Introduction
Pancreatic cancer (PC) is a biologically and
clinically challenging disease due to the limited treatment
options. Thus, early diagnosis is critical to improve treatment
outcomes (1). Circulating miRNAs
have been shown to be non-invasive diagnostic biomarkers in many
diseases (2). Earlier studies have
demonstrated the association between aberrant miRNA expression and
development and progression of human cancers (3). For example, circulating miRNAs were
proposed as potential biomarkers of diagnostic and prognostic
relevance in the context of pancreatic cancer (4).
Chronic pancreatitis (CP) is considered as a
premalignant lesion (5); however,
the relationship between CP and PC is not fully understood. Thus,
in this study, we first profiled serum miRNA levels in patients
with PC, those with CP and in normal controls. Then the overlap
miRNAs, miR-23b-3p were selected for the following in vitro
studies. We assessed the effects of miR-23b-3p knock-in on
proliferation, migration, and invasive growth properties of PC
cells.
Exosomes are small membranous vesicles (30–100 nm)
that are produced by liver cells. They represent a powerful
diagnostic tool owing to their relative stability, recoverability
in all body fluids, and composition covering a wide range of
cancer-related biomarkers including proteins, metabolites, DNA, DNA
modifications, and coding and non-coding RNAs. Cancer cells secrete
exosomes, and transfer exosomes from primary tumors to the
circulation (6). Thus, detection of
exosomic miRNA levels in body fluids may serve as novel biomarkers
for early diagnosis of tumors, therapeutic monitoring, and for
prognostic assessment. In this study, the exosomes in the serum of
PC patients were extracted, and the expression of miR-23b-3p was
determined; finally the correlation of exosomic miR-23b-3p with
CA-19-9 was studied. We analyzed the role of altered miRNAs in the
development and progression of PC. The objective was to provide
novel insights into use of miR-23b-3p as a therapeutic target and
predictor for prevention and treatment of PC in future.
Materials and methods
Ethics statement
The study was approved by the Institutional Review
Board (IRB) of Nanjing Medical University. All patients provided
written informed consent prior to their enrolment.
Collection of human body liquid
samples, RNA extraction and miRNA microarray
The diagnosis of PC was based on the National
Comprehensive Cancer Network (NCCN) clinical practice guidelines
2016 (7). CP was diagnosed based on
the American Pancreatic Association's practice guidelines for
chronic pancreatitis, 2014 (8).
Blood samples from 20 healthy controls and serum samples of 18 CP
and 16 PC patients were collected at the Wuxi People's Hospital.
All body fluid specimens were flash-frozen upon collection and
stored at −80°C until further use.
Three serum samples from each group were randomly
selected based on the shortest sampling time for miRNA microarray
analysis. Total RNA was isolated from these frozen body fluids
using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the
manufacturer's instructions. The quality and quantity of the
isolated RNA was then assessed using agarose gel electrophoresis
and a spectrophotometer (Sangon Biotech, Shanghai, China), and then
labeled with biotin and hybridized to a GeneChip miRNA 3.0 Array
from Affymetrix (Santa Clara, CA, USA), according to the
manufacturer's protocol. Following hybridization, the images were
digitized and analyzed using a laser scanner interfaced with
Affymetrix GeneChip Command Console (AGCC). The most differentially
expressed miRNAs in the CCA serum samples were identified and
compared to those from normal control and PC samples.
Quantitative real-time RT-PCR
(qRT-PCR)
miRNA microarray-identified miRNA expression was
further confirmed with qRT-PCR using total RNA from serum samples
and exosomes. In brief, RNA samples were converted into cDNA using
0.5 µg RNA sample and a reverse transcription kit (Takara, Ohtsu,
Shiga, Japan). qRT-PCR was performed using the iQ™ SYBR®
Green Supermix (Bio-Rad) in an ABI 7500 qPCR system (Applied
Biosystems, Foster City, CA, USA). The optimal dilution and melting
curves were utilized to quantify each of the amplified production
using specific primer sets. Bulge-loop™ miRNA qRT-PCR primer sets
(one RT primer and a pair of qRT-PCR primers for each miRNA) were
designed and produced by RiboBio (Guangzhou, China) and ribosome
RNA 5S was used as an internal control for miR-23b-3p level in sera
and bile. The relative quantification of each gene was measured
using the 2−∆∆CT method. We performed qRT-PCR in serum
samples from 20 healthy controls, 18 patients with CP and 16
patients with PC; all measurements were performed in
triplicate.
Cell culture and transfection
Human PC cell lines pancreatic cancer cells (PANC-1)
cultured in DMEM containing 10% fetal bovine sera, 100 g/ml
streptomycin, and 100 U/ml penicillin. The synthesized miR-23b-3p
mimic (miR-23b-3pM) and non-specific mimic (NSM) were purchased
from RiboBio. Here, 70% of confluent cells were transfected using
20 nM of miR-23b-3pM or NSM with Lipofectamine RNAiMAX (Invitrogen,
Carlsbad, CA, USA). Cells were allowed to incubate for 48 h after
transfection and then harvested for further studies.
