Introduction
Hepatocellular carcinoma (HCC) is a common cancer
worldwide (1). In China, the
incidence of HCC accounts for more than 50% of all cancer cases
(2). Only 5% of HCC patients
survive more than five years (3),
which is mainly attributed to late diagnosis. Patients who undergo
surgical therapy usually have a high recurrence rate which may be
another reason for the low survival rate. Therefore, a full
understanding of the mechanisms underlying HCC tumorigenesis and
tumor progression is needed for improving the diagnosis and
clinical therapy of HCC. Recently accumulating studies have
demonstrated that microRNAs, a type of non-coding small RNAs play
important biological roles in the tumorigenesis and tumor
progression of HCC, providing new insights into the therapy of HCC
(4–7).
MicroRNAs (miRNAs) are a series of small non-coding
RNAs, formed by ~21–24 nucleotides, and they regulated gene
expression by targeting mRNAs, which may lead to mRNA degradation
or translation suppression (8,9).
Recently, studies indicate that deregulation of miRNAs causes the
regulation of cell proliferation, differentiation and apoptosis,
and these biological functions are important in cancer development
(10,11). Several studies reported that
miR-196a expression is upregulated in many human cancers including
non-small cell lung cancer, gastric and breast cancer (12–15).
miR-196a is transcribed from miR-196a-1 and miR-196a-2; miR-196a
plays important biological roles in the development of human cancer
(16–18). However, the biological role of
miR-196a in human liver cancer is not well established. In the
present study, we evaluated the expression of miR-196a in human
liver cancer cells, investigated the biological roles of miR-196a
and aimed to ascertain the mechanisms underlying the effects of
miR-196a on human liver cancer cells.
Materials and methods
Cell lines and animals
All the liver cancer cell lines were purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells (Huh7, MHCC-97H, Hep3B, HepG2 and
SMMC-7721) were cultured in Dulbeccos modified Eagles medium (DMEM)
which was supplemented with fetal bovine serum (FBS) (1:10),
penicillin (1:100) and streptomycin (1:100) (all from Invitrogen,
Carlsbad, CA, USA). Primary human hepatocytes (PHHs) and PHH medium
were purchased from the Research Institute for Liver Diseases Co.
Ltd. (Shanghai, China). BALB/c nude mice (4–5 weeks of age)
(female) were purchased from SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). Mice were housed in an SPF animal facility for
at least 7 days before the start of the present study. All animal
studies were performed according to the requirements of the ‘Guide
for the Care and Use of Laboratory Animals’ [National Research
Council (US) Committee].
miR-196a quantification
The total RNA was extracted from the human liver
cancer cells using TRIzol. cDNA was transcribed from the extracted
total RNA using a TaqMan® MicroRNA Reverse Transcription
kit (Applied Biosystems™). qPCR assay was performed for the
quantification of miR-196a; the PCR assay was performed using
TaqMan® Universal PCR Master Mix (Applied Biosystems™).
The primers used were: miR-196a forward, CGTCAGAAGGAATGATGCACAG and
reverse, ACCTGCGTAGGTAGTTTCATGT. RNA U6 was used as an internal
control; the primers used for U6 were forward, CTCGCTTCGGCAGCACA
and reverse, AACGCT TCACGAATTTGCGT. The miR-196a expression was
normalized with U6. Real-time PCR was performed on a LightCycler
480 Real-Time PCR System (Roche Diagnostics, Basel, Switzerland) 40
cycles of 95°C for 10 sec, and 60°C for 30 sec).
Transfection assay
Human liver cancer cells were cultured for 24 h in
DMEM which was supplemented with FBS. Cultured cells were
transfected with the miR-196a or miR control inhibitor using
Lipofectamine® 3000 reagent (Invitrogen™). Both miR-196a
inhibitor and miR control inhibitor were purchased from Exiqon,
Inc. (Woburn, MA, USA). The final concentration of inhibitor used
was 10 nM. For the FOXO1 knockdown assay, the present study was
performed according to a previously described protocol (19). The siRNA was purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
MTT and colony formation assays
For the MTT assay, human liver cancer cells were
transfected with miR-196a or miR control inhibitor, and cultured
(3,500 cells/well) in a 96-well plate. The cell proliferation was
evaluated every 24 h using an MTT kit (cat. no. C0009, Beyotime
Institute of Biotechnology, Nantong, China) following the
manufacturer's protocol. For colony formation assay, human liver
cancer cells were transfected with miR-196a or control inhibitor
and seeded into a 6-well plate (500 cells/well). Cells were
cultured for another 2 weeks, and then the cells were fixed with
methanol and stained with crystal violet; the numbers of colonies
were counted. All the experiments were performed in triplicate.
Cell invasion assays
For the cell invasion assay, transfected cells were
placed into the upper chamber of an insert coated with Matrigel
(500,000 cells/well). Cell culture medium with FBS was added into
the lower culture chamber. After a 12-h incubation, the cells
remaining on the upper membrane (not migrated) were removed by
washing with cold phosphate-buffered saline (PBS) twice; while the
cells that had migrated were fixed and stained with crystal violet
solution. The number of cells was counted using an inverted
microscope. Each sample was counted in five random fields.
Cell apoptosis assay
Human liver cancer cells were transfected with
miR-196a or miR control inhibitor, and cultured for 48 h. Then, the
cells were harvested and stained with FITC-Annexin V and propidium
iodide. FACS assay was performed to evaluate cell apoptosis using
flow cytometry. Data were analyzed using CellQuest software (both
from BD Biosciences, San Jose, CA, USA). All the experiments were
performed in triplicate.
Experimental metastasis assay in
vivo
To investigate the effect of miR-196a on human liver
cancer cell metastasis in vivo, a mouse model was used. Mock
and transfected HepG2 cells were injected into 4- to 5-week-old
nude mice via tail vein (4,000,000 cells/mouse; 0.2 ml). Four weeks
later, the mice were sacrificed and the mouse liver tissues were
harvested. Then, the number of metastatic tumor nodules were
counted under a dissecting microscope.
