Introduction
Annually, 160,000 new laryngeal cancers are
diagnosed globally. In the Nordic countries, nearly 700 new cases
are reported each year (2009–2013) with ~230 laryngeal
cancer-related deaths annually (2006–2010) (1). Laryngeal cancer, of which 90%
represents squamous cell carcinoma (SCC), is subclassified
according to localization into supraglottic, glottic and subglottic
carcinoma. In glottic SCC, lymph node metastases are rare at
presentation and thus, the treatment objective is to achieve
permanent local control. Currently, the management of T2N0 and T3N0
glottic SCC consists mainly of radiotherapy (RT) which is combined
with chemotherapy (chemoradiotherapy, CRT) in locally advanced
cases.
Recent studies from Finland and Sweden show
suboptimal treatment results for patients with T2-T3 laryngeal SCC
(2,3), and poor results regarding T3N0
patients are also a concern in the United States (4). Although the reasons for these poor
results are currently unknown, it has been speculated that the use
of organ preservation treatment (RT or CRT) as an alternative to
radical surgery (often total laryngectomy) may be one of the
culprits (4,5). In this setting, there is a great need
for predictive markers to identify the patients that will respond
favorably to definitive oncological treatment. With proper
pre-treatment stratification, oncological treatment could be
directed to ‘responders’ whereas known ‘non-responders’ could
undergo primary surgical therapy for improved outcome. We have
previously explored a number of potential markers, in vitro
and in vivo, that could potentially identify head and neck
SCC (HNSCC) patients who would benefit from RT. Through these
studies we have identified two proteins, survivin and WRAP53β,
which were significantly associated with positive response to RT
and longer overall survival (OS) in patients with HNSCC (6,7).
Survivin (BIRC5), the smallest member of the
inhibitor of apoptosis family, is a multifunctional protein that
acts both as an inhibitor of apoptosis and a regulator of mitosis
(8). It is also involved in cell
proliferation, chromosome movement, and regulation of response to
cellular stress (9). Survivin
exists both in the nucleus and in the cytoplasm, and its
subcellular localization is suggested to correspond with its
different functions (10,11). In oral SCC, Lo Muzio et al
(12) have reported expression of
survivin as a negative prognostic factor, whereas Freier et
al (13) have showed a
correlation between high survivin expression and improved OS.
The scaffold protein WRAP53β (alias TCAB1, WDR79),
encoded by the WRAP53 gene (WD40 encoding RNA antisense to p53)
(14), controls the intracellular
localization of factors involved in splicing, telomere elongation
and DNA repair (15). Lower
expression of nuclear WRAP53β correlates with shorter survival not
only for HNSCC, but also for ovarian and breast cancer and in
addition, correlates with disruption of the DNA damage response in
ovarian tumors (7,16,17).
Moreover, inherited mutations in WRAP53β cause the
cancer-predisposition disorder dyskeratotis congenita where
patients mainly develop hematological malignancies and head and
neck cancer, altogether indicating that the WRAP53β protein acts as
a tumor suppressor. At the same time, overexpression of WRAP53β is
observed in different cancer types, head and neck, lung and rectal
cancer (18), however, the clinical
relevance of such overexpression remains unclear.
HPV infection has been well established as a
favorable prognostic marker in oropharyngeal SCC and the expression
of p16INK4a is used as surrogate marker for the
infection. The favorable prognostic value of p16 expression remains
irrespective of the administered treatment (19). In laryngeal SCC the incidence of HPV
or p16-positivity is low compared with oropharynx (20) and a link between p16/HPV and
treatment outcome has not been found (21,22).
The primary aim of this study was to investigate
survivin, WRAP53β and p16 expression as potential predictive tumor
biomarkers for oncological treatment outcome in a selective HNSCC
patient group: T2N0-T3N0 glottic SCC treated with definitive RT or
CRT.
Materials and methods
Patient selection
Registries of the Helsinki University Hospital,
Karolinska University Hospital, and Linköping University Hospital
were utilized to identify patients treated for primary T2N0 or T3N0
glottic laryngeal SCC during 1999–2010. The study was approved by
the Regional Ethics Review Boards at Linköping University,
Karolinska Institute, and Helsinki University Hospital.
The inclusion criteria were as follows: tumor of
glottic origin, absence of neck metastasis at presentation (i.e.
classification T2N0 or T3N0), primary curatively aimed oncological
treatment (definitive RT or CRT), completion of treatment,
availability of histological biopsy material for analysis, and
follow-up of at least six months after the treatment for living
patients. Clinical patient data extracted from the hospital
registries included tumor characteristics, details on primary
treatment (RT doses), possible salvage therapy, and follow-up
information including recurrences and causes of death. Regarding
treatment, RT was given in 2 Gy fractions 5 days a week until the
target dose was reached. The mean RT dose was 68 Gy (median, 68 Gy;
range, 46–80 Gy). All except 1 patient received a minimum dose of
62 Gy. For CRT, cisplatinum 40 mg/m2 was administered
once a week concomitantly with RT to a total of six doses.
Immunohistochemistry
Standard hematoxylin and eosin stained slides of the
clinical biopsies were first evaluated by a pathologist to confirm
diagnosis and assure tumor content in paraffin blocks. New sections
from the tumor biopsies were thereafter mounted on positively
charged slides and deparaffinized in Aqua DePar (Biocare Medical,
Pacheco, CA, USA). For WRAP53β, sections were pretreated with 10 mM
citrate buffer (DakoCytomation epitope retrieval solution) in a hot
water bath (up to 100°C) for 40 min, blocked with Envision
peroxidase block (BCPX968) for 5 min, and incubated for 30 min at
room temperature with a rabbit polyclonal anti-WRAP53-C2 antibody
diluted 1:1,000 (Innovagen AB, Lund, Sweden). WRAP53β was stained
with the EnVision System-HRP (DAB) kit (DakoCytomation), followed
by counterstaining for 1 min with Tachas hematoxylin.