Cell growth curve, proliferation,
invasion study, and wound healing assay
Ten thousand cells were transfected and placed in
24-well plates (Corning, USA). The number of cells was determined
every other day with a hemocytometer under an inverted-light
microscope.
We also performed the
5-ethynyl-20-deoxyuridine-Apollo 488 (EdU-Apollo 488) incorporation
assay to assess the cell proliferation capacity. CCA cells were
seeded into 96-well plates at a density of 3×104
cells/ml and 24 h later, cells were transfected with 20 nM of NSM
or 23b-3pM for 6 h. The cells were stained with EdU and Hoechst
33342 separately from a kit from RiboBio, according to the
manufacturer's instructions. The proliferating cells were checked
under the fluorescence microscope and the cell proliferation rate
calculated by the formula: (number of proliferating cells/total
cells) × 100%.
For the invasion assay,
2.5×104 transfected cells were placed in 24-well plates
with inserts
This established a two-chamber system divided by a
cell-permeable membrane coated with Matrigel (R&D Systems,
USA). Cells were allowed to incubate for 24 h, fixed, and stained
with crystal violet. Then invasive cells were counted and images of
10 random fields under an inverted-light microscope were
captured.
For the wound healing assay, 2.5×104
transfected cells were cultured in 24-well plates. A line was drawn
when cells reached ~90% confluence. At 0, 24, and 48 h, images of
the same 10 fields were recaptured.
Exosome isolation, characterization,
uptake, and miRNA expression detection
Serum as well as supernatant of PANC-1 cells were
transferred to ultracentrifuge tubes, and centrifuged at 16,500 × g
for 20 min at 4°C to further remove cells and cell debris; the
supernatant was filtered through a 0.2-µm filter to remove
particles sized >200 nm. The filtered supernatant was
transferred to new ultracentrifuge tubes and the tubes sealed prior
to ultracentrifugation at 120,000 × g for 70 min at 4°C to obtain
exosome pellets. The supernatant was discarded, and the exosomes
were collected. For maximal exosome retrieval, the exosome enriched
pellet was resuspended repeatedly in a small volume tube (9). Then total RNA was isolated from these
exosomes using TRIzol as described elsewhere (10).
For the cell uptake experiment, the exosomes were
stained with oil red, and washed using an ultra-filtration membrane
(10 kDa, Merck Millipore, Darmstadt, Germany) to remove any free
nucleotides.
1,1′-dioctadecyl-3,3,3′,3–tetramethylindocarbocyanineperchlorate
(Dil) was used to label the membrane component of the exosomes. The
exosomes were incubated with 10 µg/ml DiI (Beyotime, Nantong,
China) for 15 min at 37°C and then washed twice with cold
phosphate-buffered saline (PBS). The labeled exosomes were added to
PANC-1 cells grown on chamber slides of a 35-mm dish with sterile
small circular glass and incubated at 37°C. After 60 min, the cells
were harvested and washed twice with PBS. Then, the cells were
stained with 4′-6-diamidino-2-phenylindole (DAPI; Invitrogen) to
visualize the nuclei and were examined using an UltraView VoX
confocal fluorescence microscope (Perkin-Elmer, Waltham, MA, USA)
(11).
Statistical analysis
All data are presented as mean ± SEM. One-way or
two-way ANOVA was performed for statistical analysis, using
GraphPad Prism 5. Differences between group means with p-values
<0.05 were regarded as statistically significant. The
correlations were assessed by calculation of Pearson correlation
coefficients.
Results
Bodily fluid miRNA expression profiles
are significantly altered in CP and PC
miRNA microarrays were used to assess the miRNA
expression profiles of patients with CP and PC in order to assess
changes in body fluid miRNAs from chronic inflammation to cancers.