Dual-luciferase assay
To construct the FOXO1 luciferase report vector, the
wild-type (WT) or mutant (Mut) FOXO1 gene was cloned downstream of
the luciferase gene in the pLUC luciferase vector. Then, the
reporter vectors were cotransfected with the miR-196a mimic or
control mimic into HepG2 cells using Lipofectamine® 3000
reagent (Invitrogen). Cells were cultured for another 48 h, and
then harvested and lysed for the luciferase assay using a
luciferase assay kit. Relative luciferase activity was normalized
with the Renilla luciferase activity. All the experiments
were performed in triplicate.
Real-time PCR
Human liver cancer cells were transfected with
miR-196a inhibitor or control inhibitor and cultured. The total RNA
was extracted from the human liver cancer cells using TRIzol
(Invitrogen). cDNA was transcribed from the extracted total RNA
using Reverse Transcriptase MMLV (Takara, Shiga, Japan). PCR
reactions were performed as followed: 95°C for 5 min, followed by
45 cycles of 15 sec at 95°C, 30 sec at 60°C and 15 sec at 72°C. The
primers used for FOXO1 were purchased from Bio-Rad (Hercules, CA,
USA). The primers used were: GAPDH forward, GGTGATGCTGGTGCTGAGTA
and reverse, ACTGTGGTCATGAGCCCTTC. The relative expression of FOXO1
was determined using the 2−ΔΔCt method. All the
experiments were performed in triplicate.
Western blotting
Human liver cancer cells were transfected with
miR-196a or control inhibitor and cultured for 48 h, and then cells
were harvested. Protein was extracted from the cells and separated
using SDS-polyacrylamide gel. Then, the separated protein was
transferred to an NC membrane. The NC membrane was blocked with
blocking buffer for 120 min and then submitted to primary antibody
incubation at RT for another 120 min. The NC membrane was washed
with TBST three times and then the NC membrane was incubated with
the secondary antibody for 120 min. The NC membrane was washed with
Tris-buffered saline with Tween-20 (TBST) for three times again and
the target protein was examined using ECL chemiluminescence and
exposure to X-ray film. All the experiments were performed in
triplicate.
Statistical analysis
Student's t-test (two-tailed) and one-way ANOVA were
performed to analyze statistical differences using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). For in vitro study,
the data are expressed as mean ± SD; for in vivo study the
data are expressed as mean ± SEM. p<0.05 indicates a
statistically significant.
Results
miR-196a is overexpressed in human
liver cancer cells
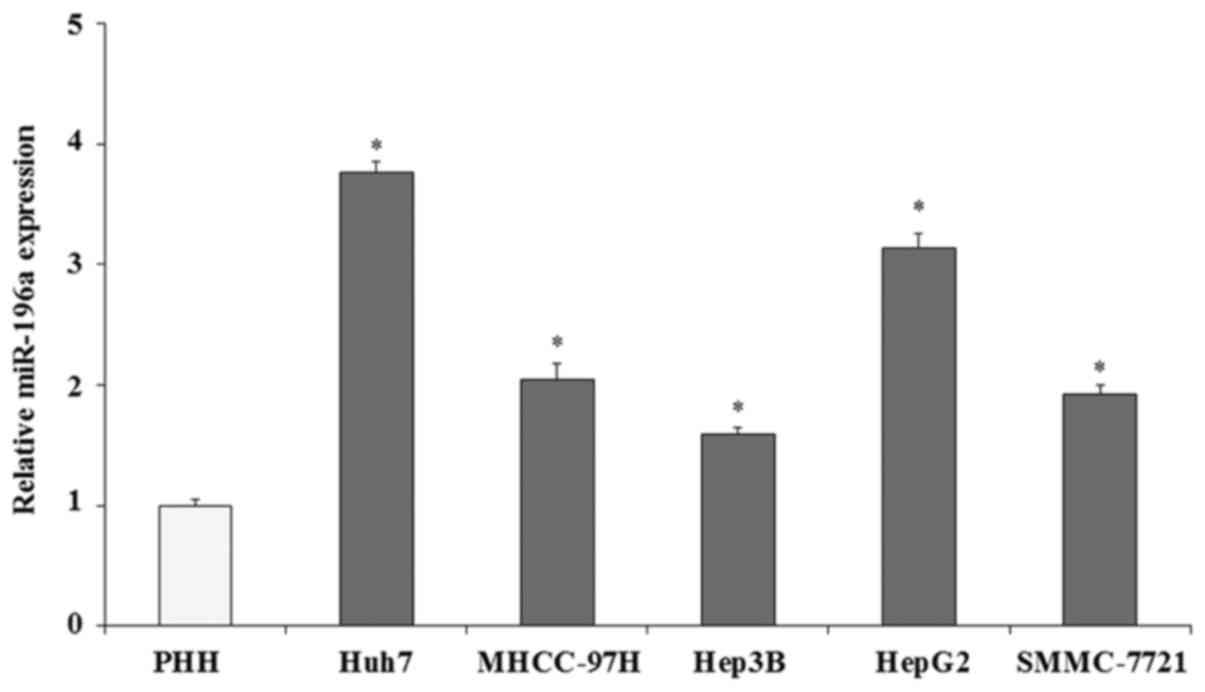
The expression of miR-196a in human liver cancer
cells was evaluated by qRT-PCR assay. The expression of miR-196a
was normalized with U6 (U6 was used as an internal control). The
qRT-PCR results demonstrated that the expression of miR-196a in
five liver cancer cell lines (Huh7, MHCC-97H, Hep3B, HepG2 and
SMMC-7721) was significantly increased compared with that in the
PHHs (Fig. 1; p<0.05),
particularly in HepG2 (3.14-fold; p<0.05) and Huh7 cells
(3.76-fold; p<0.05). These findings indicate that miR-196a is
overexpressed in human liver cancer cells. We hypothesized that
miR-196a plays important roles in the progression and development
of human liver cancer. In the present study, we selected the two
cell lines, HepG2 and Huh7, with high miR-196a expression to
investigate the biological roles of miR-196a in human liver
cancer.
