Introduction
Colorectal cancer (CRC) has become a serious concern
for public health issues, with more than million new cases each
year (1,2). The CRC morbidity keeps climbing and
its estimated figure may reach 2.4 million in 2035 globally
(3,4). Surgery assisted with chemotherapy
represents the first-line strategy for most patients (5). Currently, the management of
chemotherapy is applied using various chemoreagents including
5-fluorouracil (5-FU), leucovorin (LV), oxaliplatin, irinotecan,
capecitabine, bevacizumab and cetuximab, either as single agent or
in combination (6). However,
chemoresistance greatly limits the clinical outcome of the
treatment on CRC patients, resulting into most cancer-related
deaths.
5-FU as the most commonly used agent in many
chemotherapy regimens, may lead to acquired resistance in cancer
cells. Moreover, 5-FU-resistant cells can develop resistance to
other drugs, like those with very different acting mechanisms
and/or chemical structures, which is defined as multidrug
resistance (MDR) (7). The
mechanisms of chemoresistance are complicated, including the
increase in drug efflux, reduction in drug absorption, changes in
the targets of anticancer drugs, decrease in drug activity,
enhancement of DNA repair following damage, deregulation of
signaling pathways (8). PI3K/AKT
pathway exerts essential roles in survival, proliferation,
migration and differentiation of cells. However, once it is
aberrantly activated, it will underlie the biology of cancer and
enhance drug efflux by highly expressing ABC transporters, reducing
the response to 5-FU or other chemotherapeutic agents (9–11).
Thus, the inhibition of PI3K/AKT pathway becomes a promising
therapeutic target.
The existing chemical reversal agents targeting
different mechanisms of MDR are of poor selectivity and with
apparent side effects (12).
Besides, the genomic instability and heterogeneity of cancer cells
make it ineffective or even encounter drug resistance during the
single-target treatment (13),
which increases the urgency for developing new therapeutic
approaches. Scutellaria barbata D. Don (SB) is a well-known
traditional Chinese folk-medicine that has been widely used in the
treatment of various kinds of cancers including CRC (14). As reported previously, the ethanol
extract of SB (EESB) possesses significant antitumor activity by
promoting cell apoptosis, as well as inhibiting cell proliferation
and tumor angiogenesis via modulating several pathways (15–17).
However, its activity against cancer chemoresistance is less known.
Therefore, using a 5-FU-resistant CRC cell line HCT-8/5-FU, in this
study we evaluated the therapeutic efficacy of the ethanol extracts
of SB (EESB) against 5-FU resistance and explored the possible
molecular mechanisms.
Materials and methods
Materials and reagents
Roswell Park Memorial Institute medium-1640
(RPMI-1640, C11875500BT), fetal bovine serum (FBS, #10099-141),
0.25% trypsin-EDTA (#25200-072), penicillin-streptomycin (SV30010),
DreamTaq Green PCR Master Mix (K1081), Pierce RIPA buffer (#89901),
Pierce BCA Protein assay kit (#23227) and SuperSignal™ West Pico
Chemiluminescent Substrate (#34080) were obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Culture flask and
plates were from NEST Biotechnology Co., Ltd. (Jiangsu, China).
5-FU (#040302) were obtained from Xudong Haipu Pharmaceutical Co.,
Ltd. (Shanghai, China).
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT,
M8180) was from Solarbio Science and Technology (Beijing, China).
Annexin V-FITC apoptosis detection kit (KGA108) was obtained from
KeyGen Biotech Co., Ltd. (Jiangsu, China). DAPI staining solution
(C1005) and Rho-123 (C2007) were obtained from Beyotime (Shanghai,
China). RNAiso plus (#9109) and PrimScript RT reagent kit with gDNA
Eraser (RR047A) were from Takara Biotechnology Co., Ltd. (Dalian,
China). The nitrocellulose (NC) membrane (0.45 µm, HATF00010) was
obtained from Millipore (Billerica, MA, USA). Rabbit monoclonal
antibody against cyclin D1 (#2978), p21 (#2947S), PI3K (#4257),
p-AKT (#4058) and AKT (#4685) and rabbit polyclonal antibody
against β-actin (#4967) were obtained from Cell Signaling (Beverly,
MA, USA). Rabbit polyclonal antibody against Bcl-2 (ab59348), Bax
(ab53154), and ABCG2 (ab63907) were from Abcam (Cambridge, MA,
USA). HRP-conjugated goat anti-rabbit secondary antibody (E030120)
was from Earthox (Millbrae, CA, USA).
Preparations of EESB
EESB powder was prepared as previously described
(15) and dissolved into DMSO to
make a stock solution with a concentration of 500 mg/ml, and stored
at −20°C. Immediately before each experiment, stock solution was
diluted into culture medium to make different working
concentrations of EESB. The content of DMSO in the medium was
<0.5%.
Cell culture
Human CRC cell lines HCT-8/5-FU and the parental
HCT-8 cells were obtained from KeyGen Biotech Co., Ltd. Cells were
cultured in RPMI-1640 complete medium, containing 10% (v/v) FBS and
1% antibiotics, and at condition of 37°C, 5% CO2 in an
incubator with saturated humidity (Forma 3110; Thermo Fisher
Scientific, Inc. The HCT-8/5-FU cells were grown in the complete
medium with 15 µg/ml 5-FU.
Cell viability analysis
Cell viability of HCT-8 and HCT-8/5-FU was estimated
by MTT assay. In short, cells were plated into 96-well plates
(1×104 cells per well) in 100 µl of medium. After 12 h,
cells were dealt with different doses of 5-FU or EESB for indicated
time. Equivoluminal DMSO was used as the vehicle control. Details
for MTT assay were as described before (15). The resistance index (RI) was used to
analyze the drug resistance of the HCT-8/5-FU cells to 5-FU. RI was
calculated by dividing the dose of 5-FU required to inhibit growth
by 50% (IC50) for HCT-8/5-FU cells by the
IC50 value for the parental cells (HCT-8).
IC50 values were assessed using non-linear regression
analysis.
Cellular morphology observation
HCT-8/5-FU cells were plated into 6-well plates at a
density of 5×105 cells/well in 2 ml complete medium and
administered with EESB (0, 0.5, 1.0 and 1.5 mg/ml) for 24 h. A
phase-contrast microscope (Leica Camera AG; Leica Microsystems,
Wetzlar, Germany) was used to observe cell morphology, and images
were photographed at a magnification of ×200.
