Introduction
Estrogen plays critical roles in normal mammary
gland development, physiological processes as well as
tumorigenesis. Estrogen receptor (ER) is the major mediator of the
effect of estrogen. After binding to estrogen, ER is activated and
translocates into the cell nucleus (1). In the nucleus, ER can
transcriptionally regulate target genes either by directly binding
to estrogen response elements (EREs) or through protein-protein
interactions with other transcriptional factors such as AP1, SP1
and NF-κB (2). Moreover, ~70% of
breast cancers express ER. ER is essential for the initiation and
development of breast cancers (1,3,4). The
pro-oncogenic effect of ER is mediated primarily by
transcriptionally regulating its target genes. Various genes that
play crucial roles in cell proliferation and apoptosis such as
c-Myc, CCND1 and BCL2 have been identified as
ER-regulated genes. Hundreds of protein-coding genes have been
identified as ER-regulated genes by using microarray and RNA-seq as
well as ChIP-seq (5–9).
Long non-coding RNAs (lncRNAs) refer to non-coding
RNAs consisting of >200 nucleotides. Recently, the important
roles of lncRNAs in breast cancer cell proliferation, apoptosis,
metastasis and endocrine resistance have been reported (10–12).
Because of their crucial roles in breast cancer, we explored
ER-regulated lncRNAs. Although a few studies have been carried out
to identify ER-regulated lncRNAs very recently (13–15),
their roles in regulation of gene expression and cell phenotype
remains largely unknown. Here, we report a genome-wide study of
ER-regulated lncRNAs by conducting an integrate analysis of
ChIP-seq, strand-specific RNA-seq and TCGA clinical data. We
observed that many of these ER-regulated lncRNAs were overexpressed
in ER+ breast cancer and exhibited co-expression with
several key regulator proteins. Moreover, we found one of most
prominent lncRNAs, AP000439.3, can promote cell cycle
progression through enhancing CCDN1 expression induced by
estrogen. These findings reveal ER can regulate many lncRNAs that
exhibit important functions in regulation of gene expression and
cell phenotype in breast cancer.
Materials and methods
Datasets and computational
analysis
The 17β-estradiol (E2) and vehicle treated MCF7
RNA-seq data (pair-end, strand-specific) was previously reported by
Dago et al (16) and
obtained from GEO dataset (GSE64590). For RNA-seq analysis, the
sequenced reads were aligned to human reference genome (Hg38) and
transcriptome (GENCODE.v23) using STAR (17) and then processed by RSEM (18). Genes with FDR<0.05 generated by
both DESeq (19) and edgeR
(20) were considered as
differentially expressed genes.
ERα ChIP-seq of estrogen treated and untreated MCF7
were reported by Franco et al (21) and obtained from GEO dataset
(GSE59530). Reads of ChIP-seq were aligned to human reference
genome (Hg38) using BOWTIE (22).
The estrogen treated peaks were generated by MACS (23) using untreated as control. The ER
binding sites within ±100 kb region of transcriptional start sites
(TSS) of protein-coding genes and lncRNAs (GENCODE.v23) were
considered as potential ER regulatory sites.
The TCGA lncRNA data were downloaded from TANRIC
database (24), mRNA and RPPAs data
were downloaded from Broad Dashboard-Stddata (https://confluence.broadinstitute.org/display/GDAC/Dashboard-Stddata).
Cell culture and treatments
MCF7, ZR-75-1 and T47D cells were obtained from
ATCC. MCF7 cells were cultured in Eagle's minimum essential medium
supplemented with 10% FBS; ZR-75-1 and T47D were cultured in
RPMI-1640 media supplemented with 10% FBS. Before estrogen
treatments, the cells were grown for 72 h in phenol red-free MEM
Eagle medium supplemented with 10% charcoal-dextran-treated FBS.
The cells were then treated with ethanol (vehicle) or 10 nM E2 for
24 h. siRNAs were synthesized in GenePharma Co. (Shanghai, China).
The sequences of siRNA are listed in Table II. siRNA was transfected at a final
concentration of 50 nM using Lipofectamine 2000 reagent
(Invitrogen).
 | Table II.Primers and siRNAs used in this
study. |
Table II.
Primers and siRNAs used in this
study.
| qPCR primers | Forward | Reverse |
|---|
| AP000439.3 |
CCCCAGGCTAGGAAGATGT |
GAGCCAAGAGGTCCTCACAG |
| RP11-321G12.1 |
GGTTTGGTTCCCAATTGTTG |
TTGGAAGACCCCATCTTCAC |
| RP11-150O12.3 |
ACCATTTCCAAACTGCCAAG |
GCTCCATGCACACTCAAGAA |
| LINC01016 |
TACAGCATGGTTCCCAAATG |
GGGCCATGGTCACTCATATT |
| SIAH2-AS1 |
CTCCTCAATCCCCACACAGT |
TGCAGACGTGTATTCGGGTA |
| RP11-95G17.2 |
TTGCTGTAGTGCGGCTTAAA |
TAACCCCTTGCAATCAGCTC |
|
| ChIP PCR
primers | Forward | Reverse |
|
| ER binding site
1 |
AGGGAGAGTTCCCAGGAGTC |
CAGCCCTGTCTGAGCAATTT |
| ER binding site
2 |
CTCCACCGAGCACTCCATAC |
TGCCTCTTGTTTCCCCTAAA |
|
| siRNAs | sense | antisene |
|
| AP000439.3 siRNA
1# |
GCUAGGAAGAUGUGCACCU |
AGGUGCACAUCUUCCUAGC |
| AP000439.3 siRNA
2# |
GCAAAGCUCACAGGAAAUA |
UAUUUCCUGUGAGCUUUGC |
| ERα siRNA |
CGAGUAUGAUCCUACCAGA |
UCUGGUAGGAUCAUACUCG |
Real-time quantitative PCR and western
blotting
Cells were grown and treated as described above and
then RNA was collected using TRIzol reagent (Invitrogen), RNA was
reverse-transcribed using PrimeScript RT reagent kit (Takara
RR047A) and analyzed by LightCycler® 96 real-time PCR
thermocycler (Roche), primers are listed in Table II. The lncRNA expression was
normalized to β-actin transcript as an internal standard. The CCND1
and α-tubulin protein were detected by western blotting using
cyclin D1 mouse mAb (BD Pharmigen #556470) and α-tubulin antibody
(Santa Cruz, SC-5286).
Cell cycle analysis and colony
formation assay
Seventy-two hours after siRNA transfection, cells
were digested and washed with PBS and then fixed in 70% ethanol
overnight at 4°C, then incubated with RNase and propidium iodide
(PI) for 10 min and analyzed by flow cytometry.
