Introduction
Corilagin
(C27H22O18), a gallotannin found
in many plants, is a major active component of many
ethnopharmacological plants, such as Phyllanthus niruri L.,
P. emblica L. and P. urinaria L. Corilagin was first
isolated in 1951 by Schmidt and Lademann from Divi-divi
(Caesalpina coriaria) plants (1). In the past few decades, corilagin has
been reported to display several pharmacological activities,
including antioxidant (2),
hepatoprotective (3),
anti-inflammatory (4), neural
system protective (5) and
cardiovascular protective (6)
activities, and has been found to be beneficial in managing type 2
diabetes (7). Recently, its
antitumor effects in hepatocellular carcinoma (8,9),
ovarian cancer (10) and
cholangiocarcinoma (11) have
attracted attention.
Since 2005, we screened hundreds of herbs, among
which Phyllanthus niruri L. has the highest anticancer
potential. The present study further identified that corilagin is a
major active component from P. niruri L. extracts and has
broad-spectrum antitumor activity, a better antitumor potential but
lower toxicity to normal cells (12). It is effective in retarding the
growth of hepatocarcinoma cells by inducing G2/M phase arrest
(9); inhibiting ovarian cancer
cells via TGF-β/AKT/ERK signaling pathways (10); and suppressing cholangiocarcinoma
progression through the Notch signaling pathway (11). Recently, we found that corilagin
enhances the sensitizing effects of chemotherapy drugs in ovarian
cancer cells, through Snail epithelial-mesenchymal transition (EMT)
and glycolysis pathways.
Therefore, the present study aimed to explore the
molecular mechanisms of the sensitizing effects of corilagin in
ovarian cancer cells to chemotherapeutic drugs. The present study
may provide strong evidence to verify corilagin as a complementary
anticancer herbal drug for use in ovarian cancer therapy.
Materials and methods
Cancer cell lines, 2D and 3D
cultures
The human ovarian cancer cell lines SKOv3ip (Skip),
OVCAR5, OC316 and Hey were obtained from the MD Anderson Cancer
Center (Houston, TX, USA) and were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS). HO8910PM-Snail
(HOPM-Snail), a stable Snail-expressing cell line, and its control
cell line HO8910PM-vector (HOPM) were cultured in RPMI-1640 medium
supplemented with 10% FBS and 400 µg/ml of G418 as previously
described (9). Several ovarian
cancer cell lines: Hey, SKOv3ip, HOPM and HOPM-Snail were used for
the present study. Hey and SKOv3ip cells were used for cell growth
and signaling, HOPM-Snail cells were used for cell growth,
signaling and Snail inhibition, and HOPM was used as the parental
control. Since Hey and HOPM-Snail cells grow well in Matrigel,
these two cell lines were chosen for corilagin inhibition in 3D
culture.
3D culture was performed in Matrigel with or without
corilagin treatment. Matrigel was used to coat (80–100 µl/well) a
pre-cooled 8-well chamber slide. The chamber slide was placed in a
37°C culture incubator for at least 15 min. Cells with a final
concentration of 5×104/ml were put in each well with 4%
Matrigel (8/200 µl), and maintained in a 37°C culture incubator for
the indicated time. Corilagin was added after 24 h. Medium and
drugs were changed every 3–4 days with 2% Matrigel. Cells were
observed 7–14 days after treatment.
Reagents
Antibodies against pAKT, AKT, pERK, ERK, Snail,
STAT3 and CD44 were purchased from Cell Signaling Technology
(Danvers, MA, USA), and an anti-GAPDH antibody was purchased from
KangChen Biotech, Co., Ltd. (Shanghai, China). Matrigel was
purchased from BD Biosciences (San Jose, CA, USA).
Extraction and purification of
corilagin
Corilagin was extracted and purified by the Xiamen
Overseas Chinese Subtropical Plant Introduction Garden as
previously described (12).
Cell proliferation assay
Sulforhodamine B (SRB) was used to detect the effect
of drugs on the proliferation of ovarian cancer cell lines, as
previously described (10). For
each cell line, we tested the different concentrations of each
drugs by SRB, and defined the 10, 25 and 50% inhibitory
concentration (IC) for cells and used low (equal to
IC10), medium (equal to IC25) and high (equal
to IC50) concentrations in all experiments.
Western blot analysis
Cells were seeded into 60-mm plates
(1–2×105/plate) and incubated with corilagin (20–40 µM)
or dimethyl sulfoxide (DMSO) (as a control) for 24, 48 or 72 h.
Cell lysates were harvested with lysis buffer (1% Triton X-100, 50
mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA,
100 mM NaF, 10 mM NaPPi and 10% glycerol, to which 1 mM PMSF, 1 mM
Na3VO4, and 1X protease inhibitor were added
before use). For TGF-β stimulation assay, HOPM-snail cells were
seeded in a 60-mm plate, starved overnight and treated with TGF-β1
alone or in combination with corilagin; DMSO was used as the
control. Proteins from total cell lysates were resolved using a
10–15% SDS-PAGE gel and transferred to polyvinylidene difluoride
(PVDF) membranes (Millipore, Billerica, MA, USA). The membranes
were blocked, washed and incubated with specific primary
antibodies, followed by incubation with HRP-conjugated secondary
antibodies. The bands were detected with an enhanced
chemiluminescence assay (PerkinElmer, Waltham, MA, USA).
Reverse phase protein array (RPPA)
analysis
Untreated and corilagin-treated HOPM-Snail cells
were used for RPPA analysis at The University of Texas, MD Anderson
Cancer Center RPPA Core Facility. We followed the methods described
at the following web address: http://www.mdanderson.org/education-and-research/resources-for-professionals/scientific-resources/core-facilities-and-services/functional-proteomics-rppa-core/index.html.
Proteomics analysis
Isobaric tags for relative and absolute quantitation
(iTRAQ) proteomics analysis (OmicsBean, Shanghai, China) were
performed in corilagen-treated ovarian cancer Skip and Hey cells.