Colony formation
HCT-8/5-FU cells were seeded into 6-well plates at a
density of 5×105 cells/well in 2 ml complete medium and
intervented with EESB (0, 0.5, 1.0 and 1.5 mg/ml) for 24 h.
Subsequently, cells were collected, diluted with fresh medium
without EESB, and reseeded into 6-well plates at a density of 1,000
cells/well in 2 ml. The medium was replaced with fresh medium every
four days, and 10 days later, colonies were fixed with 4%
paraformaldehyde, stained with 0.01% crystal violet and
photographed.
Apoptosis detection with DAPI and
Annexin V-FITC/PI staining
DAPI staining was used to determine apoptosis of
HCT-8/5-FU cells after EESB treatment. Briefly, 4% paraformaldehyde
was added to fix the cells at room temperature for 15 min and
washed with PBS 3 times. Subsequently, DAPI solution was added to
stained cells at room temperature for 10 min and washed with PBS 3
times. DAPI stained cells were visualized using an inverted
fluorescence microscope (DMI4000B; Leica Microsystems) with 100 W
mercury lamp light source using UV filter cubes. Excitation was 340
nm (long pass). Furthermore, Annexin V-FITC/PI double staining
followed by FACSCalibur determination were used to verify the
apoptosis-inducing effect of EESB. Procedures were performed
according to the manufacturer's instructions. Herein, Annexin V/PI
double-negative population (in the lower left quarter of FACS
diagram) indicates living cells; Annexin V-positive/PI-negative or
Annexin V/PI double-positive population (in the lower right quarter
or upper right quarter of FACS diagram) stands for cells undergoing
early or late apoptosis, respectively. Final results are
represented by calculating the percentage of both early and late
apoptosis as total apoptosis.
Rhodamine-123 (Rh-123) exclusion
Rh-123 is the substrate of the ATP-binding cassette
(ABC) transporter, and the Rh-123 exclusion assay was used to
investigate the reversal effect according to the retention of
Rh-123 after treatment. Briefly, HCT-8/5-FU cells were treated with
EESB (0, 0.5, 1.0 and 1.5 mg/ml) for 24 h before collected, and a
total of 106 cells in 1 ml medium with 5 µg/ml Rh-123
were incubated at 37°C for 10 min. Then cells were washed twice
with pre-cold PBS and resuspended in 0.5 ml PBS, followed by 30-min
incubation at 37°C. Fluorescence intensity was detected at 488 nm
to determine the intracellular content of Rh-123 and quantitated
using the FACSCalibur flow cytometer (BD FACSCalibur;
Becton-Dickinson, CA, USA). The results were indicated as the mean
fluorescence intensity of Rh-123.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
HCT-8/5-FU cells (4×105) were plated into
6-well plates in 2 ml complete medium and treated with EESB (0,
0.5, 1.0 and 1.5 mg/ml) for 24 h. Total RNA were extracted with
RNAiso plus and reverse-transcribed by the PrimScript RT reagent
kit with gDNA Eraser, according to the manufacturer's instructions.
The cDNA was used to measure the mRNA amount of cyclin D1, p21,
Bcl-2, Bax and ABCG2 by RT-PCR. β-actin was used as an internal
control. The RT-PCR conditions were performed as follows:
denaturation at 94°C for 40 sec, annealing at 60°C for 40 sec and
extension at 72°C for 45 sec for 30 cycles. The sequences of
primers are listed in Table I.
 | Table I.Primer sequences for RT-PCR. |
Table I.
Primer sequences for RT-PCR.
| Gene | Primers
(5′-3′) |
|---|
| Cyclin D1 | F: TGG ATG CTG GAG
GTC TGC GAG GAA |
|
| R: GGC TTC GAT CTG
CTC CTG GCA GGC |
| p21 | F: GCG ACT GTG ATG
CGC TAA TGG |
|
| R: TAG AAA TCT GTC
ATG CTG GTC TGC |
| Bcl-2 | F: CAG CTG CAC CTG
ACG CCC TT |
|
| R: AGT CAG TTC CTT
GTG GAG CC |
| Bax | F: TGC TTC AGG GTT
TCA TCC AGG |
|
| R: TGG CAA AGT AGA
AAA GGG CGA |
| ABCG2 | F: GCC GTG GAA CTC
TTT GTG GTA G |
|
| R: ACA GCA AGA TGC
AAT GGT TGT |
| β-actin | F: CCA GGG CGT TAT
GGT AGG CA |
|
| R: TTC CAT ATC GTC
CCA GTT GGT |
Western blot analysis
HCT-8/5-FU cells were treated as previously
described, then Pierce RIPA buffer, which consist of several
protein inhibitors, was used to lyse the cells. The lysates were
then centrifuged for 20 min at the condition of 14,000 rpm and low
temperature, and the concentrations of supernatant were detected by
BCA Protein Assay Reagent kit. Protein (50 µg) for each sample was
loaded onto sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and resolved using 20 V for 10 min,
subsequently 80 V for 30 min followed by 120 V for 1 h, then
transferred onto nitrocellulose (NC) membranes. After blocking with
5% non-fat dry milk, membranes were incubated with cyclin D1, p21,
Bcl-2, Bax, ABCG2, PI3K, p-AKT, AKT or β-actin, respectively
(1:1,000 dilution) overnight at 4°C, and then incubated with
HRP-conjugated anti-rabbit secondary antibodies (1:5,000 dilution)
for 1 h at room temperature. The membranes were then exposed to
enhanced chemiluminescence (ECL) detection using SuperSignal™ West
Pico Chemiluminescent Substrate. Images were obtained with ChemiDoc
XRS+ imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The images were analysed by Image Lab™ Software
(Version 3.0).
Statistical analysis
All data were collected based on the mean of three
experiments. Statistical analysis was performed using SPSS software
(version 17.0) for Windows (SPSS, Inc. Chicago, IL, USA) using
one-way ANOVA. P<0.05 was considered as statistically
significant.