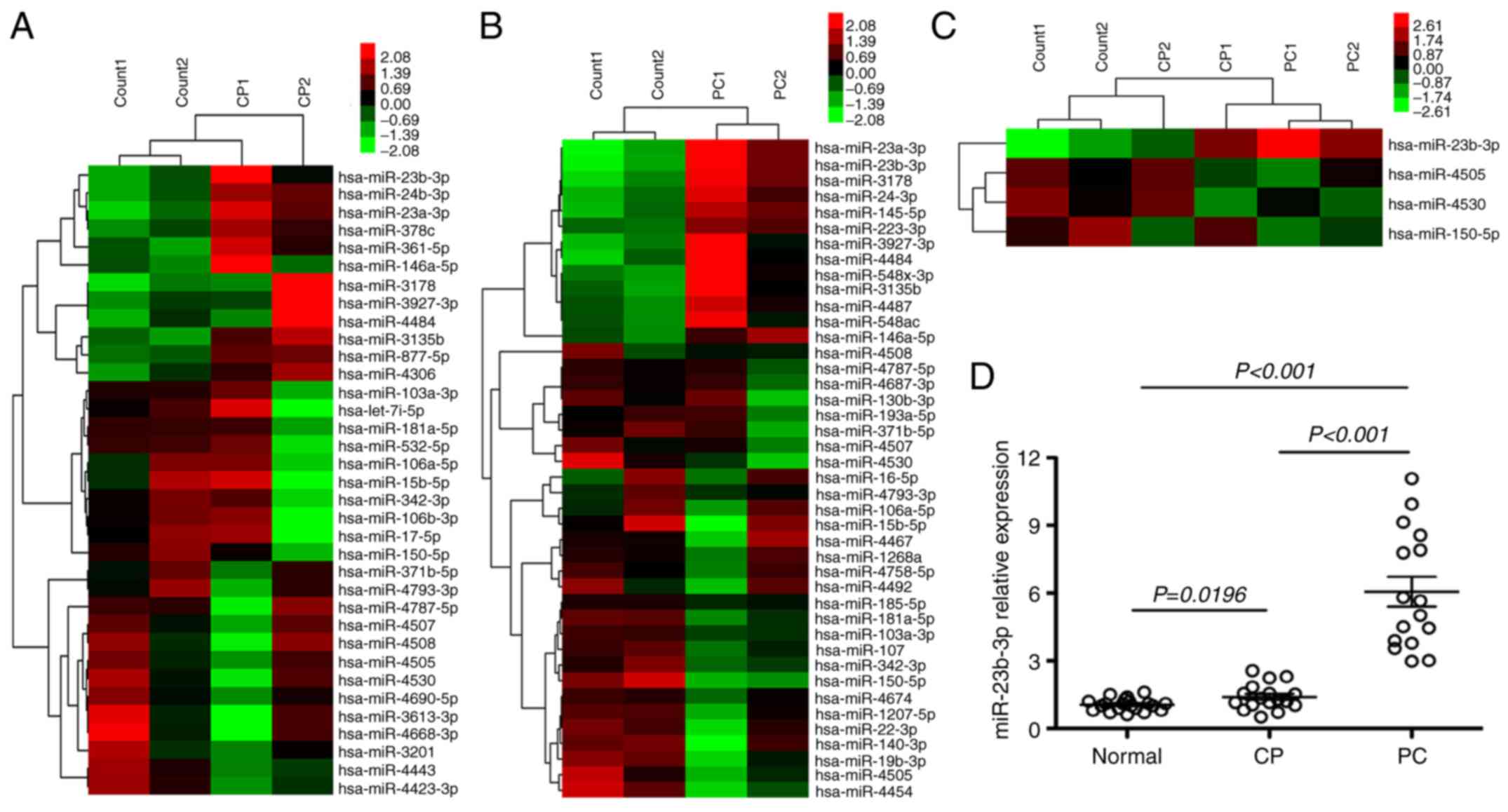
Thirty-five dysregulated serum miRNAs were found in CP, which were
not observed in healthy controls (p<0.01). Of these, 12 were
upregulated and 23 were downregulated (Fig. 1A). There were 42 dysregulated serum
miRNAs in PC that were not found in healthy controls (p<0.01).
Of these, 14 were upregulated and 28 were downregulated (Fig. 1B). Four miRNAs were verified altered
in both CP and PC; among these, miR-23b-3p was upregulated, while 3
miRNAs, miR-4505, 4530, and 150-5p were downregulated (Fig. 1C). qRT-PCR was performed to
determine the expression of miR-23b-3p in the serum of CP and PC
patients. miR-23b-3p was found upregulated in sera of both CP and
PC, and the expression level in PC was higher than that in CP
(p<0.01) (data pertaining to 3 downregulated miRNAs is not
shown). This finding was consistent with the microarray data
(Fig. 1D).
