP, Passin S, Kessler
J, Wichmann H, Kappler M, Paschke R, Kaluđerović GN, Kommera H,
Taubert H, et al: Increased betulinic acid induced cytotoxicity and
radiosensitivity in glioma cells under hypoxic conditions. Radiat
Oncol. 6:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bernier J: Current state-of-the-art for
concurrent chemoradiation. Semin Radiat Oncol. 19:3–10. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee S, Lim MJ, Kim MH, Yu CH, Yun YS, Ahn
J and Song JY: An effective strategy for increasing the
radiosensitivity of Human lung Cancer cells by blocking
Nrf2-dependent antioxidant responses. Free Radic Biol Med.
53:807–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Provencio M, Sánchez A, Garrido P and
Valcárcel F: New molecular targeted therapies integrated with
radiation therapy in lung cancer. Clin Lung Cancer. 11:91–97. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nishida Y, Mizutani N, Inoue M, Omori Y,
Tamiya-Koizumi K, Takagi A, Kojima T, Suzuki M, Nozawa Y, Minami Y,
et al: Phosphorylated Sp1 is the regulator of DNA-PKcs and DNA
ligase IV transcription of daunorubicin-resistant leukemia cell
lines. Biochim Biophys Acta. 1839:265–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W, et al: MiR-20a induces cell
radioresistance by activating the PTEN/PI3K/Akt signaling pathway
in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Eder-Czembirek C, Erovic BM, Czembirek C,
Brunner M, Selzer E, Pötter R and Thurnher D: Betulinic acid a
radiosensitizer in head and neck squamous cell carcinoma cell
lines. Strahlenther Onkol. 186:143–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stambolic V, MacPherson D, Sas D, Lin Y,
Snow B, Jang Y, Benchimol S and Mak TW: Regulation of PTEN
transcription by p53. Mol Cell. 8:317–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Virolle T, Adamson ED, Baron V, Birle D,
Mercola D, Mustelin T and de Belle I: The Egr-1 transcription
factor directly activates PTEN during irradiation-induced
signalling. Nat Cell Biol. 3:1124–1128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim DH, Xiao Z, Kwon S, Sun X, Ryerson D,
Tkac D, Ma P, Wu SY, Chiang CM, Zhou E, et al: A dysregulated
acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity.
EMBO J. 34:184–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hsu TI, Wang MC, Chen SY, Huang ST, Yeh
YM, Su WC, Chang WC and Hung JJ: Betulinic acid decreases
specificity protein 1 (Sp1) level via increasing the sumoylation of
sp1 to inhibit lung cancer growth. Mol Pharmacol. 82:1115–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|