Introduction
Osteosarcoma (OS), a cancer more precarious in
males, occurs during childhood and adolescence (1,2), with
a rate of incidence of 8.7 per million individuals. The cancer
usually occurs in a growing long bone (3), such as the humerus (10%), the distal
femur (43%), or the proximal tibia (23%). Despite trying to improve
results through higher doses, novel therapeutic targets, and new
modalities of osteosarcoma therapy, the overall survival rates for
osteosarcoma have not significantly improved over the past three
decades (4). With the emergence of
neoadjuvant chemotherapy prior to surgery, the 5-year survival rate
following surgical resection of the osteosarcoma and chemotherapy
have improved less than 20% overall since 1970, from 65% to only
70% (5,6). One key reason could be that
osteosarcoma is among the tumors most resistant to all traditional
chemotherapeutic agents. Our research was aimed at discovering more
efficient agents and reducing the resistance of cancer cells to
chemotherapy.
ABT-737 is a BH-3 mimetic that interacts and
inhibits Bcl-2, and has been previously shown to be an inducer of
cancer cell apoptosis (7,8). This mimetic can inhibit the
anti-apoptotic Bcl-2 family of proteins (i.e., Bcl-xL, Bcl-2, and
Bcl-w), but has a lower effect on Mcl-1 proteins (9,10).
Previous research has demonstrated that ABT-737 has a potent
single-agent activity or can act synergistically with other
chemotherapeutic drugs against various types of hematological
malignancies and solid tumors, such as melanoma cells, glioma cells
(11), acute myeloid leukemia,
chronic lymphocytic leukemia, malignant lymphomas, multiple
myelomas, acute lymphoblastic leukemia, and on solid tumors
(12). Studies have identified that
Bcl-2 expression in human OS cells plays a significant role in
Bcl-2-mediated tumor progression (13). Therefore, we assessed the action of
ABT-737 for OS treatment. To our knowledge, this is the first study
that uses ABT-737 for OS treatment.
In the 1960s and 1970s, cisplatin
(cis-diamminedichloroplatinum (II); DDP) was discovered to
eliminate and regress tumors on a wide spectrum (14). Neoadjuvant chemotherapy with DDP is
one of the first-line anticancer treatments and is used as a
general chemotherapy in clinical treatment to eliminate or regress
possible microscopic metastases (15,16).
DDP inhibits the anti-apoptotic Bcl-2 family of proteins Bcl-2 and
Mcl-1, whereas ABT-737 inhibits the anti-apoptotic Bcl-2 family of
protein Bcl-xL, Bcl-2, and Bcl-w, while having less effect on
Mcl-1. Previous research shows that high levels of Mcl-1 confers
resistance to ABT-737 (17), thus,
we hypothesized that ABT-737 can act synergistically with DDP and
tested the action of ABT-737 to enhance the activity of DDP on OS
cells. Moreover, DDP has the risk of severe side effects and
unpredictable efficacy, so it was imperative to develop less toxic
and more effective approaches (18).
Studies from other groups indicate that the
mitochondrial apoptotic pathway may be involved in the action of
ABT-737 and DDP (19). Apoptosis is
composed of two signaling pathways: the extrinsic or death receptor
pathway, which is regulated by combining extracellular ligands of
the tumor necrosis factor (TNF) family with death receptors, and
the intrinsic or mitochondrial pathway, which is controlled by
proteins of the B-cell lymphoma (Bcl-2) family (i.e., Bcl-xL,
Bcl-w, Mcl-1, Bax, Bak, Bid, Noxa, Puma, and Bim) (9). Interactions and the balance between
pro-survival and pro-apoptotic members could determine the cell's
fate (11). When the ratio of Bcl-2
to Bax is reduced, this can cause the severance of the
electrochemical gradient across the mitochondrial membranes
(20), which influences the
mitochondrial outer membrane permeabilization (MOMP), subsequently
causing the release of apoptogenic proteins such as cytochrome
c into the cytosol. Cytochrome c can bind to Apaf-1
and activate caspase-9, which activates the downstream caspases-3
and/or caspase-7; this triggers a cascade of caspase activations,
which in turn results in the cleavage or degradation of several key
cellular substrates, including PARP, resulting in cell
apoptosis.
In this study, in vitro experiments were
conducted to confirm the effects of ABT-737 on human U-2OS cells,
alone or in combination with DDP, and the intracellular molecular
mechanisms of its actions.
Materials and methods
Materials
Roswell Park Memorial Institute-1640 (RPMI-1640),
fetal bovine serum (FBS), phosphate-buffered saline (PBS), and
Hoechst 33258 Staining kit were provided by KeyGEN Biotech
(Nanjing, China). CCK-8 was obtained from Solarbio (Beijing,
China). Antibodies against Bcl-2, Mcl-1, Bax, cytochrome c,
caspase-3, caspase-8, caspase-9, and PARP were purchased from Abcam
(Cambridge, UK). Antibodies against β-actin were from Solarbio.
Horseradish peroxidase (HRP)-conjugated secondary antibodies were
from Transgen (Beijing, China). The Annexin V-PI/FITC Apoptosis
Detection Kit was acquired from Becton-Dickinson (San Jose, CA,
USA). ABT-737 was obtained from Nanjing ZeLang Medical Technology
Co. Ltd. (Nanjing, China). Stock solutions of ABT-737 were prepared
by dissolving the ABT-737 powder in DMSO to a concentration of 10
mM and stored at −80°C. The working concentrations of ABT-737 were
prepared by diluting the stock solution in culture medium. The
final concentration of DMSO in the medium was ≤0.5%.
Cell culture
The human osteosarcoma cell line U-2OS was obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and cultured in RPMI-1640 supplemented with 10%
(v/v) FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells
were grown in a humidified atmosphere containing 5% CO2
at 37°C. The cells used in this study were subjected to less than
20 passages and all cells used in this study were in exponential
cell growth.