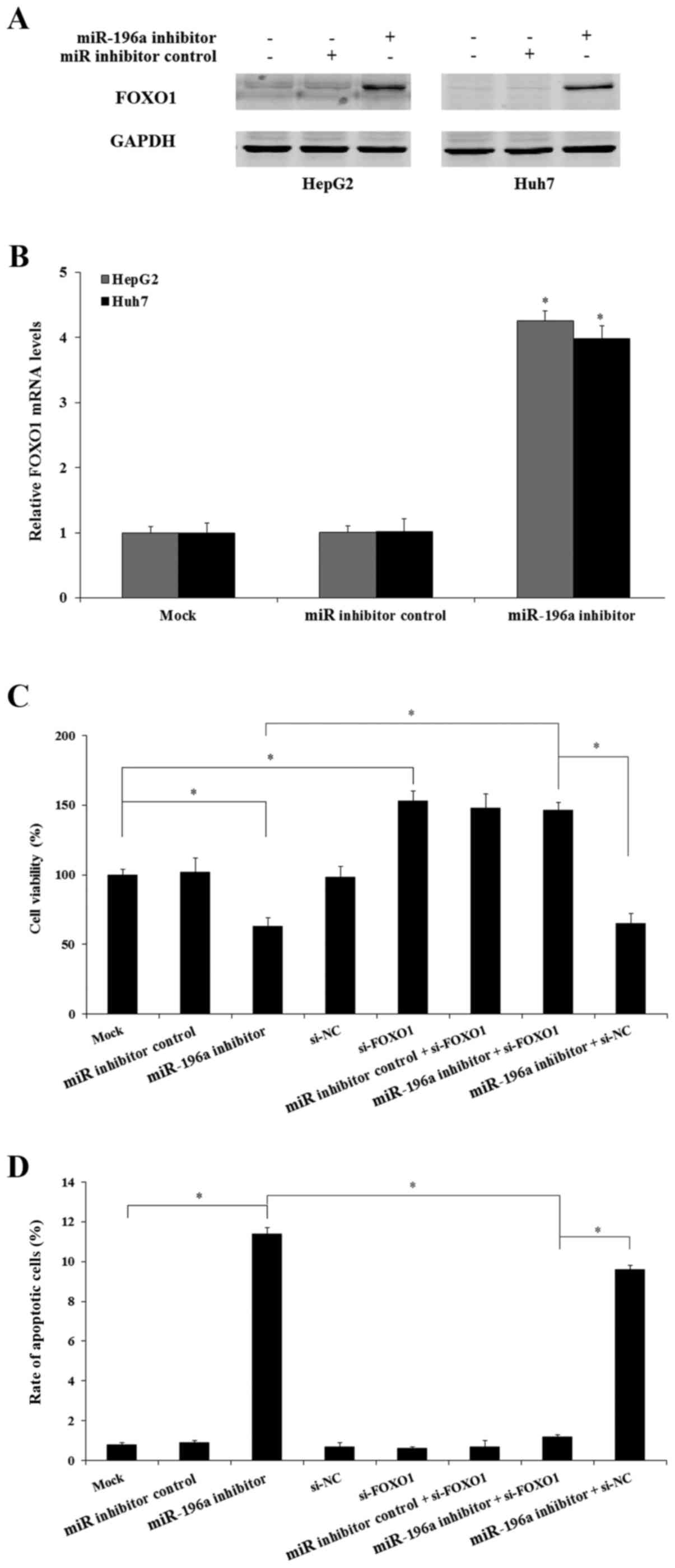
miR-196a manipulation in human liver
cancer cells
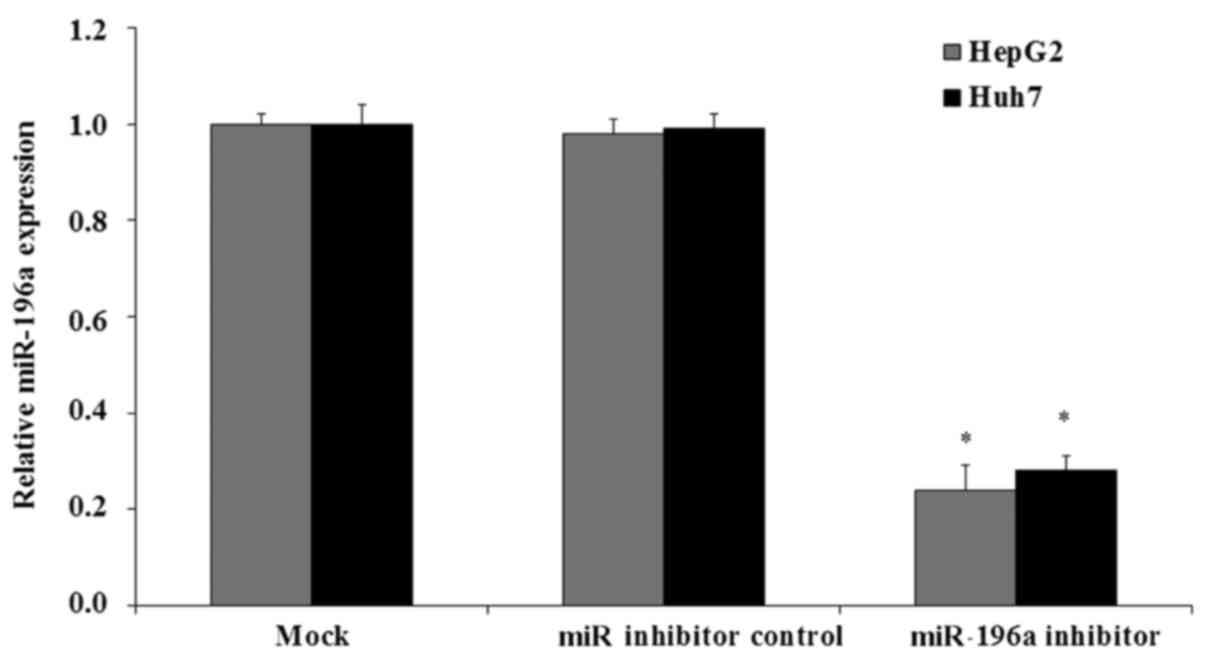
In order to selectively inhibit the expression of
miR-196a, miR-196a inhibitor transfection assay was performed.