For colony forming analysis, 1,000 cells were plated
in 6-well plates and grown for 2 weeks, colonies were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet. The number
of colonies were counted and analyzed by ImageJ software.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay were performed as previously
described (25) with MCF7 cells
using 3 µg normal IgG (CST, #2729) and ER (Santa Cruz sc-543)
antibody. The ChIP PCR primers were listed in Table II.
Statistical analysis
Wilcoxon rank-sum test was used for comparing fold
change between lncRNA and protein. Spearman's rank moment
correlation coefficient was calculated for analysis of the
co-expression of lncRNAs and RPPAs, Pearson's product moment
correlation for lncRNA-mRNA co-expression. For qRT-PCR and colony
formation assay, Student's t-test was used to test for statistical
significance of the differences between the different group
parameters. p-values of <0.05 was considered statistically
significant.
Results
Global identification and
characterization of ER-regulated lncRNAs
To identify ER-regulated lncRNAs in a genome-wide
manner, we exploited published RNA-seq of E2- and vehicle-treated
cells that were originally used for analyzing alternative mRNA
splicing (16) (GSE64590). We chose
this dataset because: i) this RNA-seq was strand-specific, which
was superb for analyzing antisense transcripts that overlapped with
host genes. ii) Three repetitions with high reproducibility
occurred in this dataset. We conducted an analysis of differential
expression (DE) transcripts using edgeR (20) and DEseq (19) processed by RSEM (18). We observed 2,869 DE transcripts,
which included 1,784 upregulated and 1,085 downregulated
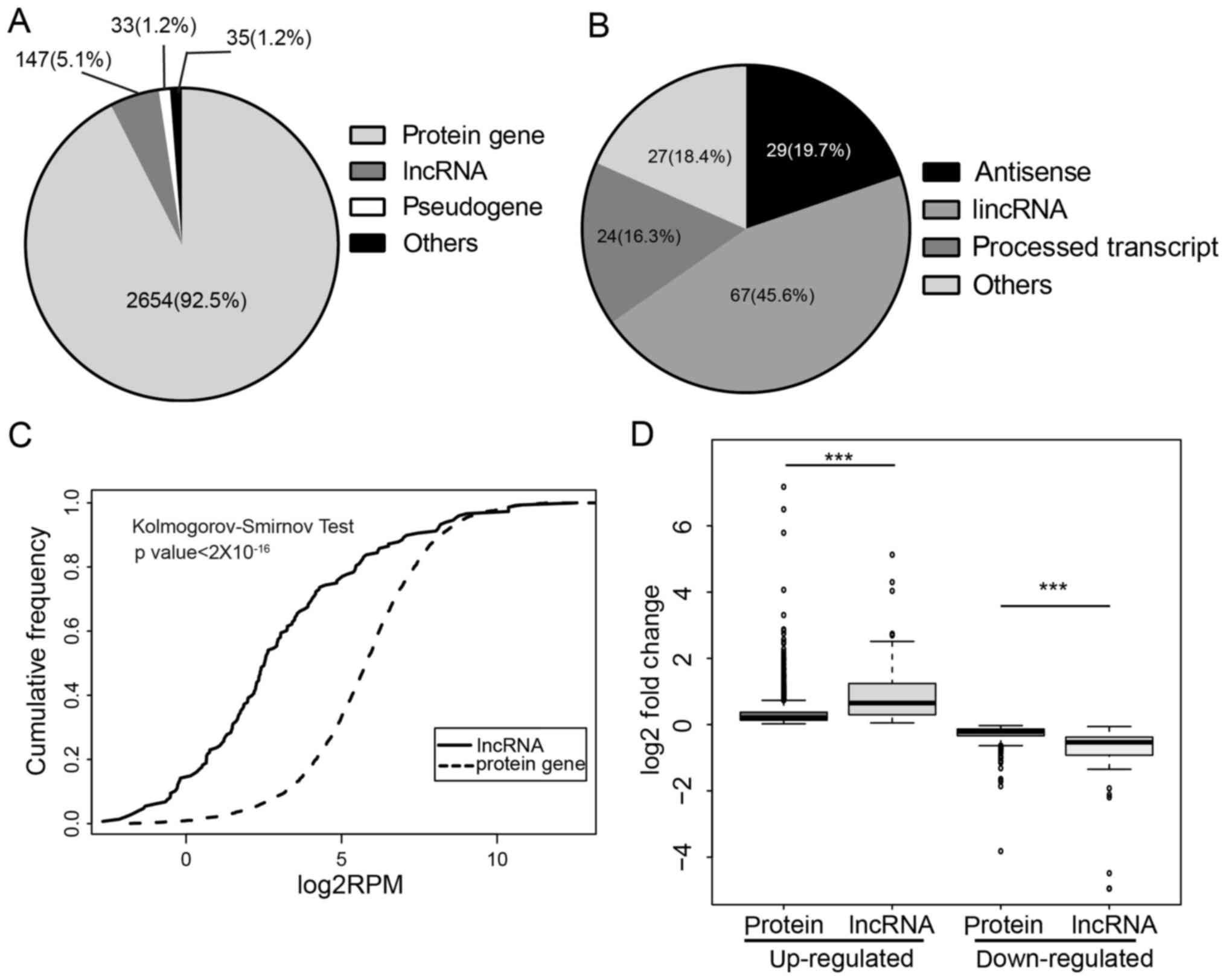
transcripts. The dominant types of DE transcripts were
protein-coding genes (92.5%); 147 lncRNAs were found to express
differentially upon E2 treatment (Fig.
1A). Of these lncRNAs, 45.6% were long intergenic RNA
(lincRNAs), 19.7% were antisense and 16.3% were processed
transcripts (Fig. 1B). DE lncRNAs
showed significantly lower expression than protein-coding genes
(Fig. 1C), which is in agreement
with previous reports (26).
Despite low expression of DE lncRNAs, we found DE lncRNAs exhibited
a higher fold-change than DE protein-coding genes in the presence
of estrogen (Fig. 1D). LncRNAs tend
to be more specifically expressed than protein (27,28);
moreover, lncRNAs differ not just between tissues, but also between
closely related cell types, which indicates lncRNAs are likely
under stricter regulations (29).
In agreement with this notion, our analysis revealed lncRNAs were
under more rigorous regulation of activated ER. To investigate
whether these DE transcripts were transcriptionally regulated by
ER, we conducted an analysis of ER binding sites using published
ChIP-seq data, and 114 of 147 (77.6%) DE lncRNAs had at least one
ER binding site within their genomic domain (within ± 100 kb from
TSS). Half of these lncRNAs were upregulated by E2 and these
lncRNAs were referred to as putative ER-upregulated lncRNAs
(Table I). To explore the potential
roles of these lncRNAs, we conducted co-expression analysis between
lncRNAs and reverse-phase protein array (RPPAs) proteomics data
(30), which includes extensively
validated antibodies to nearly 200 proteins and phosphoproteins of
TCGA clinical samples. We found most of these lncRNAs co-expressed
with some key regulators of the cell cycle (cyclin family) and
apoptosis (Bcl-2 family) as well as key components of important
signaling pathway such as IGF1R, MAPK (pT202/Y204) and JNK2. These
data suggest that ER can transcriptionally regulate hundreds of
lncRNAs that may participate in some important cellular
processes.
 | Table I.List of ER-upregluated lncRNAs. |
Table I.