miR-23b-3p promotes the ability of
growth, proliferation, invasion, and migration of PANC-1 cells in
vitro
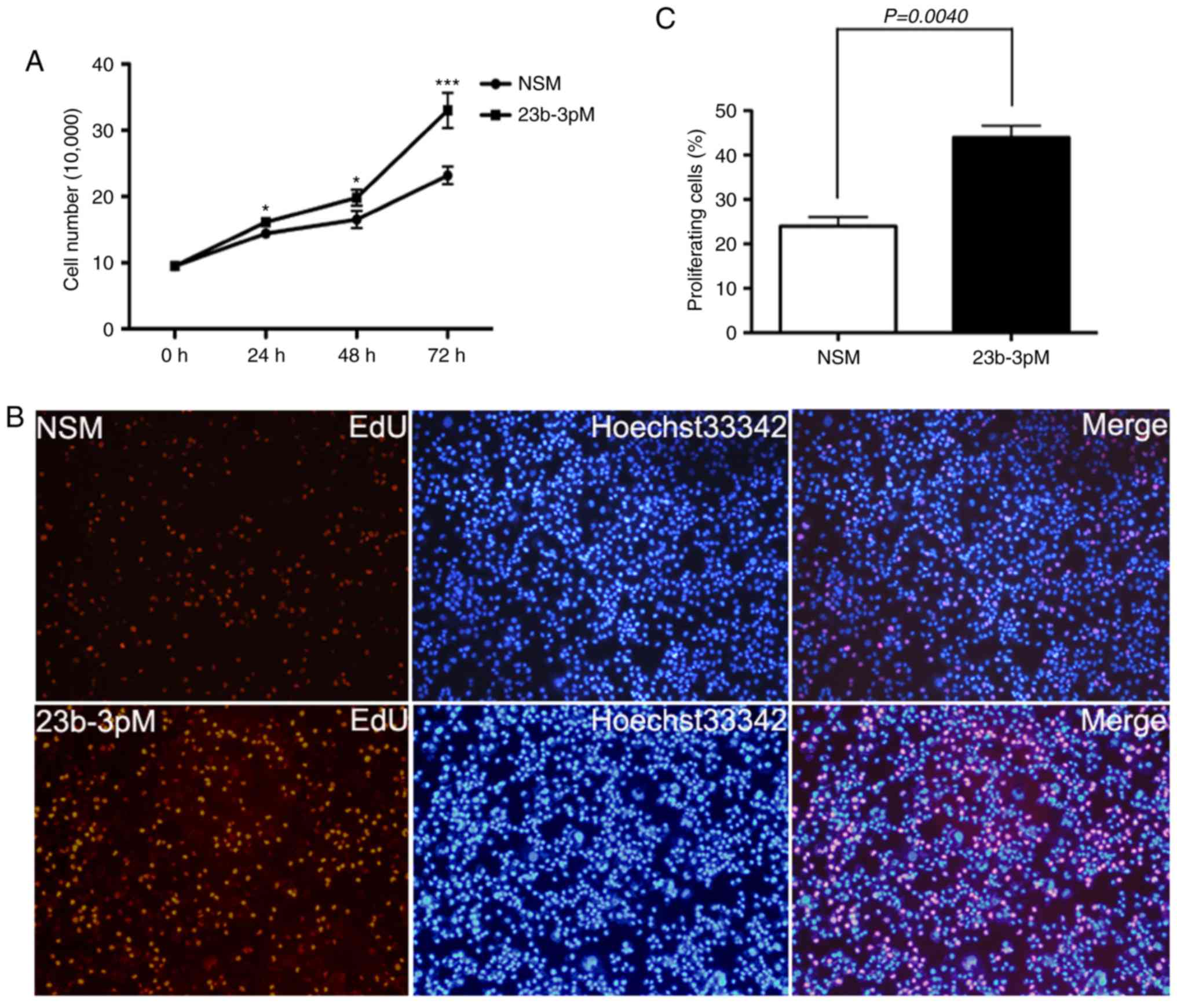
Cell counting and EdU incorporation assays showed
that transfection of PANC-1 cells with 23b-3pM increase tumor cell
growth and proliferation rate as compared to those of
NSM-transfected cells (p<0.05; Fig.
2).
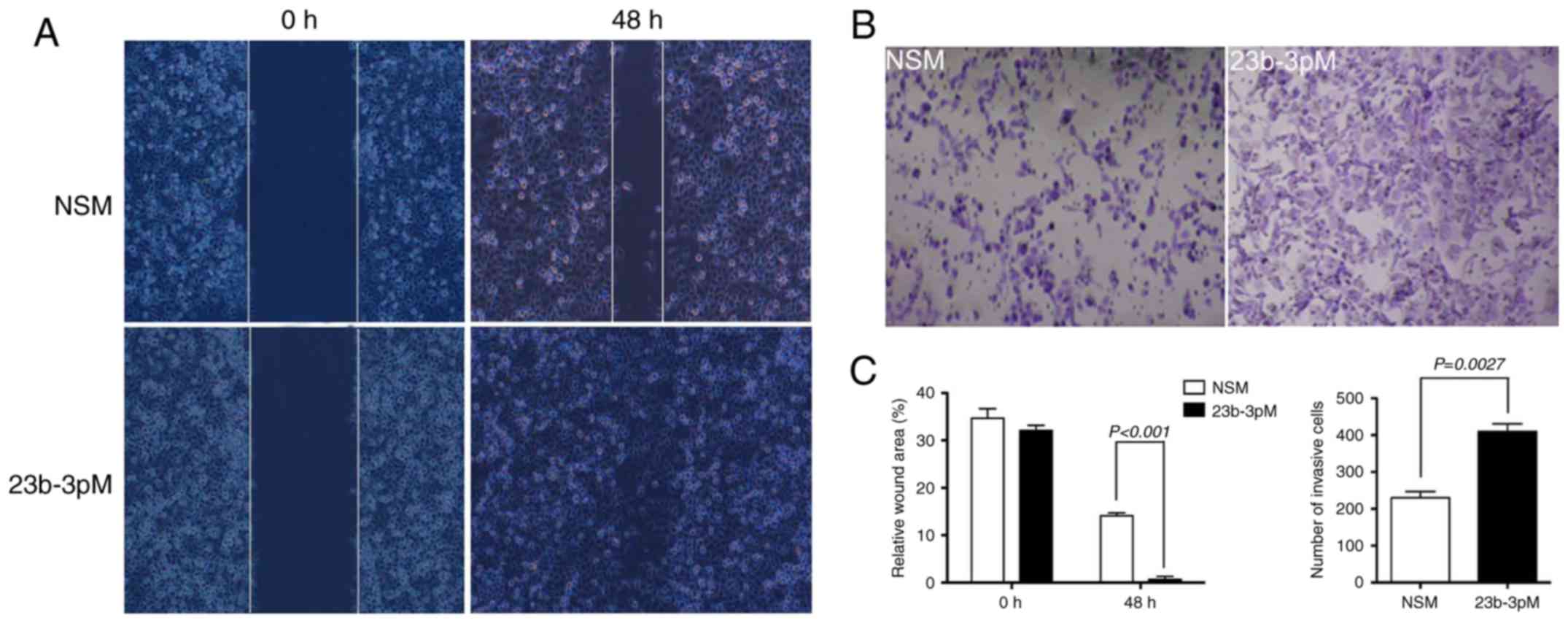
Transwell and wound healing assays were used to
analyze the effect of miR-23b-3p on in vitro invasion and
metastasis of PANC-1 cells, respectively. The overexpression of
miR-23b-3p in PANC-1 cells caused substantially greater cell
invasion through the Transwell chamber at 48 h, as compared to that
observed among cells transfected with NSM (p<0.05). The wound
healing scratch test revealed that introduction of miR-23b-3p also
accelerated the mobility of PANC-1 cells at 48 h after scratching,
relative to cells transfected with NSM (p<0.05) (Fig. 3).