For survivin, sections were blocked for endogenous
peroxidase, and thereafter subjected to heat-induced antigen
retrieval. Automated IHC was performed using a LabVision
Autostainer 480S (Thermo Fisher Scientific, Runcorn, UK). A rabbit
polyclonal anti-survivin antibody 1:400 (Thermo Fisher Scientific)
was diluted in UltraAb Diluent (Thermo Fisher Scientific, Fremont,
CA, USA) and applied to the slides for 30 min at room temperature.
The slides were further incubated with the secondary reagent
(anti-rabbit horseradish peroxidase-conjugated UltraVision; Thermo
Fisher Scientific, Runcorn, UK) for 30 min at room temperature.
Following the washing steps, the slides were developed for 10 min
using the avidin-biotin peroxidase staining technique (Vector
Elite; Vector Laboratories, Burlingame, CA, USA) using
3,3-diaminobenzidine as the substrate. The slides were
counterstained with Mayers hematoxylin for 5 min (Sigma-Aldrich,
St. Louis, MO, USA).
For p16 analysis, the CINtec Histology kit with
monoclonal mouse antibody (clone E6H4; Mtm Laboratories AG,
Heidelberg, Germany) was used according to the manufacturers
instructions. Results were evaluated independently by one
pathologist (S.G.) and two additional investigators (A.H. and
K.T./L.F.) without knowledge of patient treatment or outcome. Upon
disagreement a consensus score was agreed upon.
For WRAP53β and survivin the staining intensity was
scored as follows: 0 (none), 1 (weak), 2 (moderate), or 3 (strong).
Intensity of 0–1 was considered negative and 2–3 was considered
positive. Due to slight differences in staining frequency, the
percentage of WRAP53β-positive tumor cells was scored 0 (0%), 1
(<10%), 2 (11–50%) or 3 (>50%); for survivin 0 (0%), 1