Cell viability by CCK-8 assay
Cells were cultured in 96-well plates at a
concentration of 5×104 cells/well. Cell viability was
determined by the CCK-8 colorimetric assay. Briefly, the cells were
treated with DDP (0.75, 1.5, 3, 5, 7, 9, 11, or 13 µg/ml), ABT-737
(1, 2, 5, 10, 20, 40, or 50 µM), and DDP (0.75, 1.5, 3, 5, 7, 9,
11, or 13 µg/ml) together with ABT-737 (10 µM) for 24, 48, or 72 h,
whereas the control cells were treated with only 0.5% DMSO. After
the indicated incubation times, 10 µl of CCK-8 was added to the
plates and they were incubated at 37°C for an additional 1–4 h.
Following this, the absorbance was measured at 450 nm using an
ELISA plate reader (model EXL800; BioTek Instruments,. Inc.,
Winooski, VT, USA).
Hoechst 33258 staining of U-2OS
cells
Cells were incubated with DDP (4 µg/ml) alone or
together with ABT-737 (10 µM) for 24 h, harvested, fixed in 4%
paraformaldehyde for 30 min at 25°C, washed three times with
ice-cold PBS, and stained with 10 mg/l of Hoechst 33258 (Sigma) in
the dark at room temperature (25°C) for 10 min. Finally, the
stained nuclei were observed under a fluorescence microscope
(Olympus ×100) with the excitation filter at 350 nm and the
emission filter at 460 nm.
Analysis of cell apoptosis by the
Annexin V-PI/FITC staining assay
U-2OS cells were stained with Annexin V-PI/FITC (BD
Biosciences, San Jose, CA, USA). U-2OS cells were cultured in
24-well plates at a density of 1×105 cells/well.
Following an overnight incubation, these cells were treated with
DDP (4 µg/ml) alone or together with ABT-737 (10 µM) for 24 h,
whereas the control cells were treated with 0.1% DMSO. All cells
were collected by trypsinization without EDTA. After being washed
twice with 4°C PBS, cell pellets were suspended in 400 µl of
ice-cold 1X binding buffer at a density of nearly 1×106
cells/ml, and then incubated with 5 µl of Annexin V-PI/FITC for 15
min at room temperature (25°C) in the dark. Samples were analyzed
by a flow cytometer within 1 h after staining.
Western blot analysis
U-2OS cells were cultured in 6-well plates at a
density of 2×105 cells/well. After treatment with DDP (4
µg/ml) alone or in combination with ABT-737 (10 µM) for 24 h, cells
were washed with PBS and lysed in cell lysis buffer. Control cells
were treated with 0.1% DMSO and harvested identically. The lysates
were centrifuged at 12,000 × g at 4°C for 10 min. The supernatant
was collected and the protein concentration was determined by the
BCA method. Similar amounts of proteins from each treated cell
group were loaded and run on a 10% SDS-PAGE gel and transferred to
polyvinylidene fluoride (PVDF) membranes. The membranes were