Results
EESB overcomes 5-FU resistance in CRC
HCT-8 cells
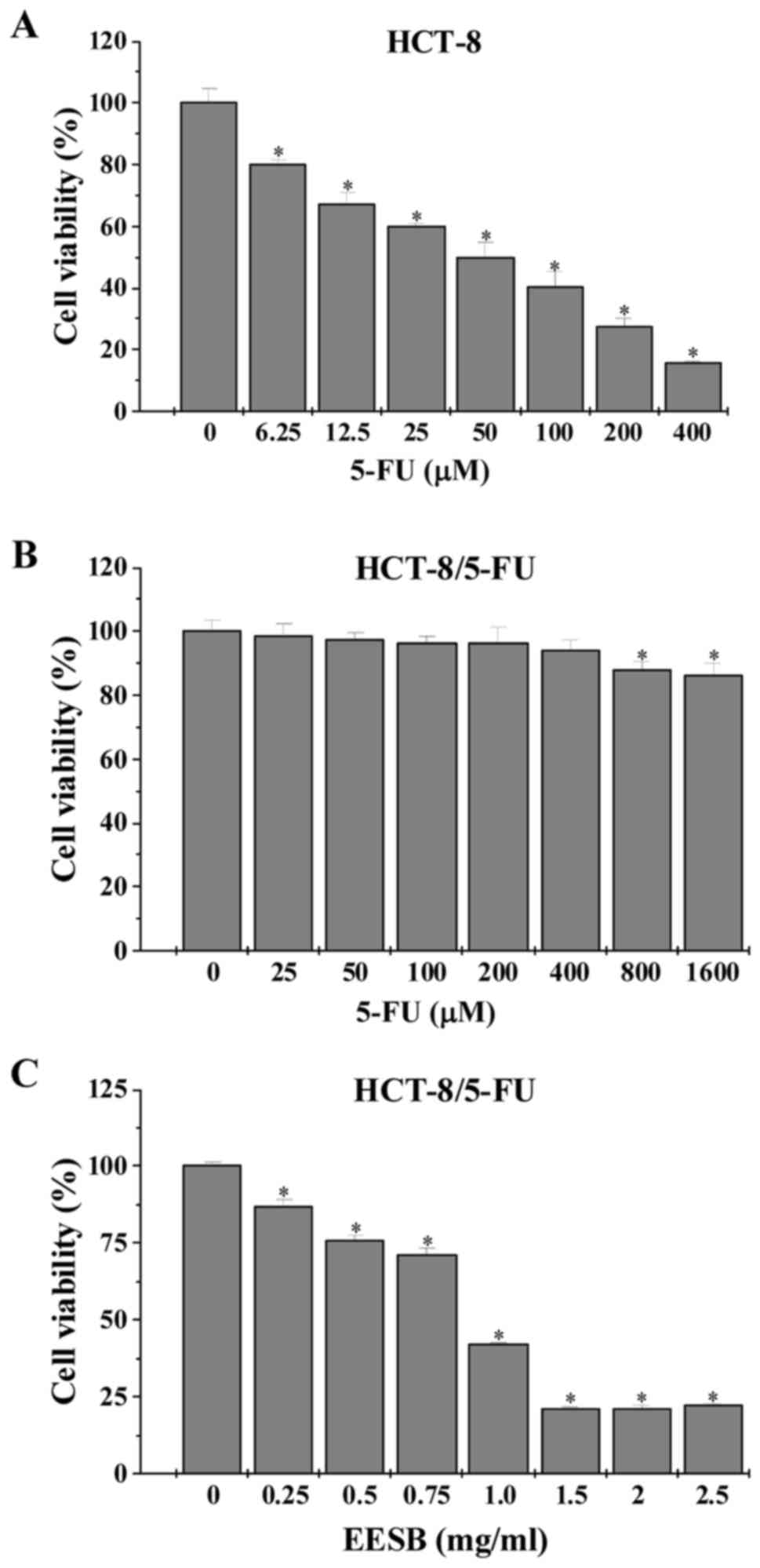
After exposure to 5-FU or EESB, the viability of
HCT-8 and HCT-8/5-FU cells was examined by MTT assay. Data in
Fig. 1 showed that the
IC50 value in HCT-8 and HCT-8/5-FU cells were 0.11 and
2.91 mM, respectively, leading to a resistance index (RI) of 25.34.
However, treatment with 0.25–2.5 mg/ml of EESB dose-dependently
reduced HCT-8/5-FU cell viability by 12.92–78.01%, as compared with
untreated cells.
EESB inhibits proliferation, promotes
apoptosis and inhibits drug efflux in HCT-8/5-FU cells
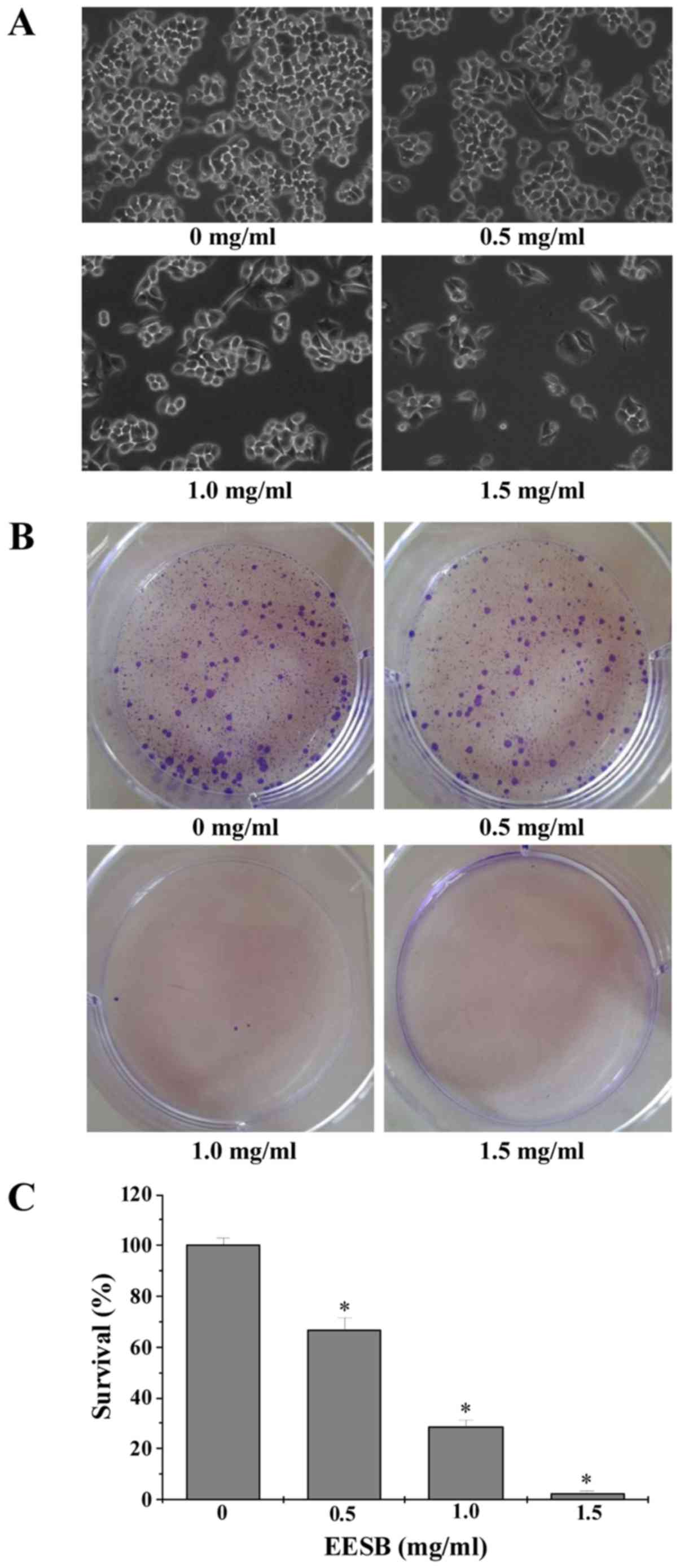
Effect of EESB on cell growth was determined by
observation of cell morphology and colony formation. As shown in
Fig. 2A, the monolayers of
untreated HCT-8/5-FU cells were crowded and disorganized, while the
cell density of treated ones showed a reduction in the confluent