(<10%), 2 (11–50%), 3 (51%-80%) or 4 (>80%). The predominant
subcellular localization was determined by the difference in
staining intensity between the nucleus and the cytoplasm. In
analyses of WRAP53β, predominantly nuclear or equal staining in the
nucleus and the cytoplasm was considered nuclear as previously
described (7). For survivin,
predominantly nuclear, equal nuclear and cytoplasmic, and
predominantly cytoplasmic were analyzed separately. Examples of
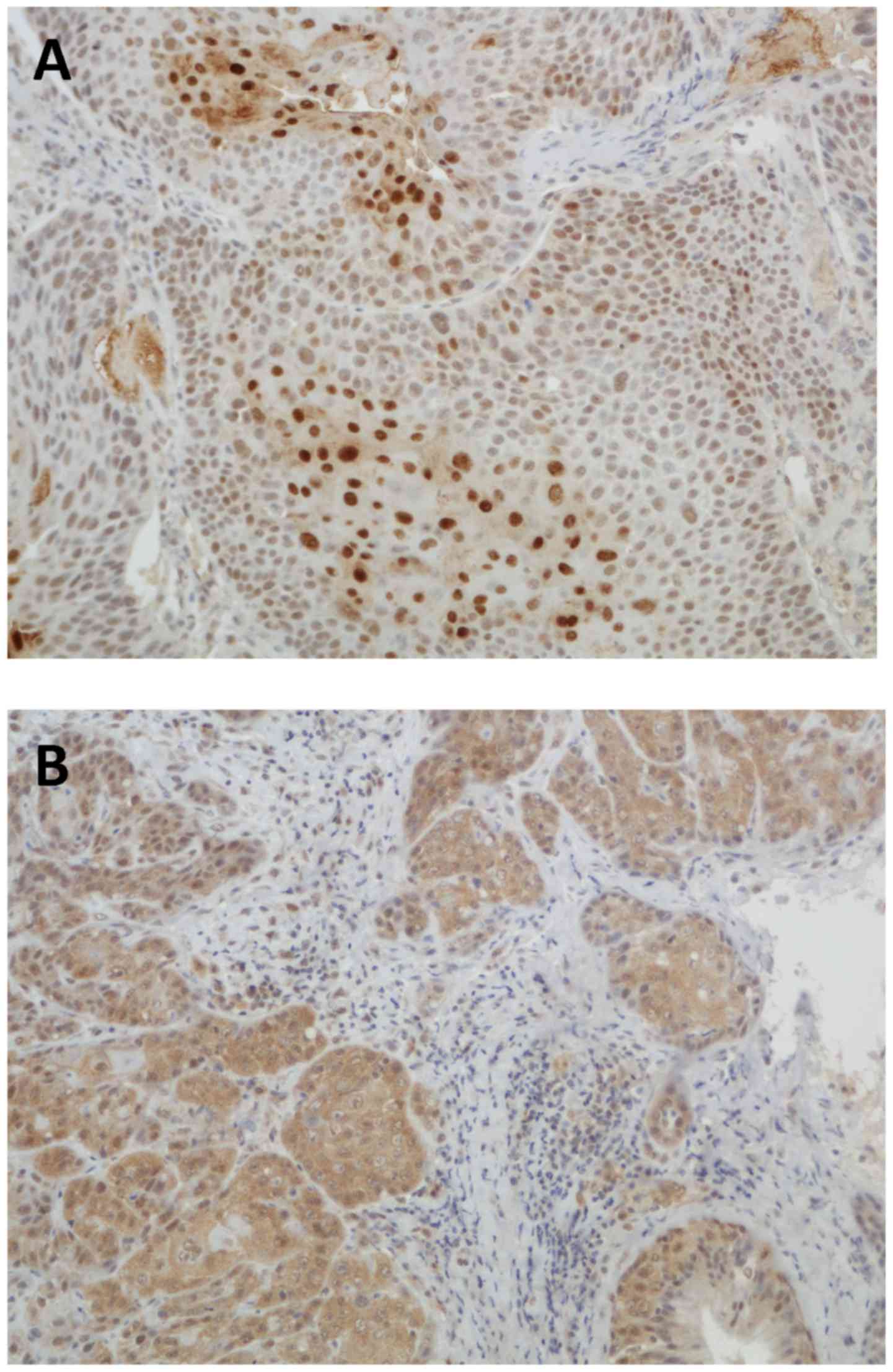
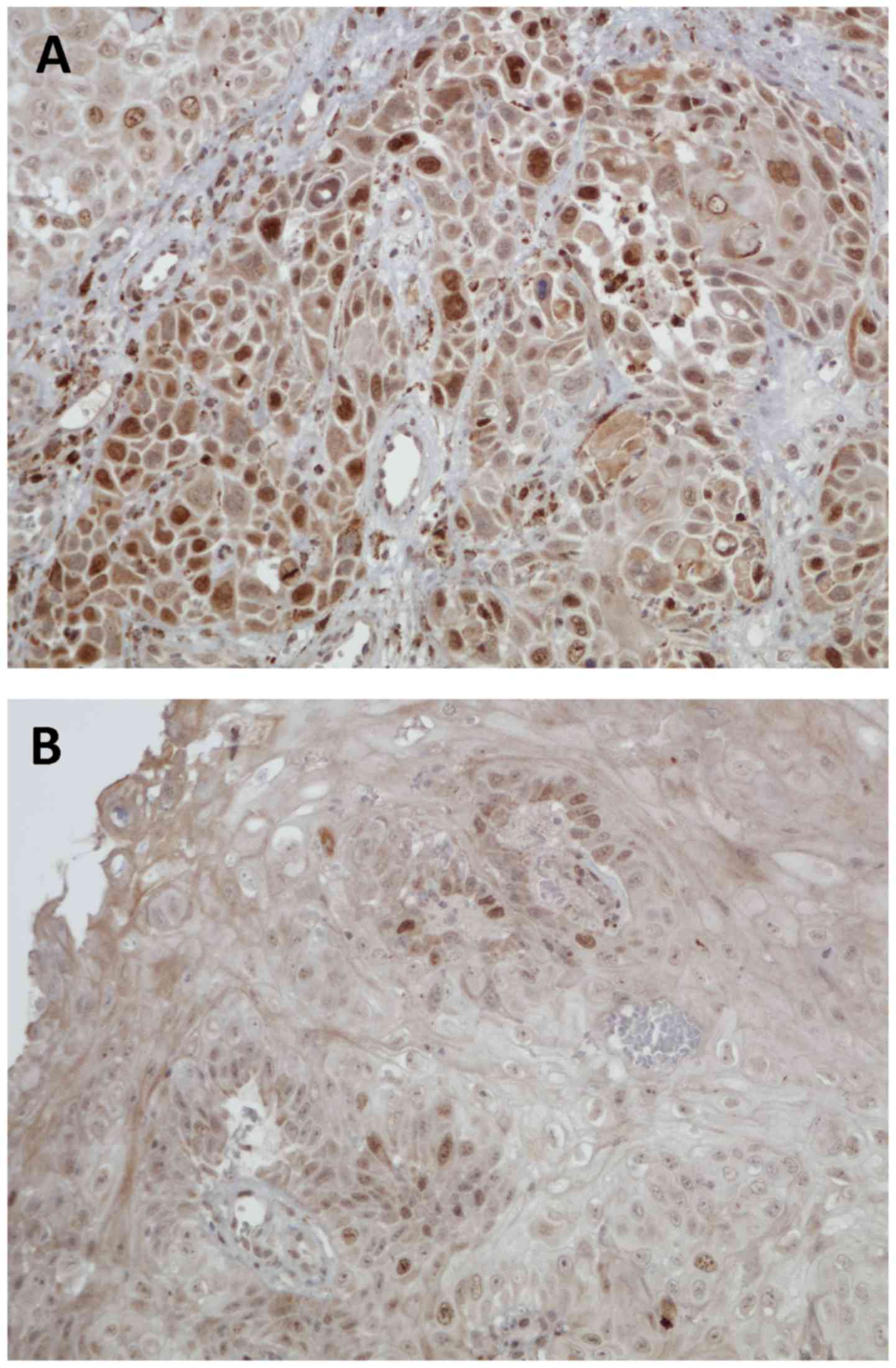
staining patterns of WRAP53β and survivin are shown in Figs. 1 and 2.
All p16 slides were scored for intensity of p16
staining in the nucleus and cytoplasm as: 0 (none), 1 (weak), 2
(moderate) or 3 (strong), with 2 or 3 being considered positive if
the majority of tumor cells (>70%) showed staining both in the
nucleus and cytoplasm (23).
Statistical methods
Comparisons of demographic factors and recurrence
with protein expression results were conducted by using the
Chi-squared test and P≤0.05 was considered significant. Overall
survival (OS, death as endpoint), disease-specific survival (DSS,
laryngeal cancer death as endpoint), disease-free survival (DFS,
recurrence or death of any cause as endpoint) and relapse-free
survival (RFS: recurrence as endpoint) were calculated using
Kaplan-Meier curves. OS and DSS times were calculated from the date
of diagnosis to the date of event or last follow-up. DFS and RFS
times were calculated from the date of treatment completion to the
date of event or last follow-up. Significance of differences
between patient groups regarding survival was determined using the
log-rank (Mantel-Cox) test. P≤0.05 was considered significant. SPSS
version 22.0 was used for all statistical analyses.