miR-23b-3p was translocated into human
PC cells in an exosome-mediated manner
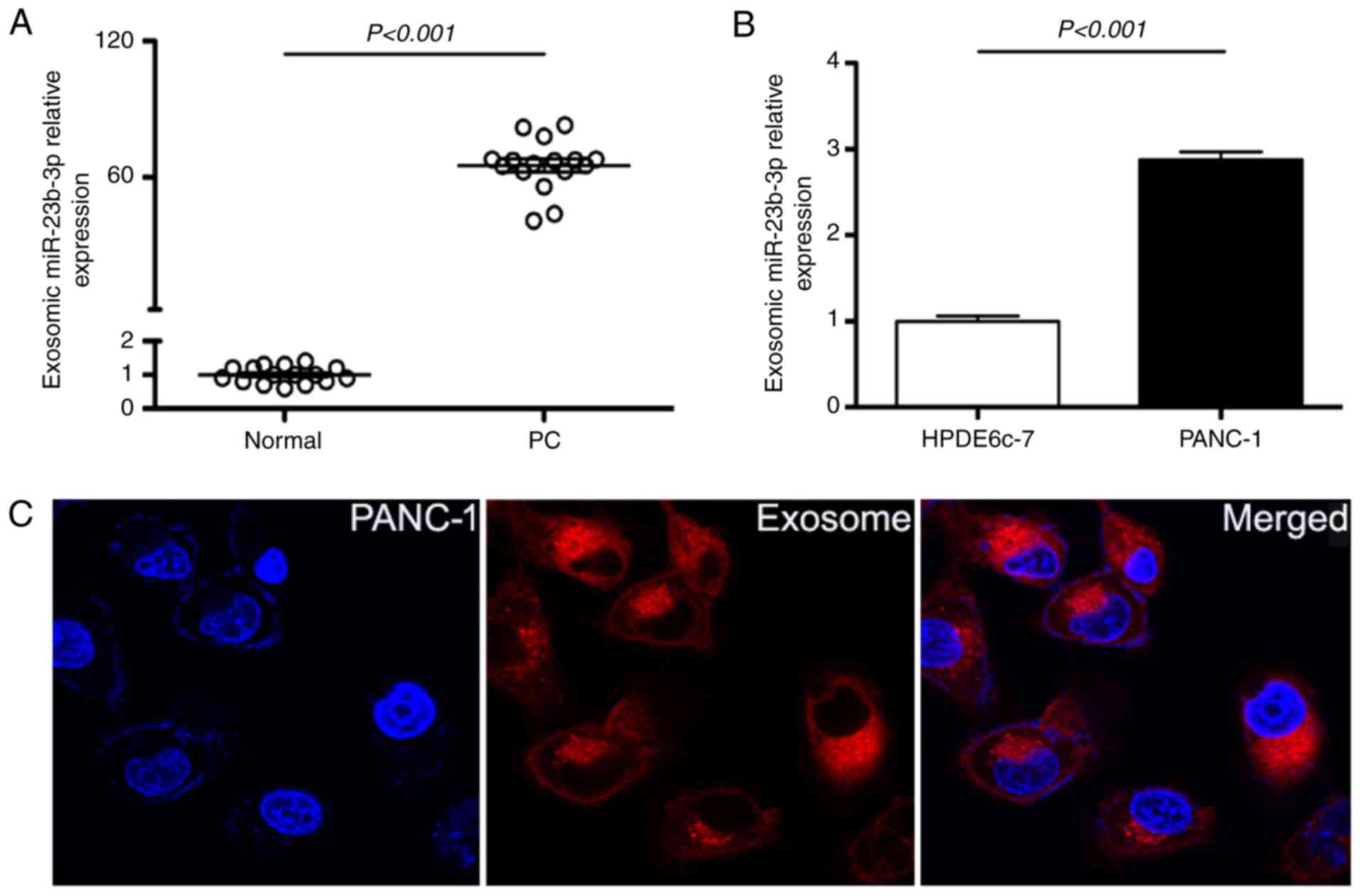
The exosomes in supernatant of PC patients sera as
well as in pancreatic cell lines were isolated; the expression of
exosomic miR-23b-3p was checked by RT-PCR. The results showed
overexpression of exosomic miR-23b-3p in sera of patients with PC
when compared with that in sera of healthy controls (Fig. 4A). Moreover, the exosomic miR-23b-3p
expression in the supernatant of PANC-1 was higher than that in the
pancreatic ductal epithelial cells (HPDE6c-7) (Fig. 4B). Furthermore, exosomes were
labeled with the membrane dye Dil and the labeled exosomes were
added to the culture medium of the PANC-1 cells. After co-culture
for 60 min with unlabeled recipient cells, the labeled exosomes as
well as PANC-1 cells were observed using a confocal microscope; it
was detected that PANC-1 cells can take up exosomes (Fig. 4C). Our results demonstrated that the
pancreatic cancer cells can release exosomes into the circulation
and that the serum exosomes containing miRNAs can in turn be taken
up by pancreatic cancer cells and may subsequently mediate cellular
functions.