monolayers. In addition, the colonies reduced after EESB treatment
in a dose-dependent manner (Fig. 2B and
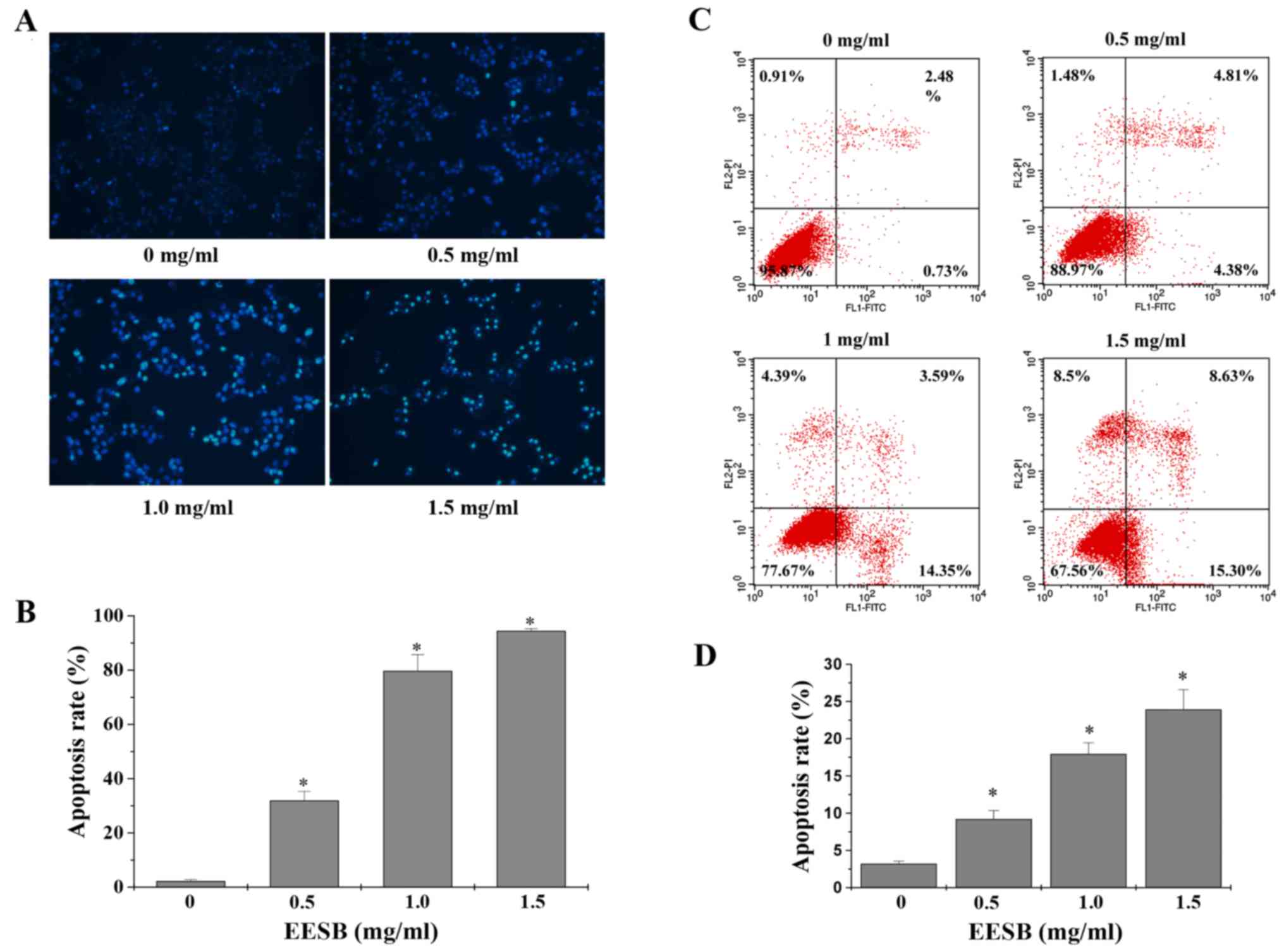
C). Cell apoptosis was assessed by DAPI and Annexin V/PI
staining, respectively. When apoptosis occurs, cells will
experience a process of chromatin condensation, the nucleus shrinks
and DNA fragments, so that they stain by DAPI with strong light. As
shown in Fig. 3A and B, the
percentage of cells with strong dye treated with 0, 0.5, 1 and 1.5
mg/ml of EESB was 2.90, 31.81, 79.59 and 94.31%, respectively
(P<0.05). Then, we used Annexin V/PI staining to verify this
result. As shown in Fig. 3C and D,
with the indicated concentration of EESB treatment, the percentage
of total apoptotic cells increased from 3.2 to 33.9%, in a
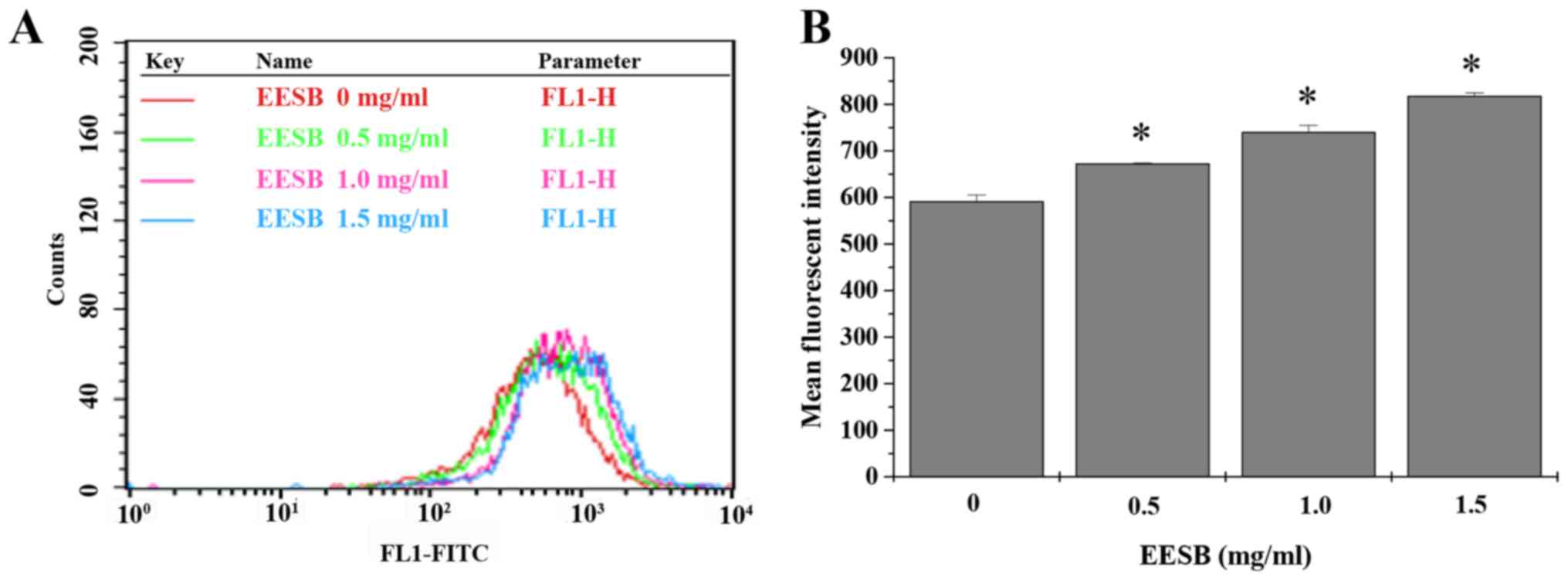
dose-dependent manner. To find out the effects of EESB on drug