Untreated cells were used as the control.
Glycolysis analysis
Glycolysis was analyzed using a Seahorse XF96
extracellular flux analyzer (Seahorse Bioscience, Shanghai, China)
by real-time measurements of the extracellular acidification rate
(ECAR) which is indicative of glycolysis. Cells (Skip and Hey,
5,000 cells/well; HOPM and HOPM-Snail, 8,000 cells/well) were
seeded in complete growth medium in the wells of 96-well plates
designed for the XF96 with quadruple repeat. Different
concentrations of corilagin were added on the following day for 24
h. Cells were incubated in basal glucose-free medium before
incubation in a CO2-free incubator after treatment with
PEG-GO. The default standard glycolysis stress-test program was
selected. Measurements were conducted using final concentrations of
10 mM glucose, 1 mM oligomycin (OM) or 100 mM 2-deoxyglucose
(2-DG).
Statistical analysis
Triplicates or duplicates were performed in each
experiment. All data were subjected to statistical analysis and
were reported as the mean ± standard deviation. The criterion for
statistical significance was taken as P<0.05 using a two-tailed
t-test and the count data were tested using Chi-square criterion
comparing the frequency of parameters. Analyses were performed
using SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Corilagin inhibits 3D cell
culture
Since the advent of routine eukaryotic cell culture
>40 years ago, the most common substrates for supporting cell
growth have been made from polystyrene or glass and have taken the
form of a flat two-dimensional surface. As we described previously,
in this 2D culture system, corilagin demonstrated significant
inhibition of ovarian cancer cell growth, but had much lower
cytotoxicity in normal ovarian surface epithelium cells (10). To overcome some disadvantages of the
2D system, a number of three-dimensional methods have been
developed. Matrigel (BD Biosciences) provides a biologically active
basement membrane model for in vitro invasion assay and drug
toxicity studies (13). We
investigated the effects of corilagin in a 3D system with BD
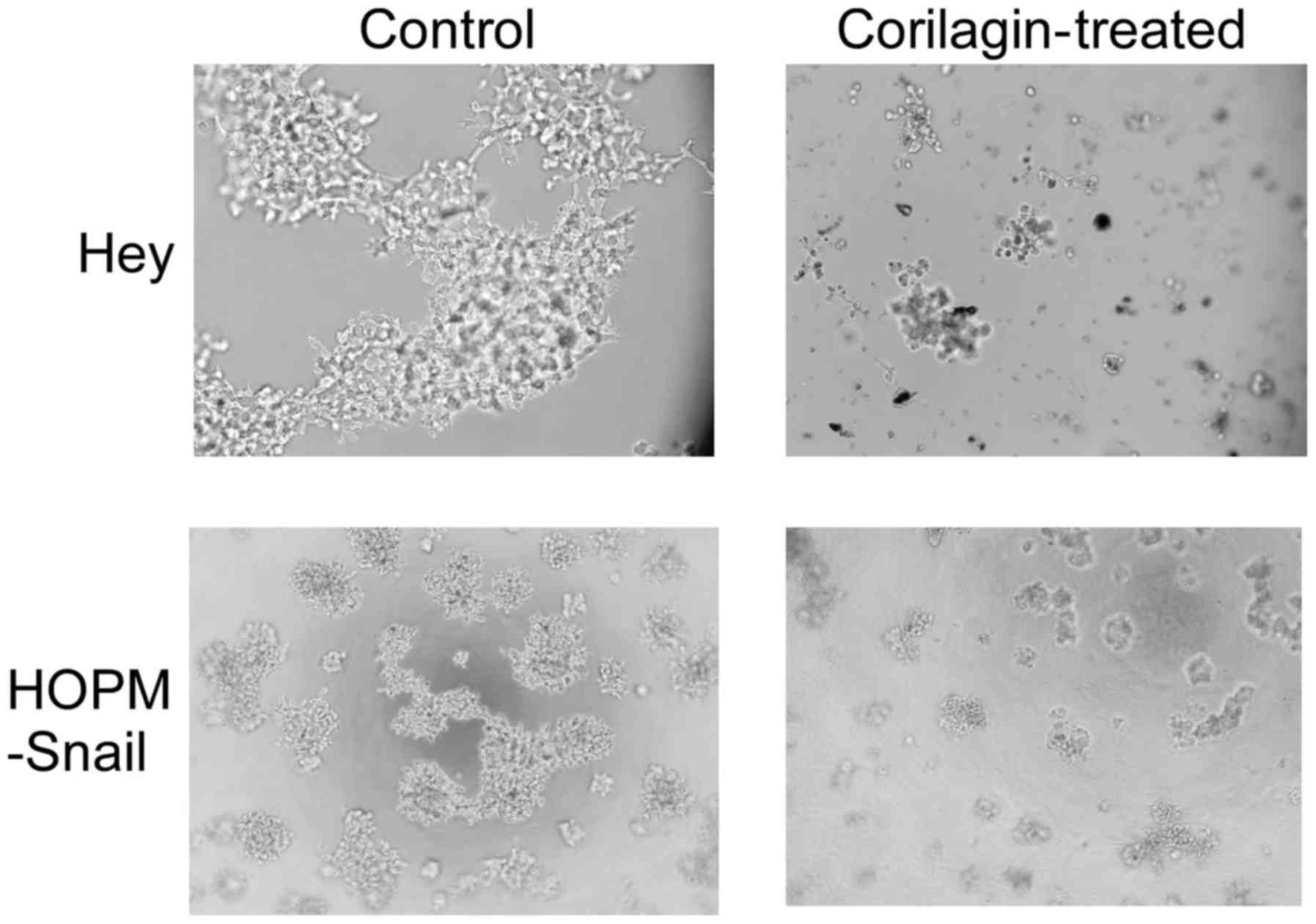
Matrigel. As shown in Fig. 1, both
Hey and HOPM-Snail cells formed extensive growth colonies and
connected bridges in the Matrigel. Corilagin treatment hampered the
colony formation in both cell lines, suggesting that corilagin not
only inhibited ovarian cancer cell growth, but also affected cell
invasion.