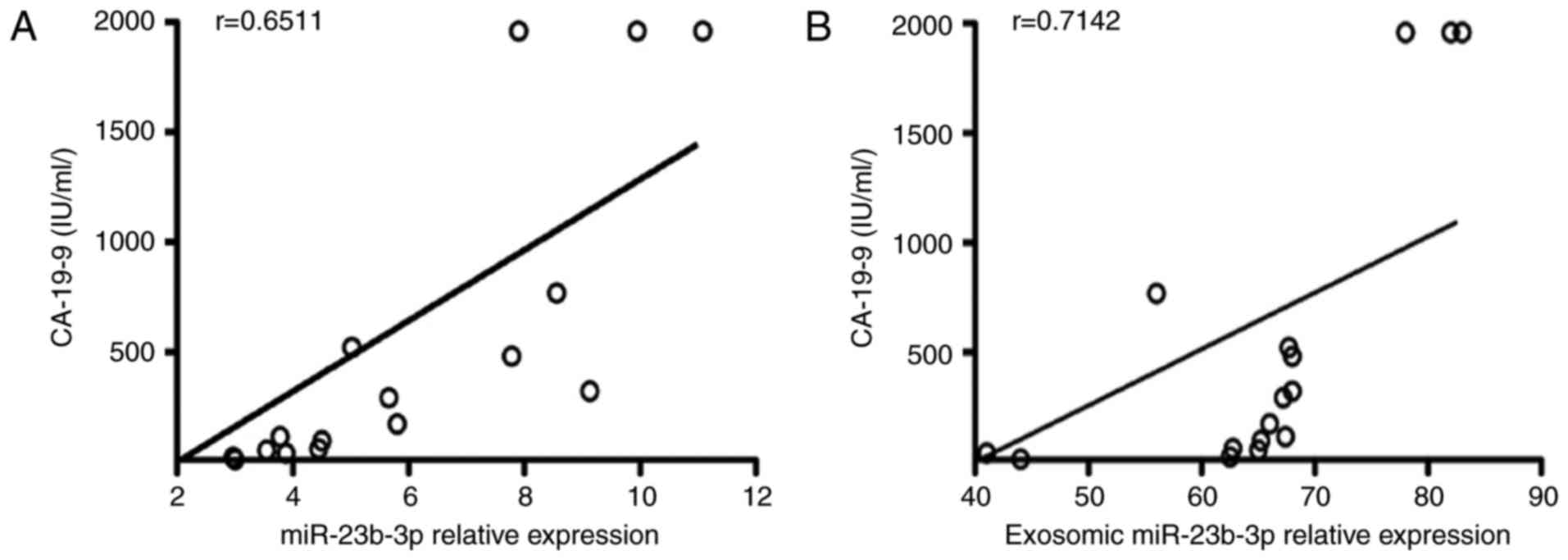
Upregulated sera as well as exosomic
miR-23b-3p was closely related to CA-19-9
In order to investigate the relationship between
miR-23b-3p and CA-19-9, we examined the correlation between the
two. miR-23b-3p in sera of PC patients showed a significant
positive correlation with CA-19-9 (R=0.6511; Fig. 5A). Moreover, the exosomic miR-23b-3p
expression in the sera of PC patients also showed a positive
correlation with that of CA-19-9 (R=0.7142; Fig. 5B).