efflux, the intracellular accumulation of Rh-123 was measured in
EESB-treated HCT-8/5-FU cells. Data in Fig. 4 show that when compared with
untreated controls, HCT-8/5-FU cells treated with EESB for 24 h had
a distinct rise in Rh-123 intracellular level.
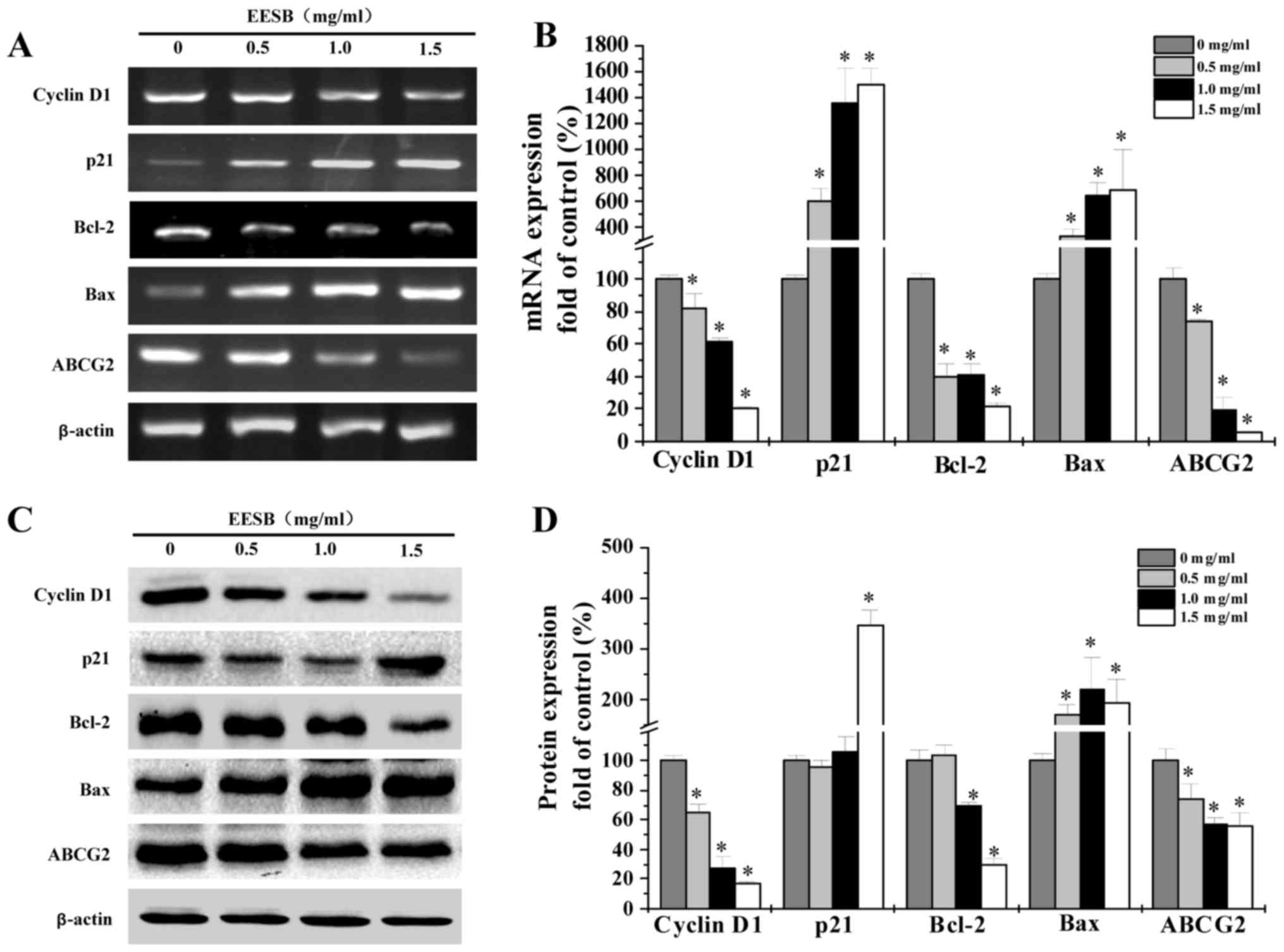
EESB regulates expression of cyclin
D1, p21, Bcl-2, Bax and ABCG2 in HCT-8/5-FU cells
RT-PCR and western blot analyses were used to
determine the mRNA and protein levels of cyclin D1, p21, Bcl-2, Bax
and ABCG2 in EESB-treated HCT-8/5-FU cells. According to results
(Fig. 5), pro-proliferative cyclin
D1 and anti-apoptotic Bcl-2 were reduced in both mRNA and protein
expression under EESB treatment while anti-proliferative p21 and
pro-apoptotic Bax showed increased levels. Besides, the ABC
transporter, ABCG2, was significantly downregulated by EESB
treatment.