List of ER-upregluated lncRNAs.
| Ensembl ID | LncRNA name | Fold change
(log2) | FDR |
|---|
|
ENSG00000273565.1 | CTD-3075F15.1 | 5.13 | 0.000258667 |
|
ENSG00000249346.6 | LINC01016 | 4.30 | 1.89E-107 |
|
ENSG00000266036.1 | RP11-452I5.2 | 4.04 | 3.29E-06 |
|
ENSG00000254290.1 | RP11-150O12.3 | 2.74 | 6.46E-08 |
|
ENSG00000258354.1 | MIR3180–1 | 2.69 | 6.93E-06 |
|
ENSG00000272472.1 | RP11-95G17.2 | 2.51 | 5.95E-15 |
|
ENSG00000244265.1 | SIAH2-AS1 | 2.48 | 0.000112224 |
|
ENSG00000227036.6 | LINC00511 | 1.97 | 5.34E-30 |
|
ENSG00000259459.5 | RP11-321G12.1 | 1.86 | 0.000182177 |
|
ENSG00000229525.1 | AC053503.4 | 1.83 | 3.17E-06 |
|
ENSG00000233885.7 | YEATS2-AS1 | 1.48 | 2.03E-14 |
|
ENSG00000259080.1 | RP11-158I13.2 | 1.46 | 1.04E-05 |
|
ENSG00000261578.1 | RP11-21L23.2 | 1.39 | 1.73E-26 |
|
ENSG00000280186.1 | RP11-483I13.6 | 1.35 | 9.59E-53 |
|
ENSG00000204792.2 | LINC01291 | 1.32 | 1.38E-21 |
|
ENSG00000253125.1 | RP11-459E5.1 | 1.24 | 6.02E-05 |
|
ENSG00000260401.1 | RP11-800A3.4 | 1.23 | 2.02E-94 |
|
ENSG00000261051.1 | RP11-274H2.5 | 1.22 | 1.07E-16 |
|
ENSG00000266709.1 | RP11-214O1.2 | 1.19 | 1.26E-05 |
|
ENSG00000223749.7 | MIR503HG | 1.18 | 2.99E-42 |
|
ENSG00000280924.1 | LINC00628 | 1.16 | 2.97E-05 |
|
ENSG00000226471.6 | CTA-292E10.6 | 1.16 | 9.45E-19 |
|
ENSG00000277159.1 | RP11-88E10.4 | 1.12 | 2.90E-09 |
|
ENSG00000281207.1 | SLFNL1-AS1 | 1.03 | 6.93E-06 |
|
ENSG00000280073.1 | CTD-2525P14.5 | 0.95 | 1.47E-05 |
|
ENSG00000232956.8 | SNHG15 | 0.93 | 1.38E-37 |
|
ENSG00000230836.1 | LINC01293 | 0.84 | 5.77E-15 |
|
ENSG00000246174.7 | KCTD21-AS1 | 0.79 | 3.09E-08 |
|
ENSG00000236144.6 | TMEM147-AS1 | 0.42 | 8.75E-07 |
|
ENSG00000213062.4 | RP1-206D15.6 | 0.75 | 1.69E-05 |
|
ENSG00000270344.2 | RP11-734K2.4 | 0.73 | 9.31E-17 |
|
ENSG00000260257.2 | RP5-1085F17.3 | 0.72 | 0.000169607 |
|
ENSG00000261226.1 | RP11-830F9.7 | 0.63 | 7.69E-06 |
|
ENSG00000249859.7 | PVT1 | 0.62 | 5.88E-46 |
|
ENSG00000271020.1 | RP11-10C24.1 | 0.61 | 6.26E-05 |
|
ENSG00000247092.6 | SNHG10 | 0.57 | 2.45E-12 |
|
ENSG00000232445.1 | RP11-132A1.4 | 0.57 | 2.35E-06 |
|
ENSG00000271643.1 | RP11-10C24.3 | 0.56 | 9.12E-08 |
|
ENSG00000262202.4 | RP11-160E2.6 | 0.56 | 5.30E-09 |
|
ENSG00000259977.1 | AL121578.2 | 0.53 | 7.72E-06 |