HepG2 or Huh7 cells were cultured and transfected with miR-196a
inhibitor or miR inhibitor control and cultured for another 48 h.
The expression of miR-196a was then examined by qRT-PCR assay. The
results showed that after miR-196a inhibitor transfection the
expression of miR-196a was significantly decreased to 0.21-fold in
the HepG2 cells and 0.23-fold in the Huh7 cells, respectively,
compared with the mock HepG2 or Huh7 cells (Fig. 2; p<0.05). In contrast, in the miR
inhibitor control-transfected cells there were no significant
changes in the expression of miR-196a (Fig. 2; p>0.05).
Effect of miR-196a on human liver
cancer cell proliferation and invasion
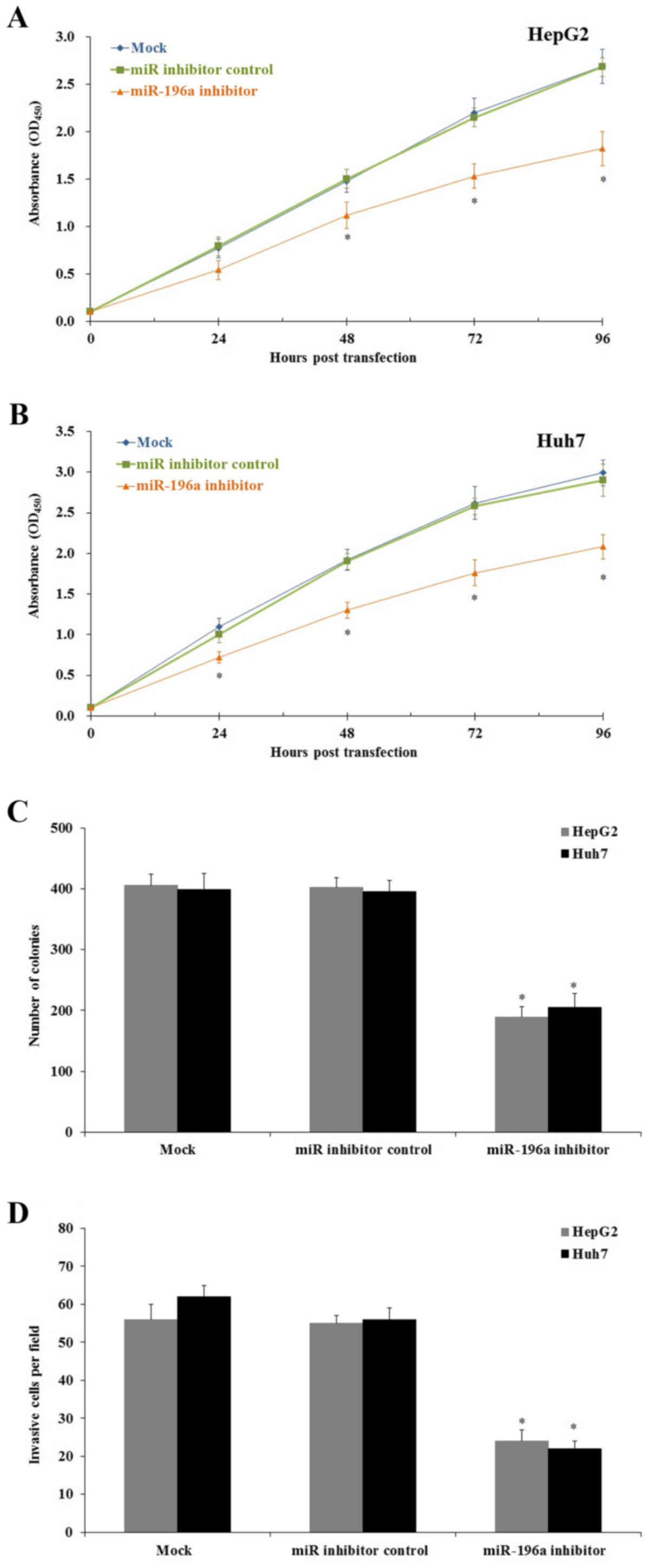
To better understand the biological roles of
miR-196a in the progression and development of human liver cancer,
we assessed the effect of miR-196a on liver cancer cell
proliferation. Human liver cancer HepG2 and Huh7 cells were seeded
into 96-well plates and the cell proliferation was evaluated by MTT
assay every 24 h. MTT results revealed that after miR-196a
inhibitor transfection the cell proliferation was significantly
inhibited when compared to that noted in the cells transfected with
the miR inhibitor control and the mock group (Fig. 3A and B; p<0.05). Then, we
performed a colony-formation assay to confirm the cell
proliferation inhibition. As we expected the results demonstrated
that downregulation of miR-196a significantly decreased the colony
formation number both in the HepG2 and Huh7 cells (Fig. 3C; p<0.05). Further study
indicated that downregulation of miR-196a significantly inhibited
human liver cancer cell invasion in vitro (Fig. 3D; p<0.05).