blocked with 5% (w/v) fat-free milk in Tris-buffered saline
containing 0.05% Tween-20 (TBS-T), followed by incubation with a
primary antibody overnight at 4°C. The following day, PVDF
membranes were washed in TBS-T three times and the membranes were
incubated with a horseradish peroxidase (HRP)-conjugated secondary
antibody. Immunoreactive proteins were detected by enhanced
chemiluminescence (ECL kit; Transgen), followed by exposure to a
X-ray film.
Statistics
All data were calculated as means ± standard
deviation (SD) and analyzed by Graphpad Prism 6.0 software.
Student's t-test or one-way analysis of variance (ANOVA) were
performed to determine the significance of differences between the
experimental conditions. All the experiments were repeated at least
three times. P-values of <0.05 were considered to be
statistically significant, P<0.05, P<0.01, P<0.001.
Results
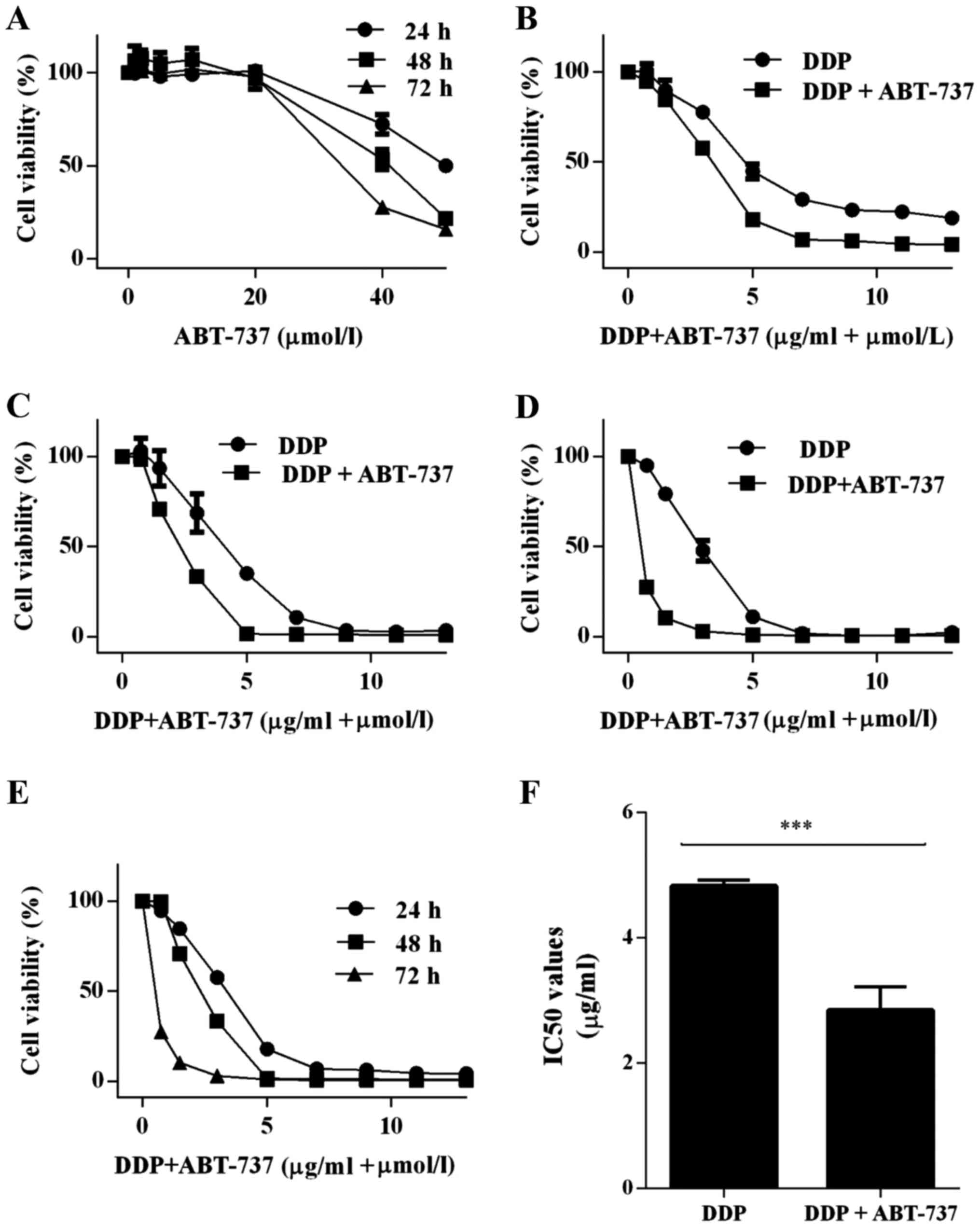
Co-treatment with ABT-737 and DDP
reduced the viability of U-2OS cells
The effects of the treatments on the viability of
U-2OS cells were determined by CCK-8 viability assay, where U-2OS
cells were treated with ABT-737 and/or DDP for 24, 48, or 72 h
(Fig. 1A-E). We found that ABT-737
alone only played a very small role in eliminating U-2OS cells at
the physiological dose (11)
(Fig. 1A). The IC50
value was 50.74±1.86 µM at 24 h. When the cells were treated with
DDP and ABT-737 together, there were substantial inhibitory effects
on the human U-2OS cells compared to when DDP was used alone in a
time- and dose-dependent manner; the IC50 values were
2.84±0.83 µg/ml (DDP+ABT-737) and 4.82±0.11 µg/ml (DDP) at 24 h,
respectively (Fig. 1E and F).
Since ABT-737 alone had a nominal effect on U-2OS
cells, the cells were treated with DDP (4 µg/ml) alone or combined
with ABT-737 (10 µM) for 24 h in the following assays.
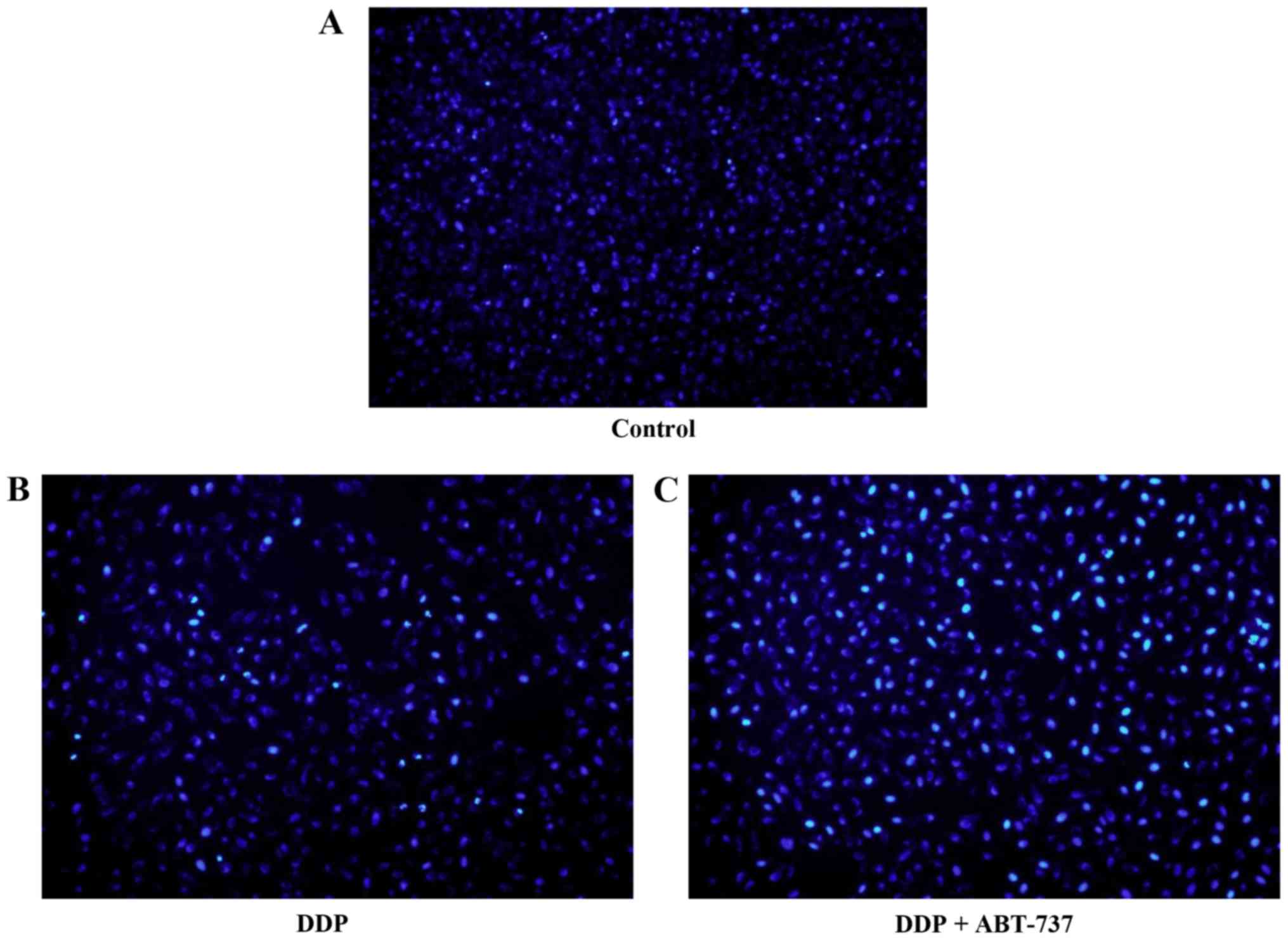
Induction of morphological changes of
U-2OS cells
Untreated U-2OS cells grew well, as seen by phase
contrast microscopy. After 24 h of treatment with DDP alone or in
combination with ABT-737, cells appeared more broken, necrosed, and
detached when compared to the control cells, which was consistent
with the growth inhibition by these treatments. U-2OS cells treated
with DDP stained with the fluorescent DNA-binding dye Hoechst 33258
revealed condensed and fragmented nuclei, which is a typical
morphological feature of apoptosed cells. In contrast,
morphological signs of apoptosis were observed with DDP combined
with ABT-737 treatment. The results indicated that DDP alone or
together with ABT-737 can induce apoptosis, where the combination
therapy had much clearer results (Fig.