Corilagin enhances the inhibitory
effects of chemotherapy drugs in ovarian cancer cells
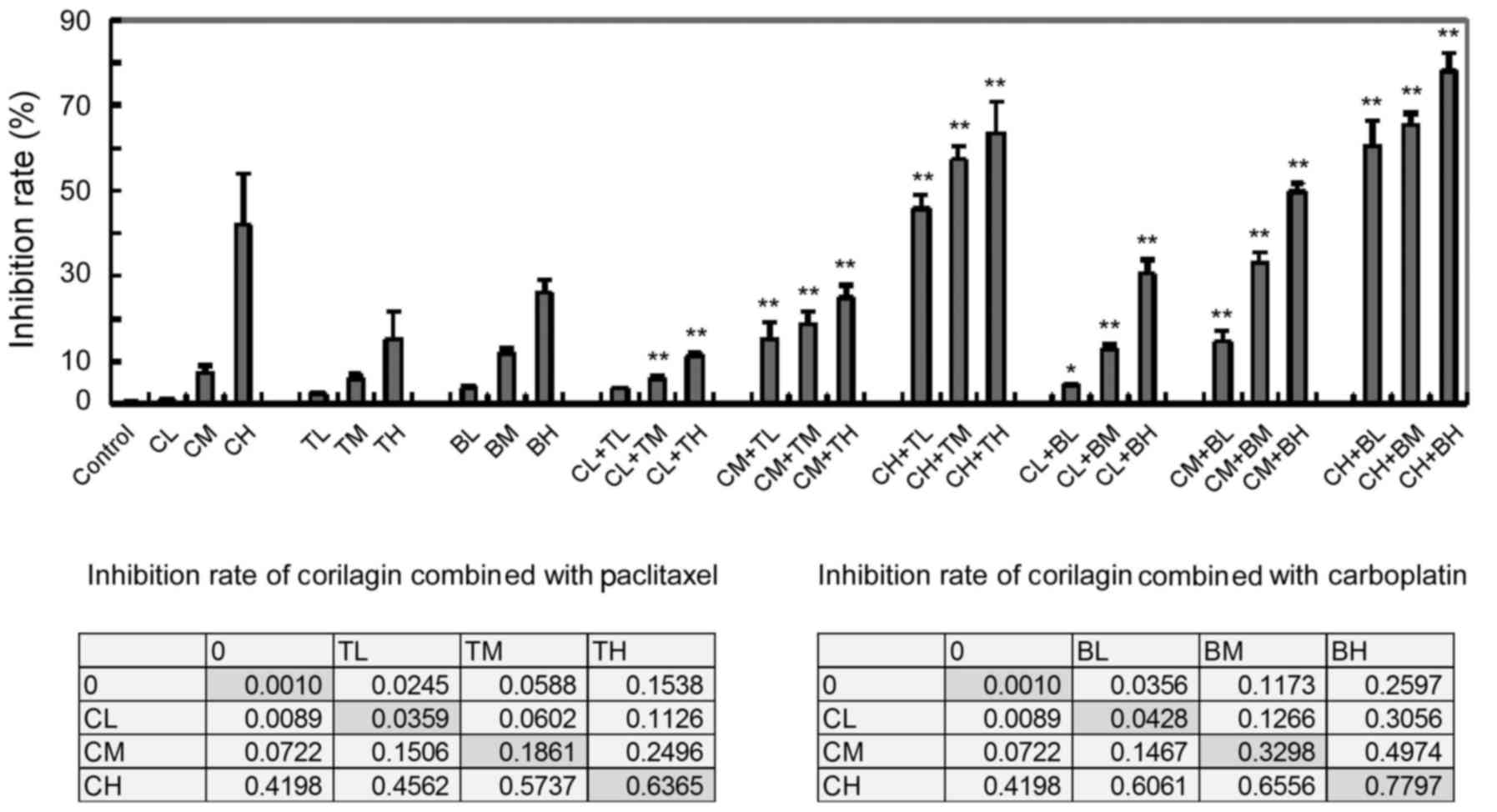
Different concentrations of corilagin were used in
combination assays with paclitaxel and carboplatin in ovarian
cancer cell lines SKip, Hey and HOPM-Snail. Corilagin distinctly
increased the inhibitory effects of paclitaxel and carboplatin
(Fig. 2). Statistical analysis
confirmed that corilagin had an additive effect when combined with
chemotherapeutic drugs. There were significant changes between low,
medium and high concentrations of corilagin (CL, CM, CH),
paclitaxel (TL, TM, TH) or carboplatin (BL, BM, BH). In contrast to
treatment with each single drug only, no difference was noted in CL
+ TL, while significant differences were noted in CL + BL
(p<0.05) and in the other combinations (p<0.01).
Corilagin acts not only on apoptotic
pathways, but also via its distinct pathways
To understand the sensitization mechanisms of
corilagin, we performed RPPA analysis to determine
corilagin-induced signaling networks, using HOPM-Snail cells,
treated with paclitaxel and carboplatin in the presence or absence
of corilagin. As presented in Fig.
3, both paclitaxel and carboplatin treatment upregulated the
levels of several apoptotic and death proteins, caspase 3, caspase
7, and PDCD4, while combined with corilagin, these apoptotic
effects were further enhanced. Meanwhile, corilagin demonstrated
distinct pathways to paclitaxel and carboplatin. Corilagin
inhibited the expression of Snail, PLK1 and RB1, and enhanced the
expression of HIF1A and NDRG1, which were opposite to paclitaxel or
carboplatin treatment. All these changes suggest that corilagin
acts not only on apoptotic pathways, but also via its distinct
pathways.