|
ENSG00000255774.1 | AP000439.3 | 0.40 | 0.000181605 |
|
ENSG00000252690.3 | SCARNA15 | 0.39 | 1.54E-08 |
|
ENSG00000255717.6 | SNHG1 | 0.34 | 6.40E-61 |
|
ENSG00000236824.1 | BCYRN1 | 0.32 | 4.03E-08 |
|
ENSG00000236830.6 | CBR3-AS1 | 0.31 | 6.86E-12 |
|
ENSG00000226950.6 | DANCR | 0.30 | 7.63E-15 |
|
ENSG00000234912.9 | SNHG20 | 0.25 | 3.78E-05 |
|
ENSG00000227051.5 | C14orf132 | 0.23 | 3.24E-16 |
|
ENSG00000235123.5 | DSCAM-AS1 | 0.22 | 2.09E-29 |
|
ENSG00000163597.14 | SNHG16 | 0.20 | 3.35E-46 |
|
ENSG00000259623.1 | RP11-156E6.1 | 0.19 | 1.91E-06 |
|
ENSG00000260032.1 | LINC00657 | 0.16 | 3.44E-37 |
|
ENSG00000224032.6 | EPB41L4A-AS1 | 0.16 | 5.66E-06 |
|
ENSG00000242125.3 | SNHG3 | 0.14 | 1.29E-05 |
|
ENSG00000244879.4 | GABPB1-AS1 | 0.11 | 1.09E-05 |
|
ENSG00000234741.7 | GAS5 | 0.06 | 6.73E-08 |
|
ENSG00000175061.17 | LRRC75A-AS1 | 0.05 | 3.01E-05 |
Detection of ER-regulated lncRNAs
To verify the DE lncRNAs analyzed by RNA-seq, we
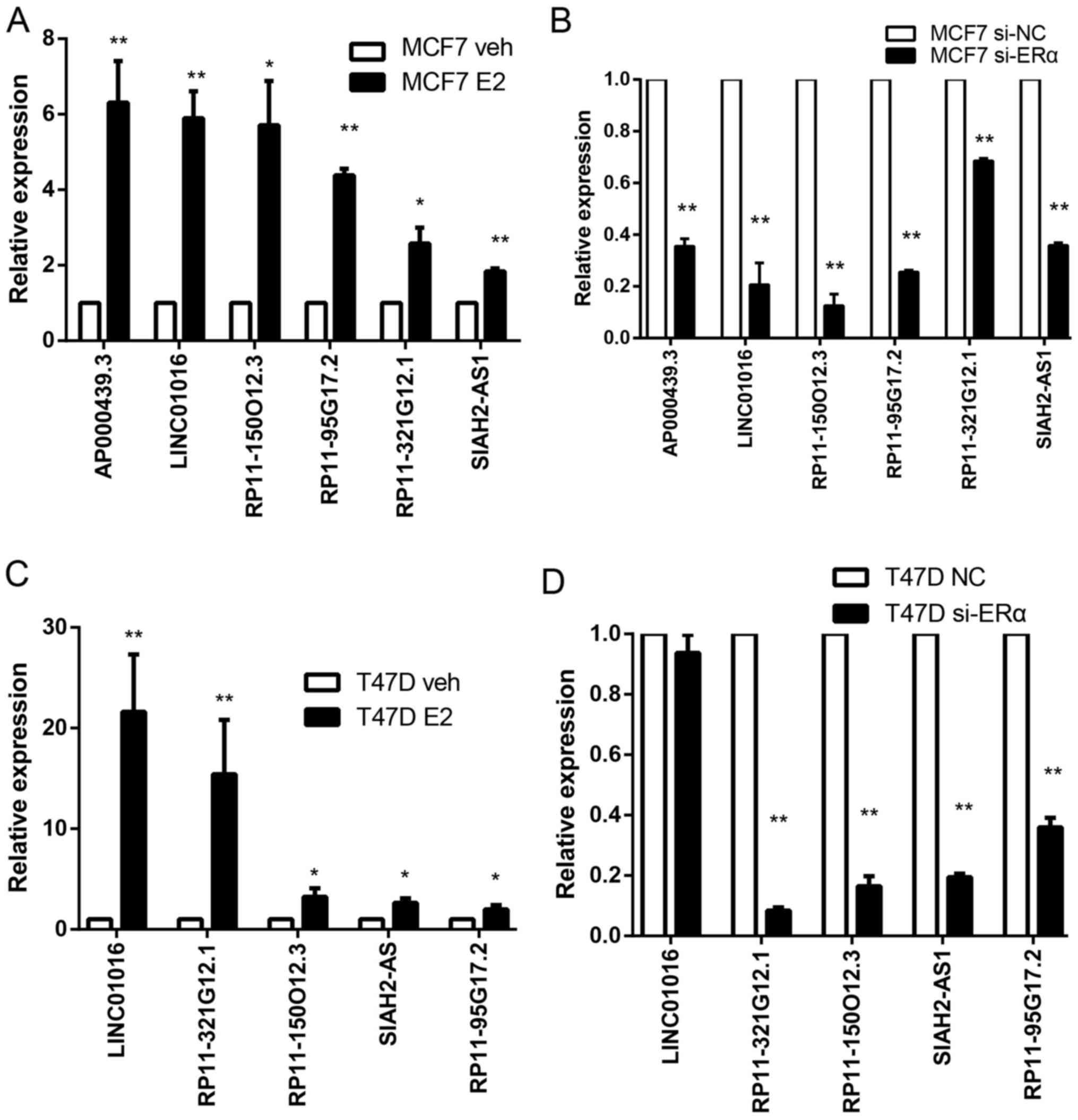
selected six lncRNAs for further analysis. We first performed qPCR
experiments after E2 treatment. Consistent with RNA-seq results. In
MCF7, the most significantly changed lncRNA is AP000439.3,
which increased 6 times. The expressions of this lncRNA in T47D was
too low to be effectively detected. We also observed significant
increase of AP000439.3 after E2 treatment in ZR-75-1 cells
(data not shown). The other lncRNAs also increased remarkably after
E2 treatment of both in MCF7 and T47D (Fig. 2A and C). To confirm that these DE
lncRNAs are regulated by ER, we silenced ERα in MCF7 and T47D by
siRNA. In MCF7 cells, all of these lncRNAs dramatically decreased
when silencing ERα, some of the lncRNAs such as RP11-150O12.3,
AP000439.3 and RP11-95G17.2 decreased by >60%
(Fig. 2B and D). Similar results
were observed when ER was knocked down in T47D. Although
LINC01016 is a significantly upregulated lncRNA in T47D,
silencing ER did not result in its reduction. LINC01016 has
been reported as an ER-target lncRNA in a previous study (13,14).
Failure to observe a decrease of LINC01016 when silencing
ERα may be due to its low expression level in T47D, hence hard to
downregulate further, but easy to upregulate.