Effect of miR-196a on human liver
cancer cell apoptosis
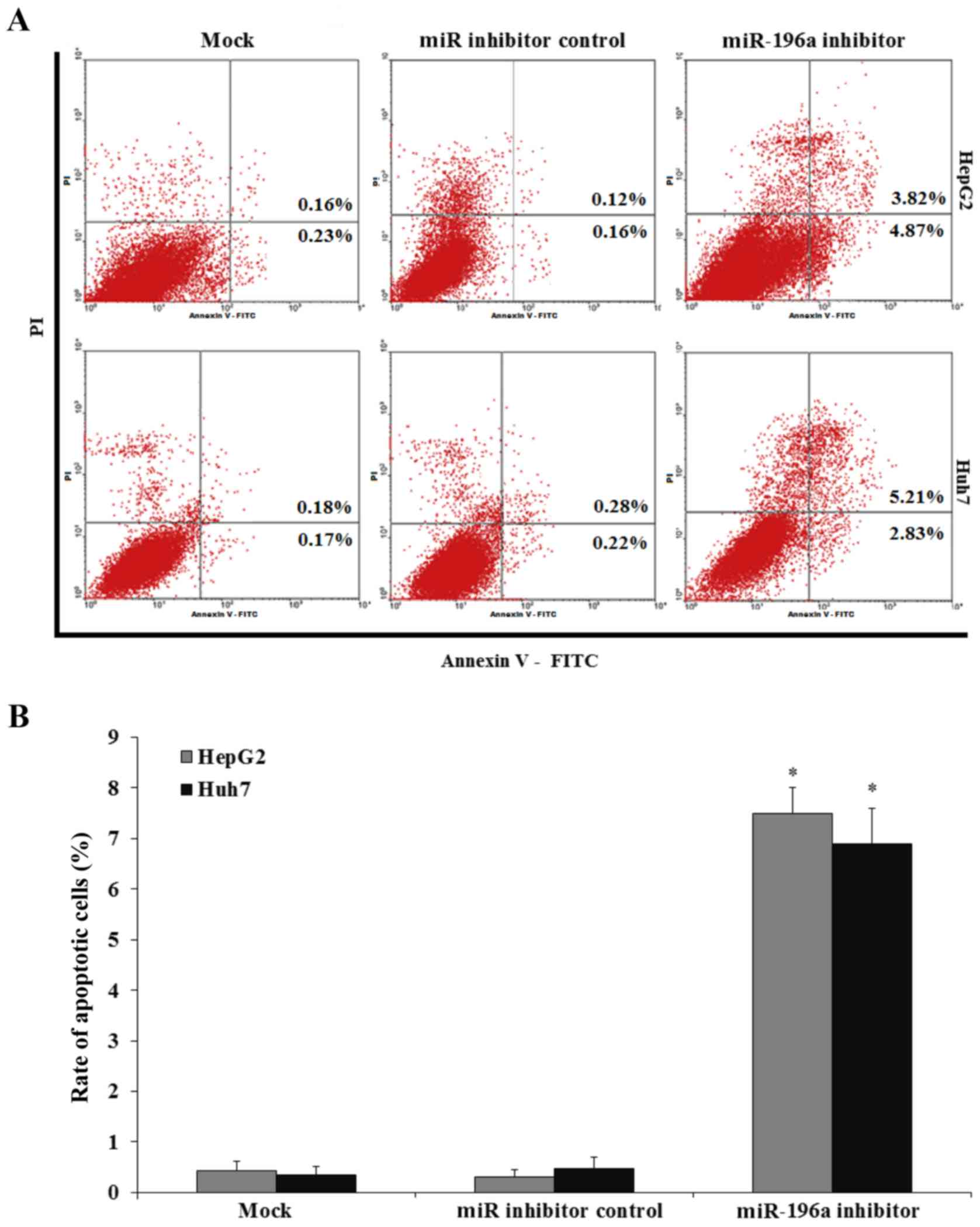
In order to investigate whether the HepG2 and Huh7
cell growth inhibition was attributed to cell apoptosis, FACS assay
was performed. HepG2 and Huh7 cells were transfected with miR-196a
or miR inhibitor control and cultured for 24 h, and then the HepG2
and Huh7 cells were harvested and submitted to a FACS assay. Our
results showed that the downregulation of miR-196a by inhibitor
transfection increased the HepG2 and Huh7 cell apoptosis, while the
miR inhibitor control transfection did not affect HepG2 and Huh7
cell apoptosis (Fig. 4; p<0.05).
Therefore, downregulation of miR-196a induced human liver cancer
cell apoptosis.
miR-196a promotes proliferation,
migration and invasion of HepG2 cells in vivo
The previous results demonstrated that inhibition of
miR-196a suppressed human liver cancer HepG2 and Huh7 cell growth
in vitro. We hypothesized that downregulation of miR-196a
would also inhibit liver cancer cell growth and migration in
vivo. We confirmed the hypothesis using an in vivo mouse
model. Mice were modeled by the injection of HepG2 cells (mock or
transfected) via tail vein; each mouse was injected with
4×106 cells. Mice were maintained in an SPF animal room
for six weeks, and then the mice were sacrificed and the mouse
livers were harvested; the metastatic nodules were counted. The
results showed that in the mock and the miR inhibitor control group
there were a few large metastatic tumors (data not shown); however,
large tumors were not observed in the miR-196a inhibitor group.
Tumor counting results showed that the number of metastatic nodules
was decreased significantly in the miR-196a inhibitor group
compared with that in the miR inhibitor control and mock group
(Fig. 5; p<0.05). Therefore, the
present study indicated that the inhibition of miR-196a decreased
the proliferation and migration of HepG2 cells in vivo.