2).
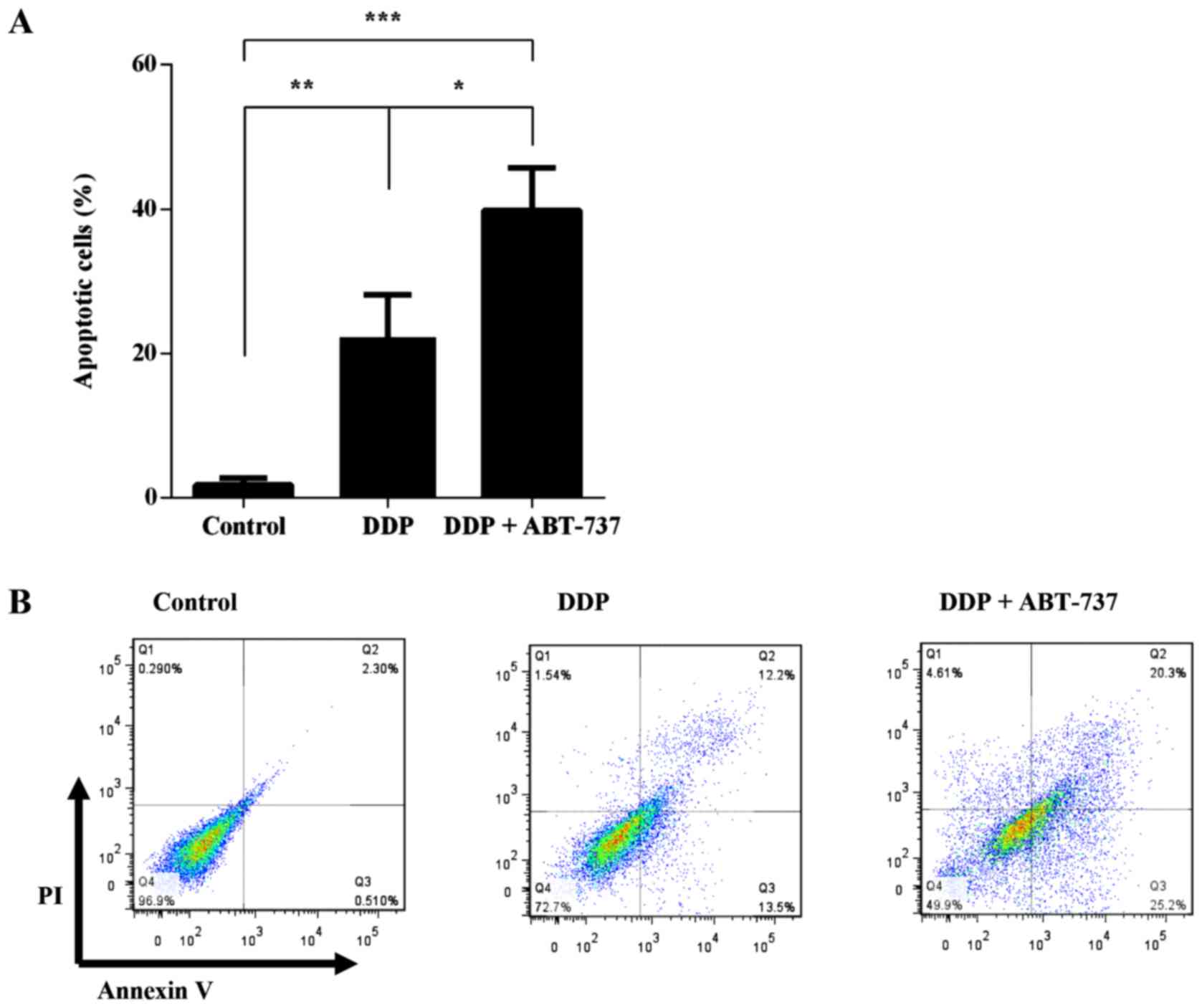
Annexin V-PI/FITC staining assay
The rate of cell apoptosis was detected by flow
cytometry by double labeling with Annexin V-PI/FITC. The apoptosis
rate in control cells was 1.78±0.96%. The rates of apoptosis were
increased to 22.23±5.91% and 39.82±5.92% following treatment with
DDP alone or in combination with ABT-737 for 24 h, respectively
(Fig. 3).