ER-regulated lncRNA AP000439.3
promotes cell cycle progression
To further characterize these ER-regulated lncRNAs,
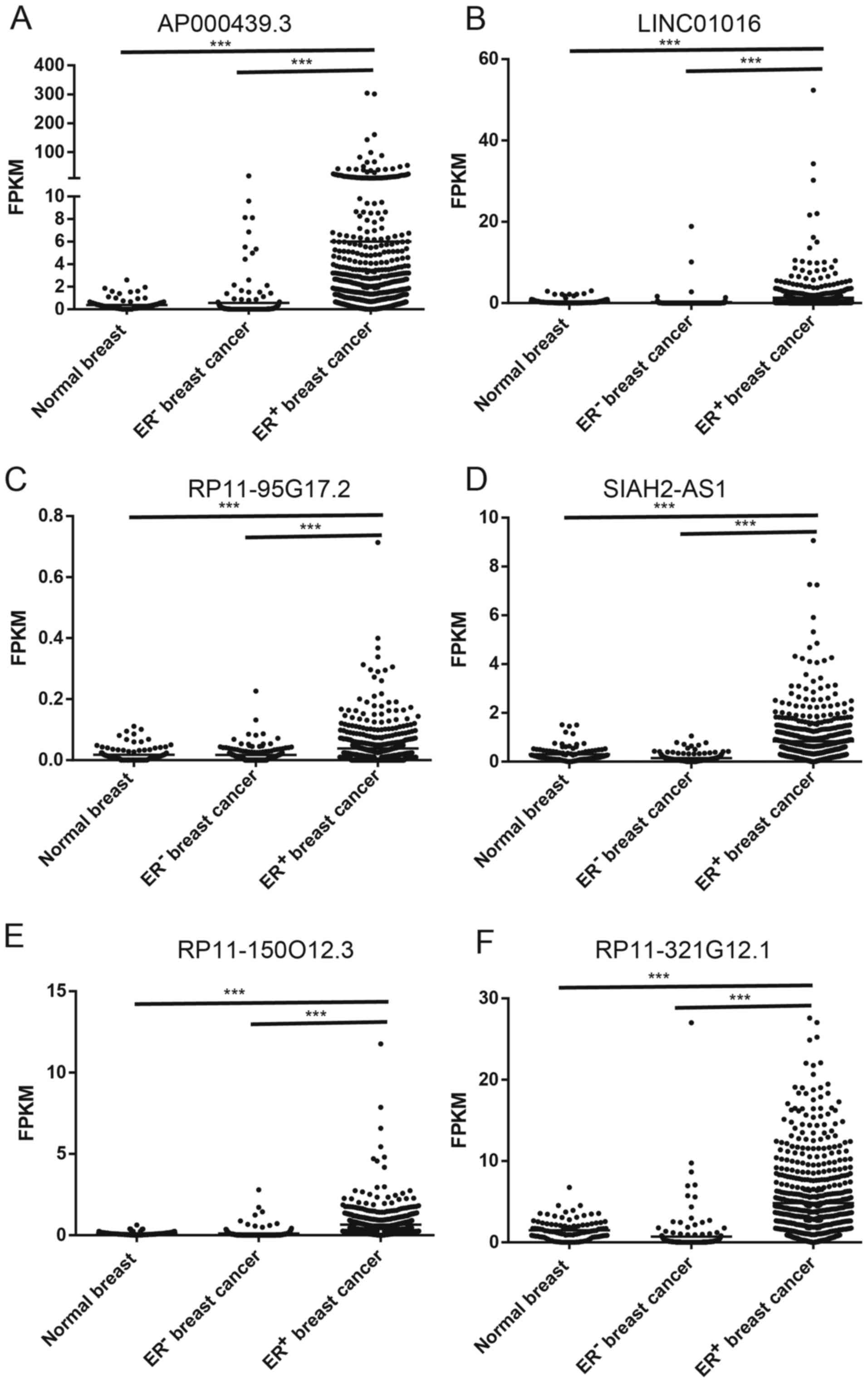
we analyzed the expression of lncRNAs in breast tissues using The
Cancer Genome Atlas (TCGA) data (31). Most of these selected lncRNAs were
dramatically overexpressed in ER+ breast cancer compared
to ER− breast cancer and normal breast tissues
(p<0.001, Fig. 3).
To explore the roles of ER target lncRNAs in breast
cancer, we selected one of the most significantly upregulated
lncRNA (AP000439.3) for further analysis. Using ChIPBase, an
integrated database for decoding transcriptional regulators of
genes and lncRNAs (32,33), we found ERα is the top ranked
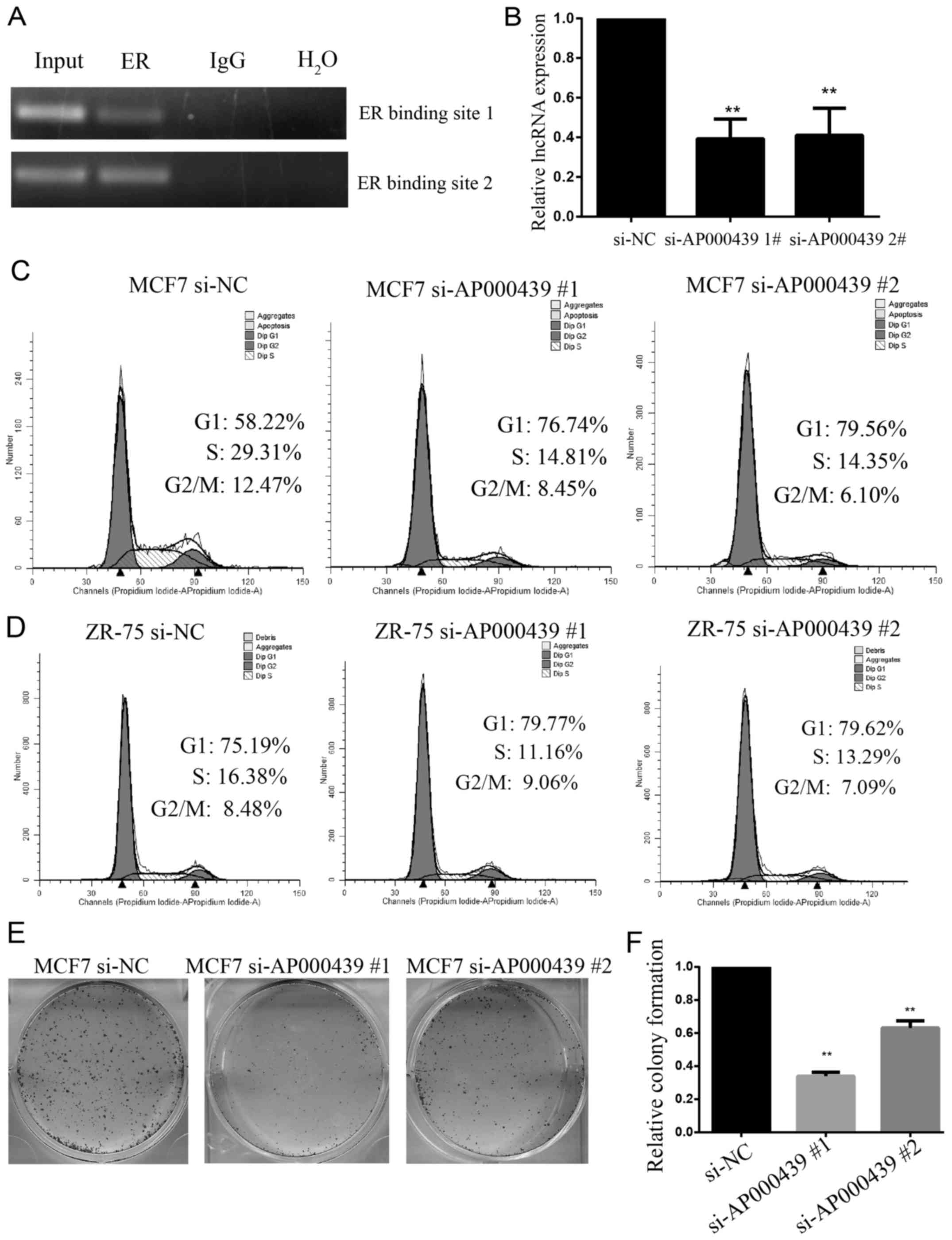
regulator. To verify that AP000439.3 is regulated by ER, ChIP PCR
assay was performed on potential ER binding sites identified by
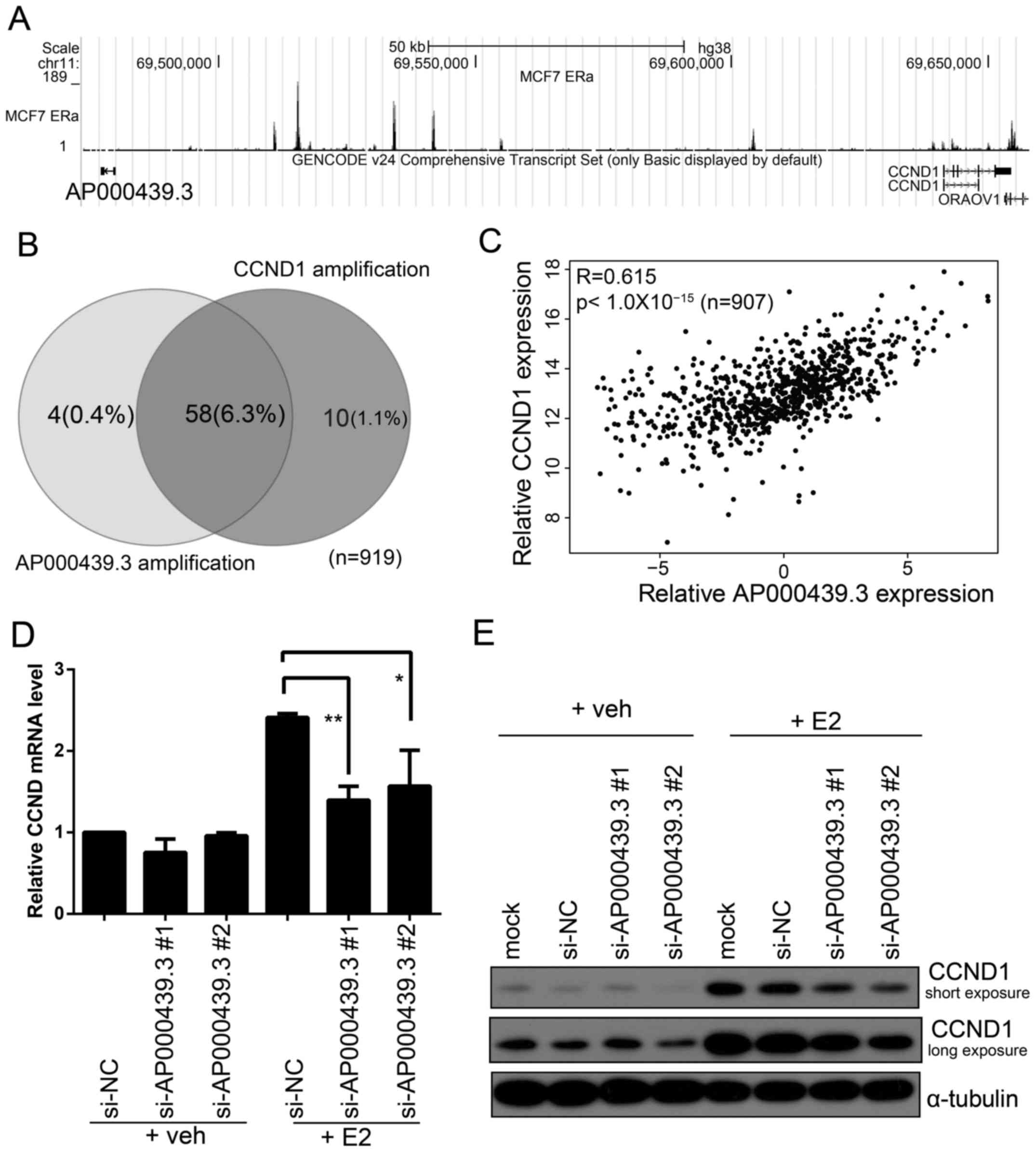
ChIP-seq data and ChIPBase. As shown in Fig. 4A, ER can directly bind upstream of
AP000439.3.
Since ER can regulate dozens of genes that play
crucial roles in breast cancer, we speculate these lncRNAs may also
have an important impact on breast cancer cell function. AP000439.3
was silenced using two individual siRNAs by >50% (Fig. 4B). The most dominant role of ER in
breast cancer is promotion of cell proliferation and cell cycle
progression (1). Hence the cell
cycle progression was detected using flow cytometry assay.
Silencing AP000439.3 resulted in a dramatic inhibition of
the G1-S transition in MCF7 cells (Fig.
4C) and in ZR-75-1 cells (Fig.
4D). Likewise, silencing AP000439.3 significantly
suppressed clonogenic proliferation (Fig. 4E and F). Taken together, lncRNA