Discussion
There is a need for more sensitive diagnostic
markers for PC as it is a highly aggressive malignancy with no
evident symptoms at early stages and a low overall 5-year survival
rate (<5%). miRNA is a desirable tool and biomarker because of
its high stability in many types of body fluids. Several
blood-circulating miRNAs have been shown to be associated with
cancer development and progression and can serve as novel potential
diagnostic and prognosis biomarkers in the context of PC (4). Earlier studies have shown that miR-224
and miR-150-5p regulate tumor invasion and migration of
cholangiocarcinoma cells and can be biomarkers for tumor diagnosis
(12,13). In this study, microarray was applied
to check the deregulation of circulating miRNAs in PC. miR-23b-3p
was verified to be the only upregulated miRNA in both PC and CP
groups, as compared to that in normal controls. Furthermore, the
extent of upregulation in PC group was higher than that in the CP
group. Previous studies have found upregulated expression of
miR-23b-3p in renal (14), gastric
(15), and non-small cell lung
cancers (16). miR-23b-3p acts as
an oncogene in these cancers; however, this was not found to be the
case in PC, which may be attributable to racial differences in the
study population (17–19). This study verified the
overexpression of serum miR-23b-3p in patients with PC and CP; the
level of upregulation in PC patients was higher than that in CP,
which was consistent with the microarray data described above.
Overexpression of miR-23b-3p in vitro promoted the PANC-1
cells proliferation, invasion, and migration ability. It is
speculated that miR-23b-3p may serve as a novel potential
diagnostic and prognostic biomarker in the context of PC. However,
the function and role of miR-23b-3p alterations in PC development
and progression needs to be further studied.
Exosomes are extracellular vesicles 30–100 nm in
size and play a key role in intercellular communications and in
regulating diverse biological processes. We sought to build on
recently reported findings and sought to establish the potential
diagnostic value of serum exosomes in the context of PC (20). In this study, PANC-1 cells were
shown to produce exosomes in the supernatant and take them up as
well; furthermore, miR-23b-3p was found to be overexpressed in the
exosomes in sera of patients with PC. Currently, CA-19-9 is a
traditional clinical biomarker for PC diagnosis (21), but several more novel biomarkers,
such as miRNAs and proteomics need to be further studied (22). Many studies have suggested the
utility of combined use of miRNAs and CA19-9 as biomarkers for
diagnosis of PC (23,24). Exosomic miRNAs have also been
proposed as potential biomarkers for the diagnosis of PC (25–27);
however, the relationship between exosomic miRNA and CA19-9 is not
clear. In this study, miR-23b-3p expression in sera or that in the
exosomes isolated from sera showed a close relationship with
CA-19-9 expression. However, larger studies on higher number of
patients are required to assess the superiority of use of
miR-23b-3p over that of CA-19-9 for diagnostic purposes, and to
determine if combined use of miR-23b-3p and CA-19-9 is more
sensitive and specific for diagnosis of early PC, as compared to
use of either of these parameters individually.
In conclusion, we determined differential miRNA
expression profiles in sera of patients with CP and PC. The
expression of miR-23b-3p was upregulated in the sera specimens, and
in vitro overexpression of miR-23b-3p promoted
proliferation, migration, and invasive growth of PC cells.
Moreover, upregulated miR-23b-3p was also detected in the exosomes
from the sera of PC patients; miR-23b-3p in both serum and exosomes
was positively associated with CA19-9 in PC patients. However, this
study is just a proof-of-principle investigation; more studies are
needed to fully disclose the function and role of exosomic miRNA
alterations in the development and progression of PC, and for the
establishment of miR-23b-3p as a novel target and diagnostic and
prognostic marker for PC.
Acknowledgements
The authors would like to thank our staff in both
Departments for their contributions to this study. This study was
supported in part by grants from the National Natural Science
Foundation of China (no. 81502038 to F.A., no. 81302104 to J.D. and
no. 81302382 to Y.J.), the Natural Science Foundation of Jiangsu
Province (no. BK2012098 to F.A.), the Youth Medical Talent of
Jiangsu Province (no. QNRC2016187 to F.A.) and Wuxi Medical
Innovation Team (no. CXTD005 to Q.Z.).
References
|
1
|
Karanikas M, Esempidis A, Chasan ZT,
Deftereou T, Antonopoulou M, Bozali F, Amarantidis K and Man YG:
Pancreatic cancer from molecular pathways to treatment opinion. J
Cancer. 7:1328–1339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghai V and Wang K: Recent progress toward
the use of circulating microRNAs as clinical biomarkers. Arch
Toxicol. 90:2959–2978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ebrahimi S, Hosseini M, Ghasemi F,
Shahidsales S, Maftouh M, Akbarzade H, Parizadeh SA, Hassanian SM
and Avan A: Circulating microRNAs as novel potential diagnostic and
prognosis biomarkers in pancreatic cancer. Curr Pharm Des.