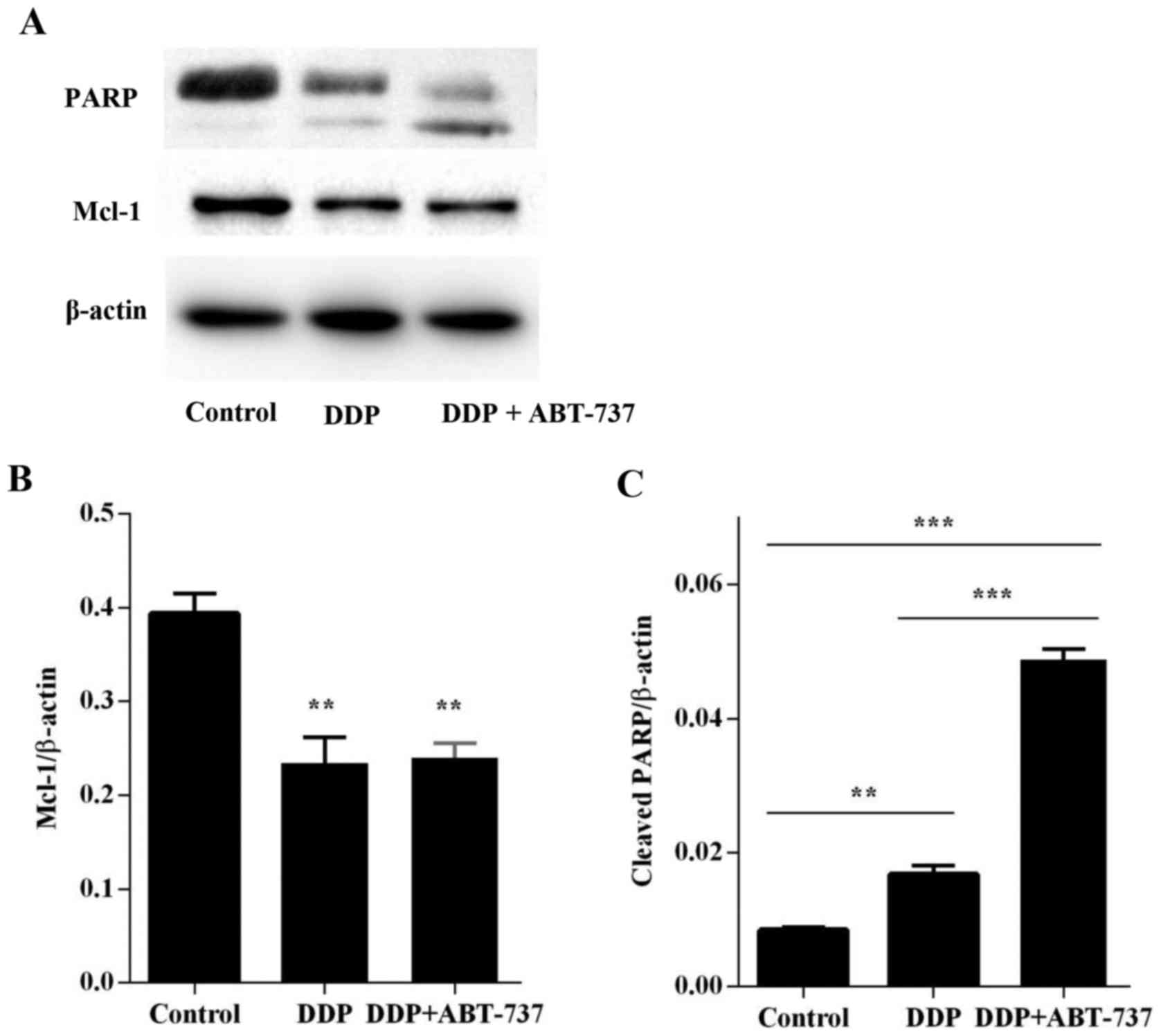
ABT-737 decreases the expression of
anti-apoptotic protein Bcl-2, whereas Mcl-1 increases pro-apoptotic
proteins Bax and cytochrome c
The results of our western blot analyses revealed
that DDP treatment combined with ABT-737 caused a marked increase
in the expression level of Bax (Fig.
4) proteins and the release of cytochrome c (Fig. 5), whereas it decreased the levels of
Bcl-2 and Mcl-1 proteins when compared to the levels in the DDP
alone and control treatments, the value of Mcl-1 was >0.05 for
the DDP alone condition vs. the co-treatment of DDP and ABT-737
(Figs. 4 and 6A). Since DDP could induce U-2OS cell
apoptosis via the mitochondrial apoptotic pathway (19), an increase in cytochrome c
level was observed, this increase was less than that in the
combined treatment group (Fig. 5).
This demonstrated that co-treatment with ABT-737 and DDP activated
the mitochondrial apoptotic pathway in U-2OS cells via regulating
the expression of the Bcl-2 family proteins.