AP000439.3 is stimulated by activated ER, it is
overexpressed in ER+ breast cancer and promotes cell
cycle progression and proliferation.
AP000439.3 facilitates estrogen
induced CCDN1 expression
To understand how AP000439.3 regulates the cell
cycle, we first investigated its location and gene structure.
AP000439.3 is a long intergenic non-coding RNA (lincRNA)
located at chromosome 11q13. AP000439.3 is 160 kb upstream
of the CCDN1 transcriptional start site (TSS) (Fig. 5A), AP000439.3 and
CCDN1 are transcribed divergently (head to head).
CCND1 encodes the cyclin D1 protein that serves as a
regulator of cyclin-dependent kinase as a crucial regulator of the
cell cycle. Amplification of CCND1 has been reported in many
kinds of tumors including breast cancer, its amplification is
associated with a poor prognosis (34). Analysis of COSMIC CNV data (35) revealed that CCND1 amplification
occurred in 68/919 (7.4%) breast cancer samples, 58 of 68 (85%) of
these also have an amplification of AP000439.3 (Fig. 5B). Expression analysis of
CCND1 and AP000439.3 showed a high correlation
between them (R=0.61, p<10−15, Fig. 5C). CCND1 is overexpressed in
~50% breast cancer, while amplification of the CCND1 gene is
present only in a minority of CCND1-overexpressed breast
cancers (36), directly regulated
by ER is another important reason for overexpression of
CCND1 (37).
LncRNAs are likely to regulate the expression of an
adjacent gene (38–40). Hence we speculated that
AP000439.3 may influence the expression of CCND1. We
silenced AP000439.3 in MCF7 and then treated these cells
with either E2 or vehicle. Both qPCR and western blotting showed
that knockdown of AP000439.3 could impair CCND1
expression induced by E2 (Fig. 5D).