Results
Clinical data
Altogether 149 patients matched the inclusion
criteria. Patient and tumor characteristics and primary treatment
are shown in Table I. The median
follow-up was 67 months (mean, 77; range, 9–163). Incomplete
response to primary treatment was observed in 13 out of the 149
patients (9%). None of the T3N0 patients who received CRT as
primary treatment had residual tumor after treatment whereas 5
patients (23%) treated with RT had residual tumor (P=0.018). This
difference was not noted in T2N0 patients. Patients with residual
tumor after primary treatment had a significantly lower 5-year DSS
compared with patients with complete response to primary treatment
(51 vs. 87%; P=0.009). The recurrence rates after primary treatment
for T2N0 and T3N0 patients were 23 and 45%, respectively (P=0.006).
The median time to recurrence was 10 months (mean, 19; range,
3–112). Recurrence expectedly led to lower 5-year OS (no
recurrence, 71%, recurrence, 42%; P=0.001). Five-year OS and DSS
for T2N0 and T3N0 patients were 69 and 91 and 45 and 69%,
respectively.
 | Table I.Patient and tumor characteristics as
well as primary treatment. |
Table I.
Patient and tumor characteristics as
well as primary treatment.
| Characteristics | No. of patients | % of patients |
|---|
| Age (years) |
|
|
|
<60 | 58 | 39 |
|
≥60 | 91 | 61 |
| Sex |
|
|
|
Male | 143 | 96 |
|
Female | 6 | 4 |
| Smoking |
|
|
|
Ever | 128 | 86 |
|
Never | 10 | 7 |
|
N/A | 11 | 7 |
| Histological
grade |
|
|
| I | 33 | 22 |
| II | 82 | 55 |
|
III | 16 | 11 |
|
N/A | 18 | 12 |
| T class |
|
|
|
T2N0 | 105 | 71 |
|
T3N0 | 44 | 30 |
| Treatment |
|
|
| T2 |
|
|
|
RT | 94 | 90 |
|
CRT | 11 | 10 |
| T3 |
|
|
|
RT | 22 | 50 |
|
CRT | 22 | 50 |
| RT dose (Gy) |
|
|
|
<60 | 1 | 1 |
|
60-69 | 99 | 66 |
|
≥70 | 49 | 33 |
In the T3N0 group, significant differences in favor
of CRT over RT were observed in RFS, DFS, OS and DSS (Table II). Moreover, patients with T3N0
tumors who received CRT showed a significantly lower recurrence