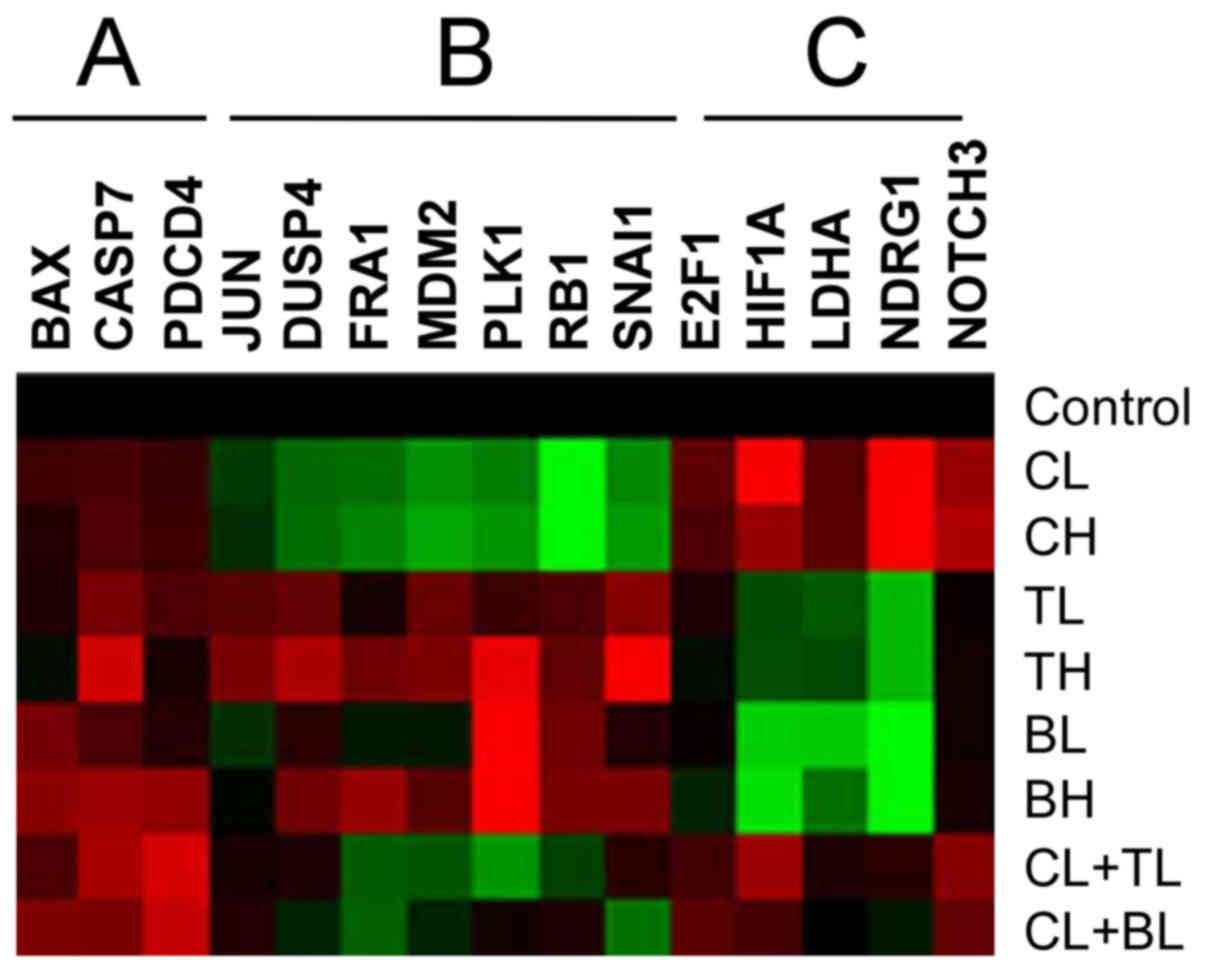 | Figure 3.RPPA analysis. HOPM-Snail cells
treated with low, high doses of corilagin (CL, CH); paclitaxel (TL,
TH); carboplatin (BL, BH) or their combinations. The figure
presents a small portion of the results. Red color indicates
enhanced expression, green color indicates reduced expression.
Untreated cells were used as the control. A, Corilagin enhances
apoptotic effects of drugs; B, Corilagin inhibits these proteins,
but drugs do not; C, Corilagin increases these proteins, but drugs
do not. |
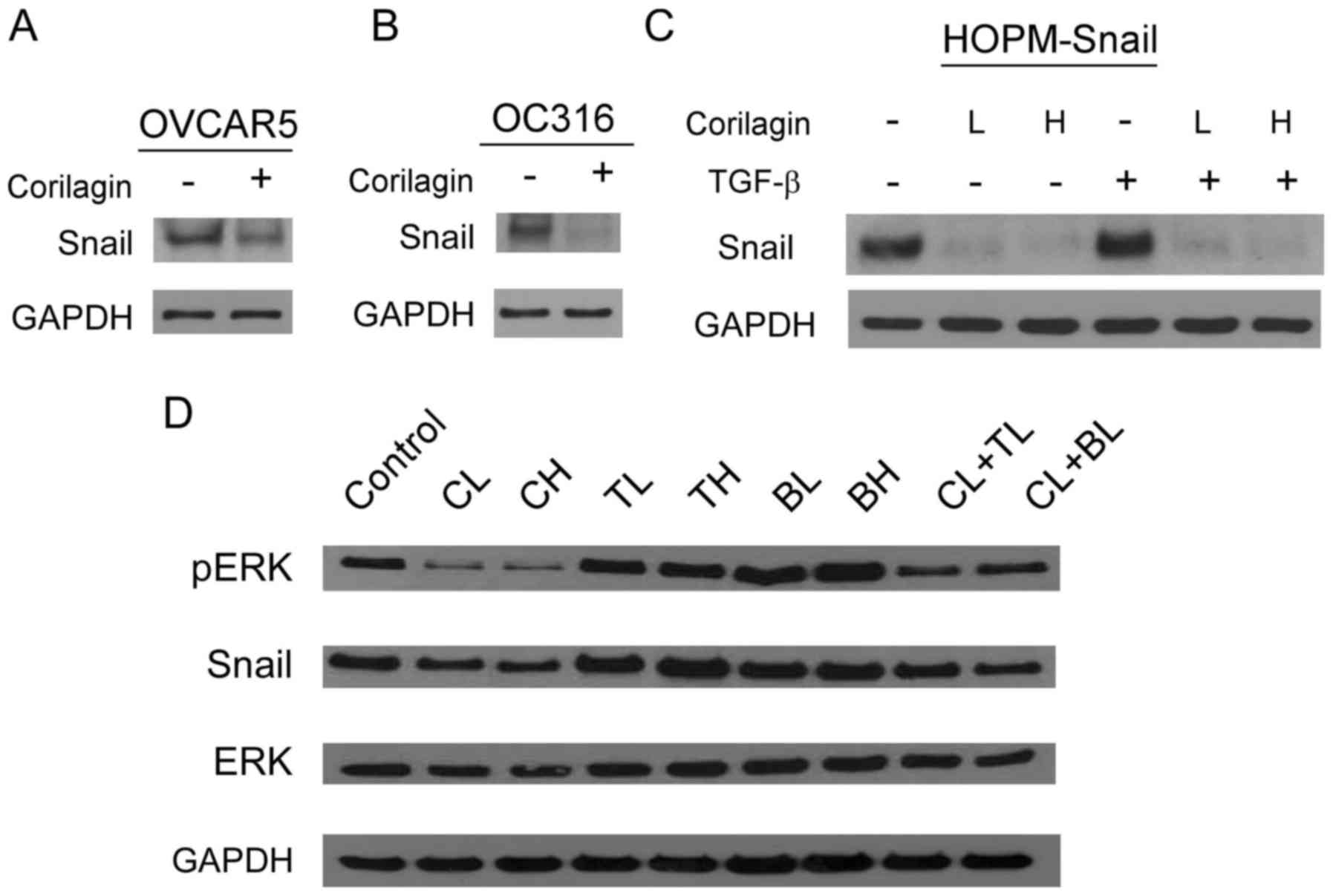
RPPA results were confirmed by western blot
analyses. We found that corilagin treatment downregulated Snail
expression in three ovarian cancer cell lines (OVCAR5, OC316 and
HOPM-Snail) (Fig. 4A-C),
particularly in the Snail-overexpressing cell line HOPM-Snail.
Corilagin blocked TGFβ-enhanced Snail in HOPM-Snail cells (Fig. 4C). Corilagin decreased the
expression of Snail and phospho-ERK, which was not observed
following paclitaxel and carboplatin treatments (Fig. 4D). All these results suggest that
corilagin is not only an apoptosis inducer, similar to
chemotherapeutic drugs, but also has its distinct pathways, such as
inhibition of Snail, which has been recognized as an EMT
inducer.
Corilagin inhibits the glycolysis
pathways via Snail