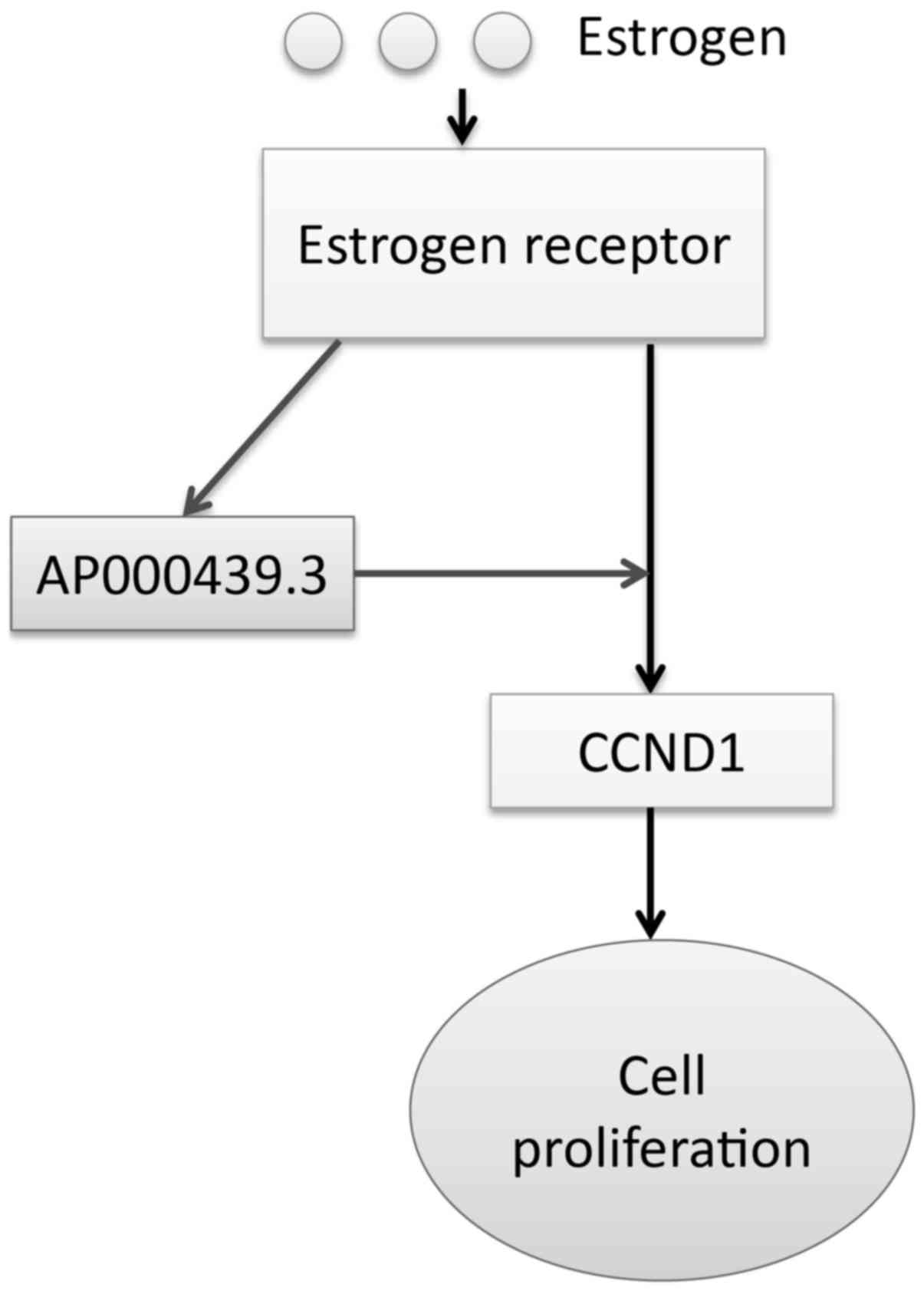
Taken together, these data suggest ER-regulated lncRNA
AP000439.3 can facilitate ER regulation of CCND1 and
thus promote cell cycle progression (Fig. 6).
Discussion
In this study, we described a genome-wide
identification and characterization of ER-regulated lncRNAs in
breast cancer cells. We found many of these lncRNAs were
overexpressed in ER+ breast cancer and co-expressed with
some key regulators. Moreover, we found one of the most prominent
lncRNA, AP000439.3, can promote cell cycle progression
through enhancing CCDN1 expression induced by estrogen.
The mechanisms of how AP000439.3 facilitate
CCND1 expression remains to be further investigated.
Considering AP000439.3 can influence transcription of
CCND1, it may involve changes in chromatin organization.
This mechanism have been reported by lncRNA CCAT1-L regulation on
its adjacent gene C-MYC in colorectal cancer (41).
The roles of ER is mediated primarily by its
downstream effectors, the ER-regulated protein-coding genes and
their roles have been well studied. However, some important issues
such as the mechanisms by which ER-mediated drug resistance remains
largely unknown (42). Study of
ER-regulated lncRNAs may provide a unique prospective to answer
these questions.
Our findings may provide another layer of estrogenic
control of gene expression: ER can promote expression of lncRNAs
that are adjacent to protein-coding genes; these lncRNAs can serve
as positive regulators that further facilitate transcriptional
regulation by ER. It remains to be further investigated how many
lncRNAs function in this way and whether these lncRNAs specifically
regulate their neighboring adjacent genes or have a more extensive
impact on ER regulation. Since these ER-regulated lncRNAs showed
highly cell-type specificity, some oncogenic lncRNAs can be
potential biomarkers and therapeutic targets.
Acknowledgements
We would like to thank Ms. Qing Wei and Ms. Fang Su
for flow cytometry analysis. We thank The Cancer Genome Atlas
(TCGA) project and their contributors for providing this valuable
public data set. This study was supported by grants from the
Natural Science Foundation of China (81572484, 81420108026 and
81621004 to D.Y.); Guangdong Science and Technology Department
(2014A050503026 to D.Y., 2015B050501004 and S2012030006287);
Guangzhou Bureau of Science and Information Technology
(201704030036 to D.Y. This study was also supported by the RNA
Biology Center at the Cancer Science Institute of Singapore, NUS,
as part of funding under the Singapore Ministry of Education's Tier
3 grants, grant no. MOE2014-T3-1-006.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
lncRNA
|
long non-coding RNA
|
|
EREs
|
estrogen response elements
|
|
lincRNA
|
long intergenic non-coding RNA
|
|
DE
|
differential expression
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
TCGA
|
The Cancer Genome Atlas
|
|
RPM
|
reads per million
|
|
FPKM
|
reads per kilobase of exon model per
million mapped reads
|
|
ChIP
|
chromatin immunoprecipitation
|
References
|
1
|
Liang J and Shang Y: Estrogen and cancer.
Annu Rev Physiol. 75:225–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marino M, Galluzzo P and Ascenzi P:
Estrogen signaling multiple pathways to impact gene transcription.
Curr Genomics. 7:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao W and Brown M: Advances in estrogen
receptor biology: Prospects for improvements in targeted breast
cancer therapy. Breast Cancer Res. 6:39–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frasor J, Danes JM, Komm B, Chang KC,
Lyttle CR and Katzenellenbogen BS: Profiling of estrogen up- and
down-regulated gene expression in human breast cancer cells:
Insights into gene networks and pathways underlying estrogenic
control of proliferation and cell phenotype. Endocrinology.