rate (1 out of 22; 5%) compared with patients who were treated with
RT alone (11 out of 22; P<0.001). For patients with T2N0, a
significant difference in 5-year DFS was observed favoring CRT, but
no statistical differences were observed in OS, DSS or RFS.
 | Table II.OS, DSS, DFS and RFS in T2N0, T3N0
patients treated with RT or CRT. |
Table II.
OS, DSS, DFS and RFS in T2N0, T3N0
patients treated with RT or CRT.
|
| T2N0 (n=105) | T3N0 (n=44) |
|---|
|
|
|
|
|---|
|
| RT (n=94) | CRT (n=11) | P-value | RT (n=22) | CRT (n=22) | P-value |
|---|
| 5-year OS | 66.9 | 90.9 | 0.147 | 18.2 | 72.4 | 0.001 |
| 5-year DSS | 89.8 | 100 | 0.302 | 49.5 | 85.7 | 0.018 |
| 5-year DFS | 54.9 | 90.9 | 0.046 | 13.6 | 54.5 | 0.001 |
| 5-year RFS | 74.8 | 100 | 0.085 | 43.6 | 66.2 | 0.039 |
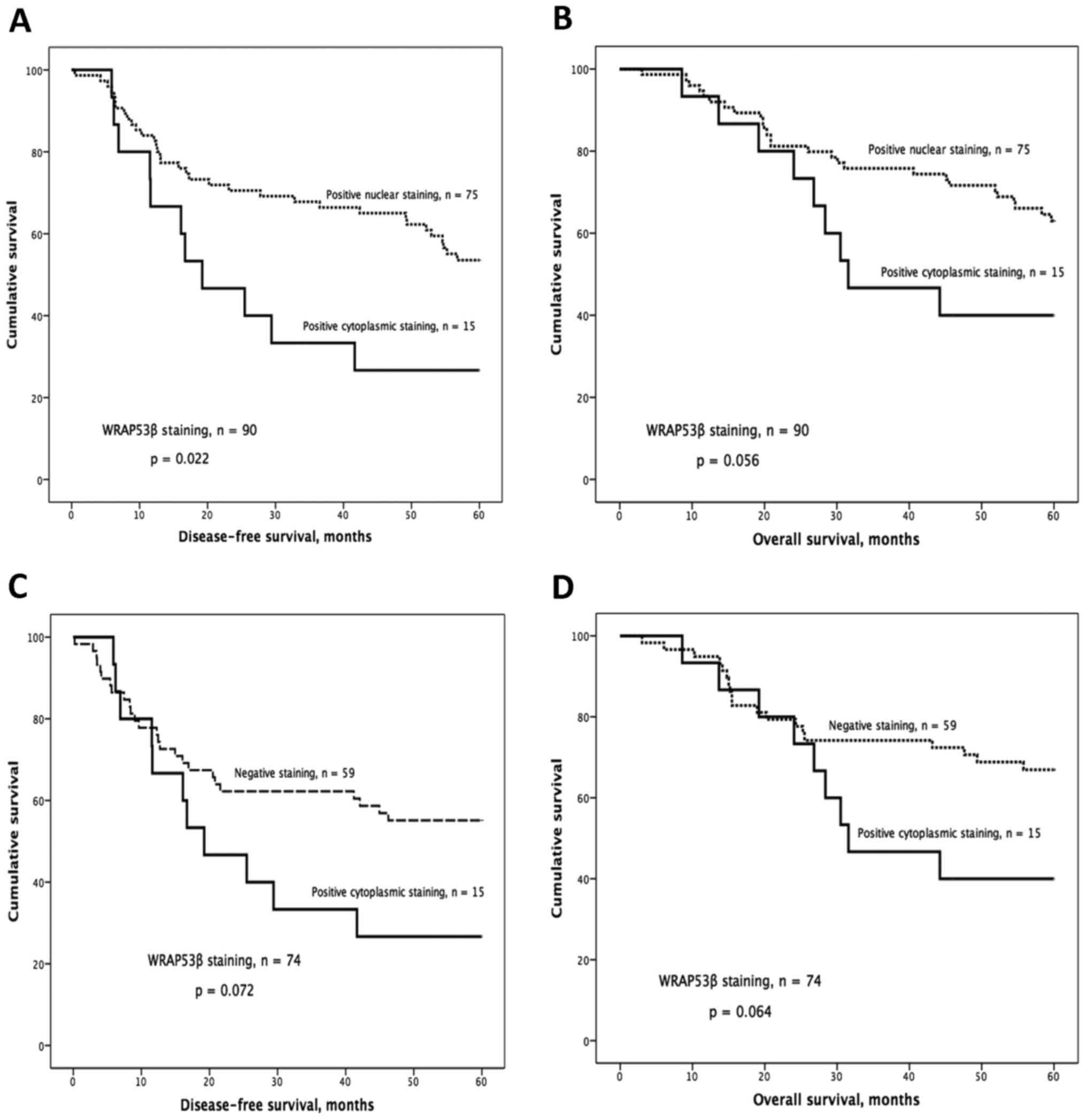
IHC staining of WRAP53β
The distribution and subcellular localization of
WRAP53β were as follows: 75 positive nuclear, 15 positive
cytoplasmic and 59 negative. Kaplan-Meier analysis showed
significantly worse DFS and a strong tendency for worse OS for
patients with tumors showing cytoplasmic staining compared with
patients with nuclear staining (P=0.022 for DFS, P=0.056 for OS;
Fig. 3A and B). This trend was also
observed when comparing patients with cytoplasmic staining to those
classified as negative (P=0.072 for DFS; P=0.064 for OS; Fig. 3C and D).
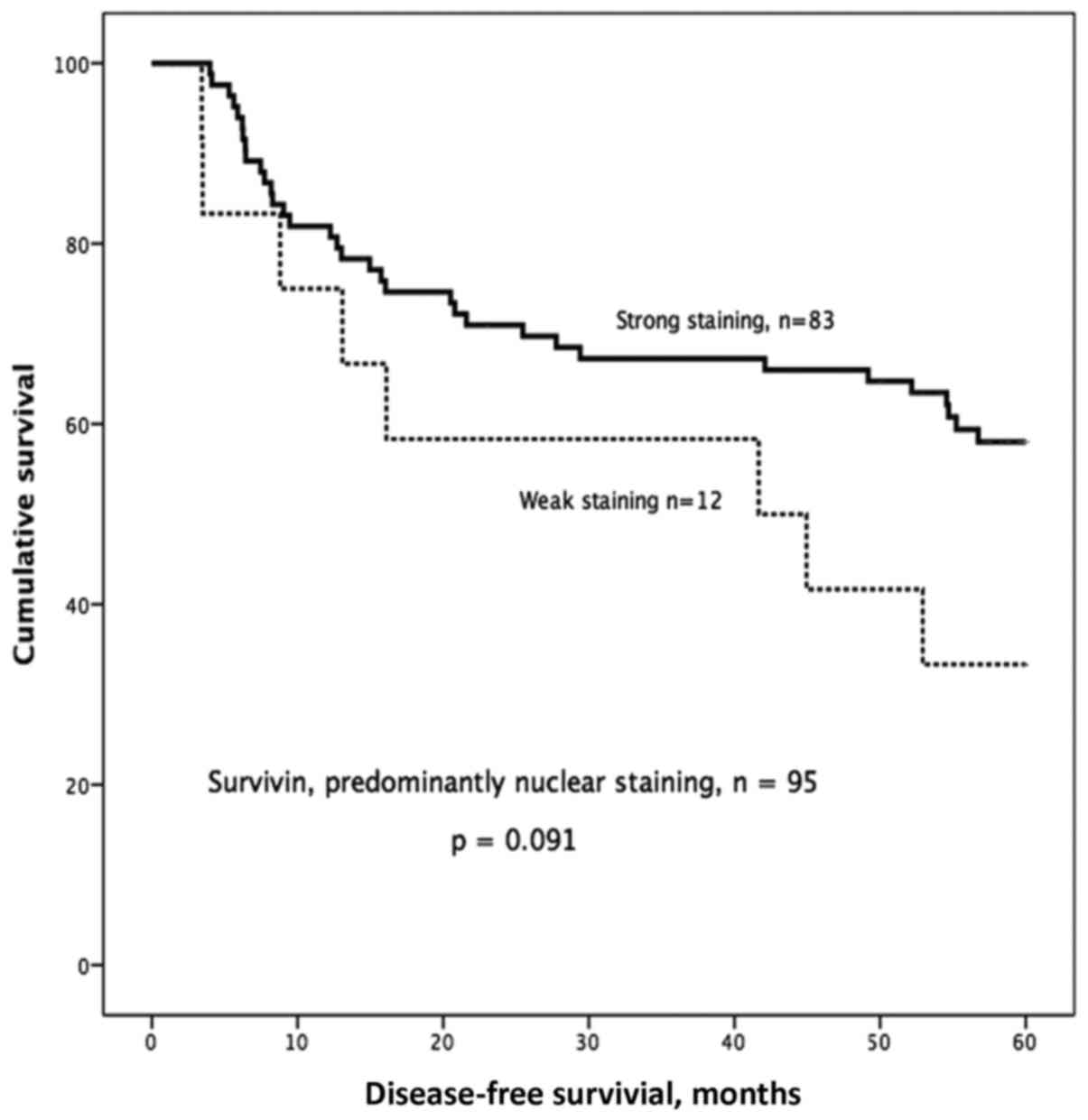
IHC staining of survivin
Based on our earlier study (6) we decided to look more specifically at
the group with predominantly nuclear staining and of the 148
samples, 95 showed predominantly nuclear staining, 35 showed
predominantly cytoplasmic staining, and 18 showed equal staining
intensity in the cytoplasm and nucleus. One sample was excluded due
to staining failure. In the group with predominantly nuclear
staining, we observed a trend towards better 5-year DFS in patients
with strong nuclear survivin expression compared with those with
weak nuclear survivin expression (P=0.091; Fig. 4).