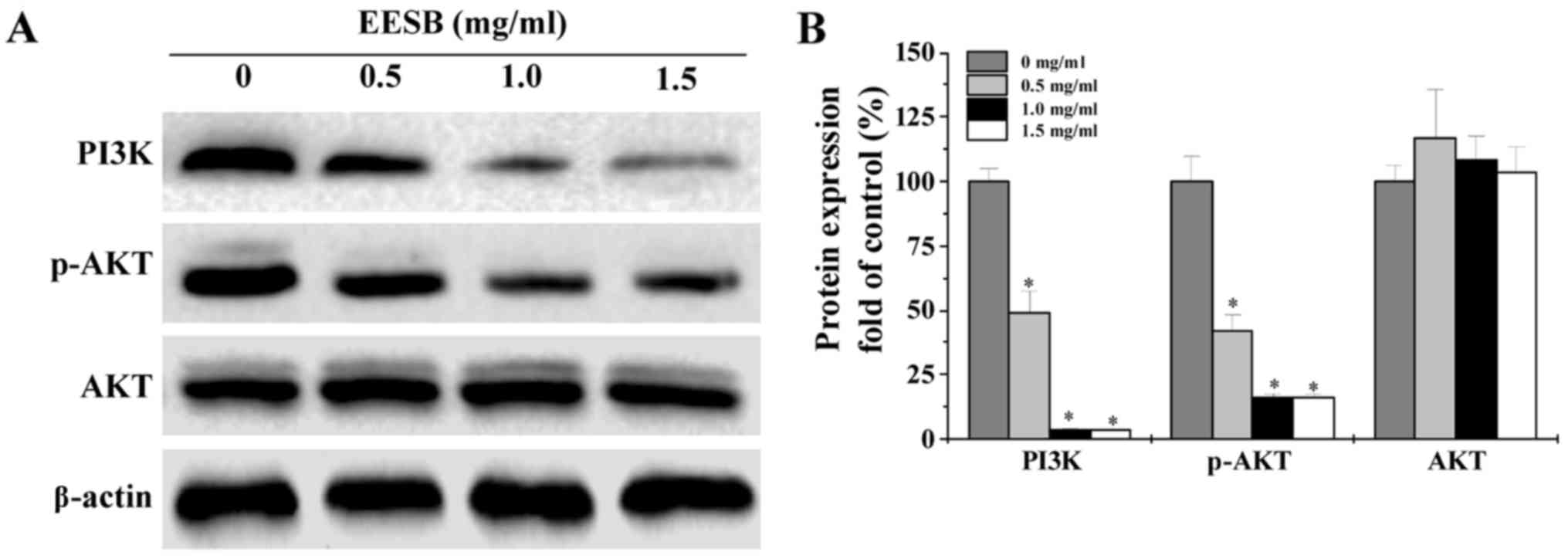
EESB suppresses the activation of
PI3K/AKT pathway in HCT-8/5-FU cells
The activation of PI3K/AKT signaling was evaluated
by western blot analysis. As shown in Fig. 6, EESB treatment remarkably decreased
PI3K protein expression and AKT phosphorylation level, while the
protein expression of total AKT was not affected.
Discussion
Currently, 5-FU-based regimens still are chosen for
most CRC patients as a therapy. However, the sensitivity to
different chemotherapeutics varies widely from individual to
individual. Due to 5-FU resistance or MDR and the fact that normal
cells cannot stand a certain level of toxicity, systemic
chemotherapy using 5-FU-based regimens shows a poor response of
10–20% (18–21). A few mechanisms have been proposed
for the resistance to chemotherapeutic agents in CRC cells.
Among those proposals, the disorder in cell
proliferation contributes to MDR. For example, cancer cells survive
by controlling the cell cycle. Under this main working mechanism,
cell cycle checkpoints may be activated when cancer cells are in
drug-toxicity, so that cell cycle cannot progress. This leads to
the enhancement of damage repair and resistance phenotype. Cyclin
D1 and p21 are crucial regulators in the cell cycle. By regulating
with these two regulators, the cell cycle has been shown arrested
by many anticancer agents at a particular checkpoint (22–24).
Therefore, to develop anticancer and MDR reversal agents, a
decisive method is to target the specific cell cycle regulators.
MDR cells often show a strong ability of apoptosis resistant due to
the imbalance of pro- and anti-apoptosis. In blocking apoptosis, a
key role is played by anti-apoptotic Bcl-2 protein, while its
overexpression also leads to evasion of apoptosis and the increase
of MDR. On the other hand, pro-apoptotic Bax can make malignant
cells more sensitive to apoptosis, which leads to overcoming MDR
(25,26), but it is always with a low
expression in MDR cells, together with the upregulated Bcl-2,
supporting the MDR phenotype. However, the gene therapy directly
targeting the cyclin D1, p21, Bcl-2 and Bax remains
controversial.
Besides, the high expression of ABC transporters
also draws an attention. ABC transporters, relating to plasma
membrane and depending on consuming energy, work as efflux pumps,
effectively putting various substrates across lipid bilayers
(27–29). Its role in fighting against
xenobiotics and their metabolites has been of great importance
(30,31) and this also worked in the
ATP-dependent export of chemotherapeutic drugs that makes
contribution to the MDR phenotype found in CRC (32,33).
When ABC transporters overexpress themselves, many drugs show a
reduced level in toxicity and the intercellular accumulation; thus,
showing promise in CRC that ABCG2 can be the target for therapeutic
treatment, and effort has been focused on the identification of
ABCG2 pharmacophores and their effective inhibition in the efflux
effect (34). Effort to develop
reversing agents that target ABC transporters has been reported,
yet in clinical application the limitations of these agents,
including the ABCG2 dual inhibitor, Elacridar, have shown poor
performance of their solubility and the reduction in oral
bioavailability (35–37).