144:4562–4574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coser KR, Chesnes J, Hur J, Ray S,
Isselbacher KJ and Shioda T: Global analysis of ligand sensitivity
of estrogen inducible and suppressible genes in MCF7/BUS breast
cancer cells by DNA microarray. Proc Natl Acad Sci USA. 100:pp.
13994–13999. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue A, Yoshida N, Omoto Y, Oguchi S,
Yamori T, Kiyama R and Hayashi S: Development of cDNA microarray
for expression profiling of estrogen-responsive genes. J Mol
Endocrinol. 29:175–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soulez M and Parker MG: Identification of
novel oestrogen receptor target genes in human ZR75-1 breast cancer
cells by expression profiling. J Mol Endocrinol. 27:259–274. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CY, Ström A, Vega VB, Kong SL, Yeo AL,
Thomsen JS, Chan WC, Doray B, Bangarusamy DK, Ramasamy A, et al:
Discovery of estrogen receptor alpha target genes and response
elements in breast tumor cells. Genome Biol. 5:R662004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tseng YY, Moriarity BS, Gong W, Akiyama R,
Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell
TC, et al: PVT1 dependence in cancer with MYC copy-number increase.
Nature. 512:82–86. 2014.PubMed/NCBI
|
|
11
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-κB interacting
long noncoding RNA blocks IκB phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue X, Yang YA, Zhang A, Fong KW, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miano V, Ferrero G, Reineri S, Caizzi L,
Annaratone L, Ricci L, Cutrupi S, Castellano I, Cordero F and De
Bortoli M: Luminal long non-coding RNAs regulated by estrogen
receptor alpha in a ligand-independent manner show functional roles
in breast cancer. Oncotarget. 7:3201–3216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jonsson P, Coarfa C, Mesmar F, Raz T,
Rajapakshe K, Thompson JF, Gunaratne PH and Williams C:
Single-molecule sequencing reveals estrogen-regulated clinically
relevant lncRNAs in breast cancer. Mol Endocrinol. 29:1634–1645.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu JJ, Ye LC, Ding JX, Feng WW, Jin HY,
Zhang Y, Li Q and Hua KQ: Expression and clinical significance of
estrogen-regulated long non-coding RNAs in estrogen receptor
α-positive ovarian cancer progression. Oncol Rep. 31:1613–1622.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dago DN, Scafoglio C, Rinaldi A, Memoli D,
Giurato G, Nassa G, Ravo M, Rizzo F, Tarallo R and Weisz A:
Estrogen receptor beta impacts hormone-induced alternative mRNA
splicing in breast cancer cells. BMC Genomics. 16:3672015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franco HL, Nagari A and Kraus WL: TNFα
signaling exposes latent estrogen receptor binding sites to alter
the breast cancer cell transcriptome. Mol Cell. 58:21–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Liu T, Meyer CA, Eeckhoute J,
Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et
al: Model-based analysis of ChIP-Seq (MACS). Genome Biol.
9:R1372008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Han L, Roebuck P, Diao L, Liu L,
Yuan Y, Weinstein JN and Liang H: TANRIC: An interactive open
platform to explore the function of lncRNAs in cancer. Cancer Res.
75:3728–3737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong S, Han J, Chen H, Liu T, Huen MS,
Yang Y, Guo C and Huang J: The human SRCAP chromatin remodeling
complex promotes DNA-end resection. Curr Biol. 24:2097–2110. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amin V, Harris RA, Onuchic V, Jackson AR,
Charnecki T, Paithankar S, Subramanian S Lakshmi, Riehle K, Coarfa
C and Milosavljevic A: Epigenomic footprints across 111 reference
epigenomes reveal tissue-specific epigenetic regulation of
lincRNAs. Nat Commun. 6:63702015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Lu Y, Akbani R, Ju Z, Roebuck PL,
Liu W, Yang JY, Broom BM, Verhaak RG, Kane DW, et al: TCPA: A
resource for cancer functional proteomics data. Nat Methods.
10:1046–1047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weinstein JN, Collisson EA, Mills GB, Shaw
KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C and Stuart JM:
Cancer Genome Atlas Research Network: The Cancer Genome Atlas
Pan-Cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang JH, Li JH, Jiang S, Zhou H and Qu LH:
ChIPBase: A database for decoding the transcriptional regulation of
long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic
Acids Res. 41D:D177–D187. 2013. View Article : Google Scholar
|
|
33
|
Zhou KR, Liu S, Sun WJ, Zheng LL, Zhou H,
Yang JH and Qu LH: ChIPBase v2.0: Decoding transcriptional
regulatory networks of non-coding RNAs and protein-coding genes
from ChIP-seq data. Nucleic Acids Res. 45D:D43–D50. 2017.
View Article : Google Scholar
|
|
34
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Forbes SA, Bindal N, Bamford S, Cole C,
Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al:
COSMIC: Mining complete cancer genomes in the Catalogue of Somatic
Mutations in Cancer. Nucleic Acids Res. 39(Database): D945–D950.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arnold A and Papanikolaou A: Cyclin D1 in
breast cancer pathogenesis. J Clin Oncol. 23:4215–4224. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eeckhoute J, Carroll JS, Geistlinger TR,
Torres-Arzayus MI and Brown M: A cell-type-specific transcriptional
network required for estrogen regulation of cyclin D1 and cell
cycle progression in breast cancer. Genes Dev. 20:2513–2526. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berghoff EG, Clark MF, Chen S, Cajigas I,
Leib DE and Kohtz JD: Evf2 (Dlx6as) lncRNA regulates ultraconserved
enhancer methylation and the differential transcriptional control
of adjacent genes. Development. 140:4407–4416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spurlock CF III, Tossberg JT, Guo Y,
Collier SP, Crooke PS III and Aune TM: Expression and functions of
long noncoding RNAs during human T helper cell differentiation. Nat
Commun. 6:69322015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo S, Lu JY, Liu L, Yin Y, Chen C, Han X,
Wu B, Xu R, Liu W, Yan P, et al: Divergent lncRNAs regulate gene
expression and lineage differentiation in pluripotent cells. Cell
Stem Cell. 18:637–652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiang JF, Yin QF, Chen T, Zhang Y, Zhang
XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, et al: Human colorectal
cancer-specific CCAT1-L lncRNA regulates long-range chromatin
interactions at the MYC locus. Cell Res. 24:513–531. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu CY, Jiang ZN, Zhou Y, Li JJ and Huang
LM: Estrogen receptor α roles in breast cancer chemoresistance.
Asian Pac J Cancer Prev. 14:4049–4052. 2013. View Article : Google Scholar : PubMed/NCBI
|