IHC staining of p16
Moderate or strong p16 staining was only found in 11
(7%) of the tumors. The rest of the 138 tumors had weak or no
staining. When the staining of all 149 samples was examined, no
significant differences in OS, DSS, DFS or RFS between patients
with p16-positive and -negative tumors were found. However, none of
the patients with p16-positive tumors had a residual tumor after
treatment compared with 9% residual tumor rate in patients with
p16-negative tumors. This finding, however, did not reach
statistical significance (P=0.287).
Regarding demographics, samples from patients under
the age of 60 years (n=58) had higher frequency of p16 positivity
compared with older patients (16 vs. 3%; P=0.017). In the cohort of
young patients, never-smokers had more commonly p16-positive tumors
than current or former smokers (50 vs. 10%; P=0.022). Furthermore,
none of the 8 p16-positive tumors in this younger patient group
recurred compared with 36% (18/50) recurrence in p16-negative
tumors (P=0.041). Regarding survival outcomes in this younger
patient group, no significant differences were observed between
p16-positive and -negative patients (OS 100 vs. 68%; P=0.083, DSS
100 vs. 86%, P=0.276 or RFS 100 vs. 65%, P=0.073). However, a
significant difference was observed in DFS (p16-positive, 100 vs.
p16-negative, 50%; P=0.021).
The associations between WRAP53β and DFS remained
statistically significant when patients with tumors staining
positive for p16 were excluded. Different combinations of
p16INK4a, WRAP53β and survivin scores gave no additional
prognostic value.
Discussion
Studies regarding prognostic/predictive markers in
HNSCC often include tumors from different sites in a single series.
However, it is widely acknowledged that etiological factors,
metastatic potential, treatment approaches and patient outcomes
vary considerably between tumor sites. Previous studies regarding
WRAP53β, survivin and p16 have been prone to confounding factors
induced by inclusion of tumors from different HNSCC sites, TNM
classes, stages and primary treatments. To the best of our
knowledge this is the first study examining these potential
prognostic biomarkers in a large (n=149), yet, consecutive patient
series of a single HNSCC subsite (glottic SCC) with selected TNM
class tumors (T2-3N0) with uniform treatment (RT or CRT).
We observed a significantly improved outcome for
T3N0 patients treated with CRT compared with those treated with RT
alone. This improvement in outcome with CRT has been previously
demonstrated in a prospective randomized trial in advanced
laryngeal cancer patients (24). In
T2N0 patients, a significant difference in 5-year DFS was observed
favoring CRT, but no statistical differences were observed in OS,
DSS, or RFS. It should be noted, however, that the number of CRT
patients in the T2N0 group (n=11; 10%) was small for comparison.
Our results are in line with those from Nishimura et al
(25) and Akimoto et al
(26), who reported improved larynx
preservation and DFS but no survival benefit for T2N0 glottic SCC
patients treated with CRT.
To the best of our knowledge, this is the first
study to investigate the expression of WRAP53β specifically in
laryngeal SCC. Our previous study on a heterogeneous HNSCC patient
population suggested that the nuclear localization of WRAP53β was
associated with improved response to RT and improved OS (7). In the present study, our results
suggest that predominant cytoplasmic localization of WRAP53β is a
potential predictive marker of poor OS and DFS in glottic SCC.
Similarly, in breast cancer, Silwal-Pandit et al (17) have reported negative
nuclear/positive cytoplasmic WRAP53β to be associated with reduced
survival. There are several possible explanations for these
findings, related to presumed decreased nuclear function of the
WRAP53β protein if potentially trapped in the cytoplasm. One
example could be related to telomerase dysfunction, an event known
to occur in WRAP53β-deficient cells and previously linked to
radioresistance (27,28). Loss of WRAP53β protein has also been
shown to disturb repair of DNA double-strand breaks, resulting in
increased genomic instability (16,29).
In the present study, survivin did not appear as
strongly associated with outcome in glottic SCC as it did in our
previous investigation of a heterogeneous HNSCC study population
(6). We have demonstrated in
vitro that downregulation of survivin in two HNSCC cell lines
led to decreased proliferation and increased radioresistance
(6). One difference between our
studies is the IHC staining pattern observed, having been
homogeneously nuclear in the former study and more heterogeneous
nuclear and cytoplasmic in the present study. It is interesting
that when isolating the patients whose tumors showed predominantly
nuclear expression, a trend for improved DFS was observed for those
with strong nuclear staining, but difficulties in standardizing the
IHC staining may reduce the utility of this marker.