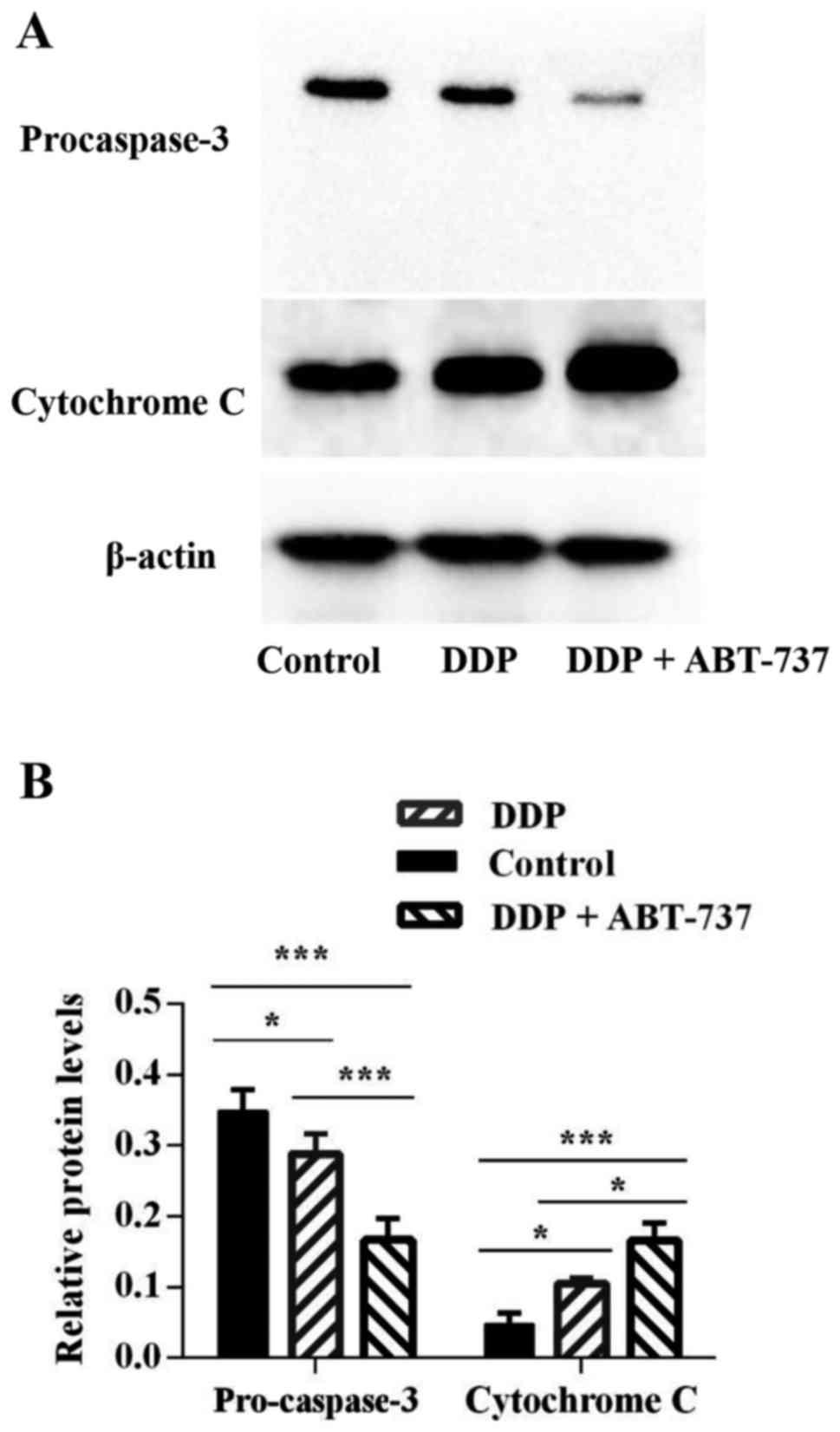
Effects of ABT-737 combined with DDP
on the expression levels of caspase proteins
The caspase cascade reaction is one of the most
important events in the process of apoptosis through the
mitochondrial pathway. Therefore, the protein expression levels of
caspase-9, caspase-8, and caspase-3 were assessed by western blot
analyses. There was no obvious change in caspase-8 expression,
however, the expression levels of pro-caspase-9 and pro-caspase-3
were downregulated in the cells treated with DDP alone or combined
with ABT-737, where the co-treatment condition showed a more
notable decrease in the protein levels. Additionally, the cleavage
of PARP, a key cellular substrate, was observed (Figs. 4–6).
These results indicated that the apoptosis induced by DDP combined
with ABT-737 treatment involved the caspase cascade and was
initiated via the mitochondrial pathway.
Discussion
Apoptosis is an innate cellular response designed to
eliminate abnormal or redundant cells (21); therefore, it is considered an
important mechanism with which to target cancer cells that evade
programmed cell death. There is accumulating evidence that ABT-737
and drugs with antitumor effects can trigger apoptosis in various
tumor cells (22). In this study,
we determined the anticancer effect and associated mechanisms of
ABT-737 in combination with DDP on human U-2OS cells in
vitro. CCK-8 viability assay results showed that ABT-737 alone
had little influence on U-2OS cells at the clinically administered
dose. Previous studies have shown that ABT-737 has the ability to
enhance the efficacy of other drugs (7,19) and
ABT-737 preferentially inhibits the anti-apoptotic Bcl-2 family
proteins Bcl-xL, Bcl-2, and Bcl-w, while having a weaker effect on
Mcl-1. Furthermore, previous research shows that high levels of
Mcl-1 confer resistance to ABT-737 (17); nevertheless, DDP inhibits the
anti-apoptotic Bcl-2 family proteins Bcl-2 and Mcl-1. Therefore, we
tried to use DDP and ABT-737 in combination therapy to treat OS. We
found ABT-737 treatment combined with DDP effectively suppressed
the proliferation of the human U-2OS cell line in a dose- and
time-dependent manner. Hoechst 33258 staining and Annexin V-PI/FITC
staining analyses further revealed that co-treatment with DDP and
ABT-737 can strongly induce apoptosis in OS cells.
Mitochondrial-mediated apoptosis has two signaling
pathways for programmed cell death: the death receptor pathway and
the mitochondrial pathway, which are regulated via caspase-9 and
caspase-8, respectively (23).
Previous research has shown that caspases play significant roles in
the apoptotic cascade (24,25). In the mitochondrial (intrinsic)
pathway, members of the Bcl-2 family can regulate apoptosis
downstream of caspase protein activation. An attenuated ratio of
Bcl-2/Bax can cause the loss of the electrochemical gradient across
the mitochondrial membranes, resulting in apoptosis-associated MOMP
that forms pores in the mitochondrial membrane. This leads to the
release of many apoptogenic proteins from the mitochondrial
intermembranous space, including cytochrome c, which can
further activate caspase-9. Active caspase-9 promotes the activity
of downstream caspase-3, which causes the cleavage or degradation
of key cellular substrates, including PARP, leading to apoptosis
(26–33). In the death receptor (extrinsic)
pathway, Fas/FasL, which are found on the cell surface, activates
the death receptor, which then activates downstream caspase-8.
Active caspase-8 can initiate the activity of downstream caspase-3,
which causes the same cleavage or degradation of key cellular
substrates as the intrinsic pathway, leading to apoptosis (34–39).