However, it has been reported that without the
PI3K/AKT signaling pathway, many cellular functions such as cell
cycle, cell proliferation and cell survival cannot regulate
normally (38,39). Also, according to several studies,
AKT mediate the regulation of ABCG2 function and localization to
the plasma membrane. Such is not the constrain in tumor development
but in the tumor's potential response to cancer treatment. Being
activated, this pathway has decreasing sensitivity to
chemotherapeutics which contribute to treatment failure. Targeting
the PI3K/AKT signaling pathway is just like the saying ‘to catch
bandits, first catch the ringleader’ and may become a promising way
to deal with the MDR obstacle. A flavonoid derivative, LY294002
(LY), inhibitor of PI3K/AKT pathway, has been reported, but still
requires clinical trial (40).
Approaches are not many to reduction of side effects and of the
risk of inducting drug resistance in CRC, thus proposal to adopt
alternative remedies, such as traditional medicines and herbs is
being supported by patients and clinicians alike (41).
The effects of EESB, a traditional Chinese herbal
medicine, in anticancer use has been revealed as potent, albeit
knowledge how EESB works in reducing drug resistance is lacking.
This study showed 5-FU effectively decrease the degree of cell
viability of HCT-8 cells, making not significant impact on the
drug-resistant HCT-8/5-FU cells (Fig.
1). Different from 5-FU, EESB treatment impressively decreased
the cell proliferation and survival of HCT-8/5-FU cells mainly by
regulating the related cyclin D1 and p21 (Figs. 2 and 5). Besides, EESB treatment significantly
promoted apoptosis of HCT-8/5-FU cells through the rebalance of the
Bcl-2 and Bax (Figs. 3 and 5). Moreover, the ABC transporters, ABCG2,
is part of the reason why there is MDR phenotype in CRC, and by
checking the accumulation assay of the Rh-123, molecularly the
efflux activity of ABC transporters in treated cells (42) can be measured. To further clarify
how EESB functions as a reversing agent targeting ABC transporters,
flow cytometry was used to evaluate the EESB's role in Rh-123 in
accumulation. EESB-treated cells presented obvious increased
retention of Rh-123 and lower level of expression of ABCG2, based
on the comparison with untreated controls (Figs. 4 and 5), which indicates that EESB may inhibit
the efflux function of ABC transporters and thus increase drug
accumulation in treated cells. Since the PI3K/AKT pathway is
closely linked to the MDR in CRC, we evaluated the effect of EESB
on HCT-8/5-FU cells, the results showed that EESB treatment
obviously inhibited the activation of this pathway (Fig. 6).
In conclusion, the results of the present study
further assistance in getting insight into EESB that can potently
inhibit CRC drug resistance, however, due to the complexity of MDR,
further mechanism studies are yet to be elucidated.
Acknowledgements
This study was supported by the Natural Science
Foundation of Fujian Province, China (2015J01337), Project Funding
for the Training of Young and Middle-aged Backbone Personnel of
Fujian Provincial Health and Family Planning Commission
(2016-ZQN-67) and the Developmental Fund of Chen Keji Integrative
Medicine (Fujian, China; CKJ2014013 and CKJ2015007).
Glossary
Abbreviations
Abbreviations:
|
EESB
|
ethanol extract of Scutellaria
Barbata D. Don
|
|
CRC
|
colorectal cancer
|
|
MDR
|
multidrug resistance
|
|
5-FU
|
5-fluorouracil
|
|
ABC transporter
|
ATP-binding cassette transporter
|
|
DAPI
|
2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride
|
|
Rh-123
|
rhodamine-123
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andersen V, Vogel LK, Kopp TI, Sæbø M,
Nonboe AW, Hamfjord J, Kure EH and Vogel U: High ABCC2 and low
ABCG2 gene expression are early events in the colorectal
adenoma-carcinoma sequence. PLoS One. 10:e01192552015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.(In Japanese).
PubMed/NCBI
|
|
5
|
Mastalier B, Tihon C, Ghiţă B, Botezatu C,
Deaconescu V, Mandisodza P, Drăghici C and Simion S: Surgical
treatment of colon cancer: Colentina surgical clinic experience. J
Med Life. 5:348–353. 2012.PubMed/NCBI
|
|
6
|
Van Cutsem E, Nordlinger B and Cervantes
A: ESMO Guidelines Working Group: Advanced colorectal cancer: ESMO
Clinical Practice Guidelines for treatment. Ann Oncol. 21 Suppl
5:v93–v97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Mo Y, Wang X, Liu J, Zhang X, Wang
J, Hu L, Yang C, Chen L and Wang Y: Conditionally replicative
adenovirus-based mda-7/IL-24 expression enhances sensitivity of
colon cancer cells to 5-fluorouracil and doxorubicin. J
Gastroenterol. 48:203–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shain KH and Dalton WS: Cell adhesion is a
key determinant in de novo multidrug resistance (MDR): New targets
for the prevention of acquired MDR. Mol Cancer Ther. 1:69–78.
2001.PubMed/NCBI
|
|
9
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tazzari PL, Cappellini A, Ricci F,
Evangelisti C, Papa V, Grafone T, Martinelli G, Conte R, Cocco L,
McCubrey JA, et al: Multidrug resistance-associated protein 1
expression is under the control of the phosphoinositide 3
kinase/Akt signal transduction network in human acute myelogenous
leukemia blasts. Leukemia. 21:427–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shekari F, Sadeghpour H, Javidnia K, Saso
L, Nazari F, Firuzi O and Miri R: Cytotoxic and multidrug
resistance reversal activities of novel 1,4-dihydropyridines
against human cancer cells. Eur J Pharmacol. 746:233–244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stepanenko AA, Andreieva SV, Korets KV,
Mykytenko DO, Baklaushev VP, Huleyuk NL, Kovalova OA, Kotsarenko
KV, Chekhonin VP, Vassetzky YS, et al: Temozolomide promotes
genomic and phenotypic changes in glioblastoma cells. Cancer Cell
Int. 16:362016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pharmacopoeia of the People's Republic of
China. China Medical Science Press; Beijing: pp. 109–110. 2010
|
|
15
|
Wei L, Lin J, Wu G, Xu W, Li H, Hong Z and
Peng J: Scutellaria barbata D. Don induces G1/S arrest via
modulation of p53 and Akt pathways in human colon carcinoma cells.