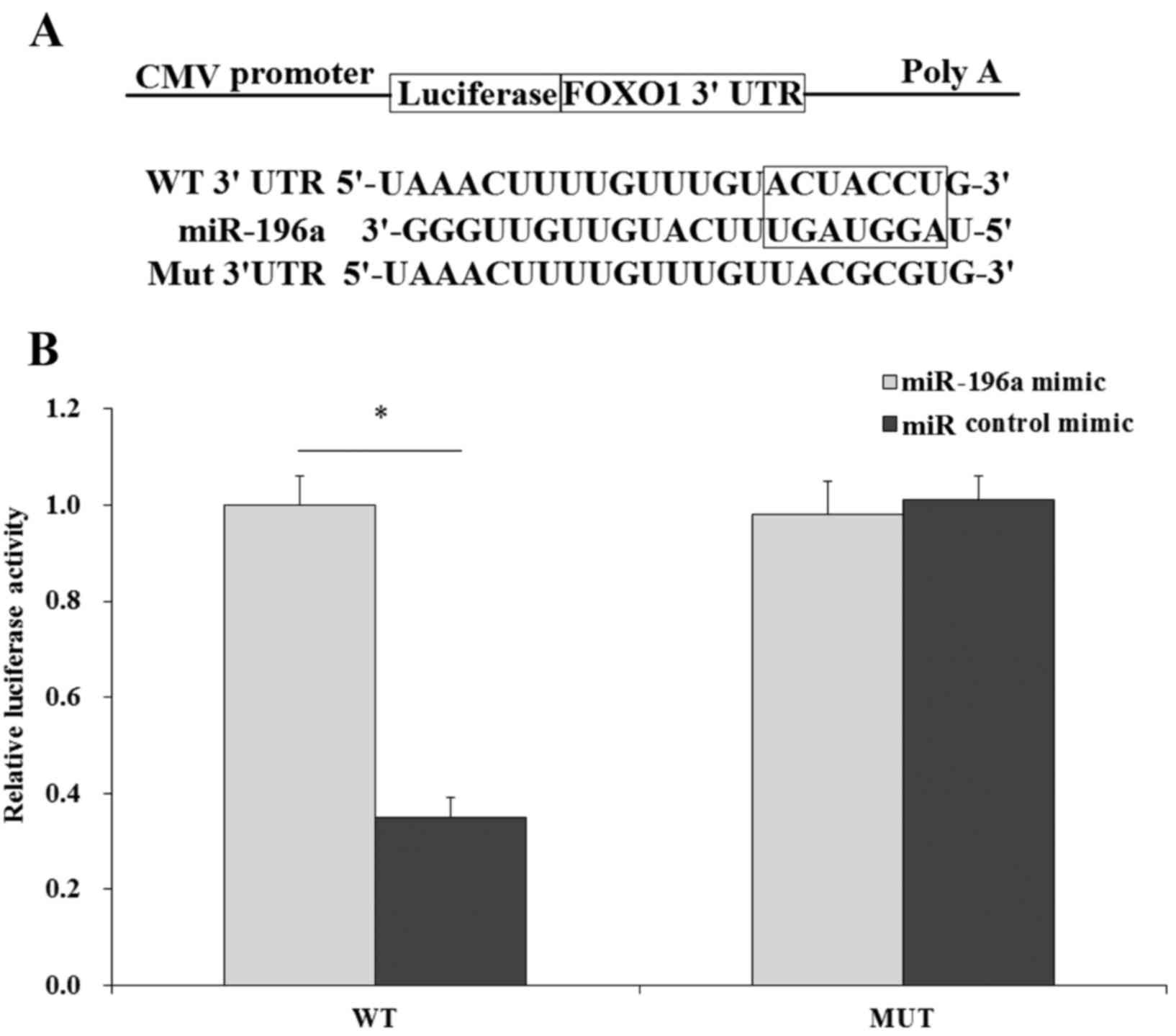
FOXO1 is a direct target of
miR-196a
To better understand the molecular mechanisms by
which miR-196a regulates the behavior of human liver cancer cells,
we aimed to identify the direct target of miR-196a. FOXO1 was found
to be a potential target of miR-196a by TargetScan program search
(Fig. 6A). Next, we investigated
whether FOXO1 is a direct target of miR-196a by dual-luciferase
assay. The WT 3 untranslated region (3′UTR) of FOXO1 was directly
fused downstream of the firefly luciferase gene, and the miR-196a
mimic and various luciferase 3′UTR constructs (WT, Mut) were
co-transfected into HepG2 cells. miR control mimic was used as the
control. Cells were cultured and luciferase activity was
determined. The results indicated that induction of miR-196a
significantly decreased the luciferase activity of the pLuc-FOXO1
3′UTR reporter (WT), while we did not observe significant
inhibition between the miR-196a mimic and miR control mimic in the
Mut group (Fig. 6B), therefore
FOXO1 was identified as a direct target of miR-196a.
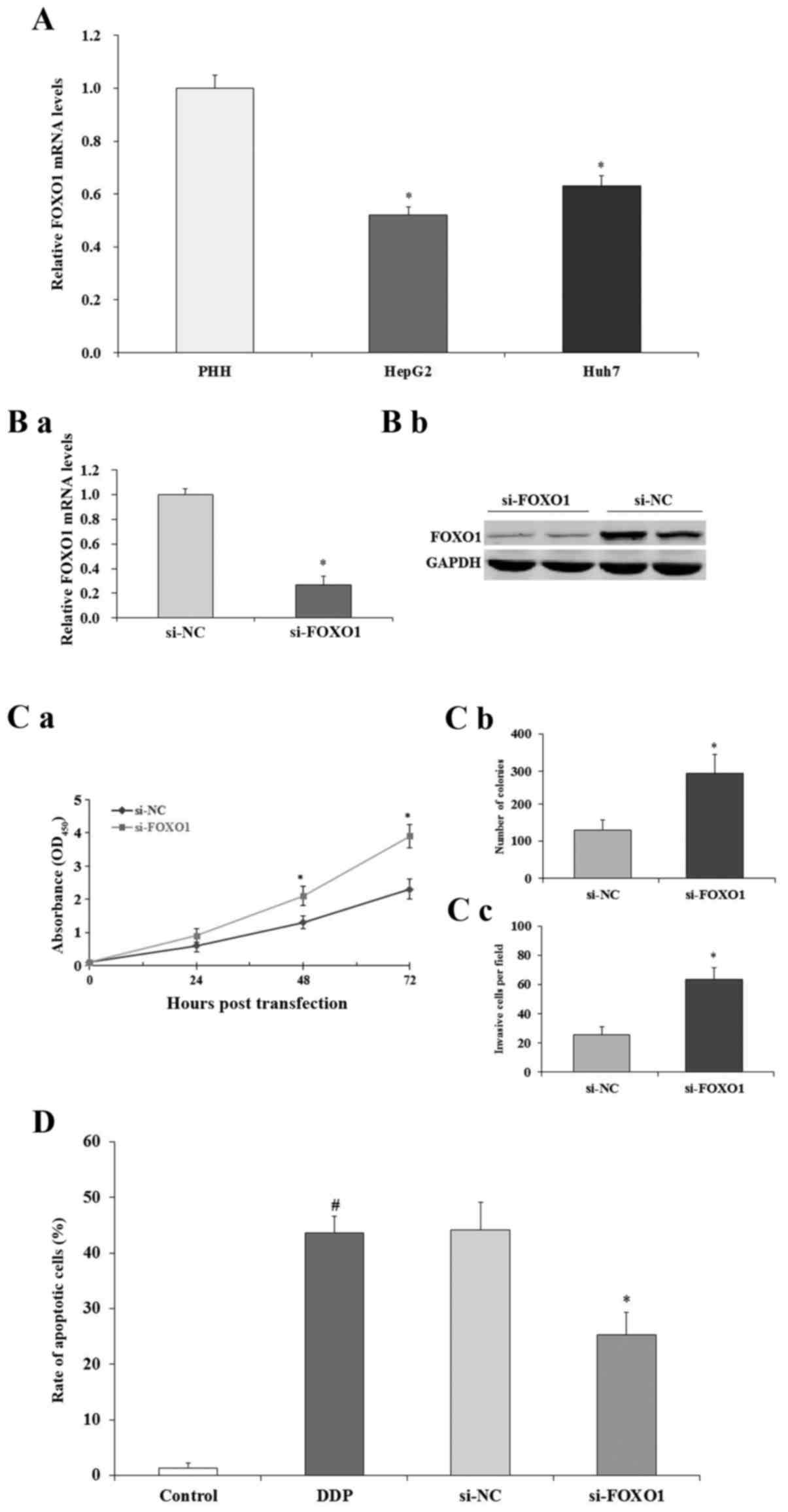
Effect of FOXO1 on human liver cancer
cells
To examine the expression of FOXO1 in human liver
cancer cells, qRT-PCR assay was performed. The expression of FOXO1
was significantly decreased in the HepG2 and Huh7 cells compared to
that noted in the PHHs (p<0.05; Fig.
7A). To selectively regulate the expression of FOXO1 in human
liver cancer cells, RNAi knockdown assay was performed. As shown in
Fig. 7B, qRT-PCR (a) and western
blotting (b) results indicated that after the transfection of
si-FOXO1, the expression of FOXO1 in the HepG2 cells was
significantly decreased both at mRNA and protein level compared to
the levels noted in the si-NC-treated HepG2 cells (p<0.05). As
shown in Fig. 7C, MTT (a) and
colony formation (b) assays demonstrated that the downregulation of
FOXO1 promoted HepG2 cell proliferation (p<0.05). Cell invasion
study indicated that downregulation of FOXO1 promoted HepG2 cell
invasion (p<0.05; Fig. 7C-c).
Then, we performed FACS assay to evaluate the effect of FOXO1 on
HepG2 cell apoptosis. FACS results indicated that downregulation of
FOXO1 decreased the apoptosis of HepG2 cells induced by cisplatin
(DDP) (p<0.05; Fig. 7D). All