To deduce the apoptotic signaling mechanism by which
DDP combined with ABT-737 acts on OS cells, the expression levels
of Bcl-2 family proteins, caspase-9, caspase-8, caspase-3, and PARP
were tested in U-2OS cells. The present data showed that apoptosis
induced by ABT-737 in combination with DDP was accompanied by
altering the Bax/Bcl-2 ratio, and activating caspase-9 and
caspase-3, but not caspase-8, The P-value of caspase-8 was >0.05
between each condition (Fig. 4B).
On the other hand, the increased cleavage of PARP was discovered
when ABT-737 and DDP were used together. Thus, these findings
showed that apoptosis induced by ABT-737 in combination with DDP in
U-2OS cells was activated by the intrinsic pathway.
Overall, we affirmed that ABT-737 alone had nominal
effects on U-2OS cells, whereas in combination with DDP, it
upregulated Bax expression and downregulated Bcl-2 expression in
human U-2OS cells. This resulted in the release of cytochrome
c into cytosol, which further activated caspase-9.
Furthermore, caspase-9 activated downstream caspase-3, which in
turn resulted in the cleavage or degradation of several key
cellular substrates, including PARP, leading to subsequent cell
death. These results indicated that ABT-737 combined with DDP could
be a new treatment for OS, while reducing the toxicity of DDP
treatment alone.
Further studies are required to elucidate whether
ABT-737 can synergize with other chemotherapy drugs, such as
doxorubicin and methotrexate. In addition, studies on the in
vivo effect of ABT-737 combined with DDP on U-2OS xenograft
tumors in nude mice are currently in progress.
Acknowledgements
This study was supported by The Foundation of Health
Department of Jiangxi Province (2016A073) and Gan-Po Talents
Project 555 of Jiangxi Province.
References
|
1
|
Delebinski CI, Georgi S, Kleinsimon S,
Twardziok M, Kopp B, Melzig MF and Seifert G: Analysis of
proliferation and apoptotic induction by 20 steroid glycosides in
143B osteosarcoma cells in vitro. Cell Prolif. 48:600–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu SY, Deng SY, He YB and Ni GX: miR-451
inhibits cell growth, migration and angiogenesis in human
osteosarcoma via down-regulating IL 6R. Biochem Biophys Res Commun.
482:987–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Hou W, Chai M, Zhao H, Jia J, Sun
X, Zhao B and Wang R: MicroRNA-127-3p inhibits proliferation and
invasion by targeting SETD8 in human osteosarcoma cells. Biochem
Biophys Res Commun. 469:1006–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bishop MW, Janeway KA and Gorlick R:
Future directions in the treatment of osteosarcoma. Curr Opin
Pediatr. 28:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao JZ, Chen FH, Wang L, Wei H and Meng
SL: YM155 inhibits tumor growth and enhances chemosensitivity to
cisplatin in osteosarcoma. Eur Rev Med Pharmacol Sci. 19:2062–2069.
2015.PubMed/NCBI
|
|
7
|
Yu T, Chen C, Sun Y, Sun H, Li TH, Meng J
and Shi X: ABT-737 sensitizes curcumin-induced anti-melanoma cell
activity through facilitating mPTP death pathway. Biochem Biophys
Res Commun. 464:286–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim LH, Shin JA, Jang B, Yang IH, Won DH,
Jeong JH, Chung TH, Cho NP and Cho SD: Sorafenib potentiates
ABT-737-induced apoptosis in human oral cancer cells. Arch Oral
Biol. 73:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broecker-Preuss M, Becher-Boveleth N,
Müller S and Mann K: The BH3 mimetic drug ABT-737 induces apoptosis
and acts synergistically with chemotherapeutic drugs in thyroid
carcinoma cells. Cancer Cell Int. 16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng R, You Z, Jia J, Lin S, Han S, Liu
A, Long H and Wang S: Curcumin enhances the antitumor effect of
ABT-737 via activation of the ROS-ASK1-JNK pathway in
hepatocellular carcinoma cells. Mol Med Rep. 13:1570–1576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Premkumar DR, Jane EP, DiDomenico JD,
Vukmer NA, Agostino NR and Pollack IF: ABT-737 synergizes with
bortezomib to induce apoptosis, mediated by Bid cleavage, Bax
activation, and mitochondrial dysfunction in an Akt-dependent
context in malignant human glioma cell lines. J Pharmacol Exp Ther.
341:859–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao J, Li F, Zhang RY, Zhou HH and Chen
GA: BH3 mimetic ABT-737 induces apoptosis in CD34+ acute
myeloid leukemia cells and shows synergistic effect with
conventional chemotherapeutic drugs. Asia Pac J Clin Oncol.
13:e144–e152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trieb K, Sulzbacher I and Kubista B: Bcl-2
correlates with localization but not outcome in human osteosarcoma.
Oncol Lett. 6:559–561. 2013.PubMed/NCBI
|
|
14
|
Pu F, Chen F, Lin S, Chen S, Zhang Z, Wang
B and Shao Z: The synergistic anticancer effect of cisplatin
combined with Oldenlandia diffusa in osteosarcoma MG-63 cell line
in vitro. Onco Targets Ther. 9:255–263. 2016.PubMed/NCBI
|
|
15
|
Yu L, Fan Z, Fang S, Yang J, Gao T, Simões
BM, Eyre R, Guo W and Clarke RB: Cisplatin selects for stem-like
cells in osteosarcoma by activating Notch signaling. Oncotarget.
7:33055–33068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han XG, Du L, Qiao H, Tu B, Wang YG, Qin
A, Dai KR, Fan QM and Tang TT: CXCR1 knockdown improves the
sensitivity of osteosarcoma to cisplatin. Cancer Lett. 369:405–415.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu XY, Hao CP, Ling M, Guo CH and Ma W:
Hypoxia-induced apoptosis is blocked by adrenomedullin via
upregulation of Bcl-2 in human osteosarcoma cells. Oncol Rep.