Our previous study demonstrated that Snail is
significantly overexpressed in metastatic lesions, and Snail is a
key inducer of EMT in ovarian cancer (14). To understand the effects of
corilagin in Snail-EMT pathways, we performed iTRAQ proteomics
analysis in corilagen-treated ovarian cancer cells. In total, with
FDR <0.01 and P-value <0.05 cut-off, we identified 108
proteins that were differentially expressed and mainly
downregulated (28 upregulated, 80 downregulated) by corilagin
treatment. The targeted proteins were found to be cytoskeletal
proteins and related enzymes (lipase, ATPase and kinase), mainly
involved in the glycolysis pathway. Enrichment results of both
biological processes and KEGG pathways revealed that several
pathways such as glycolysis pathway, focal adhesion, the HIF-1
signaling pathway and proteoglycans were inhibited. Moreover,
corilagen may block glycolysis by inhibiting several key proteins,
including ENO1, LDHA, GPI and PGK1; further downregulating CD44,
cortactin, STAT3 and filamin.
To the best of our knowledge, increased aerobic
glycolysis in cancer cells, termed the Warburg effect, represents a
potential targeting strategy for cancer treatment (15). Snail and other transcription
factors, such as STAT3 and CD44, are known to regulate glycolysis
(16–23), and then further affect tumor growth.