these data suggest that FOXO1 expression inhibits human liver
cancer HepG2 cell growth and induces cell apoptosis.
miR-196a modulates liver cancer cell
proliferation by suppressing FOXO1
As miR-196a was found to be upregulated in human
liver cancer cells and FOXO1 is a direct target of miR-196a and
regulates the growth of human liver cancer cells, we next aimed to
determine whether miR-196a regulates human liver cancer cell growth
by the regulation of FOXO1. miR-196a inhibitor and miR inhibitor
control were transfected into HepG2 and Huh7 cells, and the
expression of FOXO1 was examined. The results indicated that
downregulation of miR-196a significantly increased the expression
of FOXO1 both at the mRNA and protein levels compared with the miR
inhibitor control and mock group (p<0.05; Fig. 8A and B). Further study indicated
that following knockdown of the FOXO1 gene by si-FOXO1, suppression
of miR-196a did not inhibit Huh7 cell proliferation (Fig. 8C) and also did not induce cell
apoptosis (Fig. 8D) in the Huh7
cells. Therefore, miR-196a regulates the growth of human liver
cancer cells by targeting FOXO1 which is a direct target of
miR-196a.
Discussion
miRs are a series of small and non-coding RNAs.
Recently studies have demonstrated that miRs play important roles
in the tumorigenesis and tumor progression of HCC (4–7).
Research also indicates that miRs regulate the expression of
several genes and pathways (CDK, Ci/Kip, PI3K/AKT and mTOR) to
regulate human liver cancer cell proliferation (20). Previous studies have shown that
miR-196a is upregulated in breast, colon, gastric, pancreatic and
cervical cancer, and miR-196a promotes cancer cell proliferation by
the negative regulation of tumor-suppressor genes (FOXO1, p27,
IκBα, netrin 4 and ING5) (21–23).
FOXO1 and p27Kip1 are direct targets of miR-196a in human cervical
cancer (21). However the
biological function of miR-196a in human liver cancers are still
not well explored. In the present study, we found that the
expression of miR-196a was upregulated in human liver cancer cells
compared to that noted in normal human liver cells. Downregulation
of miR-196a inhibited human liver cancer cell proliferation and
invasion in vitro and in vivo which were attributed
to the induction of cell apoptosis.