22:6444–6450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Midha S, Sreenivas V, Kabra M,
Chattopadhyay TK, Joshi YK and Garg PK: Genetically determined
chronic pancreatitis but not alcoholic pancreatitis is a strong
risk factor for pancreatic cancer. Pancreas. 45:1478–1484. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Chen JQ, Liu JL and Tian L:
Exosomes in tumor microenvironment: Novel transporters and
biomarkers. J Transl Med. 14:2972016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Hawary M: Role of imaging in diagnosing
and staging pancreatic cancer. J Natl Compr Canc Netw. 14
(Suppl):678–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conwell DL, Lee LS, Yadav D, Longnecker
DS, Miller FH, Mortele KJ, Levy MJ, Kwon R, Lieb JG, Stevens T, et
al: American Pancreatic Association Practice Guidelines in Chronic
Pancreatitis: Evidence-based report on diagnostic guidelines.
Pancreas. 43:1143–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lässer C, Eldh M and Lötvall J: Isolation
and characterization of RNA-containing exosomes. J Vis Exp.
59:e30372012.
|
|
10
|
An F, Gong B, Wang H, Yu D, Zhao G, Lin L,
Tang W, Yu H, Bao S and Xie Q: miR-15b and miR-16 regulate TNF
mediated hepatocyte apoptosis via BCL2 in acute liver failure.
Apoptosis. 17:702–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He
Y, Chen G, Zhou Q, Wang W, Zhou X, et al: Radiation-induced
miR-208a increases the proliferation and radioresistance by
targeting p21 in human lung cancer cells. J Exp Clin Cancer Res.
35:72016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang M, Wu X, Cao H, Zhan Q, Xia M, Zhou
Q, Cai X and An F: Regulatory role of serum miR-224 in invasiveness
and metastasis of cholangiocarcinoma. Zhonghua Gan Zang Bing Za
Zhi. 23:748–753. 2015.(In Chinese). PubMed/NCBI
|
|
13
|
Wu X, Xia M, Chen D, Wu F, Lv Z, Zhan Q,
Jiao Y, Wang W, Chen G and An F: Profiling of downregulated
blood-circulating miR-150-5p as a novel tumor marker for
cholangiocarcinoma. Tumour Biol. 37:15019–15029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An Y, Zhang Z, Shang Y, Jiang X, Dong J,
Yu P, Nie Y and Zhao Q: miR-23b-3p regulates the chemoresistance of
gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis.
6:e17662015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Begum S, Hayashi M, Ogawa T, Jabboure FJ,
Brait M, Izumchenko E, Tabak S, Ahrendt SA, Westra WH, Koch W, et
al: An integrated genome-wide approach to discover deregulated
microRNAs in non-small cell lung cancer: Clinical significance of
miR-23b-3p deregulation. Sci Rep. 5:132362015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kojima M, Sudo H, Kawauchi J, Takizawa S,
Kondou S, Nobumasa H and Ochiai A: MicroRNA markers for the
diagnosis of pancreatic and biliary-tract cancers. PLoS One.
10:e01182202015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin MS, Chen WC, Huang JX, Gao HJ and
Sheng HH: Aberrant expression of microRNAs in serum may identify
individuals with pancreatic cancer. Int J Clin Exp Med.
7:5226–5234. 2014.PubMed/NCBI
|
|
19
|
Cao Z, Liu C, Xu J, You L, Wang C, Lou W,
Sun B, Miao Y, Liu X, Wang X, et al: Plasma microRNA panels to
diagnose pancreatic cancer: Results from a multicenter study.
Oncotarget. 7:41575–41583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu L and Risch HA: Exosomes: Potential for
early detection in pancreatic cancer. Future Oncol. 12:1081–1090.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao S, Hu Y, Gao X, Liao Q and Zhao Y:
Serum carbohydrate antigen 19-9 in differential diagnosis of benign
and malignant pancreatic cystic neoplasms: A meta-analysis. PLoS
One. 11:e01664062016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ballehaninna UK and Chamberlain RS:
Biomarkers for pancreatic cancer: Promising new markers and options
beyond CA 19-9. Tumour Biol. 34:3279–3292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao L, He SB and Li DC: Effects of miR-16
plus CA19-9 detections on pancreatic cancer diagnostic performance.
Clin Lab. 60:73–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang WS, Liu LX, Li GP, Chen Y, Li CY, Jin
DY and Wang XL: Combined serum CA19-9 and miR-27a-3p in peripheral
blood mononuclear cells to diagnose pancreatic cancer. Cancer Prev
Res (Phila). 6:331–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Machida T, Tomofuji T, Maruyama T, Yoneda
T, Ekuni D, Azuma T, Miyai H, Mizuno H, Kato H, Tsutsumi K, et al:
miR-1246 and miR-4644 in salivary exosome as potential biomarkers
for pancreatobiliary tract cancer. Oncol Rep. 36:2375–2381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Madhavan B, Yue S, Galli U, Rana S, Gross
W, Müller M, Giese NA, Kalthoff H, Becker T, Büchler MW, et al:
Combined evaluation of a panel of protein and miRNA serum-exosome
biomarkers for pancreatic cancer diagnosis increases sensitivity
and specificity. Int J Cancer. 136:2616–2627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zöller M: Pancreatic cancer diagnosis by
free and exosomal miRNA. World J Gastrointest Pathophysiol.
4:74–90. 2013. View Article : Google Scholar : PubMed/NCBI
|