To validate the iTRAQ proteomics results, we investigated whether
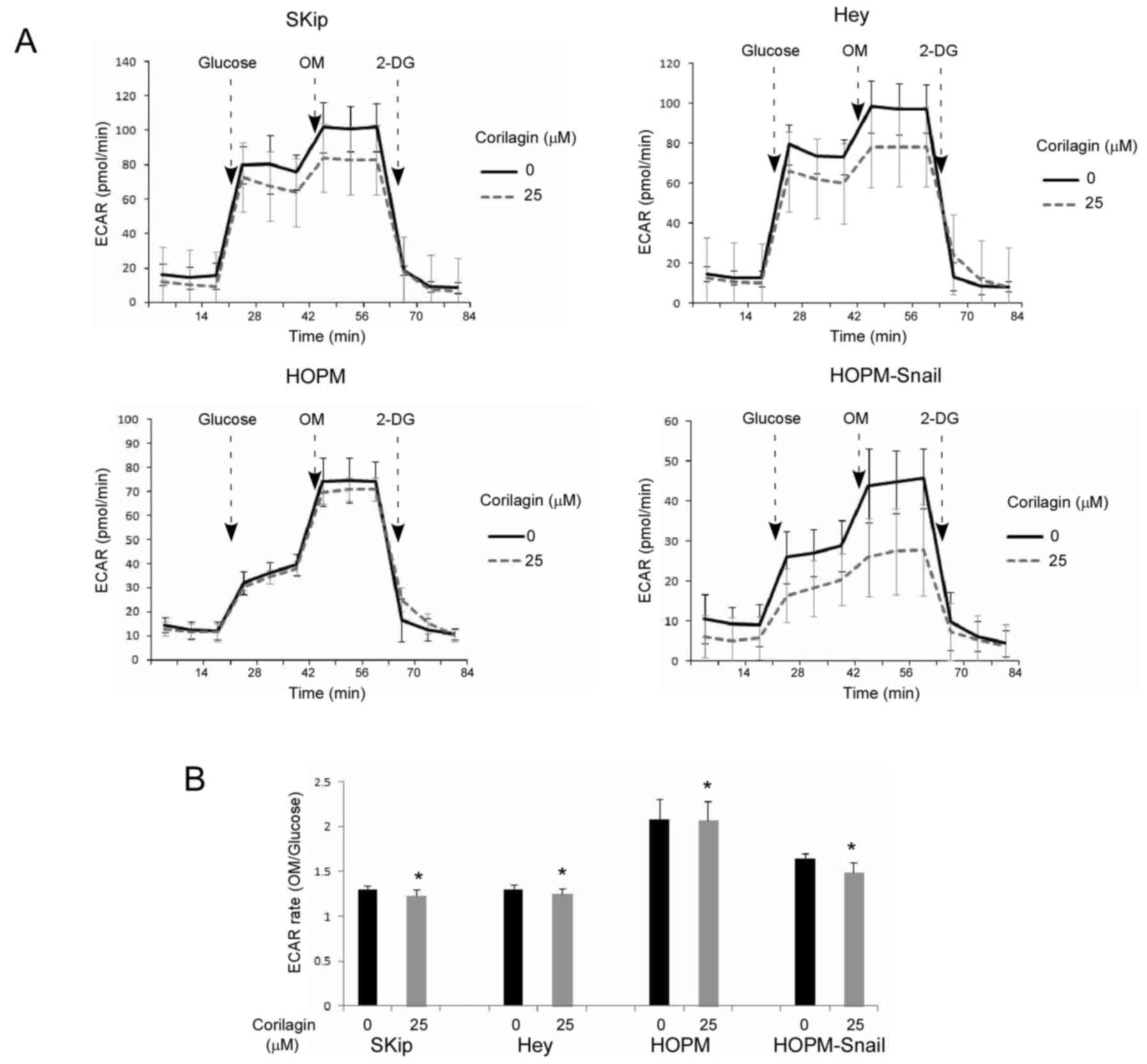
corilagin affects glycolysis. The extracellular acidification rate
(ECAR), which indicates the rate of glycolysis (basal glycolysis,
maximal glycolytic capacity and glycolytic reserve) (24), was determined using a Seahorse XF96
extracellular flux analyzer (Fig.
5A). The results showed that four ovarian cancer cell lines
(Skip, Hey, HOPM and HOPM-Snail) treated with corilagin had a lower
ECAR curve when compared with the untreated cells. Compared with
the HOPM parental cells, HOPM-Snail cells showed much higher
glycolysis inhibition, suggesting that corilagin inhibits
glycolysis via Snail. When the ECAR rate was presented as the
average value of OM injected divided by the average value of
glucose injected, all four corilagin treated cells had a lower rate
compared with the untreated cells (p<0.05) (Fig. 5B).
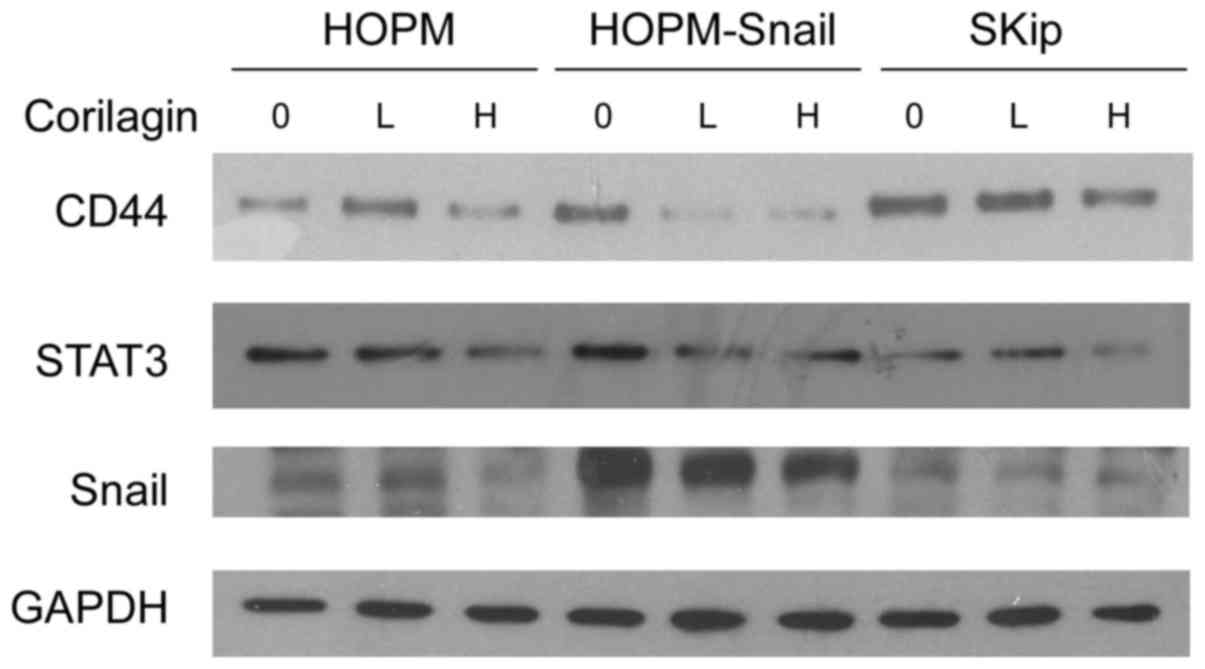
Western blot analysis also indicated that corilagin
did inhibit the expression of CD44 and STAT3 (Fig. 6), both of which have been
demonstrated to be crucial for glycolysis regulation (18–23).