Although p16 expression is well recognized as a
prognostic factor for oropharyngeal SCC, in laryngeal SCC its
prognostic value remains unclear according to largest studies
available by Morshed et al (22) (n=93) and Young et al
(21) (n=307). Unfortunately, their
series contain tumors from variable laryngeal subsites (glottic,
supraglottic and subglottic), variable stages (T1-4 and N0-N3), and
variable treatments (both surgical and non-surgical), thus,
complicating the evaluation of outcome differences between groups.
However, the lack of general prognostic significance proved true
also in our highly homogeneous patient material of T2-3N0 glottic
SCC treated with RT/CRT, when observed together as single series.
The incidence of p16 expression in this study is in agreement with
Young et al (21) and
Castellsagué et al (20) who
noted incidences of 6.5 and 3%, respectively. Higher prevalence of
p16 or HPV-positivity in laryngeal SCC has previously been
associated with female sex (21),
non-smokers (30) and younger age
(31). We also found a significant
predominance of p16-positivity in younger (<60 years) patients.
Among these patients, non-smokers were more frequently p16-positive
than smokers, albeit smoking had no significant relation to p16
positivity in the whole cohort.
Although no significant link between HPV/p16
positivity and laryngeal SCC treatment outcome has been established
in general, some authors have observed trends towards less
recurrence among HPV/p16-positive patients (30,31).
In our material, none of the p16-positive laryngeal SCCs in
patients under 60 years recurred (P=0.041), and DFS was also
significantly better for this group. Taken together, these previous
and current findings suggest that there may be a distinct subgroup
of laryngeal SCCs with HPV as a major etiological factor. This
group may also have an improved outcome compared with laryngeal
SCCs with no HPV association. Unfortunately, HPV- or p16-positive
tumors form a small minority of laryngeal SCC. Larger studies or
meta-analyses are needed to reach sufficient statistical power to
assess its utility as a prognostic factor. Also, the insufficient
sensitivity of p16 as a surrogate marker (32) calls for proper assessment of HPV
status in these p16-positive tumors, which could not be conducted
in the current study.
In conclusion, the present study demonstrates
markedly improved outcome for T3N0 glottic SCC patients treated
with CRT as compared to those treated with RT alone. Furthermore,
our results suggest that cytoplasmic WRAP53β may be a potential
predictive marker of poor response to RT/CRT in glottic laryngeal
cancer. While HPV or p16 positivity is rare in laryngeal SCC, it
may prove significant in identifying a prognostically separate
subgroup in the future. Prospective studies are warranted to test
the value of predictive markers of RT response in HNSCC such as
WRAP53β, to help us to proceed with more individualized treatment
decisions and better outcomes for our patients.
Acknowledgements
The present study was supported by the Swedish
Cancer Society (2010/545), the County Council of Östergötland, the
Research Funds of Linköping University Hospital, the Finnish Cancer
Society, the Finnish Medical Foundation, the Finnish-Norwegian
Medical Foundation and Helsinki University Hospital Research Funds
(TYH2015204).
References
|
1
|
Engholm G, Ferlay J, Christensen N, Bray
F, Gjerstorff ML, Klint A, Køtlum JE, Olafsdóttir E, Pukkala E and
Storm HH: NORDCAN - a Nordic tool for cancer information, planning,
quality control and research. Acta Oncol. 49:725–736. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haapaniemi A, Koivunen P, Saarilahti K,
Kinnunen I, Laranne J, Aaltonen LM, Närkiö M, Lindholm P, Grénman
R, Mäkitie A, et al: Finnish Head and Neck Oncology Working Group:
Laryngeal cancer in Finland: A 5-year follow-up study of 366
patients. Head Neck. 38:36–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wennerberg J: A population based
perspective on treatment and outcome of glottic laryngeal carcinoma
stage T3 and T4 - does organ preservation jeopardize survival?5th
World Congress of IFHNOS and the 2014 Annual Meeting of the AHNS.
New York: 2014
|
|
4
|
Hoffman HT, Porter K, Karnell LH, Cooper
JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A and Robinson RA:
Laryngeal cancer in the United States: Changes in demographics,
patterns of care, and survival. Laryngoscope. 116 Suppl 111:1–13.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olsen KD: Reexamining the treatment of
advanced laryngeal cancer. Head Neck. 32:1–7. 2010.PubMed/NCBI
|
|
6
|
Farnebo L, Tiefenbock K, Ansell A, Thunell
LK, Garvin S and Roberg K: Strong expression of survivin is
associated with positive response to radiotherapy and improved
overall survival in head and neck squamous cell carcinoma patients.
Int J Cancer. 133:1994–2003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garvin S, Tiefenböck K, Farnebo L, Thunell
LK, Farnebo M and Roberg K: Nuclear expression of WRAP53β is
associated with a positive response to radiotherapy and improved
overall survival in patients with head and neck squamous cell
carcinoma. Oral Oncol. 51:24–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumor therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altieri DC: Targeting survivin in cancer.
Cancer Lett. 332:225–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F, Yang J, Ramnath N, Javle MM and Tan
D: Nuclear or cytoplasmic expression of survivin: what is the
significance? Int J Cancer. 114:509–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stauber RH, Mann W and Knauer SK: Nuclear
and cytoplasmic survivin: Molecular mechanism, prognostic, and
therapeutic potential. Cancer Res. 67:5999–6002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo Muzio L, Farina A, Rubini C, Pezzetti
F, Stabellini G, Laino G, Santarelli A, Pannone G, Bufo P, de Lillo
A, et al: Survivin as prognostic factor in squamous cell carcinoma
of the oral cavity. Cancer Lett. 225:27–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Freier K, Pungs S, Sticht C,
Flechtenmacher C, Lichter P, Joos S and Hofele C: High survivin
expression is associated with favorable outcome in advanced primary
oral squamous cell carcinoma after radiation therapy. Int J Cancer.