Oncol Rep. 29:1623–1628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z
and Peng J: Scutellaria barbata D. Don inhibits tumor
angiogenesis via suppression of Hedgehog pathway in a mouse model
of colorectal cancer. Int J Mol Sci. 13:9419–9430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Lin J, Xu W, Hong Z, Liu X and Peng
J: Inhibition of tumor angiogenesis by Scutellaria barbata
D. Don via suppressing proliferation, migration and tube formation
of endothelial cells and downregulation of the expression of VEGF-A
in cancer cells. J Med Plants Res. 5:3260–3268. 2011.
|
|
18
|
Lin L, Liu Y, Li H, Li PK, Fuchs J,
Shibata H, Iwabuchi Y and Lin J: Targeting colon cancer stem cells
using a new curcumin analogue, GO-Y030. Br J Cancer. 105:212–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 3:5232006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
21
|
Van Cutsem E and Costa F: Progress in the
adjuvant treatment of colon cancer: Has it influenced clinical
practice? JAMA. 294:2758–2760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji T, Lin C, Krill LS, Eskander R, Guo Y,
Zi X and Hoang BH: Flavokawain B, a kava chalcone, inhibits growth
of human osteosarcoma cells through G2/M cell cycle arrest and
apoptosis. Mol Cancer. 12:552013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu G, Chu J, Huang Z, Ye J, Chen P, Zheng
C, Li X, Liu X and Wu M: Xiao Jin Wan, a traditional Chinese herbal
formula, inhibits proliferation via arresting cell cycle
progression at the G2/M phase and promoting apoptosis via
activating the mitochondrial-dependent pathway in U-2OS human
osteosarcoma cells. Int J Oncol. 42:1070–1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng SE, Xiong S, Lin F, Qiao GL, Feng T,
Shen Z, Min DL, Zhang CL and Yao Y: Pirarubicin inhibits
multidrug-resistant osteosarcoma cell proliferation through
induction of G2/M phase cell cycle arrest. Acta Pharmacol Sin.
33:832–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wesarg E, Hoffarth S, Wiewrodt R, Kröll M,
Biesterfeld S, Huber C and Schuler M: Targeting BCL-2 family
proteins to overcome drug resistance in non-small cell lung cancer.
Int J Cancer. 121:2387–2394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Zhang CL, Zeng BF, Wu XS, Gao TT
and Oda Y: Enhanced chemosensitivity of drug-resistant osteosarcoma
cells by lentivirus-mediated Bcl-2 silencing. Biochem Biophys Res
Commun. 390:642–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deeley RG, Westlake C and Cole SP:
Transmembrane transport of endo- and xenobiotics by mammalian
ATP-binding cassette multidrug resistance proteins. Physiol Rev.
86:849–899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao D, Tang S, Aslam S, Ahmad M, To KK,
Wang F, Huang Z, Cai J and Fu L: UMMS-4 enhanced sensitivity of
chemotherapeutic agents to ABCB1-overexpressing cells via
inhibiting function of ABCB1 transporter. Am J Cancer Res.
4:148–160. 2014.PubMed/NCBI
|
|
30
|
Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S,
Zhang L and Han Z: Suppression of ABCG2 inhibits cancer cell
proliferation. Int J Cancer. 126:841–851. 2010.PubMed/NCBI
|
|
31
|
Zhang H, Wang YJ, Zhang YK, Wang DS,
Kathawala RJ, Patel A, Talele TT, Chen ZS and Fu LW: AST1306, a
potent EGFR inhibitor, antagonizes ATP-binding cassette subfamily G
member 2-mediated multidrug resistance. Cancer Lett. 350:61–68.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ee PL, He X, Ross DD and Beck WT:
Modulation of breast cancer resistance protein (BCRP/ABCG2) gene
expression using RNA interference. Mol Cancer Ther. 3:1577–1583.
2004.PubMed/NCBI
|
|
33
|
Giampieri R, Scartozzi M, Loretelli C,
Piva F, Mandolesi A, Lezoche G, Del Prete M, Bittoni A, Faloppi L,
Bianconi M, et al: Cancer stem cell gene profile as predictor of
relapse in high risk stage II and stage III, radically resected
colon cancer patients. PLoS One. 8:e728432013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X and Morris ME: Effects of the
flavonoid chrysin on nitrofurantoin pharmacokinetics in rats:
Potential involvement of ABCG2. Drug Metab Dispos. 35:268–274.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu KJ, He JH, Su XD, Sim HM, Xie JD, Chen
XG, Wang F, Liang YJ, Singh S, Sodani K, et al: Saracatinib
(AZD0530) is a potent modulator of ABCB1-mediated multidrug
resistance in vitro and in vivo. Int J Cancer. 132:224–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang HW, Hua MY, Liu HL, Tsai RY, Pang ST,
Hsu PH, Tang HJ, Yen TC and Chuang CK: An epirubicin-conjugated
nanocarrier with MRI function to overcome lethal
multidrug-resistant bladder cancer. Biomaterials. 33:3919–3930.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sane R, Mittapalli RK and Elmquist WF:
Development and evaluation of a novel microemulsion formulation of
elacridar to improve its bioavailability. J Pharm Sci.
102:1343–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arafa SA, Zhu Q, Shah ZI, Wani G, Barakat
BM, Racoma I, El-Mahdy MA and Wani AA: Thymoquinone up-regulates
PTEN expression and induces apoptosis in doxorubicin-resistant
human breast cancer cells. Mutat Res. 706:28–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hegedüs C, Truta-Feles K, Antalffy G,
Brózik A, Kasza I, Német K, Orbán TI, Özvegy-Laczka C, Váradi A and
Sarkadi B: PI3-kinase and mTOR inhibitors differently modulate the
function of the ABCG2 multidrug transporter. Biochem Biophys Res
Commun. 420:869–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Wang J, Ren M, Li M, Chen D, Chen
J, Shi F, Wang X and Dou J: Gene therapy of ovarian cancer using
IL-21-secreting human umbilical cord mesenchymal stem cells in nude
mice. J Ovarian Res. 7:82014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kono T, Hata T, Morita S, Munemoto Y,
Matsui T, Kojima H, Takemoto H, Fukunaga M, Nagata N, Shimada M, et
al: Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): A
phase 2, multicenter, randomized, double-blind, placebo-controlled
trial of goshajinkigan to prevent oxaliplatin-induced neuropathy.
Cancer Chemother Pharmacol. 72:1283–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pallis M and Russell N: P-glycoprotein
plays a drug-efflux-independent role in augmenting cell survival in
acute myeloblastic leukemia and is associated with modulation of a
sphingomyelin-ceramide apoptotic pathway. Blood. 95:2897–2904.
2000.PubMed/NCBI
|