Corilagin showed strong inhibition of Snail in HOPM-Snail cells,
compared with HOPM cells.
Discussion
Conventional chemotherapeutic agents, such as
alkylating agents and Taxol, in ovarian cancer, shrink tumor size
but with high toxicity. In addition, repeated treatment with these
agents leads tumors to acquire resistance to the chemotherapies.
Therefore, it is necessary to discover compounds with growth
inhibitory and apoptosis induction properties in cancer cells but
causing less or no toxicity in normal cells. Ideally, such
compounds could target multiple major cellular signaling pathways
in cancer cells. Recent research has focused on herbal medicines,
among which, corilagin is a novel anticancer agent. This drug was
confirmed to inhibit cancer growth involving multiple pathways
(8–11). Corilagin was reported to have the
following characteristics: non-toxicity to normal tissues, a wide
spectrum of efficacy against multiple cancers, and ability for oral
consumption. Importantly, our experiments displayed that corilagin
enhanced the effects of paclitaxel and carboplatin in all ovarian
cancer cells, being an ideal complementary medicine in cancer
therapy. Corilagin presented different mechanisms in ovarian cancer
when compared with those of current chemotherapy. In our previous
study, we showed that corilagin inhibited TGF-β secretion into the
culture supernatant of ovarian cancer cell lines. In contrast, a
reduction in TGF-β secretion was not observed in cancer cells
treated with the cytotoxic drug paclitaxel, suggesting that
corilagin specifically targets TGF-β secretion (10). In the present study, we found that
corilagin acts not only on apoptotic pathways, but also via its
distinct pathways, such as inhibition of Snail and phospho-ERK
(Fig. 4). Conventional drugs, such
as paclitaxel and carboplatin, inhibited the phosphorylation of
AKT, but did not inhibit the phosphorylation of ERK, and also did
not inhibit Snail. These studies may explain why corilagin displays
additive effects with chemotherapeutic drugs during treatment.
Recently, numerous studies have focused on cancer
drug resistance, and the role of EMT in chemoresistance has
emerged. EMT is a highly conserved process in which polarized,
immobile epithelial cells lose tight junctions, associated
adherence and become migratory mesenchymal cells. Several
transcription factors, including the Snail/Slug family, Twist,
δEF1/ZEB1, SIP1/ZEB2 and E12/E47 respond to microenvironmental
stimuli and function as molecular switches for the EMT program
(25). Among these factors, Snail
is the most important one. Our previous study confirmed that Snail
is critical for tumor growth and metastasis of ovarian carcinoma
(14). Snail causes a metabolic
reprogramming, bestows tumor cells with cancer stem cell-like
traits and additionally, promotes drug resistance, tumor recurrence
and metastasis (25). Kurrey et
al found that Snail and Slug are critical for a cancer cell to
acquire stem cell-like characteristics toward resisting
radiotherapy- or chemotherapy-mediated cellular stress (26). In the present study, we demonstrated
that corilagin enhanced chemosensitivity by inhibition of Snail,
and also by inhibition of CD44 and Stat3, factors that may relate
to cancer stem cell development. This herbal medicine may provide a
new strategy to overcome chemoresistance.
Glycolysis is the major source of energy in cancer
cells. Warburg et al showed in the 1920s that under aerobic
conditions, tumor tissues metabolize ~10-fold more glucose to
lactate in a given time than normal tissues, a phenomenon known as
the Warburg effect. However, this increase in aerobic glycolysis in
cancer cells is often erroneously thought to occur instead of
mitochondrial respiration and has been misinterpreted as evidence
for damage to respiration instead of damage to the regulation of
glycolysis. In fact, many cancers exhibit the Warburg effect while
retaining mitochondrial respiration (15). To the best of our knowledge,
TGFβ1-induced EMT is accompanied by coordinately reduced enzyme
expression required to convert glucose into fatty acids, and
concomitant enhanced respiration (16). Snail, a transcription factor
mediating TGFβ1-induced EMT, suppresses lipogenesis and favors
energy production through carbohydrate-responsive-element-binding
protein (ChREBP, a master lipogenic regulator), and fatty acid
synthase (FASN) (16). In the
present study, corilagin treatment inhibited the ECAR curve, which
indicates the rate of glycolysis, in ovarian cancer cells. Compared
with HOPM parental cells, HOPM-Snail cells showed much higher
glycolysis inhibition when Snail was clearly downregulated by
corilagin (Fig. 6), suggesting that
the inhibition of glycolysis by corilagin could be mainly through
Snail-TGFβ inhibition.
Proteomics analysis revealed that corilagen may
block glycolysis by inhibiting several other key proteins except
Snail, such as CD44, cortactin, STAT3 and filamin. We confirmed
that corilagin inhibited CD44 and STAT3 in all ovarian cancer cells
(Fig. 6). STAT3 acts as a master
regulator of cell metabolism, inducing aerobic glycolysis and
downregulating mitochondrial activity (20). CD44, a cell surface marker for
cancer stem cells, interacts with pyruvate kinase M2 (PKM2) and
thereby enhances the glycolytic phenotype of cancer cells. Ablation
of CD44 induces glycolysis-to-oxidative phosphorylation transition
(21). Inhibition of CD44 also
sensitized prostate cancer cells to carboplatin (22). Reduction of STAT3 and CD44
expression has a significant impact on the study of corilagin
mechanisms. STAT3 and CD44 may regulate glycolysis by different
ways. Inhibition of glycolysis by corilagin could also occur
through STAT3 and CD44 pathways, which is a new area to
explore.
Acknowledgements
The present study was supported by funds from the
National Natural Science Foundation of China (grant no. 81274149 to
Y.M.). The present study was also supported by grants from the
Natural Science Foundation of Fujian Province (grant no. 2010D012
to Y.M.), the Xiamen Municipal Science and Technology Innovation
Fund Project (grant no. 3502Z20101016 to Y.M.), and the Shanghai
Pujiang Program (grant no. 15PJ1400900 to H.Z.).