120:942–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahmoudi S, Henriksson S, Corcoran M,
Méndez-Vidal C, Wiman KG and Farnebo M: Wrap53, a natural p53
antisense transcript required for p53 induction upon DNA damage.
Mol Cell. 33:462–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henriksson S and Farnebo M: On the road
with WRAP53β: Guardian of Cajal bodies and genome integrity. Front
Genet. 6:912015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hedström E, Pederiva C, Farnebo J, Nodin
B, Jirström K, Brennan DJ and Farnebo M: Downregulation of the
cancer susceptibility protein WRAP53β in epithelial ovarian cancer
leads to defective DNA repair and poor clinical outcome. Cell Death
Dis. 6:e18922015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silwal-Pandit L, Russnes H, Borgen E,
Skarpeteig V, Vollan Moen HK, Schlichting E, Kåresen R, Naume B,
Børresen-Dale AL, Farnebo M, et al: The sub-cellular Localization
of WRAP53 has prognostic impact in breast cancer. PLoS One.
10:e01399652015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rassoolzadeh H, Böhm S, Hedström E, Gad H,
Helleday T, Henriksson S and Farnebo M: Overexpression of the
scaffold WD40 protein WRAP53β enhances the repair of and cell
survival from DNA double-strand breaks. Cell Death Dis.
7:e22672016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fischer CA, Zlobec I, Green E, Probst S,
Storck C, Lugli A, Tornillo L, Wolfensberger M and Terracciano LM:
Is the improved prognosis of p16 positive oropharyngeal squamous
cell carcinoma dependent of the treatment modality? Int J Cancer.
126:1256–1262. 2010.PubMed/NCBI
|
|
20
|
Castellsagué X, Alemany L, Quer M, Halec
G, Quirós B, Tous S, Clavero O, Alòs L, Biegner T, Szafarowski T,
et al: ICO International HPV in Head and Neck Cancer Study Group:
HPV involvement in head and neck cancers: Comprehensive assessment
of biomarkers in 3680 patients. J Natl Cancer Inst. 108:djv4032016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Young RJ, Urban D, Angel C, Corry J, Lyons
B, Vallance N, Kleid S, Iseli TA, Solomon B and Rischin D:
Frequency and prognostic significance of p16INK4A
protein overexpression and transcriptionally active human
papillomavirus infection in laryngeal squamous cell carcinoma. Br J
Cancer. 112:1098–1104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morshed K, Polz-Dacewicz M, Szymański M
and Polz D: Short-fragment PCR assay for highly sensitive
broad-spectrum detection of human papillomaviruses in laryngeal
squamous cell carcinoma and normal mucosa: Clinico-pathological
evaluation. Eur Arch Otorhinolaryngol. 265 Suppl 1:S89–S96. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rischin D, Young RJ, Fisher R, Fox SB, Le
QT, Peters LJ, Solomon B, Choi J, OSullivan B, Kenny LM, et al:
Prognostic significance of p16INK4A and human papillomavirus in
patients with oropharyngeal cancer treated on TROG 02.02 phase III
trial. J Clin Oncol. 28:4142–4148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishimura G, Tsukuda M, Mikami Y, Matsuda
H, Horiuchi C, Taguchi T, Takahashi M, Kawakami M, Watanabe M, Niho
T, et al: Efficacy of concurrent chemoradiotherapy for T1 and T2
laryngeal squamous cell carcinoma regarding organ preservation.
Anticancer Res. 29:661–666. 2009.PubMed/NCBI
|
|
26
|
Akimoto T, Nonaka T, Kitamoto Y, Ishikawa
H, Ninomiya H, Chikamatsu K, Furuya N, Hayakawa K, Mitsuhashi N and
Nakano T: Radiation therapy for T2N0 laryngeal cancer: A
retrospective analysis for the impact of concurrent chemotherapy on
local control. Int J Radiat Oncol Biol Phys. 64:995–1001. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berardinelli F, Nieri D, Sgura A,
Tanzarella C and Antoccia A: Telomere loss, not average telomere
length, confers radiosensitivity to TK6-irradiated cells. Mutat
Res. 740:13–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCaul JA, Gordon KE, Minty F, Fleming J
and Parkinson EK: Telomere dysfunction is related to the intrinsic
radio-resistance of human oral cancer cells. Oral Oncol.
44:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henriksson S, Rassoolzadeh H, Hedström E,
Coucoravas C, Julner A, Goldstein M, Imreh G, Zhivotovsky B, Kastan
MB, Helleday T, et al: The scaffold protein WRAP53beta orchestrates
the ubiquitin response critical for DNA double-strand break repair.
Genes Dev. 28:2726–2738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalfert D, Celakovsky P, Laco J and
Ludvikova M: The role of protein p16INK4a in glottic
laryngeal squamous cell carcinoma. Pathol Oncol Res. 20:909–915.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baumann JL, Cohen S, Evjen AN, Law JH,
Vadivelu S, Attia A, Schindler JS, Chung CH, Wirth PS, Meijer CJ,
et al: Human papillomavirus in early laryngeal carcinoma.
Laryngoscope. 119:1531–1537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jouhi L, Hagström J, Atula T and Mäkitie
A: Is p16 an adequate surrogate for human papillomavirus status
determination? Curr Opin Otolaryngol Head Neck Surg. 25:108–112.
2017. View Article : Google Scholar : PubMed/NCBI
|