34:787–794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Zhou X, Fu C, Wang Q, Nie T, Zou F,
Guo R, Liu H, Zhang B and Dai M: Celastrol induces apoptosis of
human osteosarcoma cells via the mitochondrial apoptotic pathway.
Oncol Rep. 34:1129–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Z, Yu H, Cui N, Kong X, Liu X, Chang
Y, Wu Y, Sun L and Wang G: ABT737 enhances cholangiocarcinoma
sensitivity to cisplatin through regulation of mitochondrial
dynamics. Exp Cell Res. 335:68–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Péchery A, Fauconnet S, Bittard H and
Lascombe I: Apoptotic effect of the selective PPARβ/δ agonist
GW501516 in invasive bladder cancer cells. Tumour Biol.
37:14789–14802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Yang Z, Li Y, Xia J, Li D, Li H, Ren
M, Liao Y, Yu S, Chen Y, et al: Cell apoptosis, autophagy and
necroptosis in osteosarcoma treatment. Oncotarget. 7:44763–44778.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen J, Xu L and Zhao Q: Perifosine and
ABT-737 synergistically inhibit lung cancer cells in vitro and in
vivo. Biochem Biophys Res Commun. 473:1170–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu G, Kuang G, Jiang W, Jiang R and Jiang
D: Polydatin promotes apoptosis through upregulation the ratio of
Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin
signaling in human osteosarcoma cells. Am J Transl Res. 8:922–931.
2016.PubMed/NCBI
|
|
24
|
Xiong M, Wang L, Yu HL, Han H, Mao D, Chen
J, Zeng Y, He N, Liu ZG, Wang ZY, et al: Ginkgetin exerts growth
inhibitory and apoptotic effects on osteosarcoma cells through
inhibition of STAT3 and activation of caspase-3/9. Oncol Rep.
35:1034–1040. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YL, Sun J, Hu X, Pan YN, Yan W, Li QY,
Wang F, Lin NM and Zhang C: Epothilone B induces apoptosis and
enhances apoptotic effects of ABT-737 on human cancer cells via
PI3K/AKT/mTOR pathway. J Cancer Res Clin Oncol. 142:2281–2289.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH,
He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, et al: Pro-apoptotic and
pro-autophagic effects of the Aurora kinase A inhibitor alisertib
(MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the
activation of mitochondria-mediated pathway and inhibition of p38
MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther.
9:1555–1584. 2015.PubMed/NCBI
|
|
27
|
Lin CH, Hong YC and Kao SH: Aeroallergen
Der p 2 induces apoptosis of bronchial epithelial BEAS-2B cells via
activation of both intrinsic and extrinsic pathway. Cell Biosci.
5:712015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhe N, Chen S, Zhou Z, Liu P, Lin X, Yu M,
Cheng B, Zhang Y and Wang J: HIF-1α inhibition by
2-methoxyestradiol induces cell death via activation of the
mitochondrial apoptotic pathway in acute myeloid leukemia. Cancer
Biol Ther. 17:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YJ, Kim SA and Lee SH: Hyaluronan
suppresses lidocaine-induced apoptosis of human chondrocytes in
vitro by inhibiting the p53-dependent mitochondrial apoptotic
pathway. Acta Pharmacol Sin. 37:664–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang X, Li L, Ying Z, Pan C, Huang S, Li
L, Dai M, Yan B, Li M, Jiang H, et al: A small molecule that
protects the integrity of the electron transfer chain blocks the
mitochondrial apoptotic pathway. Mol Cell. 63:229–239. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhai X, Ding Y, Wang Q, Zhang H and Li F:
Rutin acid ameliorates neural apoptosis induced by traumatic brain
injury via mitochondrial pathways in mice. Neuroimmunomodulation.
23:179–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian X, Shi Y, Liu N, Yan Y, Li T, Hua P
and Liu B: Upregulation of DAPK contributes to homocysteine-induced
endothelial apoptosis via the modulation of Bcl2/Bax and activation
of caspase 3. Mol Med Rep. 14:4173–4179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Shamoto-Nagai M, Maruyama W, Osawa T
and Naoi M: Phytochemicals prevent mitochondrial membrane
permeabilization and protect SH-SY5Y cells against apoptosis
induced by PK11195, a ligand for outer membrane translocator
protein. J Neural Transm (Vienna). 124:89–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang F, Chen L, Jin H, Shao J, Wu L, Lu Y
and Zheng S: Activation of Fas death receptor pathway and Bid in
hepatocytes is involved in saikosaponin D induction of
hepatotoxicity. Environ Toxicol Pharmacol. 41:8–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horie Y, Nemoto H, Itoh M, Kosaka H and
Morita K: Fermented brown rice extract causes apoptotic death of
human acute lymphoblastic leukemia cells via death receptor
pathway. Appl Biochem Biotechnol. 178:1599–1611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon SB, Kim MJ, Yang JM, Lee HP, Hong JT,
Jeong HS, Kim ES and Yoon DY: Cudrania tricuspidata stem
extract induces apoptosis via the extrinsic pathway in SiHa
cervical cancer cells. PLoS One. 11:e01502352016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han MH, Lee WS, Nagappan A, Kim HJ, Park
C, Kim GY, Hong SH, Kim ND, Kim G, Ryu CH, et al: Polyphenols from
Korean prostrate spurge Euphorbia supina induce apoptosis
through the Fas-associated extrinsic pathway and activation of ERK
in human leukemic U937 cells. Oncol Rep. 36:99–107. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Tang X, Zhou B, Xu N and Wang Y:
Mechanism of Deca-BDE-induced apoptosis in Neuro-2a cells: Role of
death-receptor pathway and reactive oxygen species-mediated
mitochondrial pathway. J Environ Sci (China). 46:241–251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang S, Sun K, Wang Y, Dong S, Wang C,
Liu L and Wu Y: Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS
death receptor pathway in apoptosis induced by zinc oxide
nanoparticles in human aortic endothelial cells and the protective
effect by alpha-lipoic acid. Chem Biol Interact. 258:40–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|