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
SRB
|
sulforhodamine B
|
|
RPPA
|
reverse phase protein array
|
|
iTRAQ
|
isobaric tags for relative and
absolute quantitation
|
|
ECAR
|
extra-cellular acidification rate
|
|
OM
|
oligomycin
|
|
2-DG
|
2-deoxyglucose
|
|
IC
|
inhibitory concentration
|
References
|
1
|
Schmidt OT and Lademann R: Corilagin, ein
weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung
über natürliche Gerbstoffe. Justus Liebigs Ann Chem. 571:232–237.
1951.(In German). View Article : Google Scholar
|
|
2
|
Wu N, Zu Y, Fu Y, Kong Y, Zhao J, Li X, Li
J, Wink M and Efferth T: Antioxidant activities and xanthine
oxidase inhibitory effects of extracts and main polyphenolic
compounds obtained from Geranium sibiricum L. J Agric Food
Chem. 58:4737–4743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kinoshita S, Inoue Y, Nakama S, Ichiba T
and Aniya Y: Antioxidant and hepatoprotective actions of medicinal
herb, Terminalia catappa L. from Okinawa Island and its
tannin corilagin. Phytomedicine. 14:755–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao L, Zhang SL, Tao JY, Pang R, Jin F,
Guo YJ, Dong JH, Ye P, Zhao HY and Zheng GH: Preliminary
exploration on anti-inflammatory mechanism of corilagin
(beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-d-glucose)
in vitro. Int Immunopharmacol. 8:1059–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong XR, Luo M, Fan L, Zhang T, Liu L,
Dong JH and Wu G: corilagin inhibits the double strand
break-triggered NF-kappaB pathway in irradiated microglial cells.
Int J Mol Med. 25:531–536. 2010.PubMed/NCBI
|
|
6
|
Duan W, Yu Y and Zhang L: Antiatherogenic
effects of Phyllanthus Emblica associated with corilagin and
its analogue. Yakugaku Zasshi. 125:587–591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao H, Huang YN, Xu PY and Kawabata J:
Inhibitory effect on α-glucosidase by the fruits of Terminalia
chebula Retz. Food Chem. 105:628–634. 2007. View Article : Google Scholar
|
|
8
|
Hau DK, Zhu GY, Leung AK, Wong RS, Cheng
GY, Lai PB, Tong SW, Lau FY, Chan KW, Wong WY, et al: In vivo
anti-tumour activity of corilagin on Hep3B hepatocellular
carcinoma. Phytomedicine. 18:11–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ming Y, Zheng Z, Chen L, Zheng G, Liu S,
Yu Y and Tong Q: Corilagin inhibits hepatocellular carcinoma cell
proliferation by inducing G2/M phase arrest. Cell Biol Int.
37:1046–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia L, Jin H, Zhou J, Chen L, Lu Y, Ming Y
and Yu Y: A potential anti-tumor herbal medicine, corilagin,
inhibits ovarian cancer cell growth through blocking the TGF-β
signaling pathways. BMC Complement Altern Med. 13:332013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu Y, Xiao L, Ming Y, Zheng Z and Li W:
Corilagin suppresses cholangiocarcinoma progression through Notch
signaling pathway in vitro and in vivo. Int J Oncol.
48:1868–1876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng ZZ, Chen LH, Liu SS, Deng Y, Zheng
GH, Gu Y and Ming YL: Bioguided fraction and isolation of the
antitumor components from Phyllanthus niruri L. Biomed Res
Int. 2016:97292752016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee GY, Kenny PA, Lee EH and Bissell MJ:
Three-dimensional culture models of normal and malignant breast
epithelial cells. Nat Methods. 4:359–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia
L, Wu Y, Zhou BP and Feng Y: Snail is critical for tumor growth and
metastasis of ovarian carcinoma. Int J Cancer. 126:2102–2111.
2010.PubMed/NCBI
|
|
15
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang L, Xiao L, Sugiura H, Huang X, Ali
A, Kuro-o M, Deberardinis RJ and Boothman DA: Metabolic
reprogramming during TGFβ1-induced epithelial-to-mesenchymal
transition. Oncogene. 34:3908–3916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poli V and Camporeale A: STAT3-mediated
metabolic reprograming in cellular transformation and implications
for drug resistance. Front Oncol. 5:1212015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Liu T, Zhao L, Chen W, Hou H, Ye Z
and Li X: Ginsenoside 20(S)-Rg3 inhibits the Warburg effect through
STAT3 pathways in ovarian cancer cells. Int J Oncol. 46:775–781.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demaria M, Giorgi C, Lebiedzinska M,
Esposito G, D'Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE,
Watson CJ, et al: A STAT3-mediated metabolic switch is involved in
tumour transformation and STAT3 addiction. Aging. 2:823–842. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nam K, Oh S and Shin I: Ablation of CD44
induces glycolysis-to-oxidative phosphorylation transition via
modulation of the c-Src-Akt-LKB1-AMPKα pathway. Biochem J.
473:3013–3030. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Cohen A, Sun Y, Squires J, Braas D,
Graeber TG, Du L, Li G, Li Z, Xu X, et al: The role of CD44 in
glucose metabolism in prostatic small cell neuroendocrine
carcinoma. Mol Cancer Res. 14:344–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamada M, Nagano O, Tateyama S, Ohmura M,
Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, et
al: Modulation of glucose metabolism by CD44 contributes to
antioxidant status and drug resistance in cancer cells. Cancer Res.
72:1438–1448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou T, Zhang B, Wei P, Du Y, Zhou H, Yu
M, Yan L, Zhang W, Nie G, Chen C, et al: Energy metabolism analysis
reveals the mechanism of inhibition of breast cancer cell
metastasis by PEG-modified graphene oxide nanosheets. Biomaterials.
35:9833–9843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|