FOXO1 is an important member of the FOXO family. The
FOXO1 gene regulates several biological functions of cancer cells
including cancer cell proliferation, cancer cell differentiation,
cancer cell apoptosis and angiogenesis (24). Tumorigenesis is closely connected
with cell proliferation, cell cycle and cell apoptosis, while FOXO
regulates cell proliferation and cell cycle by the regulation of
related genes, such as: p53, p27Ki p1, cyclin B, cyclin D1/D2 and
cyclin G2 (24–26). Previous research revealed the FOXO1
is weakly expressed in liver cancer tissue, which results in
abnormal cell proliferation and cell apoptosis; while the induction
of FOXO1 expression inhibited liver cancer cell proliferation and
induced cell apoptosis by the regulation of p21, p27 and cyclin D1
(26). In the present study, we
demonstrated that FOXO1 is a direct target of miR-196a. Inhibition
of FOXO1 significantly promoted human liver cancer cell
proliferation and downregulation of miR-196a induced the expression
of FOXO1.
Taken together, the present study showed that
miR-196a is overexpressed in human liver cancer cells, and the
downregulation of miR-196a inhibited human liver cancer cell
proliferation and induced human liver cancer cell apoptosis which
was attributed to the regulation of FOXO1, a direct target of
miR-196a. Therefore, the present study suggests a new vision and
strategy for the therapy of liver cancer. However, the detailed
mechanisms underlying the effects of miR-196a on liver cancer
warrant further investigation.
References
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hao K, Luk JM, Lee NP, Mao M, Zhang C,
Ferguson MD, Lamb J, Dai H, Ng IO, Sham PC, et al: Predicting
prognosis in hepatocellular carcinoma after curative surgery with
common clinicopathologic parameters. BMC Cancer. 9:3892009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drakaki A, Hatziapostolou M and Iliopoulos
D: Therapeutically targeting microRNAs in liver cancer. Curr Pharm
Des. 19:1180–1191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He XX, Chang Y, Meng FY, Wang MY, Xie QH,
Tang F, Li PY, Song YH and Lin JS: MicroRNA-375 targets AEG-1 in
hepatocellular carcinoma and suppresses liver cancer cell growth in
vitro and in vivo. Oncogene. 31:3357–3369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang L, Wong CM, Ying Q, Fan DN, Huang S,
Ding J, Yao J, Yan M, Li J, Yao M, et al: MicroRNA-125b
suppressesed human liver cancer cell proliferation and metastasis
by directly targeting oncogene LIN28B2. Hepatology.
52:1731–1740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S and He X: The role of microRNAs in
liver cancer progression. Br J Cancer. 104:235–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chitwood DH and Timmermans MC: Small RNAs
are on the move. Nature. 467:415–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosik KS: MicroRNAs and cellular
phenotypy. Cell. 143:21–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hui AB, Shi W, Boutros PC, Miller N,
Pintilie M, Fyles T, McCready D, Wong D, Gerster K, Waldron L, et
al: Robust global micro-RNA profiling with formalin-fixed
paraffin-embedded breast cancer tissues. Lab Invest. 89:597–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen
XM and Gao HJ: Initial study of microRNA expression profiles of
colonic cancer without lymph node metastasis. J Dig Dis. 11:50–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun M, Liu XH, Li JH, Yang JS, Zhang EB,
Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ, et al: MiR-196a is
upregulated in gastric cancer and promotes cell proliferation by
downregulating p27 (kip1). Mol Cancer Ther. 11:842–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Wang ZX, Yang JS, Pan X, De W and
Chen LB: MicroRNA-451 functions as a tumor suppressor in human
non-small cell lung cancer by targeting ras-related protein 14
(RAB14). Oncogene. 30:2644–2658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braig S, Mueller DW, Rothhammer T and
Bosserhoff AK: MicroRNA miR-196a is a central regulator of HOX-B7
and BMP4 expression in malignant melanoma. Cell Mol Life Sci.
67:3535–3548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan Y, Mizoguchi M, Yoshimoto K, Hata N,
Shono T, Suzuki SO, Araki Y, Kuga D, Nakamizo A, Amano T, et al:
MiRNA-196 is upregulated in glioblastoma but not in anaplastic
astrocytoma and has prognostic significance. Clin Cancer Res.
16:4289–4297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schimanski CC, Frerichs K, Rahman F,
Berger M, Lang H, Galle PR, Moehler M and Gockel I: High
miR-196a levels promote the oncogenic phenotype of
colorectal cancer cells. World J Gastroenterol. 15:2089–2096. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du Y, Zhu M, Zhou X, Huang Z, Zhu J, Xu J,
Cheng G, Shu Y, Liu P, Zhu W, et al: MiR-20a enhances cisplatin
resistance of human gastric cancer cell line by targeting NFKBIB.
Tumour Biol. 20:1–9. 2015.
|
|
20
|
Huang F, Tang J, Zhuang X, Zhuang Y, Cheng
W, Chen W, Yao H and Zhang S: MiR-196a promotes pancreatic cancer
progression by targeting nuclear factor kappa-B-inhibitor alpha.
PLoS One. 9:e878972014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou T, Ou J, Zhao X, Huang X, Huang Y and
Zhang Y: MicroRNA-196a promotes cervical cancer proliferation
through the regulation of FOXO1 and p27Kip1. Br J
Cancer. 110:1260–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Zheng F, Yu G, Yin Y and Lu Q:
miR-196a targets netrin 4 and regulates cell proliferation and
migration of cervical cancer cells. Biochem Biophys Res Commun.
440:582–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, Du Y, Gao J, Liu J, Kong X, Gong Y,
Li Z, Wu H and Chen H: Aberrant expression miR-196a is associated
with abnormal apoptosis, invasion, and proliferation of pancreatic
cancer cells. Pancreas. 42:1169–1181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Wang Y and Zhu WG: Applications of
post-translational modifications of FoxO family proteins in
biological functions. J Mol Cell Biol. 3:276–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Gan B, Liu D and Paik JH: FoxO
family members in cancer. Cancer Biol Ther. 12:253–259. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carbajo-Pescador S, Mauriz JL,
García-Palomo A and González-Gallego J: FoxO proteins: Regulation
and molecular targets in liver cancer. Curr Med Chem. 21:1231–1246.
2014. View Article : Google Scholar : PubMed/NCBI
|